TO THE EDITOR
Activation of c-MET oncogene can be the result of amplification or activating mutations. In this letter we would like to share our experience of treating a patient with metastatic lung adenocarcinoma, whose tumor was found to harbor a MET mutation occurring at a splice site within the juxtamembrane domain.
A 71 year old Caucasian man with a 15 pack-year smoking history presented to our clinic with a biopsy-proven left lung adenocarcinoma with a 6.4 × 5 cm left pleural-based mass invading the fourth rib, left hilar lymphadenopathy and numerous bilateral sub-centimeter pulmonary nodules. He received palliative thoracic radiation to a total dose of 3000 cGy, followed by 2 cycles of chemotherapy with carboplatin AUC 5 and pemetrexed 500 mg/m2 intravenously every 3 weeks. Follow-up CT scan of the chest and abdomen revealed improvement in the previously irradiated left lung mass, mediastinal and hilar adenopathy, but new and enlarging pulmonary nodules, bilateral supraclavicular adenopathy and new sclerotic bone lesions.
Targeted next-generation sequencing of 42 cancer-related genes (Comprehensive Cancer Gene Set version 2 assay, GPS@WUSTL, St. Louis, MO) was performed on DNA derived from the formalin-fixed paraffin-embedded tumor biopsy specimen.1 A MET single nucleotide variant (SNV) was identified, chr7:g.116412043G>C, involving the terminal nucleotide of exon 14 (Figure 1). This variant could result in a p.D1028H missense mutation (NM_001127500:c.3082G>C), but is also predicted by in-silico modeling to affect the splice donor site (Figure 2). Fluorescence in-situ hybridization (FISH) analysis was performed using commercial probes (Abbott Molecular, Des Plaines, IL) for MET/CEP7 (7q31.2/7p11.1-q11.1). Polysomy of chromosome 7 was noted (CEP7 and MET average copy numbers 2.31 and 2.23, respectively), although the MET/CEP7 ratio of 0.96 was negative for MET amplification. He was started on crizotinib 250 mg orally twice daily. Restaging CT scans after 6 weeks of therapy revealed decrease in size of the pulmonary lesions with continued response at 6 months (Figure 3).
Figure 1.
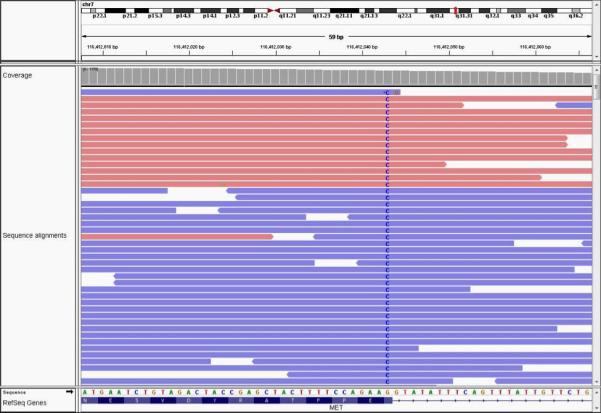
MET chr7:g.116412043G>C alteration as viewed in the Integrative Genomics Viewer (Broad Institute, Cambridge, MA). The guanine to cytosine alteration is highlighted at the terminus of MET exon 14.
Figure 2.
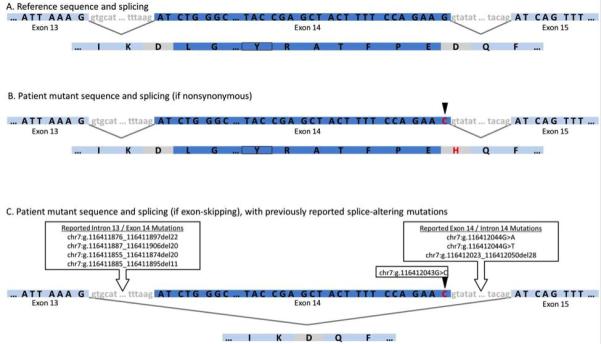
MET alteration and predicted outcomes. Each panel shows MET exon 14 and flanking DNA sequence with splicing events indicated by black lines followed by the resulting protein sequence. A) Normal MET sequence and splicing. The c-Cbl binding site (Y1021) is marked with a box. B) Effect of chr7:g.116412043G>C alteration (red, arrow) if splicing is unimpaired resulting in a p.D1028H substitution without deletion of exon 14. C) Alternately, as predicted by splice site algorithms, the variant could result in altered splicing with deletion of exon 14, as has been described for other MET splice site variants in lung cancer.
Figure 3.
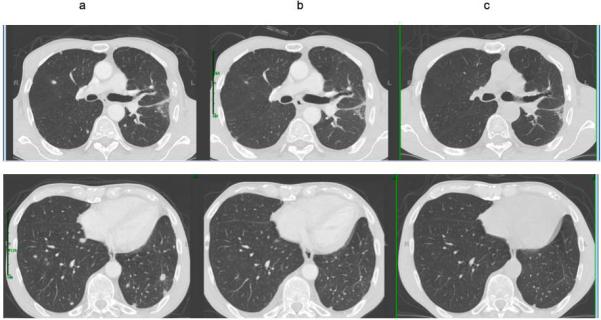
Radiographic response to crizotinib. CT scans (a) at baseline (and (b) after 6 weeks and (c) 6 months of crizotinib.
The MET receptor tyrosine kinase is a known oncogene, with a somatic mutation frequency of 8.3% in lung adenocarcinoma and 2% in lung squamous cell carcinoma, based on sequencing data from The Cancer Genome Atlas (TCGA).2 MET mutations are seen typically in smokers, and may co-occur with TP53 mutations.3 Unlike activating EGFR mutations that occur primarily in the tyrosine kinase domain, MET mutations are distributed across all domains of the gene. While mutations in the semaphorin domain may affect ligand-binding affinity, juxtamembrane domain mutations impact CBL mediated ubiquitination and MET receptor degradation. Somatic splice site mutations involving MET exon 14, resulting in deletion of the juxtamembrane domain, have been described in lung cancer.4,5 The juxtamembrane domain contains a binding site for CBL, which is required for ubiquitin-mediated MET degradation. The variant described here could result in over-activation of MET via skipping of exon 14 with loss of the CBL/ubiquitin-mediated degradation (Figure 2). To the best of our knowledge, this is the first report demonstrating successful targeting of this MET tyrosine kinase variant by crizotinib.
ACKNOWLEDGEMENT
This work was made possible by Grant Number 1K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH) and Grant Number UL1 TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCI, NCATS or NIH.
REFERENCES
- 1.Sehn JK, Hagemann IS, Pfeifer JD, Cottrell CE, Lockwood CM. Diagnostic utility of targeted next-generation sequencing in problematic cases. The American journal of surgical pathology. 2014;38:534–41. doi: 10.1097/PAS.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 2.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnaswamy S, Kanteti R, Duke-Cohan JS, et al. Ethnic differences and functional analysis of MET mutations in lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5714–23. doi: 10.1158/1078-0432.CCR-09-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer research. 2006;66:283–9. doi: 10.1158/0008-5472.CAN-05-2749. [DOI] [PubMed] [Google Scholar]
- 5.Asaoka Y, Tada M, Ikenoue T, et al. Gastric cancer cell line Hs746T harbors a splice site mutation of c-Met causing juxtamembrane domain deletion. Biochemical and biophysical research communications. 2010;394:1042–6. doi: 10.1016/j.bbrc.2010.03.120. [DOI] [PubMed] [Google Scholar]


