We thank Richard F. Spaide1 for his careful reading of our article,2 and we welcome this opportunity to address some of his concerns. Given the complexity of the relevant concepts, we have chosen to present a summary of our findings followed by a list of Spaide's relevant criticisms (in italic) along with our rebuttals. In the interest of brevity, we have chosen not to respond to criticisms that do not bear on the scientific impact of our article (e.g., the availability of raw, linear data in the Spectralis [Heidelberg Engineering, Heidelberg, Germany] software).
In cross-sectional optical coherence tomography (OCT) images (B-scans) of the retina, several bands are seen in the outer retina. Our article sought to investigate one of these bands—“band 2”—which originates from a structure confined to photoreceptor cells. We aimed to quantitatively characterize this structure by resolving it within single cones and measuring its average thickness and its position with respect to the adjacent bands 1 and 3. We hypothesized that the outer retinal band 2 would be thinner than the inner segment ellipsoid (16–20 μm) and that it would lie near the inner and outer segment (IS/OS) junction, approximately half way between the external limiting membrane (ELM) and cone outer segment tips (COST).
We imaged four healthy eyes at two retinal locations (foveal and parafoveal) using adaptive optics (AO)-OCT, which permits three-dimensional resolution of cone photoreceptors.3 From the resulting eight volumetric images, we segmented 9593 cones and measured the two parameters relevant to our hypotheses. We segmented each cone in three dimensions, using its lateral extent in en face projection and the locations of bands 1 and 3. Bands 1 and 3 were identified by identifying the A-lines comprising each cone and averaging them together to form the cone's longitudinal reflectance profile (LRP). By quantifying cellular morphology on each of the 9593 cones, we found that band 2 has a thickness of 4.7 μm, corresponding to an object thickness of 3.5 μm or less, and that it lies approximately equidistant from the ELM and COST. These findings are consistent with the interpretation that band 2 arises from the junction between IS and OS, but inconsistent with the recent hypothesis4 that the band arises from the IS ellipsoid.
Spaide's letter1 raised seven significant criticisms of our methods and our interpretation of results. These are listed below, in order of perceived relative importance, along with our responses.
Criticism 1: The mismatch between images of IS/OS and COST suggests that the cones were not segmented correctly.
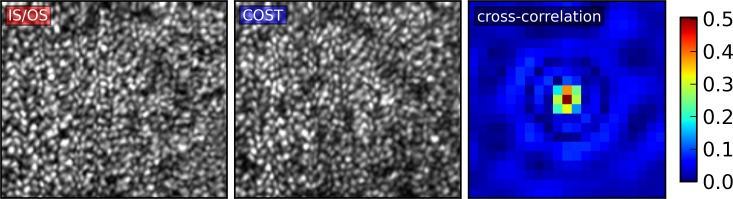
Our response: To measure cellular morphology, bands 1, 2, and 3 must be readily identifiable in the cone's LRP. Spaide points out that the IS/OS and COST images in our figure 2 are not identical, and that these differences manifest in the transverse locations of peaks (his fig. 1) and mismatch between their en face projections (his fig. 2). Moreover, he points out that the ELM is not visible in our figure 5. He reasons that if we cannot identify structures in the images, we cannot provide accurate morphological measurements.
Figure.
Projections of IS/OS and COST and their cross-correlation. Visible correspondence is apparent between projections of the IS/OS (left) and COST (center). Quantitatively, this correspondence manifests in the peak of the cross-correlation between IS/OS and COST, the central 21 × 21-pixel region of which is shown here (right). The peak value of 0.504 is equal to the Pearson correlation between the two images, and corresponds to a negligible P value. Fourier transformation of the projections had peaks at the expected spatial frequencies of the cones, derived from histological measurements.26 The high correlation and similar power spectra suggest high correspondence between the structures responsible for the images, contrary to Spaide's claim.
First and most importantly, the AO-OCT B-scan to which he refers was a conceptual illustration, not data fit for further analysis. It is a single, unaveraged, unprocessed B-scan. It represents a transverse cut through the cones, but not necessarily through their centers, as they form a somewhat irregular mosaic that is not aligned to the imaging source's scanned path across the retina. In short, each cone in the B-scan is represented by between one and five A-lines, significantly smaller than the 22 A-line volumetric image of each cone we used to compute an LRP. Worse, the image has lost four bits of dynamic range for publication. In the article, we described figure 5 as providing context for our results, and specifically not necessary for the scientific conclusions of the article, and that is because qualitative observations based on a single B-scan are of limited usefulness.
Spaide does not describe precisely how he generated his figures 1 and 2. Given the diversity of methods for generating such images, we would prefer to deal with this question quantitatively. The Pearson correlation of the two original images in our figure 2 is 0.504 (see cross-correlation in attached Figure). Considering that this coefficient is computed over 19,370 pixels, the resulting P value is less than 10−6, which is consistent with the conclusion that the images originate from corresponding features. Due to bit-depth compression and smoothing, the publication images are even more highly correlated.
Spaide is right that the images of IS/OS and COST are not identical, and this observation is confirmed by the fact that the Pearson correlation is less than 1.0. There are (at least) three possible reasons for the mismatch. First, it is known that the cone IS and OS diameter differ throughout most of the retina. There is now evidence that these differences affect the transverse waveguide modes supported by these respective segments, which can cause the IS/OS and COST reflections to differ, slightly in the fovea and dramatically in the periphery (Liu Z, Kocaoglu OP, Turner TL, Miller DT, manuscript submitted, 2015).5 Second, because our main goal was characterization of band 2, focus of the system was optimized for that band, which may have contributed to differences in their appearance as well. Third, like all images, these have some random, uncorrelated noise.
For any given cone, these factors may have caused differences in the lateral distribution of light at each layer, which explains the imperfect correspondence shown in his figure 2. The high statistical correspondence between the images (P < 10 − 6) suggests that although the noise and transverse modes may not be aligned, the bulk of the features in the images are. The low visibility of ELM in the B-scan is due, as Spaide recognized, to the linear scaling of the image, limited dynamic range of print images, and absence of any lateral integration of signal. After averaging a sufficient number of A-lines from each cone, the ELM was always identifiable in our experiment, in spite of its dark appearance in single B-scans. The ELM is comparably dark in Spaide's own linearly scaled Spectralis B-scans,4 even with the latter instrument's greater optical blur, averaging, and postprocessing. Nevertheless, we are confident that his use of it as a morphological landmark was also justified.
Criticism 2: Speckle and waveguiding made image analysis difficult.
Our response: Spaide argues that averaging produces a smooth topography that “more accurately represents” the structure of interest. He is correct that averaging adjacent B-scans can reduce speckle contrast and increase signal, but this is true only if the extent of averaging is smaller than the structure of interest and larger than the speckle. This principle guided the design of our morphological analysis, in which we averaged all the A-lines originating from each cone (i.e., the structure of interest). We specifically avoided averaging together A-lines from different cones because the axial displacement of IS/OS would cause band 2 to broaden in the multicone profile.
Spaide argues that the apparent axial displacement of IS/OS among cones and the resulting roughness of the IS/OS topography are partly due to speckle artifacts. Although he provides an interesting summary of some of the properties of speckle, he provides no rationale for believing that the topographical roughness of the IS/OS projection is due to speckle. This roughness is readily apparent in many light and electron micrographs of the retina, including those in the correlative studies he cited.6,7 Spaide says that we have confused speckle for structure. We disagree. We believe the IS/OS and COST reflections do not suffer from typical OCT speckle. Because they are confined to optical waveguides, the physical process by which any speckles are generated is simplified, and they are more akin to specular reflections than speckle.
Because the roughness of band 2 (see fig. 4 in our article2) is due to axial displacements of neighboring cones, it has a spatial scale equal to the fundamental frequency of the cones. The AO-OCT imaging spot is smaller than the cones, so at any given time it is sampling a smooth surface at the depth of band 2, possibly the distal IS membrane. In contrast, the Spectralis imaging spot is larger, causing many cones to be sampled at once. This collective reflection is rough because of the axial displacements of band 2. The former situation, measurement of an optically flat surface with a coherent source, does not generate speckle, whereas the latter situation, simultaneous measurement of axially and laterally displaced cones, is the classic arrangement for speckle generation. Thus, for imaging band 2, AO-OCT is less affected by speckle than the Spectralis (or any OCT system with lateral resolution larger than the cones).
We agree that the cones consist of two waveguides stacked on top of each other, but we don't agree with Spaide that localization of reflections is more difficult in waveguides than it is elsewhere. Indeed, given the potential role of mode mismatch and the simplification of speckle in waveguides, it is probably easier to localize reflections within waveguides. Moreover, such complexities would affect any attempt, whether by commercial OCT or AO-OCT, to localize reflections within cones, and more severely if the photoreceptors cannot be individually resolved.
Criticism 3: High specificity in our cone identification procedure caused a selection bias.
Our response: In our study, we analyzed 80% to 90% of the cones in the imaged patches, nearly 10,000 cones in all. It is theoretically possible that excluding 20% of the cones could introduce a bias. However, to explain the discrepancy between our thickness measurement (4.7 μm) and Spaide's own measurements (16–20 μm), the excluded cones would have to have an average band 2 thickness of nearly 100 μm, which exceeds the length of the cell. As such, we do not believe selection bias could possibly explain our results. Spaide's measurements4 were taken from 10 Spectralis A-scans, probably integrating over fewer than 40 cones in total, but even in this small number of cones we do not believe that selection bias was the key factor in the discrepancy.
Criticism 4: In previous studies6,7 structures identified as IS/OS in histology were nonreflective in OCT images of the same tissue. As such, we must perform correlative OCT/histology ourselves, and “show how the two previous studies were somehow defective.”
Our response: We hesitate to call those studies “defective,’’ because they advanced the field of OCT-based anatomical work. Correlation of retinal layers requires precise alignment of the micrograph and OCT B-scan, which presents a number of significant technical challenges. First, the physical axial sampling frequency of the OCT must be precisely determined. In spectral-domain OCT this requires calibration of the spectrometer, as described in our article.2 Analogous methods exist for the time-domain OCT systems used for the cited correlative studies. Second, because OCT measures optical path length (the product of physical length and refractive index), investigators must transform the OCT image accordingly; no small feat, given the variation in reported refractive indices of the relevant retinal layers,4 as well as potential variability among human subjects. Third, histological preparation is widely reported to result in deformations of tissue. Worse, the composition of retinal tissue, which varies with depth, may affect the refractive index and the amount and type of deformation,7 affecting both absolute and relative layer thicknesses. Correlative histology has often relied on “unequivocal landmarks” to compare images,7 but a small, finite number of such landmarks would likely underdetermine the nonlinear, discontinuous transformation necessary to align the images. In short, we believe the alignment of images in those studies permitted accurate correlation of some retinal features, but was not sufficiently precise for the purposes of the present investigation. Indeed, the authors of those studies adopted the IS/OS nomenclature for the second band,8 as did other authors who had done correlative histology.9,10 We do not believe these technical challenges to be insurmountable. As we stated in the Conclusions of our article, we concur that further correlative studies may be useful in settling the question of the band's origin. We disagree with Spaide, however, that this question, or any scientific question for that matter, will only yield to one approach.
Criticism 5: Rods play a role in the OCT image, but were not considered in our study.
Our response: Spaide is correct that rods contribute to the OCT image. This has been shown using conventional OCT11 and AO-OCT.12,13 The structures responsible for bright reflections in the cones appear to be axially displaced in rods, as evidenced by confocal micrographs of monkey retina (from Nicolas Cuenca14,15), stained light micrographs of human retina,16 and shifts in focus required for AO imaging of the rod mosaic.17 Moreover, it has been shown that the OS tips of cones and rods lie at different depths in the outer retina, and that cone and rod OS tips are not visible in the same en face slices of AO-OCT volumes.12,13 The axial displacement between rod and cone reflections may be another factor contributing to broadening of the IS/OS and COST bands in commercial OCT systems. We hesitated to raise this issue, as a complete picture of these displacements is not yet in place. Our article sought to determine morphological characteristics of cones, so we excluded rods intentionally.
However, it is possible, as Spaide suggests, that we mistakenly included A-lines originating from rods in the LRPs of cones. We took careful measures to avoid this, as it would artificially broaden the band 2 reflection and introduce a bias in our results. If, however, we inadvertently included rods, band 2 in cones may be even thinner than our estimate.
Criticism 6: There is no sharp transition in refractive index between IS/OS and COST.
Our response: In his article,4 Spaide argued that the refractive index mismatch between IS and OS is not sufficient to produce a bright band in the OCT image. In our article, we explained how the sensitivity of OCT is indeed high enough to detect such a mismatch. In his letter, he casts doubt on our argument by saying that if it were true, the 43 dB of the Spectralis would be insufficient for detecting an IS/OS reflection, which requires 57 dB. This point requires some clarification. The dynamic range of the Spectralis image is 43 dB, and 57 dB is the sensitivity required to see the IS/OS junction; both were clearly described in our article. Dynamic range and sensitivity are both typically reported in dB, but they are distinct parameters. A complete explanation is beyond the scope of this response, but can be found in many articles in the OCT literature.
In his letter, Spaide argues that there is no sharp transition between IS and OS, but the electron micrograph we reproduced in our article, which is representative of what we found in the literature, shows a sharp transition, in spite of the complex arrangement of new discs at the basal OS. The fact that the myoid-ellipsoid index mismatch is greater than that of the ellipsoid-OS might cause us to expect the former boundary to generate a brighter reflection than the latter. That it doesn't, in our view, suggests three possibilities. First, it may be that the small gap between IS and OS, often observed in electron micrographs of the retina,2 plays a pivotal role in generating the OCT image; that is, the lower index extracellular matrix increases the reflectance of the boundary. Second, it may be that the myoid-ellipsoid boundary is rough, scattering more photons than IS/OS, but with more multiple scattering; multiply-scattered light does not contribute effectively to the OCT signal. Third, it may be, as we speculated in our article, that mode mismatch between IS and OS, which has significantly different diameters, causes a reflection brighter than that predicted by the index mismatch.
Criticism 7: The COSTs are different from Verhoeff's membrane.
Our response: Spaide is correct that the University of California Davis OCT group has referred to the COSTs as Verhoeff's membrane. He has referred to this misnomer in this letter and several other articles,4,18 which might make us seem unqualified to study the origins of the OCT image. This was a misnomer that appeared in one of the first demonstrations of the AO-OCT method,19 an article that was not meant to present any anatomical findings, but rather to showcase a powerful tool we had developed. Although the misnomer persisted through several of our publications, we have continually referred to that nomenclature as “tentative,” and described “Verhoeff's membrane” as an alternative term for COST. We described it as equivalent to COST,20 “tentative” and equivalent to COST,21 “tentatively labeled” and “due to the interface between the cone photoreceptors and the RPE,”22,23 and synonymous with COST.24
A critical difference between those erroneous applications of the term and the present study, however, is that the identity of COST was not a critical aspect of any of those studies. Moreover, as the first to have observed numerous retinal structures in the living human eye, we would be wise to expect an occasional preliminary misattribution. Indeed, Spaide himself referred to band 3 as Verhoeff's membrane,25 following us, until further research revealed this to be a misnomer. In short, we feel that although his allegation is true, it should not color the reader's opinion of the current article.
Finally, we concluded our article by saying that reattribution of the IS/OS layer might require a number of previous studies to be reevaluated. We were explaining the stakes of the debate, not providing confirmation of our hypothesis, as Spaide suggests. Although we have tried here to address his concerns, the best way to fully understand the scope and implications of our study is to read it.
Acknowledgments
Supported by grants from the National Institutes of Health (R01 EY 024239, R01 AG 004058, P30 EY 012576, R01 EY 018339).
Disclosure: R.S. Jonnal, P; O.P. Kocaoglu, None; R.J. Zawadzki, None; S.-H. Lee, None; J.S. Werner, None; D.T. Miller, P
References
- 1.Spaide RF.Outer retinal bands. Invest Ophthalmol Vis Sci. 2015; 56: 2505–2506. [DOI] [PubMed] [Google Scholar]
- 2.Jonnal RS,, Kocaoglu OP,, Zawadzki RJ,, Lee S-H,, Werner JS,, Miller DT.The cellular origins of the outer retinal bands in optical coherence tomography images. Invest Ophthalmol Vis Sci. 2014; 55: 7904–7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y,, Cense B,, Rha J,, et al. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. Opt Express. 2006; 14: 4380–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spaide RF,, Curcio CA.Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011; 31: 1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z,, Kocaoglu OP,, Miller DT.In-the-plane design of an off-axis ophthalmic adaptive optics system using toroidal mirrors. Biomed Opt Express. 2013; 4: 3007–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gloesmann M,, Hermann B,, Schubert C,, Sattmann H,, Ahnelt PK,, Drexler W.Histologic correlation of pig retina radial stratification with ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2003; 44: 1696–1703. [DOI] [PubMed] [Google Scholar]
- 7.Anger EM,, Unterhuber A,, Hermann B,, et al. Ultrahigh resolution optical coherence tomography of the monkey fovea. identification of retinal sublayers by correlation with semithin histology sections. Exp Eye Res. 2004; 78: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 8.Ko TH,, Fujimoto JG,, Duker JS,, et al. Comparison of ultrahigh-and standard-resolution optical coherence tomography for imaging macular hole pathology and repair. Ophthalmology. 2004; 111: 2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y,, Cideciyan AV,, Papastergiou GI,, et al. Relation of optical coherence tomography to microanatomy in normal and rd chickens. Invest Ophthalmol Vis Sci. 1998; 39: 2405–2416. [PubMed] [Google Scholar]
- 10.Cideciyan AV,, Jacobson SG,, Aleman TS,, et al. In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa. Proc Natl Acad Sci U S A. 2005; 102: 5233–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan VJ,, Monson BK,, Wojtkowski M,, et al. Characterization of outer retinal morphology with high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2008; 49: 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S-H,, Werner JS,, Zawadzki RJ.Improved visualization of outer retinal morphology with aberration cancelling reflective optical design for adaptive optics-optical coherence tomography. Biomed Opt Express. 2013; 4: 2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felberer F,, Kroisamer J-S,, Baumann B,, et al. Adaptive optics SLO/OCT for 3D imaging of human photoreceptors in vivo. Biomed Opt Express. 2014; 5: 439–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb H.Webvision: Cone Pathways Through the Retina. Available at: http://webvision.med.utah.edu/book/part-iii-retinal-circuits/cone-pathways-through-the-retina/ Accessed February 23, 2015. [PubMed]
- 15.Cuenca N.Retinal Microscopy. Available at: http://www.retinalmicroscopy.com/ Accessed February 23, 2015.
- 16.Ahnelt PK,, Kolb H,, Pflug R.Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. J Comp Neurol. 1987; 255: 18–34. [DOI] [PubMed] [Google Scholar]
- 17.Dubra A,, Sulai Y,, Norris J,, et al. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011; 2: 1864–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staurenghi G,, Sadda S,, Chakravarthy U,, Spaide RF.Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN-OCT consensus. Ophthalmology. 2014; 121: 1572–1578. [DOI] [PubMed] [Google Scholar]
- 19.Zawadzki R,, Jones S,, Olivier S,, et al. Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in vivo imaging. Opt Express. 2005; 13: 8532–8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pircher M,, Zawadzki R,, Evans J,, Werner J,, Hitzenberger C.Simultaneous imaging of human cone mosaic with adaptive optics enhanced scanning laser ophthalmoscopy and high-speed transversal scanning optical coherence tomography. Opt Lett. 2008; 33: 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SS,, Zawadzki RJ,, Greiner MA,, Werner JS,, Keltner JL.Fourier-domain optical coherence tomography and adaptive optics reveal nerve fiber layer loss and photoreceptor changes in a patient with optic nerve drusen. J Neuroophthalmol. 2008; 28: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerth C,, Zawadzki RJ,, Héon E,, Werner JS.High-resolution retinal imaging in young children using a handheld scanner and Fourier-domain optical coherence tomography. J AAPOS. 2009; 13: 72–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerth C,, Zawadzki RJ,, Werner JS,, Héon E.Detailed analysis of retinal function and morphology in a patient with autosomal recessive bestrophinopathy (ARB). Doc Ophthalmol. 2009; 118: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner J,, Keltner J,, Zawadzki R,, Choi S.Outer retinal abnormalities associated with inner retinal pathology in nonglaucomatous and glaucomatous optic neuropathies. Eye. 2011; 25: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Switzer DW, Jr,, Mendonça LS,, Saito M,, Zweifel SA,, Spaide RF.Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina. 2012; 32: 1265–1271. [DOI] [PubMed] [Google Scholar]
- 26.Curcio C,, Sloan K,, Kalina R,, Hendrickson A.Human photoreceptor topography. J Comp Neurol. 1990; 292: 497–523. [DOI] [PubMed] [Google Scholar]