Abstract
Purpose.
Purified Clostridium botulinum exoenzyme C3 transferase (C3) effects on the actin cytoskeleton in human trabecular meshwork cells (HTM) and on the outflow facility response in monkey organ-cultured anterior segments (MOCAS) were determined in the presence or absence of viral vectors.
Methods.
Human adenovirus type 5 (AdV) and feline immunodeficiency virus (FIV) vectors were produced using kits. Cell soluble purified C3 (C3cs) was purchased commercially. Recombinant C3 (C3rec) cDNA was overexpressed in Escherichia coli and purified. The HTM cells were incubated with up to 10 μg/mL C3cs or with 5 μg of C3rec and/or viral vector (multiplicity of infection [MOI] = 25). Cells then were fixed and stained for actin. Outflow facility in MOCAS was measured at baseline, 4 hours, 24 hours, and 3 to 4 days following bolus injection of AdV (1.6 × 107 transducing units) and/or 2.5 μg C3rec.
Results.
The HTM cells treated for 4 hours with C3cs (all doses) or for 24 hours with C3rec developed a rounded morphology and lost stress fibers. Cells transduced with vectors alone showed no changes at any time point. Cells exposed to C3rec and cotransduced with either viral vector showed significant disruption of the actin cytoskeleton within 4 hours after exposure, which persisted at 24 hours. In MOCAS, the AdV vector alone had no effect on outflow facility, but enhanced the response to C3rec at 4 hours.
Conclusions.
Coadministration of viral vectors enhances the ability of C3 transferase to disrupt actin stress fiber formation in HTM cells and increase outflow facility in MOCAS. Viral vectors potentially could be used to increase the bioavailability of proteins for cells that are difficult to transfect.
Keywords: viral vector, C3 transferase, trabecular meshwork, actin, outflow facility
Cotreatment of HTM cells with viral vectors and purified exoenzyme C3 transferase protein increased the intracellular actin disrupting activity of the protein and the in situ outflow facility response, most likely by enhancing cellular uptake of the protein into cells.
In the human eye, the main pathway of aqueous humor outflow from the anterior chamber involves filtering through intercellular spaces in the trabecular meshwork before reaching Schlemm's canal, which drains into the aqueous veins.1 Increased resistance to outflow through this pathway results in an increase in IOP, often leading to development of open-angle glaucoma. Cellular relaxation and other architectural modifications to the trabecular meshwork that reduce its stiffness and increase intercellular spaces have the potential to facilitate outflow and alleviate chronically elevated IOP.2 Rho signaling pathways regulate the assembly and contractility of the actomyosin network, and Rho guanosine triphosphatases (GTPases) specifically regulate actin dynamics, actomyosin contractility, and cellular adhesions.3 The Clostridium botulinum exoenzyme C3 transferase (C3) inactivates the Rho GTPases RhoA, RhoB, and RhoC by adenosine diphosphate (ADP)–ribosylation, resulting in disruption of actin stress fibers, cellular relaxation, and loss of focal adhesions.4
We previously showed the ability of adenoviral (AdV)–mediated delivery of C3 to the trabecular meshwork by intracameral injection to increase outflow facility in monkey organ cultured anterior segments (MOCAS).5 Transduction of differentiated human trabecular meshwork (HTM) cell monolayers with AdV.C3 resulted in a loss of stress fibers as observed by actin fluorescence. By phase contrast imaging, cells treated with AdV.C3 appeared to be partially retracted or rounded up compared to cells treated with media or control AdV.5
Interestingly, it has been shown previously that purified C3 protein is taken up by cells, resulting in a physiological effect.6,7 The uptake mechanism for C3 toxin is thought to be by a specific endocytic mechanism involving acidified endosomal vesicles.8 The fact that purified C3 is taken up by cells afforded us the opportunity to separate the effect of C3 expression in TM cells from the potential cytoskeletal altering activities of viral vectors.
Both AdV and lentiviral infection involve temporary rearrangement of the actin cytoskeleton.9 The AdV receptor (CAR)10 or CD4611 serve as the major primary human AdV (HAdV) receptors on most cell types. Subsequent AdV internalization via clathrin-mediated endocytosis is facilitated by secondary interactions of the penton base with αv integrins.12 This event is accompanied by partial disassembly of the virus particle in which the vertex proteins (i.e., penton base, pIIIa, fiber, peripentonal hexons) are released in the acidified environment of the early or late endosome. The precise molecular events involved in HAdV disassembly are not very well defined, but it is thought that the release of the internal capsid protein pVI at this stage facilitates endosomalysis13 allowing entry of the uncoated particle into the cytoplasm. Entry of VSV-G envelope pseudotyped lentiviral vectors activates the PI3 kinase pathway, which also results in alterations in the actin cytoskeleton.14 Thus, the viral vectors themselves could be having a role in the actin disrupting activity of C3 transferase.
The current studies sought to determine whether or not coadministration of viral vectors with recombinant proteins could alter the delivery and cell uptake of potential therapeutic proteins in the eye. We determined in primary differentiated HTM cells if cotransduction of recombinant C3 (C3rec) with AdV or lentivirus vectors enhanced the effect of C3rec on the actin cytoskeleton. Two different vectors were used to determine if the effects were vector-specific. In MOCAS, we determined whether coadministration of C3rec and AdV altered outflow facility.
Methods
Purified C. botulinumExoenzyme C3 Transferase
Commercially available cell soluble C3 transferase (C3cs, cat#CT04; Cytoskeleton, Inc., Denver, CO, USA) consists of highly purified C3 transferase covalently linked to a proprietary cell-penetrating peptide (CPP) via a disulfide bond. The cell-penetrating moiety allows rapid and efficient uptake into the cell. Once in the cytosol, the cell-penetrating moiety is released, thereby allowing C3 transferase to freely diffuse intracellularly and inactivate RhoA, RhoB, and RhoC, but not related GTPases, such as Cdc42 or Rac1. For all experiments, a stock solution of 0.1 mg/mL C3cs in 50% glycerol/sterile water was made and stored at −20°C. Working solutions were made up in serum-free media.
A recombinant C. botulinum exoenzyme C3 transferase lacking a CPP (C3rec) also was used. The C3rec was expressed as a glutathionine-S-transferase (GST) fusion protein using the pGEX-2t plasmid15 and was generously provided by Alan Rapraeger, PhD (University of Wisconsin). It was expressed in Escherichia coli and purified as previously described using a glutathione sepharose and elution by thrombin cleavage.
Vectors
An AdV type 2 vector expressing green fluorescent protein (GFP, AdV; Stratagene, La Jolla, CA, USA) was packaged as described previously5,16 and was purified using the Adenopure adenovirus purification kit (Puresyn, Inc., Malvern, PA, USA) according to manufacturer's instructions. The titers of the AdV stocks were approximately 1 × 1012 per mL.
A feline immunodeficiency virus (FIV) vector (System Biosciences, Mountain View, CA, USA) expressing GFP and pseudotyped with the VSV-G protein was packaged by cotransfecting the plasmids pCDF1-MSC1-EF1-copGFP (1.736 mg), PFIV-34N (326 μg), and pVSVG (408 μg) into HEK293TN cells in eight 500-cm2 culture plates (#431110; Corning, Corning, NY, USA). Culture supernatants were harvested 48 hours later and centrifuged at 24,000g for 30 minutes to pellet viral particles followed by pelleting through a 36% sucrose (PBS) cushion. Viral pellets were resuspended in Hank's Balanced Salt Solution (HBBS; Mediatech, Manassas, VA, USA). The titers of the FIV vector stocks were approximately 1 × 109 per mL.
Cell Culture
All studies were conducted in adherence to the tenets of the Declaration of Helsinki. Differentiated HTM primary cells were grown on coverslips in low-glucose Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich Corp., St. Louis, MO, USA) containing 15% fetal bovine serum (FBS), 2 mM L-glutamine (Sigma-Aldrich Corp.), 1% amphotericin B (Mediatech), 0.05% gentamicin (Mediatech), and 1 ng/mL FGF-2 (PeproTech, Rocky Hill, NJ, USA).17–20 Cell strains were established using tissue from a 27-year-old donor.17,19 The immortalized human TM-1 cell lines were established using tissue from a 30-year-old donor17–20 and were cultured in low-glucose DMEM (Sigma-Aldrich Corp.) containing 10% FBS, 2 mM L-glutamine (Sigma-Aldrich Corp.), 1% amphotericin B (Mediatech), and 0.05% gentamicin (Mediatech). Neither donor had a history of ocular diseases. Both HTM and TM-1 cells were treated with 0, 2, 5, or 10 μg/mL C3cs for 2 or 4 hours. Cells treated with vehicle (50% glycerol/water) were used as controls.
In other experiments, HTM cells on coverslips were treated with 5 μg (12.5 μg/mL) of C3rec and/or virus (AdV or FIV) at a multiplicity of infection (MOI) of 25. Cells were incubated at 37°C in 5% CO2, and fixed and stained for actin with phalloidin-Alexa-488 conjugated goat-anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) 4 and 24 hours after treatment. Nuclear staining was with Hoechst 33342 (Invitrogen).
Fluorescent Microscopy Studies
Cultured HTM cells and TM-1 were washed with 2-(N-morpholino)ethane sulfonic acid (MES), permeabilized with 0.5% Triton X-100 for 2 minutes, and then fixed with 4.0% paraformaldehyde for 20 minutes at room temperature. Cells were washed with 0.05% BSA in ×1 PBS and blocked with 1% BSA for 1 hour. Fixed cells were labeled with Alexa 488 phalloidin (1:300) and Hoescht 33342 nuclear dye for 40 minutes. Coverslips were mounted with Immumount and imaged with an epifluorescence microscope (Axioplan 2; Carl Zeiss, Inc., Thornwood, NY, USA). Image acquisition software (Axiovision 4.6; Carl Zeiss, Inc.) was used to image the tissue sections. To quantify the actin disrupting activity, photographs were taken of 3 random fields in each condition at 4 or 24 hours after treatment. The total number of cells was determined by counting the number of 4′6-diamidino-2-phenylindole (DAPI)–stained nuclei. Cells showing rounding or disruption of the cytoskeleton then were counted by a masked observer and the percentage of cells with actin disruption for each image were calculated.
Monkey Organ-Cultured Anterior Segments (MOCAS)
Studies were conduced in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eyes were obtained from 6 cynomolgus (Macaca fascicularis) and 16 rhesus (M. mulatta) monkeys that were euthanized for nonocular studies at the Wisconsin National Primate Research Center. The MOCAS were established according to the method of Hu et al.21 Following two days of equilibration at 2.5 μL/min, baseline outflow facility was measured by 2-level constant pressure perfusion.22
For C3cs studies, segments then were exchanged with 3 mL of DMEM containing 2.5 (n = 1) or 10 (n = 2) μg/mL C3cs. The contralateral segment was exchanged with vehicle (1.25–5% glycerol/DMEM). Infusion was stopped for 3 hours, then infusion was resumed and outflow facility measured at 3 to 4 (10 μg/mL) or 6 (2.5 μg/mL) hours. Infusion was continued with media, 1 or 2.5 μg/mL C3cs for 18 to 26 hours and outflow facility measured.
For C3rec ± AdV studies, preparations of C3rec were diluted with PBS to 2.5 μg/5 μL and aliquots were frozen until use. Following measurement of the baseline outflow facility on day 2 of equilibration, 2.5 μg of C3rec was injected as a bolus into contralateral segments of a pair of MOCAS. One segment of each pair also received 5 μL of AdV (1.6 × 107 TU). Infusion was stopped for 3 hours, then infusion with DMEM was resumed for 1 hour, and outflow facility was then measured. The infusion was continued and outflow facility measured again at 20 to 28 hours and at 3 to 4 days after injection. Additional MOCAS were treated with only AdV to one segment and no treatment to the other segment. In some cases, only one segment was usable after baseline. These were used as untreated controls with outflow facility measurements done according to the same schedule as injected segments.
At the conclusion of each experiment, anterior segments that had been treated with AdV with or without C3rec were fixed in 4% paraformaldehyde. The presence of GFP, indicating successful transduction, was verified in flat mounts by fluorescent microscopy. Tissues then were processed for hematoxylin and eosin (H&E) staining and examined by light microscopy.
Statistical Analysis
Outflow facility results were analyzed using the 2-tailed paired t-test for ratios compared to 1.0. The percentages of cells showing actin disruption were analyzed by ANOVA to determine if statistically significant differences were present.
Results
Differentiated HTM cells treated with C3cs developed a rounded morphology at all doses within 2 hours after exposure. When the cells were fixed and stained for actin at 4 hours after treatment, there was a loss of stress fibers in cells exposed to 2 μg/mL of C3cs, and the disruption increased in cells treated with 5 and 10 μg/mL. The degree of filament disruption appeared to be similar in cells exposed to both of the higher concentrations of C3cs (Fig. 1).
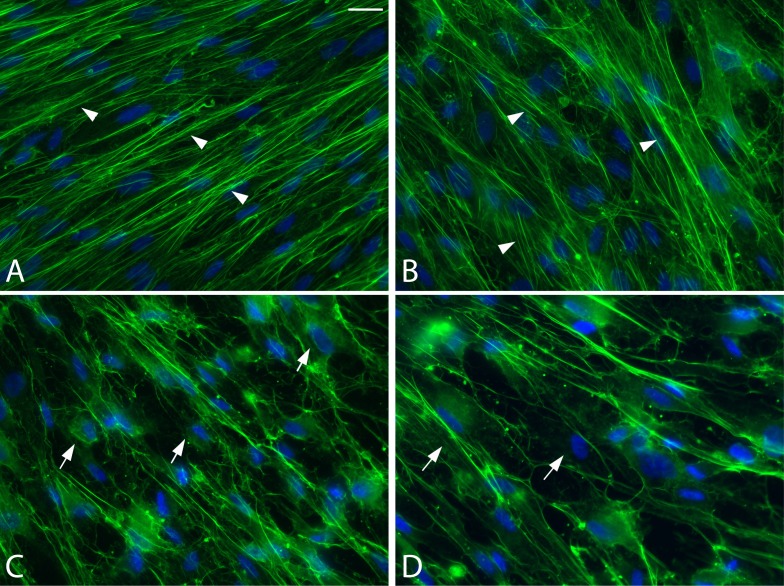
Figure 1.
Actin organization in confluent monolayers of differentiated primary HTM cells 4 hours after transfection with C3cs. Cells were treated for 4 hours with vehicle (A), or with 2 (B), 5 (C) or 10 (D) μg/mL C3cs. In the presence of the vehicle alone or 2 μg/mL C3cs, well-formed actin stress fibers (arrowheads) were observed throughout the culture. As the concentration of C3cs was increased, fewer actin stress fibers were observed in the cytoplasm (arrows). At the highest concentration (10 μg/mL), very few actin stress fibers were observed and mainly cortical actin filaments appeared intact, suggesting that the cells were less well spread. Cells were double-labeled with alexa-488 phalloidin and Hoescht 33342. Magnification: ×40.
Differentiated HTM cells incubated with 12.5 μg/mL C3rec alone did not display a disruption of actin stress fibers 4 hours after exposure (Fig. 2A), but there was significant stress fiber disruption and cell rounding 24 hours after treatment (Fig. 2B). Cells treated with AdV or FIV vectors alone showed no effect on actin organization at either 4 or 24 hours after treatment. In contrast, cells incubated with C3rec and treated with either viral vector showed disruption of the actin cytoskeleton within 4 hours after treatment, which persisted at 24 hours (Figs. 2A, 2B). However, quantitatively significant enhancement in actin cytoskeleton disruption compared to C3rec alone was detected only with AdV + C3rec (Table 1).
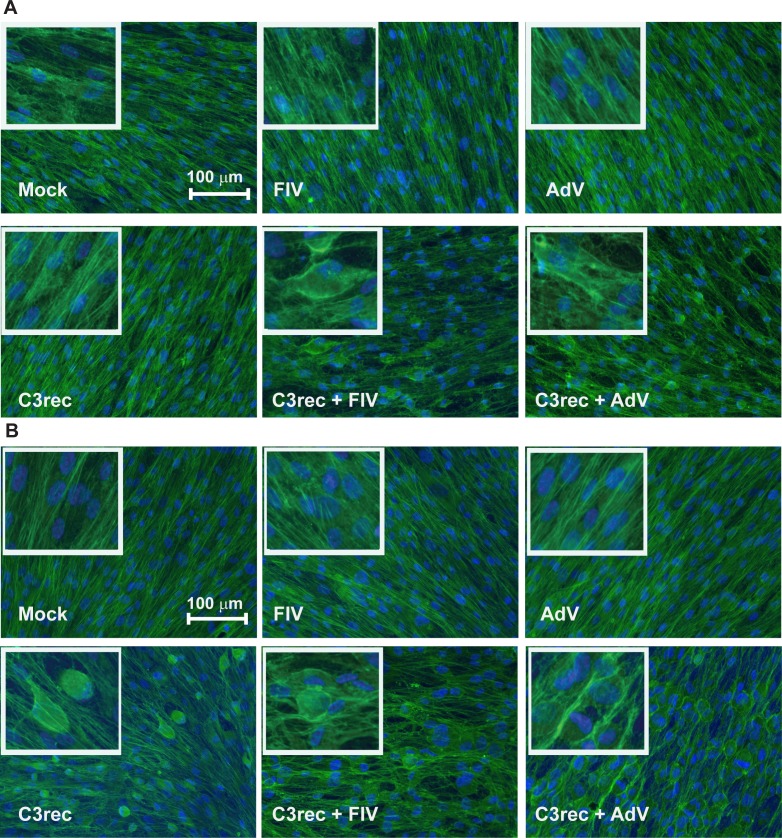
Figure 2.
Actin organization in confluent monolayers of differentiated primary HTM cells receiving C3rec with or without viral vectors. (A) At 4 hours after transfection, no change in actin organization was seen in cells receiving media, virus, or C3rec alone, but cells receiving C3rec and virus simultaneously showed significant actin reorganization and cell rounding. (B) At 24 hours after transfection, cells receiving media or virus alone still showed no change in actin organization, but cells receiving C3rec now showed significant actin reorganization and cell rounding. Cells receiving C3rec and virus simultaneously showed the most widespread actin reorganization and cell rounding. The percentage of cells in each condition exhibiting actin disruption is shown in Table 1.
Table 1.
Percentage of Cells With Actin Disruption
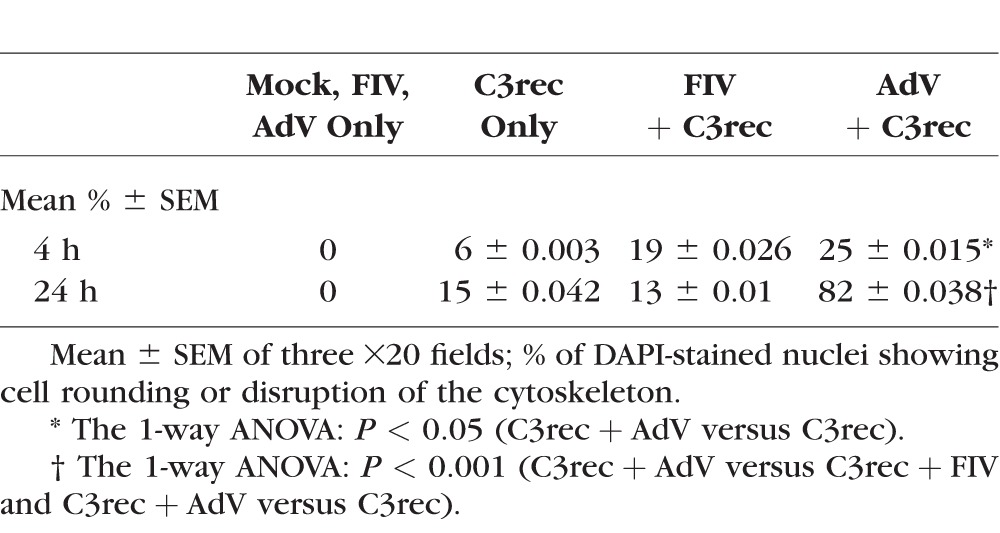
In TM-1 cells, no change in actin organization was seen at 4 hours after treatment with media, AdV, or C3rec alone. Cells receiving C3rec and AdV simultaneously showed actin aggregation near the nucleus (data not shown), but the cells remained adherent and no cell rounding was seen. At 24 hours after treatment, cells receiving media or AdV showed some loss of actin stress fibers and an increase of actin at the cell periphery, but no cell rounding. Cells receiving C3rec only showed significant actin aggregation near the nucleus and some cell rounding at 24 hours after exposure. Cells receiving C3rec and AdV simultaneously showed actin aggregation and widespread cell rounding. Thus, immortalized TM-1 cells behaved similarly to the HTM cells.
In MOCAS, C3cs produced a transient 38% increase in outflow facility at 3 to 4 hours after treatment with 10 μg/mL compared to vehicle-treated control in one pair. No outflow facility effect was apparent with the 2.5 μg/mL dose of C3cs. Since the response at 10 μg/mL was not large and not persistent and there was great expense to administer and maintain the concentration of C3cs in the anterior chamber, no additional studies were conducted with C3cs.
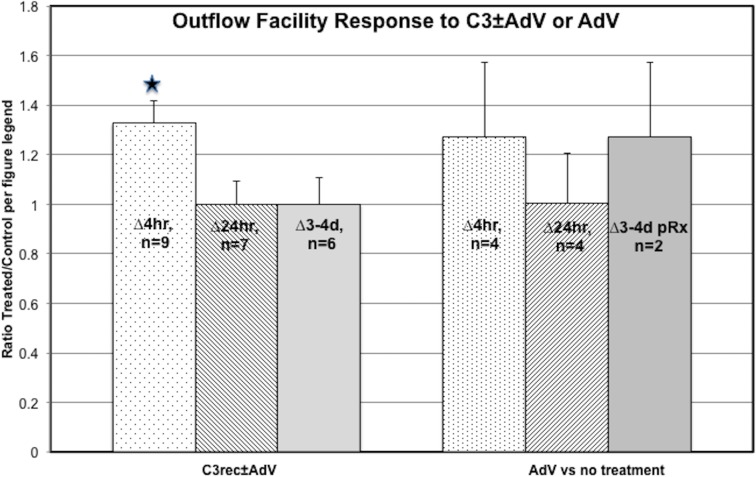
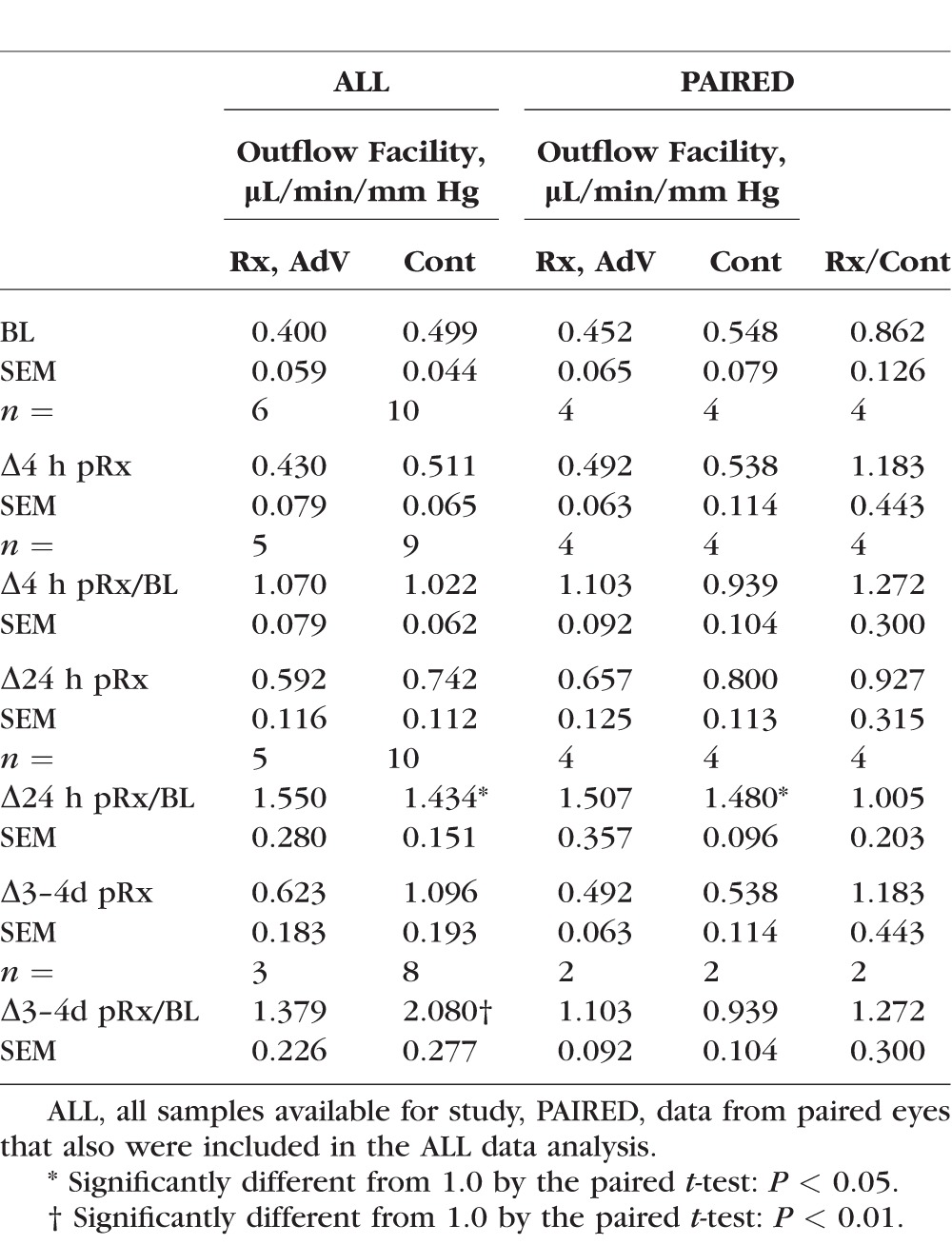
The MOCAS studies with C3rec ± AdV showed that the outflow facility response to a bolus injection of 2.5 μg C3rec was enhanced by AdV coadministration at 4 hours after treatment by 33% (P < 0.01, paired t-test) compared to C3rec alone after correction for baseline. There was no apparent sustained enhancement of outflow facility after C3rec ± AdV at any later time points (Table 2, Fig. 3). The C3rec alone did not significantly increase outflow facility at any time point compared to AdV alone or untreated controls. There was no difference in outflow facility at any time point produced by AdV alone compared to untreated controls (Table 3, Fig. 3).
Table 2.
Outflow Facility Response to C3rec With or Without Coadministration of AdV
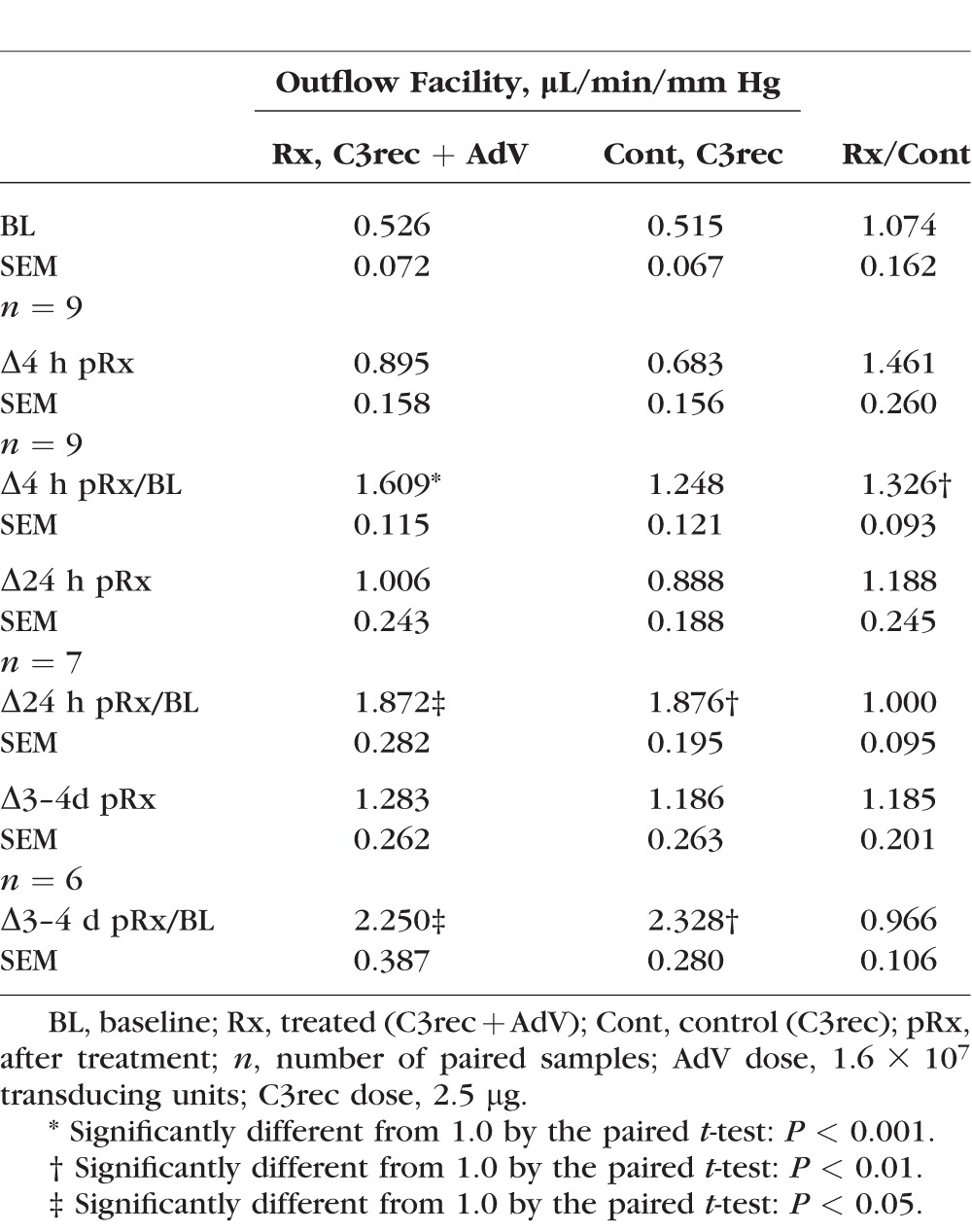
Figure 3.
Graphical representation of the outflow facility results from Tables 1 and 2 for paired anterior segments. Data are the ratio ± SEM of treated to control after correction for baseline (i.e., [C3rec + AdV/BL]/[C3rec/BL] or [AdV/BL]/[untreated/BL] for each of the various time points). *Significantly different from 1.0 by the 2-tailed paired t-test.
Table 3.
Effect of AdV on Outflow Facility in MOCAS Compared to Untreated Controls
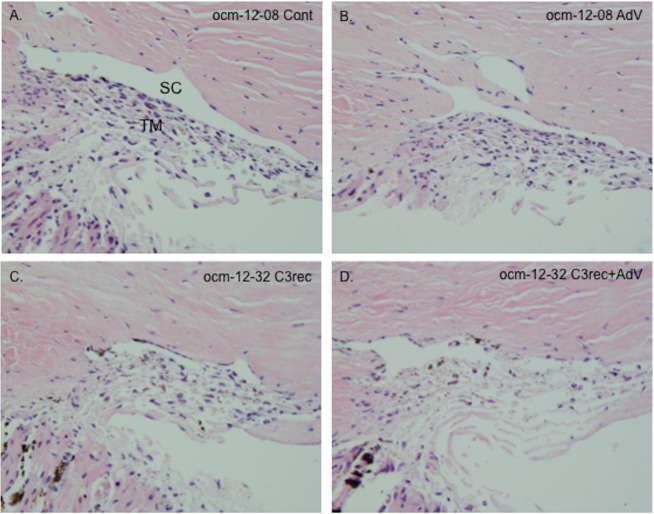
The effect of the various treatments on gross morphology of the anterior segment at the 24-hour time point was evaluated by light microscopy (Fig. 4). There were no apparent qualitative morphological differences between paired control and AdV-treated segments (Figs. 4A, 4B) in terms of cellularity of the juxtacanalicular regions and along the collagen beams, the integrity of Schlemm's canal, and the organization of the collagen beams. In paired C3rec and AdV + C3rec segments (Figs. 4C, 4D) there was reduced cellularity, disorganized beams, and discontinuity of the inner wall of Schlemm's canal. These alterations appeared to be more prominent in the AdV + C3rec segment than in the C3rec segment.
Figure 4.
H&E staining showing gross morphology of segments at 24 hours after the various treatment regimens. (A, B) Untreated control (Cont) and AdV treated segments from MOCAS ocm-12-08. There were no apparent qualitative morphological differences between paired control and AdV-treated segments in terms of cellularity of the juxtacanalicular regions and along the collagen beams, the integrity of Schlemm's canal (SC), and the organization of the collagen beams. (C, D) The C3rec- and C3rec + AdV–treated segments from MOCAS ocm-12-32. There was reduced cellularity, disorganized beams, and discontinuity of the inner wall of SC. These alterations appeared to be more prominent in the AdV + C3rec segment than in the C3rec segment. TM, trabecular meshwork.
Discussion
Our previous studies5 suggested that expression of the C3 transferase gene delivered by AdV transduction in HTM cells and in MOCAS altered cellular adhesions and increased outflow facility. Given that viral entry is known to at least locally alter the actin cytoskeleton, our results raised the possibility that the viral vector, in particular AdV vectors, were having a role in altering the physiology. This study had two goals. First, we wished to determine in the current study whether C3 alone could produce a physiologic response suggesting a potential therapeutic use. Second, we wanted to know if the AdV vector itself may have affected the response to the transduced C3 gene in our previous studies.
We chose C3cs, which has an attached cell penetrating peptide, for our initial studies given its superior cell penetrating properties compared to C3 alone (Cytoskeleton, Inc.). Our studies with C3cs in HTM cell cultures demonstrated strong disruption of the actin cytoskeleton. However, C3cs was deemed unsuitable for in vivo studies due to the high cost of treatments and the small transient effect on outflow facility in MOCAS. To determine the effect of viral vectors on the C3 response in cells and in MOCAS, we also used a purified C3 (C3rec) lacking cell permeability enhancement moieties.
The presence of the cell penetrating peptide on C3cs clearly enhanced the ability of the protein to disrupt the actin cytoskeleton as C3rec was found to disrupt actin stress fiber formation only at 24 hours after exposure in HTM cells. In cells treated with C3rec, this activity was enhanced when cells were transduced simultaneously with AdV or FIV vectors, as the stress fiber disruption and cell rounding were observed much earlier. The effect was not specific to AdV, since the FIV vector also enhanced the C3rec effect. Similar responses and time-lines were obtained in immortalized TM-1 cells cotreated with C3rec and AdV, suggesting that cell transformation did not alter the responses.
We also found that the outflow facility response in MOCAS was transiently enhanced by cotreatment with C3rec and AdV suggesting that the cell cultures adequately mimic the in vivo situation. Although we obtained a significant increase in outflow facility, the effect was not of sufficient duration to be considered useful for animal studies.
Adenoviruses are endocytosed into cells using αv integrins that activate the PI3 kinase pathway,23 resulting in increased phagocytic activity by the cell. The FIV vector was pseudotyped with the VSV G protein. Although the receptor for VSV G has not been determined, it is known that VSV enters cells via clathrin-mediated endocytosis and that activation of the PI3 kinase pathway is involved.14 Both vector viruses, therefore, activate the PI3 kinase pathway, which would increase endocytic activity of the cells. This is a likely explanation for the viral enhancement of the C3 transferase protein–mediated cytoskeletal disruption we observed. The transient change in outflow facility we observed in the MOCAS also would be consistent with this mechanism. Since we found that neither of the GFP-expressing viral vectors alone altered the cytoskeleton in HTM cells, the effect is specific for the C3 protein. Our findings also are consistent with the known role of integrins in the biology of TM cells,24 as various integrins regulate endocytosis, phagocytosis, and the actin cytoskeleton in these cells. Although the viral vectors are taken up via endocytosis, we cannot rule out the possibility that pinocytosis or other cellular uptake mechanisms are responsible for the uptake of C3. Regardless of the mechanism, it is clear that the viral vectors enhance the cytoskeletal disruption ability of the C3 protein, most likely by increasing the uptake of the protein.
A phenomenon termed pseudotransduction has been described previously for retroviral-based vectors.25,26 During pseudotransduction, proteins contaminating the viral vector preparation enter cells, giving the false impression that viral vector–mediated delivery has occurred. In this respect, our findings that exposure of cells to C3 transferase in the presence of the vector enhances uptake may be similar to a pseudotransduction event, although this has not been described previously for AdV based vectors. In this study, pseudotransduction of protein from the vector preparation would not be an issue as the transgene delivered by both vectors is GFP and not C3 transferase. In addition, the lentiviral vector preparations were purified by centrifugation through two sucrose gradient cushion steps which would have removed most if not all of any contaminating proteins and the AdV vectors were purified using an affinity-based method with extensive washing of the affinity columns before elution.
Our observation that the viral vectors alone did not disrupt the cytoskeleton is consistent with previous observations that viruses induce actin polymerization locally at the site of attachment.27 This has been described for adenovirus9 and lentiviruses.28,29 Thus, it is somewhat of a paradox that viral vectors would enhance the cytoskeletal-disrupting response, because the induction of actin polymerization would counteract the activity of C3rec. However, the actin polymerization is induced locally at the site of endocytosis and it is transient, lasting only as long as it takes the viruses to enter the cell; thus, we would not expect to see a generalized effect on the cytoskeleton. There also did not appear to be any qualitative alterations of the gross morphology of the anterior segment in our control compared to AdV-treated segments at 24 hours after transduction (Fig. 4). However, morphological changes found at 24 hours after treatment with C3rec alone suggested that some C3rec was able to penetrate the anterior segments, but resulted in a lesser degree of change than observed in segments treated with the combined AdV and C3rec.
In our previous study, where we used an adenoviral vector to deliver C3 transferase and showed that this increased outflow facility in our MOCAS model,5 it was not clear that the effect was due only to the C3 transferase, since the binding of the vector to integrins could have altered the cytoskeleton. The results presented here strongly suggested that the effect was due only to the C3 transferase, because the effect was transient in the MOCAS and adenovirus is known to induce only local actin polymerization.9
Conclusions
In summary, we have shown that treatment of HTM cells with viral vectors and a potentially aqueous outflow-therapeutic protein increases the intracellular activity of the therapeutic protein and the in situ outflow tissue response, most likely by enhancing cellular uptake of the protein into cells. This strategy could be used to improve the effect of other therapeutic proteins that might be useful for treating the outflow pathways in glaucoma.
Acknowledgments
Supported by National Institutes of Health (NIH; Bethesda, MD, USA) Grants P30 EY016665, P51 RR000167, EY017006, EY0020490, and EY018274; Research to Prevent Blindness, Inc. (New York, NY, USA); unrestricted departmental and Physician-Scientist awards; Ocular Physiology Research and Education Foundation; and Walter Helmerich Chair from the Retina Research Foundation.
Disclosure: S.R. Slauson, None; D.M. Peters, None; M.K. Schwinn, None; P.L. Kaufman, P; B.T. Gabelt, None; C.R. Brandt, None
References
- 1.Llobet A,, Gasull X,, Gual A.Understanding trabecular meshwork physiology: a key to the control of intraocular pressure? News Physiol Sci. 2003; 18: 205–209. [DOI] [PubMed] [Google Scholar]
- 2.Tian B,, Kaufman PL.Comparisons of actin filament disruptors and Rho kinase inhibitors as potential antiglaucoma medications. Expert Rev Ophthalmol. 2012; 7: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall A.Rho GTPases and the actin cytoskeleton. Science. 1998; 279: 509–514. [DOI] [PubMed] [Google Scholar]
- 4.Aktories K,, Mohr C,, Koch G.Clostridium botulinum C3 ADP-ribosyltransferase. Curr Top Microbiol Immunol. 1992; 175: 115–131. [DOI] [PubMed] [Google Scholar]
- 5.Liu X,, Hu Y,, Filla MS,, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005; 11: 1112–1121. [PubMed] [Google Scholar]
- 6.Zhong C,, Chrzanowska-Wodnicka M,, Brown J,, et al. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998; 141: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q,, Peyruchaud O,, French KJ,, Magnusson MK,, Mosher DF.Sphingosine 1-phosphate stimulates fibronectin matrix assembly through a Rho-dependent signal pathway. Blood. 1999; 93: 2984–2990. [PubMed] [Google Scholar]
- 8.Fahrer J,, Kuban J,, Heine K,, et al. Selective and specific internalization of clostridial C3 ADP-ribosyltransferases into macrophages and monocytes. Cell Microbiol. 2010; 12: 233–247. [DOI] [PubMed] [Google Scholar]
- 9.Nemerow GR,, Pache L,, Reddy V,, Stewart PL.Insights into adenovirus host cell interactions from structural studies. Virology. 2009; 384: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergelson JM,, Cunningham JA,, Droguett G,, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997; 275: 1320–1323. [DOI] [PubMed] [Google Scholar]
- 11.Gaggar A,, Shayakhmetov DM,, Lieber A.CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003; 9: 1408–1412. [DOI] [PubMed] [Google Scholar]
- 12.Wickham TJ,, Mathias P,, Cheresh DA,, Nemerow GR.Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993; 73: 309–319. [DOI] [PubMed] [Google Scholar]
- 13.Wiethoff CM,, Wodrich H,, Gerace L,, Nemerow GR.Adenovirus protein VI mediates membrane disruption following capsid disassembly. J Virol. 2005; 79: 1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer J,, Schelhaas M,, Helenius A.Virus entry by endocytosis. Annu Rev Biochem. 2010; 79: 803–833. [DOI] [PubMed] [Google Scholar]
- 15.Dillon ST,, Feig LA.Purification and assay of recombinant C3 transferase. Methods Enzymol. 1995; 256: 174–184. [DOI] [PubMed] [Google Scholar]
- 16.Lee ES,, Gabelt BT,, Faralli JA,, et al. COCH transgene expression in cultured human trabecular meshwork cells and its effect on outflow facility in monkey organ cultured anterior segments. Invest Ophthalmol Vis Sci. 2010; 51: 2060–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filla MS,, Liu X,, Nguyen TD,, et al. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibronectin. Invest Ophthalmol Vis Sci. 2002; 43: 151–161. [PubMed] [Google Scholar]
- 18.Filla MS,, Schwinn MK,, Nosie AK,, Clark RW,, Peters DM.Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves β3 integrin signaling. Invest Ophthalmol Vis Sci. 2011; 52: 2952–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filla MS,, Schwinn MK,, Sheibani N,, Kaufman PL,, Peters DM.Regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct beta1 and beta3 integrin pathways. Invest Ophthalmol Vis Sci. 2009; 50: 5723–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwinn MK,, Gonzalez JM, Jr,, Gabelt BT,, et al. Heparin II domain of fibronectin mediates contractility through an alpha4beta1 co-signaling pathway. Exp Cell Res. 2010; 316: 1500–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y,, Gabelt BT,, Kaufman PL.Monkey organ-cultured anterior segments: technique and response to H-7. Exp Eye Res. 2006; 82: 1100–1108. [DOI] [PubMed] [Google Scholar]
- 22.Bárány EH.Simultaneous measurements of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest Ophthalmol. 1964; 3: 135–143. [PubMed] [Google Scholar]
- 23.Li E,, Stupack D,, Klemke R,, Cheresh DA,, Nemerow GR.Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J Virol. 1998; 72: 2055–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagen J,, Faralli JA,, Filla MS,, Peters DM.The role of integrins in the trabecular meshwork. J Ocul Pharmacol Ther. 2014; 30: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M-L,, Winther BL,, Kay MA.Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)-moloney murine leukemia virus-derived retrovirus vectors: comparison of VSV-G and amphotropic vectors for hepatic gene transfer. J Virol. 1996; 70: 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geering B,, Schmidt-Mende J,, Federzoni E,, Stoeckle C,, Simon H-U.Protein overexpression following lentiviral infection of primary neutrophils is due to pseudotransduction. J Immunol Methods. 2011; 373: 209–218. [DOI] [PubMed] [Google Scholar]
- 27.Delorme-Axford E,, Coyne CB.The actin cytoskeleton as a barrier to virus infection of polarized epithelial cells. Viruses. 2011; 3: 2462–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon-Alonso M,, Rocha-Perugini V,, Alvarez S,, et al. Actin-binding protein drebrin regulates HIV-1-triggered actin polymerization and viral infection. J Biol Chem. 2013; 288: 28382–28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolp B,, Fackler OT.How HIV takes advantage of the cytoskeleton in entry and replication. Viruses. 2011; 3: 293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]