Rituximab is an approved B-lymphocyte depleting agent for induction of remission in patients with granulomatosis with polyangiitis and microscopic polyangiitis.1,2 Unlike cyclophosphamide, rituximab is not known to interfere with fertility and appears to be a safe and effective alternative. Pregnancy outcomes after maternal exposure to rituximab have been described,3 primarily in women with lymphoma, rheumatoid arthritis and lupus, but little is known about the impact of rituximab exposure on fetal outcomes, and more specifically, fetal B-lymphocyte populations among women with vasculitis. We performed a retrospective analysis of women with vasculitis who received rituximab in our centre and who achieved pregnancy, and their resultant offspring.
While receiving rituximab, women were counselled extensively to avoid pregnancy. Urine levels of human chorionic gonadotropin were measured and negative before each dose. When patients became pregnant, maintenance immunosuppression was minimised to prednisone and/or azathioprine and high-risk obstetrical care at our centre was initiated. Where possible, maternal and fetal cord blood was tested for CD20+ B-lymphocytes at delivery.
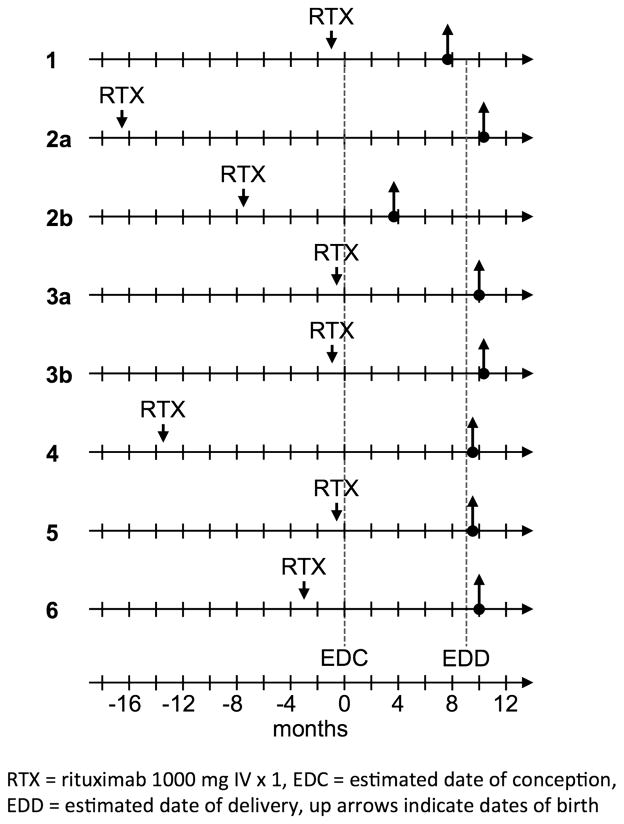
A total of 157 women (age range 16–93 years, mean (SD) 58.9 years (17.3)), including 22 women under 40 years of age, were treated with rituximab from 2002 to 2012. Only one patient (39 years) who desired pregnancy was unable to conceive. Eight pregnancies were achieved between six of the women (four planned and four unplanned) as represented in figure 1. Patient 2b had a miscarriage due to intrauterine fetal demise at 15 weeks. Histopathologic fetal tissue analysis was consistent with Beckwith–Wiedemann syndrome and genotyping of the parents was negative, indicating a non-familial aetiology. Patient 3a had progressive airway disease despite absence of B-lymphocytes, but she improved with increased prednisone. Remaining pregnancies were uneventful. At delivery, maternal CD20+ B-lymphocytes were absent in six of eight patients; however, B-lymphocytes were present in fetal cord blood in three of three (100%) samples collected (table 1).
Figure 1.
Timelines of women treated with rituximab who achieved pregnancy.
Table 1.
Characteristics of women with autoimmune vasculitis exposed to rituximab and their offspring
| Patient | 1 | 2a | 2b | 3a | 3b | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 31 | 25 | 27 | 20 | 22 | 29 | 40 | 32 |
| Self-reported ethnicity | W | W | W | W | W | I | ||
| Tobacco use during pregnancy | none | none | none | none* | none | none | none | none |
| Diagnosis | PAN | GPA | GPA | GPA | MPA | GPA | ||
| Induction medications | CYC | CYC | CYC | CYC | CYC | CYC | ||
| PRED | PRED | PRED | AZA | PRED | PRED | |||
| MMF | RTX | PRED | ||||||
| RTX | ||||||||
| Disease duration (years) | 13 | 5 | 7 | 2 | 4 | 9 | 3 | 4 |
| Medications at delivery† | AZA50 PRED7.5 |
none | none | PRED20 | PRED5 | PRED10 | none | none |
| RTX exposure prior to EDC (months) | 0.5 | 16.5 | 7.5 | 0.25 | 0.5 | 13.5 | 0.25 | 2.8 |
| Weeks gestation (days) | 31 (217) | 41 (288) | 15 (104)‡ | 40 (281) | 41 (288) | 38 (269) | 38 (266) | 40 (280) |
| Child’s sex | M | F | N/A | M | F | F | M | M |
| Child’s Apgar scores | 8/9 | 9/9 | N/A | 9/9 | 9/9 | 8/9 | 8/10 | 9/9 |
| Child’s birth weight (grams) | 1625 | 3790 | N/A | 2945 | 3500 | 3270 | 3515 | 2693 |
| Maternal parity after delivery | G2P2 | G1P1 | G2P1 | G1P1 | G2P2 | G1P1 | G10P10 | G1P1 |
| Maternal IgG at delivery (mg/dL)§ | 760 | 662 | 542 | NR | 521 | 558 | 745 | 638 |
| Maternal B cells at delivery (%) | <0.01 | 2.79 | <0.01 | <0.01 | 0.54 | <0.01 | <0.01 | <0.01 |
| Fetal cord blood B cells at delivery (%) | NR | NR | N/A | 3 | NR | 5 | NR | 7 |
Patient 3 was a social smoker, but quit smoking 4 years prior to first pregnancy.
Numbers indicate daily dose in milligrams. Of note, none of the women receiving corticosteroids during pregnancy developed gestational diabetes.
Patient 3a pregnancy ended in miscarriage.
IgG normal reference range=614–1295 mg/dL.
AZA, azathioprine; CYC, cyclophosphamide; EDC, estimated date of conception; F, female; G, gestation; GPA, granulomatosis with polyangiitis (formerly Wegener’s); I, Indian; M, male; MMF, mycophenolate mofetil; MPA, microscopic polyangiitis; N/A, not applicable; NR, not recorded; P, parturition; PAN, polyarteritis nodosa; PRED, prednisone; RTX, rituximab; W, White.
Most women were able to achieve pregnancy after rituximab treatment. We attribute this high fertility rate to decreased use of cyclophosphamide with improved disease control and quality of life on rituximab. Interestingly, in women with anti-neutrophil cytoplasmic autoantibody vasculitis who are in remission after standard induction therapy, pregnancy is not typically associated with relapse and outcomes are excellent.4 One of our patients had progressive airway disease despite improvement of other disease manifestations; however, remaining patients had no return of disease despite a reduction of immunosuppression. It appears likely that the sole pregnancy with congenital anomalies and associated miscarriage was unrelated to rituximab use.5 Surprisingly, CD20+B-lymphocytes measured in fetal cord blood at delivery did not appear to be affected despite efficient placental transfer of immunoglobulin G and presumably rituximab.6
Our findings suggest that rituximab use prior to pregnancy results in a low rate of adverse effects even when used close to conception. The ability of these offspring to mount an effective immune response to vaccination warrants investigation. Further study is needed to more precisely determine perinatal safety of rituximab given its increasing use in women of childbearing age.
Acknowledgments
The authors wish to thank the patients and their children as well as the clinic nurses involved in their care, including Donna Hagstrom, Laura Chambers White, Eleanor Coughlin and Kate Cosgrove.
Footnotes
Contributors WFP, MMM, MFG and JLN provided the clinical care and data interpretation for the patients described herein. APM and PM assisted with data collection and interpretation. KAL serves as manager of the MGH Vasculitis and Glomerulonephritis Clinic, obtained institutional review board approval for this study and provided invaluable insight and recommendations regarding this work as well as interpretation of the data. WFP wrote and prepared the manuscript. All authors participated in reviewing the revised version of the manuscript and approved the final revised version.
Competing interests None.
Patient consent Obtained.
Ethics approval Partners Healthcare System Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–20. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 2.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499–506. doi: 10.1182/blood-2010-07-295444. [DOI] [PubMed] [Google Scholar]
- 4.Tuin J, Sanders JS, de Joode AA, et al. Pregnancy in women diagnosed with antineutrophil cytoplasmic antibody-associated vasculitis: outcome for the mother and the child. Arthritis Care Res (Hoboken) 2012;64:539–45. doi: 10.1002/acr.21556. [DOI] [PubMed] [Google Scholar]
- 5.Weksberg R, Shuman C, Beckwith JB. Beckwith-Widemann syndrome. Eur J Hum Genet. 2010;18:8–14. doi: 10.1038/ejhg.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmeira P, Quinello C, Silveira-Lessa AL, et al. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]