Abstract
Here we describe the gross and microscopic findings of naturally occurring, β-hemolytic Escherichia coli peritonitis in B6.129-Myd88tm1Aki male and female mice. Over approximately 5 mo, 10 homozygous mutant mice deficient in myeloid differentiation factor 88 (C57BL/6 strain; male and female) that had not been used in research protocols developed rapid-onset abdominal swelling associated with copious viscous ascites. Each mouse developed an anterior peritonitis, primarily involving the parietal peritoneum and the visceral surface of the spleen, liver, diaphragm, and stomach. Inflammation was confined to the organ surfaces, with no indication of septicemia or grossly apparent gastrointestinal perforation or other tissue compromise that would initiate peritonitis. Peritonitis was likely attributable to compromised antibacterial innate immunity; cohoused, similarly immunodeficient littermates did not develop similar clinical signs. An unusual finding in all cases was mesothelial cell hyperplasia and hypertrophy. Although the underlying innate immune deficiency accounts for much of the observed pathology, the remarkable mesothelial cell morphology and the episodic nature of the peritonitis in some littermates and not others remain unexplained.
Abbreviations: MyD88, myeloid differentiation response 88; TLR, Toll-like receptor
Mice deficient in myeloid differentiation factor 88 (myD88) are commonly studied in immunologic research as models of various diseases, including inflammatory bowel disease and diabetes.2,3 MyD88 is a key signal transduction molecule for most of the Toll-like receptors (TLR) and IL1 family receptors, initiating cytokine release essential for effective innate immunity.18 The loss of this adapter protein impairs production of IL1, IL6, IL18, macrophage inhibitory proteins 1 and 2, and various chemokines.1,12,14 Knockout mutant mice are especially susceptible to gram-negative bacteria, because TLR4, which triggers signaling through MyD88, mediates responses to LPS.7,17 These immunologic mutants are common in research animal colonies, but their development of clinical signs and lesions consistent with Escherichia coli peritonitis, which arose at different times and affected only some of the immunodeficient mice, was previously unknown.
Case Reports
Over several months, 3 B6.129-Myd88tm1Aki mice and an additional unaffected litter-mate were provided to the diagnostic laboratory for necropsy. The initial case involved a 19-wk-old male B6.129-Myd88tm1Aki mouse that was removed from the colony because of abdominal distension and was euthanized by CO2 inhalation. The mouse weighed 44 g, and approximately 10 mL of viscous exudative ascites was aspirated at necropsy. The diaphragm was thickened by inflammation and firmly adhered to the capsular surface of the liver and the serosa of the stomach. There was hepatomegaly and splenomegaly. Gram-stained cytology of the ascites identified gram-negative bacilli, and cultures of the ascites and diaphragm grew β-hemolytic E. coli.
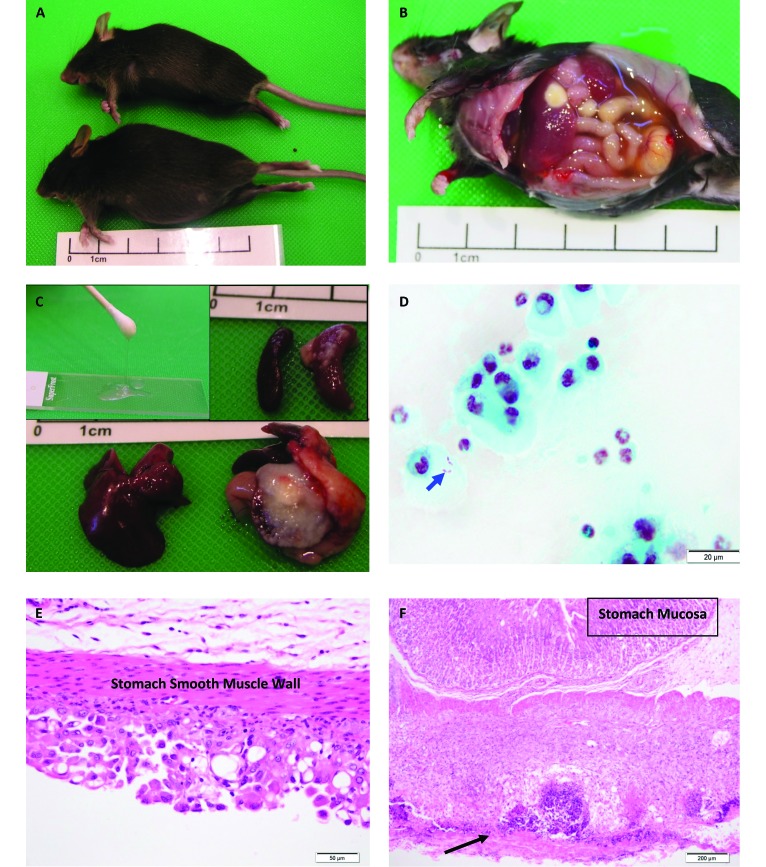
One month after the initial case, animal care staff noted that a 6-wk-old female B6.129-Myd88tm1Aki mouse had developed abdominal swelling (Figure 1 A). The affected mouse weighed 20.1 g, whereas a mutant littermate control female mouse weighed 17.3 g. Gross necropsy of the affected mouse was similar to the previous case: 3 mL of copious viscous ascites, hepatosplenomegaly, and inflammation and adhesions to the diaphragm, liver, stomach, and spleen (Figure 1 B and C). The total protein concentration of the ascites was 3 g/dL. Bacterial cultures of the ascites grew β-hemolytic E. coli (Figure 1 D). The mutant, littermate control female lacked gross or microscopic lesions.
Figure 1.
(A) Swollen abdomen of an affected female MyD88-deficient mouse (a) compared with her MyD88-deficient littermate control (b). (B) Ascites and liver inflammation in MyD88−/− mouse. (C) Littermate control liver (left) and MyD88-deficient affected liver (right); inset, viscous ascites (left). Littermate control spleen (left) and affected MyD88−/− spleen (right). (D) Gram-stained ascites cytology with gram negative bacilli within the cytoplasm (arrow). (modified gram stain). (E) Mesothelial cell hyperplasia and hypertrophy of stomach serosa . Hematoxylin and eosin stain. (F) Necrotizing serositis (arrow) and extensive inflammation and thickening of the stomach muscularis. Note that the gastric mucosa is not inflamed. All affected tissues are from case 13-613.
Two weeks later, animal husbandry staff reported another 7-wk-old female B6.129-Myd88tm1Aki mouse with abdominal distension. She weighed 35 g and in poor condition, according to palpation of her lumbar spine and pelvis. Approximately 5 mL of viscous exudate was aspirated from the abdomen. Bacterial cultures of the ascites and inflamed tissues grew β-hemolytic E. coli. Like the other 2 mice, she had hepatosplenomegaly, adherent capsulitis of the liver lobes to the diaphragm, and peritonitis, primarily associated with the stomach, spleen, pancreas, and anterior intestine.
Mice
MyD88-deficient mice were from the Immunogenetics Research Facility at James Cook University. The genetic background and husbandry of these mice had previously been described.9 Founder B6.129-Myd88tm1Akimice, backcrossed to C57BL/6 mice for 10 generations, were kindly provided by Dr Shizuo Akira (Osaka University, Osaka, Japan). All mice were bred and maintained under SPF conditions at the Immunogenetics Research Facility of James Cook University. The microbial interface is further limited by a unidirectional operator flow path and shower-in access, a 4-pass air-filtration system, and advanced drinking water sterilization and purification. Mouse holding rooms are environmentally controlled, HEPA-filter–ventilated, and maintained at 19 ± 1.5 °C and 40% to 50% relative humidity. Mice are housed in static filter-top microisolation cages (Airlaw, Smithfield, New South Wales, Australia). A dirty-bedding sentinel program is in place. Each cage is visually checked daily, and each mouse is health-checked during weekly cage-changing. A commercial chow (Specialty Feeds, Glen Forrest, WA) is γ-irradiated for sterilization; sourcing of grain is restricted to a limited number of farms. The facility is reviewed regularly by the James Cook University Animal Care and Ethics Committee, and all research studies must obtain approval.
Complete necropsies, including photos, histopathology and microbiology, were performed on 1 male mouse (case 13-514), 2 female mice (case 13-613), and 1 other female mouse (case 13-640). One of the female mice (case 13-613) is a MyD88-deficient littermate mouse control that was cohoused with an affected female. Mice were not ill at the same time and were not cohoused, except for the affected and control female mice of case 13-613. Samples derived from tissues and ascites were cultured on blood agar and MacConkey agar and then incubated at 37 °C for 24 h. Bacterial cultures were indole-positive and oxidase-negative, and additional identification from API 20E test strips (bioMérieux Vitek, Hazelwood, MO) confirmed that the bacilli were β-hemolytic E. coli.
Histopathology
In all cases, the abdominal surface of the diaphragm had skeletal muscle necrosis and extensive pyogranulomatous inflammation, including lymphocytes and plasma cells. Limited inflammation through the muscular membrane to the parietal pleura was noted microscopically in 2 cases. Inflammation within the thorax was mild and grossly not visible. The livers had characteristic capsular inflammation, with areas of necrosis, peripheral fibrosis, and predominately pyogranulomatous inflammation. Necrosis and inflammation did not extend to portal or centrilobular regions or distend sinusoids within the parenchyma. The spleens had white-pulp hyperplasia. In all cases, the mesentery was expanded with edema, fibrin, fibrous tissue, and pyogranulomatous inflammation, including infrequent plasma cells and lymphocytes.
Histopathology identified mesothelial cell proliferation and hypertrophy in the cranial abdomen, which corresponded to the primary area of inflammation (Figure 1 E and 1F). Additional histologic staining (alcian blue, pH 2.5) was performed to better characterize the presumed secretory nature of the prominent mesothelial cells and demonstrated high-molecular–weight glycosaminoglycan secretion by mesothelial cells from all affected mice. The tissues from the unaffected, littermate control mouse had only the thinnest surface coating and often appeared to be negative for any mucin secretion. In addition, mesothelial cells lining visceral and parietal surfaces of the distal abdomen had infrequent, much less, or no hyperplasia or hypertrophy.
Gross and microscopic findings were similar among all mutant mice. In contrast, the MyD88-deficient, unaffected, littermate control did not have any gross or microscopic lesions.
Discussion
The heterogenous intestinal microbiota contains an estimated 100 trillion microorganisms.6 This commensal bacterial population is located within the intestinal lumen and is largely excluded from the intestinal epithelial cells by the mucus layer. In addition to the mucus, specialized epithelial and mesenchymal cells of the intestinal epithelium and lamina propria act as a barrier to bacterial entry into deeper host tissues.6,8
The microbiota and intestinal epithelial cells communicate via pattern-recognition receptors, which include the TLR family.4,10 Previous studies report that the loss of MyD88 signaling increases host susceptibility to noninvasive enteric bacterial pathogens.6 Targeted knockout mutation studies of MyD88 restricted to intestinal epithelial cells indicate that the loss of MyD88 results in increased numbers of mucus-associated bacteria; attachment between bacteria and epithelial cells; modification of the mucus, including down-regulated expression of antimicrobial peptides and immunoglobulin receptors; and increased intestinal permeability; these features ultimately lead to increased translocation of intraintestinal bacteria to extraintestinal host tissues.5 Bacterial translocation is believed to be facilitated by dendritic cells within the lamina propria that extend processes through epithelial cell tight junctions to phagocytose intestinal bacteria; these dendritic cells then migrate to mesenteric lymph nodes where antigen presentation to T cells occurs.11,12 A study in humans confirmed the gut origin of translocating bacteria by examining E. coli DNA fingerprints and determined that bacterial translocation is more dependent on the gut epithelial cells than on the virulence properties of resident enteric bacteria.11
Gastrointestinal histopathology of these 3 cases did not identify intestinal mucosal inflammation or provide any indication of gastroenteritis or of colitis. Gastric transmural inflammation (2 cases) and necrosis (1 case) were present in the region of the pylorus. The pancreatic and mesenteric lymph nodes were enlarged in all cases but lacked significant cortical follicular hyperplasia and inflammation. Gram-stained sections from the 3 cases yielded few E. coli bacilli. Bacilli were found in areas of necrosis, associated with the serosal surfaces, or adjacent to hypertrophied and hyperplastic mesothelial cells, phagocytosed by inflammatory cells, including mesothelial cells, but were not apparent within mesenteric lymph nodes or organ parenchyma.
We have been unable to discern why these MyD88-deficient mice developed β-hemolytic E. coli peritonitis. The anterior abdominal location in each of the mice suggested a similar site and route for bacterial translocation to anterior abdominal lymph nodes. The husbandry staff identified affected mice rapidly, prior to wide bacterial and inflammatory dissemination. Husbandry staff were unable to recall any incident or conditions that might have triggered stress in the colony or any reasons for sporadic distress. A literature search focused on intestinal permeability or altered intestinal barrier function in MyD88-deficient mice revealed that loss of mucosal barriers may develop without intestinal pathology and can occur even with normal intestinal barrier function.14-16
The purpose of this report is to describe the gross and histopathologic presentation of these cases of β-hemolytic E. coli peritonitis in MyD88 deficient mice. An environmental event that may have incited this condition was not documented in any of the occurrences. The similarity of the lesions and the identical microbiology obtained in the cases warrants alerting researchers and diagnosticians. Of particular interest are the unusual viscous ascites and mesothelial cell hyperplasia and hypertrophy associated with E. coli peritonitis in this immunocompromised mutant mouse model.
Acknowledgments
We thank Dr Jackie Picard, veterinary microbiologist, and Kerryn McEachren, for sharing their expertise in bacterial identification and Laurie Reilly, Chris Wright, and Virna Duffy, for their instruction, patience, and histologic assistance. We also thank Ian Johnson for his technical skills and assistance, as well as the staff at the Comparative Genomics Centre.
References
- 1.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagam M, Nakanishi K, Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL1- and IL18-mediated function. Immunity 9:143–150. [DOI] [PubMed] [Google Scholar]
- 2.Alkanani AK, Hara N, Lien E, Ir D, Kotter CV, Robertson CE, Wagner BD, Frank DN, Zipris D. 2014. Induction of diabetes in the RIP-B7.1 mouse model is critically dependent on TLR3 and MyD88 pathways and is associated with alterations in the intestinal microbiome. Diabetes 63:619–631. [DOI] [PubMed] [Google Scholar]
- 3.Asquith MJ, Boulard O, Powrie F, Maloy KJ. 2010. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology 139:519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cario E. 2010. Heads up! How the intestinal epithelium safeguards mucosal barrier immunity through the inflammasome and beyond. Curr Opin Gastroenterol 26:583–590. [DOI] [PubMed] [Google Scholar]
- 5.Frantz AL, Rogier EW, Weber CR, Shen L, Cohen DA, Fenton LA, Bruno ME, Kaetzel CS. 2012. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin 2, and antibacterial peptides. Mucosal Immunol 5:501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper LV, Macpherson AJ. 2010. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 10:159–169. [DOI] [PubMed] [Google Scholar]
- 7.Janeway CA Jr, Medzhitov R. 2002. Innate immune recognition. Annu Rev Immunol 20:197–216. [DOI] [PubMed] [Google Scholar]
- 8.Magalhaes JG, Tattoli I, Girardin SE. 2007. The intestinal epithelial barrier: how to distinguish between the microbial flora and pathogens. Semin Immunol 19:106–115. [DOI] [PubMed] [Google Scholar]
- 9.Miranda-Hernandez S, Gerlach N, Fletcher JM, Biros E, Mack M, Körner H, Baxter AG. 2011. Role for MyD88, TLR2, and TLR9 but not TLR1, TLR4, or TLR6 in experimental autoimmune encephalomyelitis. J Immunol 187:791–804. [DOI] [PubMed] [Google Scholar]
- 10.Mowat AM. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 3:331–341. [DOI] [PubMed] [Google Scholar]
- 11.Reddy BS, MacFie J, Gatt M, Macfarlane-Smith L, Bitzopoulou K, Snelling AM. 2007. Commensal bacteria do translocate across the intestinal barrier in surgical patients. Clin Nutr 26:208–215. [DOI] [PubMed] [Google Scholar]
- 12.Rescigno M, Urgbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2:361–367. [DOI] [PubMed] [Google Scholar]
- 13.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. 2009. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci USA 106:2348–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, Bercik P, Verdu EF, McCoy KD, Macpherson AJ. 2009. Innate and adaptive immunity cooperate flexibly to maintain host–microbiota mutualism. Science 325:617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su L, Qi Y, Zhang M, Weng M, Zhang X, Su C, Shi HN. 2014. Development of fatal intestinal inflammation in MyD88-deficient mice coinfected with helminth and bacterial enteropathogens. PLoS Negl Trop Dis 8:e2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi O, Hoshino K, Akira S. 2000. TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcs aureus infection. J Immunol 165:5392–5396. [DOI] [PubMed] [Google Scholar]
- 17.van't Veer C, van den Pangaart PS, Kruijswijk D, Florquin S, de Vos AF, van der Poll T. 2011. Delineation of the role of Toll-like receptor signaling during peritonitis by a gradually growing pathogenic Escherichia coli. J Biol Chem 286:36603–36618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Arostegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yagüe J, Antón J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Maródi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]