Abstract
Diabetes is characterized by hyperglycaemia and perturbations in intermediary metabolism. In particular, diabetes can augment flux through accessory pathways of glucose metabolism, such as the hexosamine biosynthetic pathway (HBP), which produces the sugar donor for the β-O-linked-N-acetylglucosamine (O-GlcNAc) post-translational modification of proteins. Diabetes also promotes mitochondrial dysfunction. Nevertheless, the relationships among diabetes, hyperglycaemia, mitochondrial dysfunction and O-GlcNAc modifications remain unclear. In the present study, we tested whether high-induced increases in O-GlcNAc modifications directly regulate mitochondrial function in isolated cardiomyocytes. Augmentation of O-GlcNAcylation with high glucose (33 mM) was associated with diminished basal and maximal cardiomyocyte respiration, a decreased mitochondrial reserve capacity and lower Complex II-dependent respiration (P < 0.05); however, pharmacological or genetic modulation of O-GlcNAc modifications under normal or high glucose conditions showed few significant effects on mitochondrial respiration, suggesting that O-GlcNAc does not play a major role in regulating cardiomyocyte mitochondrial function. Furthermore, an osmotic control recapitulated high-glucose-induced changes to mitochondrial metabolism (P < 0.05) without increasing O-GlcNAcylation. Thus, increased O-GlcNAcylation is neither sufficient nor necessary for high-glucose-induced suppression of mitochondrial metabolism in isolated cardiomyocytes.
Keywords: cardiomyocytes, diabetes, hexosamine biosynthetic pathway, mitochondrial function
INTRODUCTION
Diabetes can promote cardiac dysfunction independently of coronary disease [1–3]. Although the mechanisms of diabetic cardiomyopathy [4,5] remain unknown, dysregulated cardiac metabolism is a likely contributor. Mitochondria, central elements in metabolism, become dysfunctional during diabetes [6,7] and the diabetic heart demonstrates lower levels of glucose utilization [8]. Despite decreases in glucose uptake and oxidation [9], diabetes may alter the fate of glucose and increase flux of glucose-derived carbons through accessory pathways, such as the hexosamine biosynthetic pathway (HBP). Indeed, this is commonly observed in diabetic tissues and in cells cultured under diabetic conditions [10–17]. Hence, some have argued that the HBP plays a causal role in diabetic pathophysiology of the cardiovascular system [18].
Under resting physiological conditions, a minor fraction of intracellular glucose is committed to the HBP, which culminates in the formation of UDP-GlcNAc. UDP-GlcNAc is the monosaccharide donor for the β-O-linked-N-acetylglucosamine (O-GlcNAc) post-translational modification of proteins [19–21]. O-GlcNAcylation of target proteins can induce functional changes and alter transcription and translation. In this evolutionarily conserved system, only two antagonistic enzymes catalyse O-GlcNAc modification of proteins: O-GlcNAc transferase (OGT) catalyses the O-linkage of GlcNAc to proteins at serine or threonine residues, whereas O-GlcNAcase (OGA) removes GlcNAc from proteins. Thus, hyperglycaemia, a hallmark of diabetes, may increase flux through the HBP and consequently promote protein O-GlcNAcylation [22–25]. Indeed, several studies suggest that protein O-GlcNAcylation contributes to various aspects of cellular dysfunction during diabetes [10–14,16– 18,25–31]. In contrast, acute protein O-GlcNAcylation confers protection in the context of cell stress [20,32–43].
Given the importance of understanding the mechanisms of diabetic pathophysiology, particularly metabolism and mitochondrial function, and the diverse roles of O-GlcNAc in various diseases, we investigated the influence of O-GlcNAcylation on mitochondrial bioenergetics. We used extracellular flux (XF) analysis and genetic/pharmacological manipulation of O-GlcNAc levels to address the potential relationship between mitochondrial dysfunction, high glucose and O-GlcNAc. Our results suggest that high-glucose-induced mitochondrial dysfunction can occur independently of increased protein O-GlcNAcylation.
MATERIALS AND METHODS
All animals were used in compliance with the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health. The experimental protocols for the present study have been reviewed and approved by the University of Louisville Institutional Animal Care and Use Committee.
Neonatal rat cardiomyocyte isolation and culture
Neonatal rat cardiomyocytes (NRCMs) were isolated as previously described [36,37,40,44–51] from 1–2-day-old Sprague–Dawley rats. NRCMs were plated at a density of 850 000 cells/ml in six-well plates for protein isolation or 75 000 cells per well in Seahorse plates for bioenergetic assay. For the first 4 days following isolation, NRCMs were cultured in medium containing BrdU (bromodeoxyuridine; 0.1 mM), 5% FBS, penicillin (100 units/ml), streptomycin (100 mg/ml) and vitamin B12 (2 µg/ml). On day 4 (post-isolation), NRCM medium was changed to Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 4 mM glutamine, 1 mM pyruvate and the corresponding treatment (5 mM d-glucose, 33 mM d-glucose or 5 mM d-glucose + 28 mM mannitol). A 5 mM d-glucose + 28 mM mannitol treatment served as the osmotic control. Cells were cultured in their respective treatment for 48 h prior to protein or bioenergetics assays.
Gene transfer and OGA inhibition
For gene transfer experiments, NRCMs were serum-starved overnight and then transduced with 100 multiplicity of infection (MOI) of replication-deficient adenoviruses carrying the OGT gene (Ad-OGT) or the OGA gene (Ad-OGA) or null virus (Ad-Null) in medium containing 5% FBS for 5 h [36,37,40,42,44,48]. After 5 h, the medium was replenished with medium lacking virus. Bioenergetics profiling and protein isolation occurred 48 h post-transduction.
To pharmacologically augment O-GlcNAc levels, we used Thiamet G (TMG; Cayman Chemicals), which inhibits OGA and increases protein O-GlcNAcylation. NRCMs were treated with 1 µM TMG or vehicle (DMSO). Bioenergetics profiling of intact NRCMs was conducted 48 h after TMG treatment. Bioenergetics profiling of permeabilized NRCMs was conducted 24 h after TMG treatment.
Bioenergetic profiling of intact adherent cells
The bioenergetics of intact adherent NRCMs that were seeded at 75000 cells per well was measured using a Seahorse Bioscience XF24 Flux Analyzer [42,47,50,52,53]. For these experiments, the treatment medium was replaced with 675 µl of assay medium: unbuffered DMEM supplemented with glucose (5 or 33 mM; or appropriate concentration of mannitol osmotic control), 4 mM glutamine and 1 mM pyruvate, and plates were placed in a non-CO2 supplemented incubator 1 h before assay. Following microplate insertion, the XF24 automated protocol consisted of a 10 min delay followed by baseline oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) measurements [3 × (3 min mixing, 2 min wait and 3 min measure)]. To interrogate mitochondrial function, the following compounds were injected following three baseline measurements: Port A, oligomycin; Port B, FCCP (carbonilcyanide p-triflouromethoxyphenylhydrazone); and Port C, antimycin A (AA). After each injection, one measurement was recorded, with each having a 3 min mixing, 2 min wait and 3 min measure cycle. Stocks (1 mM) of oligomycin (Sigma), FCCP (Sigma) and AA (Sigma) were prepared in 100% DMSO (Sigma). Prior to assay, stocks were diluted in assay medium to yield 0.01 mM oligomycin, 0.01 mM FCCP and 0.1 mM AA, which, after injection, yielded final concentrations of 1.0 µM oligomycin, 1.0 µM FCCP and 10.0 µM AA. All experiments were conducted at 37°C. Parameters of mitochondrial function were calculated as previously described [47,54–56]. Protein concentration measured following XF analysis was not significantly different between groups.
Bioenergetic profiling of permeabilized, adherent cells
Bioenergetic profiling for electron transport chain activity was performed as recently described [57]. Prior to bioenergetic profiling, NRCMs were changed to mannitol and sucrose (MAS) medium {220 mM mannitol, 70 mM sucrose, 5 mM MOPS [3-(N-morpholino)propansulfonic acid] and 4% fatty-acid-free BSA, pH 7.2}. NRCMs were permeabilized following a Port A injection of 25 µg/ml saponin. Complex II-specific substrate [10 mM succinate, 1 µM rotenone (Rot) and 1 mM ADP] was also contained in Port A to support cellular respiration. Following State 3 rate measurements, oligomycin (1 µg/ml) was injected from Port B; following State 4o measurement, AA (10 µM) and Rot (1 µM) were injected from Port C to inhibit mitochondrial oxygen consumption. Protein concentration measured following XF analysis was not significantly different between groups.
Cell fractionation
NRCMs (n = 3) were fractionated to assess O-GlcNAcylation of mitochondrial proteins following treatment with 5 or 33 mM glucose, osmotic control (5 mM glucose + 28 mM mannitol), TMG, Ad-Null or Ad-OGA. Cardiomyocytes were trypsinized with 0.25% trypsin/EDTA (Thermo Fisher) for 5 min at 37°C. Trypsin was neutralized and cells were counted. Cardiomyocyte fractionation was performed with a standard Cell Fractionation Kit (Abcam).
Immunoblotting
Total cellular protein was harvested and quantified from NRCMs using established protocols [20,36,37,39–42,45–48,50,53,58]. Standard Western blotting procedures were used to probe for O-GlcNAc (anti-O-GlcNAc, CTD110.6; Biolegend), OGT (DM-17; Sigma) and OGA (anti-NCOAT; Sigma). Antibodies for α-tubulin (Sigma) and ATP5A (Abcam) were used as loading controls for whole-cell and mitochondrial protein respectively. Protein expression levels were quantified by densitometric analysis. For O-GlcNAc Western blots, the entire lane was quantified to determine overall protein O-GlcNAcylation [20,36,37,39–42,44,45,48].
Statistical analyses
Results are shown as means + S.D. (not S.E.M.). Statistical analyses (GraphPad Prism 5.0d) were conducted using a paired Student’s t test or by one-way ANOVA followed by Newman– Keuls Multiple Comparison Test, as appropriate. Each ‘n’ represents a separate NRCM isolation; treatments/interventions were always performed with their respective controls. Differences were considered statistically significant if P < 0.05.
RESULTS
High glucose increases protein O-GlcNAcylation
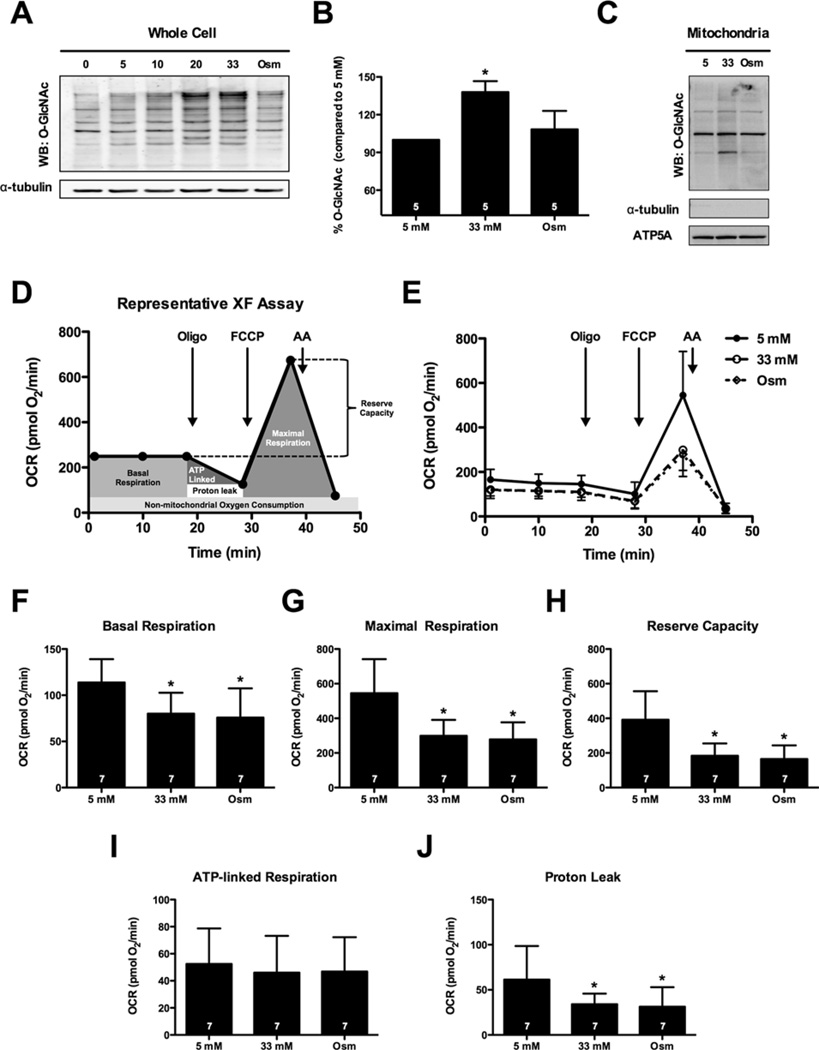
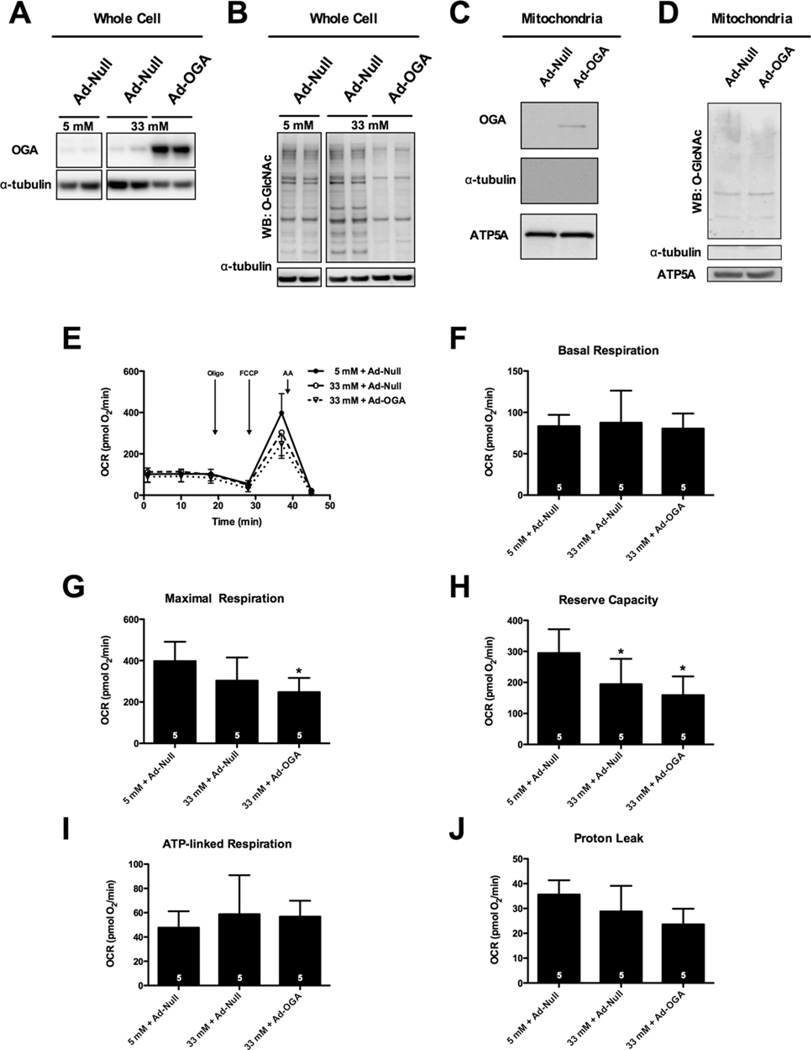
To determine whether high-glucose promotes protein O-GlcNAcylation, NRCMs were cultured for 48 h in medium supplemented with 0, 5, 10, 20 or 33 mM glucose (the osmotic control group was 5 mM glucose + 28 mM mannitol) and O-GlcNAc levels were assessed via immunoblotting (Figure 1A). Quantification of protein O-GlcNAc levels in whole cells demonstrated that the 33 mM glucose treatment resulted in a significant increase in O-GlcNAcylation when compared with 5 mM glucose (Figure 1B); however, when we isolated the mitochondrial fraction from similarly treated cells and probed for mitochondrial protein O-GlcNAcylation, we observed a much smaller effect (Figure 1C). Because we confirmed that high glucose induced protein O-GlcNAcylation in our system (similar to others [17,59]), we next queried whether high glucose compromises mitochondrial function.
Figure 1. High glucose depresses the bioenergetic reserve capacity of cardiomyocytes.
(A) Immunoblot of whole-cell protein O-GlcNAcylation following 0, 5, 10, 20 and 33 mM glucose treatment. An osmotic control (Osm, 5 mM glucose + 28 mM mannitol) was also used. (B) Densitometric measurement of O-GlcNAcylation in relation to 5 mM control demonstrated a significant induction of protein O-GlcNAcylation following 33 mM glucose. (C) Immunoblotting for mitochondrial protein O-GlcNAcylation. NRCMs were fractionated to isolate mitochondrial protein following treatment with 5 mM glucose, 33 mM glucose and the osmotic control. (D) Representative XF assay and diagram of how mitochondrial measurements were calculated. (E) Mitochondrial function assay following 48 h of high glucose treatment. From (E) parameters of mitochondrial function were assessed (as described in D): (F) basal respiration, (G) maximal respiration, (H) reserve capacity, (I) ATP-linked respiration, and (J) proton leak. As indicated in bars, n = 7 independent experiments; *P < 0.05, compared with 5 mM.
High glucose depresses mitochondrial bioenergetic reserve capacity
We performed XF analysis of NRCMs to determine whether high-glucose-mediated increases in O-GlcNAc could affect mitochondrial bioenergetics in the intact cell (Figure 1D, E). Protein concentration measured following XF analysis was not significantly different between groups. High glucose (33 mM, n = 7) depressed basal respiration (Figure 1F; P < 0.05), maximal respiration (Figure 1G; P < 0.05), reserve capacity (Figure 1H; P < 0.05) and proton leak (Figure 1J; P <0.05) compared with the normal glucose control (5 mM, n = 7). ATP-linked respiration remained unaltered following hyperglycaemic treatment (Figure 1I). To address the contribution of an osmotic influence in the depression of mitochondrial function, we used an osmotic control (5 mM glucose + 28 mM mannitol, n = 7), which recapitulated the depression (P < 0.05) in basal respiration, maximal respiration, reserve capacity and proton leak (Figures 1E–1J). We also queried whether the osmotic stress also augmented O-GlcNAc levels (similar to the high glucose treatment) and found that the osmotic control (see lane ‘Osm’ in Figure 1A) did not affect O-GlcNAc levels. Similarly, mitochondrial O-GlcNAcylation was largely unaffected by the osmotic control treatment (Figure 1C). Collectively, these findings indicate that the high-glucose-induced suppression of basal respiration, maximal respiration and reserve capacity were associated with an increase in osmolarity and not the high-glucose-induced increase in O-GlcNAcylation (Figure 1A). Interestingly, much of the basal differences in OCR were attributable to reductions in proton leak, which was reduced in both the high glucose and the osmotic control groups (P < 0.05) (Figure 1J) compared with the normal glucose group. Despite this apparent improvement in mitochondrial coupling, cells that were treated with high glucose or exposed to osmotic stress showed diminished mitochondrial reserve capacity. Thus, we suggest that osmotic stress caused by high glucose instigates loss of mitochondrial reserve capacity.
OGA or OGT overexpression does not alter bioenergetic reserve
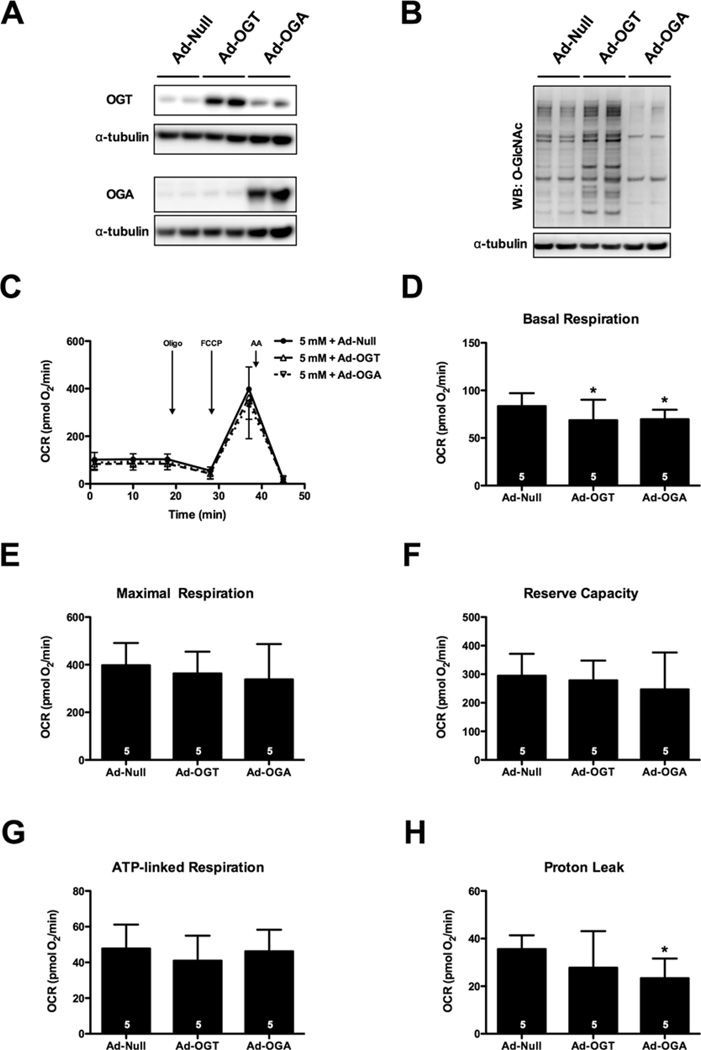
Although high-glucose-induced suppression of mitochondrial bioenergetics seemed O-GlcNAc-independent, we next asked whether it was possible for alterations in O-GlcNAc levels to influence mitochondrial bioenergetics. To this end, we transduced cells with Ad-OGT or Ad-OGA prior to normal glucose treatment for 48 h (Figure 2). Transduction resulted in a corresponding increase in OGT and OGA protein expression (Figure 2A) and commensurate changes in protein O-GlcNAcylation (Figure 2B). Overexpression of either OGT (n = 5) or OGA (n = 5) induced a small but statistically significant depression in basal respiration (Figures 2C and 2D) and OGA overexpression decreased proton leak (Figure 2H; P < 0.05). Overexpression of neither OGT nor OGA affected maximal respiration or reserve capacity (Figures 2E and 2F). Importantly, none of these modest changes are consistent with the high glucose-induced suppression of mitochondrial reserve capacity (Figure 1). Thus, neither promoting nor antagonizing O-GlcNAcylation via genetic means is sufficient to recapitulate the high-glucose-induced bioenergetic defect.
Figure 2. Overexpression of OGT or OGA does not affect bioenergetic reserve.
NRCMs were subjected to adenoviral overexpression of OGT and OGA. (A) Immunoblot for OGT and OGA protein expression following adenoviral treatment. (B) Immunoblot for protein O-GlcNAcylation demonstrated an induction of O-GlcNAc in response to Ad-OGT and a reduction in response to Ad-OGA. (C) Mitochondrial function assay of NRCMs 48 h post-transfection. Parameters of mitochondrial function were calculated from (C), including: (D) basal respiration, (E) maximal respiration, (F) reserve capacity, (G) ATP-linked respiration, and (H) proton leak. As indicated in bars, n = 5 independent experiments; *P < 0.05, compared with Ad-Null.
Inhibition of OGA does not affect oxygen consumption in NRCMs
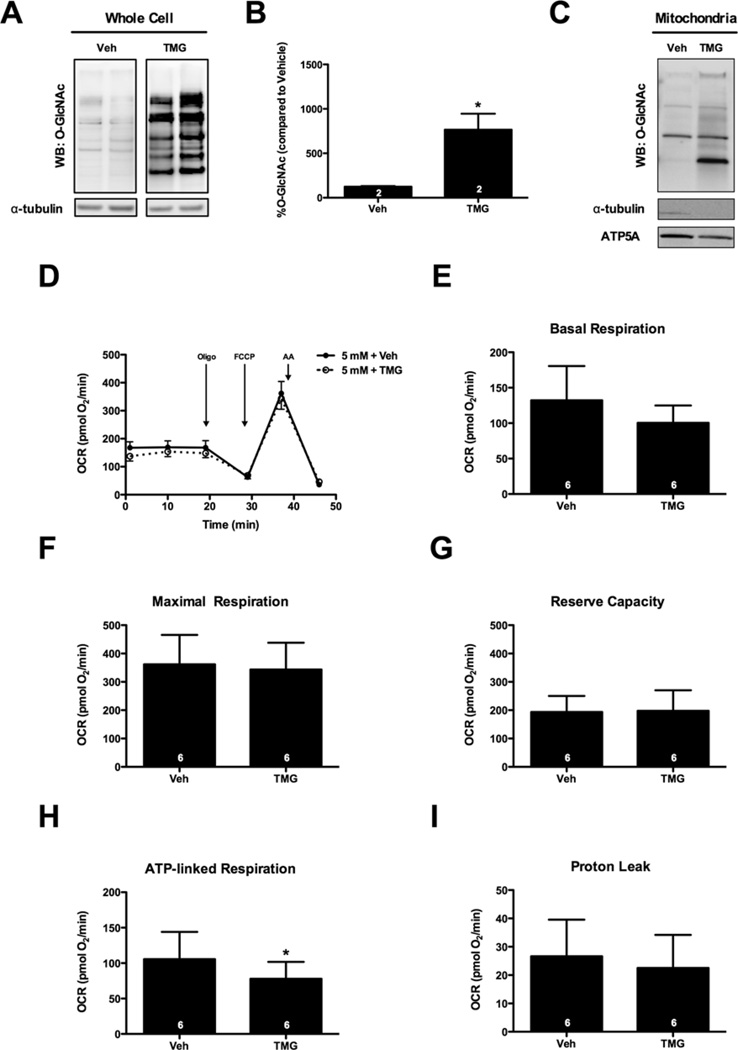
To further investigate whether protein O-GlcNAcylation is responsible for the bioenergetics depression observed with high glucose treatment, we used a potent pharmacological inhibitor of OGA, TMG, to increase robustly whole-cell protein O-GlcNAcylation (Figures 3A and 3B). Isolated mitochondrial fractions from similarly treated NRCMs showed no net significant increases in protein O-GlcNAcylation, although one band did appear to show elevated O-GlcNAc modification (n= 3; Figure 3C). Regardless, XF analysis in the intact cell preparations indicated that TMG did not significantly affect basal respiration, maximal respiration or reserve capacity (Figures 3D–3F). TMG treatment slightly but significantly decreased ATP-linked respiration (Figure 3H); however, proton leak (Figure 3I) and non-mitochondrial OCR (results not shown) were not different. Even inhibiting OGA with PUGNAc, a less potent inhibitor of OGA, or DON, an inhibitor of GFAT, the rate-limiting enzyme in formation of the O-GlcNAc moiety, did not alter mitochondrial bioenergetics (Supplementary Figure S1). Thus, inhibition of OGA (via TMG) increased O-GlcNAcylation but, unlike high glucose, did not suppress mitochondrial reserve capacity.
Figure 3. Inhibition of OGA has negligible effects on mitochondrial function.
NRCMs were treated with TMG (1 µM) for 48 h to induce protein O-GlcNAcylation. (A) Immunoblot for whole-cell protein O-GlcNAcylation. (B) Densitometric measurement of O-GlcNAcylation. (C) Immunoblot for mitochondrial protein O-GlcNAcylation following treatment with 1 µM TMG. (D) Mitochondrial function assay of NRCMs following 48 h treatment with TMG. Parameters of mitochondrial function were measured from (D), including: (E) basal respiration, (F) maximal respiration, (G) reserve capacity, (H) ATP-linked respiration, and (I) proton leak. As indicated in bars, n = 6 independent experiments; *P < 0.05.
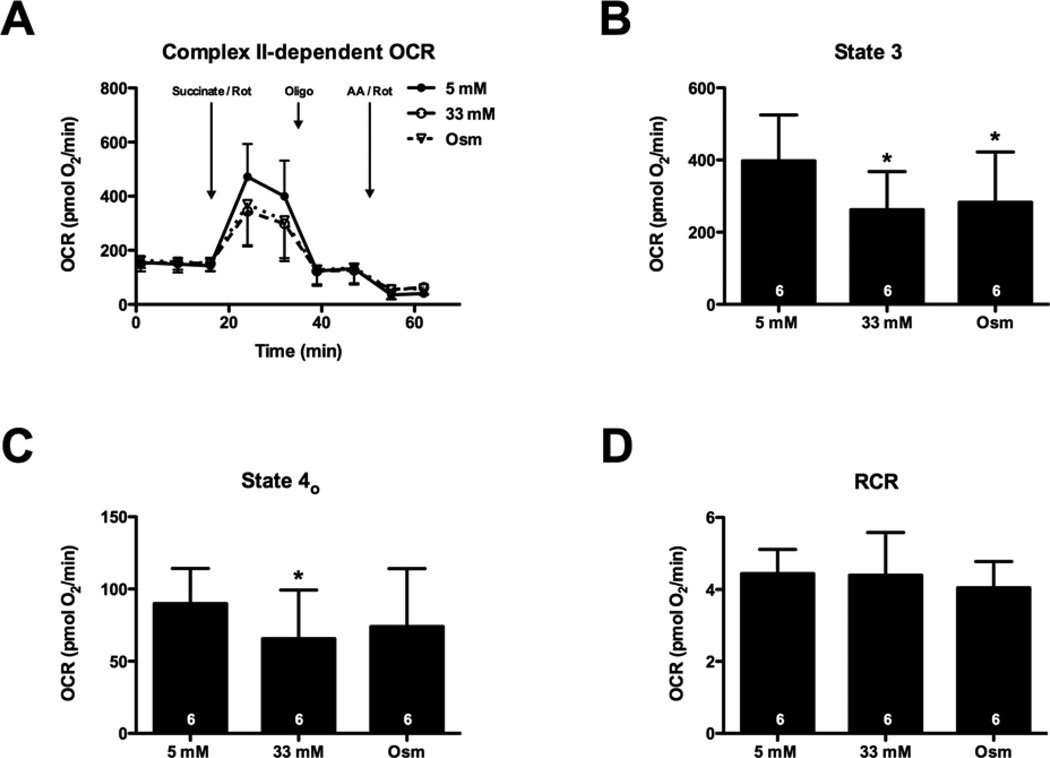
High glucose suppresses Complex II-dependent respiration
To determine the specific sites at which high glucose (and osmotic stress) might depress mitochondrial function, we subjected cardiomyocytes to conditions mimicking euglycaemia or hyperglycaemia for 48 h and then examined mitochondrial activity in permeabilized cells. We found Complex II-dependent respiration was depressed by high glucose (Figure 4). Succinate-supported State 3 and State 4o respiration was approximately 40% lower in cardiomyocytes incubated in high glucose (P < 0.05; Figures 4A–4C). Similar to the findings in intact cells, an osmotic control recapitulated the high-glucose-induced suppression of State 3 respiration (P < 0.05; Figure 4B). No change in respiratory control ratio (RCR) was found after high glucose treatment or in the osmotic control (Figure 4D). These data are consistent with intact cell data, suggesting that high osmolarity mediates depression in mitochondrial function.
Figure 4. High glucose depresses Complex II-dependent State 3 and 4o respiration.
NRCMs were treated for 48 h with 5 or 33 mM glucose or an osmotic control. During XF analysis, NRCMs were permeabilized and provided with succinate (to support Complex II-dependent respiration) as well as Rot (to inhibit Complex I activity). (A) XF assay of Complex II-dependent respiration: following three baseline OCR measurements in MAS buffer, the permeabilization agent, saponin, and succinate + Rot were injected. After two measurements, oligomycin (Oligo), then AA + Rot (AA–Rot) were injected sequentially, with measurements recorded after each injection. (B) State 3 OCR: The AA + Rot rate was subtracted from the succinate-stimulated rate to determine the State 3 rates. (C) State 4o OCR: The AA + Rot rate was subtracted from the oligomycin rate to obtain State 4o rates. (D) RCR: State 3/State 4o. As indicated in bars, n = 6 independent experiments; *P < 0.05, compared with 5 mM glucose.
OGA overexpression does not rescue the high-glucose-induced bioenergetic defect
Next, we reasoned that, if enhanced protein O-GlcNAcylation were necessary (though clearly insufficient) for the high-glucose-induced depression in bioenergetic reserve capacity, then decreasing protein O-GlcNAcylation should rescue this defect. To this end, we overexpressed OGA, which as expected decreased O-GlcNAcylation (Figures 5A and 5B). OGA overexpression produced detectable OGA in mitochondrial fractions; however, there was not a significant difference in O-GlcNAcylation in the mitochondrial fraction (Figures 5C and 5D). OGA overexpression did not rescue suppression of mitochondrial reserve capacity induced by high glucose (P < 0.05; Figure 5H). In fact, in cells incubated with high glucose, overexpression of OGA depressed both maximal respiration (P < 0.05; Figure 5G) and reserve capacity (P < 0.05; Figure 5H). Overexpression of OGA did not affect ATP-linked respiration or proton leak (Figures 5I and 5J). These results suggest that an elevated level of O-GlcNAcylation is neither necessary nor sufficient to explain high-glucose-induced suppression of mitochondrial bioenergetics.
Figure 5. Overexpression of OGA does not rescue high-glucose-induced suppression of bioenergetic reserve.
NRCMs were transduced with virus to overexpress OGA prior to treatment with hyperglycaemia. Ad-Null was used as a vector control. (A) Immunoblot for whole-cell OGA protein expression following viral transduction. (B) Whole-cell protein O-GlcNAcylation levels following adenoviral transduction. (C) Immunoblot for mitochondrial OGA following adenoviral overexpression. (D) Immunoblot for mitochondrial protein O-GlcNAcylation following adenoviral overexpression of OGA. (E) Mitochondrial function assay following 48 h of hyperglycaemic treatment. From assay in (E) parameters of mitochondrial function were measured: (F) basal respiration, (G) maximal respiration, (H) reserve capacity, (I) ATP-linked respiration, and (J) proton leak. As indicated in bars, n = 5 independent experiments; *P < 0.05, compared with 5 mM glucose + Ad-Null.
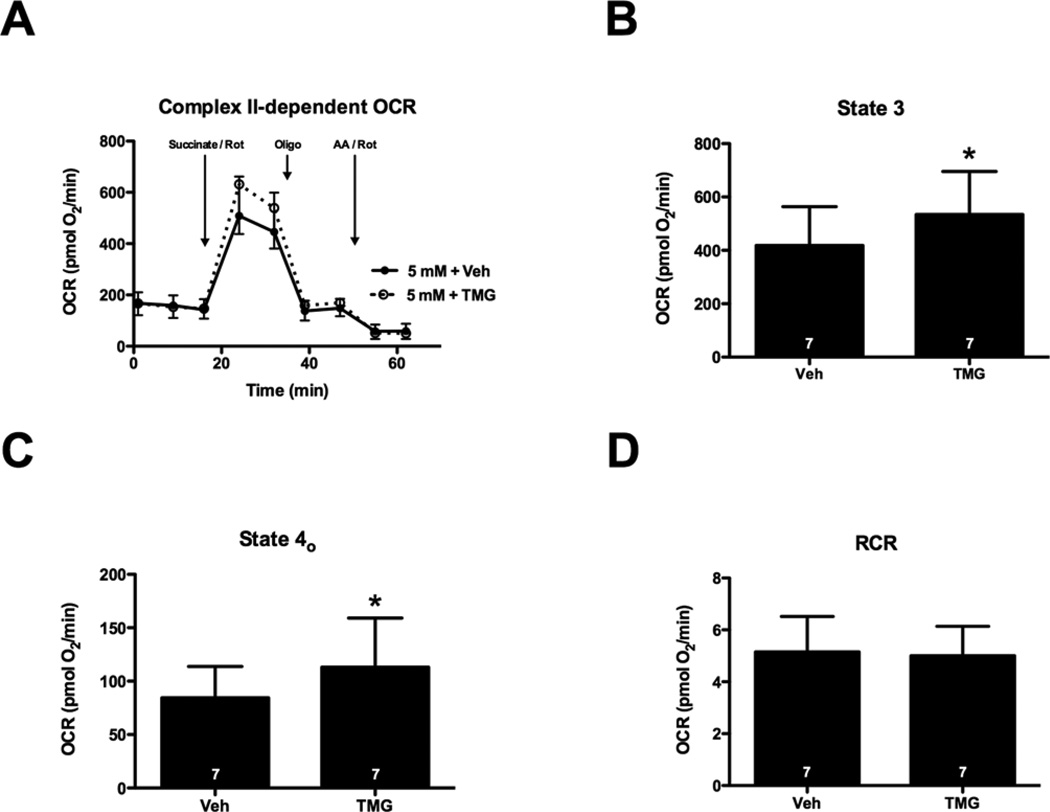
Inhibition of OGA increases Complex II-dependent State 3 respiration
Because high glucose impaired Complex II-dependent respiration (and induced epiphenomenal O-GlcNAcylation), we determined whether increasing O-GlcNAcylation with TMG (which inhibits OGA) might affect Complex II-dependent oxygen consumption in the permeabilized cell assay. Inhibition of OGA significantly increased protein O-GlcNAcylation (Figures 3A and 3B) and Complex II-dependent State 3 and 4o respiration (Figures 6A–6C); however, OGA inhibition did not significantly change respiration stimulated by provision of pyruvate and malate (Complex I) and it did not affect Complex III + IV-dependent OCR (Supplementary Figure S2). Thus, augmenting protein O-GlcNAcylation pharmacologically is not sufficient to depress mitochondrial function and may actually increase Complex II-dependent mitochondrial respiration.
Figure 6. Inhibition of OGA increases Complex II-dependent State 3 respiration.
NRCMs were treated for 24 h with 1 µM TMG to induce protein O-GlcNAcylation. During XF analysis NRCMs were permeabilized and provided with succinate (to support Complex II-dependent respiration), as well as Rot (to inhibit Complex I activity). (A) XF assay of Complex II-dependent respiration: following three baseline OCR measurements in MAS buffer, the permeabilization agent, saponin, and succinate + Rot were injected. After two measurements, oligomycin (Oligo) and AA + Rot (AA–Rot) were injected sequentially, with two measurements recorded after each injection. (B) State 3 OCR: the AA + Rot rate was subtracted from the succinate-stimulated rate to determine the State 3 rates. (C) State 4o OCR: the AA + Rot rate was subtracted from the oligomycin rate to obtain State 4o rates. (D) RCR: State 3/State 4o. As indicated in bars, n = 7 independent experiments; *P < 0.05.
DISCUSSION
In the present study, we directly addressed an emerging question regarding the relationship between high glucose, mitochondrial dysfunction and O-GlcNAcylation. First, we confirmed that high glucose could induce mitochondrial dysfunction and increase protein O-GlcNAcylation. Although impaired mitochondrial function was evident in high glucose conditions, an osmotic control exerted a similar suppressive effect on bioenergetic reserve; yet, the osmotic control did not increase O-GlcNAcylation. Furthermore, increasing O-GlcNAcylation through either OGA inhibition or OGT overexpression was insufficient to recapitulate the deleterious effects of high glucose on mitochondrial function. Finally overexpressing OGA in the context of hyperglycemia to reduce protein O-GlcNAcylation did not rescue the hyperglycemia-induced suppression of reserve capacity. Thus, increased O-GlcNAcylation is neither sufficient nor necessary for high-glucose-induced mitochondrial dysfunction in isolated cardiomyocytes.
Considering the manifold changes in metabolism and metabolic signalling that occur in diabetes, there are numerous options for targeted investigations. In particular, protein O-GlcNAcylation has received growing attention as a candidate component of diabetic pathophysiology [18]. This idea holds that elevations in extracellular glucose floods accessory pathways of glucose metabolism, one of which is the HBP, which produces the sugar donor for the O-GlcNAc modification of proteins. Indeed, elevated extracellular glucose concentration enhances OGT activity [10] and promotes protein O-GlcNAcylation [10,16,59]. Moreover, O-GlcNAcylation is enhanced in patients with diabetes [29,60]. Animal models of diabetes, such as streptozotocin-treated mice, Zucker rats and db/db mice, all demonstrate elevated O-GlcNAcylation of at least some proteins in the heart [13,14,16]. Thus, the coincident observations of increased O-GlcNAcylation and cardiac dysfunction during diabetes are consistent with the notion that O-GlcNAc plays a role in diabetic cardiac dysfunction.
Given the critical role of mitochondria in metabolism in general and the centrality of high mitochondrial activity to cardiac function specifically, several groups have pondered the potential role of hyperglycaemia/high glucose in mitochondrial dysfunction [61]. Moreover, some have attempted to identify a connection between O-GlcNAcylation and mitochondrial dysfunction [59,62]. In studies of suspended permeabilized NRCMs, Hu et al. [59] identified high-glucose-induced O-GlcNAcylation of cardiac mitochondrial Complexes I, III and IV and associated it with impaired mitochondrial function. Overexpression of OGA (to reverse the high-glucose-induced increase in O-GlcNAcylation) largely rescued the observed defects in mitochondrial complex activities. Interestingly, the study by Hu et al. [59] and our present work share several similarities: (1) both focused on mitochondrial function in NRCMs and assessed it, in part, via respirometry; (2) both used mannitol as an osmotic control; (3) both attempted adenovirus-mediated rescue of high-glucose-induced mitochondrial dysfunction and (4) both studies (at least in part) assessed cell function in permeabilized cells. Nevertheless, there were key differences in methodology between the two reports: (1) we evaluated mitochondrial function in adherent cardiomyocytes (intact and permeabilized); (2) we recapitulated the increase in O-GlcNAcylation via genetic overexpression of OGT and pharmacological inhibition of OGA (in the absence of high glucose); and (3) we maintained the intact cells in the respective glucose conditions (i.e. the high glucose cells were subjected to the XF assay in high glucose conditions). Any number of these relatively subtle study differences may partially explain the discrepancies in our findings.
In a related and recent study, Tan et al. [62] used a proteomics approach to study the effect of altering O-GlcNAc on mitochondrial protein expression, morphology and function in neuroblastoma cells. They observed that overexpression of either OGT or OGA resulted in altered mitochondrial expression of proteins involved in transport, translation and respiratory activity [62]. Furthermore, these changes resulted in alterations in mitochondrial morphology and function. On face value, it may appear that the results of Tan et al. [62] also disagree with our present results; however, the differences may be fewer than they appear. We too found that overexpression of either OGT or OGA reduced OCR (including maximal OCR) in the context of high glucose. Yet, when we overexpressed OGT or OGA in euglycaemic conditions, only basal respiration was slightly depressed. Interestingly, TMG, which remarkably increases O-GlcNAc levels, did not negatively affect OCR. Thus, these data indicate that there is not a clear and deleterious relationship between O-GlcNAcylation and mitochondrial dysfunction.
The fact that high-glucose-induced O-GlcNAcylation had little effect on mitochondrial energetics suggests an alternative mechanism by which hyperglycaemia impairs mitochondrial function. Interestingly, the defect in reserve capacity caused by the hyperglycaemic condition was recapitulated with the osmotic control, mannitol. Although we did not study the mechanism by which osmotic stress may have mediated changes in the mitochondrial function, this observation could be relevant for the response of myocytes to stress. For example, elevated by-products of anaerobic metabolism occurring during ischaemia create an osmotic load that has been estimated to be ∼60 mOsm [63–65]. This acute change in osmolality affects cell volume, cation/anion transport and other phenomena such as ischaemic pre-conditioning [66]. Hence, because cell volume is tightly linked with cation/anion transport and cation transport is a key regulator of ATP demand and mitochondrial activity, it is possible that the osmotic stress induced by high glucose reduces reserve capacity in a manner dependent on changes in these critical processes. Further studies are required to assess the full significance of osmotic stress to hyperglycaemia-induced myocyte stress.
A major question in the field relates to a mitochondrial O-GlcNAcylation system. Evidence of a mitochondrial OGT [(m)OGT] [67,68] would appear to suggest that a mitochondrial OGA [(m)OGA] should exist as well; however, our studies demonstrate that under normal conditions, mitochondria in myocytes have little, if any, detectable OGA and we only detected (m)OGA when we overexpressed OGA using adenoviruses. Because this is an overexpression system and fractionation procedures do not yield 100 % pure subcellular fractions, it is possible that the OGA we found in the mitochondrial fraction was a contaminant from the cytoplasm. Also, the transport mechanism for the essential substrate, UDP–GlcNAc, has never been identified in the mitochondrion. Hence, the nature of how mitochondrial O-GlcNAcylation occurs and whether it is due to a veritable mitochondrial O-GlcNAc-modification system, comprising a UDP–GlcNAc transporter, (m)OGA and (m)OGT, is still unclear. It is possible that O-GlcNAcylation of mitochondrial proteins predominately occurs in the cytosol prior to mitochondrial protein targeting.
In conclusion, several approaches, including overexpression of OGA or OGT, exposure to high glucose and pharmacological manipulation, were used to assess the role of O-GlcNAc in regulating cardiomyocyte bioenergetics. We demonstrate that O-GlcNAcylation is probably not responsible for mitochondrial dysfunction occurring during hyperglycaemic conditions and that osmotic stress due to high glucose appears to underlie depression of mitochondrial reserve capacity. Although these findings do not entirely rule out the involvement of O-GlcNAc in modulating bioenergetics in diabetes, they show that increasing O-GlcNAc levels are not sufficient or necessary to cause high-glucose-induced bioenergetic dysfunction.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Linda Harrison for her expert technical assistance with cardiomyocyte isolations.
FUNDING
This work was supported by the National Institutes of Health [grant numbers R01 HL083320, R01 HL094419, P20 GM103492 and P01 HL078825 (to S.P.J. and B.G.H.)]; an American Heart Association Postdoctoral Fellowship [grant number 0825643D (to H.T.F.)]; and an American Heart Association Predoctoral Fellowship [grant number 14PRE19710015 (to S.D.)].
Glossary
Abbreviations
- (m)OGA
mitochondrial OGA
- (m)OGT
mitochondrial OGT
- AA
antimycin A
- Ad-Null
null adeno virus
- Ad-OGA
adenoviruses carrying OGA gene
- Ad-OGT
adenoviruses carrying OGT gene
- DMEM
Dulbecco’s modified Eagle’s medium
- HBP
hexosamine biosynthetic pathway
- MAS
mannitol and sucrose
- NRCM
neonatal rat cardiomyocyte
- OCR
oxygen consumption rate
- OGA
O-GlcNAcase
- O-GlcNAc
β-O-linked-N-acetylglucosamine
- OGT
O-GlcNAc transferase
- Rot
rotenone
- TMG
Thiamet G
- XF
extracellular flux
Footnotes
AUTHOR CONTRIBUTION
Sujith Dassanayaka performed the experiments, analysed the data, generated the figures, wrote the draft and revised the manuscript. Ryan Readnower performed experiments, analysed data, wrote the draft and designed the experiments. Joshua Salabei performed the experiments and analysed data. Bethany Long performed the experiments and analysed data. Allison Aird performed the experiments and analysed the data. Yu-Ting Zheng performed the experiments and analysed the data. Senthilkumar Muthusamy performed and designed the experiments. Heberty Facundo performed and designed the experiments. Bradford Hill designed the experiments, revised the manuscript and provided funding. Steven Jones designed the experiments, wrote the draft, revised the manuscript and provided funding.
REFERENCES
- 1.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Hamby RI, Zoneraich S, Sherman L. Diabetic cardiomyopathy. JAMA. 1974;229:1749–1754. CrossRef PubMed. [PubMed] [Google Scholar]
- 3.Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. J. Clin. Invest. 1977;60:884–899. doi: 10.1172/JCI108843. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1489–H1506. doi: 10.1152/ajpheart.00278.2006. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 6.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reference deleted. [Google Scholar]
- 9.Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ. Heart Fail. 2013;6:1039–1048. doi: 10.1161/CIRCHEARTFAILURE.112.000228. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Akimoto Y, Kreppel LK, Hirano H, Hart GW. Increased O-GlcNAc transferase in pancreas of rats with streptozotocin-induced diabetes. Diabetologia. 2000;43:1239–1247. doi: 10.1007/s001250051519. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 11.Akimoto Y, Kreppel LK, Hirano H, Hart GW. Hyperglycemia and the O-GlcNAc transferase in rat aortic smooth muscle cells: elevated expression and altered patterns of O-GlcNAcylation. Arch. Biochem. Biophys. 2001;389:166–175. doi: 10.1006/abbi.2001.2331. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Buse MG, Robinson KA, Marshall BA, Hresko RC, Mueckler MM. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am. J. Physiol. Endocrinol. Metab. 2002;283:E241–E250. doi: 10.1152/ajpendo.00060.2002. PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC. Impact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am. J. Physiol. Cell Physiol. 2007;292:C1370–C1378. doi: 10.1152/ajpcell.00422.2006. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ. Res. 2005;96:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 15.Marsh SA, Dell’italia LJ, Chatham JC. Interaction of diet and diabetes on cardiovascular function in rats. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H282–H292. doi: 10.1152/ajpheart.00421.2008. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh SA, Dell’Italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids. 2011;40:819–828. doi: 10.1007/s00726-010-0699-8. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J. Biol. Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ. Res. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dassanayaka S, Jones SP. O-GlcNAc and the cardiovascular system. Pharmacol. Ther. 2014;142:62–71. doi: 10.1016/j.pharmthera.2013.11.005. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatham JC, Not LG, Fulop N, Marchase RB. Hexosamine biosynthesis and protein O-glycosylation: the first line of defense against stress, ischemia, and trauma. Shock. 2008;29:431–440. doi: 10.1097/shk.0b013e3181598bad. PubMed. [DOI] [PubMed] [Google Scholar]
- 23.Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim. Biophys. Acta. 2010;1800:80–95. doi: 10.1016/j.bbagen.2009.07.017. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci. STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Wells L, Vosseller K, Hart GW. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell. Mol. Life Sci. 2003;60:222–228. doi: 10.1007/s000180300017. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J. Biol. Chem. 1991;266:4706–4712. PubMed. [PubMed] [Google Scholar]
- 27.McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10695–10699. doi: 10.1073/pnas.152346899. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5313–5318. doi: 10.1073/pnas.072072399. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Park K, Comer F, Hsieh-Wilson LC, Saudek CD, Hart GW. Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes. Diabetes. 2009;58:309–317. doi: 10.2337/db08-0994. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoe S, Asahi M, Takeda T, Otsu K, Taniguchi N, Miyoshi E, Suzuki K. Inhibition of phospholamban phosphorylation by O-GlcNAcylation: implications for diabetic cardiomyopathy. Glycobiology. 2010;20:1217–1226. doi: 10.1093/glycob/cwq071. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 31.McLarty JL, Marsh SA, Chatham JC. Post-translational protein modification by O-linked N-acetyl-glucosamine: its role in mediating the adverse effects of diabetes on the heart. Life Sci. 2013;92:621–627. doi: 10.1016/j.lfs.2012.08.006. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am. J. Physiol. Cell. Physiol. 2007;292:C178–C187. doi: 10.1152/ajpcell.00162.2006. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 33.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am. J. Physiol. Cell. Physiol. 2008;294:C1509–C1520. doi: 10.1152/ajpcell.00456.2007. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2227–H2236. doi: 10.1152/ajpheart.01091.2006. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J. Mol. Cell. Cardiol. 2006;40:303–312. doi: 10.1016/j.yjmcc.2005.11.003. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 36.Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1711–H1719. doi: 10.1152/ajpheart.00553.2009. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J. Mol. Cell. Cardiol. 2008;45:313–325. doi: 10.1016/j.yjmcc.2008.04.009. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 39.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ. Res. 2009;104:41–49. doi: 10.1161/CIRCRESAHA.108.189431. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngoh GA, Jones SP. New insights into metabolic signaling and cell survival: the role of beta-O-linkage of N-acetylglucosamine. J. Pharmacol. Exp. Ther. 2008;327:602–609. doi: 10.1124/jpet.108.143263. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zafir A, Readnower R, Long BW, McCracken J, Aird A, Alvarez A, Cummins TD, Li Q, Hill BG, Bhatnagar A, Prabhu SD, et al. Protein O-GlcNAcylation is a novel cytoprotective signal in cardiac stem cells. Stem Cells. 2013;31:765–775. doi: 10.1002/stem.1325. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vibjerg Jensen R, Johnsen J, Buus Kristiansen S, Zachara NE, Botker HE. Ischemic preconditioning increases myocardial O-GlcNAc glycosylation. Scand. Cardiovasc. J. 2013;47:168–174. doi: 10.3109/14017431.2012.756984. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 44.Facundo HT, Brainard RE, Watson LJ, Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H2122–H2130. doi: 10.1152/ajpheart.00775.2011. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 46.Teshima Y, Akao M, Jones SP, Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ. Res. 2003;93:192–200. doi: 10.1161/01.RES.0000085581.60197.4D. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 47.Sansbury BE, Jones SP, Riggs DW, Darley-Usmar VM, Hill BG. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem. Biol. Interact. 2011;191:288–295. doi: 10.1016/j.cbi.2010.12.002. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. doi: 10.1007/s00726-010-0728-7. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sansbury BE, Jones SP, Riggs DW, Darley-Usmar VM, Hill BG. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem. Biol. Interact. 2011;191:288–295. doi: 10.1016/j.cbi.2010.12.002. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sansbury BE, Riggs DW, Brainard RE, Salabei JK, Jones SP, Hill BG. Responses of hypertrophied myocytes to reactive species: implications for glycolysis and electrophile metabolism. Biochem. J. 2011;435:519–528. doi: 10.1042/BJ20101390. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akao M, Ohler A, O’Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ. Res. 2001;88:1267–1275. doi: 10.1161/hh1201.092094. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 52.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Readnower RD, Brainard RE, Hill BG, Jones SP. Standardized bioenergetic profiling of adult mouse cardiomyocytes. Physiol. Genomics. 2012;44:1208–1213. doi: 10.1152/physiolgenomics.00129.2012. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill BG, Benavides GA, Lancaster JR, Jr, Ballinger S, Dell’Italia L, Jianhua Z, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 2009;424:99–107. doi: 10.1042/BJ20090934. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salabei JK, Gibb AA, Hill BG. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat. Protoc. 2014;9:421–438. doi: 10.1038/nprot.2014.018. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 59.Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Hu Y, Oyeleye MO, Dillmann WH. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J. Biol. Chem. 2009;284:547–555. doi: 10.1074/jbc.M808518200. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park K, Saudek CD, Hart GW. Increased expression of beta-N-acetylglucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes. 2010;59:1845–1850. doi: 10.2337/db09-1086. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovasc. Res. 2010;88:229–240. doi: 10.1093/cvr/cvq239. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan EP, Villar MT, E L, Lu J, Selfridge JE, Artigues A, Swerdlow RH, Slawson C. Altering O-Linked beta-N-Acetylglucosamine cycling disrupts mitochondrial function. J. Biol. Chem. 2014;289:14719–14730. doi: 10.1074/jbc.M113.525790. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steenbergen C, Hill ML, Jennings RB. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ. Res. 1985;57:864–875. doi: 10.1161/01.res.57.6.864. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 64.Tranum-Jensen J, Janse MJ, Fiolet WT, Krieger WJ, D’Alnoncourt CN, Durrer D. Tissue osmolality, cell swelling, and reperfusion in acute regional myocardial ischemia in the isolated porcine heart. Circ. Res. 1981;49:364–381. doi: 10.1161/01.res.49.2.364. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 65.Jennings RB, Reimer KA, Steenbergen C. Myocardial ischemia revisited. The osmolar load, membrane damage, and reperfusion. J. Mol. Cell. Cardiol. 1986;18:769–780. doi: 10.1016/s0022-2828(86)80952-x. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 66.Diaz RJ, Armstrong SC, Batthish M, Backx PH, Ganote CE, Wilson GJ. Enhanced cell volume regulation: a key protective mechanism of ischemic preconditioning in rabbit ventricular myocytes. J. Mol. Cell. Cardiol. 2003;35:45–58. doi: 10.1016/s0022-2828(02)00277-8. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 67.Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, Kochran J, Love DC. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch. Biochem. Biophys. 2003;409:287–297. doi: 10.1016/s0003-9861(02)00578-7. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- 68.Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J. Cell Sci. 2003;116:647–654. doi: 10.1242/jcs.00246. CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.