Abstract
Stress can promote palatable food intake, and consumption of palatable foods may dampen psychological and physiological responses to stress. Here we develop a rat model of daily limited sweetened drink intake to further examine the linkage between consumption of preferred foods and HPA axis responses to acute and chronic stress. Adult male rats with free access to water were given additional twice daily access to 4 ml of sucrose (30%), saccharin (0.1%; a noncaloric sweetener), or water. After 14d of training, rats readily learned to drink sucrose and saccharin solutions. One-half the rats were then given chronic variable stress (CVS) for 14d immediately following each drink exposure; the remaining rats (nonhandled controls) consumed their appropriate drinking solution at the same time. On the morning following CVS, responses to a novel restraint stress were assessed in all rats. Multiple indices of chronic stress adaptation were effectively altered by CVS. Sucrose consumption decreased the plasma corticosterone response to restraint stress in CVS rats and nonhandled controls; these reductions were less pronounced in rats drinking saccharin. Sucrose or saccharin consumption decreased corticotropin releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus. Moreover, sucrose attenuated restraint-induced c-fos mRNA expression in the basolateral amygdala, infralimbic cortex, and claustrum. These data suggest that limited consumption of sweetened drink attenuates HPA axis stress responses, and calories contribute, but are not necessary for this effect. Collectively the results support the hypothesis that the intake of palatable substances represents an endogenous mechanism to dampen physiological stress responses.
Keywords: sucrose, saccharin, CRH, c-fos, ACTH, corticosterone, rat
Introduction
Mounting evidence demonstrates that there is strong relationship between the intake of palatable food (e.g. calorically-dense food containing high amounts of carbohydrates and/or fats) and stress. Humans under stress generally increase consumption of palatable sweet and fatty foods (1–6). Rats under stress decrease total food intake, but increase consumption of palatable food when given this as a free access option (7–9). It has been hypothesized that individuals may consume more palatable “comfort” food during stress in an attempt to alleviate the emotional and/or physiological response to stress (5, 10, 11). In fact, intake of comfort food in humans is linked with improved emotional states (12), and a high carbohydrate diet is associated with reduced resting and stress-evoked cortisol levels (13–16).
One of the body’s physiological responses to stress (a real or perceived threat to homeostasis) is activation of the hypothalamic-pituitary-adrenocortical (HPA) axis, a neuroendocrine system that acts to maintain the constancy of the internal state (reviewed in (17)). In this system, information regarding a stressor is processed by numerous brainstem and limbic brain regions (e.g. the hippocampus, amygdala, and medial prefrontal cortex), and ultimately activates hypophysiotrophic neurons in the paraventricular nucleus of the hypothalamus (PVN). These PVN neurons then secrete releasing hormones (e.g. corticotropin releasing hormone (CRH) and arginine-vasopressin) that promote adrenocorticotropic hormone (ACTH) secretion by the anterior pituitary into systemic blood. ACTH subsequently evokes glucocorticoid (i.e. cortisol in humans and corticosterone in rats) release from the adrenal cortex. Glucocorticoids then exert many actions throughout the body including mobilization of stored energy and maintenance of blood pressure; glucocorticoids also exert negative-feedback onto the axis to assist in the termination of the acute stress response. However, during chronic stress, continual or repeated activation of the HPA axis results in marked changes in the tone of the HPA axis. For example, in rats chronic stress generally produces increased expression of CRH and/or arginine-vasopressin mRNA in the PVN, enlarged adrenal glands in response to adrenocortical stimulation, thymic involution in response to sustained elevations of plasma corticosterone levels, and diminished rate of body weight gain (18–30). Chronic stress also affects HPA axis hormone levels, typically including increased basal secretion (18, 20, 22, 30–32), habituated responses to homotypic (familiar) stressors (23, 30, 33, 34), and facilitated responses to heterotypic (novel) stressors (21, 31, 35).
Emerging evidence suggests that palatable food intake affects HPA axis activity. For example, free access to sucrose drink reverses the stimulatory effects of adrenalectomy on the HPA axis, whereas the noncaloric artificial sweetener saccharin is without effect, suggesting that the metabolic consequences of sucrose consumption down-regulate the HPA axis (36, 37). Rats given free access to sucrose drink also attenuate HPA axis responses to acute and chronic stress (8, 38, 39). However, rats given free access to sucrose drink consume a large proportion of their daily calories as sucrose (~30–40%), resulting in dramatically decreased (~30%) intake of chow (8, 38, 39). The marked decrease in chow intake may confound comparisons to saccharin and water control groups, since it is difficult to resolve the extent to which potential effects are due to the intake of sucrose versus the decreased intake of other nutrients. Moreover, while consumption of such large amounts of sucrose may reflect behavior in certain groups of people, it may not be reflective of palatable food intake by the general population. While meal and snack patterns and composition vary greatly between individuals and cultures, Americans and Europeans typically consume 3 meals and 2 snacks per day; snacks are generally high in carbohydrates and contribute roughly 11–18% of daily caloric intake (1, 40–44). In the present work we develop a paradigm of rat palatable food intake that is intended to model more typical human snacking behavior. Rats with free access to normal chow are given small amounts of sucrose or saccharin drink twice daily for several weeks both in the presence and absence of chronic stress. This model is then applied to test the hypothesis that a history of limited access to small amounts of palatable drink attenuates the HPA axis response to both acute and chronic stress. The results indicate that daily limited access to palatable drink attenuates HPA responses to stress, and while calories amplify this effect, they are not necessary.
Materials and Methods
Animals
Adult male Long-Evans rats (250g; Harlan, supplied by the Indianapolis facility) were used. Rats arrived at least one week prior to the onset of experiments and were housed individually on a 12-hour light-dark cycle (06:00–18:00 h) with ad libitum access to normal rat chow (LM-485; Harlan Teklad, Madison, WI) and water. All procedures were approved by the University of Cincinnati Animal Care and Use Committee. Food intake and body weight were monitored periodically throughout the study.
Drink training
Rats (n= 16–17/group) with free access to water were given additional twice daily (at approximately 09:00 and 15:00 h) access to 4 ml of sucrose (30%; Sigma-Aldrich Co., St. Louis, MO), sodium saccharin (0.1%; Sigma-Aldrich Co.), or water. After two weeks of drink training, half of each group of rats was then given chronic variable stress (CVS) for 14d, with the rats receiving their respective solutions immediately prior to each stress exposure; solutions were given just before each stress exposure in an effort to maximize the potential effects of drink on stress responses. The remaining rats (nonhandled controls) consumed their appropriate drink solution at the same time, but were not given CVS.
A relatively small maximal volume of drink intake (4 ml/session) was selected to insure that control rats would reliably drink the full volume, thereby minimizing variations in the amount consumed as a factor contributing to increased variability of effects. The relatively high concentration of sucrose (30%) was used to maximize the number of calories contained in the volume of drink permitted (8 ml/day with about 9 calories/day). Saccharin was included as a noncaloric sweet drink (45) to determine whether calories are necessary for the effects of sweet drink; the concentration of 0.1% was chosen based on literature reports (45, 46), and our own preliminary finding that the acquisition of training was optimal at this concentration.
Stress exposure
The present study used CVS, a well-characterized rodent model that minimizes stressor-specific habituation and produces typical features of chronic stress exposure, including diminished body weight gain, adrenal enlargement, thymic involution, elevated morning nonstress plasma corticosterone levels, and facilitated plasma ACTH and corticosterone responses to a novel stressor (18, 25, 47). CVS consisted of twice daily exposure to one of several stressors presented in an unpredictable order. All CVS rats were given the same schedule of CVS stress exposure. Stressors included: 20-min hypoxia (8% oxygen, 92% nitrogen), 20-min warm swim (26–30°C), 10-min cold swim (17–18°C), 1 hour in cold room (4°C), 5-min novel environment, and 1 hour on rotating platform (90 rpm). In addition, CVS rats were housed overnight in novel guinea pig cages (on the evenings of experiment days 17, 19, 22 and 25) or mouse cages (on the evenings of experiment days 16, 21, 24 and 27). On the morning (08:00) following completion of the CVS paradigm (day 29), rats did not receive their respective drink solutions and were given a novel restraint stress challenge. All rats, both control and CVS, were placed into well-ventilated restraint tubes for 20 min with tail clip blood sampling (200 µl) into chilled tubes containing EDTA at 0, 20, and 40 min after initiation of restraint. Rats were decapitated at 60 min after initiation of restraint and trunk blood was collected. Adrenal and thymus glands were removed, cleaned and weighed. Carcasses were frozen at −20°C until subsequent dissection and weighing of fat depots (retroperitoneal, mesenteric, epidydimal, inguinal, and interscapular brown). Blood samples were centrifuged (3000 g, 15 min, 4°C) and plasma was stored at −20°C until measurement of immunoreactive ACTH and corticosterone concentrations by radioimmunoassay as described previously (48). Brains were removed and frozen in isopentane cooled on dry ice (−45°C) and stored at −80°C. Brains were then cryosectioned (14 µm) on a Microm cryostat, thaw-mounted on Gold Seal Ultrastick slides (Portsmouth, NH), and stored at −20°C until assessment of CRH and c-fos mRNA expression by in situ hybridization.
CRH and c-fos in situ hybridizations
A one-in-ten series of brain sections was fixed in 4% phosphate-buffered paraformaldehyde for 10 min and rinsed twice in 5 mM potassium phosphate-buffered saline (KPBS) for 5 min, twice in KPBS containing 0.2% glycine for 5 min, and twice in KPBS for 5 min. Sections were then acetylated in 0.25% acetic anhydride (suspended in 0.1 M triethanolamine, pH 8) for 10 min, rinsed twice in 2× saline sodium citrate (SSC) for 5 min, and dehydrated through graded alcohols.
Antisense cRNA probes complementary to CRH (765 bp)(49) and c-fos (587bp)(49) were generated by in vitro transcription using 35S-UTP. The CRH fragment was cloned into a pGem3 vector, linearized with HindIII, and transcribed with T7 RNA polymerase. The c-fos fragment (original full-length cDNA from T. Curran, St. Jude Children’s Research Hospital, Memphis, TN) was cloned into a pGEM 4z vector, linearized with HindIII, and transcribed with SP6 RNA polymerase. Each transcription reaction (15 µl) consisted of 1× transcription buffer, 62.5 µCi 35S-UTP, 330 µM ATP, 330 µM GTP, 330 µM CTP, 10 µM cold UTP, 66.6 mM dithiothreitol, 40 U ribonuclease inhibitor, 20 U T7 or SP6 RNA polymerase, and 2.5 µg linearized DNA. The transcription reaction was incubated at 37°C for 60 min, and the labeled probe was separated from free nucleotide by ammonium acetate precipitation.
35S-probe was diluted in hybridization buffer (50% formamide, 20 mM Tris-HCl at pH 7.5, 1 mM EDTA, 335 mM NaCl, 1× Denhardt’s solution, 200 µg/ml herring sperm DNA, 100 µg/ml yeast tRNA, 20 mM dithiothreitol, and 10% dextran sulfate) to yield 1,000,000 cpm/50 µl buffer. A 50 µl aliquot of diluted probe was applied to each slide. Slides were then coverslipped and incubated overnight at 50°C in humidified chambers containing 50% formamide. The next morning, coverslips were removed in 2× SSC and slides were incubated in 100 µg/ml ribonuclease A for 30 min at 37°C. Slides were rinsed in 2× SSC, rinsed and incubated in 0.2× SSC (65°C) for 1 hour, dehydrated through graded alcohols, and exposed to Kodak Biomax MR-2 film for 7 or 13 days for CRH and c-fos probes, respectively. All tissue sections hybridized for CRH were processed in a single assay; tissue hybridized for c-fos was divided into two assays containing either anterior brain regions (prior to the decussation of the anterior commissure) or posterior brain regions. Slides hybridized with sense probe were used as a negative control. In addition, all autoradiographs included ARC 146-14C standard slides (American Radiolabeled Chemicals, Inc., St. Louis, MO) as internal controls to verify that film exposure was not saturated and was equal between films.
This study uses c-fos mRNA induction as an indirect index of recent neuronal activation. It is well-established that basal, non-stimulated levels of c-fos mRNA expression are very low (e.g. near the limit of detection) in most brain regions (50–55); these levels were not assessed in the present experiment in an attempt to limit the number of rats required. Following acute stress exposure, c-fos mRNA can be detected as early as 15 min after stress onset, with levels peaking at 30–60 min and returning to baseline at 90–180 min after stress onset (50–53, 55). Our selection of a 60-min post-stress collection time was based on our primary goal of assessing plasma hormone levels through this time point, and the expression of c-fos mRNA at this time is likely near the peak of the response to stress. It should also be noted that while analysis of c-fos mRNA expression has been validated as a tool for neuronal mapping, it has certain limitations. For instance, c-fos mRNA is not up-regulated in all neurons following activation and neurons that are inhibited in a circuit cannot be identified. Lastly, basal levels of CRH mRNA in the PVN are relatively high and show either no, or a slight, increase at 60 min after acute stress (51–53), suggesting that the CRH mRNA assessed at this time point is predominantly reflective of expression prior to the acute stress challenge.
Image analysis
Semi-quantitative analyses of in situ hybridization autoradiographs were conducted using Scion Image 1.62 software (Scion, Frederick, MD). Anatomical regions of interest were determined based on the Paxinos and Watson and Swanson rat brain atlases (56, 57). CRH mRNA expression was assessed in the PVN, central amygdala (CeA), and oval (ovBST) and fusiform (fuBST) subregions of the bed nucleus of the stria terminalis (BST). c-fos mRNA expression was utilized as an indirect index of neuronal activation, and was measured in the cortex (anterior cingulate, anterior gustatory, infralimbic, orbitofrontal, piriform, posterior gustatory, and prelimbic); hippocampus (CA1, CA3 and dentate gyrus (DG)); lateral septum (intermediate and ventral); hypothalamus (anterodorsal preoptic nucleus (ADP), dorsomedial hypothalamic nucleus (DMH), lateral hypothalamic area (LHA), PVN, and the ventrolateral portion of the medial preoptic area (vlMPOA)); amygdala (basolateral amygdala (BLA) and medial amygdala (MeA)); claustrum; lateral habenula; posterior portion of the BST (Post. BST); and posterior paraventricular nucleus of the thalamus (Post. PVThal). We also attempted to measure c-fos mRNA expression in the nucleus accumbens core and shell, however restraint stress did not evoke c-fos mRNA expression above background in these regions. Background signal was determined over a non-hybridized area of tissue (e.g. white matter) and subtracted from total gray level to obtain corrected gray level units. The mean value of 2–4 sections through a given region (approximately 4–8 individual measurements) was calculated for each rat and used in the statistical analysis. All in situ hybridization analyses were performed by an observer unaware of group assignments.
Statistical analyses
Data are shown as mean ± SEM. All of the statistical analysis methods employed in this work were determined prior to the experiment. Organ and fat pad weights are reported as actual and adjusted (i.e. normalized to body weight) weights. Organ and fat pad weight data were each analyzed by two-way analysis of variance (ANOVA) with CVS (Control, CVS) and DRINK (water, saccharin, sucrose) as between-subjects factors. Body weight gain (calculated as the percent increase from the initial body weight) and food intake were each analyzed by three-way ANOVA with CVS and DRINK as between-subject factors and DAY (1–14; 15–29) as a within-subjects factor. Average daily drink intake from the sippers was analyzed by three-way ANOVA with CVS and DRINK as between-subject factors and DAY (1–7; 8–14; 15–21; 22–28) as a within-subject measure. Time courses of plasma ACTH and corticosterone responses to restraint were each analyzed by three-way ANOVA with CVS and DRINK as between-subject factors and TIME (0, 20, 40, 60 min) as a within subject factor. Integrated plasma hormone responses were calculated as the area under the curve (AUC) of the time course data. The effects of drink type and CVS on integrated hormone responses and unstressed AM hormone levels were each determined by two-way ANOVA with DRINK and CVS as between-subject factors. CRH and c-fos mRNA expression were analyzed by a two-way ANOVA with DRINK and CVS as between-subject factors for each brain region examined. When the variance was not homogenous, analyses were performed after square-root transform. To determine individual group differences, specific planned pair-wise comparisons were determined by protected Fisher’s LSD procedure; no further adjustments were made to control for the experimentwise error rate. Potential outliers were assessed using two different tests: 1) outliers were values that differed from the mean by more than 1.96 times the standard deviation, and 2) outliers were values that were below the lower quartile or above the upper quartile by more than 1.5 times the interquartile range (58). A positive identification by both outlier tests was required before a value was removed from the analysis. Statistical significance was taken as p<0.05.
Results
Organ weights
CVS decreased both actual thymus weight (CVS, F1,49 = 49.24, p<0.05) and adjusted thymus weight (CVS, F1,49 = 43.32, p<0.05), suggesting that CVS effectively induced thymic atrophy (Table 1). Neither actual nor adjusted thymus weights were affected by type of drink and no CVS-DRINK interactions were observed.
Table 1.
CVS decreased actual and adjusted thymus weight and increased adjusted adrenal weight. CVS also decreased final body weight and the amount of body weight gained over the course of the experiment.
| Control | CVS | |||||
|---|---|---|---|---|---|---|
| Water | Saccharin | Sucrose | Water | Saccharin | Sucrose | |
| Thymus weight (mg) | 343 ± 34 | 409 ± 25 | 359 ± 23 | 238 ± 18# | 237 ± 17# | 229 ± 23# |
| Thymus weight (mg)/100g body weight | 95 ± 7 | 106 ± 6 | 97 ± 4 | 71 ± 4# | 72 ± 5# | 68 ± 6# |
| Adrenal weight (mg) | 48.7 ± 0.9 | 48.3 ± 1.6 | 46.4 ± 1.9 | 47.7 ± 3.0 | 50.3 ± 2.2 | 49.8 ± 2.8 |
| Adrenal weight (mg)/100 g body weight | 13.7 ± 0.4 | 12.8 ± 0.5 | 12.6 ± 0.4 | 14.3 ± 0.9 | 15.3 ± 0.7# | 15.0 ± 0.6# |
| Final body weight (g) | 356 ± 9 | 379 ± 8 | 368 ± 10 | 335 ± 8 | 330 ± 6# | 333 ± 10# |
| Body weight gained (g) | 97 ± 5 | 116 ± 7 | 103 ± 6 | 71 ± 6# | 66 ± 3# | 70 ± 7# |
Data are shown as mean±SEM. n= 8–9/group.
p<0.05 vs Control.
Actual adrenal weight was not affected by CVS, whereas adjusted adrenal weight was increased by CVS (CVS, F1,49 = 12.20, p<0.05), suggesting that CVS induced modest adrenal hypertrophy (Table 1). Neither actual nor adjusted adrenal weights were affected by type of drink and no CVS-DRINK interactions were observed.
Drink Intake
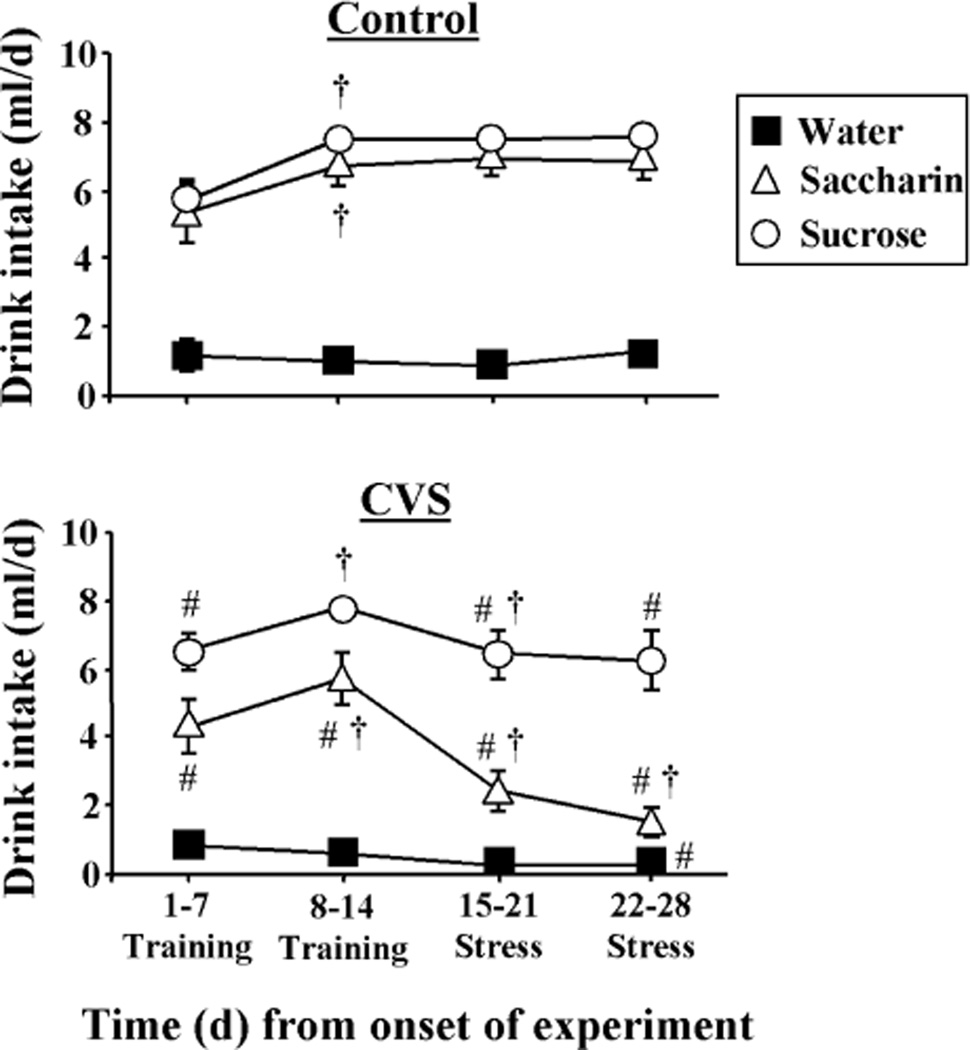
Daily intake from the sippers (Figure 2) was affected by the type of drink (DRINK, F2,199 = 101.76, p<0.05), the day of study (DAY, F3,199 = 14.66, p<0.05), and exposure to CVS (CVS, F1,199 = 12.87, p<0.05). There were also significant DRINK-CVS (F2,199 = 5.57, p<0.05), DRINK-DAY (F6,199 = 6.87, p<0.05), CVS-DAY (F3,199 = 25.71, p<0.05), and DRINK-DAY- CVS (F6,199 = 6.20, p<0.05) interactions. More specifically, multiple comparison analysis revealed that rats drank very little water from the sippers, and the amount consumed did not vary with time or exposure to CVS. In contrast, rats drinking sucrose and saccharin rapidly learned to drink the solutions at amounts greater than that for water, and intake further increased through days 8–14. In control rats, sucrose and saccharin consumption remained high through the remainder of study. In CVS rats, sucrose consumption decreased slightly with the onset of CVS and then stabilized at this lower level throughout the remainder of the study, whereas saccharin consumption decreased markedly at onset of CVS and continued to decrease throughout the end of the study. Lastly, the percentage of daily calories obtained from sucrose per 100 g body weight on days 15–28 was not affected by CVS (Control= 2.94 ± 0.18, CVS= 3.05 ± 0.39).
Figure 2.
Rats readily learned to drink sucrose and saccharin and intake was reduced during chronic stress. Time course of mean daily drink intake (8 ml/day maximum) for Control (top) and CVS (bottom) rats receiving sucrose, saccharin or water twice daily via sippers (in addition to ad lib access to food and water). #p<0.05 vs Control. †p<0.05 vs previous time point. Not noted on figure: all sucrose and saccharin values are different from water. n=8–9/group.
In order to determine whether the effects of CVS on drink intake correlated with CVS-induced reductions in body weight, we also analyzed the average daily sipper intake for days 22–28 normalized to the final body weight (data not shown). This analysis revealed a main effect of drink type (DRINK, F2,53 = 102.40, p<0.05), exposure to CVS (CVS, F1,53 = 24.86, p<0.05), and an interaction (DRINK-CVS, F2,53 = 13.93, p<0.05). Multiple comparison analysis showed that normalized sipper intake in control rats was minimal in those drinking water, and increased to the same extent in those drinking sucrose or saccharin. Normalized saccharin intake was dramatically reduced by CVS exposure, whereas normalized sucrose and water intake were not affected by CVS.
Plasma hormones
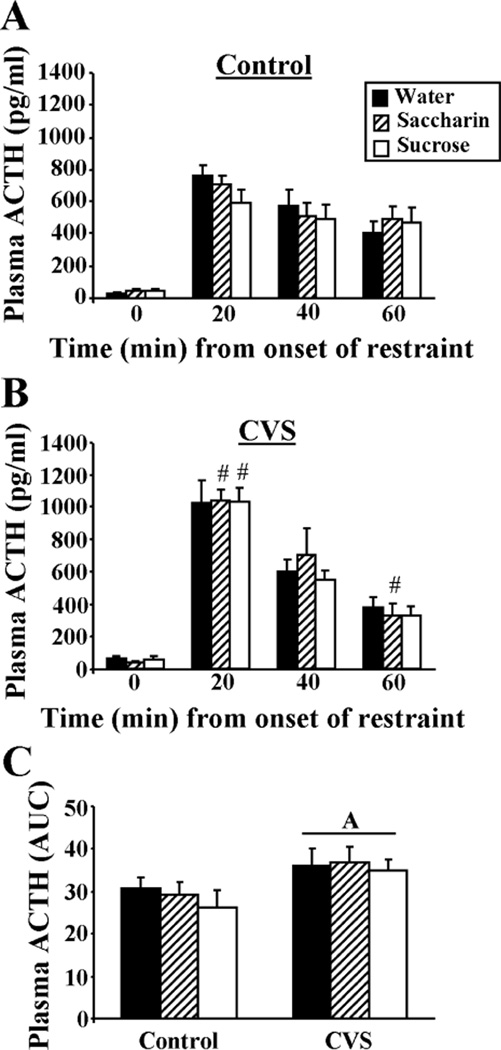
The time course of the plasma ACTH response (Figure 3A,B) to acute restraint stress challenge showed a main effect of time from onset of restraint (TIME, F3,199 = 257.40, p<0.05), but no main effects of CVS or type of drink. Moreover, there was a CVS-TIME interaction (F3,199 = 9.29, p<0.05), but no other interactions. Unstressed AM plasma ACTH (Figure 3A,B; time 0 min.) showed no main effects of CVS or type of drink and no CVS-DRINK interaction. The integrated plasma ACTH response to restraint (Figure 3C) was affected by prior CVS experience (CVS, F1,49 = 6.63, p<0.05), but not type of drink, and there was no CVS-DRINK interaction. In summary, multiple comparison analyses revealed that plasma ACTH increased after restraint with a peak at 20 min after onset of restraint and then decreased throughout 60 min regardless of type of drink. Prior CVS did not affect basal plasma ACTH, but increased the plasma ACTH response to restraint (by both time course and integrated plasma ACTH data), suggestive of chronic stress-induced facilitation; this facilitation occurred regardless of type of drink.
Figure 3.
The plasma ACTH response to restraint (20-min) was augmented by prior CVS exposure and not affected by type of drink. The time course of restraint-stress induced plasma ACTH for Control (A) and CVS (B) rats receiving sucrose, saccharin or water twice daily via sippers (in addition to ad lib access to food and water). #p<0.05 vs Control. Not noted on figure: all values at 20, 40, and 60 min are greater than 0 min. (C) The integrated (area under the curve, AUC) plasma ACTH response to restraint. A= main effect of CVS vs Control. n=7–9/group.
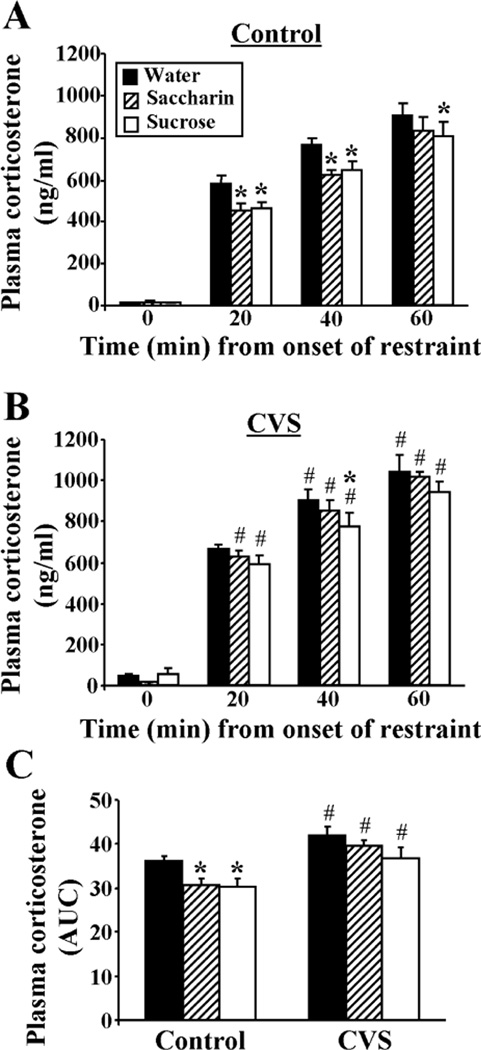
The time course of the plasma corticosterone response (Figure 4A,B) to acute restraint stress challenge showed main effects of type of drink (DRINK, F2,199 = 4.11, p<0.05), prior CVS exposure (CVS, F1,199 = 23.23, p<0.05), and time from onset of restraint (TIME, F3,199 = 794.32, p<0.05), in addition to a CVS-TIME interaction (F3,199 = 5.08, p<0.05). Unstressed AM plasma corticosterone (Figure 4A,B; time 0 min.) was increased by prior CVS (CVS, F1,45 = 4.80, p<0.05), but was not affected by type of drink, and there was no CVS-DRINK interaction. The integrated plasma corticosterone response to restraint (Figure 4C) had a main effect of drink type (DRINK, F2,49 = 4.63, p<0.05) and prior CVS (CVS, F1,49 = 22.57, p<0.05). Collectively, multiple comparison analyses revealed that plasma corticosterone increased through 60 min after onset of a 20-min restraint stress. The corticosterone response in Controls was reduced by a history of sucrose or saccharin drink, as shown in both the time course and integrated plasma corticosterone data. This observed reduction in the integrated plasma corticosterone response to restraint suggests that the magnitude of attenuation by saccharin and sucrose was physiologically relevant. The plasma corticosterone response in CVS rats was also reduced slightly by sucrose, but not by saccharin; these attenuated effects may reflect that CVS rats drink slightly less total sucrose and much less saccharin than Control rats. Lastly, prior CVS increased (1) unstressed AM plasma corticosterone, and (2) the corticosterone response to restraint (by both time course and integrated plasma corticosterone data), suggestive of chronic stress-induced facilitation; this facilitation occurred regardless of type of drink.
Figure 4.
The plasma corticosterone response to restraint was attenuated by sucrose and saccharin drink in Control rats, and CVS facilitated the plasma corticosterone response to restraint regardless of type of drink. The time course of restraint-stress induced plasma corticosterone for Control (A) and CVS (B) rats receiving sucrose, saccharin or water twice daily via sippers (in addition to ad lib access to food and water). (C) The integrated (area under the curve, AUC) plasma corticosterone response to restraint. *p<0.05 vs water, #p<0.05 vs Control. Not noted on figure: all values at 20, 40, and 60 min are greater than 0 min. n=7–9/group. Please note that the statistics depicted on the figure resulted from a three-way ANOVA comparing DRINK, CVS, and TIME. A separate planned 2-way ANOVA at the 0-min time point revealed a main effect of CVS to increase basal plasma corticosterone, which is not indicated on the figure.
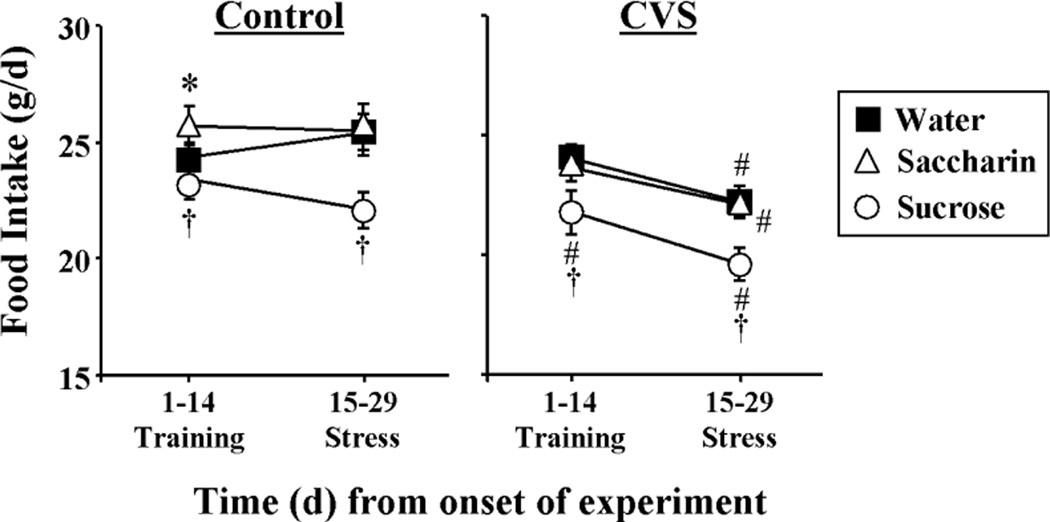
Food intake
Food intake (Figure 5) was affected by type of drink (DRINK, F2,99 = 7.72, p<0.05), the day of study (TIME, F1,99 = 29.28, p<0.05), and prior exposure to CVS (CVS, F1,99 = 13.90, p<0.05). There were also DRINK-TIME (F2,99 = 4.85, p<0.05) and CVS-TIME (F1,99 = 22.99, p<0.05) interactions. More specifically, multiple comparison analysis showed that sucrose drink decreased food intake regardless of experimental day or CVS exposure. Also, CVS during days 15–28 decreased food intake relative to (1) intake prior to the onset of CVS (days 1–14) and (2) intake of the respective Controls during the same time period (days 15–28). This CVS-induced reduction of food intake occurred regardless of type of drink.
Figure 5.
Food intake was decreased by sucrose drink, and CVS reduced food intake regardless of type of drink. The time course of average daily food intake for Control (left) and CVS (right) rats receiving sucrose, saccharin or water twice daily via sippers (in addition to ad lib access to food and water). *p<0.05 vs water, #p<0.05 vs Control, †p<0.05 vs water and saccharin. Not noted on figure: 15–29 days is different from 1–14 days for all groups except Saccharin Control. n=8–9/group.
Body weight
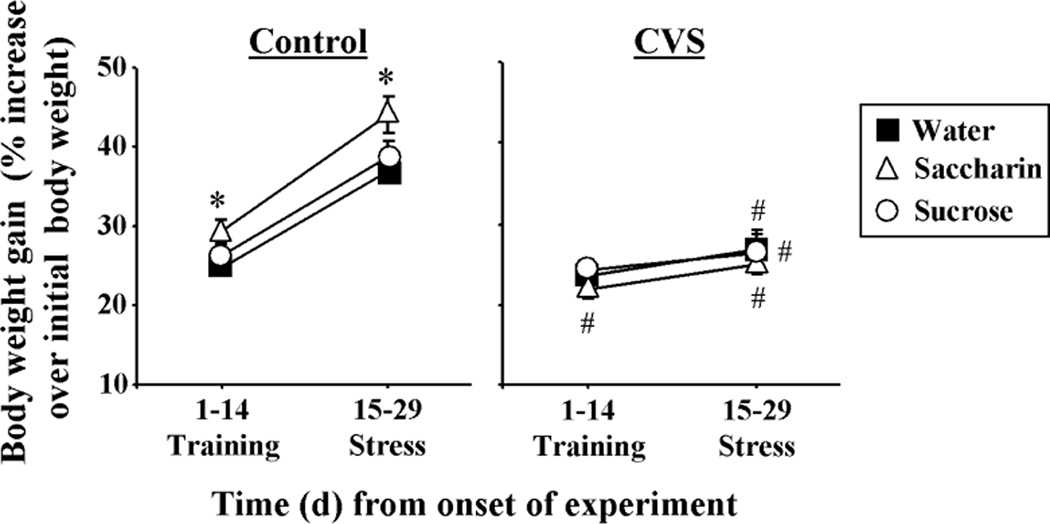
Final body weight was reduced by chronic stress (F1,49 = 24.88, p<0.05), with no effect of drink type or DRINK-CVS interaction (Table 1). In addition, total body weight gained through the experiment was reduced by chronic stress (F1,49 = 58.39, p<0.05), with no effect of drink type or DRINK-CVS interaction (Table 1). Percent body weight gain (Figure 6) showed main effects of CVS (CVS, F1,99 = 51.08, p<0.05) and the day of study (DAY, F1,99 = 338.09, p<0.05), but no main effect of type of drink. There were also DRINK-CVS (F2,99 = 3.75, p<0.05) and CVS-DAY interactions (F1,99 = 141.82, p<0.05). Multiple comparison analysis revealed that all Control rats gained body weight during days 1–14, with further increases during days 15–29. Moreover, Control rats drinking saccharin gained more body weight during days 1–14 than those drinking water or sucrose, and maintained this increase at 15–29 days. CVS rats drinking saccharin gained less body weight during training days 1–14 than their nonstress controls. Also, for all types of drink, body weight gained during CVS (days 15–29) was less than the respective nonstress Controls, suggesting that CVS attenuated body weight gain regardless of type of drink.
Figure 6.
Body weight gain was attenuated by CVS regardless of type of drink. The time course of increased body weight for Control (left) and CVS (right) rats receiving sucrose, saccharin or water twice daily via sippers (in addition to ad lib access to food and water). *p<0.05 vs water and sucrose, # p<0.05 vs Control. Not noted on figure: 15–29 days is different from 1–14 days for all groups except Sucrose CVS. n= 8–9/group.
Fat pad weights
There was a main effect of CVS to decrease the actual weight of all white fat depots examined (Table 2): retroperitoneal (F1,49 = 22.91, p<0.05), mesenteric (F1,49 = 30.32, p<0.05), epidydimal (F1,49 = 17.12, p<0.05), inguinal (F1,49 = 21.14, p<0.05), and total (F1,49 = 28.87, p<0.05). In contrast, there was no effect of CVS on the actual weight of the interscapular brown fat, and there was no main effect of drink type and no DRINK-CVS interaction for the actual weights of any of the fat pads.
Table 2.
CVS decreased the individual (retroperitoneal, mesenteric, epidydimal, and inguinal) and total weight of white fat depots regardless of type of drink. CVS did not affect actual interscapular brown fat weight and modestly increased interscapular brown fat weight normalized to body weight.
| Control | CVS | |||||
|---|---|---|---|---|---|---|
| Water | Saccharin | Sucrose | Water | Saccharin | Sucrose | |
| Retroperitoneal (g) | 4.1 ± 0.6 | 4.4 ± 0.5 | 3.9 ± 0.6 | 2.5 ± 0.2# | 2.5 ± 0.2# | 2.6 ± 0.2# |
| Mesenteric (g) | 3.7 ± 0.2 | 4.2 ± 0.3 | 3.6 ± 0.3 | 2.7 ± 0.2# | 3.0 ± 0.2# | 2.7 ± 0.1# |
| Epidydimal (g) | 3.9 ± 0.3 | 4.1 ± 0.3 | 4.0 ± 0.5 | 3.0 ± 0.3# | 3.0 ± 0.2# | 3.0 ± 0.1# |
| Inguinal (g) | 4.4 ± 0.4 | 4.5 ± 0.4 | 4.1 ± 0.4 | 3.1 ± 0.2# | 2.8 ± 0.1# | 3.2 ± 0.2# |
| Total WAT (g) | 16.0 ± 1.3 | 17.2 ± 1.3 | 15.6 ± 1.7 | 11.2 ± 0.8# | 11.3 ± 0.5# | 11.4 ± 0.5# |
| Total WAT (g)/100g body weight | 4.5 ± 0.3 | 4.5 ± 0.3 | 4.2 ± 0.4 | 3.3 ± 0.2# | 3.4 ± 0.2# | 3.4 ± 0.1# |
| Interscapular BAT (g ×10) | 4.0 ± 0.3 | 3.8 ± 0.4 | 3.3 ± 0.2 | 4.2 ± 0.5 | 4.3 ± 0.5 | 3.9 ± 0.2 |
| Interscapular BAT (g ×10)/1000g body weight | 11.2 ± 0.6 | 9.9 ± 0.9 | 9.1 ± 0.4 | 12.5 ± 1.2 | 13.1 ± 1.4# | 11.7 ± 0.8 |
All data are shown as mean ± SEM. n= 8–9/group.
p<0.05 vs. Control. WAT= white adipose tissue, BAT= brown adipose tissue.
When fat pad weights were normalized to body weight (Table 2 and data not shown), the adjusted weights of the retroperitoneal (F1,49 = 19.31, p<0.05), mesenteric (F1,49 = 19.22, p<0.05), epidydimal (F1,49 = 5.93, p<0.05), inguinal (F1,49 = 19.95, p<0.05) and total white adipose (F1,49 = 21.90, p<0.05) depots were decreased by CVS. The adjusted interscapular brown fat weight was increased by CVS (F1,49 = 9.21, p<0.05) and multiple comparison analysis showed that this occurred primarily in the rats drinking saccharin. There was no main effect of drink type and no DRINK-CVS interaction for the adjusted weights of any of the fat pads.
CRH mRNA expression
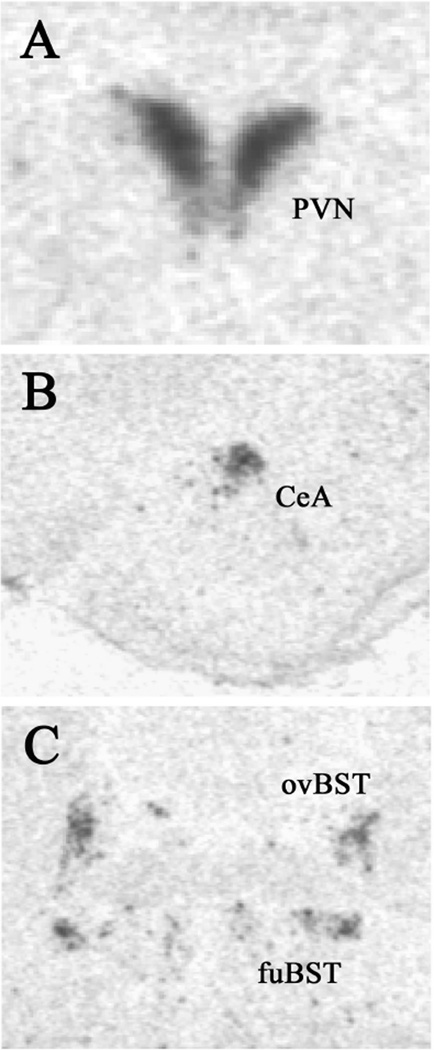
CRH mRNA expression in the PVN (Figure 7A; Table 3) was not affected by a history of CVS (F1,46 = 3.69, p=0.06) or type of drink, but there was a CVS-DRINK interaction (F2,46 = 3.75, p<0.05). Multiple comparison analyses showed that sucrose and saccharin drink decreased CRH mRNA expression in nonstress Controls, and CVS increased CRH mRNA expression in rats drinking saccharin. In contrast, CRH mRNA expression in the CeA, fuBST, and ovBST (Figure 7B,C; Table 3) was not affected by type of drink or CVS, and there was no DRINK-CVS interaction.
Figure 7.
Example images of CRH mRNA expression as assessed by in situ hybridization. (A) paraventricular nucleus of the hypothalamus (PVN), (B) central nucleus of the amygdala (CeA), and (C) oval and fusiform subregions of the bed nucleus of the stria terminalis (ovBST and fuBST, respectively).
Table 3.
Sucrose and saccharin decreased CRH mRNA expression in the PVN of nonstress controls, but not CVS rats. CVS increased CRH mRNA in the PVN of saccharin rats. CRH mRNA expression in the CeA, fuBST, and ovBST was not affected by type of drink or CVS.
| Control | CVS | |||||
|---|---|---|---|---|---|---|
| Water | Saccharin | Sucrose | Water | Saccharin | Sucrose | |
| PVN | 102 ± 7 | 80 ± 7* | 85 ± 6* | 92 ± 6 | 102 ± 3# | 100 ± 7 |
| CeA | 54 ± 5 | 55 ± 9 | 52 ± 7 | 55 ± 5 | 48 ± 5 | 50 ± 6 |
| fuBST | 56 ± 4 | 50 ± 3 | 57 ± 3 | 58 ± 6 | 55 ± 4 | 54 ± 6 |
| ovBST | 66 ± 2 | 65 ± 5 | 68 ± 4 | 70 ± 2 | 65 ± 5 | 62 ± 8 |
Units= Corrected gray levels. Data are shown as mean ± SEM. n= 7–9/group.
p<0.05 vs Water.
p<0.05 vs Control.
c-fos mRNA expression
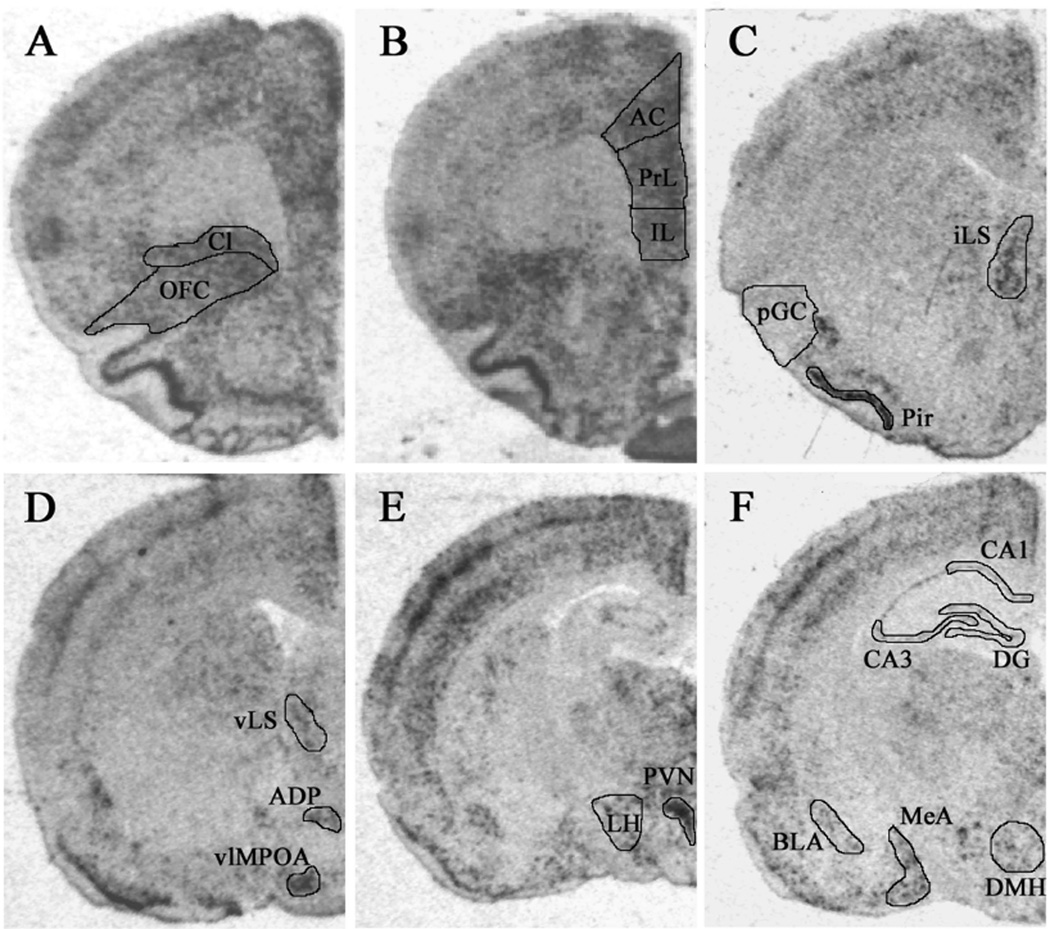
The expression of c-fos mRNA was assessed in various brain regions following acute restraint stress challenge (Figure 8; Table 4). For ease of comparison, the results described below are grouped into four different categories: regions where expression was affected only by CVS; regions where expression was affected only by type of drink; regions where expression was affected by both type of drink and CVS; and regions where expression was not affected by drink or CVS.
Figure 8.
Example images of c-fos mRNA expression as assessed by in situ hybridization. (A) claustrum (Cl) and orbitofrontal cortex (OFC); (B) anterior cingulate (AC), prelimbic (PrL), and infralimbic (IL) subregions of medial prefrontal cortex; (C) intermediate lateral septum (iLS), posterior gustatory cortex (pGC), and piriform cortex (Pir); (D) ventral lateral septum (vLS), anterodorsal preoptic nucleus (ADP), and ventrolateral medial preoptic area (vlMPOA); (E) paraventricular nucleus of the hypothalamus (PVN) and lateral hypothalamic area (LH); (F) CA1, CA3 and dentate gyrus (DG) subregions of the hippocampus, basolateral amygdala (BLA), medial amygdala (MeA) and dorsomedial nucleus of the hypothalamus (DMH).
Table 4.
c-fos mRNA expression (corrected gray levels).
| Control | CVS | |||||
|---|---|---|---|---|---|---|
| Water | Saccharin | Sucrose | Water | Saccharin | Sucrose | |
| Cortex: | ||||||
| Ant. Cingulate | 63 ± 2 | 70 ± 1 | 61 ± 2 | 52 ± 3# | 52 ± 2# | 50 ± 3# |
| Ant. gustatory | 21 ± 3 | 20 ± 3 | 19 ± 2 | 17 ± 1 | 17 ± 3 | 18 ± 3 |
| Infralimbic | 61 ± 2 | 63 ± 2 | 52 ± 3* | 45 ± 3# | 43 ± 2# | 42 ± 3# |
| Orbitofrontal | 71 ± 3 | 71 ± 4 | 67 ± 3 | 59 ± 2# | 61 ± 3# | 56 ± 3# |
| Piriform | 88 ± 3 | 84 ± 3 | 84 ± 3 | 78 ± 3# | 79 ± 3 | 78 ± 2 |
| Post. gustatory | 20 ± 3 | 18 ± 1 | 15 ± 1 | 16 ± 1 | 17 ± 1 | 16 ± 1 |
| Prelimbic | 68 ± 3 | 71 ± 1 | 65 ± 2 | 57 ± 3# | 53 ± 1# | 51 ± 2# |
| Hippocampus: | ||||||
| CA1 | 30 ± 2 | 30 ± 1 | 26 ± 2 | 23 ± 1# | 21 ± 1# | 24 ± 1 |
| CA3 | 24 ± 1 | 24 ± 1 | 22 ± 1 | 20 ± 1 | 19 ± 1# | 22 ± 1 |
| DG | 21 ± 1 | 19 ± 1 | 18 ± 1 | 19 ± 1 | 17 ± 1 | 18 ± 1 |
| Lateral septum: | ||||||
| Intermediate | 46 ± 3 | 40 ± 2 | 41 ± 2 | 31 ± 1# | 23 ± 3#† | 29 ± 3# |
| Ventral | 39 ± 6 | 34 ± 2 | 34 ± 4 | 25 ± 2# | 27 ± 3 | 28 ± 3 |
| Hypothalamus: | ||||||
| ADP | 37 ± 5 | 37 ± 4 | 36 ± 4 | 38±4 | 33 ± 3 | 36 ± 3 |
| DMH | 47 ± 4 | 48 ± 1 | 47 ± 3 | 49 ± 4 | 42 ± 3 | 43 ± 3 |
| LHA | 35 ± 4 | 38 ± 3 | 35 ± 3 | 35 ± 2 | 30 ± 1 | 33 ± 1 |
| PVN | 83 ± 4 | 85 ± 4 | 82 ± 3 | 78 ± 3 | 71 ± 4# | 73 ± 2 |
| vlMPOA | 41+5 | 43 ± 4 | 45 ± 4 | 39 ± 3 | 35 ± 3 | 34 ± 2# |
| Amygdala: | ||||||
| BLA | 30 ± 2 | 25 ± 3 | 23 ± 3† | 27 ± 1 | 22 ± 1 | 25 ± 2 |
| MeA | 48 ± 4 | 47 ± 3 | 47 ± 3 | 35 ± 4# | 40 ± 3 | 40 ± 1 |
| Other: | ||||||
| Claustrum | 78 ± 2 | 74 ± 2 | 72 ± 1† | 67 ± 2# | 66 ± 1# | 63 ± 2# |
| Lat. habenula | 41 ± 3 | 40 ± 2 | 45 ± 3 | 39 ± 3 | 35 ± 2 | 41 ± 3 |
| Post. BST | 17 ± 3 | 14 ± 2 | 20 ± 2 | 10 ± 2 | 11 ± 3 | 12 ± 2 |
| Post. PVThal | 51±4 | 54 ± 3 | 53 ± 4 | 47 ± 3 | 44 ± 2 | 50 ± 4 |
All data are shown as mean ± SEM. n= 7–9/group.
p<0.05 vs. water and saccharin,
p<0.05 vs water.
p<0.05 vs Control.
Regions affected only by CVS
Several of the examined brain regions exhibited a main effect for CVS to decrease the restraint-induced c-fos mRNA expression, whereas there was no effect of drink type and no DRINK-CVS interaction (Table 4). These brain regions included the anterior cingulate cortex (F1,48 = 42.25, p<0.05), orbitofrontal cortex (F1,48 = 20.67, p<0.05), piriform cortex (F1,48 = 8.59, p<0.05), prelimbic cortex (F1,48 = 61.86, p<0.05), CA1 (F1,48 = 21.58, p<0.05) and CA3 (F1,48 = 7.01, p<0.05) subregions of the hippocampus, ventral lateral septum (F1,46 = 8.15, p<0.05), PVN (F1,48 = 12.81, p<0.05), vlMPOA (F1,47 = 6.59, p<0.05), MeA (F1,48 = 12.02, p<0.05), and Post. BST (F1,44 = 4.89, p<0.05).
Regions affected only by drink type
Restraint-induced c-fos mRNA expression in the BLA was affected by type of drink (F2,48 = 3.29, p<0.05), but was not affected by CVS and there was no DRINK-CVS interaction. More specifically, sucrose drink decreased BLA c-fos mRNA expression relative to water in Control rats (Table 4).
Regions affected by both CVS and drink type
There was a main effect for CVS to decrease restraint-induced c-fos mRNA expression in the infralimbic cortex (F1,48 = 51.37, p<0.05), claustrum (F1,48 = 36.84, p<0.05), and intermediate lateral septum (F1,48 = 51.12, p<0.05). These brain regions also showed main effects of type of drink [infralimbic cortex (F2,48 = 3.39, p<0.05); claustrum (F2,48 = 3.35, p<0.05); intermediate lateral septum (F2,48 = 4.71, p<0.05)]. Multiple comparison analyses (Table 4) revealed that c-fos mRNA expression in the infralimbic cortex and claustrum was reduced by sucrose drink in Control, but not CVS, rats. Saccharin drink reduced c-fos mRNA expression in the intermediate lateral septum of CVS rats only. In addition, CVS decreased c-fos mRNA expression regardless of type of drink for all three regions.
Regions not affected by drink or CVS
The c-fos mRNA expression after acute restraint challenge was not affected by type of drink or a history of CVS, and there were no DRINK-CVS interactions for several brain regions (Table 4). These brain regions included the ADP, Post. PVThal, lateral habenula, DG of hippocampus, anterior gustatory cortex, posterior gustatory cortex, DMH, and LHA.
Discussion
The present study has two principle purposes. First the work seeks to develop and characterize a model of daily limited palatable drink intake in rats. Second, this model is applied to determine the effect of limited palatable drink on central and peripheral indices of HPA axis responses to acute and chronic stress. The results are discussed below in relation to these primary objectives.
Characterization of limited palatable drink model
Rats with free access to normal chow and water readily learned to drink sucrose (30%) and saccharin (0.1%) solutions during the first several days of drink exposure, consistent with reports that sucrose and saccharin are sweet substances that are palatable to rats (45, 46). Saccharin intake (both actual and normalized to body weight) dropped dramatically with the onset of CVS, suggestive of chronic stress-induced anhedonia, as seen with similar chronic stress paradigms (59), or perhaps of increased perception of aversive taste properties (60). In contrast, actual sucrose drink intake was more modestly reduced during CVS in an amount proportional to the CVS-induced reduction in body weight, suggesting a resistance to CVS-induced anhedonia for 30% sucrose (61). The more limited CVS-induced anhedonia with 30% sucrose is likely due to a higher palatability compared to 0.1% saccharin (46). The more modest effect of palatable drink on HPA axis responses to acute restraint in CVS rats (particularly the saccharin group, as described further below) may result, at least in part, from the reduced drink intake in these rats.
Food intake was reduced by roughly 10% in rats drinking sucrose regardless of time point and CVS exposure. This reduction in chow intake approximates the calories received from sucrose drink (roughly 8–9 kcal/d), resulting in no effect on body weight gained. The effects of saccharin drink on food intake and body weight gain were less consistent. Control rats drinking saccharin showed a temporary (days 1–14) increase in food intake that increased body weight gained at this time point; this weight gain was then maintained through the remainder of the study. However, CVS rats drinking saccharin did not show this initial increase in food intake and body weight gain during their training phase (prior to the onset of CVS). We would expect that CVS and control groups would look similar prior to the onset of CVS; we have no explanation for the inconsistent effects of saccharin in these groups.
Lastly, CVS decreased food intake, as seen by others after chronic stress (32, 33), regardless of type of drink. There was no effect of CVS on percentage of total calories obtained from palatable drink, likely because the intake of sucrose drink was limited by design. The CVS-induced hypophagia was accompanied by a reduction in body weight gain and reduced white fat depot weight, as seen previously after chronic stress (62). More specifically, the decreases in white adipose tissue weight occurred to roughly the same extent in subcutaneous (30% in inguinal) and visceral (27% in mesenteric; 39% in retroperitoneal; 23% in epidydimal) depots, suggesting that a marked shift towards visceral adiposity did not occur during CVS. However, it is possible that a more subtle increase in visceral adiposity may have been revealed if comparisons had been made to pair-fed controls, as suggested by others (10, 63). Interestingly, interscapular brown fat weight was not decreased by CVS and was increased by CVS when normalized to body weight, as seen previously after chronic hindlimb suspension stress (64), perhaps as result of increased circulating glucocorticoids (65). Notably, no fat depot weights were affected by type of drink, suggesting that the effects of drink on HPA axis responses to stress (see below) are not mediated via changes in mesenteric fat depot size, as has been proposed for models using unlimited access to palatable substances (10, 66).
Effects of limited palatable drink on HPA axis stress responses
A history of CVS elevated morning basal plasma corticosterone levels and potentiated plasma ACTH and corticosterone responses to a novel restraint stress, suggestive of chronic stress-induced facilitation, as seen previously with this and other chronic stress paradigms (21, 31, 35, 47). Moreover, the plasma corticosterone response to restraint was reduced by sucrose in control and CVS rats, and by saccharin in control rats; the lack of saccharin effect in CVS rats may reflect the dramatically reduced saccharin intake during CVS. In contrast, the plasma ACTH response to restraint was not affected by type of drink in either control or CVS rats. Dissociations between plasma ACTH and corticosterone responses after stress are relatively common (7, 31, 47, 67). In this case, it is possible that sucrose and saccharin reduced adrenal sensitivity to ACTH, possibly via modified neural input to the adrenal (68). Alternatively, the immunoreactive ACTH measured in the RIA may not be completely reflective of the amount of bioactive ACTH, as suggested by some (69).
Attenuation of the plasma corticosterone response to restraint by sucrose and saccharin was associated with alterations in CRH mRNA expression in the PVN, suggesting, at least in part, a central site of action for palatable drink. In particular, both sucrose and saccharin decreased PVN CRH mRNA levels in control rats but not CVS rats, while CVS modestly increased PVN CRH mRNA particularly in rats drinking saccharin. These results indicate that sucrose and saccharin may attenuate HPA responses by reducing PVN tone or the amount of CRH available for release.
We have previously seen more robust increases in CRH mRNA expression in the PVN after CVS in adult male Sprague-Dawley rats (18, 47). We speculate that the modest and somewhat inconsistent effect of CVS in the present study may be due to inter-strain differences. In support of this idea, we and others (23) have observed that adult male Long-Evans rats generally show greater CVS-induced thymic atrophy relative to adrenal growth (as seen in the present data), whereas this pattern is generally reversed in Sprague-Dawley rats (18, 47).
Collectively, analyses of HPA axis function suggest that limited sweet drink intake reduced responses to acute stress, as hypothesized. However, the hypothesized reduction in HPA axis adaptation to chronic stress was not supported. Recent work using other manipulations have also shown specific effects on the HPA axis response to acute versus chronic stress (35, 70). The present results underscore the emerging idea that acute and chronic stress responses may be modulated by largely distinct neural mechanisms (66, 70).
Lastly, as an initial attempt to identify brain regions that may mediate the effects of sweet drink on HPA axis responsivity, we assessed c-fos mRNA expression after novel restraint stress in numerous brain areas associated with HPA axis modulation. A history of CVS decreased restraint-induced c-fos mRNA expression in numerous brain regions, including the medial prefrontal cortex, orbitofrontal cortex, piriform cortex, CA1 and CA3 subregions of hippocampus, lateral septum, PVN, vlMPOA, MeA, and Post. BST. This data is similar to reports of widespread reductions in brain c-fos mRNA/protein responses to repeated homotypic stressors (71–75), and may be reflective of habituated c-fos responses following repeated activation during CVS. Moreover, reduced neuronal activation in regions known to dampen HPA axis activity, such as prelimbic cortex, hippocampus, and lateral septum (17) may contribute to facilitated HPA axis responses after chronic stress exposure. However, facilitated hormonal responses to novel restraint despite attenuated PVN c-fos mRNA induction suggests that there may have been a switch from c-fos to other immediate early gene signaling molecules, such as deltaFosB, following repeated activation (76).
Chronic limited sucrose drink diminished the c-fos mRNA response to restraint in control, but not CVS, rats consistent with a more pronounced effect of sucrose on plasma corticosterone response in control versus CVS rats. Saccharin drink showed an intermediate and non-significant reduction in c-fos mRNA in the BLA and claustrum, which correlates with a more modest effect of saccharin on plasma corticosterone responses to restraint. These initial results suggest that the BLA, infralimbic cortex, and claustrum are candidates for mediation of sweet drink effects on the HPA axis. The BLA and infralimbic cortex provide excitatory drive to the HPA axis under some circumstances (77–81), so reduced neuronal activation in these regions may limit HPA axis activation. It is not known whether the claustrum modulates HPA axis activity. Future work is planned to evaluate whether these candidate brain regions contribute to the HPA-inhibiting effects of sweet drink.
Role of metabolic versus non-metabolic properties of sweetened drink
Sucrose drink has multiple attributes which can be crudely divided into two categories: metabolic properties, which generally occur post-ingestion and include factors such as calories and osmolarity, and non-metabolic properties, such as taste, the act of drinking, motivation and the choice to drink, hedonics, and reward. In the present work, saccharin drink was included as a sweet substance that is palatable and rewarding to rats similar to sucrose, but devoid of calories (45, 46). The presently observed HPA axis hyporesponsivity following saccharin drink suggests that the metabolic properties of sucrose are not necessary, and that hedonics and reward play a critical role, likely via activation of brain opioid and dopamine systems (82, 83). The larger effect of 30% sucrose relative to 0.1% saccharin may occur because the sucrose is more rewarding, due both to its increased palatability (46) and the rewarding effects of its metabolic properties (84). Alternatively, the more robust effects of sucrose versus saccharin drink may indicate that the metabolic consequences of sucrose, such as effects on plasma glucose and/or energy regulation, contribute to the dampening of the HPA axis. Future work will use intragastric infusion of sucrose to address whether the metabolic attributes of sucrose are sufficient for altering HPA axis stress responses.
Comparison to unlimited palatable food paradigms
Previous models of palatable food intake have allowed rats with ad libitum access to chow, additional unlimited access to calorically-dense sucrose and/or lard. In these studies, rats generally consume a large amount of the palatable substances (~30–55% of total daily caloric intake), markedly reduce chow intake (~30–45%), increase total caloric intake (~10–20%), and increase white adipose tissue weight, but show no increase in total body weight (7, 8, 38, 85). Under these conditions, ad libitum sucrose consumption in adrenalectomized rats normalizes indices of HPA axis activation, including plasma ACTH and CRH mRNA expression in the PVN (36, 37). These effects of sucrose in adrenalectomized rats are primarily due to the metabolic consequences of sucrose ingestion since unlimited saccharin intake is not sufficient (36, 86). In adrenal-intact rats with free access to chow, unlimited access to sucrose and/or lard reduces basal CRH mRNA expression in the PVN and attenuates HPA axis responses to acute and repeated restraint stress and chronic cold stress (7, 8, 38, 85). Noncaloric palatable substances were not tested in these studies, so the potential contribution of the non-metabolic properties of sucrose (e.g. hedonics, reward, etc.) is not clear. Notably, in other studies the unlimited intake of sucrose or saccharin reduces the plasma ACTH and corticosterone response to paradoxical sleep deprivation in rats to the same extent (39), suggesting that non-metabolic properties of palatable substances can modulate HPA axis response in some circumstances.
In the present work, rats consumed at most 9 cal/day from sucrose, representing approximately 10% of their daily caloric intake. This sucrose intake reduced chow intake isocalorically and resulted in no change in body weight. Collectively, these data suggest that we fulfilled our intent to develop a model of daily limited palatable drink intake with minimal effects on chow intake and body weight. Importantly, even this limited intake of sucrose (and saccharin) was effective at dampening the HPA axis response to acute stress. The presently observed lack of effect of sucrose and saccharin on chronic stress adaptation may be “dose-related,” such that larger amounts of sweet drink may be required to alter responses to chronic stress.
Perspectives
A history of limited, intermittent sucrose drink, and to a lesser extent saccharin drink, diminished the plasma corticosterone response to acute restraint stress regardless of prior chronic stress exposure. In non-CVS rats, the expression of CRH mRNA in the PVN was reduced by both sucrose and saccharin drink, and the c-fos mRNA response to restraint was reduced by sucrose drink in the BLA, infralimbic cortex and claustrum. While the CVS paradigm induced indices of HPA axis adaptation to chronic stress, such as adrenal growth, thymic atrophy, and facilitated plasma ACTH and corticosterone stress responses, none of these indices were affected by type of drink. Collectively, these results demonstrate that only minimal amounts of sweetened drink are needed to attenuate HPA axis responses to acute stress. Also, sweetened drink does not need to contain calories to produce this effect. Humans under stress generally increase palatable food intake (1–6). Also, the intake of palatable/sweet food in humans during stress may help individuals “cope” (15). However, excessive palatable snacking may be maladaptive, as intake of large amounts of calorically-dense palatable foods is associated with increased risk for obesity (87). The present work provides additional insight into the relationship between the consumption of palatable food and responses to stress, and demonstrates that limited intake of caloric or noncaloric palatable substances can dampen physiological responses to stress.
Figure 1.
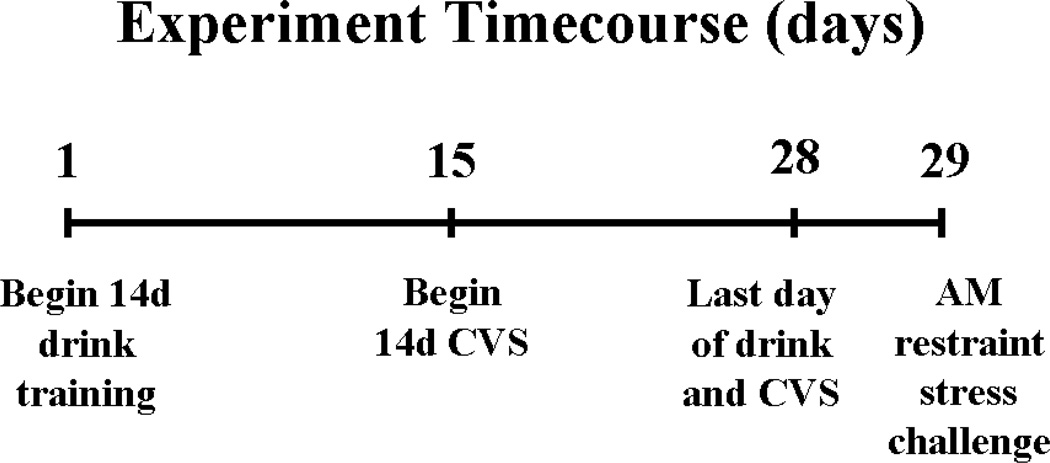
Timeline of the experimental design. On day 1, rats began drink training consisting of twice daily access (09:00 and 15:00) to sippers containing 4 ml of water, saccharin (0.1%), or sucrose (30%). On days 15–28, half of the rats were given twice daily exposure to an unpredictable stressor (chronic variable stress; CVS) immediately after receiving their respective drink solutions. Nonhandled control rats continued to receive their respective drink solutions but did not receive CVS. On the morning of day 29, rats did not receive their drink solutions and were challenged with a novel restraint stress.
Acknowledgements
The authors would like to thank Laurie Burck, Neil Dazet, and Jenny Gibson for their technical assistance. This work was supported by MH049698 (JPH), MH069725 (JPH), DK059803 (YMU, ARF), DK067820 (YMU), and DA016466 (MMO).
Footnotes
This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.
Disclosure summary: JPH consults for Phase 2 Discovery, Inc. All other authors have nothing to disclose.
References
- 1.Michaud C, Kahn J, Musse N, Burlet C, Nicolas J, Mejean L. Relationships between a critical life event and eating behaviour in high-school students. Stress Med. 1990;6:57–64. [Google Scholar]
- 2.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav. 1999;66:511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- 3.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res. 2000;48:195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 4.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 5.Leigh Gibson E. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol Behav. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 6.McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosom Med. 1990;52:97–108. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 8.Bell ME, Bhargava A, Soriano L, Laugero K, Akana SF, Dallman MF. Sucrose intake and corticosterone interact with cold to modulate ingestive behaviour, energy balance, autonomic outflow and neuroendocrine responses during chronic stress. J Neuroendocrinol. 2002;14:330–342. doi: 10.1046/j.1365-2826.2002.00784.x. [DOI] [PubMed] [Google Scholar]
- 9.Bertiere MC, Sy TM, Baigts F, Mandenoff A, Apfelbaum M. Stress and sucrose hyperphagia: role of endogenous opiates. Pharmacol Biochem Behav. 1984;20:675–679. doi: 10.1016/0091-3057(84)90183-7. [DOI] [PubMed] [Google Scholar]
- 10.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Dube L, LeBel JL, Lu J. Affect asymmetry and comfort food consumption. Physiol Behav. 2005;86:559–567. doi: 10.1016/j.physbeh.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Utter AC, Kang J, Nieman DC, Williams F, Robertson RJ, Henson DA, Davis JM, Butterworth DE. Effect of carbohydrate ingestion and hormonal responses on ratings of perceived exertion during prolonged cycling and running. Eur J Appl Physiol Occup Physiol. 1999;80:92–99. doi: 10.1007/s004210050563. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KE, Rosner W, Khan MS, New MI, Pang SY, Wissel PS, Kappas A. Diet-hormone interactions: protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sci. 1987;40:1761–1768. doi: 10.1016/0024-3205(87)90086-5. [DOI] [PubMed] [Google Scholar]
- 15.Markus R, Panhuysen G, Tuiten A, Koppeschaar H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav. 2000;70:333–342. doi: 10.1016/s0031-9384(00)00265-1. [DOI] [PubMed] [Google Scholar]
- 16.Deuster P, Singh A, Hofmann A, Moses F, Chrousos G. Hormonal responses to ingesting water or a carbohydrate beverage during a 2 h run. Med Sci Sports Exerc. 1992;24:72–79. [PubMed] [Google Scholar]
- 17.Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 18.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 19.Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- 21.Armario A, Restrepo C, Castellanos JM, Balasch J. Dissociation between adrenocorticotropin and corticosterone responses to restraint after previous chronic exposure to stress. Life Sci. 1985;36:2085–2092. doi: 10.1016/0024-3205(85)90304-2. [DOI] [PubMed] [Google Scholar]
- 22.Marti O, Gavalda A, Jolin T, Armario A. Effect of regularity of exposure to chronic immobilization stress on the circadian pattern of pituitary adrenal hormones, growth hormone, and thyroid stimulating hormone in the adult male rat. Psychoneuroendocrinology. 1993;18:67–77. doi: 10.1016/0306-4530(93)90056-q. [DOI] [PubMed] [Google Scholar]
- 23.Simpkiss JL, Devine DP. Responses of the HPA axis after chronic variable stress: effects of novel and familiar stressors. Neuroendocrinol Lett. 2003;24:97–103. [PubMed] [Google Scholar]
- 24.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- 25.Paskitti ME, McCreary BJ, Herman JP. Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res Mol Brain Res. 2000;80:142–152. doi: 10.1016/s0169-328x(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 26.Bartolomucci A, Palanza P, Parmigiani S, Pederzani T, Merlot E, Neveu PJ, Dantzer R. Chronic psychosocial stress down-regulates central cytokines mRNA. Brain Res Bull. 2003;62:173–178. doi: 10.1016/j.brainresbull.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 28.Gomez F, Lahmame A, Kloet ERd, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- 29.Kitraki E, Karandrea D, Kittas C. Long-lasting effects of stress on glucocorticoid receptor gene expression in the rat brain. Neuroendocrinology. 1999;69:331–338. doi: 10.1159/000054435. [DOI] [PubMed] [Google Scholar]
- 30.Burchfield SR, Woods SC, Elich MS. Pituitary adrenocortical response to chronic intermittent stress. Physiol Behav. 1980;24:297–302. doi: 10.1016/0031-9384(80)90090-6. [DOI] [PubMed] [Google Scholar]
- 31.Kiss A, Aguilera G. Regulation of the hypothalamic pituitary adrenal axis during chronic stress: responses to repeated intraperitoneal hypertonic saline injection. Brain Res. 1993;630:262–270. doi: 10.1016/0006-8993(93)90665-a. [DOI] [PubMed] [Google Scholar]
- 32.Marti O, Gavalda A, Gomez F, Armario A. Direct evidence for chronic stress-induced facilitation of the adrenocorticotropin response to a novel acute stressor. Neuroendocrinology. 1994;60:1–7. doi: 10.1159/000126713. [DOI] [PubMed] [Google Scholar]
- 33.Armario A, Hidalgo J, Giralt M. Evidence that the pituitary-adrenal axis does not cross-adapt to stressors: comparison to other physiological variables. Neuroendocrinology. 1988;47:263–267. doi: 10.1159/000124921. [DOI] [PubMed] [Google Scholar]
- 34.Odio M, Brodish A. Age-related adaptation of pituitary-adrenocortical responses to stress. Neuroendocrinology. 1989;49:382–388. doi: 10.1159/000125142. [DOI] [PubMed] [Google Scholar]
- 35.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 36.Laugero KD, Bell ME, Bhatnagar S, Soriano L, Dallman MF. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis? Endocrinology. 2001;142:2796–2804. doi: 10.1210/endo.142.7.8250. [DOI] [PubMed] [Google Scholar]
- 37.Bell ME, Bhatnagar S, Liang J, Soriano L, Nagy TR, Dallman MF. Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy. J Neuroendocrinol. 2000;12:461–470. doi: 10.1046/j.1365-2826.2000.00488.x. [DOI] [PubMed] [Google Scholar]
- 38.Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol. 1997;272:R840–R848. doi: 10.1152/ajpregu.1997.272.3.R840. [DOI] [PubMed] [Google Scholar]
- 39.Suchecki D, Antunes J, Tufik S. Palatable solutions during paradoxical sleep deprivation: reduction of hypothalamic-pituitary-adrenal axis activity and lack of effect on energy imbalance. J Neuroendocrinol. 2003;15:815–821. doi: 10.1046/j.1365-2826.2003.01067.x. [DOI] [PubMed] [Google Scholar]
- 40.Haveman-Nies A, de Groot LP, van Staveren WA. Snack patterns of older Europeans. J Am Diet Assoc. 1998;98:1297–1302. doi: 10.1016/s0002-8223(98)00290-9. [DOI] [PubMed] [Google Scholar]
- 41.Phillips SM, Bandini LG, Naumova EN, Cyr H, Colclough S, Dietz WH, Must A. Energy-dense snack food intake in adolescence: longitudinal relationship to weight and fatness. Obes Res. 2004;12:461–472. doi: 10.1038/oby.2004.52. [DOI] [PubMed] [Google Scholar]
- 42.Bellisle F, Dalix AM, Mennen L, Galan P, Hercberg S, de Castro JM, Gausseres N. Contribution of snacks and meals in the diet of French adults: a diet-diary study. Physiol Behav. 2003;79:183–189. doi: 10.1016/s0031-9384(03)00088-x. [DOI] [PubMed] [Google Scholar]
- 43.Huang TT, Howarth NC, Lin BH, Roberts SB, McCrory MA. Energy intake and meal portions: associations with BMI percentile in U.S. children. Obes Res. 2004;12:1875–1885. doi: 10.1038/oby.2004.233. [DOI] [PubMed] [Google Scholar]
- 44.Kerver JM, Yang EJ, Obayashi S, Bianchi L, Song WO. Meal and snack patterns are associated with dietary intake of energy and nutrients in US adults. J Am Diet Assoc. 2006;106:46–53. doi: 10.1016/j.jada.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 45.Collier G, Novell K. Saccharin as a sugar surrogate. J Comp Physiol Psychol. 1967;64:401–408. [PubMed] [Google Scholar]
- 46.Smith JC, Sclafani A. Saccharin as a sugar surrogate revisited. Appetite. 2002;38:155–160. doi: 10.1006/appe.2001.0467. [DOI] [PubMed] [Google Scholar]
- 47.Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulrich-Lai YM, Engeland WC. Hyperinnervation during adrenal regeneration influences the rate of functional recovery. Neuroendocrinology. 2000;71:107–123. doi: 10.1159/000054527. [DOI] [PubMed] [Google Scholar]
- 49.Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- 50.Chan RK, Brown ER, Ericsson A, Kovacs KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imaki T, Shibasaki T, Hotta M, Demura H. Early induction of c-fos precedes increased expression of corticotropin-releasing factor messenger ribonucleic acid in the paraventricular nucleus after immobilization stress. Endocrinology. 1992;131:240–246. doi: 10.1210/endo.131.1.1612001. [DOI] [PubMed] [Google Scholar]
- 52.Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herman JP, Schafer MK, Thompson RC, Watson SJ. Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol. 1992;6:1061–1069. doi: 10.1210/mend.6.7.1324419. [DOI] [PubMed] [Google Scholar]
- 54.Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- 55.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 56.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- 57.Swanson LW. Brain maps: structure of the rat brain. Amsterdam: Elsevier; 1998. [Google Scholar]
- 58.McClave J, Dietrich F. Statistics. 6 ed. Englewood Cliffs, NJ: Macmillan College Publishing Company, Inc.; 1994. [Google Scholar]
- 59.Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- 60.Dess NK. Saccharin’s aversive taste in rats: evidence and implications. Neurosci Biobehav Rev. 1993;17:359–372. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- 61.Matthews K, Forbes N, Reid IC. Sucrose consumption as an hedonic measure following chronic unpredictable mild stress. Physiol Behav. 1995;57:241–248. doi: 10.1016/0031-9384(94)00286-e. [DOI] [PubMed] [Google Scholar]
- 62.Akana SF, Hanson ES, Horsley CJ, Strack AM, Bhatnagar S, Bradbury MJ, Milligan ED, Dallman MF. Clamped Corticosterone (B) Reveals the Effect of Endogenous B on Both Facilitated Responsivity to Acute Restraint and Metabolic Responses to Chronic Stress. Stress. 1996;1:33–49. doi: 10.3109/10253899609001094. [DOI] [PubMed] [Google Scholar]
- 63.Rebuffe-Scrive M, Walsh UA, McEwen B, Rodin J. Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Physiol Behav. 1992;52:583–590. doi: 10.1016/0031-9384(92)90351-2. [DOI] [PubMed] [Google Scholar]
- 64.Yamashita H, Ohira Y, Wakatsuki T, Yamamoto M, Kizaki T, Oh-ishi S, Sato Y, Ohno H. Responses of brown adipose tissue activity to unloading in rats. J Appl Physiol. 1995;78:384–387. doi: 10.1152/jappl.1995.78.2.384. [DOI] [PubMed] [Google Scholar]
- 65.Strack AM, Bradbury MJ, Dallman MF. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am J Physiol. 1995;268:R183–R191. doi: 10.1152/ajpregu.1995.268.1.R183. [DOI] [PubMed] [Google Scholar]
- 66.Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, Houshyar H, Gomez F. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann N Y Acad Sci. 2004;1018:141–150. doi: 10.1196/annals.1296.017. [DOI] [PubMed] [Google Scholar]
- 67.Dempsher DP, Gann DS. Increased cortisol secretion after small hemorrhage is not attributable to changes in adrenocorticotropin. Endocrinology. 1983;113:86–93. doi: 10.1210/endo-113-1-86. [DOI] [PubMed] [Google Scholar]
- 68.Ulrich-Lai Y, Engeland W. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology. 2002;76:79–92. doi: 10.1159/000064426. [DOI] [PubMed] [Google Scholar]
- 69.Engeland WC, Miller P, Gann DS. Dissociation between changes in plasma bioactive and immunoreactive adrenocorticotropin after hemorrhage in awake dogs. Endocrinology. 1989;124:2978–2985. doi: 10.1210/endo-124-6-2978. [DOI] [PubMed] [Google Scholar]
- 70.Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci. 2000;20:5564–5573. doi: 10.1523/JNEUROSCI.20-14-05564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Umemoto S, Noguchi K, Kawai Y, Senba E. Repeated stress reduces the subsequent stress-induced expression of Fos in rat brain. Neurosci Lett. 1994;167:101–104. doi: 10.1016/0304-3940(94)91037-5. [DOI] [PubMed] [Google Scholar]
- 72.Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- 73.Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- 74.Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–319. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- 78.Coover G, Ursin H, Levine S. Corticosterone and avoidance in rats with basolateral amygdala lesions. J Comp Physiol Psychol. 1973;85:111–122. doi: 10.1037/h0034858. [DOI] [PubMed] [Google Scholar]
- 79.Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feldman S, Siegel RA, Conforti N. Differential effects of medial forebrain bundle lesions on adrenocortical responses following limbic stimulation. Neuroscience. 1983;9:157–163. doi: 10.1016/0306-4522(83)90053-2. [DOI] [PubMed] [Google Scholar]
- 81.Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 82.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 83.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 85.la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- 86.Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR, Dallman MF. Corticosterone facilitates saccharin intake in adrenalectomized rats: does corticosterone increase stimulus salience? J Neuroendocrinol. 2000;12:453–460. doi: 10.1046/j.1365-2826.2000.00487.x. [DOI] [PubMed] [Google Scholar]
- 87.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]