Abstract
Major depression is a complex multi-factorial disorder with a lifetime diagnosis of nearly 1 out of 6. We used the Flinders Sensitive Line (FSL) of rats, a model of depression, and the parent Sprague–Dawley (SD) rats to identify genes, gene ontology categories and pathways associated with depression. Depression-like behavior was verified in the FSL line by forced swim testing, with FSL animals exhibiting greater immobility compared to SD rats. RNA samples from the hippocampus were isolated from a group of experimentally naïve FSL and SD rats for microarray analysis. Microarray analysis yielded a total of 361 genes that were differentially regulated between FSL and SD rats, with catechol-O-methyltransferase (COMT) being the most up-regulated. The genes that were differentially regulated between FSL and SD rats were subjected to bioinformatic analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID), which yielded several gene ontology categories that were overrepresented. Subsequent pathway analysis indicated dysregulation of the adipocytokine signaling pathway. To test the translational impact of this pathway, metabolic factors and psychiatric symptoms were evaluated in a sample of human research participants. Results from our human subjects indicated that anxiety and a subset of depressive symptoms were correlated with adiponectin levels (but not leptin levels). Our results and those of others suggest that disruption of the adipocytokine signaling pathway may be a critical component of the depressive-like behaviors observed in the FSL rats and may also be an important indicator of depressive and anxiety symptoms in humans.
Keywords: Depression, Forced swim test, Hippocampus, Behavioral despair, Adipocytokine
1. Introduction
Major depressive disorder (MDD) is a serious illness characterized by deep sadness, feelings of hopelessness and despair, and increased somatic symptoms (e.g., disturbed sleep, appetite changes, and pain). By the year 2020, depression will be the 2nd most disabling health problem in the world. Up to 15% of individuals with severe MDD die by suicide (American Psychiatric Association, 2000). Current medications to treat depression exhibit limited efficacy (e.g. Fountoulakis and Möller, 2011). Residual depressive symptoms are common, increase the risk for relapse, and may result in a more chronic course of illness (reviewed in Kurian et al., 2009). Thus, new treatments for major depression are needed to address the high rates of resistance to current interventions and the chronic residual symptoms in many patients treated for depression. A better understanding of the pathophysiological mechanisms associated with depression will contribute to this need for improved therapeutic strategies.
The heterogeneity of MDD suggests that multiple neurocircuits and neurochemicals are involved in its pathogenesis. The most widely accepted neurochemical theory of depression is the monoamine hypothesis which postulates that depression is a pathology caused by alterations in brain serotonergic and noradrenergic systems. However, this theory is incomplete (Lee et al., 2010), and the role of the immune system in depression is increasingly appreciated and supported (reviewed in Loftis et al., 2010; Raedler, 2011; Leonard and Maes, 2012).
To identify genes that contribute to depression and the biological mechanisms through which they act, we used the Sprague Dawley (SD) derived Flinders Sensitive Line (FSL) rats to perform genome-wide expression profiling. The FSL rats, a well-validated genetic model for depression, were previously generated by selective breeding of out-bred SD rats for differences in the effects of the anticholin-esterase agent diisopropylfluorophosphate (DFP) (Overstreet and Russell, 1982; Overstreet, 1986). FSL rats are more sensitive to DFP and cholinergic agonists, a feature shared by depressed humans (Janowsky et al., 1994). Subsequent evaluations of the FSL rats revealed that, in addition to their cholinergic hypersensitivity, they express behavioral (e.g., reduced locomotor activity, increased immobility, and cognitive deficits (Overstreet, 1993)) and physiological (e.g., psychomotor retardation, lower body weight, and reduced appetite (Overstreet et al., 2005)) features similar to those found in MDD. Recent proteomic analyses found that a number of analytes previously associated with MDD were similarly altered in hippocampus and prefrontal cortex of FSL rats (Carboni et al., 2010; Piubelli et al., 2011), including alterations in proteins associated with energy metabolism, cellular localization and transport, cytoskeleton organization, and apoptosis.
The hippocampus is increasingly thought to be involved in the patho-physiological mechanisms of depression (Duman, 2002; Santarelli et al., 2003; Campbell et al., 2004; Videbach and Ravnkilde, 2004), and therefore was selected for gene expression profiling. Repeated intra-hippocampal administration of prednisolone (a commonly prescribed glucocorticoid) increased anxiety and depression-like behavior in mice, and altered expression of genes associated with cell death and inflammation (Kajiyama et al., 2010). Imaging studies show that hippocampal volumes are reduced in some patients with MDD (Bremner et al., 2000; MacQueen et al., 2003; Sheline et al., 1996), possibly due to reduced neuropil sizes (Rosoklija et al., 2000). Early life stress may underlie the reduced hippocampal volumes observed in some patients with MDD (see Frodl and O'Keane, in press for review). Efficacious antidepressant treatments function in part, by normalizing disturbed neuroplasticity (Michael-Titus et al., 2008) and facilitating axonal and dendritic sprouting (Vaidya et al., 1999) — processes that can help restore synaptic connections within the neuropil. Although the role of hippocampal neurogenesis in the development and persistence of depression is not completely understood, its requirement for antidepressant efficacy is well accepted (Lewitus et al., 2009; Santarelli et al., 2003; Malberg et al., 2000).
In the present study we identified associations with several genes and found dysregulation of the adipocytokine signaling pathway in the FSL rat model of depression.We chose to conduct the microarray investigation under resting conditions (i.e., using experimentally naïve rats) to conform to studies involving depressed patients (e.g., Shelton et al., 2011) and to determine whether differences in gene expression would be evident in the absence of stress or pharmacological manipulation. We found significant gene expression differences at all levels of analysis, including at the single gene level, at the biological process level (gene ontology) and at the pathway level. We followed up these preclinical findings with a study in humans to determine whether disrupted adipocytokine signaling was similarly associated with symptoms of depression or anxiety. Our translational findings highlight important new directions for depression research and diagnosis and further support the utility and relevance of the FSL rat model.
2. Materials and methods
2.1. Animals
Male FSL rats (302.9±23.4 g; Dr. Amir H. Rezvani, Duke University) and male SD rats (294.9±17.6 g; Harlan Laboratories) were housed in the same room for 3 months prior to the start of the experiments. Rats were pair-housed under conditions of constant temperature (20–22 °C) and humidity (30–45%) with free access to food and water. The room was maintained on a 12:12 h light:dark cycle with lights off at 1800 h. All animal studies were approved by the Institutional Animal Care and Use Committee at the Portland VA Medical Center and were performed in accordance with the guidelines of the National Institutes of Health.
2.2. Forced swim test
To evaluate depressive-like behavior in FSL and SD rats, forced swim testing (FST) was performed to assess behavioral despair (immobility). Rats participating in the behavioral testing were not used for the microarray experiment because the stress associated with the swim test could alter gene expression (e.g. Drossopoulou et al., 2004). The FST was performed as previously described (Loftis et al., 2006; Wilhelm et al., 2011). Briefly, rats were placed in a clear acrylic cylinder (40 cm height; 18 cm diameter) filled to 30 cm high with 25 °C (±2 °C) water. The water was sufficiently deep that the rats would swim or float in the water without limbs or tail touching the floor of the container. Rats were exposed to a 15-min practice swim (training session), followed 24 h later by a 5-min test swim. Both the training and test sessions were video recorded, and the test sessions were scored by an independent observer. Three behaviors were measured: swimming, climbing and immobility. These behaviors were defined by Cryan et al. (2002):
Immobility — floating in the water without struggling and using only small movements to keep the head above water
Swimming — moving limbs in an active manner (more than required to keep head above water) causing movement around the cylinder
Climbing — making active movements with the forepaws in and out of the water, usually directed against the wall.
The predominant behavior during 5-s intervals of the 5-min test swim was assessed. The main dependent variables for this task were time spent immobile and latency to start floating. The total time spent immobile was calculated and subjected to a t-test (two-tailed, unpaired).
2.3. Flow cytometry
Animals (n=7 from each group) were euthanized via CO2 asphyxiation and whole brains were rapidly removed. A single-cell suspension of mononuclear cells was prepared using a 100 µm cell strainer (BD Biosciences, San Jose, CA, USA). Recovered cells were washed in RPMI 1640, resuspended in 8 ml of 40% Percoll (Pharmacia LKB Biotechnology AB, Uppsala, Sweden) underlaid with 3 ml of 80% Percoll to form a discontinuous gradient in a 15-ml centrifuge tube. The gradient was centrifuged at 500 ×g for 30 min, and the cells at the 40% to 80% interface harvested. Cells were washed, stained for flow cyotmetric analysis (antibodies for CD11b/c-APC, CD45-PE, CD3-FITC, and CD161a–PE), and acquired on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
2.4. RNA isolation, amplification and microarray analysis
Animals were euthanized by rapid decapitation and hippocampal sections were removed on dry ice, as previously described (Loftis and Janowsky, 2002). Hippocampi were placed in RNAlater (Qiagen, Inc., Valencia, CA, USA) and stored at −80 °C until processed for microarray analysis. RNA was isolated from tissue samples using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) as described in the product manual.
Microarray experiments were conducted in the Affymetrix Microarray Core of the Oregon Health & Science University Gene Microarray Shared Resource. Messenger RNA was amplified and labeled from 200 ng of total RNA in two steps using the MessageAMP Premier RNA Amplification kit (Ambion, Inc., Austin, TX, USA) as described in the product manual. Target yield was measured by UV260 absor-bance and quality was assessed by examining yield and size distribution of the in vitro synthesis reaction products using a 2100 Bioanalyzer and RNA 6000 LabChip (Agilent Technologies, Santa Clara, CA, USA). Labeled target was fragmented at 95 °C in the presence of high magnesium concentration to produce a uniform distribution of short cRNAs. Ten micrograms of the fragmented material was combined with biotinylated hybridization control oligomer and biotinylated control cRNAs for BioB, BioC, BioD and CreX (Affymetrix, Santa Clara, CA, USA) in hybridization buffer and hybridized on the Affymetrix GeneChip Rat Genome 230 2.0 array for 18 h as described in the Affymetrix expression analysis technical manual. The array image scan was processed with Affymetrix GeneChip Command Console (AGCC).
2.5. Statistical analysis of microarray data
The “affy” and “gcrma” packages of Bioconductor (http://www.bioconductor.org) were employed to normalize the intensities following import of CEL files into the R statistical program. Then, the GeneChip Robust Multiarray Analysis (GCRMA) was used to adjust perfect match (PM) probe data for background noise and further normalized by quantile normalization. Gene expression values were determined using a linear model estimated by the median polish algorithm. The normalized intensities between the SD and FSL lines were compared by using the Significant Analysis of Microarrays with q-value less than 5% and up- or down-regulated by 1.5 fold changes or more (corresponding to fold changes of >1.5 for genes up-regulated or <0.67 (1/1.5) for genes down-regulated). These cutoffs make the gene selection process symmetric on a log2 scale. The q value is a Bayesian equivalent to the false discovery rate adjusted p-value. This allowed us to identify a large set of genes that were differentially regulated between FSL animals and SD animals which could then be subjected to pathway enrichment analyses. Functional analysis of the data was performed using the DAVID (the Database for Annotation, Visualization and Integrated Discovery v6.7) Bioinformatics Resources (http://david.abcc.ncifcrf.gov/home.jsp) (Huang da et al., 2009a, 2009b). Genes identified as differentially expressed between the FSL and SD rats were assessed for significant enrichment of biological processes using the terms of the fifth level of Gene Ontology (GO). Pathway analysis was carried out using the Kyoto Encyclopedia of Genes and Genomes (KEGG) module within DAVID. Statistical analysis for GO and pathway analyses were carried out using a modified Fisher Exact Test (Expression Analysis Systematic Explorer (EASE); Reviewed in Hosack et al., 2003).
2.6. Human research participants
A total of 40 adults were recruited from the Portland Veterans Affairs Medical Center (PVAMC) and community hospitals via study advertisements posted throughout the hospital, or word of mouth. Participants were excluded if they met any of the following criteria: 1) History of antiviral therapy or chemotherapy for any purpose, 2) History of a major medical condition, or currently unstable medical condition, that is likely to be associated with severe neurological or immune dysfunction (e.g., stroke, seizures, brain tumors, Parkinson's disease, neurode-generative dementia, mental retardation, hepatic encephalopathy, and HIV). Participants with common well-controlled or stable conditions were included as long as severe cognitive or immunological effects (conditions that were excluded: stroke, seizure, brain tumors, Parkinson's disease, neurodegenerative dementia, mental retardation, hepatic encephalopathy, and human immunodeficiency virus (HIV)) were not currently suspected (conditions that were allowed: well-controlled diabetes, hypertension, or asthma). 3) History of traumatic brain injury with known loss of consciousness≥30 min. 4) Use of alcohol, illicit substances, or medications with acute cognitive effects such as sedation or intoxication (e.g., benzodiazepines, opiates, and muscle relaxants) on the day of testing, or chronic use of medications with long-term cognitive effects (e.g. topiramate, remicade, anticholinergics and steroids). 5) Advanced liver disease as indicated by any of the following:a)classified as having stage 4 liver disease or grade 4 inflammation upon biopsy, OR, b) classified by a hepatologist as having probable decompensated cirrhosis based on clinical indicators and standard liver labs, OR, and c) aspartate aminotransferase (AST) to platelet (PLT) ratio index (APRI)≥1.5. 6) Current pregnancy. 7) History of schizophrenia or schizoaffective disorder, OR, current psychotic or manic episode, OR currently unstable and severe psychiatric disorder. Patients with mild but stable depression, anxiety, or post-traumatic stress disorder were included. 8) Alcohol or drug abuse or dependence within the past 90 days (except nicotine or caffeine), based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (American Psychiatric Association, 2000), confirmed with the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998).
2.7. Procedures for human participants
All research was conducted with permission from the PVAMC's Institutional Review Board and in accordance with the Helsinki Declaration. All participants gave written informed consent and were paid $75 to complete the following study procedures: clinical interview, comprehensive medical record review, questionnaires to evaluate symptoms of anxiety and depression [i.e., Generalized Anxiety Disorder Inventory (GADI) (Argyropoulos et al., 2007); Beck Depression Inventory (BDI-II) (Beck et al., 1996)], and blood sample collection. Questionnaires and interviews were administered by one of four advanced doctoral candidates in clinical psychology; all graduate students were trained and supervised by a clinical neuropsychologist (M.H.). Study data was entered into a database initially and then double-checked by separate study personnel prior to analyses.
Clinical interviews were conducted using a structured case report form (developed specifically for this study) including prompts to screen patients based on each inclusion criteria, gather relevant demographic data, assess for a full range of current and past Axis I psychiatric and substance use disorders using DSM-IV (American Psychiatric Association, 2000) criteria (confirmed with the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998)), evaluate for history of head injuries, and record a comprehensive list of current and previous medical conditions. Study personnel additionally reviewed each participant's complete electronic medical record to collect recent medical laboratory results and to cross-validate the psychiatric, substance use, and medical history gathered in the clinical interview.
Symptoms of depression were assessed using the Beck Depression Inventory, Second Edition (BDI-II) (Beck et al., 1996). This is a well-validated and widely used measure of depression. Symptoms of anxiety were assessed using the Generalized Anxiety Disorder Inventory (GADI) (Argyropoulos et al., 2007). This brief inventory measures severity of current anxiety symptoms.
2.8. Multiplex metabolic factor assessments
Following collection of the neuropsychiatric data, blood was drawn in the afternoon (mean time was 12:57 PM, SD=1:45 h) by one-time venipuncture into cell preparation tubes (BD Vacutainer Systems, Franklin Lakes, NJ) containing 1 ml of 0.1 M sodium citrate solution. The blood was then centrifuged at 1500 RCF for 20 min at room temperature (22–25 °C). Plasma was separated, collected and immediately aliquoted in polypropylene tubes (Phoenix Research Products, Hayward, CA) and frozen at −80 °C until assayed. Metabolic factors (adiponectin and leptin) were measured by Myriad Rules-Based Medicine, Inc., (Austin, TX) using multi-analyte testing technology. Myriad Rules-Based Medicine, Inc. operates using Good Laboratory Practices (GLP) and has maintained CLIA (Clinical Laboratory Improvement Amendments) accreditation from the Commission on Office Laboratory Accreditation continuously since 2005. The lower limits of detection were as follows: adiponectin≤0.02 µg/ml; leptin=0.074 ng/ml.
2.9. Statistical analyses of human data
Analyses were conducted with SPSS or Microsoft Excel, and p-values<0.05 were considered significant unless otherwise noted. Correlations between questionnaire measures (i.e., BDI-II and GADI) and metabolic factors were carried out using Pearson's correlation coefficient unless data were non-normal, in which case Spearman's Rho was used. For the BDI-II, in addition to BDI-II total scores, the Cognitive–Affective and Somatic factor scores derived from our recent BDI-II factor analysis were also used to evaluate depressive symptoms in our participants (Patterson et al., 2011). The Cognitive–Affective factor consists of 11 BDI-II items and the Somatic factor consists of 7 items (Supplementary Table S4).
3. Results
3.1. Behavioral assessment
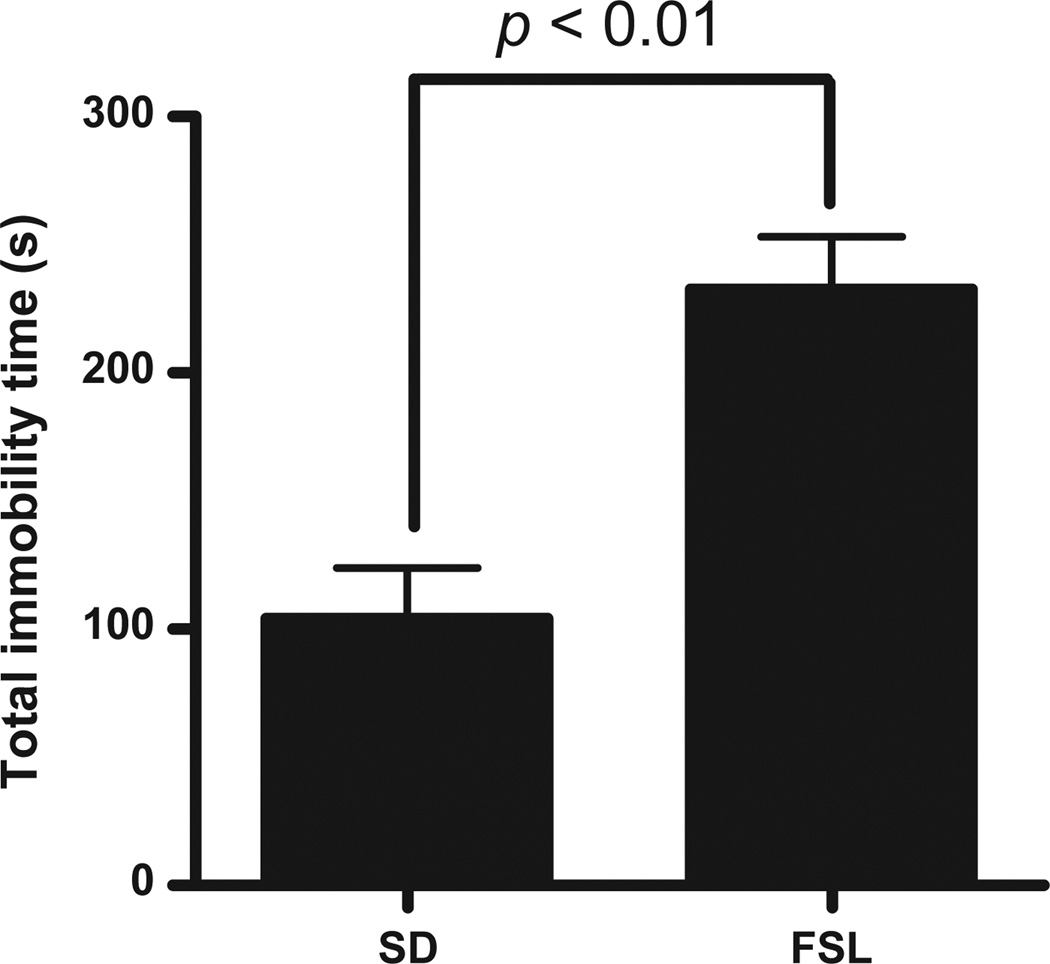
As expected FSL animals spent more time immobile in the forced swim test (FST; a measure of behavioral despair often used as an antidepressant screen) relative to SD rats (t=4.45, p<0.01; Fig. 1). This test confirms increased depression-like behavior in FSL animals relative to SD animals and is consistent with previous studies examining this behavior in FSL rats (e.g. Overstreet, 1986; Wegener et al., 2011).
Fig. 1.
Forced swim test results for FSL (n=4) and SD rats (n=5). FSL rats exhibit greater immobility compared with SD rats.
3.2. Brain immune cell expression
To determine whether phenotypic immune cell differences play a role in differential gene expression, we conducted flow cytometry of brain monocytes from FSL (n=7) and SD (n=7) rats. We found no significant differences between FSL and SD animals in proportions of CD3 (T cells), CD161a (natural killer cells), or CD45/CD11b/c (macro-phages) (Supplementary Table S1) indicating that differences in immune cell expression in brain is not likely to be a source of differential gene expression.
3.3. Gene expression and validation
Gene expression studies were carried out on five FSL rats and five SD rats that were experimentally naïve. We identified 130 genes that were up-regulated and 231 genes that were down-regulated in FSL rats relative to SD rats (Supplementary Table S2). The ten most up-or down-regulated named transcripts are listed in Table 1.
Table 1.
The most highly up- (A) or down- (B) regulated named sequences were identified from the list of 361 regulated transcripts. Transcripts are listed from most to least regulated. Log2 (Fold change (FC)) represents the log2 of the difference in log2 probe intensity between FSL animals relative to SD animals. Positive log2 (FC) values indicate increased gene expression within FSL animals relative to SD animals, while negative log2 (FC) values indicate decreased gene expression within FSL animals relative to SD animals. Probe set ID represents the proprietary Affymetrix identification method associated with the specific microarray chip.
| Probe set ID | Gene name | Gene symbol | Log2 (FC) |
|---|---|---|---|
| A. Up-regulated sequences | |||
| 1368826_at | Catechol-O-methyltransferase | COMT | 6.14 |
| 1371970_at | Family with sequence similarity 111, member A | Fam111a | 5.51 |
| 1383211_at | Tuftelin 1 | Tuft1 | 5.26 |
| 1395020_at | Pleckstrin homology domain containing, family H (with MyTH4 domain) member 1 | Plekhh1 | 5.25 |
| 1387839_at | RT1 class Ib gene, H2-TL-like, grc region (N1)///RT1 class Ib gene, H2-TL-like, grc region(N2)///RT1 class Ib gene, H2-TL-like, grc region (N3) | RT1-N1///RT1-N2///RT1-N3 | 4.62 |
| 1369144_a_at | Potassium voltage gated channel, Shal-related family, member 3 | Kcnd3 | 3.61 |
| 1390777_at | Sterol-C5-desaturase (ERG3 delta-5-desaturase homolog, Saccharomyces cerevisiae)-like | Sc5dl | 2.69 |
| 1393842_at | Coiled-coil domain containing 77 | Ccdc77 | 2.54 |
| 1380577_at | ATP-binding cassette, sub-family G (WHITE), member 2 | Abcg2 | 2.53 |
| 1378346_at | Lipin 2 | Lpin2 | 2.27 |
| B. Down-regulated sequences | |||
| 1391262_at | Similar to SUMO/sentrin specific protease 5///SUMO/sentrin specific protease 5 | RGD1564247///Senp5 | −6.21 |
| 1393623_at | Tripartite motif-containing 25 | TRIM25 | −5.72 |
| 1397439_at | Similar to diacylglycerol kinase epsilon | LOC497978 | −4.81 |
| 1377133_at | Hypothetical protein LOC680687 | LOC680687 | −4.81 |
| 1372120_at | Ubiquitin-like modifier activating enzyme 5 | Uba5 | −3.98 |
| 1377442_at | HtrA serine peptidase 4 | HTRA4 | −3.81 |
| 1368943_at | Ribonuclease, RNase A family 4 | Rnase4 | −3.72 |
| 1395126_at | Fc receptor-like S, scavenger receptor | Fcrls | −3.66 |
| 1388071_x_at | RT1 class Ib, locus Aw2 | RT1-Aw2 | −3.43 |
| 1388060_at | Synaptotagmin XII | Syt12 | −2.74 |
3.4. Bioinformatic functional analysis
We used the database for annotation, visualization and integrated discovery (DAVID) to perform systematic and integrative gene expression analysis as outlined in Huang da et al. (2009a, 2009b). Using this technique, we subjected genes that were up- or down-regulated to GO-based enrichment and pathway analyses. The GO enrichment analysis identified several overrepresented categories for both up- and down-regulated genes (Table 2). We then used functional annotation to determine whether distinct physiological pathways were differentially regulated between FSL and SD rats. No pathways were identified for genes that were down-regulated. The adipocytokine signaling pathway was identified for genes that were up-regulated (p=0.06). Within this pathway, three genes were differentially regulated between FSL and SD rats: tumor necrosis factor receptor superfamily, member 1A (TNFRSF1A)-associated via death domain (TRADD; ratio 2.02), acyl-CoA synthetase long-chain family member 1 (Acsl1; ratio 1.73), and mitogen activated protein kinase 10 (MAPK10, JNK; ratio 1.59). The ratio value is the average intensity of the gene derived from the microarray for FSL animals relative to the average gene intensity measured for the SD animals. No other pathways were identified from the up-regulated genes. Quantitative real-time PCR validated the microarray findings on four (C3, TRADD, Acsl1, and Rnase4) of the genes of interest (Supplementary Table S3; Supplementary methods).
Table 2.
Overrepresented GO categories identified by DAVID analysis for the group of significantly regulated transcripts between FSL and SD rats. Counts represent the number of genes from the up- (A) or down- (B) regulated gene lists submitted that were identified as components of the noted GO term. Statistical testing for significant enrichment was carried out using EASE, a modified, more conservative version of the Fisher Exact Test.
| Term | Count | p-Value | Gene symbols |
|---|---|---|---|
| A. FSL up-regulated | |||
| Protein complex assembly | 7 | 0.014 | CNTF, P2RX4, pfdn6, TRADD, MIS12, polr2i, ATL1 |
| Protein complex biogenesis | 7 | 0.014 | CNTF, P2RX4, pfdn6, TRADD, MIS12, polr2i, ATL1 |
| Response to drug | 6 | 0.020 | mgst1, sst, COMT, SLC1A3, Abcg2, ACSL1 |
| Negative regulation of cell proliferation | 5 | 0.038 | COMT, THY1, GTPBP4, Fntb, CDH13 |
| Regulation of axonogenesis | 3 | 0.042 | CNTF, THY1, MBP |
| Regulation of cell migration | 4 | 0.045 | sst, THY1, GTPBP4, CDH13 |
| Macromolecular complex assembly | 7 | 0.045 | CNTF, P2RX4, pfdn6, TRADD, MIS12, polr2i, ATL1 |
| Cellular amino acid derivative metabolic process | 4 | 0.047 | mgst1, ddc, COMT, SLC1A3 |
| B. FSL down-regulated | |||
| Oxidation reduction | 16 | 0.001 | DPYD, cat, Cyp4f1, retsat, NQO2, RTN4IP1, PXDN, ME3, RGD1304982, PYROXD2, etfB, FMO5, AOX1, Cyp4f4, DEGS1, Aldh3b1 |
| Regulation of myeloid leukocyte mediated immunity | 3 | 0.009 | C3, PLD2, Fcgr2b |
| Immunoglobulin mediated immune response | 4 | 0.011 | C3, MLH1, Fcgr2b, C1qa |
| B cell mediated immunity | 4 | 0.013 | C3, MLH1, Fcgr2b, C1qa |
| Positive regulation of immune effector process | 4 | 0.013 | C3, PLD2, Fcgr2b, NP |
| Antigen processing and presentation of peptide antigen via MHC class I | 3 | 0.021 | Fcgr2b, RT1-S3, RT1-EC2 |
| Phosphoinositide-mediated signaling | 4 | 0.022 | F2R, PIK3C3, Pthr1, HCRT |
| Lymphocyte mediated immunity | 4 | 0.022 | C3, MLH1, Fcgr2b, C1qa |
| Regulation of endocytosis | 4 | 0.024 | C3, PLD2, Fcgr2b, STON2 |
| Positive regulation of transport | 7 | 0.026 | F2R, C3, PLD2, Fcgr2b, HCRT, AKAP5, ACSL5 |
| Adaptive immune response | 4 | 0.027 | C3, MLH1, Fcgr2b, C1qa |
| Adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains | 4 | 0.027 | C3, MLH1, Fcgr2b, C1qa |
| Activation of protein kinase C activity by G-protein coupled receptor protein signaling pathway | 3 | 0.028 | F2R, HCRT, LOC497978 |
| dGTP metabolic process | 2 | 0.029 | Dguok, NP |
| Regulation of leukocyte mediated immunity | 4 | 0.036 | C3, PLD2, Fcgr2b, NP |
| Leukocyte mediated immunity | 4 | 0.037 | C3, MLH1, Fcgr2b, C1qa |
| Deoxyribonucleoside catabolic process | 2 | 0.038 | DPYD, NP |
| Positive regulation of calcium ion transport | 3 | 0.041 | F2R. HCRT, AKAP5 |
| Positive regulation of type IIa hypersensitivity | 2 | 0.048 | C3, Fcgr2b |
| Regulation of type IIa hypersensitivity | 2 | 0.048 | C3, Fcgr2b |
| Positive regulation of myeloid leukocyte mediated immunity | 2 | 0.048 | C3, Fcgr2b |
| Regulation of type II hypersensitivity | 2 | 0.048 | C3, Fcgr2b |
| Positive regulation of type II hypersensitivity | 2 | 0.048 | C3, Fcgr2b |
| Positive regulation of endocytosis | 3 | 0.048 | C3, PLD2, Fcgr2b |
Abbreviations: Ciliary neurotropic factor, CNTF; purinergic receptor P2X, ligand-gated ion channel 4, P2RX4; prefoldin 6, pfdn6; TNFRSF1A–associated via death domain, TRADD; MIS12, MIND kinetochore complex component, homolog (yeast), MIS12; polymerase (RNA) II (DNA directed) polypeptide I, 14.5 kDa, polr2i; spastic paraplegia 3A homolog (human), ATL1; microsomal glutathione S-transferase 1, mgst1; somatostatin, sst; catechol-O-methyltransferase, COMT; solute carrier family 1 (glial high affinity glutamate transporter), member 3, SLC1A3; ATP-binding cassette, sub-family G (WHITE), member 2, Abcg2; acyl-CoA synthetase long-chain family member 1, ACSL1; Thy-1 cell surface antigen, THY1; similar to Nucleolar GTP-binding protein 1 (chronic renal failure gene protein) (GTP-binding protein NGB); similar to G protein-binding protein CRFG; GTP binding protein 4; similar to isopentenyl diphosphate delta-isomerase type 2, GTPBP4; farnesyltransferase, CAAX box, beta, Fntb; cadherin 13, CDH13; myelin basic protein, MBP; dopa decarboxylase (aromatic L-amino acid decarboxylase), ddc; dihydropyrimidine dehydrogenase, DPYD; catalase, cat; cytochrome P450, family 4, subfamily f, polypeptide 1, Cyp4f1; retinol saturase (all trans retinol 13,14 reductase), retsat; NAD(P)H dehydrogenase, quinone 2, NQO2; reticulon 4 interacting protein 1, RTN4IP1; peroxidasin homolog (Drosophila), PXDN; malic enzyme 3, NADP(+)-dependent, mitochondrial, ME3; similar to RIKEN cDNA 2810025M15, RGD1304982; pyridine nucleotide-disulphide oxidoreductase domain 2, PYROXD2; electron-transfer-flavoprotein, beta polypeptide, etfB; flavin containing monooxygenase 5, FMO5; aldehyde oxidase 1, AOX1; cytochrome P450, family 4, subfamily f, polypeptide 4, Cyp4f4; degenerative spermatocyte homolog 1, lipid desaturase (Drosophila), DEGS1; aldehyde dehydrogenase 3 family, member B1, Aldh3b1; complement component 3, C3; phospholipase D2, PLD2; Fc fragment of IgG, low affinity IIb, receptor (CD32); Fc fragment of IgG, low affinity IIa, receptor (CD32), Fcgr2b; mutL homolog 1 (E. coli), MLH1; complement component 1, q subcomponent, alpha polypeptide, C1qa; nucleoside phosphorylase, NP; histocompatibility 2, T region locus 23; histocompatibility 2, T region locus 24, RT1-S3; RT1 class Ib, locus Aw2, RT1-EC2; coagulation factor II (thrombin) receptor, F2R; phosphoinositide-3-kinase, class 3, PIK3C3; parathyroid hormone receptor 1, Pthr1; hypocretin, HCRT; stonin 2, STON2; A kinase (PRKA) anchor protein 5, AKAP5; acyl-CoA synthetase long-chain family member 5, ACSL5; similar to diacylglycerol kinase epsilon, LOC497978; deoxyguanosine kinase, Dguok.
3.5. Translational impact of metabolic factors
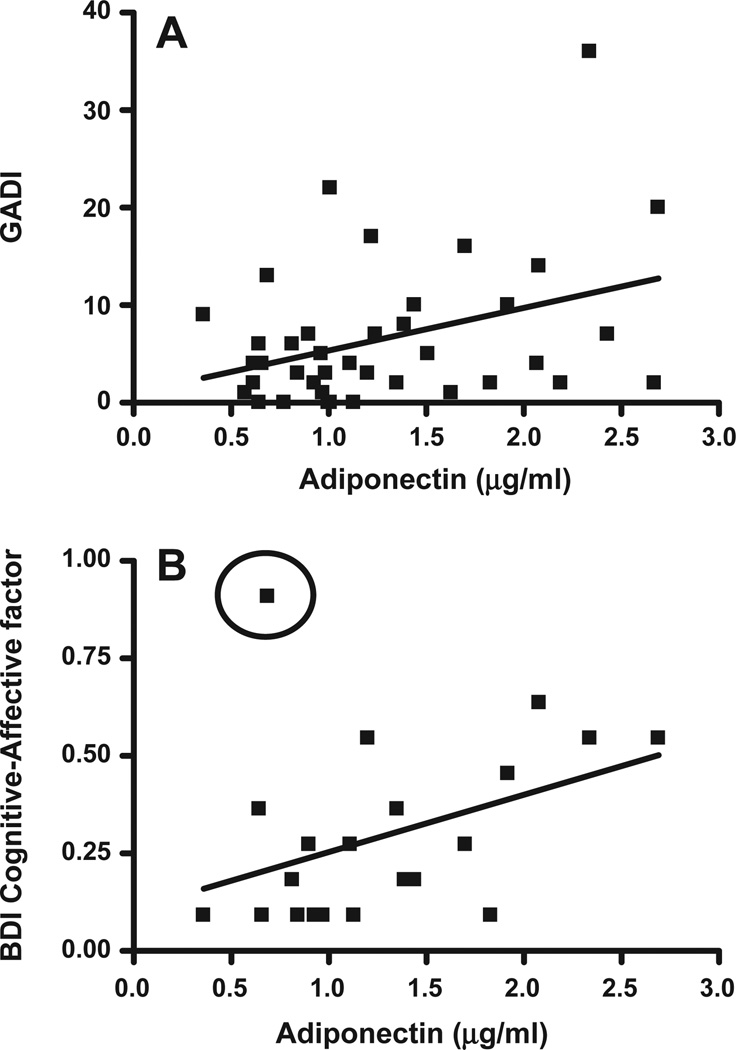
To assess the potential role of components of the adipocytokine signaling pathway (leptin and adiponectin) on symptoms of depression and anxiety, we collected blood samples and questionnaire data from 40 adults. Table 3 shows the demographic, psychiatric and medical characteristics of the research participants. Leptin and adiponectin levels were not significantly correlated (p=0.75). There was also no significant correlation between leptin levels and symptoms of anxiety or depression. Adiponectin, however, was significantly correlated with GADI (r2=0.13; p<0.05) (Fig. 2). To specifically examine individuals with some symptoms of depression, we removed individuals with BDI-II Cognitive–Affective factor scores of zero (n=16), which resulted in a marginal correlation (r2=0.15; p=0.07). Subsequent removal of a single outlier individual with a history of alcohol dependence lead to a very strong correlation (r2=0.44, p<0.001) (Fig. 2). It should be noted that the individuals with BDI-II Cognitive–Affective factor score of zero had plasma adiponectin levels that ranged from 0.5 to 2.8 µg/ml. Nevertheless, the presence of a strong correlation among those with non-zero BDI-II Cognitive–Affective factor scores is strongly suggestive of a role for adiponectin in depressive symptoms.
Table 3.
Demographic, psychiatric and medical characteristicsa
| N=40 | |
|---|---|
| Demographics | |
| Age (mean years ±SD)b | 47.9 ±13.4 |
| Body mass index | 28.1 ±5.1 |
| Male gender | 29 (72.5%) |
| Caucasian | 28 (70.0%) |
| Veteran status | 20 (50.0%) |
| Years of education (mean ± SD)b | 13.8 ±2.3 |
| Current psychiatric status | |
| Current psychiatric diagnosis (any) | 4 (10.0%) |
| Major depressive disorder | 3 (7.5%) |
| PTSDc | 1 (2.5%) |
| Other anxiety disorder | 2 (5.0%) |
| Neuropsychiatric symptom severity | |
| Depression (BDI-II) | 4.5 ± 5.1 |
| Anxiety (GADI) | 6.6 ± 7.5 |
| Medical history | |
| Past medical diagnoses (any) | 17 (42.5%) |
| Diabetes | 5 (12.5%) |
| Hyperlipidemia | 4 (10.0%) |
| Hypertension | 9 (22.5%) |
| Other cardiovascular | 3 (7.5%) |
| Asthma/pulmonary | 5 (12.5%) |
Data are expressed as n, with (%) in terms of n over total N unless otherwise stated.
Psychiatric diagnoses were based on DSM-IV criteria verified using the MINI (Mini-International Neuropsychiatric Interview), medical records, and clinical interviewing. Abbreviations: PTSD, posttraumatic stress disorder; BDI-II, Beck Depression Inventory, II; GADI, Generalized Anxiety Disorder Inventory. Data shown are mean±standard deviation.
Fig. 2.
(A) Significant positive correlation between plasma levels of adiponectin and GADI (Generalized Anxiety Disorder Inventory) anxiety measure (Pearson’s correlation p<0.05). (B) Significant positive correlation between plasma levels of adiponectin and BDI-II Cognitive–Affective factor measure. The point circled was excluded. All subjects with BDI-II Cognitive–Affective factor scores of 0 were removed. Pearson’s correlation (p=0.07 with outlier) (p<0.001 with outlier removed).
4. Discussion
We identified 361 transcripts that were differentially regulated between experimentally naïve FSL and SD control rats (Supplementary Table S2). The benefit of using naïve animals is that they have not been exposed to the stress induced by depression-related tests such as the FST (Drossopoulou et al., 2004). The limitation of this design is that measures of depressive-like behavior were not directly correlated with microarray expression findings. The gene most up-regulated in the FSL rats compared to SD rats was catechol-O-methyltransferase (COMT) (Table 1). COMT is an enzyme involved in the degradation of dopamine, epinephrine and norepinephrine. Increased COMT activity in FSL rats would imply reductions in levels of catecholamine neuro-transmitters. In agreement with this finding are the results from Roth-Deri et al. (2009) who found sub-sensitivity to cocaine, a dopa-mine transporter inhibitor, in FSL rats. Characterization of neurotrans-mitter signaling in FSL rats revealed low extracellular levels of dopamine within the nucleus accumbens following cocaine administration, which was a consequence of attenuated dopamine release (Roth-Deri et al., 2009). Similarly, reduced dopamine signaling was observed in the ventral tegmental area of FSL rats (Friedman et al., 2008). Clinical studies have examined the val158met polymorphism of COMT to determine whether it is associated with MDD. These results have largely been equivocal, however (see Opmeer et al., 2010 for review), suggesting that the val158met polymorphism does not have a large effect on the development of MDD. Nevertheless, treatment with the COMT inhibitor tolcapone reduced symptoms of depression on several depression scales among subjects with MDD (Fava et al., 1999). Clearly, the role of COMT in MDD and in FSL rats has yet to be fully elucidated and should be examined in future studies.
In addition to COMT and consistent with Blaveri et al. (2010), we also found differential expression of the genes, TMEM176A and RNase 4 (Supplementary Table S2), with reduced expression in FSL rats as compared to SD rats (or Flinders Resistant Line (FRL) rats in Blaveri et al., 2010). We found the transcript for FAM111A to be one of the most up-regulated transcripts in FSL rats compared with SD rats (Table 1). This compares with Blaveri et al., who found FAM111A to be one of the most down-regulated transcripts in the hippocampus and prefrontal/frontal cortex between FSL and FRL rats. It is unclear what underlies the apparent differences in gene expression between our study and that of Blaveri et al. (2010). One possibility is that the rats used by Blaveri et al. had previously been stressed in the FST one week prior to collection of samples leading to the possibility that swim stress may have impacted the gene expression data. An additional difference is that Blaveri et al. used the FRL rats for comparison as opposed to SD (parent line) rats. Thus, the experimental design of the two studies varied considerably and therefore it is not surprising that some profiling differences exist.
To better understand how differential expression of neurotrans-mitter and immune system genes may affect biological mechanisms and contribute to depression, we used DAVID to identify biological processes that were overrepresented in our sample of regulated transcripts. We found several GO categories that exhibited enrichment in FSL rats compared with SD rats (Table 2). The list of biological processes associated with genes exhibiting reduced expression was dominated by immune-related processes (e.g., regulation of myeloid leukocyte mediated immunity; immunoglobulin mediated immune response; B cell mediated immunity; and positive regulation of immune effector process). Interestingly, complement component 3 (C3) was found to be down-regulated in three different animal models of treatment- or surgery-induced depression (Uriquen et al., 2008). C3 levels were significantly elevated in plasma from schizophrenic or depressed subjects relative to healthy controls, and were normalized in medicated patients with the same disorders (Maes et al., 1997). A sibling-pair linkage study also found a significant association with the C3 locus and depression spectrum disease (Tanna et al., 1976). The role of C3 and other immune related molecules in MDD remains unclear; however increasing evidence indicates that the immune system is centrally involved in depression.
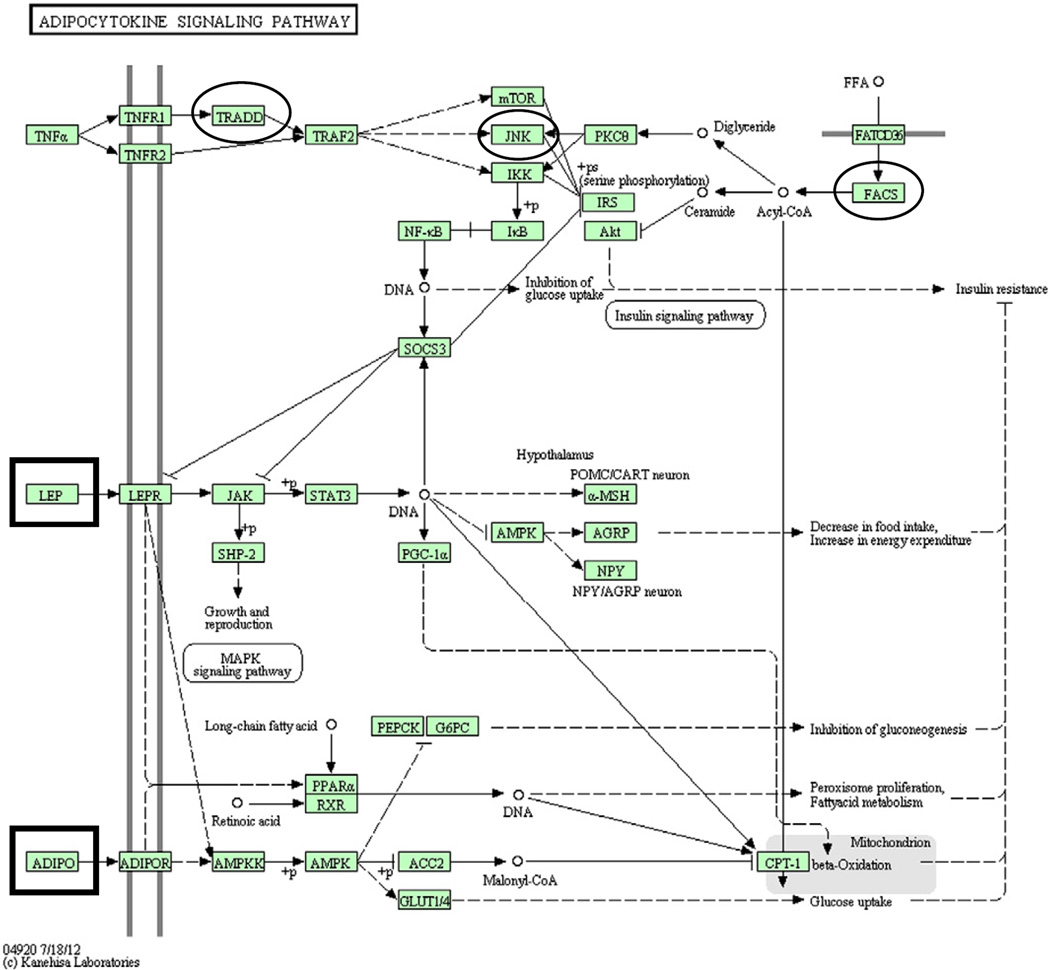
To examine overrepresentation and dysregulation of gene networks, we used the functional annotation chart feature in DAVID. The adipocytokine signaling pathway was identified as overrepre-sented, albeit with a marginal p value of 0.06 (Fig. 3). Adipocytes (a component of white adipose tissue) are primarily localized to the subcutaneous region and the viscera and combined with infiltrating immune cells (mostly macrophages and T cells) are the primary cells involved in adipocytokine signaling (Guzik et al., 2006). Impaired adipocytokine signaling, as in the case of obesity, is associated with generalized inflammation and altered cytokine production (Ouchi et al., 2003).
Fig. 3.
The adipocytokine signaling pathway as represented in the Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/kegg1.html) (Kanehisa and Goto, 2000; Kanehisa et al., 2006, 2010). Connections represent relations between adjacent molecules, with arrows representing activation and blunted lines representing inhibition. Dashed lines indicate an indirect effect. Microarray results found the following genes from this pathway to be up-regulated in FSL rats relative to SD rats: TRADD, acyl-CoA synthetase long-chain family member 1 (FACS; Acsl1), and mitogen activated protein kinase 10 (MAPK10, JNK). A conservative statistical analysis (EASE) indicated overrepresen-tation of this pathway (p=0.06). Results from our human studies indicated significant correlations between adiponectin (Adipo) levels and questionnaire measures of anxiety and depression. Significant correlations were not found between these measures and leptin (Lep) levels. Factors examined in the human component of this study are outlined in rectangles, while those factors found to be differentially regulated in FSL rats are circled.
Leptin and adiponectin are the two best-studied adipocytokines, withleptin playingakey role in satiety and generally promoting inflammation and adiponectin being anti-inflammatory (reviewed in Guzik et al., 2006). FSL rats have reduced leptin levels when compared with FRL rats (Husum et al., 2003). Interestingly, leptin levels are reduced following chronic unpredictable stress, or chronic social defeat (models of depression) in rats and injection of leptin reduces immobility time in the FST in both rats (Lu et al., 2006) and mice (Liu et al., 2009; Yamada et al., 2011). Recent studies have found reduced leptin receptor expression in men with MDD who committed suicide (Lalovic et al., 2010) andseveral studies report reduced serum or plasma leptin in subjects with depression (Pasco et al., 2008; Yang et al., 2007; Eikelis et al., 2006; Jow et al., 2006; Kraus et al., 2002). Furthermore a ghrelin polymorphism (ghrelin enhances appetite thereby acting in opposition to leptin) has been associated with depression (Nakashima et al., 2008). Also, though not a component of the adipocytokine signaling pathway, RNase4 (a.k.a. angiogenin) was found to be significantly down-regulated (Table 1) in FSL animals, and along with adiponectin, has been associated with coronary disease and may be an additional component of inflammatory signaling within adipose tissue (Krecki et al., 2010). Thus, adipocytokine signaling may play a critical role in depression. Future studies are needed to determine the role of leptin and adiponectin signaling: 1) in depression, and 2) in relation to putative neurotransmitter and immune system alterations associated with depression.
As a preliminary step toward confirming the findings from our animal experiments, we evaluated adipocytokine levels (e.g., leptin and adiponectin) and psychiatric symptoms (e.g., anxiety and depressive symptoms) in a sample of human subjects. The relationship between central gene expression, peripheral factors and disease states is unclear. A microarray analysis of tissue from the brain and blood of schizophrenics revealed 177 and 123 putative schizophrenia bio-markers respectively. Of these, six were present in both the blood and the brain (Glatt et al., 2005). A recent study by Savitz et al. (in press) using whole genome expression analysis of mRNA derived from peripheral blood mononuclear cells found significant correlations between genes associated with depression and brain activity assessed by fMRI within the amygdala, ventromedial prefrontal cortex and hippocampus, supporting the influence of peripheral factors on neurological function. Future studies will be needed to identify disease-associated genes that generalize across tissues as well as across species, specifically within a context of mood disorders.
Human studies of associations between leptin and depression have been inconsistent, with studies finding elevations (Antonijevic et al., 1998; Rubin et al., 2002), reductions (Jow et al., 2006; Kraus et al., 2002), or no differences (Deuschle et al., 1996) in leptin levels between control and depressed patients. We did not find significant associations between leptin levels and measures of depression or related factors. The inconsistency between leptin studies of depression could be due to circadian cycle variations in leptin levels or failure to control for body mass index differences. We cannot rule out the possibility that similar circadian variations obscured an association between leptin and our behavioral measures. In contrast, we identified significant correlations between levels of adiponectin and a measure of anxiety (GADI) and the Cognitive–Affective BDI-II factor. It should be noted that the correlation between adiponectin and GADI has a relatively low r2=0.13, suggesting that this relationship is fairly weak. Furthermore, the correlation between adiponectin and the Cognitive–Affective BDI-II factor was only observed following removal of participants with scores of 0 on this measure as well as the removal of an outlier with a history of alcohol dependence.
Despite our encouraging initial results, caution should be taken in interpreting these findings, as generalizing gene expression data obtained from the rat hippocampus to the human condition of major depression will require extensive validation which is outside the realm of the current study. In addition, depression is a stress-related disorder, and behavioral differences between FSL and SD rats are seen exclusively in the stressed state. Thus, our study may identify factors underlying risk for depression (or depressive-like behaviors), such as poor stress management.
Depression and anxiety are common co-morbid conditions with significant symptom overlap (e.g. Starr and Davila, 2012). Recent studies suggest that more than half of individuals with major depression exhibit a co-morbid anxiety disorder (Kessler et al., 2007). Previous studies have identified similar increases in adiponectin associated with symptoms of depression (Jeong et al., 2012), however, like lep-tin, the relationship between adiponectin and depression remains complex. Others have found reductions in adiponectin levels in subjects with major depression (Diniz et al., 2012; Cizza et al., 2010). The adipocytokine signaling pathway brings together immune system and metabolic factors that provide an intriguing integration of factors known to be associated with depression. Future studies identifying the specific factors that underlie depression will provide critical new targets for the development of more effective therapies.
Supplementary Material
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Portland Veterans Affairs Medical Center. JML, CJW and MH are supported by career development awards from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Clinical Sciences Research and Development. We thank Dr. Amir H.Rezvani, Departmentof Psychiatry, Duke University Medical Center for providing the Flinders Sensitive Line rats and Dr. David Overstreet, Department of Psychiatry, University of North Carolina at Chapel Hill, for review of this manuscript. We also thank Nikki Walter and the Portland Alcohol Research Center Molecular and Bioinformatics Core for technical assistance with the quantitative real-time PCR.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.pbb.2012.11.001.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. text rev. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Antonijevic IA, Murck H, Frieboes RM, Horn R, Brabant G, Steiger A. Elevated nocturnal profiles of serum leptin in patients with depression. J Psychiatr Res. 1998;32:403–410. doi: 10.1016/s0022-3956(98)00032-6. [DOI] [PubMed] [Google Scholar]
- Argyropoulos SV, Ploubidis GB, Wright TS, Palm ME, Hood SD, Nash JR, et al. Development and validation of the Generalized Anxiety Disorder Inventory (GADI) J Psychopharmacol. 2007;21(2):145–152. doi: 10.1177/0269881107069944. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Blaveri E, Kelly F, Mallei A, Harris K, Taylor A, Reid J, et al. Expression profiling of a genetic animal model of depression reveals novel molecular pathways underlying depressive-like behaviours. PLoS One. 2010;5:e12596. doi: 10.1371/journal.pone.0012596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Carboni L, Becchi S, Piubelli C, Mallei A, Giambelli R, Razzoli M, et al. Early-life stress and antidepressants modulate peripheral biomarkers in a gene-environment rat model of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1037–1048. doi: 10.1016/j.pnpbp.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Cizza G, Nguyen VT, Eskandari F, Duan Z, Wright EC, Reynolds JC, Ahima RS, Blackman MR. POWER Study Group Low 24-hour adiponectin and high nocturnal leptin concentrations in a case-control study of community-dwelling premenopausal women with major depressive disorder: the Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression (POWER) study. J Clin Psychiatry. 2010;71(8):1079–1087. doi: 10.4088/JCP.09m05314blu. [Epub 2010 May 18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Blum WF, Englaro P, Schweiger U, Weber B, Pflaum CD, Heuser I. Plasma leptin in depressed patients and healthy controls. Horm Metab Res. 1996;28(12):714–717. doi: 10.1055/s-2007-979885. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Campos AC, Miranda AS, Rocha NP, Talib LL, Gattaz WF, Forlenza OV. Reduced serum levels of adiponectin in elderly patients with major depression. J Psychiatr Res. 2012;46(8):1081–1085. doi: 10.1016/j.jpsychires.2012.04.028. [Epub 2012 May 25] [DOI] [PubMed] [Google Scholar]
- Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, et al. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience. 2004;126:849–857. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;17(Suppl.3):306–310. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- Eikelis N, Esler M, Barton D, Dawood T, Wiesner G, Lambert G. Reduced brain leptin in patients with major depressive disorder and in suicide victims. Mol Psychiatry. 2006;11:800–801. doi: 10.1038/sj.mp.4001862. [DOI] [PubMed] [Google Scholar]
- Fava M, Rosenbaum JF, Kolsky AR, Alpert JE, Nierenberg AA, Spillmann M, et al. Open study of the catechol-O-methyltransferase inhibitor tolcopane in major depressive disorder. J Clin Psychopharmacol. 1999;19:329–335. doi: 10.1097/00004714-199908000-00008. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Möller HJ. Efficacy of antidepressants: a re-analysis and re-interpretation of theKirsch data. IntJ Neuropsychopharmacol. 2011;14:405–412. doi: 10.1017/S1461145710000957. [DOI] [PubMed] [Google Scholar]
- Friedman A, Friedman Y, Dremencov E, Yadid G. VTA dopamine neuron bursting is altered in an animal model of depression and corrected by desipramine. J Mol Neurosci. 2008;34:201–209. doi: 10.1007/s12031-007-9016-8. [DOI] [PubMed] [Google Scholar]
- Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis in press. doi: 10.1016/j.nbd.2012.03.012. PMID: 22426398 [Epub ahead of print PubMed] [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Everall IP, Kremen WS, Corbelil J, Sasik R, Khanlou N, et al. Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc Natl Acad Sci U S A. 2005;25:15533–15538. doi: 10.1073/pnas.0507666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Mangalat D, Korbut R. Adipocytokines — novel link between inflammation and vascular function? J Physiol Pharmacol. 2006;57:505–528. [PubMed] [Google Scholar]
- Hosack DA, Dennis G, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009a;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths towards the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009b;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husum H, Van Kammen D, Termeer E, Bolwig G, Mathe A. Topiramate normalizes hippocampal NPY-LI in Flinders sensitive line ‘depressed’ rats and upregulates NPY, galanin, and CRH-LI in the hypothalamus: implications for mood-stabilizing and weight loss-inducing effects. Neuropsychopharmacology. 2003;28:1292–1299. doi: 10.1038/sj.npp.1300178. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Overstreet DH, Nurnberger JI., Jr Is cholinergic sensitivity a genetic marker for the affective disorders? Am J Med Genet. 1994;54:335–344. doi: 10.1002/ajmg.1320540412. [DOI] [PubMed] [Google Scholar]
- Jeong HG, Min BJ, Lim S, Kim TH, Lee JJ, Park JH, Lee SB, Han JW, Choi SH, Park YJ, Jang HC, Kim KW. Plasma adiponectin elevation in elderly individuals with subsyndromal depression. Psychoneuroendocrinology. 2012;37(7):948–955. doi: 10.1016/j.psyneuen.2011.11.002. [Epub 2011 Nov 29] [DOI] [PubMed] [Google Scholar]
- Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90:21–27. doi: 10.1016/j.jad.2005.09.015. [Epub 2011 Nov 29] [DOI] [PubMed] [Google Scholar]
- Kajiyama Y, Lijima Y, Chiab S, Furuta M, Ninomiya M, Izumi A, et al. Prednisolone causes anxiety- and depression-like behaviors and altered expression of apoptotic genes in mice hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:159–165. doi: 10.1016/j.pnpbp.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, et al. From ge-nomics to chemical genomics: new developments in KEGG. Nucleic Acids Res. 2006;34:D354–D357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010;38:D355–D360. doi: 10.1093/nar/gkp896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol. 2007;3:137–158. doi: 10.1146/annurev.clinpsy.3.022806.091444. [DOI] [PubMed] [Google Scholar]
- Kraus T, Haack M, Schuld A, Hinze-Selch D, Koethe D, Pollmacher T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neu-roendocrinology. 2002;73:243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- Krecki R, Krzeminska-Pakula M, Drozdz J, Szczesniak P, Peruga JZ, Lipiec P, et al. Relationship between serum angiogenin, adiponectin and resistin levels with biochemical risk factors and the angiographic severity of three-vessel coronary disease. Cardiol J. 2010;17:599–606. [PubMed] [Google Scholar]
- Kurian BT, Greer TL, Trivedi MH. Strategies to enhance the therapeutic efficacy of anti-depressants: targeting residual symptoms. Expert Rev Neurother. 2009;9:975–984. doi: 10.1586/ERN.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalovic A, Klempan T, Sequeira A, Luheshi G, Turecki G. Altered expression of lipid metabolism and immune response genes in the frontal cortex of suicide completers. J Affect Disord. 2010;120:24–31. doi: 10.1016/j.jad.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong J, Kwak Y, Park SK. Depression research: where are we now? Mol Brain. 2010;3:8. doi: 10.1186/1756-6606-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012 Feb;36(2):764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, et al. Vaccination as a novel approach for treating depressive behavior. Biol Psychiatry. 2009;65:283–288. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Liu J, Graza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl) 2009;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Cocaine treatment- and withdrawal-induced alterations in the expression and serine phosphorylation of the NR1 NMDA receptor subunit. Psy-chopharmacology (Berl) 2002;164:349–359. doi: 10.1007/s00213-002-1209-9. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Wall JM, Pagel RL, Hauser P. Administration of pegylated interferon-alpha-2a or −2b does not induce sickness behavior in Lewis rats. Psychoneuroendocrinology. 2006;31:1289–1294. doi: 10.1016/j.psyneuen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Huckans M, Morasco BJ. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol Dis. 2010;37:519–533. doi: 10.1016/j.nbd.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macqueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotro-pic drugs. Psychiatry Res. 1997;66:1–11. doi: 10.1016/s0165-1781(96)02915-0. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Titus AT, Albert M, Michael GJ, Michaelis T, Watanabe T, Frahm J, et al. SONU20176289, a compound comining partial dopamine D(2) receptor agonism with specific serotonin reuptake inhibitor activity, affects neuroplasticity in an animal model for depression. Eur J Pharmacol. 2008;598:43–50. doi: 10.1016/j.ejphar.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Akiyoshi J, Hatano H, Tanaka Y, Tsuru J, Matsushita H, et al. Ghrelin gene polymorphism is associated with depression, but not panic disorder. Psychiatr Genet. 2008;18:257. doi: 10.1097/YPG.0b013e328306c979. [DOI] [PubMed] [Google Scholar]
- Opmeer DEM, Kortekaas R, Aleman A. Depression and the role of genes involved in do-pamine metabolism and signaling. Prog Neurobiol. 2010;92:112–133. doi: 10.1016/j.pneurobio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. Selective breeding for increased cholinergic function: development of a new animal model of depression. Biol Psychiatry. 1986;21:49–58. doi: 10.1016/0006-3223(86)90007-7. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev. 1993;17:51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Russell RW. Selective breeding for diisopropyl fluorophosphates-sensitivity: behavioural effects of cholinergic agonists and antagonists. Psychophar-macology (Berl) 1982;78:150–155. doi: 10.1007/BF00432254. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders sensitive line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, et al. Leptin in depressed women: cross-sectional and longitudinal data from an epidemiologic study. J Affect Disord. 2008;107:221–225. doi: 10.1016/j.jad.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Patterson AL, Morasco BJ, Fuller BE, Indest DW, Loftis JM, Hauser P. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33:354–362. doi: 10.1016/j.genhosppsych.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piubelli C, Carboni L, Becchi S, Mathe AA, Domenici E. Regulation of cytoskeleton machinery, neurogenesis and energy metabolism pathways in a rat gene-environment model of depression revealed by proteomic analysis. Neuroscience. 2011;176:349–380. doi: 10.1016/j.neuroscience.2010.12.043. [DOI] [PubMed] [Google Scholar]
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24(6):519–525. doi: 10.1097/YCO.0b013e32834b9db6. [DOI] [PubMed] [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Friedman A, Abraham L, Lax E, Flaumenhaft Y, Dikshtein Y, et al. Antide-pressant treatment facilitates dopamine release and drug seeking behavior in a genetic animal model of depression. Eur J Neurosci. 2009;30:485–492. doi: 10.1111/j.1460-9568.2009.06840.x. [DOI] [PubMed] [Google Scholar]
- Rubin RT, Rhodes ME, Czambel RK. Sexual diergism of baseline plasma leptin and leptin suppression by arginine vasopressin in major depressives and matched controls. Psychiatry Res. 2002;113:255–268. doi: 10.1016/s0165-1781(02)00263-9. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hip-pocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS, McKinney BA, Bodurka J, Kent Teague T, Drevets WC. Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain Behav Immun in press. doi: 10.1016/j.bbi.2012.10.007. pii: S0889-1591(12)00469-2. PubMed PMID: 23064081. [Electronic publication ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl.20):22–33. [quiz 34–57] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16:751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr LR, Davila J. Responding to anxiety with rumination and hopelessness: mechanism of anxiety–depression symptom co-occurrence? Cogn Ther Res. 2012;36(4):321–337. doi: 10.1007/s10608-011-9363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna VL, Winokur G, Elston RC, Go RC. A linkage study of depression spectrum disease: the use of the sib-pair method. Neuropsychobiology. 1976;2:52–62. doi: 10.1159/000117529. [DOI] [PubMed] [Google Scholar]
- Uriquen L, Arteta D, Diez-Alarcia R, Ferrer-Alcon M, Diaz A, Pazos A, et al. Gene expression patterns in brain cortex of three different animal models of depression. Genes Brain Behav. 2008;7:649–658. doi: 10.1111/j.1601-183X.2008.00402.x. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89:157–166. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- Videbach P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wegener G, Finger BC, Elfving B, Keller K, Liebenberg N, Fischer CW. Neuropeptide S alters anxiety, but not depression-like behavior in Flinders sensitive line rats: a genetic animal model of depression. Int J Neuropsychopharmacol. 2011;9:1–13. doi: 10.1017/S1461145711000678. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Murphy-Crews A, Menasco DJ, Huckans MS, Loftis JM. Corticotropin releasing factor-1 receptor antagonism alters the biochemical, but not behavioral effects of repeated interleukin-1β administration. Neuropharmacology. 2011;62:313–321. doi: 10.1016/j.neuropharm.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, et al. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152:2634–2643. doi: 10.1210/en.2011-0004. [DOI] [PubMed] [Google Scholar]
- Yang K, Xie G, Zhang Z, Wang C, Li W, Zhou W, et al. Levels of serum interleukin (IL)-6, IL-1beta, tumour necrosis factor-alpha and leptin and their correlation in depression. Aust N Z J Psychiatry. 2007;41:266–273. doi: 10.1080/00048670601057759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.