Abstract
The increased use of flammable plastics and electronic devices along with stricter fire safety standards has led to the heavy use of flame retardant chemicals in many consumer, commercial, and industrial products. Although flame retardant use has increased, a great deal of uncertainty surrounds their safety with some evidence showing toxicity and risk to human and environmental health. Recent efforts have focused on designing high-throughput biological platforms with nonmammalian models to evaluate and prioritize chemicals with limited hazard information. To complement these efforts, this study used a new morphological and behavioral testing platform with embryonic zebrafish to characterize the developmental toxicity of 44 halogenated and organophosphate flame retardants, including several of their known metabolites. Zebrafish were exposed to flame retardants from 6 to 120 h post fertilization (hpf) across concentrations spanning 4 orders of magnitude (eg, 6.4 nM to 64 µM). Flame retardant effects on survival and development were evaluated at 24 and 120 hpf, and neurobehavioral changes were measured using 2 photomotor response (PMR) assays. Compared to controls, 93% (41/44) of flame retardants studied elicited adverse effects among one or more of the bioassays and concentrations tested with the aryl phosphate ester (APE)-based mono-isopropylated triaryl phosphate and the brominated-bisphenol-A analog tetrabromobisphenol-A producing the greatest array of malformations. Hierarchical clustering showed that APE flame retardants with isopropyl, butyl, and cresyl substituents on phenyl rings clustered tightly and were particularly potent. Both PMR assays were highly predictive of morphological defects supporting their use as nonlethal means of evaluating teratogenicity that could allow for additional evaluations of long-term or delayed effects in older animals. Taken together, evidence presented here indicates that zebrafish neurodevelopment is highly sensitive to many flame retardants currently in use and can be used to understand potential vulnerabilities to human health.
Keywords: Firemaster 550, neurotoxicity, PBDE, TBBPA, TDCPP, TCPP, TCEP, TPP, teratogenicity
Dramatic increases in the use of flammable plastics and electronics coupled with stricter fire safety standards have resulted in the heavy use of flame retardant chemicals. Flame retardants today represent a diverse array of chemicals with differing structural characteristics and fire suppressing properties. Increased public, media, and government scrutiny of flame retardants in recent years has called attention to their design, use, and safety. Two of the commonly used classes of flame retardants, brominated flame retardants (BFRs) and organophosphate-based flame retardants, which may include both halogenated and nonhalogenated structural forms, are widely used in a variety of consumer products, such as furniture, textiles, electronics, and building materials.
Polybrominated diphenyl ether (PBDE) flame retardants (Table 1) were additive BFRs used in furniture and electronic products. They were marketed as 3 major commercial mixtures: PentaBDE, OctaBDE, and DecaBDE. PentaBDE was a heterogeneous mixture of tetra-, penta-, and hexaBDEs that was added mostly to polyurethane foams and textiles, and to a lesser extent in epoxy and phenolic resins and polyesters. The vast majority (approximately 95%) of PentaBDE was used in North America (United States and Canada) in the manufacture of polyurethane foams in cushioning. As a result of this greater use in North America, PentaBDE congeners have been detected at higher levels in the U.S. population than in European and Asian populations (Johnson-Restrepo and Kannan, 2009; Toms et al., 2011). The production and use of Penta and OctaBDE has been phased out in the United States and banned in the EU since the mid-2000s due to concerns about their persistence, bioaccumulation, and toxicity. In 2009, these products were also listed as persistent organic pollutants under the UN Stockholm Convention (UNEP, 2009). DecaBDE contains the fully brominated congener decabromodiphenyl ether (BDE-209; approximately 97%) with trace amounts of nonaBDEs. It is an additive in high impact polystyrene, polyolefins, and polypropylene used in electronic equipment (eg, plastic housing), automobiles, airplanes, construction and building materials (eg, wires, cables, pipes), and textiles. In the United States, DecaBDE was subject to a voluntary phase-out at the end of 2013. It has also been restricted from use in electrical and electronic equipment since 2008 under the EU Restriction of Hazardous Substances Directive. At present, DecaBDE is not subject to restrictions in any Asian countries.
TABLE 1.
Structures of Targeted BFRs
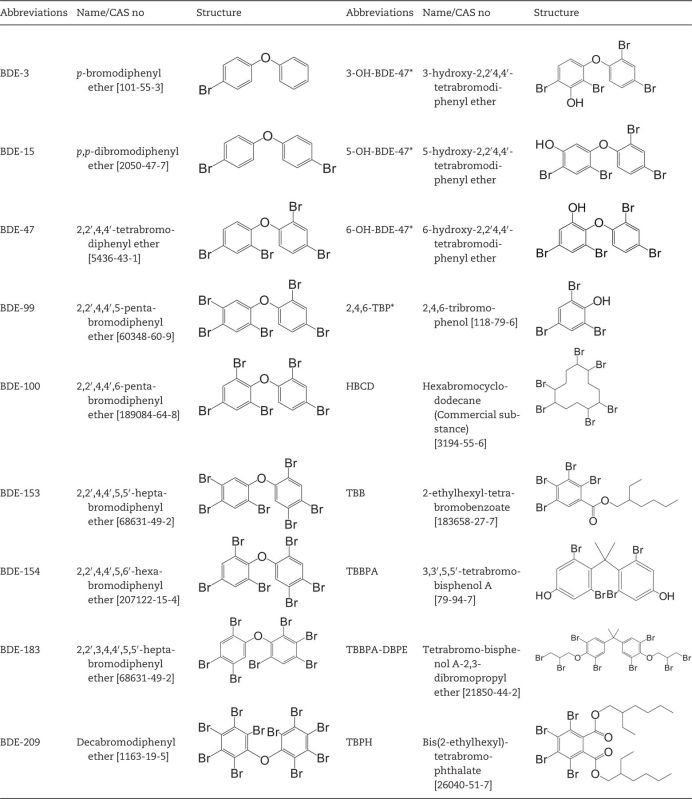 |
Metabolites are denoted with asterisks.
With the phase-out of Penta and OctaBDEs, decreasing trends or a leveling off of some PBDEs are now being measured in some biota and environmental media (Law et al., 2014). Nonetheless, constituents of PentaBDE, including BDE-47 (2,2′4,4′-tetraBDE), BDE-99 (2,2′,4,4′,5-pentaBDE), BDE-100 (2,2′,4,4′,6-pentaBDE), BDE-153 (2,2′,4,4′,5,5′-hexaBDE), and BDE-154 (2,2′,4,4′,5,6′-hexaBDE), continue to be dominant PBDEs detected in humans and wildlife worldwide despite the generally more limited use of PentaBDE outside the United States (Law et al., 2014). Sources of these congeners are likely related to the ongoing use and recycling of products that contain PentaBDE as well as their high environmental persistence and long-range global transport potential (de Wit et al., 2010). Another source of lower MW PBDEs may be attributable to the breakdown of higher PBDEs, such as BDE-209 which can undergo photolytic (Stapleton and Dodder, 2008) and metabolic breakdown (Noyes et al., 2011; Stapleton et al., 2004) to yield lower MW congeners. BDE-209 is now the dominant PBDE measured in abiotic compartments, typically at ppb to low ppm levels in dust (Stapleton et al., 2012a), soils and sediments (Marvin et al., 2013), and biosolids (Peng et al., 2009). Human body burdens of BDE-209 also appear to be on the rise in some populations, notably among E-waste workers (Bi et al., 2007; He et al., 2013) and in the general population, particularly young children in the United States (Lunder et al., 2010; Stapleton et al., 2012a).
Restrictions on the use of PBDEs have resulted in the increased use of alternate flame retardants, notably organophosphate flame retardants (OPFRs; Table 2). The OPFRs have been used for many years with a diversity of applications that may extend beyond their use as flame retardants, including as plasticizers and lubricants in industrial, commercial, and consumer products. Likewise, they can also be used in a range of polymers depending on the types of side chains present. For example, nonhalogenated OPFRs, such as triphenyl phosphate (TPP) and tricresyl phosphate (TCP) may be used as flame retardant plasticizers in PVC, thermoplastics, and synthetic rubbers, with TPP also being present in the flame retardant mixture Firemaster 550 (FM550), which is a replacement for PentaBDE that is added to polyurethane foams. Similarly, the chlorinated OPFR tris (1,3-dichloro-2-propyl) phosphate (TDCPP) is also an important replacement for PentaBDE and is used in polyurethane foams in residential furniture. Despite the increased and varied uses of OPFRs, information on their exposure and environmental contamination profiles is still limited. Much of the literature focuses on rising levels of OPFRs in abiotic environments (Klosterhaus et al., 2012; Shoeib et al., 2014) and indoor environments, especially dust (Meeker et al., 2013; Stapleton et al., 2009; van den Eede et al., 2011). More recent studies have also begun to document increasing exposures and bioaccumulation of OPFRs in humans (Butt et al., 2014; Cooper et al., 2011; Fromme et al., 2014; Kim et al., 2014) and wildlife (Greaves and Letcher, 2014; McGoldrick et al., 2014; Sundkvist et al., 2010).
TABLE 2.
Structures of Targeted APE, CPE, and Dechlorane Plus Flame Retardants
 |
Metabolites are denoted with asterisks.
One ongoing challenge with the production and use of flame retardants is that many, including PBDEs and most OPFRs are not incorporated into polymers until after polymerization and so are not chemically bound but are rather mixed into parent polymers. This additive practice presents exposure concerns as these compounds may volatilize into the air and migrate into surrounding environments, notably dust, with the breakdown of the parent polymer. Another important issue with many flame retardants currently used or considered as replacement options is that there is often little data available on their toxicity potential prior to deployment into products. Thus, there is a need to expand our understanding of the toxicity of flame retardants that are environmentally widespread and to better assess the suitability of chemicals being used/considered as replacements for those that have been banned or phased-out.
The zebrafish (Danio rerio) is an increasingly important biological sensor and model for screening chemicals for human health hazard and disease (Padilla et al., 2012; Perkins et al., 2013; Truong et al., 2014). They are small prolific spawners that are easy to manipulate genetically and pharmacologically (Granato et al., 1996; Howe et al., 2013). Moreover, the zebrafish and mammalian brain share many anatomical and functional features, including well-conserved neuronal morphology and neurotransmitter systems, although neuroanatomical differences exist between fishes and mammals (eg, comparatively small telencephalon in fish that lack characteristic structures of a cerebral cortex) (Kalueff et al., 2014; Panula et al., 2010). A number of neurobehavioral tests of locomotion, anxiety, and exploration have been modeled in zebrafish, and increasing evidence appears to support well-conserved responses resembling those of rodents (Champagne et al., 2010; Panula et al., 2006). For instance, zebrafish display anxiety-like behaviors, such as dark avoidance and thigmotaxis, when placed in novel test environments and these responses are consistent with observations in rodents, thereby providing promising approaches for evaluating chemical hazard in nonmammalian models (Champagne et al., 2010; Levin et al., 2007; Steenbergen et al., 2011). To this end, our laboratory and others have designed high-throughput methodologies with embryonic zebrafish to rapidly screen chemicals for neurological and developmental toxicity. These types of screening platforms have greatly expanded our capacity to assess large chemical libraries for teratogenicity, including for example those under the U.S. EPA ToxCast program (Padilla et al., 2012; Truong et al., 2014). They provide a systematic means of testing large, structurally diverse classes of compounds like the flame retardants. Importantly, these types of approaches represent an attractive option for in vivo screening early in R&D processes to help identify promising chemistries that elicit reduced bioactivity and abandon others with unwanted side effects.
The purpose of this study was to use this type of screening platform with embryonic zebrafish to increase our understanding of flame retardant bioactivity and toxicity potential. It had 2 major components: (1) evaluations of survival and 20 other teratogenicity endpoints in embryos at 24 and 120 hpf; and characterizations of locomotor behavior using 2 photomotor response (PMR) assay tests at 24 and 120 hpf (Fig. 1). Each flame retardant was tested across a range of nominal concentrations that spanned 4 orders of magnitude (eg, 6.4 nM to 64 µM) and included 32 replicates at each concentration. The flame retardants tested (Tables 1 and 2) included aryl phosphate ester (APE) and chlorinated phosphate ester (CPE) OPFR chemicals that are increasing in use with still insufficient toxicity testing. A number of BFRs were also examined, including the PBDEs and heavily used flame retardants tetrabromobisphenol-A (TBBPA) and hexabromocyclododecane (HBCD; Table 1). TBBPA is the most widely used BFR with early reports of increasing global market demand ranging from 120 000 to 170 000 metric tons between 1999 and 2004 (Guerra et al., 2011). It is a brominated analog of BPA that is used primarily as a reactive flame retardant in printed circuit boards and also as an additive flame retardant in polymers. HBCD is an additive brominated cyclic alkane added to polystyrene thermoplastic polymers in furniture, appliances, and construction materials. Although TBBPA has been used in greater volumes than other BFRs, reported concentrations of TBBPA in biota and the environment appear to be less than the PBDEs (ECB, 2006; Kemmlein et al., 2009). However, the EU concluded in its risk characterization of TBBPA that additional information and testing were needed with some questions raised due to its primary biodegradation to several products including BPA. Components of the commercial mixture FM550, which have been frequently detected in furniture foam since the 2005 phase-out of PentaBDE, were also studied (Dodson et al., 2012; Stapleton et al., 2012b). Although FM550 is proprietary, studies have identified its major components as TPP, 2-ethylhexyl-tetrabromobenzoate (TBB), bis(2-ethylhexyl)-tetrabromophthalate (TBPH), and isopropylated triaryl phosphates (ITPs) (Stapleton et al., 2008). Recent studies also report frequent detections of TDCPP in indoor environments and its primary metabolite bis(1,3-dichloroisopropyl)phosphate (BDCPP) in human urine since replacement of PentaBDE, and so both these chemicals were examined (Cooper et al., 2011; Meeker et al., 2013). In addition to testing TDCPP and BDCPP, several other chlorinated-tris flame retardants and known metabolites were targeted.
FIG 1.
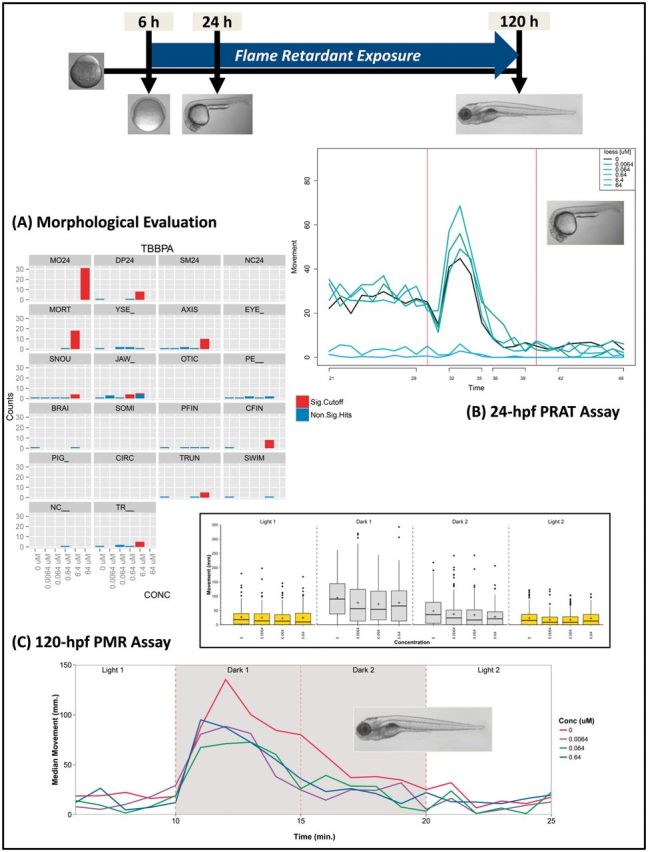
Schematic of A, morphological and B and C, behavioral testing platform used to characterize the effects of flame retardants on zebrafish neurological and morphological development at 24 and 120 hpf. Embryos were exposed continuously to chemical from 6 to 120 hpf under static nonrenewal conditions.
MATERIALS AND METHODS
Chemicals
Table 3 provides a list of flame retardants tested along with suppliers, stock purity, and concentration ranges evaluated. Three different formulations of isopropylated triphenyl phosphate (IPP) were tested from different manufacturers as this chemical is a complex mixture of numerous positional isomers and the phenol groups may be mono-, di-, or tri-isopropylated. The toxicity of the polychlorinated flame retardant Dechlorane Plus was also characterized, and although not an organophosphate, it was grouped with the CPEs because of shared chlorination. A number of metabolites were also tested, including BDCPP (TDCPP metabolite), bis(2-chloropropyl)1-carboxyethyl phosphate (BCPCP; tris (2-chloroethyl) phosphate [TCEP] metabolite), diphenyl phosphate (DPP; TPP metabolite), bis(2-chloroisopropyl)phosphate and mono(2-chloroisopropyl)phosphate (BCPP, MCPP; tris (1-chloropropyl) phosphate [TCPP] metabolites), and some of the major hydroxylated PBDE metabolites (Burka et al., 1991; Malmberg et al., 2005; Nomeir et al., 1981). The high MW PBDE decabromodiphenyl ether (BDE-209) was dissolved in acetone prior to diluting in DMSO to prevent it from precipitating out of solution. Standard stock solutions and covered dilution plates were stored at −20°C. All solvents used were high-performance liquid chromatography grade.
TABLE 3.
Sources, Purities, and Test Concentrations (N = 32) of Targeted Flame Retardant Parents and Metabolites.
| Flame retardant | Supplier | Purity (%) | Concentration ranges tested (µM) |
|---|---|---|---|
| APE FRs | |||
| BDP | Toronto Research | 98 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| BPDP | Ubichem PLC | NP | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| DPP* | Sigma-Aldrich | 99 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| EHDP | TCI Americas | 92.8 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| IDDP | Ferro Corp | NP | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| IPP-1 | Ameribrom | NP | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| IPP-2 | Chemtura | NP | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| IPP-3 | Amfinecom Inc | NP | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| mITP | Chemtura and Wellington | > 90 | 6.4, 0.64, 0.064, 0.0064,0.00064 |
| RDP | Toronto Research | 98 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| TBP | Sigma-Aldrich | 99 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| TBEP | Chiron AS | 95.5 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| TCP | Acros Organics | 99 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| o-TCP | Acros Organics | 96.8 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| TEHP | Sigma-Aldrich | 97 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| TPP | Acros Organics | 99.3 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| CPE FRs and Dechlorane | |||
| BCPCP* | MRI Global | 96 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| BDCPP* | Toronto Research | 95 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| BCPP* | MRI Global | 99.5 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| Dechlorane | Toronto Research | 98 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| MCPP* | MRI Global | 95.1 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| TCPP | Albemarle Corp | NP | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| TCEP | Sigma-Aldrich | 98.8 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| TDCPP | Sigma-Aldrich | 99 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| PBDE FRs | |||
| DE-71 (PentaBDE commercial mix) | Great Lakes Chem | 98 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| DE-79 (OctaBDE commercial mix) | Great Lakes Chem | 99 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| BDE-3 | Sigma-Aldrich | 98.6 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| BDE-15 | Sigma-Aldrich | 99.6 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| BDE-47 | Cerilliant Corp | 96 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| BDE-99 | Cerilliant Corp | 96 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| BDE-100 | AccuStandard | 100 | 0.0064, 0.00064, 6.4E-05, 6.4E-06, 0 |
| BDE-153 | Cerilliant Corp | 98 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| BDE-154 | AccuStandard | 100 | 0.0064, 0.00064, 6.4E-05, 6.4E-06, 0 |
| BDE-183 | AccuStandard | 100 | 0.0064, 0.00064, 6.4E-05, 6.4E-06, 0 |
| BDE-209 | Sigma-Aldrich | 99.9 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| 3-OH-BDE-47* | AccuStandard | 97.1 | 0.64, 0.064, 0.0064, 0.00064, 6.4E-05, 0 |
| 5-OH-BDE-47* | AccuStandard | 98 | 0.64, 0.064, 0.0064, 0.00064, 6.4E-05, 0 |
| 6-OH-BDE-47* | AccuStandard | 98 | 0.0064, 0.00064, 6.4E-05, 6.4E-06, 0 |
| 2,4,6-TBP* | Sigma-Aldrich | 99.8 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| Other brominated FRs | |||
| HBCD (commercial substance) | Sigma-Aldrich | 95 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| TBB | Toronto Research | 96 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| TBBPA | Sigma-Aldrich | 99.2 | 64, 6.4, 0.64, 0.064, 0.0064, 0 |
| TBBPA-DBPE | TCI Americas | 95 | 6.4, 0.64, 0.064, 0.0064, 0.00064, 0 |
| TBPH | Accustandard | 98.7 | 0.64, 0.064, 0064, 0.00064, 6.4E-05, 0 |
Chemicals with asterisks are metabolites measured in toxicokinetic studies. NP, not provided.
Fish husbandry
Wild-type zebrafish (Tropical 5D) were maintained under a 14:10 h light/dark photoperiod at the Sinnhuber Aquatic Research Laboratory (SARL), Oregon State University (Corvallis, Oregon) at densities of approximately 500 fish/50 gal tank in recirculating filtered water (carbon, reverse osmosis) maintained at 28°C and supplemented with salts (Instant Ocean). Spawning funnels were placed in tanks the evening prior to chemical exposures, and the next morning after spawning, embryos were collected, staged, and maintained in sterile incubation dishes under the same conditions as adults (Kimmel et al., 1995). Adult care and breeding were conducted in accordance with protocols under Oregon State University’s Institutional Animal Care and Use Committee.
Chemical exposures
At 5 hpf, embryos were enzymatically dechorionated using pronase (90 µl at 25.3 U/µl; Roche, Indianapolis, Indiana) and an automated mechanical dechorionator developed at SARL (Mandrell et al., 2012). Dechorionation procedures followed those outlined in Mandrell et al. (2012). Dechorionated embryos (1 embryo/well) were placed in polystyrene 96-well plates (BD Falcon, Corning, Lowell, Massachusetts) containing 90 µl of E2 embryo media using SARL’s automated embryo placement system. Embryos were visually inspected under a light microscope after dechorionation and robotic plating to ensure embryo viability and proper staging. For chemical exposures, 2 dilution plates were made per chemical. Plate 1 contained serially diluted chemical in 100% DMSO. Plate 2 was prepared from Plate 1 and contained chemical in E2 embryo media at 10-fold higher concentrations (eg, 0–640 µM at 6.4% DMSO) than the final concentration. Ten microliters of this second dilution plate was spiked into two 90 µl exposure plates containing embryos (eg, final concentration = 0–64 µM at 0.64% DMSO; n = 32). Embryos were exposed continuously to flame retardants from 6 to 120 hpf. For some of the flame retardants, low aqueous solubility or limitations in stock concentrations available required that the concentration range to be shifted downward.
Developmental malformation evaluations
At 24 hpf, embryos were evaluated for survival, delays in developmental progression, notochord deformities, and altered spontaneous movements. Embryos that did not move (no body flexions, tail contractions) after 60 s were scored as having altered spontaneous movements. At 120 hpf, larvae were evaluated for survival (MORT) and 17 developmental malformations, including yolk sac edema (YSE) and pericardial edema (PE); body axis (AXIS), trunk length (TRUN), caudal fin (CFIN), pectoral fin (PFIN), pigmentation (PIG), and somite (SOMI) deformities; eye (EYE), snout (SNOU), jaw (JAW), and otolith (OTIC) malformations; gross brain development (BRAIN); notochord (NC) and circulatory (CIRC) deformities; swim bladder presence and inflation (SWIM); and touch-responses (TR). For the TR endpoint, larvae were touched with a probe on the head, body, and tail to test for normal rapid swimming and touch-escape responses. Fish that did not move were scored as having impaired TRs even if they showed no other overt malformations. Binary responses were recorded as either absent (0) or present (1) for each endpoint. Data collection was undertaken using a custom barcoding and tracking system (Zebrafish Acquisition and Analysis Program) to facilitate reliable management of the large amounts of data collected. Statistical analyses were performed using R code with testing methodologies used by Truong et al. (2014) to evaluate developmental toxicity of chemicals under the ToxCast program (RCoreTeam, 2014; Truong et al., 2014). Briefly, a binomial test was performed that calculated lowest effect levels (LELs) for each endpoint to identify incidences that exceeded a significant threshold above controls. This test was preferable to a logistic regression as it accounted for the observed nonmonotonicity of flame retardant toxicity.
Embryonic PMRs
PMRs in 24 hpf embryonic zebrafish exposed to flame retardants were measured using a custom built PMR assay tool (PRAT). Starting at approximately 17–19 hpf, embryonic zebrafish begin to spontaneously contract their tails in a reflexive manner with advancing development of sensory-motor neuron interactions and muscle enervations (Kimmel et al., 1995; Kokel and Peterson, 2011; Kokel et al., 2010). This response in embryonic zebrafish has been shown to be highly sensitive to light through nonocular photoreceptors located in the hindbrain (Kokel et al., 2013). The PRAT platform uses PMR light from 2 white L300 Linear Lights (Smart Vision Lights, Muskegon, Michigan) and a high resolution Prosilica GX3300 camera (Allied Vision, Stradtroda, Germany) that is mounted under a 96-well plate holder and coupled to a near-infrared filter to remove image and light distortions. The light cycle consists of following: 30 s period of darkness (Background); pulse of PMR light (Excitation 1); 9 s of darkness; second light pulse (Excitation 2); and 10 s of darkness (Refractory). Within approximately 2 s of the initial pulse of light, embryonic fish will undergo vigorous, high frequency body flexions and tail oscillations. Embryos fail to respond to the second PMR light pulse as basal responses of the neuronal circuitry are nonresponsive or suppressed. Digital video recordings of 17 frames per sec captured 850 frames through the light cycle.
Video analyses were conducted using a custom Matlab program (Mathworks, Natick, Massachusetts) that calculated an index of movement based on pixel differences across each video frame stamp. The Matlab output was further processed and analyzed using custom scripts developed in R language (RCoreTeam, 2014). Specifically, overall patterns of activity within each cycle interval (ie, baseline, excitation, refractory) were compared with those in vehicle controls by (1) estimating the 50% peak difference from controls in either direction and (2) performing a Kolmogorov–Smirnov test that compared the empirical cumulative distribution function between chemical treatments and controls. A Bonferroni-corrected p-value threshold of .01 (0.05/5 treatments = .01) was used to determine statistical significance. Embryos that were dead or malformed at 24 hpf, including those with altered spontaneous movements, were not included. Sample sizes after the removal of dead and malformed animals are provided in the Supplementary material.
Larvae PMR
To further evaluate flame retardant effects on neurological and locomotor behavior, larvae at 120 hpf were subjected to a light–dark PMR assay test using a ViewPoint Zebrabox system and video tracking software (ViewPoint Life Sciences, Lyon, France). Zebrafish larvae display consistent patterns of visual locomotor activity upon alterations between periods of light and dark (Emran et al., 2008; Irons et al., 2010; Kimmel et al., 1974). When light is applied, larvae slow or stop moving, and when light is removed a pronounced increase in locomotion occurs that gradually subsides as darkness continues. These visual motor response behaviors may be evolutionary-linked adaptive responses to catch prey and avoid predation. For example, evidence suggests that the increased locomotor hyperactivity in response to darkness may be a tractable measure of anxiety (dark avoidance behavior) in zebrafish with decreased activity to continuing darkness proposed to represent habituation (Ali et al., 2011; MacPhail et al., 2009; Rihel et al., 2010; Steenbergen et al., 2011).
The movement of treatment and control larvae was tracked using automated video recordings with a Zebrabox equipped with a 96-well plate holder, internal LED lights for light recordings, and mounted camera. The light–dark cycling consisted of the following: 5-min light acclimation; 5-min dark stimulation; 5-min dark acclimation; 5-min light acclimation. The assay was conducted in the morning of day 5 to help protect against temporal variations. The software tracked short and large distance movements (mm) of larvae every 40 ms and integrated these data in 60 s intervals over the 25-min assay. These integrated data were then further compiled and analyzed using custom R scripts to exclude both dead and malformed larvae, determine total movement (mm; short + large distances) of fish over time, and quantify statistical differences in total motion between treatments and controls (RCoreTeam, 2014). Sample sizes after the removal of dead and malformed animals are provided in the Supplementary material. As larval activity did not meet parametric assumptions of normality, Kruskal–Wallis analyses of variance and Dunn’s multiple comparison post tests were used to compare median locomotor activity per minute in treatment versus controls in each of the 5-min light/dark phases. Integrated locomotion measured at each minute was retained as an independent observation to account for the large variation in fish-to-fish movement that is still not well understood in the presence of light/dark stimuli with the PMR assay. Statistical significance was defined at p < .05.
Heatmap/hierarchical clustering
A heatmap of flame retardant bioactivity was rendered using the ggplot2 plotting package for R based on chemical groupings and LELs (Wickham, 2009). LELs were considered optimal for use in this situation because they represented shared values among each of the 3 bioassays and the lowest concentration that elicited a significant effect above background. The chemical groupings were organized based on their dominant structural features. Hence, for example, dechlorane while not an organophosphate was including in the CPE group because it is also a polychlorinated flame retardant. Likewise, non-PBDE BFRs were grouped together even though they have other important structural attributes that could be influencing bioactivity. Hierarchical clustering analyses were conducted using a custom R script (RCoreTeam, 2014). In brief, Euclidean distance-based dissimilarity matrices were computed for each chemical group, and the resulting matrices were clustered using a complete-linkage agglomerative clustering algorithm (“bottom up” approach). With this method, objects were assigned initially to their own clusters with the algorithm proceeding iteratively to join similar clusters until there was just 1 single cluster for each grouping.
Principal component analysis
The R language was implemented with ggplot2 to construct a principal components analysis (PCA) using a covariance matrix as a dimension reduction tool to find principal components and characterize relationships between individual flame retardants, structures, and toxicity endpoints measured as LELs (RCoreTeam, 2014; Wickham, 2009). Bootstrapped k-means clustering algorithms (1000 simulations) were applied to the PCA to find clustering with the highest Jaccard similarity coefficients (cluster 1 = 0.706; cluster 2 = 0.714; cluster 3 = 0.669; cluster 4 = 0.694; cluster 5 = 0.835; cluster 6 = 0.613; cluster 7 = 0.770). Jaccard values that are > 0.75 are considered stable clusters, whereas those > 0.60 suggest clustering patterns. Ellipses drawn for each cluster represent the 95% confidence interval of each cluster center. Flame retardants are identified individually and are colored to denote their chemical class.
RESULTS
Developmental Malformations
Table 4 provides a summary of mortality and developmental malformations observed in 24 and 120 hpf fish exposed to flame retardants. Detailed results for all compounds can be found in the Supplementary material. Of the 44 chemicals targeted, 31 caused significant mortality and morbidity while several elicited no effects, including: DPP, dechlorane, BCPCP, TCPP, MCPP, BDE-3, BDE-183, BDE-209, DE-79 (OctaBDE mixture), 3-OH-BDE-47, TBB, TBPH, and TBBPA-DBPE. In contrast, TBBPA caused the greatest array of teratogenic effects at both 24 and 120 hpf, and mITP was an equally potent toxicant at 120 hpf that also caused multiple defects but was inactive at 24 hpf (Fig. 2). The PBDE congener BDE-100 was one of the more potent flame retardants examined with delayed embryonic development at 6.4E-06 µM exposures that led to high mortality by 120 hpf.
TABLE 4.
LELs Measured in Embryonic Zebrafish Exposed to Flame Retardant Chemicals
| 24 hpf embryos (LEL; µM) |
120 hpf larvae (LEL; µM) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mort | DP | Mort | YSE | Ax | E | S | J | PE | PF | CF | T | SB | TR | |
| APE FRs | ||||||||||||||
| BDP | - | - | 0.064 | - | - | - | - | - | - | - | - | - | - | - |
| BPDP | - | 64 | 0.064 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | - | - | - |
| EHDP | - | - | 64 | - | 64 | - | - | - | 64 | - | - | - | - | - |
| IDDP | 64 | 64 | 0.064 | - | - | - | - | - | - | - | - | - | - | - |
| IPP-1 | - | 64 | 64 | 64 | 64 | - | - | - | 64 | 64 | 64 | - | - | 64 |
| IPP-2 | - | - | 0.0064 | 64 | 64 | - | - | - | 64 | - | - | - | - | 64 |
| IPP-3 | - | 64 | 0.064 | 64 | 64 | - | - | - | 64 | 64 | 64 | - | - | - |
| mITP | - | - | - | 0.64 | 0.64 | - | 0.64 | 0.64 | 0.64 | 0.64 | - | - | 0.64 | 0.64 |
| RDP | - | - | 0.64 | - | - | - | - | - | - | - | - | - | - | - |
| TBEP | - | - | 6.4 | 6.4E-04 | - | - | - | - | - | - | - | - | - | - |
| TBP | - | - | 6.4E-04 | - | - | - | - | - | 0.0064 | - | - | - | - | - |
| TCP | 0.0064 | 64 | 0.0064 | 64 | 64 | - | - | - | 64 | - | - | - | - | 64 |
| o-TCP | - | - | 64 | - | - | - | - | - | - | - | - | - | - | - |
| TEHP | - | - | 6.4 | - | - | - | - | - | - | - | - | - | - | - |
| TPP | 0.64 | - | 0.0064 | 64 | - | - | - | - | - | - | - | - | - | - |
| CPE FRs | ||||||||||||||
| TCEP | - | - | 0.0064 | - | - | - | - | - | - | - | - | - | - | - |
| TDCPP | 64 | 64 | 64 | - | - | - | - | - | - | - | 64 | - | - | - |
| BCPP* | - | - | - | - | - | - | - | - | - | - | 0.064 | - | - | - |
| BDCPP* | 0.0064 | 0.0064 | 0.0064 | - | - | - | - | - | - | - | - | - | - | - |
| PBDE FRs | ||||||||||||||
| BDE-15 | - | - | - | - | 64 | - | - | - | - | - | - | - | - | - |
| BDE-47 | 0.064 | - | 0.0064 | - | 64 | - | - | - | - | - | - | - | - | - |
| BDE-99 | 6.4 | - | 0.064 | - | - | - | - | - | - | - | - | - | - | - |
| BDE-100 | - | 6.40E-06 | 6.40E-06 | - | - | - | - | - | - | - | - | - | - | - |
| BDE-153 | - | - | 0.0064 | - | - | - | - | - | - | - | - | - | - | - |
| BDE-154 | - | - | 0.0064 | - | - | - | - | - | - | - | - | - | - | - |
| DE-71 | 0.064 | - | 0.064 | - | 64 | - | - | - | - | - | - | - | - | - |
| 2,4,6-TBP* | - | - | 6.40E-04 | - | - | - | - | - | 0.0064 | - | - | - | - | - |
| 5-OH-BDE-47* | - | - | - | - | - | - | - | - | - | - | 6.40E-04 | - | - | - |
| 6-OH-BDE-47* | - | - | - | - | - | - | - | - | 0.0064 | - | - | - | - | - |
| Other brominated FRs | ||||||||||||||
| HBCD | - | - | - | - | 6.4 | - | - | - | - | - | - | - | - | - |
| TBBPA | 64 | 6.4 | 6.4 | - | 6.4 | - | 6.4 | 0.64 | - | - | 6.4 | 6.4 | - | 6.4 |
Only flame retardants with adverse effects are reported. Raw data for all the flame retardants are provided in the Supplementary material.
Mort, mortality; DP, delayed progression; Ax, axis; E, eyes; S, snout; J, jaw; PF, pectoral fin; CF, caudal fin; T, trunk; SB, swim bladder. *Metabolites.
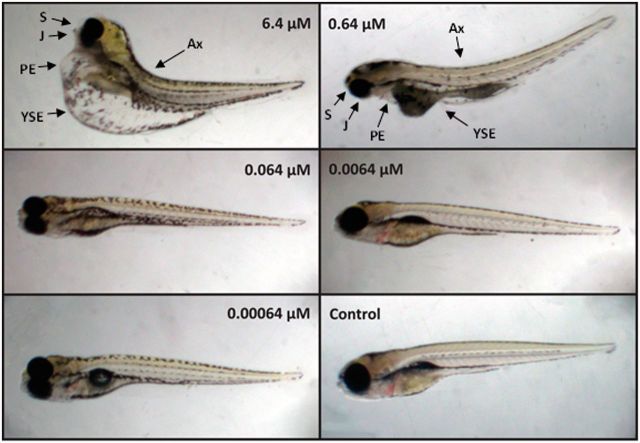
FIG 2.
Morphological deformities observed in zebrafish larvae exposed to mITP flame retardant at 0, 0.00064, 0.0064, 0.064, 0.64, and 6.4 µM for 5 days. Deformities denoted as: Ax, axis; S, snout; J, jaw; PE, pericardial edema; YSE, yolk sac edema.
Embryonic PMRs at 24 hpf
Flame retardant effects on PMRs of embryos at 24 hpf are summarized in Table 5 and are depicted as significant hyperactive (↑) or hypoactive (↓) body and tail contractions relative to controls. Detailed PRAT results for each flame retardant are also provided in the Supplementary material. Data are only shown for the initial baseline phase (darkness) and first excitation phase (rapid pulse of light) as these are the only phases in which altered activity was observed in this study. This low baseline activity in the second excitation and refractory intervals helps to further validate the assay as basal activity has been shown to be suppressed in embryos after an initial pulse of light. Compared to controls, 12/16 (75%) of the APE-based flame retardants significantly altered locomotor behavior at 24 hpf with the dominant response being hypoactivity. Significantly reduced PMRs were also measured among fish exposed to some concentrations of the CPE-based chemicals, including TDCPP, BDCPP, and TCPP. Both bisphenol A bis-(diphenyl phosphate) (BDP) and TCEP were the only OPFRs tested that caused a hyperactive response. For the brominated compounds, hypoactive responses also dominated with DE-71 (PentaBDE mixture), BDE-15, BDE-99, BDE-153, and BDE-154, as well as HBCD and TBPH, causing reduced activity compared to controls, whereas BDE-3, BDE-100, and TBBPA exposures resulted in significant hyperactivity. Embryos at the highest concentrations tested (64 µM) were also unable to acclimate normally to baseline conditions of darkness, including those exposed to the APE-based TCP, o-TCP, and TPP as well as the PBDE congener BDE-153. Embryos exposed to BDP at the lowest concentration tested (0.00064 µM) were hyperactive during the baseline dark acclimation relative to controls.
TABLE 5.
Hyperactivity (↑) and Hypoactivity (↓) Measured as LELs in 24 hpf Zebrafish Exposed to Flame Retardants
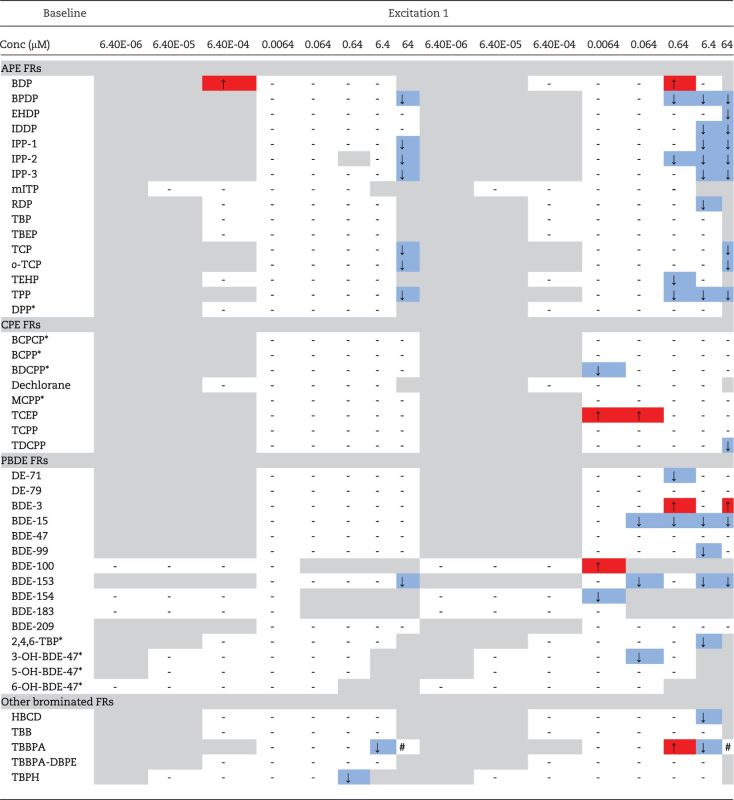 |
Dashes (-) indicate no effects compared with controls. Hash tags (#) represent concentrations that caused 100% mortality/morbidity and so were not included. Asterisks (*) denote metabolites.
Larval PMRs at 120 hpf
Table 6 summarizes flame retardant effects on larval zebrafish photomotor behavior at 120 hpf as significant hyperactive (↑) or hypoactive (↓) movement based on integrated locomotor activity in each of the light/dark phases relative to controls. Additional time series and boxplot data for individual chemicals are provided in the Supplementary material. For the OPFRs, compared to controls, exposure to BDP, t-butylphenyl diphenyl phosphate (BPDP), IPP, mITP, resorcinol A bis-(diphenyl phosphate) (RDP), tris (2-butoxyethyl) phosphate (TBEP), TPP, TCEP, TCPP, and TDCPP, as well as some metabolites (BCPP, BDCPP, and MCPP), caused significant hypoactivity under dark stimulation. This hypoactivity extended into the dark acclimation phase for IPP, RDP, TBP, TPP, BCPP, TCEP, TCPP, and TDCPP. In contrast, hyperactive responses were measured for several OPFRs in the dark startle phase (TCP, tris (2-ethylhexyl) phosphate [TEHP], and dechlorane) and dark acclimation phase (BPDP, 2-ethylhexyl diphenyl phosphate [EHDP], TEHP, and BCPCP). TDCPP, BCPCP, TCP, IPP, and BPDP also elicited potent hyperactivity compared with controls during the initial or both light acclimations. Fish were less sensitive to the polychlorinated Dechlorane Plus compound than the CPE-based flame retardants. The PentaBDE commercial mixture DE-71 and PBDE metabolites 6-OH-BDE-47 and 5-OH-BDE-47 caused significant hyperactivity in larvae subjected to dark stimulation, which for DE-71 and 5-OH-BDE-47 was preceded by hyperactivity in both the initial light and dark acclimation phases. For the other PBDE congeners as well as the PBDE metabolites 3-OH-BDE-47 and 2,4,6-TBP, depressed locomotor activity was detected in the dark startle phase as well as in some cases during light and dark acclimations (3-OH-BDE-47, BDE-47, BDE-99, BDE-100, BDE-154, and BDE-209). In addition, compared to controls, TBBPA caused significant reductions in movement during both the dark stimulatory and acclimation phases. Fish that had been exposed to TBB and TBPH were unable to acclimate to light. This inability to acclimate was also observed in the dark acclimation phase among fish exposed to TBPH and HBCD. No significant effects on behavior were observed in larvae exposed to BDE-153, BDE-183, DE-79, or TBBPA-DBPE.
TABLE 6.
Hyperactivity (↑) and Hypoactivity (↓) Measured as LELs in 120 hpf Zebrafish Exposed to Flame Retardants
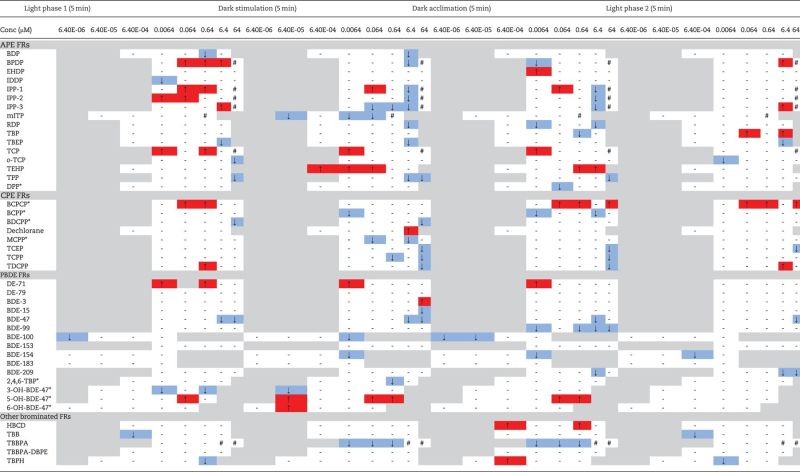 |
Dashes (-) indicate no effects compared with controls, hash tags (#) represent concentrations that caused complete or near 100% mortality/morbidity, and asterisks (*) denote metabolites.
DISCUSSION
The vast majority of flame retardants and metabolites tested in this study were bioactive with most (93%; 41/44) causing disrupted development in one or more of the 3 bioassays and across one or more concentrations tested (Fig. 3). These findings are noteworthy because the health consequences of exposures to many of these compounds, particularly the APE- and CPE-based chemicals, are poorly understood. The APE-based constituent of FM550 mITP and the brominated-BPA derivative TBBPA elicited the greatest number and variety of developmental malformations, including YSEs, PEs, impaired TRs, and deformities of the trunk, body axis, snout, jaw, caudal fin, and pectoral fins. These results are consistent with studies in rodent models and other research showing that a central mechanism of TBBPA and HBCD developmental toxicity may proceed through disruption of thyroid homeostasis (Eriksson et al., 2006; Kitamura et al., 2002; Mariussen and Fonnum, 2003). The importance of thyroid hormone in brain and somatic development is well established, and small changes in maternal or fetal thyroid hormone can cause severe deformities, motor skill deficiencies, and cognitive impairments (Haddow et al., 1999). In contrast to TBBPA, TBBPA-DBPE was inactive in all 3 bioassays. Additional toxicokinetic studies would be useful to understand TBBPA-DBPE metabolic and elimination profiles as little is known about the toxicity of this chemical. In addition, no effects were measured among embryos exposed to the OctaBDE mixture DE-79 and one of its major components BDE-183. However, the other PBDE parent and metabolites tested were bioactive in one or more of the bioassays with BDE-100 being one of the most potent toxicants tested. The potential for PBDE-induced neurodevelopmental toxicity and thyroid dysfunction are important toxicological endpoints of concern and the data findings here echo these concerns. A number of recent reviews have been written that describe PBDE-induced developmental toxicity and current knowledge of their mechanisms of action (Costa et al., 2014; Noyes and Stapleton, 2014; Staskal and Birnbaum, 2011).
FIG 3.
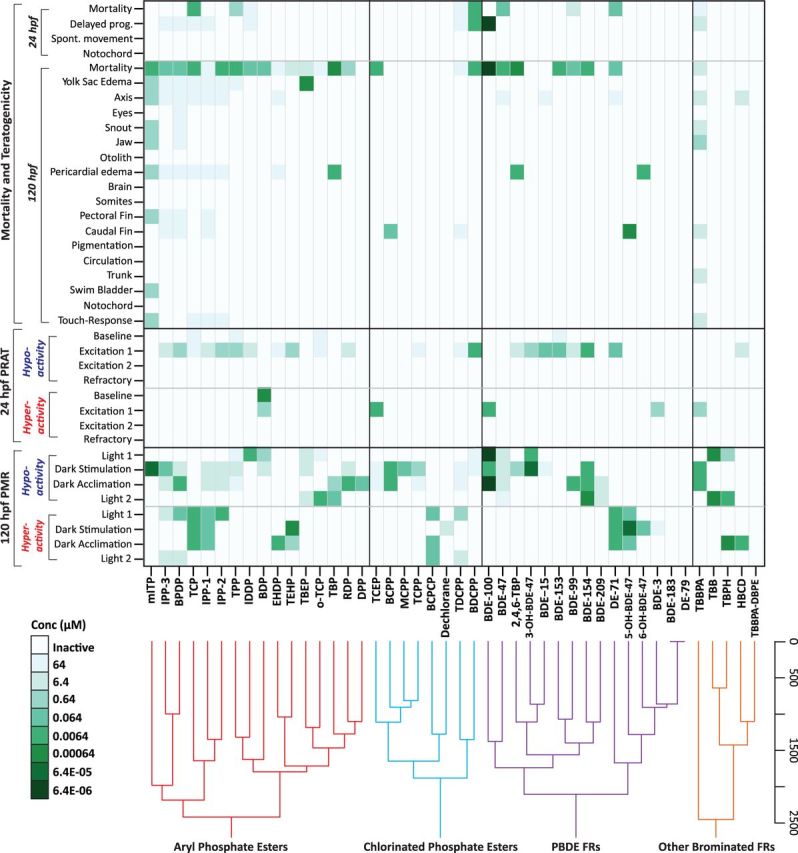
Heatmap and hierarchical clustering of morphological and behavioral responses of zebrafish embryos exposed to halogenated and APE flame retardants. Each square represents the LEL with response profiles hierarchically clustered to link flame retardant structures to bioactivity within chemical groupings.
Aryl and Chlorinated Phosphate Esters
All the APE- and CPE-based flame retardants altered 120 hpf larval locomotor behavior at one or more of the concentrations and light/dark epochs examined (Table 6), whereas 75% of the APEs impaired spontaneous motor functioning of embryos at 24 hpf (Table 5). The dominant 24 hpf PRAT response in APE-exposed embryos was hypoactivity that was also detected at baseline for some of these formulations (IPP-2, IPP-3, TCP, o-TCP, and TPP), suggesting that early development prior to 24 hpf may be an important period of heightened susceptibility to this class of flame retardants. The CPEs, in some contrast, were less bioactive at 24 hpf than the APEs, but all impaired behavior by 120 hpf suggesting potentially important target windows for the CPEs after embryonic gastrulation and segmentation. An exception to this finding for the CPEs related to TDCPP and its major metabolite BDCPP, both of which impaired development and depressed movement in 24 hpf embryos. Indeed, this is the first toxicity testing with BDCPP and results here suggest metabolic bioactivation. BDCPP was a substantially more potent teratogen by 4 orders of magnitude (LELs = 0.0064 µM) than TDCPP (LEL = 64 µM), causing reduced survival and impaired development at 24 hpf that led to high mortality by 120 hpf. Moreover, embryonic spontaneous movement at 24 hpf was also significantly depressed among 0.0064 µM concentration groups, suggesting that BDCPP could be an important driver of TDCPP developmental toxicity observed here and in other studies (Dishaw et al., 2011; Fu et al., 2013; McGee et al., 2012). By 120 hpf, both TDCPP and BDCPP elicited similar depressed locomotor phenotypes in fish under dark stimulation, although maladaptation in TDCPP-exposed fish was measured across all 4 light/dark epochs. This difference may be partly attributable to the higher mortality and teratogenicity observed among fish exposed to BDCPP.
The APE-based mITP component of FM550, which has been an important replacement for PentaBDE, was also highly bioactive with results that are consistent with recent studies showing PE and heart malformations (Gerlach et al., 2014; McGee et al., 2013). In this study, no effects were observed in 24 hpf zebrafish exposed to mITP, whereas by 120 hpf, low concentration mITP-exposed larvae presented with multiple morphological and behavioral abnormalities, suggesting that its impacts on neurodevelopment may extend across multiple early life stages with important targets later in development that have yet to be fully described. As for effects of other major components of FM550, TPP also reduced survival with high concentration edemas, and caused hypoactive locomotor responses at both 24 and 120 hpf, whereas TBB and TBPH caused no significant morphological defects (Tables 4–6, Fig. 3, and Supplementary material). However, compared to controls 120 hpf larvae exposed to TBB and TBPH were unable to acclimate to either light or dark stimuli, and this inability to acclimate was observed earlier in 24 hpf embryos exposed to TBPH. Results here with TPP contrast older screens in adult rodents that generally indicated a lack of neurotoxicity (Sobotka et al., 1986). However, this is one of only a couple developmental neurotoxicity examinations of TPP (McGee et al., 2013) and data suggest that younger animals may be more susceptible to this chemical thereby warranting further study.
Common Responses/Mechanisms of Bioactivity
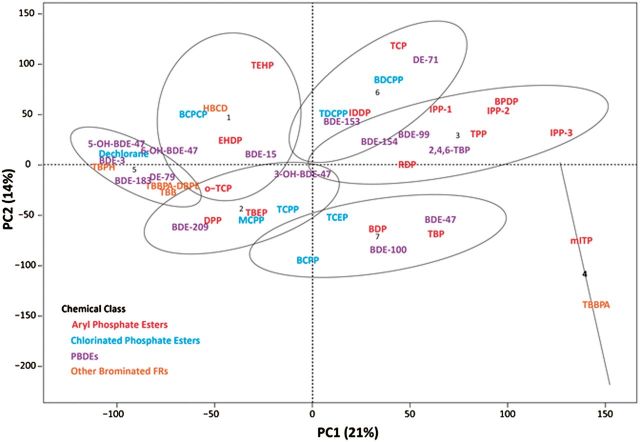
One of the ongoing challenges with characterizing toxicity results from larger chemical data sets such as this flame retardant grouping relates to effectively visualizing and dissecting potentially related responses and common toxicity mechanisms among a high-dimensional, complex set of morphological and behavioral phenotypes. For this study, 2 approaches were adopted to examine relationships of bioactivity within and across individual chemical classes. The first approach (Fig. 3) used a heatmap and hierarchical cluster analysis to evaluate interactions and differences in bioactivity within chemical groupings based on LELs. The second approach used a PCA test as a dimension reduction tool to further address whether and how compounds clustered based on their structures, teratogenicity, and neurobehavioral activity. Figure 4 shows a 2-dimensional PCA of the first 2 principal components. Loadings for each component are provided in the Supplementary material.
FIG 4.
Two-dimensional PCA and covariance matrix identifying regional clustering patterns among flame retardants based on their developmental and behavioral toxicity measured as LELs in 24 and 120 hpf zebrafish exposed to chemical from 6 to 120 hpf.
While all the APE-based chemicals tested had effects on development, those APEs with isopropyl (ie, mITP, IPP), butyl (BPDP), and cresyl (TCP) substituents on phenyl rings clustered tightly and were particularly potent across the 3 bioassays (Fig. 3). This clustering pattern suggests that these types of structural moieties and substitution patterns on phenyl may enhance the overall biological reactivity of this chemical class. As for the CPEs, TDCPP and BDCPP clustered tightly and may be indicative of the greater chlorination of TDCPP and BDCPP as well as hydroxylation of BDCPP that could be influencing their bioactivity and earlier target window at 24 hpf. The other chlorinated-tris compounds characterized, TCPP and TCEP, also clustered, with the TCPP metabolites BCPP and MCPP clustering with TCPP suggesting similar reactive features. In contrast, TCEP and its metabolite BCPCP did not cluster tightly, as BCPCP elicited potent hyperactivity in both the light and dark acclimation phases of the larval PMR assay, whereas TCEP was bioactive across several endpoints. PBDE clustering appeared to be based on patterns of bromination with congeners having between 2 and 6 bromines generally demonstrating the greatest bioactivity. BDE-100 and BDE-47 clustered tightly based on comparatively potent teratogenicity and hypoactivity in the larval PMR assay regardless of whether light or dark stimulation was applied. These compounds are structurally identical except that BDE-100 contains an additional ortho-substituted bromine atom on diphenyl ether. Indeed, a number of parent PBDEs (BDE-47, -15, -153, -99, -154, -209) and metabolites (2,4,6-TBP, 3-OH-BDE-47) clustered due to hypoactivity in one or both PMR assays and elevated mortality/morbidity. In contrast, PentaBDE (DE-71), which is composed of some of these parent PBDEs (BDE-47, -99, -153, -154), elicited a hyperactive response suggesting that zebrafish are highly sensitive to other components of DE-71.
Results of the 2-dimensional PCA (Fig. 4) and k-means derived clusters support that flame retardant bioactivity was not driven by major structural groupings, which was somewhat expected as nearly all (41/44) of the flame retardants and metabolites tested were deleterious in one or more of the bioassays across multiple endpoints and concentrations. Nonetheless, it is possible from the pattern of clustering in the PCA to discern groupings with levels of reduced and enhanced activity. Cluster 5 is notable because it includes several compounds, all brominated except for dechlorane, that produced little to no effect. The exception to this group 5 clustering was the PBDE metabolites 5-OH-BDE-47 and 6-OH-BDE-47 that caused low concentration hyperactivity in the 120 hpf PMR assay along with low concentration caudal fin and PEs, respectively, but elicited no effects in the 24 hpf-PRAT assay. These metabolites appeared to group with this cluster of comparatively inactive compounds because the 120 hpf-PMR data did not contribute substantially to the principal components, whereas the PRAT data were important to the loadings (see Supplementary material for PCA loadings).
At the other extreme, cluster 4, which is widely separated from cluster 5, contained only mITP and TBBPA as these were the most developmentally toxic flame retardants measured in this study. Clusters 3, 6, and 7 were notable because they contained generally the next most highly bioactive flame retardants after cluster 4. PentaBDE (DE-71) and all of its major components (BDE-47, -99, -100, -153, and -154) clustered in one of these 3 groupings along with one of its major hydroxy metabolites 2,4,6-TBP. This is concerning as these congeners continue to be highly detected PBDEs in humans and the environment (Toms et al., 2011). It is also notable that TPP, which along with mITP are major components of the PentaBDE replacement FM550, also clustered in group 3 along with the 3 bioactive IPP formulations, RDP, and BPDP. This is contrasted by the other major constituents of FM550, TBB and TBPH, that clustered with other less bioactive flame retardants in group 5. As for the CPEs and their metabolites, both TDCPP and its metabolite BDCPP clustered in group 6 further supporting the potential importance of BDCPP in the toxicity of TDCPP. Similarly, BCPP appeared to be a more potent bioactive metabolite than its parent TCPP.
Concordance of Bioassay Results
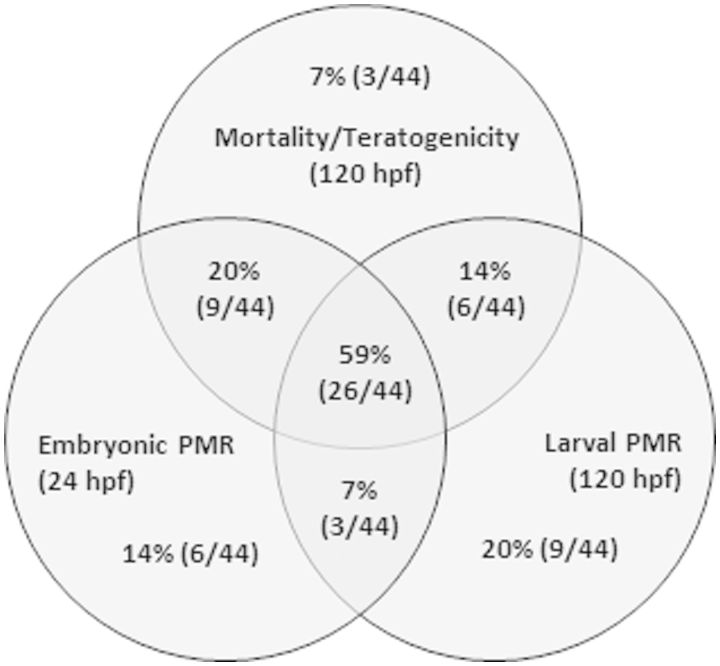
There was generally a high degree of concordance among the 3 assays in that a “hit” or lack thereof in 1 assay was typically predictive of the presence and absence of effects in another assay with some exceptions (Fig. 5). Both the 24 and 120 hpf PMR assays predicted the presence and absence of morphological defects at 120 hpf for 93% (41/44) of flame retardants tested. The exceptions to these interactions were for TCPP, 3-OH-BDE-47, and BDE-3 that elicited effects in both behavioral assays but were not morphologically sensitive. These results support the use of these types of PMR assays as promising nonlethal means to efficiently characterize developmental abnormalities in young zebrafish. This attribute may be particularly meaningful for future studies seeking to understand the long-term consequences of exposures to these and other hazardous compounds in older animals.
FIG 5.
Venn diagram showing distribution and concordance of flame retardant bioactivity and inactivity measured in 3 zebrafish bioassays used to characterize developmental toxicity.
Another observation pertains to a comparison of potency observed in the morphological and behavioral assays. Figure 3 shows that there were some chemicals that elicited significant behavioral changes at lower concentrations than those concentrations reducing survival or causing other morphological deformities. Conversely, there were also instances where survival and morphology were the most sensitive targets. These differences in relative potencies across the different bioassays could be meaningful to understanding windows of susceptibility and mechanisms of action but could also be linked to conditions of the study design. Although the PMR assays were conducted in animals that appeared to be visually normal and healthy with otherwise unaltered morphology or TRs, it is possible that some of these animals were experiencing systemic toxicity or other unobservable deformities (eg, musculoskeletal impairments) that were not readily discernable with the morphological evaluation. This could have skewed the behavioral assays to identify positive hits if animals were experiencing systemic toxicity that was not detected during the morphological evaluations. It is also possible that our testing conferred some “survivor” bias. That is, for compounds and test concentrations that caused death, it is possible that there could be a subsample of animals being tested in the PMR assays that were more resistant to the chemical and so effects on behavior might not be observed or could be reduced.
This is the first study to use the 24 hpf PRAT assay to evaluate flame retardant effects at initial stages of the developing zebrafish nervous system. There were several examples of compounds (BDP, EHDP, TEHP, TDCPP, BDE-100, DE-71, TBBPA, and HBCD) whereby directionally opposite behavioral responses were measured at 24 and 120 hpf. For instance, the APE-based flame retardant BDP caused low concentration mortality at 120 hpf in concert with increased and decreased locomotor responses at 24 and 120 hpf, respectively. There were also several instances where chemical sensitivities were detected in the 120 hpf PMR assay but not in the 24 hpf PRAT assay (mITP, TBEP, TBP, DPP, BCPP, MCPP, BCPCP, dechlorane, BDE-47, DE-71, 5-OH-BDE-47, 6-OH-BDE-47, TBB, TBPH), and vice-a-versa (BDE-153). These opposing responses are notable because they could reveal important differences in targets and mechanisms for these compounds. Compelling evidence now supports that 24 hpf PRAT excitatory motor responses in zebrafish are linked to nonocular PMR photoreceptors and distinct neuronal pathways activated in the caudal hindbrain—but not in the forebrain and midbrain—that may involve opsin-based phototransduction pathways (Kokel et al., 2013). Thus, PRAT can reveal important chemical sensitivities at some of the earliest stages of anatomical and functional patterning of the vertebrate nervous system.
While the underlying mechanisms driving larval PMR responses to light and dark and other extrinsic/intrinsic stimuli are still not well understood, it is readily evident that the central and peripheral nervous system of zebrafish is much more complex by 120 hpf than at 24 hpf. With this advancing development, a variety of toxicity mechanisms could be operating that might include chemical-induced changes in biochemical levels/activity, electrical signaling, receptor-mediated functioning, cell–cell communications, and the responsiveness and plasticity of developing organ systems. Another important difference that could be impacting the directionality of behavioral results is that the exposure duration is longer in the 120 hpf fish and so differing patterns of uptake and toxicokinetics in older larvae could elicit different patterns of toxicity and chemical sensitivities. Pairing these PMR assays together at 24 and 120 hpf is advantageous because we are able to derive a more complete picture of flame retardant effects on early development that are homologous to fundamental processes of development in humans (Selderslaghs et al., 2013). For instance, mechanistic data suggest that PBDE neurotoxicity may operate by several pathways that include disrupted thyroid hormone signaling (Ibhazehiebo et al., 2011; Noyes et al., 2013), altered cholinergic neurotransmissions (Dufault et al., 2005; Johansson et al., 2008), impaired neuronal proliferation and plasticity (Ibhazehiebo et al., 2011; Xing et al., 2009), and oxidative stress (Tagliaferri et al., 2010). Furthermore, consistent with results here, a substantial number of human epidemiology studies and neurodevelopmental toxicity studies in rodents and other models, which have been summarized in recent reviews (Costa et al., 2014; Staskal and Birnbaum, 2011), have shown that PBDEs can elicit adverse neurobehavioral outcomes in early development. Data generated here are consistent with and complement these other studies in humans and laboratory models. However, while PBDE biological disposition and toxicity are increasingly well-described, our understanding of the underlying mechanisms of toxicity for the other BFRs and OPFRs remains limited. Data generated here may be particularly helpful in future research. Moreover, other classes of organophosphates, such as organophosphate pesticides like chlorpyrifos, which have been shown to interfere with neurodevelopment by cholinergic and nonchlorinergic pathways, have been subjected to more testing than the OPFRs and may provide helpful insights into future testing that could complement data generated here and elsewhere (Levin et al., 2004; Yang et al., 2011).
CONCLUSIONS
Taken together, results of this study indicate that zebrafish neurological and morphological development appears to be highly sensitive to many of the flame retardants currently in use and present in humans and wildlife, as well as many being considered and used as replacements. This finding takes on heightened importance because these chemicals are not typically detected in humans and the environment in isolation but are present rather as complex mixtures. This type of high throughput screening methodology in zebrafish provides a meaningful opportunity moving forward to design flame retardants that impart reduced human health and environmental hazard potential. Not only do these assays detect initiating events of neurological impairments but also effects on gross morphological development. These types of readouts allow for the anchoring of developmental toxicity to morphological and behavioral phenotypes that in turn can be used to understand toxicity pathways for ultimate translation to humans. Future work will involve using this in vivo testing platform in young zebrafish to identify important mechanistic targets for these compounds and developmental windows of susceptibility. Finally, this research platform is well suited to examine the interactive effects of complex mixtures to discern differential or synergistic developmental toxicity.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
The National Institutes of Health (T32 ES007060 and P30 ES000210).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the technical support provided by Dr David Reif, N.C. State University, and Dr Lisa Truong, Sinnhuber Aquatic Research Laboratory, OSU, during the design and conduct of the data analyses. We are also grateful to Carrie Barton, Sinnhuber Aquatic Research Laboratory, OSU, for her support with fish husbandry and spawning. We would like to thank Dr Arlene Blum, Green Science Policy Institute, EPA’s National Toxicology Program, OSU Chemical Standards Store, and MRI Global for help with identifying and obtaining the flame retardants tested. We also thank Dr Susan Klosterhaus, Cradle to Cradle Products Innovation Institute, and Dr David Volz, University of South Carolina, for providing the Wellington-purified mono-ITP (mITP) used in this study.
REFERENCES
- Ali S., Champagne D. L., Spaink H. P., Richardson M. K. (2011). Zebrafish embryos and larvae: A new generation of disease models and drug screens. Birth Defects Res. C 93, 115–133. [DOI] [PubMed] [Google Scholar]
- Bi X. H., Thomas G. O., Jones K. C., Qu W. Y., Sheng G. Y., Martin F. L., Fu J. (2007). Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ. Sci. Technol. 41, 5647–5653. [DOI] [PubMed] [Google Scholar]
- Burka L. T., Sanders J. M., Herr D. W., Matthews H. B. (1991). Metabolism of tris(2-chloroethyl) phosphate in rats and mice. Drug Metab. Dispos. 19, 443–447. [PubMed] [Google Scholar]
- Butt C. M., Congleton J., Hoffman K., Fang M. L., Stapleton H. M. (2014). Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 48, 10432–10438. [DOI] [PubMed] [Google Scholar]
- Champagne D. L., Hoefnagels C. C., de Kloet R. E., Richardson M. K. (2010). Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav. Brain Res. 214, 332–342. [DOI] [PubMed] [Google Scholar]
- Cooper E. M., Covaci A., van Nuijs A. L. N., Webster T. F., Stapleton H. M. (2011). Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 401, 2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L. G., de Laat R., Tagliaferri S., Pellacani C. (2014). A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett. 230, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C. A., Herzke D., Vorkamp K. (2010). Brominated flame retardants in the Arctic environment—Trends and new candidates. Sci. Total Environ. 408, 2885–2918. [DOI] [PubMed] [Google Scholar]
- Dishaw L. V., Powers C. M., Ryde I. T., Roberts S. C., Seidler F. J., Slotkin T. A., Stapleton H. M. (2011). Is the PentaBDE replacement, tris (1,3-dichloropropyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol. Appl. Pharm. 256, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson R. E., Perovich L. J., Covaci A., Van den Eede N., Ionas A. C., Dirtu A. C., Brody J. G., Rudel R. A. (2012). After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 46, 13056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufault C., Poles G., Driscoll L. L. (2005). Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol. Sci. 88, 172–180. [DOI] [PubMed] [Google Scholar]
- ECB (2006). European Union Risk Assessment Report, 2,2,6,6-tetrabromo-4,4- isopropylidenediphenol (tetrabromobisphenol-A or TBBP-A). Part II. Human health European Chemicals Bureau, 4th priority list, EUR 22161 EN, 63, 2006. [Google Scholar]
- Emran F., Rihel J., Dowling J. E. (2008). A behavioral assay to measure responsiveness of zebrafish to changes in light intensities. J. Visual Exp. 20, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P., Fischer C., Wallin M., Jakobsson E., Fredriksson A. (2006). Impaired behaviour, learning and memory, in adult mice neonatally exposed to hexabromocyclododecane (HBCDD). Environ. Toxicol. Pharm. 21, 317–322. [DOI] [PubMed] [Google Scholar]
- Fromme H., Lahrz T., Kraft M., Fembacher L., Mach C., Dietrich S., Burkardt R., Völkel W., Göen T. (2014). Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environ. Int. 71, 158–163. [DOI] [PubMed] [Google Scholar]
- Fu J., Han J., Zhou B. S., Gong Z. Y., Santos E. M., Huo X. J., Zheng W., Liu H., Yu H., Liu C. (2013). Toxicogenomic responses of zebrafish embryos/larvae to tris(1,3-dichloro-2-propyl) phosphate (TDCPP) reveal possible molecular mechanisms of developmental toxicity. Environ. Sci. Technol. 47, 10574–10582. [DOI] [PubMed] [Google Scholar]
- Gerlach C. V., Das S. R., Volz D. C., Bisson W. H., Kolluri S. K., Tanguay R. L. (2014). Mono-substituted isopropylated triaryl phosphate, a major component of Firemaster 550, is an AHR agonist that exhibits AHR-independent cardiotoxicity in zebrafish. Aquat. Toxicol. 154, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M., vanEeden F. J. M., Schach U., Trowe T., Brand M., FurutaniSeiki M., Haffter P., Hammerschmidt M., Heisenberg C. P., Jiang Y. J., et al. (1996). Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123, 399–413. [DOI] [PubMed] [Google Scholar]
- Greaves A. K., Letcher R. J. (2014). Comparative body compartment composition and in ovo transfer of organophosphate flame retardants in North American Great Lakes Herring Gulls. Environ. Sci. Technol. 48, 7942–7950. [DOI] [PubMed] [Google Scholar]
- Guerra P., Alaee M., Ellis-Hutchings R. G., Barcelo D. (2011). Introduction to brominated flame retardants: Commercially products, applications, and physicochemical properties. In The Handbook of Environmental Chemistry; Brominated FlameRetardants (Barcelo D., Kostianoy A. G., Eds.), Vol. 16, pp. 1–17. Springer Publishing Services, Heidelberg, Germany. [Google Scholar]
- Haddow J. E., Palomaki G. E., Allan W. C., Williams J. R., Knight G. J., Gagnon J., O'Heir C. E., Mitchell M. L., Hermos R. J., Waisbren S. E., et al. (1999). Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 341, 549–555. [DOI] [PubMed] [Google Scholar]
- He S. J., Li M. Y., Jin J., Wang Y., Bu Y. J., Xu M., Yang X., Liu A. (2013). Concentrations and trends of halogenated flame retardants in the pooled serum of residents of Laizhou Bay, China. Environ. Toxicol. Chem. 32, 1242–1247. [DOI] [PubMed] [Google Scholar]
- Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., Muffato M., Collins J. E., Humphray S., McLaren K., Matthews L., et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibhazehiebo K., Iwasaki T., Kimura-Kuroda J., Miyazaki W., Shimokawa N., Koibuchi N. (2011). Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ. Health Perspect. 119, 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons T. D., MacPhail R. C., Hunter D. L., Padilla S. (2010). Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 32, 84–90. [DOI] [PubMed] [Google Scholar]
- Johansson N., Viberg H., Fredriksson A., Eriksson P. (2008). Neonatal exposure to deca-brominated diphenyl ether (PBDE 209) causes dose-response changes in spontaneous behaviour and cholinergic susceptibility in adult mice. Neurotoxicology 29, 911–919. [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B., Kannan K. (2009). An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere 76, 542–548. [DOI] [PubMed] [Google Scholar]
- Kalueff A. V., Stewart A. M., Gerlai R. (2014). Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 35, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmlein S., Herzke D., Law R. J. (2009). Brominated flame retardants in the European chemicals policy of REACH—Regulation and determination in materials. J. Chromatogr. A 1216, 320–333. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Isobe T., Muto M., Tue N. M., Katsura K., Malarvannan G., Sudaryanto A., Chang K. H., Prudente M., Viet P. H., et al. (2014). Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere 116, 91–97. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic-development of the zebrafish. Dev. Dyn. 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Patterso J., Kimmel R. O. (1974). Development and behavioral characteristics of startle response in zebrafish. Dev. Psychobiol. 7, 47–60. [DOI] [PubMed] [Google Scholar]
- Kitamura S., Jinno N., Ohta S., Kuroki H., Fujimoto N. (2002). Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem. Biophys. Res. Commun. 293, 554–559. [DOI] [PubMed] [Google Scholar]
- Klosterhaus S. L., Stapleton H. M., La Guardia M. J., Greig D. J. (2012). Brominated and chlorinated flame retardants in San Francisco Bay sediments and wildlife. Environ. Int. 47, 56–65. [DOI] [PubMed] [Google Scholar]
- Kokel D., Peterson R. T. (2011). Chapter 22 - Using the Zebrafish Photomotor Response for Psychotropic Drug Screening. Methods in Cell Biology. William Detrich M. W. H., Leonard I. Z., Academic Press, Waltham, MA, USA. Vol 105, pp. 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokel D., Bryan J., Laggner C., White R., Cheung C. Y. J., Mateus R., Healey D., Kim S., Werdich A. A., Haggarty S. J., et al. (2010). Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 6, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokel D., Dunn T. W., Ahrens M. B., Alshut R., Cheung C. Y. J., Saint-Amant L., Bruni G., Mateus R., van Ham T. J., Shiraki T., et al. (2013). Identification of nonvisual photomotor response cells in the vertebrate hindbrain. J. Neurosci. 33, 3834–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. J., Covaci A., Harrad S., Herzke D., Abdallah M. A., Fernie K., Toms L. M., Takigami H. (2014). Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environ. Int. 65, 147–158. [DOI] [PubMed] [Google Scholar]
- Levin E. D., Bencan Z., Cerutti D. T. (2007). Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 90, 54–58. [DOI] [PubMed] [Google Scholar]
- Levin E. D., Swain H. A., Donerly S., Linney E. (2004). Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol. Teratol. 26, 719–723. [DOI] [PubMed] [Google Scholar]
- Lunder S., Hovander L., Athanassiadis I., Bergman A. (2010). Significantly higher polybrominated diphenyl ether levels in young US children than in their mothers. Environ. Sci. Technol. 44, 5256–5262. [DOI] [PubMed] [Google Scholar]
- MacPhail R. C., Brooks J., Hunter D. L., Padnos B., Irons T. D., Padilla S. (2009). Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 30, 52–58. [DOI] [PubMed] [Google Scholar]
- Malmberg T., Athanasiadou M., Marsh G., Brandt I., Bergmant A. (2005). Identification of hydroxylated polybrominated diphenyl ether metabolites in blood plasma from polybrominated diphenyl ether exposed rats. Environ. Sci. Technol. 39, 5342–5348. [DOI] [PubMed] [Google Scholar]
- Mandrell D., Truong L., Jephson C., Sarker M. R., Moore A., Lang C., Simonich M. T., Tanguay R. L. (2012). Automated zebrafish chorion removal and single embryo placement: Optimizing throughput of zebrafish developmental toxicity screens. J. Lab. Autom. 17, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E., Fonnum F. (2003). The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem. Int. 43, 533–542. [DOI] [PubMed] [Google Scholar]
- Marvin C., Waltho J., Jia J., Burniston D. (2013). Spatial distributions and temporal trends in polybrominated diphenyl ethers in Detroit River suspended sediments. Chemosphere 91, 778–783. [DOI] [PubMed] [Google Scholar]
- McGee S. P., Cooper E. M., Stapleton H. M., Volz D. C. (2012). Early zebrafish embryogenesis is susceptible to developmental TDCPP exposure. Environ. Health Perspect. 120, 1585–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee S. P., Konstantinov A., Stapleton H. M., Volz D. C. (2013). Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: Potential role of the aryl hydrocarbon receptor. Toxicol. Sci. 133, 144–156. [DOI] [PubMed] [Google Scholar]
- McGoldrick D. J., Letcher R. J., Barresi E., Keir M. J., Small J., Clark M. G., Sverko E., Backus S. M. (2014). Organophosphate flame retardants and organosiloxanes in predatory freshwater fish from locations across Canada. Environ. Pollut. 193, 254–261. [DOI] [PubMed] [Google Scholar]
- Meeker J. D., Cooper E. M., Stapleton H. M., Hauser R. (2013). Urinary metabolites of organophosphate flame retardants: Temporal variability and correlations with house dust concentrations. Environ. Health Perspect. 121, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomeir A. A., Kato S., Matthews H. B. (1981). The metabolism and disposition of tris(1,3-dichloro-2-propyl) phosphate (Fyrol FR-2) in the rat. Toxicol. Appl. Pharm. 57, 401–413. [DOI] [PubMed] [Google Scholar]
- Noyes P. D., Stapleton H. M. (2014). PBDE flame retardants: Toxicokinetics and thyroid hormone endocrine disruption in fish. Endocr. Disruptors 2, e29430. [Google Scholar]
- Noyes P. D., Hinton D. E., Stapleton H. M. (2011). Accumulation and debromination of decabromodiphenyl ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicol. Sci. 122, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes P. D., Lema S. C., Macaulay L. J., Douglas N. K., Stapleton H. M. (2013). Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ. Sci. Technol. 47, 10012–10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla S., Corum D., Padnos B., Hunter D. L., Beam A., Houck K. A., Sipes N., Kleinstreuer N., Knudsen T., Dix D. J., et al. (2012). Zebrafish developmental screening of the ToxCast (TM) Phase I chemical library. Reprod. Toxicol. 33, 174–187. [DOI] [PubMed] [Google Scholar]
- Panula P., Chen Y. C., Priyadarshini M., Kudo H., Semenova S., Sundvik M., Sallinen V. (2010). The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 40, 46–57. [DOI] [PubMed] [Google Scholar]
- Panula P., Sallinen V., Sundvik M., Kolehmainen J., Torkko V., Tiittula A., Moshnyakov M., Podlasz P. (2006). Modulatory neurotransmitter systems and behavior: Towards zebrafish models of neurodegenerative diseases. Zebrafish 3, 235–247. [DOI] [PubMed] [Google Scholar]
- Peng X. Z., Tang C. M., Yu Y. Y., Tan J. H., Huang Q. X., Wu J. P., Chen S., Mai B. (2009). Concentrations, transport, fate, and releases of polybrominated diphenyl ethers in sewage treatment plants in the Pearl River Delta, South China. Environ. Int. 35, 303–309. [DOI] [PubMed] [Google Scholar]
- Perkins E. J., Ankley G. T., Crofton K. M., Garcia-Reyero N., LaLone C. A., Johnson M. S., Tietge J. E., Villeneuve D. L. (2013). Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environ. Health Perspect. 121, 1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org. [Google Scholar]
- Rihel J., Prober D. A., Arvanites A., Lam K., Zimmerman S., Jang S., Haggarty S. J., Kokel D., Rubin L. L., Peterson R. T., et al. (2010). Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 327, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selderslaghs I. W. T., Hooyberghs J., Blust R., Witters H. E. (2013). Assessment of the developmental neurotoxicity of compounds by measuring locomotor activity in zebrafish embryos and larvae. Neurotoxicol. Teratol. 37, 44–56. [DOI] [PubMed] [Google Scholar]
- Shoeib M., Ahrens L., Jantunen L., Harner T. (2014). Concentrations in air of organobromine, organochlorine and organophosphate flame retardants in Toronto, Canada. Atmos. Environ. 99, 140–147. [Google Scholar]
- Sobotka T. J., Brodie R. E., Arnold A., West G. L., Odonnell M. W. (1986). Neuromotor function in rats during subchronic dietary exposure to triphenyl phosphate. Neurobehav. Toxicol. Teratol. 8, 7–10. [PubMed] [Google Scholar]
- Stapleton H. M., Alaee M., Letcher R. J., Baker J. E. (2004). Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ. Sci. Technol. 38, 112–119. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Allen J. G., Kelly S. M., Konstantinov A., Klosterhaus S., Watkins D., McClean M. D., Webster T. F. (2008). Alternate and new brominated flame retardants detected in US house dust. Environ. Sci. Technol. 42, 6910–6916. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Dodder N. G. (2008). Photodegradation of decabromodiphenyl ether in house dust by natural sunlight. Environ. Toxicol. Chem. 27, 306–312. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Eagle S., Sjodin A., Webster T. F. (2012a). Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 120, 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Klosterhaus S., Eagle S., Fuh J., Meeker J. D., Blum A., Webster T. F. (2009). Detection of organophosphate flame retardants in furniture foam and US house dust. Environ. Sci. Technol. 43, 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Sharma S., Getzinger G., Ferguson P. L., Gabriel M., Webster T. F., Blum A. (2012b). Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol. 46, 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal D., Birnbaum L. (2011). Human health effects of brominated flame retardants. In The Handbook of Environmental Chemistry; Brominated Flame Retardants (Barcelo D., Kostianoy A. G., Eds.), Vol. 16, pp. 19–54. Springer Publishing Services, Heidelberg, Germany. [Google Scholar]
- Steenbergen P. J., Richardson M. K., Champagne D. L. (2011). Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: A pharmacological study. Behav. Brain Res. 222, 15–25. [DOI] [PubMed] [Google Scholar]
- Sundkvist A. M., Olofsson U., Haglund P. (2010). Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monitor. 12, 943–951. [DOI] [PubMed] [Google Scholar]
- Tagliaferri S., Caglieri A., Goldoni M., Pinelli S., Alinnovi R., Poli D., Pellacani C., Giordano G., Mutti A., Costa L. G. (2010). Low concentrations of the brominated flame retardants BDE-47 and BDE-99 induce synergistic oxidative stress-mediated neurotoxicity in human neuroblastoma cells. Toxicol. Vitro 24, 116–122. [DOI] [PubMed] [Google Scholar]
- Toms L. M. L., Hearn L., Sjodin A., Mueller J. F. (2011). Human exposure to brominated flame retardants. In The Handbook of Environmental Chemistry; Brominated Flame Retardants (Barcelo D., Kostianoy A. G., Eds.), Vol. 16, pp. 203–241. Springer Publishing Services, Heidelberg, Germany. [Google Scholar]
- Truong L., Reif D. M., St Mary L., Geier M. C., Truong H. D., Tanguay R. L. (2014). Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 137, 212–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP (2009). Listing of commercial pentabromodiphenyl ether and commercial octabromodiphenyl ether. United Nations Environment Programme; Stockholm Convention. UNEP-POPS-COP.4-SC-4-18. Available at: http://chm.pops.int/Implementation/NewPOPs/TheNewPOPs/tabid/672/Default.aspx. Accessed November 10, 2014. [Google Scholar]
- van den Eede N., Dirtu A. C., Neels H., Covaci A. (2011). Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int. 37, 454–461. [DOI] [PubMed] [Google Scholar]
- Wickham H. (2009). Ggplot2: Elegant Graphics for Data Analysis. Springer, New York, NY. [Google Scholar]
- Xing T. R., Chen L., Tao Y. A., Wang M., Chen J. T., Ruan D. Y. (2009). Effects of decabrominated diphenyl ether (PBDE 209) exposure at different developmental periods on synaptic plasticity in the dentate gyrus of adult rats in vivo. Toxicol. Sci. 110, 401–410. [DOI] [PubMed] [Google Scholar]
- Yang D., Lauridsen H., Buels K., Chi L.-H., La Du J., Bruun D. A., Olson J. R., Tanguay R. L., Lein P. J. (2011). Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol. Sci. 121, 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.