Abstract
The deleterious effects of emotional distractors on attention are well demonstrated. However, it is unclear if emotional distractors inevitably disrupt task-relevant attention. Using multilevel modeling (MLM), the present study examined the impact of valence and arousal dimensions of distracting emotional stimuli and individual differences in anxiety on task-relevant processing. Consistent with prior literature, high-arousal negative distractors were associated with poor task-relevant attention compared to positive and neutral distractors. However, low-arousal negative distractors were associated with better task-relevant performance than were positive and neutral distractors. Furthermore, these effects were accentuated by individual differences in worry. These findings challenge assumptions that distraction and worry must be minimized for augmented attentional performance. Overall, these results emphasize the importance of taking into account emotional dimensions of arousal and valence as well as individual differences in anxiety when examining attention in the presence of emotional distractors.
Keywords: emotion, attention, distractors, valence, arousal, anxiety
Introduction
Attention serves to prioritize information that has survival value, preferentially allocating resources to stimuli that indicate threat or reward (for review, see Bradley, 2009). A consequence of this preferential allocation of attention is that performance on concomitant tasks may be impaired (Algom, Chujat & Lev, 2004). For example, a weapon in a scene captures attention at the cost of attention to peripheral details such as the perpetrator's facial characteristics and clothing (Loftus, Loftus & Messo, 1987). In a search task, target detection is slow when one of the distractors is an emotional face compared to conditions without an emotional distractor, or conditions in which the target is emotional and the distractors are neutral (Hodsoll, Viding, & Lavie, 2011).
Although the deleterious effect of emotional distractors on attention is well demonstrated, three lines of research led us to question that emotional distractors inevitably worsen task-relevant processing. First, dimensions of arousal and valence of a stimulus can differentially alter attention to a subsequent target, prompting us to examine whether these dimensions may also differentially impact attention to concurrently displayed attentional targets. High emotional arousal can enhance subsequent cognitive performance by improving perception of high-priority information and weakening perception of low-priority information (Mather & Sutherland, 2011). Low-arousal (LA) images presented prior to target identification broaden and high-arousal (HA) images narrow attentional scope (Gable & Harmon-Jones, 2010). Levels of valence also affect attention differently, with negative moods encouraging focus on the finer details of a scene at the expense of gist, and positive moods promoting attention to the gist at the expense of details (Gasper & Clore, 2002). Finally, arousal interacts with valence to affect attention in unique ways, with negative LA mood improving and negative HA mood impairing target identification accuracy, with no difference between LA and HA positive mood conditions (Jefferies, Smilek, Eich & Enns, 2008).
A second line of research demonstrates that task-irrelevant distractors can improve performance. Participants instructed to focus on music, their last vacation, or planning for a dinner party while detecting targets performed better than those asked to focus on the task (Olivers & Nieuwenhuis, 2005). These performance-enhancing effects could be due to modulation of attentional focus by arousal induced by the distractors (Olivers & Nieuwenhuis, 2005; Easterbrook, 1959). The effects of task-irrelevant distractors on performance have been examined more directly in research on the impact of irrelevant noise on task performance. Visual search and vigilance have been shown to improve in the presence of mild acoustic distraction (Broadbent, 1971). It has been hypothesized that these performance improvements occur due to the arousing nature of noise, as optimum levels of arousal have been shown to enhance performance (Broadbent, 1971). Since arousal is a key dimension of emotion it is possible that, rather than universally impairing performance, under certain conditions emotional distractors actually enhance attentional performance.
A third line of research indicates that individual differences in anxiety moderate the impact of arousal and valence on task performance. For example, poorer attentional performance in the presence of task-irrelevant emotional distractors has been found across many types of anxiety (Williams, Mathews & MacLeod, 1996). However, compared to other types of anxiety, worry has been hypothesized to enhance effort allocation and implementation of attentional control strategies, thereby improving task performance (Eysenck & Calvo, 1992). Additional support for the multifaceted nature of anxiety comes from findings of distinct patterns of brain activation for different types of anxiety. For example, participants high in anxious apprehension (e.g., worry) displayed greater activation in left lateralized brain regions implicated in verbal processing, including the left inferior frontal gyrus (IFG) and left inferior temporal gyrus (ITG), while ignoring negative words. In contrast, participants high in anxious arousal (e.g., panic) displayed greater activation in a right posterior brain region implicated in vigilance and arousal (Engels et al., 2007). Differences in the cognitive and brain mechanisms of anxiety dimensions (Nitschke, Heller, & Miller, 2000) suggest that individual differences in anxiety differentially impact attentional performance.
In summary, several lines of evidence demonstrate a complex relationship between arousal, valence, anxiety, and attention, calling into question whether emotional distractors always impair attentional task performance. Using MLM, the present study examined how dimensions of emotional distractors and individual differences in anxiety affect task-relevant processing. Results show that emotional distractors do not inevitably impair task-relevant processing. Rather, the valence and arousal of distractors interact to affect task-relevant processing. Hence, compared to positive and neutral distractors, high-arousal negative distractors are associated with poor performance, whereas low-arousal negative distractors are associated with improved task-relevant performance. This effect is accentuated in participants who score high on a measure of worry. These findings indicate that it is critical to consider the arousal and valence of the distractors as well as individual differences in anxiety when examining attention and cognitive control in the presence of emotional distractors.
Methods & Results
One hundred forty-nine undergraduates (82 female, mean age = 18.33, SD = .76) participating for course credit, completed an attention task in which they viewed 256 images from the International Affective Picture System (IAPS; Lang, Bradley & Cuthbert, 2005) with a colored dot near a corner of the image. Images were presented in 16 blocks of positive, neutral, and negative trials. Studies using blocked designs are generally more effective at eliciting emotion-related interference and are more ecologically valid than event-related designs (Compton et al., 2003). In the present study, there were 16 trials in each block with a new trial every 2000 ms. Each trial presented an IAPS image with a superimposed dot for 1500 ms followed by a fixation cross for 500 ms. The dots were red, yellow, green, or blue; no color occurred more than two consecutive times. Participants were asked to ignore each image and identify the dot-color as quickly and accurately as possible (Figure 1) using a response pad with two-buttons , one for each hand. Each button corresponded to a color response. The mapping of color-response buttons to the left and right hand was counterbalanced across subjects to control for laterality-related effects in reaction time. STIM software from James Long Co was used for task presentation and data collection.
Figure 1.

Dot-color identification task: presenting task-irrelevant images (distracters) varying along dimensions of arousal and valence while responding to the color of a superimposed dot.
Distractor stimuli were 64 positive, 128 neutral, and 64 negative, images with superimposed dots. The color and position (upper left or right, lower left or right corner) of the dots were randomly assigned to the images. The dot-color identification task was relatively low in perceptual load, allowing sufficient perceptual resources to process the task-irrelevant images (Pessoa, 2009) and permitting examination of variance associated with emotional dimensions of the task-irrelevant stimuli. Anxiety was assessed using the Penn State Worry Questionnaire (PSWQ), a 16-item instrument designed to measure the trait of worry (Meyer, Miller, Metzger & Borkovec 1990). Anxious Arousal (17 items measuring physiological arousal) and Anhedonic Depression (8-item subset) were assessed using the Mood and Anxiety Symptom Questionnaire (MASQ-AA and MASQ-AD, respectively; Watson et al, 1995).
MLM is a form of advanced regression analysis that allows the incorporation of both fixed and random effects in a nested model. Neither aggregation across trials nor subjects is required (Linck, Kroll & Sunderman, 2009), allowing the full dimensions of each variable – arousal, valence, worry, anxious arousal, and anhedonic depression – to be employed as fixed effects and the subject as a random effect predicting reaction time for dot-color identification. Data from each subject and for each trial was included in the analyses without aggregation. MLM analyses performed dimensionally in this manner avoid the loss of information that results from categorical analyses (Haines, Stansfeld & Job, 2002; Segerstrom & Sephton, 2010; Linck, Kroll & Sunderman, 2009). Arousal and valence characteristics relied on normative ratings from Lang, Bradley and Cuthbert (2005), and anxiety and depression dimensions consisted of scores described above. All predictor variables were tested simultaneously. These analyses resulted in main effects for arousal and valence; non-significant linear and quadratic effects of worry; two-way interactions between arousal and valence and valence and worry; and a three-way interaction between arousal and valence and worry (Table 1, Figure 2). Anxious arousal and anhedonic depression did not show significant effects. To probe the structure of the three-way interaction, the significance of simple slopes and differences between the slopes were computed separately for arousal and valence (Figure 2) at lower and higher levels of worry (Aiken & West, 1991).
Table 1.
Summary of distractor arousal, valence and worry predicting reaction time (rt) for dot-color identification.
| β (std. error) | t | p | |
|---|---|---|---|
| Arousal | .0269 (.0001) | 28.28 | .001 |
| Valence | .0027 (.0012) | 2.34 | .019 |
| Worry | -.0001 (.0005) | -0.24 | .813 |
| Arousal*Valence | -.0070 (.0006) | -10.76 | .001 |
| Arousal*Worry | .0000 (.0001) | .034 | .734 |
| Valence*Worry | .0002 (.0001) | 2.41 | .016 |
| Arousal*Valence*Worry | -.0001 (<.0001) | -2.23 | .026 |
Figure 2.
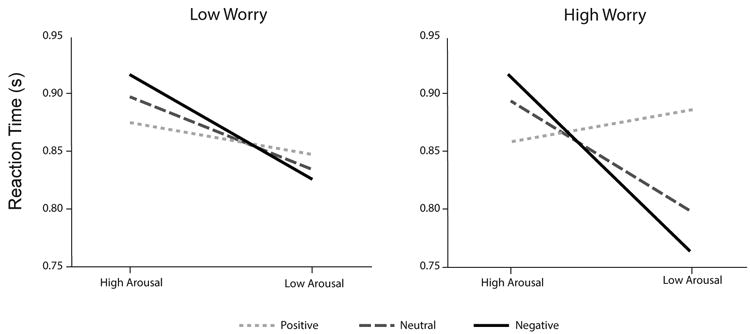
The relationship between reaction time and the level of arousal of distractor images varies as a function of distractor valence at lower and higher levels of worry. While all analyses were performed using arousal, valence and worry as continuous variables, the three-way interaction depicted below was based on predictor values one standard deviation above and below the mean, for purposes of illustration (Aiken & West, 1991).
At lower levels of worry, scenes lower in arousal were associated with faster dot-color identification for negative, neutral and positive distractors (Table 2). The relationship between arousal and reaction time was stronger for more negative than neutral distractors and for more neutral than positive distractors. This pattern of results was exaggerated at higher levels of worry. Lower arousal was associated with faster reaction times, more so for negative distractors than for neutral distractors in higher levels of worry. The relationship between arousal and reaction time was again stronger for more negative than neutral distractors and for more neutral than positive distractors. Finally, the relationship between arousal and reaction time for negative distractors was stronger in higher compared to lower levels of worry, t = 181.89, p <.001. These results were not mediated by visual clutter (Rosenholtz, Li, & Nakaano, 2007) or spatial frequency (Delplanque, N'Diaye, Scherer & Grandjean, 2007) of the images.
Table 2.
Simple slopes of positive, neutral and negative images predicting reaction time in higher and lower levels of worry. While all MLM analyses were performed using arousal, valence and worry as continuous variables, the results of slope analyses probing the nature of the three-way interaction (presented below) were based on valence and worry values one standard deviation above and below the mean (Aiken & West, 1991).
| Group | Valence | Slope | Std. error | t | p | t slope difference | p |
|---|---|---|---|---|---|---|---|
| High Worry | Positive | .01 | .002 | 5.03 | <.001 | ] -17.30 ] 16.40 |
<.001 <.001 |
| Neutral | .03 | .001 | 21.40 | <.001 | |||
| Negative | .04 | .002 | 19.22 | <.001 | |||
| Low Worry | Positive | .02 | .002 | 6.97 | <.001 | ] -10.36 ] 12.53 |
<.001 <.001 |
| Neutral | .03 | .001 | 18.64 | <.001 | |||
| Negative | .04 | .002 | 16.46 | <.001 |
Discussion
Present findings demonstrate that emotional distractors do not universally impair task-relevant processing. Distractor valence and arousal interact such that low-arousal negative distractors are associated with enhanced performance, and high-arousal negative distractors are associated with degraded performance, compared to positive and neutral distractors. This relationship between arousal and valence is exaggerated by worry. The enhancing effect of emotional arousal on task-relevant processing has been demonstrated in past studies, but only when the arousing stimulus did not compete directly with the task-relevant stimulus, for example when the arousing stimulus preceded the task-relevant stimulus or was presented in a different modality (Teichner et al., 1963; Broadbent, 1971; Mather & Sutherland, 2011). However, present results demonstrate that, even when a negative stimulus is in direct competition with the task-relevant stimulus, lower arousal is associated with improved task-relevant processing.
According to Scherer (1994), emotion has evolved as a relevance detection and response-preparation system. The former allows organisms to perceive and evaluate surrounding stimuli (some may be irrelevant for survival), and the latter allows for rapid, appropriate responses. Scherer reconciled the apparent contradiction between these two functions by considering the intensity (arousal) of the emotional stimulus. Presented with an intense emotional stimulus, the organism cannot take a risk and responds in a rapid, automatic manner. When presented with a less intense stimulus, the organism can appraise the context and gather information. In the present task, intense, high-arousal negative distractors may have been processed relatively automatically, resulting in poorer task-relevant processing. However, low-arousal negative distractors may have promoted exploration, resulting in faster detection of task-relevant stimuli. This explanation is consistent both with Pessoa's (2009) dual-competition model, which posits that low-threat stimuli enhance processing of emotional items, and with Whalen's (1998) view that low-arousal information is difficult to interpret, promoting vigilance and enhanced processing of the surrounding context. Research supports these theories, demonstrating that preceding low-arousal information widens the scope of attention (Gable & Harmon-Jones, 2010) and enhances subsequent attentional performance (Pessoa, Padmala, Kenzer & Bauer, 2012; Jefferies et al., 2008). The present study demonstrates that low-arousal information enhances task-relevant processing even when in direct competition with the task-relevant stimulus.
Present findings are also consistent with literature showing that high-arousal, task-irrelevant stimuli and negative distractors impair performance (Easterbrook, 1959; Loftus et al., 1987; Algom et al., 2004). Here, higher arousal was associated with poorer task-relevant performance, more so for negative than for neutral and positive distractors. This could be because high negative arousal narrows the focus of attention to the arousal-eliciting stimulus, resulting in reduced processing of peripheral, task-relevant information (Easterbrook, 1959; Gable & Harmon-Jones, 2010). For example, eye movement studies show that participants direct greater attention to negative images and less to peripheral details (Riggs, McQuiggan, Farb, Anderson, & Ryan, 2011).
The absence of arousal-related differences for positive stimuli in the present study is consistent with other studies finding no attentional difference between positive and neutral mood (Bruyneel et al., 2012) or between low- and high-arousal positive mood (Jefferies et al., 2008). However, these findings are inconsistent with studies demonstrating that attention is modulated by motivational intensity such that positive stimuli high in approach motivation narrow the focus of attention, and positive stimuli low in approach motivation widen the focus of attention (Gable & Harmon-Jones, 2008). Although related to emotional arousal, the dimension of motivational intensity is distinct, because it requires an impulse to move toward or away from a stimulus (Gable & Harmon-Jones, 2008). In the present paradigm, greater arousal-related differences for negative than for positive stimuli may be due to a ‘negativity bias’ in processing (Cacioppo & Berntson, 1994) or because the positive stimuli did not vary greatly in approach motivation, which may largely mediate the attentional effects of positive stimuli (Gable & Harmon-Jones, 2008).
Finally, anxiety exaggerated the arousal x valence interaction effect on attentional processing. Anxiety biases attention towards threat and slows disengagement from threatening but not neutral or happy stimuli (Fox et al., 2007). This may explain why higher arousal negative distractors were associated with poor task-relevant processing compared to neutral or positive distractors. Worry and low-arousal negative distractors both encourage a generic vigilance towards threat in the environment (Mathews, 1990; Whalen, 1998), and worry is also hypothesized to improve effort allocation and attentional control strategies in some contexts (Eysenck & Calvo, 1992), which could explain why these distractors were associated with faster target detection in high- than in low-worry participants.
Furthermore, these relationships between distractor arousal and performance were not associated with anxious arousal or depression. This is consistent with studies that have found attentional bias to threat for participants high in trait anxiety, but not for those with elevated depression scores (Bradley, Mogg, Falla & Hamilton, 1998). Different types of threat may encourage vigilance in different kinds of anxiety, with non-specific threats promoting vigilance in worry (Mathews, 1990), and immediate threats promoting vigilance in anxious arousal (Nitschke, Heller, and Miller, 2000). The IAPS images used in this experiment may not pose sufficiently immediate threat to produce an interaction between anxious arousal scores and arousal and valence ratings.
Overall, present results indicate that low-arousal negative information enhances task-relevant processing even when in direct competition with the task-relevant stimulus, a novel finding that could be applicable in diverse situations requiring attention. A commonly held view is that environments should be distraction-free and individuals worry-free, to reach top performance. Because the present study suggests that low-arousal distractors are associated with enhanced task performance, it is important to determine whether this commonly held view is based solely on distractors that are high in emotional arousal or cognitive demand. Furthermore, worry is associated with greater impairment in the presence of high-arousal distractors and with better performance in the presence of low-arousal distractors, challenging the notion that minimizing worry enhances performance. Present findings highlight the need for future research examining whether low-arousal negative distractors are associated with improved performance across a range of attentional tasks and settings, as well as the clinical relevance of present findings for anxiety. In sum, our findings suggest that a more nuanced view of emotion, incorporating dimensions of arousal and valence as well as individual differences in anxiety is necessary for a complete understanding of how emotional distractors affect attention.
Acknowledgments
This research was supported by grants from the National Institute of Health, R01 MH61358, R21 DA14111, and T32 MH19554. We thank Gerard Sanders for his help with data management.
Footnotes
Authorship: A.M. developed the study design and collected data. T.S. performed data analyses and drafted the paper. A.M., W.H. and G.A.M. provided supervision and critical revisions. All authors approved the final version of the paper for submission.
Declaration of Conflicting Interests: The authors declare that they have no conflicts of interest with respect to authorship or the publication of this article.
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks: Sage Publications; 1991. [Google Scholar]
- Algom D, Chajut E, Lev S. A rational look at the emotional Stroop phenomenon: a generic slowdown, not a Stroop effect. Journal of Experimental Psychology: General. 2004;133(3):232–338. doi: 10.1037/0096-3445.133.3.323. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Falla SJ, Hamilton LR. Attentional bias for threatening facial expressions in anxiety: Manipulation of stimulus duration. Cognition & Emotion. 1998;12:737–753. [Google Scholar]
- Bradley MM. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent DE. Decision and Stress. London: Academic Press; 1971. [Google Scholar]
- Bruyneel L, van Steenbergen H, Hommel B, Band GP, De Raedt R, Koster EH. Happy but still focused: failures to find evidence for a mood-induced widening of visual attention. Psychological Research. 2012 doi: 10.1007/s00426-012-0432-1. published online March 31, 2012. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: A critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–423. [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, Scalf PE, Webb A, Heller W. Paying attention to emotion: An fMRI investigation of cognitive and emotional Stroop tasks. Cognitive, Affective and BehavioralNeuroscience. 2003;3(2):81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Delplanque S, N'Diaye K, Scherer K, Grandjean D. Spatial frequencies or emotional effects? A systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. Journal of Neuroscience Methods. 2007;165(1):144–150. doi: 10.1016/j.jneumeth.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66(3):183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, Miller GA. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44(3):352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and Performance: The Processing Efficiency Theory. Cognition and Emotion. 1992;6(6):409–434. [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do Threatening Stimuli Draw or Hold Visual Attention in Subclinical Anxiety? Journal of Experimental Psychology: General. 2007;130(4):681–700. [PMC free article] [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. Approach-motivated positive affect reduces breadth of attention. Psychological Science. 2008;19(5):476–482. doi: 10.1111/j.1467-9280.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- Gable P, Harmon-Jones E. The Blues Broaden, but the Nasty Narrows: Attentional Consequences of Negative Affects Low and High in Motivational Intensity. Psychological Science. 2010;21(2):211–215. doi: 10.1177/0956797609359622. [DOI] [PubMed] [Google Scholar]
- Gasper K, Clore GL. Attending to the big picture: mood and global versus local processing of visual information. Psychological Science. 2002;13(1):34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- Haines MM, Stansfeld SA, Head J, Job RFS. Multilevel modeling of aircraft noise on performance tests in schools around Heathrow Airport London. Journal of Epidemiology, Community and Health. 2002;56:139–144. doi: 10.1136/jech.56.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodsoll S, Viding E, Lavie N. Attentional Capture by Irrelevant Emotional Distractor Faces. Emotion. 2011;11(2):346–353. doi: 10.1037/a0022771. [DOI] [PubMed] [Google Scholar]
- Jefferies LN, Smilek D, Eich E, Enns JT. Emotional valence and arousal interact in attentional control. Psychological Science. 2008;19(3):290–295. doi: 10.1111/j.1467-9280.2008.02082.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective ratings of pictures and instructional manual. NIMH Center for the Study of Emotion & Attention, University of Florida; 2005. [Google Scholar]
- Linck JA, Kroll JF, Sunderman G. Losing access to the native language while immersed in a second language, evidence for the role of inhibition in second-language learning. Psychological Science. 2009;20(12):1507–1515. doi: 10.1111/j.1467-9280.2009.02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EF, Loftus GR, Messo J. Some facts about weapon focus. Law and Human Behavior. 1987;11:55–62. [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives in Psychological Science. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A. Why worry? The cognitive function of anxiety. Behaviour Research and Therapy. 1990;28(6):455–468. doi: 10.1016/0005-7967(90)90132-3. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Miller GA. Anxiety, stress, and cortical brain function. In: Borod JC, editor. The neuropsychology of emotion. New York: Oxford University Press; 2000. pp. 298–319. [Google Scholar]
- Olivers CN, Nieuwenhuis S. The beneficial effect of concurrent task-irrelevant mental activity on temporal attention. Psychological Science. 2005;16(4):265–269. doi: 10.1111/j.0956-7976.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13(4):160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Kenzer A, Bauer A. Interactions between cognition and emotion during response inhibition. Emotion. 2012;12(1):192–197. doi: 10.1037/a0024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs L, McQuiggan DA, Farb N, Anderson AK, Ryan JD. The role of overt attention in emotion-modulated memory. Emotion. 2011;11(4):776–785. doi: 10.1037/a0022591. [DOI] [PubMed] [Google Scholar]
- Rosenholtz R, Li Y, Nakaano L. Measuring Visual Clutter. Journal of Vision. 2007;7(2):17, 1–22. doi: 10.1167/7.2.17. [DOI] [PubMed] [Google Scholar]
- Sass SM, Heller W, Stewart JL, Silton RL, Edgar C, Fisher JE, Miller GA. Time course of attentional bias to threat in anxiety: Emotion and gender specificity. Psychophysiology. 2010;47:247–259. doi: 10.1111/j.1469-8986.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer KR. Emotion serves to decouple stimulus and response. In: Ekman P, Davidson RJ, editors. The nature of Emotion: Fundamental Questions. New York: Oxford University Press; 1994. pp. 127–130. [Google Scholar]
- Segerstrom SC, Sephton SE. Optimistic expectancies and cell-mediated immunity: The role of positive affect. Psychological Science. 2010;21(3):448–455. doi: 10.1177/0956797610362061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a Tripartite Model: II. Exploring the Symptom Structure of Anxiety and Depression in Student, Adult, and Patient Samples. Journal of Abnormal Psychology. 1995;104(1):15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, Vigilance, and Ambiguity: Initial Neuroimaging Studies of the Human Amygdala. Current Directions in Psychological Science. 1998;7(6):177–188. [Google Scholar]


