Abstract
Chromatin remodelers use the energy of ATP hydrolysis to reposition or evict nucleosomes or to replace canonical histones with histone variants. By regulating nucleosome dynamics, remodelers gate access to the underlying DNA for replication, repair, and transcription. Nucleosomes are subject to extensive post-translational modifications that can recruit regulatory proteins or alter the local chromatin structure. Just as extensive cross-talk has been observed between different histone post-translational modifications, there is growing evidence for both coordinated and antagonistic functional relationships between nucleosome remodeling and modifying machineries. Defining the combined functions of the complexes that alter nucleosome interactions, position, and stability is key to understanding processes that require access to DNA, particularly with growing appreciation of their contributions to human health and disease. Here, we highlight recent advances in the interactions between histone modifications and the ISWI and CHD1 chromatin remodelers from studies in budding yeast, fission yeast, flies, and mammalian cells, with a focus on yeast.
Keywords: chromatin, nucleosome, histone modification, ISWI, Chd1, yeast
Regulation of chromatin by different classes of enzymes
Chromatin remodeling and histone modifying enzymes are two large classes of chromatin regulators that have distinct, fundamental roles in chromatin organization (Box 1). The misregulation of chromatin remodeling and modification is implicated in diabetes, neurodegenerative diseases, and many cancers [1–3]. Histone modifying enzymes that can add or remove modifications are targeted in cancer therapeutics as well [1]. Because of their implications in the pathogenesis and treatment of human diseases, a deeper understanding of the coordinated and antagonistic functions of chromatin remodeling and modifying enzymes is likely to have significant human health impacts.
Box 1. Dynamic functions of chromatin remodeling and modifying enzymes.
Chromatin remodelers use the energy of ATP to mobilize DNA around nucleosomes. Remodeler activity can result in nucleosome sliding, eviction, or replacement of canonical histones with specific histone variants. The gene encoding the founding chromatin remodeler was identified in two genetic screens in S. cerevisiae for mutations disrupting mating type switching (named switching-defective 2 or SWI2) and inability to grow with sucrose as a carbon source (named sucrose non-fermenting 2 or SNF2) [105]. SWI2/SNF2 was subsequently shown to alter chromatin structure and contribute to transcriptional activation [105]. Since these pioneering studies, many remodelers were identified biochemically and by homology searches based on the high degree of conservation within the enzymatic ATPase-helicase domains. Most, but not all, chromatin remodeling enzymes function in large macromolecular complexes (Fig. 1). The combination of unique remodeler domains and complex subunits results in distinct, even opposing, biochemical and genetic functions. Remodelers play key roles in DNA replication and repair, homologous recombination, and regulation of gene expression, all of which require the dynamic assembly, reassembly, movement, and replacement of nucleosomes.
Chromatin modifying enzymes are distinct from chromatin remodelers. The modifiers act to add and remove post-translational modifications from the core histones and other chromatin proteins. Post-translational modifications include the addition of small chemical groups, as in acetylation, methylation, and phosphorylation, as well as the conjugation of proteins, such as ubiquitin and SUMO (small ubiquitin-like modifier). Such modifications are predicted to serve as a signal to other proteins that bind the resulting structure, alter nucleosome stability, and change local chromatin organization. They can also affect further addition and removal of other modifications. Like remodeling enzymes, histone modifiers commonly function in macromolecular complexes that contribute to their genetic and biochemical specificity. Histone modification machinery and the modifications themselves are implicated in essentially all chromatin-templated events. Because of the rapid and responsive nature of processes such as DNA repair and gene expression, modifications themselves are highly dynamic. Thus, the active removal of histone modifications can be a key regulatory step in chromatin mechanisms. Although histones have been the best studied substrates of the modifying enzymes, ongoing proteomic, biochemical, and genetic studies are revealing non-histone targets that are also important for chromatin functions.

There are four families of chromatin remodeling enzymes with diverse functions in regulating nucleosome dynamics in eukaryotes (see Box 2, reviewed by [4]). The roles of chromatin remodelers and histone modifications in DNA repair and replication have recently been reviewed [5, 6]. Regulation of nucleosome dynamics by chromatin remodelers and modifiers is also important throughout the transcription process, which we discuss here [7–9]. Transcription begins with the binding of sequence-specific activators, followed by recruitment of general transcription factors and assembly of the pre-initiation complex (PIC) [10, 11]. After initiation, there is a transition to productive elongation and finally, termination [12, 13]. Nucleosomes are repositioned to expose cis-regulatory sequences, removed to relieve the physical barrier they pose to the transcribing RNA polymerase, and reassembled and repositioned in the wake of polymerase passage [4, 9]. Chromatin modifying enzymes add and remove chemical and protein modifications to histones coincident with different steps in transcription as well [14]. The modified histones are associated with changes in nucleosome assembly, stability, local chromatin organization, and interactions with chromatin binding proteins [14].
Box 2. Chromatin remodeling enzyme families have diverse functions.
Most remodelers described to date are grouped into four families based on shared structural or functional domains outside the enzymatic core: SWI/SNF (switching-defective/sucrose-non-fermenting, ISWI (imitation-switch), CHD (chromodomain helicase DNA-binding), and INO80 (inositol-requiring 80). The SWI/SNF family includes RSC (remodels the structure of chromatin) and functions in the sliding and eviction of nucleosomes. ISWI remodelers function in nucleosome assembly and spacing. ISWI is also proposed to have functions in higher-level chromatin organization. CHD remodelers are associated with nucleosome sliding, eviction, spacing, and nucleosome assembly. The INO80 family is specialized for restructuring the nucleosome, including the SWR1 (sick with rat8ts) family member which does so by replacement of H2A-H2B dimers with dimers containing the histone variant H2A.Z and H2B. Remodeler functions are defined through in vitro positioning assays and in vivo by analysis of mutant phenotypes. The diagram below illustrates modification activities and their end results on nucleosome placement or composition (effects on higher-order chromatin organization are not illustrated).
In this review we discuss emerging data on the transcriptional interplay of ISWI and CHD1 remodeling enzymes with histone modifiers. There are two ISWI catalytic subunits (Isw1 and Isw2) comprising three distinct enzyme complexes in budding yeast, two ISWI catalytic subunits in mammals (SMARCA5 and SMARCA1), and a single ISWI enzyme in flies. In all cases in which ISWI family remodelers have been studied, the remodelers function with regulatory subunits in multiple complexes. The specific complexes highlighted herein and shown in Figure 1 are ISW1a, ISW1b, ACF (ATP-utilizing chromatin assembly and remodeling factor), CHRAC (chromatin accessibility complex), and NURF (nucleosome remodeling factor). In contrast to these complexes, CHD1 enzymes generally function as monomers. ISWI and CHD1 remodelers are alike in that both require extranucleosomal DNA to move nucleosomes and both enzymes position nucleosomes in the center of DNA templates in vitro [15–19].
Figure 1.
Molecular cartoons of some of the Snf2 chromatin remodeling enzymes and complex subunits in S. cerevisiae, Drosophila, and mammals. CHD1 is found primarily as a monomer. ISWI remodelers exist in distinct complexes: ISW1a and ISW1b complexes are in budding yeast, ACF and CHRAC complexes are found in yeast, flies, and mammals, whereas NURF complexes are defined in Drosophila and mammals. Orthologous subunits in different organisms are shown as the same color. SMARCA5 and SMARCA1 are also known as SNF2H and SNF2L, respectively. Some of these complexes, such as ACF, function as dimers. ISWI complexes not discussed include RSF in Drosophila and mammals, and several mammalian-specific complexes. Remodelers and their complexes also include RSC, SWI/SNF, INO80, and SWR. Although not discussed here, these are considered in an earlier comprehensive review [4].
ISWI and CHD1 make multiple contacts with DNA
Early biochemical and genetic advances in the study of chromatin remodelers revealed that specific domains outside the ATPase and Helicase domains of the enzymatic core contribute to their regulation and function. CHD1 and ISWI proteins contain domains important for binding DNA and nucleosomes, mechanistic details of which have emerged from recent enzymatic and structural studies (Fig. 2). The HAND-SANT-SLIDE (HSS) structure of Drosophila ISWI, the HSS of budding yeast Isw1, as well as the C-terminus of budding yeast Chd1 (including SANT, Helical Linker 1 and SLIDE domains), have each been solved in complex with DNA [20–22]. Multiple contact points between these domains and DNA are observed, consistent with photocrosslinking studies in vitro and exonuclease digestion patterns of remodeler-bound promoters in vivo [19, 22–24]. This work and further in vitro structure-function analyses of S. cerevisiae Chd1 and, most recently Drosophila ISWI, point to a model where binding to extranucleosomal DNA relieves negative inhibition by the regulatory NegC domain (Fig. 2), which is found between the enzymatic and DNA-binding domains of the proteins [22, 25–29].
Figure 2.
CHD1 and ISWI family remodelers share functional domains. Common domains are color-coded. The N-terminal double chromodomains of CHD1 and AutoN ISWI enzymes both function to prevent ATP hydrolysis when the enzyme is not bound to nucleosomes. The chromodomain (chromatin organization modifier) is a highly conserved sequence found in many eukaryotic proteins and enzymes with chromatin functions. The DEXD ATPase /Helicase domains form the enzymatic core of these remodelers that is deeply conserved both in sequence and function and is found in all chromatin remodelers studied to date. The Neg C domains of ISWI and CHD1 prevent translocation when the enzyme is not engaged with extranucleosomal DNA. The C-terminal domains participate in extranucleosomal DNA binding. SANT (Swi3, Ada2, N-Cor, and TFIIIB) is a DNA binding domain originally identified based on homology with Myb proteins and found in many chromatin regulatory proteins [106]. SLIDE (SANT-Like ISWI Domain) is very similar to SANT, structurally, and these two DNA-binding domains are shared in CHD1 and ISWI proteins [20, 23, 106]. CHD1 contains a unique domain called HL1 (Helical Linker 1) and contains residues important for DNA-binding [22, 23]. The ISWI C-terminus also contains a HAND domain (named because the structure resembles an open hand) which is connected to the ATPase domain through a long linker [20].
Unlike monomeric CHD1, ISWI family proteins function in diverse, multi-subunit complexes that include DNA-binding regulatory subunits [30]. ISWI binds to ACF1 in Drosophila melanogaster and mammals, and the N-terminus of Drosophila ACF1 functions in DNA binding in vitro [31]. The budding yeast Isw2 binding partner Itc1 has limited homology with human ACF1 but also binds extranucleosomal DNA [17]. In addition, ACF1/Itc1 can interact with two CHRAC subunits that bind extranucleosomal DNA [17, 32]. Budding yeast Isw1 associates with the regulatory subunit Ioc3, forming ISW1a [33]. Structural analyses show two domains in Ioc3 that contact extranucleosomal DNA [15, 21]. Separately, Isw1 associates with Ioc2 and Ioc4 subunits to form ISW1b [33]. Ioc2’s PHD finger suggests DNA or nucleosome binding [33] although, to our knowledge, the function of this domain has not yet been demonstrated.
It is important to note that ISWI and its regulatory subunits and CHD1 lack strong DNA sequence-specific binding characteristic of transcription factors. Therefore, the multiple points of contact with extranucleosomal and nucleosomal DNA may allow these remodelers to associate broadly throughout the genome with enough stability to withstand the physical forces generated during nucleosome movement. In support of this view, reduced DNA binding by alterations to the DNA binding domains of budding yeast Chd1 disrupts both the directionality and extent of nucleosome repositioning in vitro [19]. Application of technical advances in nucleosome mapping to base-pair resolution will further refine understanding of contributions by individual ISWI complex subunits and their DNA binding domains to remodeling function in vivo.
ISWI and CHD1 contain domains important for nucleosome targeting
The N-terminal regions of ISWI and CHD1 proteins contain nucleosome binding or regulatory domains (Fig. 2). The chromodomains of human CHD1 specifically recognize H3K4 methylation (H3K4me) [34, 35]. It remains unresolved whether CHD1 in flies and Chd1 in yeast specifically recognize H3K4me, as opposing results have been reported [34–36]. Although Drosophila chromodomain mutants do not affect global localization of CHD1, proper induction of heat shock response genes is disrupted [37]. Thus, CHD1 chromodomains may contribute to remodeler function in transcription independently of H3K4me recognition.
The ATPase domain of ISWI binds to a nucleosomal region from which the histone H4 N-terminal tail is predicted to emerge [38, 39]. The H4 tail is required for enhanced ATP catalysis of nucleosome substrates over naked DNA [40]. Drosophila ISWI itself contains a domain that is similar to a region of the H4 N-terminal tail, and mutations in this region can greatly increase catalytic activity [28]. Combined, these data support a model of self-inhibition of ISWI by an H4-tail-like domain (AutoN) [28], which prevents full activation of the enzyme unless bound to a nucleosome substrate. Additionally, acetylation of the H4 tail at lysine 16 (H4K16) disrupts ISWI remodeling in vitro [41]. Perhaps H4K16ac diminishes activity because this modified H4 tail fails to fully disrupt ISWI auto-inhibition either through reduced binding to the ATPase domain or altering a conformational change of ISWI that would enhance its enzymatic activity.
The BPTF (bromodomain PHD finger transcription factor) subunit of the human NURF complex simultaneously recognizes two different histone modifications. The PHD finger of BPTF interacts specifically with di- and trimethylated H3K4 (H3K4me2/3) in human cells [42, 43]. The adjacent bromodomain binds H4 peptides acetylated at K12, K16, or K20 in vitro, with preference for H4K16ac [44]. BPTF also binds dinucleosome substrates where the two different modifications are on neighboring nucleosomes. In vivo, BPTF is enriched in regions with both H3K4me and H4K16ac. These observations suggest that NURF is targeted to chromatin by recognition of multiple histone modifications.
Remodelers and histone modifications have cooperative and antagonistic functions during transcription initiation
Loss of individual ISWI- or CHD-type remodelers in budding yeast does not grossly alter global nucleosome position or gene expression [45]. Rather, there are small changes in the positions of specific nucleosomes both nearby and within genes in single remodeler mutants [24, 45–47].
Based on these genome-wide data and other studies of Isw2 function, Isw1 and Isw2 move the +1 nucleosome, which is immediately downstream of the Nucleosome-Depleted Region (NDR, shown in Fig. 3), toward the intergenic regions [24, 46, 48]. The +1 nucleosome of many yeast genes partially obstructs the transcription start site and the repressive effects of Isw2 on gene expression, anti-sense and cyrptic transcription are likely linked to maintaining such repressive nucleosome positions [46, 49, 50]. ISW1b and Chd1, on the other hand, move nucleosomes in the opposite direction, away from the NDR. Isw1 and Chd1 predominantly remodel nucleosomes within the gene body, and their combined loss synergistically disrupts global gene body chromatin organization [24, 45, 47]. Loss of ISWI does not alter global nucleosome positioning in Drosophila larvae [51]. Rather, ISWI-bound genes show strongest effects on the two highly positioned nucleosomes just downstream of the transcription start site of target genes in ISWI mutants [51]. Loss of ISWI also has a striking effect on the appearance of the dosage compensated X chromosome in Drosophila males [52, 53], however global changes in nucleosome position are not observed [51]. Genes on the male X chromosome normally bound by the dosage compensation complex and ISWI accumulate a positioned nucleosome at the transcription start site in ISWI mutant males [51]. These data point to a conserved role for ISWI family remodelers in the positioning of specific nucleosomes at the 5’ ends of target genes.
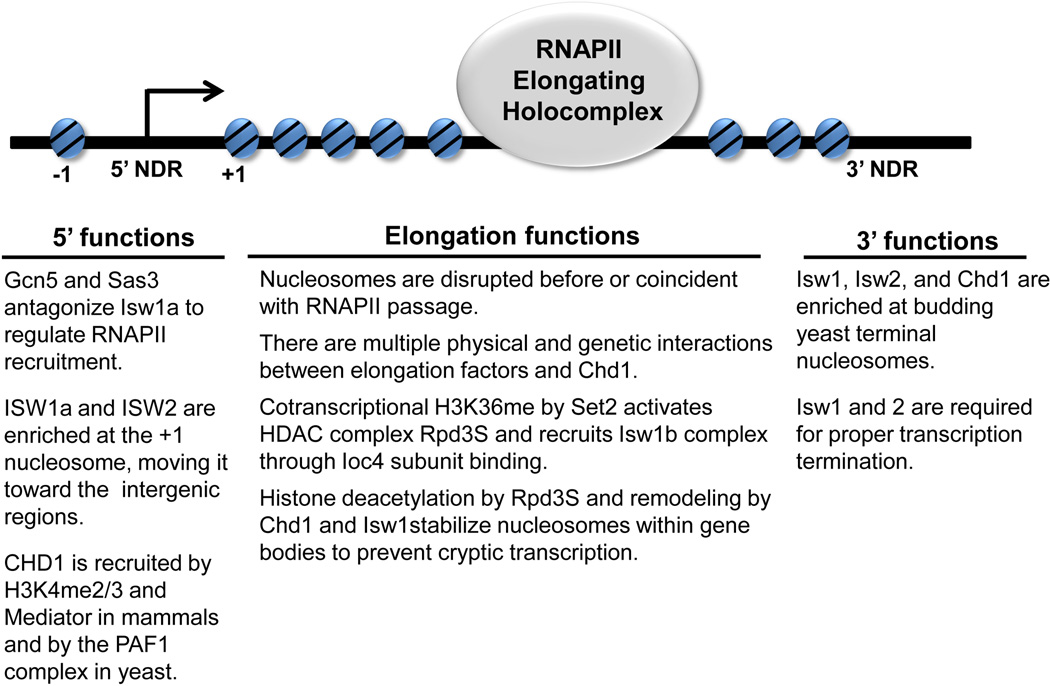
Figure 3.
A summary of SNF2 chromatin remodeler functions with histone modifiers during transcription. NDR indicates the Nucleosome Depleted Region commonly found at the 5’ and 3’ ends of genes. Although the functions are shown below the schematic of a generic gene, it is likely, and in some cases has been observed, that the reported functions and physical associations are restricted to specific genes.
Given the nucleosome-specific function of remodelers in and around genes, it is perhaps not surprising that there are numerous functional interactions between remodelers and histone modification complexes. Long-standing genetic analyses between major histone acetyltransferase (HAT) enzymes and remodelers have revealed severe sickness or lethality of many double mutants. For example, deletion of the gene encoding the HAT Gcn5, is synthetically lethal upon loss of RSC or SWI/SNF function [54, 55]. Gcn5 is a member of several transcriptional coactivator complexes, including SAGA (for Spt5-Ada-Gcn5-acetyltransferase), and targets H3K14 for acetylation. RSC is recruited to the 5’ ends of genes through recognition of H3K14ac by its bromodomains, and SWI/SNF binding is stabilized and enhanced by H3K14ac [54, 56–60]. These results have been interpreted as demonstrating parallel or cooperative functions of Gcn5 with these remodelers to regulate transcription.
By contrast, it was recently reported that Gcn5 and Sas3 have an antagonistic relationship with Isw1 and Isw2 [61]. GCN5 and SAS3 have a combined, essential function in S. cerevisiae [62], where Sas3 is the enzymatic subunit of NuA3 (nucleosome acetylation of histone H3) and is also associated with transcriptional activation with Gcn5. The two HATs contribute to global H3K9 and H3K14 acetylation, and the enzymes themselves and corresponding histone modifications map to the 5’ ends of overlapping genes [62, 63]. The gcn5Δ sas3ts mutant dies at elevated temperatures, but loss of Isw1 or Isw2 restores growth [61]. Isw1 antagonism is mediated specifically through the ISW1a complex. Loss of H3K14ac in gcn5Δ sas3ts did not strongly increase ISW1a binding at several active genes indicating that H3K14ac does not antagonize ISW1a primarily by blocking its binding to chromatin.
Loss of ISW1a partially rescues RNAPII recruitment to active genes tested in gcn5Δ sas3ts cells [61]. Given that transcriptional coactivators and chromatin remodelers relocate rapidly to responsive genes upon heat shock, it will be important to test whether dynamics of activation are altered in gcn5Δ sas3ts cells and whether loss of ISW1a function partially restores rapid activation of gene expression [24, 64]. In Drosophila, ISWI is acetylated by Gcn5 in a region with sequence similarity to the H3 N-terminal tail [65]; the function of this developmentally regulated modification, however, is not yet clear [65]. A reduction of Isw1 acetylation in gcn5Δ sas3ts cells has been observed [61]. The mechanistic significance of which will be clarified with future mutational analyses. These studies will be important to understand a potential direct mechanism of coordinate regulation by remodeling and modification enzymes.
CHD1 localization at the 5’ ends of active genes is deeply conserved, although the mechanisms of recruitment appear to differ between species. The double chromodomains of mammalian CHD1 (Fig. 2) specifically recognize H3K4me3 nucleosomes and tail peptides in vitro [36, 66]. In mice, CHD1 is associated with promoters of active genes, although not solely through recognition of H3K4me [67]. During PIC formation, recruitment of CHD1 to active promoters relies on a subunit of Mediator [68]. CHD1 also physically interacts with key players in productive transcription such as TFIIH and subunits of the PAF1 complex, which coordinates activator-dependent transcription initiation with elongation [68]. Therefore, CHD1 is targeted to active genes in mammals through interactions with multiple factors associated with transcriptional activation. In budding yeast, Chd1 recruitment to chromatin is largely independent of H3K4me, but relies on a subunit of the PAF1 complex [69]. The function of Chd1 at promoters is likely in nucleosome disassembly as chd1Δ cells have increased nucleosome stability at promoters [70, 71]. Similarly, Drosophila CHD1-deficient larvae have increased nucleosome occupancy in the 5’ region of Hsp70 upon heat shock when compared to wild type larvae [37]. Because mammalian CHD1 is tightly associated with active promoters and plays a considerable role in developmental gene expression [67], it would be useful to determine if the mechanism of action is through a conserved function in promoter nucleosome eviction.
Alternatively, there is growing evidence to support the observation that although Chd1 and ISWI remodelers are enriched at the 5’ ends of genes, they function further downstream in coding regions in budding yeast [72]. Recruitment of these remodelers to NDRs is associated with transcription factor binding [72, 73]. It has been proposed that ISWI and CHD remodelers bind to the extended extranucleosomal DNA of NDRs created by stably bound transcription factors but transiently associate with the gene body as nucleosomes are lost during transcription to reposition those newly assembled [72]. The transcription factor Ume6 is also important for targeting ISW2 to regions unbound by Ume6 through a chromatin looping mechanism [73]. Future work should determine the extent to which histone modifications contribute to transcription factor recruitment through the newly described looping mechanism.
Histone modifications target chromatin remodeling during transcriptional elongation
A number of studies describe a key role for CHD1 and ISWI remodelers in regulating nucleosome stability within the coding regions of genes during elongation. Nucleosomes are a physical barrier to RNAPII, so they may be modified by removal of one of the H2A/H2B dimers or by displacement of the entire octamer to accommodate passage [74]. In the wake of transcribing polymerases, nucleosomes are reassembled [74]. Free histones can be pre-acetylated [75, 76] which may result in an inappropriate “open” structure or signal for transcription initiation when incorporated into chromatin [77–79]. Set2-mediated methylation of H3 at K36 activates the histone deacetylase complex, Rpd3S resulting in hypoacetylation of reassembled nucleosomes [77, 80–82]. Set2 normally travels with RNAPII, and its activity results in increased H3K36me toward the 3’ end of active genes [83–85]. Loss of Set2-dependent H3K36me causes reduced Rpd3S activity, increased acetylation, and increased cryptic transcription within gene bodies [77, 79, 86]. SET2 mutants have increased H3 turnover within genes, suggesting that increased cryptic transcription results from loss of nucleosome stability [78]. Compromised Chd1 function in budding yeast suppresses growth phenotypes of several elongation factor mutants, supporting a general role for this remodeler in elongation [87]. Loss of CHD1 causes increased cryptic transcription and nucleosome turnover within gene bodies [71, 87, 88]. Therefore, whereas Chd1 may contribute to nucleosome loss at promoters, within gene bodies, Chd1 contributes to the stability of nucleosomes that are reassembled during transcriptional elongation in budding yeast.
Two recent studies reported that the Ioc4 subunit recognizes and targets ISW1b to regions enriched for H3K36me, corresponding to gene bodies [88, 89]. It was found that Ioc4 colocalizes with H3K36me throughout the genome [89]. It was hypothesized that the PWWP domain of Ioc4 functions in H3K36me binding as the PWWP domain of a human protein bRPF1 was found to specifically bind this modified form of H3 [90]. Mutation to the PWWP domain significantly reduced binding of Ioc4 to chromatin in vivo and immobilized nucleosomes with methylated H3K36 [89]. These results indicate that Ioc4 facilitates binding of ISW1b to chromatin and that it has a high affinity for H3K36me in vivo and in vitro.
In an independent proteomics approach, it was found that a number of chromatin remodelers and complex subunits physically interact with H3K36me [88]. Among the proteins identified were Chd1, Isw1, and remodeler subunits. The Ioc4 subunit was shown to interact with mononucleosomes in vivo, with enhanced interaction with H3K36me3-containing nucleosomes [88]. Interestingly, the combined loss of Isw1 and Chd1 increases cryptic transcription, histone turnover, and histone acetylation within transcribed genes, similar to loss of Set2 [88]. Ioc3 was also identified in proteomic analysis of H3K36me interactors and has expanded ORF occupancy in set2Δ cells. Therefore, it is possible that ISW1a facilitates increased turnover in the absence of H3K36me. Future genetic and biochemical studies will establish whether there is antagonism between ISW1a and ISW1b chromatin binding or activity.
Schizosaccharomyces pombe has similar nucleosome-stabilizing mechanisms within active genes. Its genome encodes three CHD1 family enzymes, Hrp3, Hrp1, and Mit1, but interestingly does not encode an ISWI family member; whether other remodelers compensate for the absence of ISWI family enzymes has not yet been established. Loss of either Hrp1 or Hrp3 leads to increased cryptic transcription, similar to that observed upon loss of Isw1 and Chd1 in budding yeast [91–93]. Regulation of cryptic transcription by CHD family remodelers in S. pombe also involves an Rpd3S-like complex (Clr6 complex-II) and Set2-mediated H3K36me [91, 93]. Whereas remodeler targeting is attributed to H3K36me in budding yeast, the recruitment mechanism for Hrp1 and 3 is not yet clear.
Loss of Hrp3 in S. pombe has a stronger effect on nucleosome positioning in the bodies of genes that have the highest expression, and Chd1 in S. cerevisiae is enriched at highly transcribed genes [88, 91]. Loss of Chd1 has strong effects on histone turnover in both lowly and highly expressed genes and particularly genes over 1kb in length [71, 88]. Loss of Isw1, on the other hand results in strongest increased nucleosome turnover in the bodies of lowly expressed genes [88]. Therefore, CHD1 and ISWI family remodelers may function within the bodies of both overlapping and distinct classes of genes defined, in part, by transcriptional frequency and length.
CHD1 regulation of nucleosome turnover during transcription is likely conserved in flies and mammals. For example, Drosophila CHD1 is required for accumulation of H3.3, a histone variant that is incorporated into nucleosomes with high turnover [94]. Yeast Chd1 promotes nucleosome turnover in the 5’ region of genes, which is where H3.3 enrichment is often observed in metazoans [71]. Also, recent studies indicate that there is a conserved effect of Chd1 on H2B monoubiquitylation [95]. H2B is ubiquitylated by the Rad6 and Bre1 E2/E3 ligases of the PAF1 complex, and this modification is associated with gene activation in yeast and mammals [96–100]. Loss of Chd1 in yeast or CHD1 in human cells reduces global H2B ubiquitylation at K123 and K120, respectively [95]. Additionally, cells lacking Chd1 or H2BK123ub have very similar effects on nucleosome occupancy within genes [95, 101]. Loss of H2BK123ub by deletion of RAD6, mutation of H2BK123 to alanine, or loss of Chd1 similarly results in reduced nucleosome occupancy within gene bodies, with effects strongest at highly expressed genes [95, 101]. Given the enrichment of H2Bub within the bodies of highly expressed genes in human cells [102], the function of this modification in regulating nucleosome turnover may also be conserved through humans.
Remodeler regulation of transcriptional termination
Genetic studies in fission and budding yeast have revealed that Chd1 and ISWI family remodelers contribute to termination of transcription. In S. pombe, Hrp1 was identified in a genetic screen for factors required for proper RNAPII termination [103]. Further study demonstrated that Chd1 in S. cerevisiae also functions in termination of RNAPII transcription [103]. Chd1 was found to work both alone and with Isw1 and Isw2 in termination of GAL10 transcripts [103]. All three proteins localized to the 3’ end of GAL10 by ChIP and deletions of the genes encoding the three remodelers altered nucleosome positioning at the end of GAL10. Localization of remodelers to gene termini is not limited to GAL10 as Isw1 and regulatory subunits Ioc3 and Ioc4, as well as Isw2 are found enriched on nucleosomes at the very 3’ end of RNAPII-transcribed genes throughout the genome [24]. Additionally, Chd1, Isw1, and Isw2 in budding yeast are required for proper termination of rDNA transcription by RNAPI [104]. Both Chd1 and Isw1 bind along the length of transcribed rDNA genes, which contain nucleosomes that are largely unpositioned. These results suggest that chromatin remodeling may produce a particular nucleosome arrangement at the very 3’ ends of genes to contribute to effective termination of RNA polymerase activity. It has not yet been explored whether the role of these remodelers in termination is conserved in other eukaryotes, nor is it currently understood whether histone modification crosstalk contributes to this function.
Concluding remarks
Regulation of gene expression is coordinated through multiple steps and enzymatic activities. We have focused here on the functional intersections between ISWI and CHD1 chromatin remodelers with histone modifiers and modified histones during transcription (Fig. 3). Studies of remodeler activity in budding and fission yeast will continue to yield a detailed understanding of biochemical and genetic mechanisms of these interactions. These details will guide work in developmental and disease models, and combined with structural studies, will promote understanding of dynamic gene regulation. Such concerted efforts may ultimately lead to the identification of new drug targets that affect the enzymatic activity of chromatin modifiers and remodelers, and their interactions that contribute to progression of human disease.
Highlights.
ISWI and CHD1 chromatin remodelers have similar biochemical structure and function.
ISWI and CHD1 interact with histone modifications and modifiers.
These interactions can be either synergistic or antagonistic.
ISWI and CHD1 function with histone modifications throughout transcription.
Acknowledgements
We apologize to those whose work we could not cite directly due to limitations on references. We thank A. Lafon, T. Tsukiyama, J. McKnight, G. Csankovszki, S. Torigoe, A.L. Torres Macchorro, M. Eustice, R.M. Garza, L. Clark, M. Koch, and three anonymous reviewers for their suggestions and corrections. Our work was initiated with support from the University of California Cancer Research Coordinating Committee and NIH GM090177 and GM056469 and continues with support from the American Cancer Society PF-13-283-01-TBE (E.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Keating ST, El-Osta A. Chromatin modifications associated with diabetes. J Cardiovasc Transl Res. 2012;5:399–412. doi: 10.1007/s12265-012-9380-9. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Carmon D, Meshorer E. Polyglutamine (polyQ) disorders: the chromatin connection. Nucleus. 2012;3:433–441. doi: 10.4161/nucl.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 5.Price BD, D'Andrea AD. Chromatin remodeling at DNA double-strand breaks. Cell. 2013;152:1344–1354. doi: 10.1016/j.cell.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papamichos-Chronakis M, Peterson CL. Chromatin and the genome integrity network. Nat Rev Genet. 2013;14:62–75. doi: 10.1038/nrg3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petesch SJ, Lis JT. Overcoming the nucleosome barrier during transcript elongation. Trends Genet. 2012;28:285–294. doi: 10.1016/j.tig.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B, et al. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Cairns BR. The logic of chromatin architecture and remodelling at promoters. Nature. 2009;461:193–198. doi: 10.1038/nature08450. [DOI] [PubMed] [Google Scholar]
- 10.Hahn S, Young ET. Transcriptional regulation in Saccharomyces cerevisiae: transcription factor regulation and function, mechanisms of initiation, and roles of activators and coactivators. Genetics. 2011;189:705–736. doi: 10.1534/genetics.111.127019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas MC, Chiang CM. The general transcription machinery and general cofactors. Crit Rev Biochem Mol Biol. 2006;41:105–178. doi: 10.1080/10409230600648736. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, et al. RNA polymerase II elongation control. Annu Rev Biochem. 2012;81:119–143. doi: 10.1146/annurev-biochem-052610-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mischo HE, Proudfoot NJ. Disengaging polymerase: terminating RNA polymerase II transcription in budding yeast. Biochim Biophys Acta. 2013;1829:174–185. doi: 10.1016/j.bbagrm.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Gangaraju VK, Bartholomew B. Dependency of ISW1a chromatin remodeling on extranucleosomal DNA. Mol Cell Biol. 2007;27:3217–3225. doi: 10.1128/MCB.01731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zofall M, et al. Functional role of extranucleosomal DNA and the entry site of the nucleosome in chromatin remodeling by ISW2. Mol Cell Biol. 2004;24:10047–10057. doi: 10.1128/MCB.24.22.10047-10057.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dang W, et al. The Dpb4 subunit of ISW2 is anchored to extranucleosomal DNA. J Biol Chem. 2007;282:19418–19425. doi: 10.1074/jbc.M700640200. [DOI] [PubMed] [Google Scholar]
- 18.Kagalwala MN, et al. Topography of the ISW2-nucleosome complex: insights into nucleosome spacing and chromatin remodeling. Embo J. 2004;23:2092–2104. doi: 10.1038/sj.emboj.7600220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKnight JN, et al. Extranucleosomal DNA binding directs nucleosome sliding by Chd1. Mol Cell Biol. 2011;31:4746–4759. doi: 10.1128/MCB.05735-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grune T, et al. Crystal structure and functional analysis of a nucleosome recognition module of the remodeling factor ISWI. Mol Cell. 2003;12:449–460. doi: 10.1016/s1097-2765(03)00273-9. [DOI] [PubMed] [Google Scholar]
- 21.Yamada K, et al. Structure and mechanism of the chromatin remodelling factor ISW1a. Nature. 2011;472:448–453. doi: 10.1038/nature09947. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, et al. Crystal structure of the chromodomain helicase DNA-binding protein 1 (Chd1) DNA-binding domain in complex with DNA. J Biol Chem. 2011;286:42099–42104. doi: 10.1074/jbc.C111.294462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan DP, et al. The DNA-binding domain of the Chd1 chromatin-remodelling enzyme contains SANT and SLIDE domains. Embo J. 2011;30:2596–2609. doi: 10.1038/emboj.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen K, et al. Genome-wide nucleosome specificity and directionality of chromatin remodelers. Cell. 2012;149:1461–1473. doi: 10.1016/j.cell.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauk G, et al. The chromodomains of the Chd1 chromatin remodeler regulate DNA access to the ATPase motor. Mol Cell. 2010;39:711–723. doi: 10.1016/j.molcel.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel A, et al. Decoupling nucleosome recognition from DNA binding dramatically alters the properties of the Chd1 chromatin remodeler. Nucleic Acids Res. 2013;41:1637–1648. doi: 10.1093/nar/gks1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel A, et al. Identification of residues in chromodomain helicase DNA-binding protein 1 (Chd1) required for coupling ATP hydrolysis to nucleosome sliding. J Biol Chem. 2011;286:43984–43993. doi: 10.1074/jbc.M111.282970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapier CR, Cairns BR. Regulation of ISWI involves inhibitory modules antagonized by nucleosomal epitopes. Nature. 2012;492:280–284. doi: 10.1038/nature11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller-Planitz F, et al. The ATPase domain of ISWI is an autonomous nucleosome remodeling machine. Nat Struct Mol Biol. 2013;20:82–89. doi: 10.1038/nsmb.2457. [DOI] [PubMed] [Google Scholar]
- 30.Yadon AN, Tsukiyama T. SnapShot: Chromatin remodeling: ISWI. Cell. 2011;144:453–453. doi: 10.1016/j.cell.2011.01.019. e451. [DOI] [PubMed] [Google Scholar]
- 31.Fyodorov DV, Kadonaga JT. Binding of Acf1 to DNA involves a WAC motif and is important for ACF-mediated chromatin assembly. Mol Cell Biol. 2002;22:6344–6353. doi: 10.1128/MCB.22.18.6344-6353.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kukimoto I, et al. The histone-fold protein complex CHRAC-15/17 enhances nucleosome sliding and assembly mediated by ACF. Mol Cell. 2004;13:265–277. doi: 10.1016/s1097-2765(03)00523-9. [DOI] [PubMed] [Google Scholar]
- 33.Vary JC, Jr, et al. Yeast Isw1p forms two separable complexes in vivo. Mol Cell Biol. 2003;23:80–91. doi: 10.1128/MCB.23.1.80-91.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pray-Grant MG, et al. Chd1 chromodomain links histone H3 methylation with SAGA- and SLIK-dependent acetylation. Nature. 2005;433:434–438. doi: 10.1038/nature03242. [DOI] [PubMed] [Google Scholar]
- 35.Flanagan JF, et al. Double chromodomains cooperate to recognize the methylated histone H3 tail. Nature. 2005;438:1181–1185. doi: 10.1038/nature04290. [DOI] [PubMed] [Google Scholar]
- 36.Sims RJ, 3rd, et al. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J Biol Chem. 2005;280:41789–41792. doi: 10.1074/jbc.C500395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morettini S, et al. The chromodomains of CHD1 are critical for enzymatic activity but less important for chromatin localization. Nucleic Acids Res. 2011;39:3103–3115. doi: 10.1093/nar/gkq1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saha A, et al. Chromatin remodeling through directional DNA translocation from an internal nucleosomal site. Nat Struct Mol Biol. 2005;12:747–755. doi: 10.1038/nsmb973. [DOI] [PubMed] [Google Scholar]
- 39.Whitehouse I, et al. Evidence for DNA translocation by the ISWI chromatin-remodeling enzyme. Mol Cell Biol. 2003;23:1935–1945. doi: 10.1128/MCB.23.6.1935-1945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clapier CR, et al. Critical role for the histone H4 N terminus in nucleosome remodeling by ISWI. Mol Cell Biol. 2001;21:875–883. doi: 10.1128/MCB.21.3.875-883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shogren-Knaak M, et al. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 42.Wysocka J, et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- 43.Li H, et al. Molecular basis for site-specific read-out of histone H3K4me3 by the BPTF PHD finger of NURF. Nature. 2006;442:91–95. doi: 10.1038/nature04802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruthenburg AJ, et al. Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell. 2011;145:692–706. doi: 10.1016/j.cell.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gkikopoulos T, et al. A role for Snf2-related nucleosome-spacing enzymes in genome-wide nucleosome organization. Science. 2011;333:1758–1760. doi: 10.1126/science.1206097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehouse I, et al. Chromatin remodelling at promoters suppresses antisense transcription. Nature. 2007;450:1031–1035. doi: 10.1038/nature06391. [DOI] [PubMed] [Google Scholar]
- 47.Tirosh I, et al. Widespread remodeling of mid-coding sequence nucleosomes by Isw1. Genome Biol. 2010 doi: 10.1186/gb-2010-11-5-r49. ( http://genomebiology.com) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitehouse I, Tsukiyama T. Antagonistic forces that position nucleosomes in vivo. Nat Struct Mol Biol. 2006;13:633–640. doi: 10.1038/nsmb1111. [DOI] [PubMed] [Google Scholar]
- 49.Goldmark JP, et al. The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell. 2000;103:423–433. doi: 10.1016/s0092-8674(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 50.Fazzio TG, et al. Widespread collaboration of Isw2 and Sin3-Rpd3 chromatin remodeling complexes in transcriptional repression. Mol Cell Biol. 2001;21:6450–6460. doi: 10.1128/MCB.21.19.6450-6460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sala A, et al. Genome-wide characterization of chromatin binding and nucleosome spacing activity of the nucleosome remodelling ATPase ISWI. Embo J. 2011;30:1766–1777. doi: 10.1038/emboj.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corona DF, et al. Modulation of ISWI function by site-specific histone acetylation. EMBO Rep. 2002;3:242–247. doi: 10.1093/embo-reports/kvf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deuring R, et al. The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol Cell. 2000;5:355–365. doi: 10.1016/s1097-2765(00)80430-x. [DOI] [PubMed] [Google Scholar]
- 54.Kasten M, et al. Tandem bromodomains in the chromatin remodeler RSC recognize acetylated histone H3 Lys14. Embo J. 2004;23:1348–1359. doi: 10.1038/sj.emboj.7600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts SM, Winston F. Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics. 1997;147:451–465. doi: 10.1093/genetics/147.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hassan AH, et al. Histone acetyltransferase complexes stabilize swi/snf binding to promoter nucleosomes. Cell. 2001;104:817–827. doi: 10.1016/s0092-8674(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 57.Syntichaki P, et al. The Gcn5 bromodomain co-ordinates nucleosome remodelling. Nature. 2000;404:414–417. doi: 10.1038/35006136. [DOI] [PubMed] [Google Scholar]
- 58.Hassan AH, et al. Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell. 2002;111:369–379. doi: 10.1016/s0092-8674(02)01005-x. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira H, et al. Histone modifications influence the action of Snf2 family remodelling enzymes by different mechanisms. J Mol Biol. 2007;374:563–579. doi: 10.1016/j.jmb.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatterjee N, et al. Histone H3 tail acetylation modulates ATP-dependent remodeling through multiple mechanisms. Nucleic Acids Res. 2011;39:8378–8391. doi: 10.1093/nar/gkr535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lafon A, et al. Functional Antagonism between Sas3 and Gcn5 Acetyltransferases and ISWI Chromatin Remodelers. PLoS Genet. 2012 doi: 10.1371/journal.pgen.1002994. ( http://www.plosgenetics.org) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howe L, et al. Histone H3 specific acetyltransferases are essential for cell cycle progression. Genes Dev. 2001;15:3144–3154. doi: 10.1101/gad.931401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosaleny LE, et al. The Sas3p and Gcn5p histone acetyltransferases are recruited to similar genes. Genome Biol. 2007 doi: 10.1186/gb-2007-8-6-r119. ( http://genomebiology.com/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Venters BJ, et al. A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces. Mol Cell. 2011;41:480–492. doi: 10.1016/j.molcel.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreira R, et al. Site-specific acetylation of ISWI by GCN5. BMC Mol Biol. 2007;8:73. doi: 10.1186/1471-2199-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartke T, et al. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell. 2010;143:470–484. doi: 10.1016/j.cell.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaspar-Maia A, et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature. 2009;460:863–868. doi: 10.1038/nature08212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin JJ, et al. Mediator coordinates PIC assembly with recruitment of CHD1. Genes Dev. 2011;25:2198–2209. doi: 10.1101/gad.17554711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simic R, et al. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. Embo J. 2003;22:1846–1856. doi: 10.1093/emboj/cdg179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ehrensberger AH, Kornberg RD. Isolation of an activator-dependent, promoter-specific chromatin remodeling factor. Proc Natl Acad Sci U S A. 2011;108:10115–10120. doi: 10.1073/pnas.1101449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radman-Livaja M, et al. A key role for Chd1 in histone H3 dynamics at the 3' ends of long genes in yeast. PLoS Genet. 2012 doi: 10.1371/journal.pgen.1002811. ( http://www.plosgenetics.org) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zentner GE, et al. ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies. PLoS Genet. 2013 doi: 10.1371/journal.pgen.1003317. ( http://www.plosgenetics.org) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yadon AN, et al. DNA looping facilitates targeting of a chromatin remodeling enzyme. Mol Cell. 2013;50:93–103. doi: 10.1016/j.molcel.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams SK, Tyler JK. Transcriptional regulation by chromatin disassembly and reassembly. Curr Opin Genet Dev. 2007;17:88–93. doi: 10.1016/j.gde.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Campos EI, et al. The program for processing newly synthesized histones H3.1 and H4. Nat Struct Mol Biol. 2010;17:1343–1351. doi: 10.1038/nsmb.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsubota T, et al. Histone H3-K56 acetylation is catalyzed by histone chaperone-dependent complexes. Mol Cell. 2007;25:703–712. doi: 10.1016/j.molcel.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carrozza MJ, et al. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123:581–592. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 78.Venkatesh S, et al. Set2 methylation of histone H3 lysine 36 suppresses histone exchange on transcribed genes. Nature. 2012;489:452–455. doi: 10.1038/nature11326. [DOI] [PubMed] [Google Scholar]
- 79.Li B, et al. Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription. Genes Dev. 2007;21:1422–1430. doi: 10.1101/gad.1539307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Govind CK, et al. Phosphorylated Pol II CTD recruits multiple HDACs, including Rpd3C(S), for methylation-dependent deacetylation of ORF nucleosomes. Mol Cell. 2010;39:234–246. doi: 10.1016/j.molcel.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drouin S, et al. DSIF and RNA polymerase II CTD phosphorylation coordinate the recruitment of Rpd3S to actively transcribed genes. PLoS Genet. 2010;6:e1001173. doi: 10.1371/journal.pgen.1001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huh JW, et al. Multivalent di-nucleosome recognition enables the Rpd3S histone deacetylase complex to tolerate decreased H3K36 methylation levels. Embo J. 2012;31:3564–3574. doi: 10.1038/emboj.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kizer KO, et al. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol Cell Biol. 2005;25:3305–3316. doi: 10.1128/MCB.25.8.3305-3316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krogan NJ, et al. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol Cell Biol. 2003;23:4207–4218. doi: 10.1128/MCB.23.12.4207-4218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li B, et al. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem. 2003;278:8897–8903. doi: 10.1074/jbc.M212134200. [DOI] [PubMed] [Google Scholar]
- 86.Selth LA, et al. Transcript Elongation by RNA Polymerase II. Annu Rev Biochem. 2010;79:271–293. doi: 10.1146/annurev.biochem.78.062807.091425. [DOI] [PubMed] [Google Scholar]
- 87.Quan TK, Hartzog GA. Histone H3K4 and K36 methylation, Chd1 and Rpd3S oppose the functions of Saccharomyces cerevisiae Spt4-Spt5 in transcription. Genetics. 2010;184:321–334. doi: 10.1534/genetics.109.111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smolle M, et al. Chromatin remodelers Isw1 and Chd1 maintain chromatin structure during transcription by preventing histone exchange. Nat Struct Mol Biol. 2012;19:884–892. doi: 10.1038/nsmb.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maltby VE, et al. Histone H3 lysine 36 methylation targets the Isw1b remodeling complex to chromatin. Mol Cell Biol. 2012;32:3479–3485. doi: 10.1128/MCB.00389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vezzoli A, et al. Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nat Struct Mol Biol. 2010;17:617–619. doi: 10.1038/nsmb.1797. [DOI] [PubMed] [Google Scholar]
- 91.Shim YS, et al. Hrp3 controls nucleosome positioning to suppress non-coding transcription in eu- and heterochromatin. Embo J. 2012;31:4375–4387. doi: 10.1038/emboj.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pointner J, et al. CHD1 remodelers regulate nucleosome spacing in vitro and align nucleosomal arrays over gene coding regions in S. pombe. Embo J. 2012;31:4388–4403. doi: 10.1038/emboj.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hennig BP, et al. Chd1 chromatin remodelers maintain nucleosome organization and repress cryptic transcription. EMBO Rep. 2012;13:997–1003. doi: 10.1038/embor.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Konev AY, et al. CHD1 motor protein is required for deposition of histone variant H3.3 into chromatin in vivo. Science. 2007;317:1087–1090. doi: 10.1126/science.1145339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee JS, et al. Codependency of H2B monoubiquitination and nucleosome reassembly on Chd1. Genes Dev. 2012;26:914–919. doi: 10.1101/gad.186841.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pavri R, et al. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125:703–717. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 97.Robzyk K, et al. Rad6-dependent ubiquitination of histone H2B in yeast. Science. 2000;287:501–504. doi: 10.1126/science.287.5452.501. [DOI] [PubMed] [Google Scholar]
- 98.Wood A, et al. The Bur1/Bur2 complex is required for histone H2B monoubiquitination by Rad6/Bre1 and histone methylation by COMPASS. Mol Cell. 2005;20:589–599. doi: 10.1016/j.molcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 99.Henry KW, et al. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 2003;17:2648–2663. doi: 10.1101/gad.1144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim J, et al. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell. 2009;137:459–471. doi: 10.1016/j.cell.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Batta K, et al. Genome-wide function of H2B ubiquitylation in promoter and genic regions. Genes Dev. 2011;25:2254–2265. doi: 10.1101/gad.177238.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Minsky N, et al. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol. 2008;10:483–488. doi: 10.1038/ncb1712. [DOI] [PubMed] [Google Scholar]
- 103.Alen C, et al. A role for chromatin remodeling in transcriptional termination by RNA polymerase II. Mol Cell. 2002;10:1441–1452. doi: 10.1016/s1097-2765(02)00778-5. [DOI] [PubMed] [Google Scholar]
- 104.Jones HS, et al. RNA polymerase I in yeast transcribes dynamic nucleosomal rDNA. Nat Struct Mol Biol. 2007;14:123–130. doi: 10.1038/nsmb1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Winston F, Carlson M. Yeast SNF/SWI transcriptional activators and the SPT/SIN chromatin connection. Trends Genet. 1992;8:387–391. doi: 10.1016/0168-9525(92)90300-s. [DOI] [PubMed] [Google Scholar]
- 106.Aasland R, et al. The SANT domain: a putative DNA-binding domain in the SWI-SNF and ADA complexes, the transcriptional co-repressor N-CoR and TFIIIB. Trends Biochem Sci. 1996;21:87–88. [PubMed] [Google Scholar]