Abstract
IMPORTANCE
Bariatric surgery is an accepted treatment for obesity. Despite extensive literature, few studies report long-term follow-up in cohorts with adequate retention rates.
OBJECTIVE
To assess the quality of evidence and treatment effectiveness 2 years after bariatric procedures for weight loss, type 2 diabetes, hypertension, and hyperlipidemia in severely obese adults.
EVIDENCE REVIEW
MEDLINE and Cochrane databases were searched from 1946 through May 15, 2014. Search terms included bariatric surgery, individual bariatric procedures, and obesity. Studies were included if they described outcomes for gastric bypass, gastric band, or sleeve gastrectomy performed on patients with a body mass index of 35 or greater, had more than 2 years of outcome information, and had follow-up measures for at least 80% of the initial cohort. Two investigators reviewed each study and a third resolved study inclusion disagreements.
FINDINGS
Of 7371 clinical studies reviewed, 29 studies (0.4%, 7971 patients) met inclusion criteria. All gastric bypass studies (6 prospective cohorts, 5 retrospective cohorts) and sleeve gastrectomy studies (2 retrospective cohorts) had 95% confidence intervals for the reported mean, median, or both exceeding 50% excess weight loss. This amount of excess weight loss occurred in 31% of gastric band studies (9 prospective cohorts, 5 retrospective cohorts). The mean sample-size–weighted percentage of excess weight loss for gastric bypass was 65.7% (n = 3544) vs 45.0% (n = 4109) for gastric band. Nine studies measured comorbidity improvement. For type 2 diabetes (glycated hemoglobin <6.5% without medication), sample-size–weighted remission rates were 66.7% for gastric bypass (n = 428) and 28.6% for gastric band (n = 96). For hypertension (blood pressure <140/90 mm Hg without medication), remission rates were 38.2% for gastric bypass (n = 808) and 17.4% for gastric band (n = 247). For hyperlipidemia (cholesterol <200 mg/dL, high-density lipoprotein >40 mg/dL, low-density lipoprotein <160 mg/dL, and triglycerides <200 mg/dL), remission rates were 60.4% for gastric bypass (n = 477) and 22.7% for gastric band (n = 97).
CONCLUSIONS AND RELEVANCE
Very few bariatric surgery studies report long-term results with sufficient patient follow-up to minimize biased results. Gastric bypass has better outcomes than gastric band procedures for long-term weight loss, type 2 diabetes control and remission, hypertension, and hyperlipidemia. Insufficient evidence exists regarding long-term outcomes for gastric sleeve resections.
Although bariatric surgery is commonly performed, it is not universally accepted as an obesity treatment. In 2009, a Cochrane systematic review advised caution before accepting the effectiveness of bariatric surgery because of limited high-quality evidence supporting its use.1 Most published studies of bariatric surgery are retrospective, short-term studies with insufficient follow-up.2 Substantial missing data in these studies preclude definitive conclusions about the procedures’ outcomes. Although there is ample short-term evidence about the benefits and risks of bariatric surgery up to 1 year after surgery, few data are available about long-term outcomes or groups.
Obesity is a chronic disease, as are its complications. Treatment success and groups should be assessed in long-term studies, particularly when the treatment is as invasive as major surgery. To ensure that outcomes are accurately assessed, researchers should follow up patients until the study’s end, particularly when treatment failure is a common reason for patients to not complete the study. If not adequately accounted for, loss to follow-up attributable to treatment failure may cause overestimation of treatment success.
We performed a systematic review of the literature to determine the association of bariatric surgery with outcomes of weight loss, diabetes, hypertension, and hyperlipidemia in studies of at least 2 years’ duration and with at least 80% follow-up of patients.
Methods
The Ovid MEDLINE (1946), Cochrane Central Register of Controlled Trials (1996), and Cochrane Systematic Reviews (1993) databases were searched from their inception dates, noted in parentheses, to May 15, 2014. ClinicalTrials.gov was searched and bibliographies of articles that met inclusion criteria were reviewed. Only published articles in the English language were included. Search terms for laparoscopic and open bariatric operations included the following Medical Subject Headings: bariatric surgery, Roux-en-Y gastric bypass (gastric bypass), adjustable gastric band (gastric band), sleeve gastrectomy, jejunoileal bypass, gastroplasty, and obesity surgery. A text-word search for the concept of the aforementioned procedures in addition to biliopancreatic bypass, biliopancreatic diversion, and duodenal switch was also conducted. The search was screened for the following outcome terms: weight loss (expressed as absolute or percentage of excess weight loss [%EWL]), type 2 diabetes (defined by glycated hemoglobin [HbA1c] and medication usage), hypertension (defined by systolic/diastolic blood pressure and medication usage), and hyperlipidemia (defined by lipid panel and medication usage). A prespecified study protocol was developed prior to the literature review using PRISMA3 criteria and followed. The protocol was not registered.
Study Inclusion
Original research reports of cohorts from randomized clinical trials (RCTs) and observational studies with at least 50 adult patients (aged ≥18 years), with a minimum body mass index (BMI) of 35 (calculated as weight in kilograms divided by height in meters squared), who were undergoing gastric bypass, gastric band, or sleeve gastrectomy were included for weight loss outcomes. We required each study to have at least 2 years of follow-up for the entire cohort and follow-up of at least 80% of the treated patients. Percentage of EWL, when not reported, was calculated using ([preoperative weight − postoperative weight] × 100) ÷ (preoperative weight − [weight at BMI 25]), where [weight at BMI 25] was for mean height of the cohort4 at baseline, either reported or derived from reported baseline weight for baseline BMI (height = [weight/BMI]1/2). Weight was in kilograms and height in meters.
Two reviewers evaluated each publication independently. Differences regarding study inclusion were resolved with input from a third reviewer. To maximize the number of studies assessing comorbidity outcomes, we decreased the minimum baseline sample size to at least 20 patients. Comorbidity outcome cohorts had to be diagnosed with type 2 diabetes, hypertension, or hyperlipidemia (defined in each methods section) at the start of the study.
Study Exclusion
Review articles, meta-analyses, case-control studies, and editorials were excluded. We evaluated only the highest-volume procedures worldwide.5 Thus, articles reporting on jejunoileal bypass, vertical banded gastroplasty, biliopancreatic diversion/duodenal switch, mini-gastric bypass, and gastric plication were excluded.
Data Abstraction and Synthesis of Results
Results from the included studies were abstracted into data tables. Data pooling was precluded due to observed heterogeneity in patients, interventions, or outcome measures. Results were summarized separately for weight loss, type 2 diabetes, hypertension, and hyperlipidemia. Long-term complications were extracted from studies meeting inclusion criteria and summarized, providing a context of risk for surgical treatment.
When there was more than 1 report from the same study population, we used only the publication having the longest postsurgery follow-up for the entire cohort and reporting outcomes on 80% or more of the cohort. If inclusion criteria were not met in the publication with the longest postsurgery follow-up (eg, 100% of the cohort a minimum of 3 years after surgery, but only 50% follow-up for outcome measures) and were met in an earlier publication (eg, 100% of the cohort a minimum of 2 years after surgery with 90% follow-up), the earlier publication was used. Likewise, when a publication reported data from multiple postsurgery years, the longest postsurgery follow-up with 80% or more of the cohort was used. When duplicate data from the same cohort were encountered in multiple publications, only 1 publication was included. To ensure the entire cohort in each study was at the reported postsurgery follow-up interval, we defined the follow-up interval as the minimum value of the follow-up range.
Study Quality Assessment
An aim of this study was to limit bias by setting a minimum 80% follow-up threshold and include as many cohorts as possible meeting this criterion. We included the maximum number of cohorts meeting this threshold regardless of study design or comparator group. Thus, any group of a prospective trial testing gastric bypass, gastric band, or sleeve gastrectomy was included, even if the comparator group was an excluded procedure or nonsurgical group. For example, the gastric bypass group of a trial comparing gastric bypass to vertical banded gastroplasty was included in our analysis. Outcomes from prospective cohorts were considered stronger evidence than retrospective cohorts. Differences in outcomes from prospective vs retrospective cohorts were evaluated. We delineated bariatric surgery outcomes of interest as being primary or secondary outcomes of the original study.
Statistical Analyses
Ninety-five percent confidence intervals for %EWL were calculated when standard deviations were provided (confidence intervals = ±[1.96 × standard deviation] ÷ [samplesize]1/2). Sample-size–weighted outcome means were compared by t tests using SAS version 9.4 (SAS Institute). All reported P values are 2-sided and considered significant if less than .05.
Results
We identified 7371 references including 184 review articles and 7187 clinical studies. Clinical studies were excluded after reviewing titles (5728; 80%), abstracts (1132; 16%), and the complete journal articles (327; 4%). Twenty-nine clinical studies (<1%) were included in this review (eFigure in the Supplement), reporting on the following: weight loss (n = 22 studies; 9 after gastric bypass, 11 after gastric band, 2 after both gastric bypass and band, and 2 after sleeve gastrectomy), type 2 diabetes (n = 6 studies; 2 after gastric bypass, 3 after gastric band, 1 after both gastric bypass and band), hypertension (n = 3 studies; 2 after gastric bypass, 1 after gastric bypass and band), and hyperlipidemia (n = 3 studies; 2 after gastric bypass, 1 after both gastric bypass and band). No studies meeting the inclusion criteria evaluated the comorbidities of interest after sleeve gastrectomy. Designs of the included studies for all outcomes were RCTs (n = 10), matched cohort (n = 1), prospective cohort (n = 6), retrospective cohort (n = 1), and case series (n = 11).
Three studies reported more than 1 outcome; 2 studies reported all 4 outcomes of interest (weight loss, type 2 diabetes, hypertension, and hyperlipidemia)6,7; 2 studies reported 2 outcomes8,9; and 26 studies reported only 1 outcome (weight loss, 21 studies; type 2 diabetes, 3 studies10–12; hypertension, 1 study9; and hyperlipidemia, 1 study13). Three of 29 studies reported on primary outcomes other than those of interest to this study. We retained these studies because they included secondary outcomes of weight loss or improvement of type 2 diabetes, hypertension, or hyperlipidemia.
Twenty-four studies reported weight loss outcomes. Most expressed mean weight loss as %EWL (20/24 studies), followed by change in BMI (16/24) and change in absolute weight (11/24). Of these, 16 included sufficient information (mean %EWL or mean percentage of absolute weight loss and standard deviation) to calculate 95% confidence intervals. Point estimates of the mean or median %EWL, without 95% confidence intervals, were provided for the remaining 7 studies.
Comorbidity improvement was reported most frequently as rate of remission (6/6 studies for type 2 diabetes, 2/3 studies for hypertension, 3/3 studies for hyperlipidemia). Remission was uniformly defined in the studies as HbA1c less than 6.5% without medications for type 2 diabetes; blood pressure less than 140/90 without medications for hypertension; and cholesterol less than 200 mg/dL, high-density lipoprotein (HDL) greater than 40 mg/dL, low-density lipoprotein (LDL) less than 160 mg/dL, and triglycerides (TG) less than 200 mg/dL for hyperlipidemia. (To convert total, HDL, and LDL cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.) One study examining hyperlipidemia measured only hypertriglyceridemia. Values for HbA1c with standard deviations before and after bariatric surgery were reported in 50% (3/6: 1 RCT,11 2 prospective cohorts8,10) of type 2 diabetes studies. Systolic and diastolic blood pressures with standard deviations before and after bariatric surgery were reported in 67% (2/3: 1 RCT,9 1 matched cohort14) of hypertension studies. Triglycerides with standard deviations before and after bariatric surgery were reported in 25% (1/4: 1 prospective cohort13) of hyperlipidemia studies. No studies evaluating comorbidities after sleeve gastrectomy met inclusion criteria.
Weight Loss
Eleven gastric bypass (n = 3544 patients), 13 gastric band (n = 4109 patients), and 2 sleeve gastrectomy (n = 115 patients) studies with weight loss outcomes met inclusion criteria (Table 1). Seventy-eight percent (7/9) of gastric bypass, 75% (9/12) of gastric band, and 50% (1/2) of sleeve gastrectomy studies reporting mean %EWL provided standard deviations, enabling calculation of confidence intervals. Approximately half of the studies (gastric bypass, 45%, 5/11; gastric band, 54%, 6/13; sleeve gastrectomy, 50%, 1/2) had follow-up time exceeding 3 years. Four studies (2 gastric bypass, 2 gastric band) had at least 5 years of postsurgery follow-up.
Table 1.
Weight Loss After Bariatric Surgerya
| Source | Primary Outcome | Secondary Outcomes | No. of Patients Entering Study | Follow-up, y | No. (%) of Patients Followed Up | Mean or Median %EWLb | EWL SD or (IQR) | Baseline BMI, Mean (Range)c | Mean Decrease in BMI | Mean or Median Weight Loss, kgb |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomized Clinical Trial | ||||||||||
| Gastric bypass | ||||||||||
| Hall et al,15 1990 | Weight loss | Complications | 99 | 3 | 85 (85) | 63.6d | (65–86) | 49d (IQR, 40–56.2) | NR | 39d |
| Skroubis et al,16 2006 | Safety, weight loss | Comorbidities | 65 | 2 | 62 (95) | 72.6 | 16.1 | 44.6 (NR) | 16.6 | NR |
| Nguyen et al,17 200917 | Weight loss | QOL | 111 | 2 | 94 (85) | 68.9 | 16.1 | 47.5 (NR) | 17 | NR |
| Gastric band | ||||||||||
| van Dielan et al,18 2005 | Weight loss, complications | Comorbidities | 50 | 2 | 50 (100) | 54.9 | 23.3 | 46.7 (NR) | 12.1 | NR |
| O’Brien et al,19 2005 | Prolapse | Weight loss, QOL | 202 | 2 | 198 (98) | 49.5e | 19 | 44.7 (NR) | NR | 29 |
| Suter et al,20 2005 | Weight loss | QOL | 180 | 2 | 156 (87) | 54.5e | NR | 46.2 (NR) | 11 | NR |
| Gravante et al,21 2007 | Device differences | Weight loss | 400 | 2 | 356 (89) | 56.0e | 35.6 | 46.2 (NR) | 11 | NR |
| Mathus-Vliegen et al,22 2007 | QOL | Weight loss | 50 | 5 | 44 (88) | 41.6e | 26.2 | 50.7 (NR) | 11.4 | 34.6 |
| Nguyen et al,17 2009 | Weight loss | QOL | 86 | 2 | 79 (92) | 41.8 | 20.0 | 45.5 (NR) | 9.8 | NR |
| Cohort | ||||||||||
| Gastric bypass | ||||||||||
| Kalfarentzos et al,23 2006 | Weight loss | Complications | 68 | 3 | 59 (87) | 68.3 | 17.3 | 44.9 (NR) | 14.2 | NR |
| Adams et al,6 2012 | Weight loss | Comorbidities | 418 | 5 | 379 (91) | 58.0 | 25.1 | 47.7 (NR) | 13 | 36.8 |
| Courcoulas et al,7 2013 | Weight loss | Comorbidities | 1738 | 3 | 1581 (91) | 69.9d | (NR) | 44.4d (IQR, 33–61) | NR | 31.5d |
| Gastric band | ||||||||||
| Basdevant et al,24 2007 | Weight loss | Complications | 946 | 2 | 876 (93) | 46.1 | (NR) | 43.6 (NR) | 9.9 | 27 |
| Phillips et al,8 2009 | Weight loss | Complications | 276 | 3 | 228 (83) | 42.8 | 25.4 | 44.5 (35–58.1) | 8.2 | NR |
| Van Nieuwenhove et al,25 2011 | Weight loss | Complications | 745 | 5 | 656 (88) | 46.2 | 36.5 | 41 (NR) | 7.8 | 22 |
| Courcoulas et al,7 2013 | Weight loss | Comorbidities | 610 | 3 | 567 (93) | 35d | (NR) | 43.9d (IQR, 40.4–48) | NR | 15.9d |
| Case Series | ||||||||||
| Gastric bypass | ||||||||||
| Pories et al,26 1995 | Weight loss | Diabetes, complications | 333 | 5 | 317 (91)b | 57.7 | 25.3 | 49.7 (33.9–101.6) | 16 | 44.6 |
| Czupryniak et al,27 2007 | Weight loss | NR | 68 | 2 | 68 (100) | 77.8 | 22.8 | 44.4 (33–61) | NR | 45.2 |
| Sekhar et al,28 2007 | Weight loss | Complications | 967 | 2 | 783 (81) | 69.3 | 17.0 | 53.1 (NR) | NR | NR |
| Yan et al,29 2008 | Weight loss | Comorbidities | 60 | 2 | 50 (83) | 51.1 | NR | 47.9 (NR) | NR | 37 |
| Hauser et al,30 2010 | Weight loss | Comorbidities | 70 | 2 | 66 (94) | 56.0 | NR | 50 (38–58) | NR | NR |
| Gastric band | ||||||||||
| Ponce et al,31 2005 | Weight loss | Complications | 77 | 3 | 68 (88) | 62.0 | 20.9 | 47.7 (35–84.4) | 16.1 | NR |
| Favretti et al,32 2007 | Weight loss | Complications | 843 | 5 | 765 (90) | 37.3 | 25.3 | 46.2 (NR) | 8.1 | 22.7 |
| Ray and Ray,33 2011 | Weight loss | NR | 82 | 5 | 66 (80) | 58 | NR | 47 (35–78) | NR | NR |
| Sleeve gastrectomy | ||||||||||
| Weiner et al,34 2007 | Weight loss | Complications | 69 | 2 | 60 (87) | 64 | 25.4 | 60.7 (NR) | 24.7 | NR |
| Kehagias et al,35 2013 | Weight loss | Complications, comorbidities | 65 | 4 | 55 (85) | 65 | NR | 43.2 (NR) | NR | NR |
Abbreviations: BMI, body mass index; EWL, excess weight loss; IQR, interquartile range; NR, not reported; QOL, quality of life.
The sample size, % follow-up, and %EWL apply to all patients in the treatment group listed and for the longest duration (column labeled “Follow-up, y”) having >80% follow-up.
Follow-up % for study overall; specific 5-year rate not reported.
Calculated as weight in kilograms divided by height in meters squared.
Median.
Weight loss data pooled for both band groups.
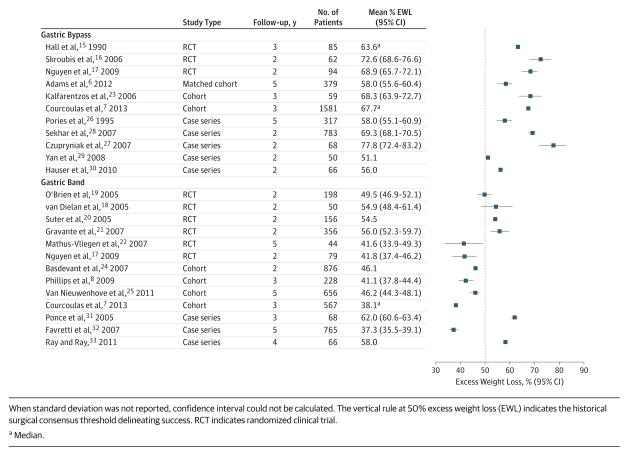
Gastric bypass resulted in greater weight loss than the gastric band (Figure). All gastric bypass (11/11) and sleeve gastrectomy (2/2) cohorts had 95% confidence intervals of the reported mean or median exceeding 50% excess weight loss. This only occurred in 31% (4/13) of gastric band cohorts. The sample-size–weighted mean excess weight loss was 65.7% after gastric bypass (n = 3544 patients, 6/11 prospective cohorts) compared with 45.0% after gastric band (n = 4109 patients, 9/13 prospective cohorts). The sample-size–weighted mean excess weight loss after sleeve gastrectomy was 64.5% (n = 115 patients, 2/2 retrospective cohorts). The sample-size–weighted mean EWL between 2 years vs 3 years or longer after surgery was significant within both gastric bypass (68.4% vs 64.5%; P < .001) and gastric band (49.4% vs 41.8%; P < .001).
Figure.
Long-term Excess Weight Loss After Gastric Bypass and Gastric Band Procedures
Gastric band studies reporting more than 50% excess weight loss by median, means, and 95% confidence intervals included 2 RCTs20,21 and 2 case series.31,33 The RCTs had shorter follow-up (2–2.9 years postsurgery) than the case series (≥3 years postsurgery). The remaining 9 studies had median or mean excess weight loss and 95% confidence intervals that were less than 50% excess weight loss.* Four gastric band studies did not provide either standard deviations needed to calculate confidence intervals20,33 or the 5% and 95% range7,24 for median weight loss.
Two case series studies described sleeve gastrectomy outcomes.34,35 One of these reported a standard deviation for calculation of a confidence interval. Sample-size–weighted mean %EWL was 64.5% for sleeve gastrectomy. There was no difference in %EWL between 2 vs 4 years after sleeve gastrectomy.
Improvement or Remission of Type 2 Diabetes
Six studies reporting on type 2 diabetes following bariatric surgery met inclusion criteria (Table 2). All studies reported remission rates defined as HbA1c less than 6.5% without medications (Table 3). Sample-size–weighted remission rates were 66.7% after gastric bypass (n = 428) and 28.6% after gastric band (n = 96) for type 2 diabetes. Half of the studies (3/6; 1 RCT after gastric bypass,11 2 prospective cohort after gastric band8,10) reported mean HbA1c levels with standard deviations before and after surgery. There was no overlap of confidence intervals for mean HbA1c values before and after surgery. The sample-size–weighted mean decrease in HbA1c was 2.2% after gastric bypass (n = 20 patients) and 1.5% after gastric band (n = 54 patients). The 2 studies reporting mean fasting blood glucose (1 gastric bypass,11 1 gastric band10) showed reduction to less than 126 mg/dL at least 2 years after surgery.
Table 2.
Improvement or Remission of Type 2 Diabetes After Bariatric Surgery: Study Characteristics and Laboratory Data
| Source | Study Type | No. of Patients With Diabetes at Baseline |
Follow- up, y |
Diabetes Diagnostic Criteria |
Mean (SD) [95% CI] | |||
|---|---|---|---|---|---|---|---|---|
| Baseline HbA1c, % |
Follow-up HbA1c, % |
Baseline FBG or FPG, mg/dL |
Follow-up FBG or FPG, mg/dL |
|||||
| Roux-en-Y Gastric Bypass | ||||||||
| Mingrone et al,11 2012 | RCT | 20 | 2 | History of diabetes for ≥5 y with HbA1c ≥7% | 8.6 (1.4) [8.0–9.2] | 6.4 (1.8) [5.6–7.2] | 172.0 (60.3) [145.6–198.4] | 114.7 (13) [108.7–120.7] |
| Adams et al,6 2012 | Matched cohort | 88 | 5 | FBG ≥126 mg/dL, HbA1c ≥6.5%, or medication | NR | NR | NR | NR |
| Courcoulas et al,7,2013 | Prospective cohort | 320 | 3 | FBG ≥126 mg/dL, HbA1c ≥6.5%, or medication | NR | NR | NR | NR |
| Laparoscopic Adjustable Gastric Band | ||||||||
| Caiazzo et al,10 2010 | Prospective cohort | 23 | 5 | ADA criteria; FBG >126 mg/dL ×2 and/or medication | 8.3 (1.6) [7.7–9.0] | 6.6 (0.9) [6.2–7.0]0 | 173.0 (54.1) [150.9–195.1]0 | 118.9 (19.8) [110.8–127.0]0 |
| Phillips et al,8 2009 | Prospective cohort | 31 | 3 | Documented diabetes and elevated HbA1c at baseline | 8.0 (1.3) [7.5–8.5]0 | 6.7 (1.0) [6.4–7.0]0 | NR | NR |
| Courcoulas et al,7 2013 | Prospective cohort | 98 | 3 | FBG ≥126 mg/dL, HbA1c ≥6.5%, or medication | NR | NR | NR | NR |
| Sultan et al,12 2010 | Case series | 95 | 4 | ADA 2003 criteria | 7.5 | 6.6 | 146 | 118.5 |
Abbreviations: ADA, American Diabetes Association; FBG, fasting blood glucose; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; NR, not reported; RCT, randomized clinical trial.
SI conversion factor: To convert glucose to mmol/L, multiply by 0.0555.
Table 3.
Type 2 Diabetes After Bariatric Surgery: Remission Characteristics of Each Study
| Source | No. of Patients With Diabetes at Baseline | Mean Change in HbA1c, % | Change in Mean FBG or FPG, mg/dL | No. (%) of Patients in Remission at Study End and Study Definition of Remission |
|---|---|---|---|---|
| Roux-en-Y Gastric Bypass | ||||
| Mingrone et al,11 2012 | 20 | 2.2 | 57.3 | 15/20 (75) patients in remission; HbA1c <6.5% and FPG <100 mg/dL without medication |
| Adams et al,6 2012 | 88 | NR | NR | 54/87 (62) patients in remission; normal HbA1c and FBG without medication |
| Courcoulas et al,7,2013 | 320 | NR | NR | 216/320 (68) patients in remission |
| Laparoscopic Adjustable Gastric Band | ||||
| Caiazzo et al,10 2010 | 23 | 1.7 | 54.1 | Preop: 43% patients with 1 medication, 43% with ≥2 medications; postop: 23% with 1 medication, 50% with ≥2 medications |
| Phillips et al,8 2009 | 31 | 1.3 | NR | NR |
| Courcoulas et al,7 2013 | 98 | NR | NR | 28/98 (29) patients in remission |
| Sultan et al,12 2010 | 95 | 0.9 | 27.5 | Significant decrease in use of oral medications and insulin for group; 23% required ≥1 fewer medications; 54% without medication requirement |
Abbreviations: preop, preoperative; FBG, fasting blood glucose; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; NR, not reported; postop, postoperative.
SI conversion factor: To convert glucose to mmol/L, multiply by 0.0555.
The single RCT measuring remission of type 2 diabetes 2 years after gastric bypass used a composite primary end point of HbA1c less than 6.5% for at least 1 year without pharmacologic therapy and fasting plasma glucose less than 100 mg/dL (to convert glucose to mmol/L, multiply by 0.0555). The trial’s remission rate was 75% with a mean baseline HbA1c 8.6% decreasing to 6.4% after surgery (no overlap of confidence intervals). All patients had type 2 diabetes for a minimum of 5 years.
Improvement or Remission of Hypertension
Three studies reporting on hypertension after bariatric surgery (1RCT after gastric bypass, 2 prospective cohorts after gastric band) met inclusion criteria.7,9,14 Two of 3 studies reported remission rates for hypertension of 38.2% after gastric bypass (n = 808 patients)7,14 and 17.4% after gastric band (n = 247 patients).7 Remission was defined as blood pressure less than 140/90 without medications. Two studies after gastric bypass (n = 132 patients) reported mean systolic and diastolic blood pressures with standard deviations before and after surgery. One of the studies showed no overlap of confidence intervals for systolic blood pressures,14 and both studies showed overlap of confidence intervals for diastolic blood pressures,9,14 before vs after surgery.
Improvement or Remission of Hyperlipidemia
Three studies reporting on hyperlipidemia after bariatric surgery (2 prospective cohorts after gastric bypass, 1 prospective cohort after gastric bypass and band) met inclusion criteria.7,9,13,14 The studies reported remission rates of 60.4% after gastric bypass (n = 477 patients) and 22.7% after gastric band (n = 97). Remission of hyperlipidemia was defined as cholesterol less than 200 mg/dL, HDL greater than 40 mg/dL, LDL less than 160 mg/dL, and TG less than 200 mg/dL. Studies (except 1 reporting on hypertriglyceridemia) did not report lipid panel laboratory values. No studies meeting criteria reported lipid-lowering medication usage.
Complications
Half of the studies included for weight loss (4 gastric bypass, 8 gastric band, and 2 sleeve gastrectomy) reported on complications at least 2 years after surgery. Complications were the primary outcome in 4 studies. Prospective cohorts of gastric bypass (n = 1796 patients) and gastric band (n = 2510 patients) reported long-term deaths of 1% and 0.2% respectively. Complications rates after gastric bypass of incisional hernia, internal hernia, or marginal ulcer were 1% each; anemia, iron deficiency requiring transfusion, or vitamin B12 deficiency were 2% each. Operative revision rates for abdominal pain or nonhealing ulcer were each 0.1%. The gastrointestinal bleeding rate was less than 1%. Complications rates after gastric band were port leak/revision, 6%; band slip/obstruction, 5%; erosion, 1%; treatment failure requiring revision, 3%; band removal, 2%; and esophageal dilation or esophagitis, 1%. Retrospective cohorts showed higher complication rates for gastric bypass (3- to 20-fold; n = 674 patients) and gastric band (2.5- to 5-fold; n = 1489 patients). The retrospective gastric bypass cohorts were largely performed by the open technique (90% vs 12% in the prospective cohorts) and much earlier (1995 vs after 2006). The retrospective gastric band cohorts reported on greater numbers of bands placed by the perigastric technique (43% vs 5% in the prospective cohorts). Retrospective sleeve gastrectomy cohorts (n = 174 patients) reported late complication rates of death, 5%; incisional hernia, 4%; treatment failure requiring operative revision, 7%; and gastroesophageal reflux, 2%.
Study Quality
Thirteen cohorts included for weight loss outcomes were studied prospectively (8 RCTs, 1 matched cohort, 4 prospective cohort), and 11 were studied retrospectively (1 cohort, 10 case series). There was no meaningful difference in sample-size–weighted mean %EWL between prospective and retrospective cohorts within either gastric bypass (66.1% vs 65.0%) or gastric band (46.2% vs 43.0%). Weight loss was the primary outcome in 20 of 24 studies (83%).
Diabetes improvement or remission was the primary outcome for half of the studies (3/6) included. Eighty-three percent of the cohorts were studied prospectively (5/6 studies: 1 RCT, 1 matched cohort, 3 prospective cohorts, 1 case series). No comparison could be made between prospective vs retrospective results secondary to heterogeneous reporting of outcomes. All cohorts included for hypertension and hyperlipidemia outcomes were prospective. The outcomes of interest in all these studies were secondary.
Discussion
Eleven hundred thirty-six of 7371 studies (16%) reported outcomes more than 2 years after bariatric surgery. Of the 1136 studies, 29 (<3%) reported weight loss outcomes for more than 80% of the original cohort. Obesity is a chronic disease, and because bariatric surgery is a major and often times irreversible intervention, out-comes from these procedures should be assessed for long-term effects. To reliably assess how bariatric surgery performs over time, researchers must follow up the majority of a study group to minimize bias toward overly optimistic estimates of the interventions’ effectiveness.
Weight regain may be a factor associated with drop out from weight loss studies, highlighting the importance of maintaining near complete follow-up. For example, a bariatric surgery outcome study reported treatment failure rates of 42% when 61% of the initial cohort was followed up 8 years after surgery.37 After implementing unusually intense efforts to locate patients who had dropped out of the study, the investigators found a 60% treatment failure rate for patients initially classified as lost to follow-up. Substantial risks exist for arriving at overly optimistic conclusions regarding the effect of a weight loss intervention when follow-up is incomplete. Because of incomplete follow-up, most bariatric surgery studies may report overly optimistic estimates for these operations’ effects.
The ideal follow-up is 80% or greater of any original cohort,38,39 and this is rarely achieved in bariatric surgery outcome studies. Very few bariatric surgery studies were found with 80% or greater follow-up at its longest follow-up duration, including the most cited bariatric cohort in the literature.40,41 The extent of attrition may or may not bias weight loss outcome studies.39 If attrition occurs randomly, it can be modeled to minimize the effect of attrition on study results. Protocols for handling missing data and dropouts should be developed and adopted for weight loss studies.
We identified 184 systematic reviews of bariatric surgery outcomes. Three reviews included only studies with greater than 3 to 5 years’ duration. None of these reviews accounted for completeness of follow-up when evaluating study quality. It is likely these reviews overestimated the efficacy of bariatric surgery because these parameters were not accounted for. Incorporating completeness and duration of follow-up as a study quality assessment in systematic reviews of weight loss may help limit the substantial risk of bias introduced by incomplete follow-up.
Proponents of gastric band claim equivalent weight loss to gastric bypass if sufficient follow-up duration is available.42 Published evidence suggests otherwise. When the procedures were compared in RCTs with relatively short follow-up to cohort and case series studies with longer follow-up, weight loss for gastric bypass was consistently greater than for gastric band. Irrespective of study design (eg, prospective or retrospective), mean %EWL 2 to 5 years after gastric bypass was more than 50% in all 11 studies examined. In contrast, for gastric band, mean %EWL after 2 to 5 years was less than 50% in 9 of 13 studies (69%). Limiting the evidence to studies with reliable long-term follow-up suggests long-term weight loss for gastric bypass is greater than for gastric band in the long-term. Despite the increasing popularity of sleeve gastrectomy, we found only 2 studies reporting weight loss outcomes for more than 2 years in sufficiently large cohorts with adequate follow-up to assess sleeve gastrectomy outcomes.
Improvements in the obesity-related comorbidities type 2 diabetes, hypertension, and hyperlipidemia were mostly reported as secondary outcomes in bariatric surgery studies. Secondary outcome analyses may be a reliable measure of an intervention’s effect when studies are sufficiently powered to answer secondary questions. Even when comorbidity remission is the primary outcome, other design problems may weaken a conclusion. For example, 1 randomized trial having a primary end point of type 2 diabetes remission rates showed gastric bypass was more effective than conventional medical therapy 2 years after surgery.11 Gastric bypass yielded a 75% compared with zero remission rate of diabetes in the medical group. The clinical relevance of this comparison is uncertain because the medical group treatments were not intended to result in diabetes remission.
Hypertension or hyperlipidemia remission was observed for some patients 2 years or later after gastric bypass and band. Most of the studies we reviewed reporting hyperlipidemia outcomes did not report laboratory information, and none assessed medication usage. Similar to assessing true diabetes remission outcomes, lack of knowledge of medication usage precludes definitive conclusions being made for the long-term effects of bariatric surgery on hyperlipidemia. Moreover, medication usage when reported for hyperlipidemia may not indicate lipid disease. Statins are frequently prescribed irrespective of lipid levels for other beneficial effects.43
Long-term complications requiring treatment were relatively low (≤3% after gastric bypass,≤6% after gastric band) for the studies included. Long-term mortality was similar to published short-term mortality for gastric bypass and band (1% and 0.2%, respectively). Mortality and morbidity rates reported for sleeve gastrectomy were assessed in a smaller sample size and earlier in the use of the procedure compared with gastric bypass or band. The short-term morbidity and mortality (≤1 year) of bariatric surgery have been extensively documented.44,45
Randomized clinical trials establish the effect size of treatments.46 Matched cohorts, prospective cohorts, and case series designs, in contrast, may yield less accurate treatment effect sizes.47 We did not find differences attributable to study design in effect sizes for the cohorts included in this review. Conceivably, when an effect size is large, as is the weight loss from bariatric surgery, its required demonstration in RCTs to substantiate widespread use of the intervention becomes less important. A large effect size on the short-term, however, requires testing long-term to assess true treatment value when the disease is chronic.
Conclusions
Studies of bariatric surgery long-term outcomes demonstrate substantial and sustained weight loss for gastric bypass procedures exceeding that for gastric band. There are few long-term studies with similar reliable follow-up for gastric sleeve operations. Flawed study design and incomplete assessment and reporting limit conclusions being drawn from most studies that had reasonable follow-up. To fully characterize the efficacy of bariatric surgery, long-term outcomes studies should report results for at least 80% of initial cohorts and with follow-up exceeding 2 years.
Supplementary Material
Acknowledgments
Funding/Support: The Department of Surgery of the University of Texas Southwestern Medical Center, Dallas, provided funding for all aspects of the preparation of this manuscript. A grant from the National Institutes of Health National Center for Advancing Translational Sciences (NIH/NCATS) (KL2TR001103) supported part of the primary author’s salary, allowing time to conceive and prepare the manuscript.
Role of Funders/Sponsors: The Department of Surgery, University of Texas Southwestern Medical Center, Dallas, provided administrative assistance and protected time to conduct all aspects of this project. NIH/NCATS provided salary support for protected time to conduct all aspects of this project in its initial year. NIH/NCATS also provided funding for training in all aspects of conducting clinical research culminating in attainment of a Master’s of Clinical Science degree. The training enabled competency required for the conception, data collection, analyses, interpretation of results, and writing of this manuscript.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Publisher's Disclaimer: Disclaimer: Dr Livingston, a JAMA Deputy Editor, did not participate in the review of or decision to publish this article.
Additional Contributions: We thank John Rush, MD, and Milton Packer, MD, Department of Clinical Science, Internal Medicine, University of Texas Southwestern Medical Center, Dallas, for consulting on bias within clinical study designs and analyses of outcome measures. He received no compensation for his contribution.
Author Contributions: Dr Puzziferri had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Puzziferri, Livingston.
Acquisition, analysis, or interpretation of data: Puzziferri, Roshek, Mayo, Gallagher, Belle.
Drafting of the manuscript: Puzziferri, Mayo, Gallagher, Livingston.
Critical revision of the manuscript for important intellectual content: Puzziferri, Roshek, Mayo, Belle, Livingston.
Statistical analysis: Puzziferri, Gallagher.
Obtained funding: Puzziferri.
Administrative, technical, or material support: Puzziferri, Roshek, Mayo, Gallagher, Livingston.
Study supervision: Puzziferri, Livingston.
References
- 1.Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev. 2009;(2):CD003641. doi: 10.1002/14651858.CD003641.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Graefen M. Low quality of evidence for robot-assisted laparoscopic prostatectomy: a problem not only in the robotic literature. Eur Urol. 2010;57(6):938–940. doi: 10.1016/j.eururo.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 4.Body mass index table 1. National Heart, Lung, and Blood Institute; [Accessed July 29, 2014]. http://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_tbl.htm. [Google Scholar]
- 5.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4):427–436. doi: 10.1007/s11695-012-0864-0. [DOI] [PubMed] [Google Scholar]
- 6.Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courcoulas AP, Christian NJ, Belle SH, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips E, Ponce J, Cunneen SA, et al. Safety and effectiveness of Realize adjustable gastric band: 3-year prospective study in the United States. Surg Obes Relat Dis. 2009;5(5):588–597. doi: 10.1016/j.soard.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Søvik TT, Aasheim ET, Taha O, et al. Weight loss, cardiovascular risk factors, and quality of life after gastric bypass and duodenal switch: a randomized trial. Ann Intern Med. 2011;155(5):281–291. doi: 10.7326/0003-4819-155-5-201109060-00005. [DOI] [PubMed] [Google Scholar]
- 10.Caiazzo R, Arnalsteen L, Pigeyre M, et al. Long-term metabolic outcome and quality of life after laparoscopic adjustable gastric banding in obese patients with type 2 diabetes mellitus or impaired fasting glucose. Br J Surg. 2010;97(6):884–891. doi: 10.1002/bjs.6993. [DOI] [PubMed] [Google Scholar]
- 11.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 12.Sultan S, Gupta D, Parikh M, et al. Five-year outcomes of patients with type 2 diabetes who underwent laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2010;6(4):373–376. doi: 10.1016/j.soard.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 13.Vagenas K, Panagiotopoulos S, Kehagias I, Karamanakos SN, Mead N, Kalfarentzos F. Prospective evaluation of laparoscopic Roux en Y gastric bypass in patients with clinically severe obesity. World J Gastroenterol. 2008;14(39):6024–6029. doi: 10.3748/wjg.14.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams TD, Pendleton RC, Strong MB, et al. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity (Silver Spring) 2010;18(1):121–130. doi: 10.1038/oby.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall JC, Watts JM, O’Brien PE, et al. Gastric surgery for morbid obesity: the Adelaide Study. Ann Surg. 1990;211(4):419–427. doi: 10.1097/00000658-199004000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skroubis G, Anesidis S, Kehagias I, Mead N, Vagenas K, Kalfarentzos F. Roux-en-Y gastric bypass versus a variant of biliopancreatic diversion in a non-superobese population: prospective comparison of the efficacy and the incidence of metabolic deficiencies. Obes Surg. 2006;16(4):488–495. doi: 10.1381/096089206776327251. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen NT, Slone JA, Nguyen XM, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg. 2009;250(4):631–641. doi: 10.1097/SLA.0b013e3181b92480. [DOI] [PubMed] [Google Scholar]
- 18.van Dielen FM, Soeters PB, de Brauw LM, Greve JW. Laparoscopic adjustable gastric banding versus open vertical banded gastroplasty: a prospective randomized trial. Obes Surg. 2005;15(9):1292–1298. doi: 10.1381/096089205774512456. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien PE, Dixon JB, Laurie C, Anderson M. A prospective randomized trial of placement of the laparoscopic adjustable gastric band: comparison of the perigastric and pars flaccida pathways. Obes Surg. 2005;15(6):820–826. doi: 10.1381/0960892054222858. [DOI] [PubMed] [Google Scholar]
- 20.Suter M, Giusti V, Worreth M, Héraief E, Calmes JM. Laparoscopic gastric banding: a prospective, randomized study comparing the Lapband and the SAGB: early results. Ann Surg. 2005;241(1):55–62. doi: 10.1097/01.sla.0000150071.86934.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravante G, Araco A, Araco F, Delogu D, De Lorenzo A, Cervelli V. Laparoscopic adjustable gastric bandings: a prospective randomized study of 400 operations performed with 2 different devices. Arch Surg. 2007;142(10):958–961. doi: 10.1001/archsurg.142.10.958. [DOI] [PubMed] [Google Scholar]
- 22.Mathus-Vliegen EM, de Wit LT. Health-related quality of life after gastric banding. Br J Surg. 2007;94(4):457–465. doi: 10.1002/bjs.5607. [DOI] [PubMed] [Google Scholar]
- 23.Kalfarentzos F, Skroubis G, Kehagias I, Mead N, Vagenas K. A prospective comparison of vertical banded gastroplasty and Roux-en-Y gastric bypass in a non-superobese population. Obes Surg. 2006;16(2):151–158. doi: 10.1381/096089206775565096. [DOI] [PubMed] [Google Scholar]
- 24.Basdevant A, Paita M, Rodde-Dunet MH, et al. A nationwide survey on bariatric surgery in France: two years prospective follow-up. Obes Surg. 2007;17(1):39–44. doi: 10.1007/s11695-007-9004-7. [DOI] [PubMed] [Google Scholar]
- 25.Van Nieuwenhove Y, Ceelen W, Stockman A, et al. Long-term results of a prospective study on laparoscopic adjustable gastric banding for morbid obesity. Obes Surg. 2011;21(5):582–587. doi: 10.1007/s11695-010-0341-6. [DOI] [PubMed] [Google Scholar]
- 26.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? an operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–350. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czupryniak L, Pawlowski M, Kumor A, Szymanski D, Loba J, Strzelczyk J. Predicting maximum Roux-en-Y gastric bypass-induced weight reduction: preoperative plasma leptin or body weight? Obes Surg. 2007;17(2):162–167. doi: 10.1007/s11695-007-9042-1. [DOI] [PubMed] [Google Scholar]
- 28.Sekhar N, Torquati A, Youssef Y, Wright JK, Richards WO. A comparison of 399 open and 568 laparoscopic gastric bypasses performed during a 4-year period. Surg Endosc. 2007;21(4):665–668. doi: 10.1007/s00464-006-9151-2. [DOI] [PubMed] [Google Scholar]
- 29.Yan E, Ko E, Luong V, Wang HJ, Romanova M, Li Z. Long-term changes in weight loss and obesity-related comorbidities after Roux-en-Y gastric bypass: a primary care experience. Am J Surg. 2008;195(1):94–98. doi: 10.1016/j.amjsurg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 30.Hauser DL, Titchner RL, Wilson MA, Eid GM. Long-term outcomes of laparoscopic Roux-en-Y gastric bypass in US veterans. Obes Surg. 2010;20(3):283–289. doi: 10.1007/s11695-009-0042-1. [DOI] [PubMed] [Google Scholar]
- 31.Ponce J, Paynter S, Fromm R. Laparoscopic adjustable gastric banding: 1,014 consecutive cases. J Am Coll Surg. 2005;201(4):529–535. doi: 10.1016/j.jamcollsurg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Favretti F, Segato G, Ashton D, et al. Laparoscopic adjustable gastric banding in 1,791 consecutive obese patients: 12-year results. Obes Surg. 2007;17(2):168–175. doi: 10.1007/s11695-007-9043-0. [DOI] [PubMed] [Google Scholar]
- 33.Ray JB, Ray S. Safety, efficacy, and durability of laparoscopic adjustable gastric banding in a single surgeon US community practice. Surg Obes Relat Dis. 2011;7(2):140–144. doi: 10.1016/j.soard.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Weiner RA, Weiner S, Pomhoff I, Jacobi C, Makarewicz W, Weigand G. Laparoscopic sleeve gastrectomy: influence of sleeve size and resected gastric volume. Obes Surg. 2007;17(10):1297–1305. doi: 10.1007/s11695-007-9232-x. [DOI] [PubMed] [Google Scholar]
- 35.Kehagias I, Spyropoulos C, Karamanakos S, Kalfarentzos F. Efficacy of sleeve gastrectomy as sole procedure in patients with clinically severe obesity (BMI ≤50 kg/m(2)) Surg Obes Relat Dis. 2013;9(3):363–369. doi: 10.1016/j.soard.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Suter M, Calmes JM, Paroz A, Giusti V. A 10-year experience with laparoscopic gastric banding for morbid obesity: high long-term complication and failure rates. Obes Surg. 2006;16(7):829–835. doi: 10.1381/096089206777822359. [DOI] [PubMed] [Google Scholar]
- 37.te Riele WW, Boerma D, Wiezer MJ, Borel Rinkes IH, van Ramshorst B. Long-term results of laparoscopic adjustable gastric banding in patients lost to follow-up. Br J Surg. 2010;97(10):1535–1540. doi: 10.1002/bjs.7130. [DOI] [PubMed] [Google Scholar]
- 38.Fewtrell MS, Kennedy K, Singhal A, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child. 2008;93(6):458–461. doi: 10.1136/adc.2007.127316. [DOI] [PubMed] [Google Scholar]
- 39.Kristman V, Manno M, Côté P. Loss to follow-up in cohort studies: how much is too much? Eur J Epidemiol. 2004;19(8):751–760. doi: 10.1023/b:ejep.0000036568.02655.f8. [DOI] [PubMed] [Google Scholar]
- 40.Sjöström L, Narbro K, Sjöström CD, et al. Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 41.Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257(1):87–94. doi: 10.1097/SLA.0b013e31827b6c02. [DOI] [PubMed] [Google Scholar]
- 43.Fleisher LA, Beckman JA, Brown KA, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine; Society for Vascular Surgery. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54(22):e13–e118. doi: 10.1016/j.jacc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Flum DR, Belle SH, King WC, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–420. doi: 10.1097/SLA.0b013e31822c9dac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalmers TC, Matta RJ, Smith H, Jr, Kunzler AM. Evidence favoring the use of anticoagulants in the hospital phase of acute myocardial infarction. N Engl J Med. 1977;297(20):1091–1096. doi: 10.1056/NEJM197711172972004. [DOI] [PubMed] [Google Scholar]
- 47.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.