Abstract
Muscles induce large forces in the tibiofemoral joint during walking and thereby influence the health of tissues like articular cartilage and menisci. It is possible to walk with a wide variety of muscle coordination patterns, but the effect of varied muscle coordination on tibiofemoral contact forces remains unclear. The goal of this study was to determine the effect of varied muscle coordination on tibiofemoral contact forces. We developed a musculoskeletal model of a subject walking with an instrumented knee implant. Using an optimization framework, we calculated the tibiofemoral forces resulting from muscle coordination that reproduced the subject’s walking dynamics. We performed a large set of optimizations in which we systematically varied the coordination of muscles to determine the influence on tibiofemoral force. Model-predicted tibiofemoral forces arising with minimum muscle activation matched in vivo forces measured during early stance, but were greater than in vivo forces during late stance. Peak tibiofemoral forces during late stance could be reduced by increasing the activation of the gluteus medius, uniarticular hip flexors, and soleus, and by decreasing the activation of the gastrocnemius and rectus femoris. These results suggest that retraining of muscle coordination could substantially reduce tibiofemoral forces during late stance.
Keywords: tibiofemoral force, walking, coordination, muscle activity, knee
The knee experiences large mechanical loads during activities of daily living. Walking, for example, induces forces as large as three bodyweights at the knee.1,2 These loads affect the development, maintenance, and health of the joint tissues.3 The onset and progression of osteoarthritis can be associated with large loads at the knee,4,5 and increased knee loads have been linked to pain in patients with osteoarthritis.6 Since tibiofemoral loads during walking are produced primarily by muscle forces,7,8 muscle coordination plays a pivotal role in determining tibiofemoral loads. Identifying muscle coordination patterns that alter tibiofemoral loads may assist in the design of rehabilitation programs to restore and maintain the health of the knee.
Training and rehabilitation programs can reduce tibiofemoral loads during walking by altering gait kinematics. Fregly et al.9 demonstrated that adopting a “medial thrust” gait reduced medial compartment forces measured in vivo using instrumented knee replacements. Strategies altering foot progression angle and medio-lateral foot placement during the stance phase of walking reduce the net knee adduction moment10–12 and knee pain.13 Exaggerated trunk sway in the mediolateral direction during walking can reduce net knee adduction moments.14 While previous work demonstrates that altering gait kinematics can reduce knee loads during walking, the effects of altered muscle coordination on tibiofemoral loads remain unclear.
Studying the effects of altered muscle coordination on tibiofemoral loads is challenging. Direct measurement of tibiofemoral loads during walking requires implanting instrumented knee prostheses in living subjects.1,2,15 This technique provides valuable data, but is highly invasive, making measurement of tibiofemoral loads impractical in healthy subjects and limiting the number of subjects in which knee loads can be measured. An alternative to direct measurement is calculating tibiofemoral loads using musculoskeletal modeling. Model-based studies have estimated tibiofemoral loads using a variety of muscle coordination strategies, including minimizing muscle activity,16,17 muscle stress,18 or energy consumption.19 Previous studies have determined a single set of muscle forces during walking and the resulting tibiofemoral loads, but the changes in tibiofemoral loads arising from variations in muscle activations remains unknown.
The purpose of this study was to determine the changes in tibiofemoral forces due to variations in muscle activation patterns. We first evaluated whether a commonly assumed muscle coordination strategy, minimizing the sum of muscle activations squared,20 produced tibiofemoral forces that were consistent with in vivo measurements. We next determined the potential for a subject to decrease tibiofemoral forces during walking by adopting a muscle coordination strategy that minimized tibiofemoral forces. Finally, we determined the changes in tibiofemoral forces due to varied activations of individual muscles of the lower limb and identified the muscles with the greatest potential to alter tibiofemoral loading.
MATERIALS AND METHODS
Human Subject Data
We used walking data of a subject implanted with an instrumented total knee replacement (TKR). These data are available from the ASME Grand Challenge Project.1 The subject (83-year-old male, 64 kg, 166 cm tall) had received bilateral TKR. The right TKR was instrumented to measure tibiofemoral forces normal to the tibial plateau.15 The data include three-dimensional marker positions, ground reaction forces, and tibiofemoral forces measured simultaneously during walking at the subject’s self-selected speed (1.3 m/s).
OpenSim Model
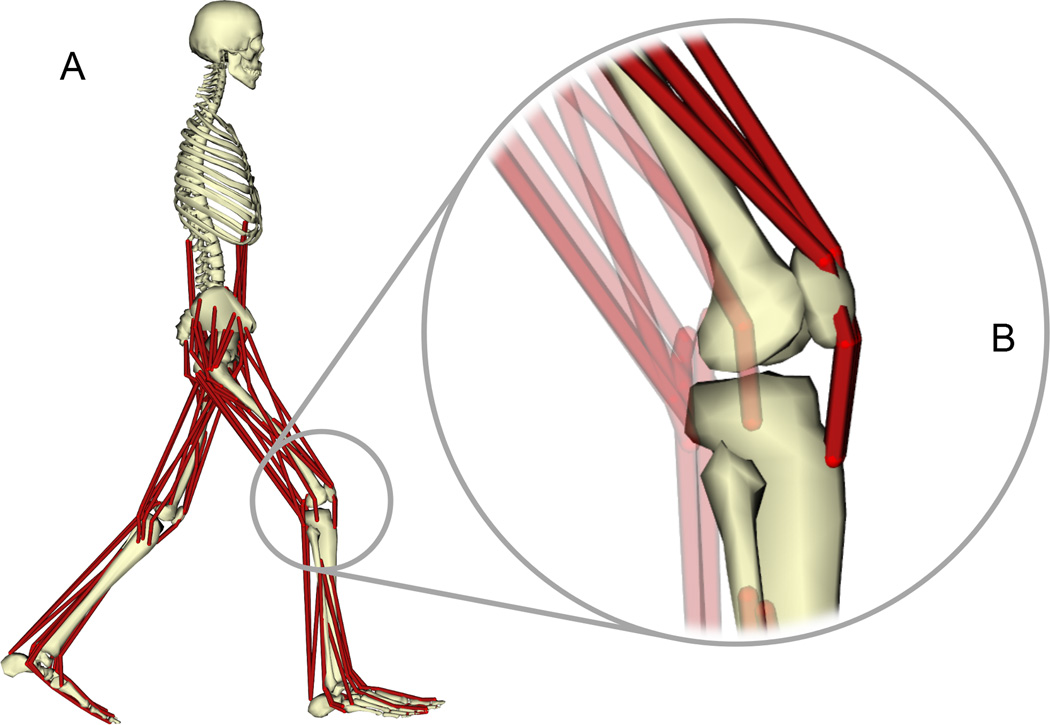
We created a full-body gait model in OpenSim21 to analyze knee loads. The 10 segment, 19 degree of freedom (dof) model was adapted from a musculoskeletal model of the lower limb published by Delp et al.22 (Fig. 1A). The model was driven by 92 muscle-tendon actuators20 that captured force-length-velocity properties, with muscle geometry and architecture based on adult cadaver data.22 A ball-and-socket joint connected the torso to the pelvis. The right and left lower limbs consisted of a ball-and-socket hip joint, a revolute ankle joint, and a coupled knee mechanism (1 dof) with translations of the tibia and patella prescribed by the knee flexion angle. We refined the knee mechanism of the generic model so that the patella articulated with the femur, and the quadriceps wrapped around the patella before attaching to the tibial tuberosity (Fig. 1B). The patella functioned as a frictionless pulley that redirected the quadriceps forces to act along the line of action of the patellar ligament. This refined knee mechanism reproduced the patellofemoral kinematics in Delp et al.22 and enabled resultant tibiofemoral forces to be computed.
Figure 1.
(A) Musculoskeletal model of the human legs and torso. The tibiofemoral and patellofemoral joints were modeled as planar joints with translations and rotations coupled to the knee flexion angle (B). Forces in the quadriceps (B, dark red) were transmitted through the patella to the tibia (see Methods section for details).
We used the full-body model to simulate 3D walking dynamics of the instrumented subject. All joint kinematics, muscle attachments, and the resulting muscle moment arms were scaled to match the segment lengths of the subject. Additionally, the optimal fiber length and tendon slack length of each muscle were scaled according to the muscle’s total change in muscle-tendon length. Other muscle parameters, including peak isometric forces and pennation angles, were not altered. We determined joint kinematics for five trials of normal walking by minimizing error between the experimentally measured marker positions and the corresponding markers on the model. A residual reduction algorithm 21 adjusted the model mass properties and joint kinematics for each trial to ensure that the ground reaction forces and body segment accelerations were dynamically consistent. After residual reduction, the model segment masses differed by less than 2% from the scaled model and the resulting joint kinematics differed by less than 2° from the kinematics tracked by the residual reduction algorithm.
Optimization
We developed a static optimization framework in OpenSim to calculate individual muscle forces and resulting tibiofemoral forces for each trial. This optimization minimized a sum of muscle activations and joint loads by combining them in a single objective function:
| (1) |
subject to the constraint
| (2) |
In the objective function, ai was the activation of the ith muscle, which could vary between 0 and 1. The activation weight, wi, was a weighting constant set to penalize activation of the ith muscle. The joint force weighting constants, , and were set to penalize the vector components of the jth joint reaction force, F⃑j. Similarly, the joint moment weighting constants, , and were set to penalize the vector components of the jth joint reaction moment, M⃑j. The joint reaction forces and moments represented the resultant loads carried by the articulating joint structures, and were calculated using the joint reaction analysis in OpenSim.17
We constrained the optimization such that the calculated muscle forces and measured external forces balanced all inertial forces to reproduce the measured walking motion (Equation 2). F⃑i(ai) represented the force applied by muscle i due to its activation, ai. The external forces included forces due to gravity and ground reactions at the feet. The system mass matrix, M(q⃑), was a function of the measured generalized coordinates, q⃑. The velocity-dependent forces, , included centripetal and Coriolis forces. The kinematic constraint forces included forces due to coupling between patellofemoral and tibiofemoral kinematics. Equation (2) guaranteed that the optimized muscle activations reproduced the walking kinematics and ground reaction forces measured from the instrumented TKR subject.
A Muscle Coordination Pattern Minimizing Muscle Activations
We simulated five trials of normal walking with a muscle coordination pattern that minimized muscle activations. In this case, the generalized objective function (Equation 1) simplified to
| (3) |
The activation weight, w, was set to 1 for all muscles to penalize all muscle activations uniformly. The joint force and moment weighting constants were set to zero so that joint loads were not penalized. This optimization strategy has been used in previous studies.16,23
A Muscle Coordination Pattern Minimizing Compressive Tibiofemoral Force
We simulated the five trials of normal walking with a muscle coordination pattern that minimized the compressive force in the tibiofemoral joint of the instrumented leg. In this case, the generalized objective function (Equation 1) simplified to
| (4) |
which is equivalent to
| (5) |
The activation weight, w, was set to zero for all muscles so that muscle activations were not penalized. The compressive tibiofemoral force was defined as the vector component of the tibiofemoral force acting normal to the tibial plateau, FTF,y. The compressive tibiofemoral force was penalized by setting its weighting constant, wFy, equal to 1; all other joint force and moment weighting constants were set to zero. This strategy determined the muscle coordination pattern that minimized the tibiofemoral forces and matched the measured walking dynamics.
Change in Tibiofemoral Forces Due to Varied Activations of Individual Muscles
We determined the change in tibiofemoral forces due to varied activations of individual muscles of the lower limb by performing optimizations with varied activation weighting constants, wi, for each muscle. For these optimizations, the generalized objective function (Equation 1) was simplified:
| (6) |
In Equation 6, w = 0 represented no penalty to activate a muscle during walking, while w = 100 prohibited activation of a muscle.
To investigate the change in tibiofemoral forces due to varied activation of a muscle, we performed two static optimizations for each trial of normal walking. First, to prohibit activation of a particular muscle, we performed a static optimization with w = 100 for that muscle while w for all other muscles was held at 1. Second, to promote activation of a particular muscle, we performed a static optimization with w = 0 for that muscle while w for all other muscles was held at 1. Performing two static optimizations for each muscle of the lower limb determined the range of tibiofemoral forces due to varying activation of that muscle. We determined the change in peak tibiofemoral force due to activation of a muscle by calculating the difference between peak tibiofemoral forces obtained from the static optimizations with w = 0 and w = 100.
Our methods produced similar joint moments, muscle activations, and tibiofemoral forces for all five walking trials; thus, we have included results from one representative trial for clarity.
RESULTS
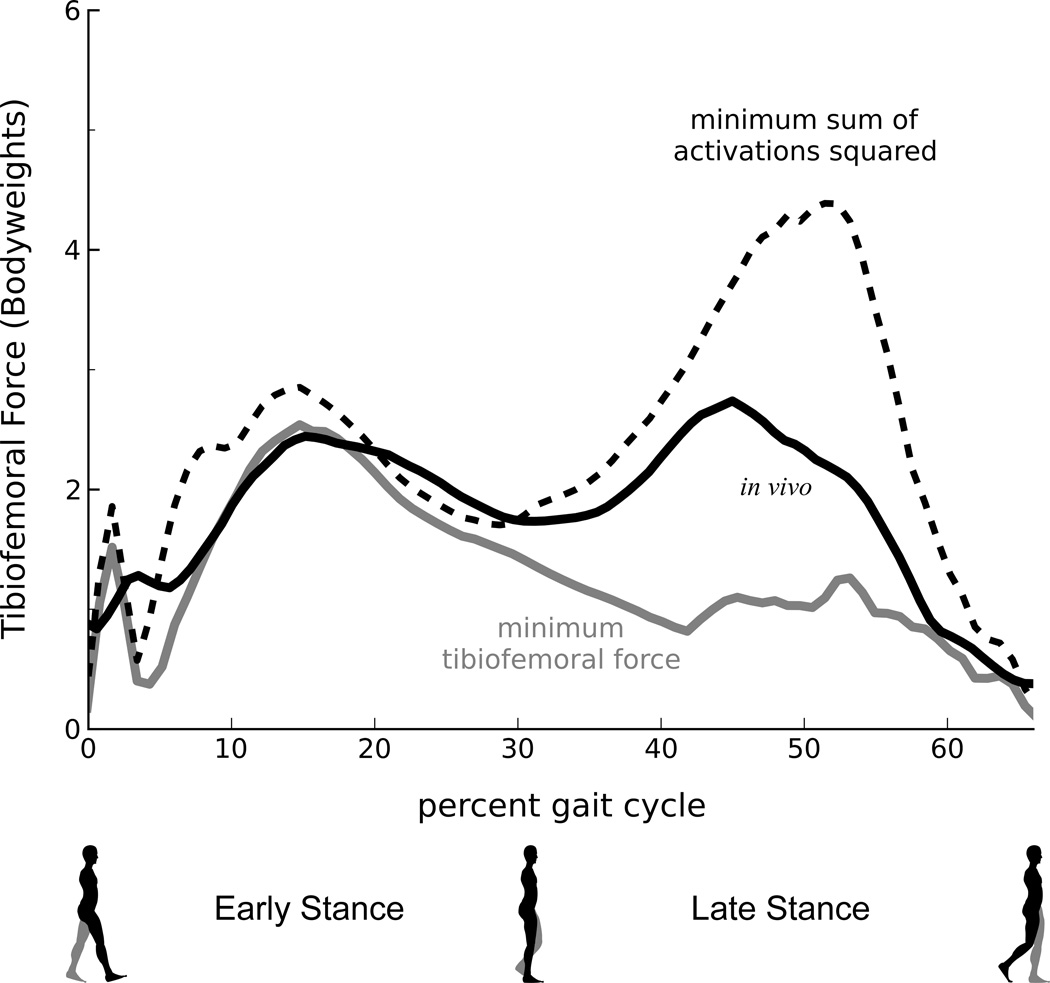
A muscle coordination strategy that minimized muscle activations produced greater tibiofemoral forces than forces measured in vivo (Fig. 2). During late stance (33–66% gait), a muscle coordination strategy that minimized the sum of muscle activations squared produced a peak tibiofemoral force that was 1.7 bodyweights larger than the peak force measured in vivo. This difference was less pronounced during early stance (0–33% gait), when minimizing muscle activations squared produced a peak tibiofemoral force that was 0.4 bodyweights larger than the peak force measured in vivo. During the swing phase (not shown), differences between the model-predicted and measured forces were less than 0.2 bodyweights.
Figure 2.
Stance-phase tibiofemoral forces predicted using a musculoskeletal model and muscle coordination strategies that minimize muscle activation squared (dashed black line) and tibiofemoral forces (solid gray line). The minimum tibiofemoral force represents the smallest compressive tibiofemoral force the model generated while still reproducing the measured walking kinematics and kinetics. Measured in vivo forces (solid black line) are shown.
A muscle coordination strategy that minimized tibiofemoral forces produced lower model-predicted forces than forces measured in vivo (Fig. 2). During late stance, a muscle coordination strategy that minimized tibiofemoral force produced a peak model-predicted force that was 1.5 bodyweights lower than the peak force measured in vivo. During early stance, this strategy produced a peak tibiofemoral force that was similar to the peak force measured in vivo.
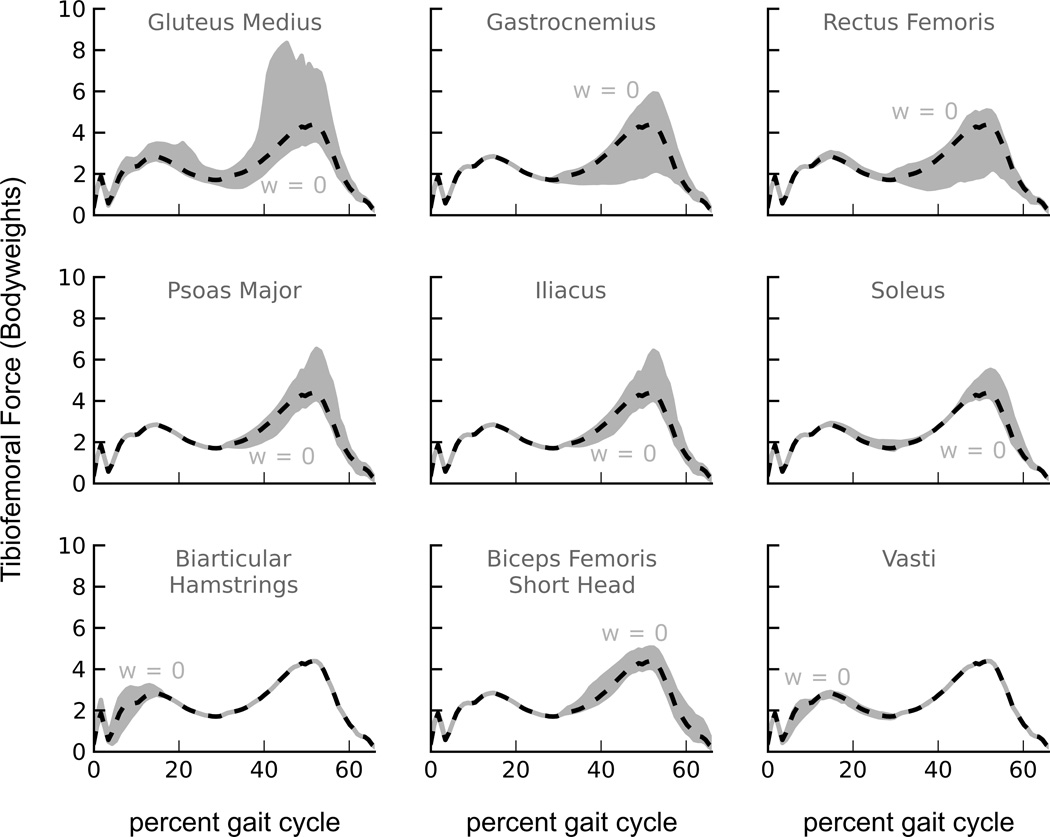
Tibiofemoral forces were sensitive to activations of muscles of the lower limb, especially during the late stance phase of walking (Fig. 3). Tibiofemoral forces were sensitive to activations of the gastrocnemius and the rectus femoris, but only during late stance. Tibiofemoral forces were also sensitive to activations of the psoas major, iliacus, and soleus muscles during late stance. Tibiofemoral forces were sensitive to activations of the biarticular hamstrings during early stance, and were sensitive to activations of the biceps femoris short head during late stance. Tibiofemoral forces were insensitive to activations of the vasti muscles; this occurred because producing the dynamics of the subject’s walking required activation of the vasti, even when activation of these muscles was penalized in the optimization. Varying activations of the gluteus medius muscle produced large changes in tibiofemoral forces throughout stance phase.
Figure 3.
The effect of varying activation of individual muscles on predicted tibiofemoral forces shown for the most influential muscles. The shaded area represents the range of predicted tibiofemoral forces due to varying the activation of each muscle. For each muscle, the boundary indicated by w = 0 corresponded to the optimization for which the muscle activity weight of that muscle was set to zero in the objective function. This objective function permitted the muscle to activate without penalty. The model predictions that minimize uniformly-weighted muscle activations squared (dashed black lines) are shown.
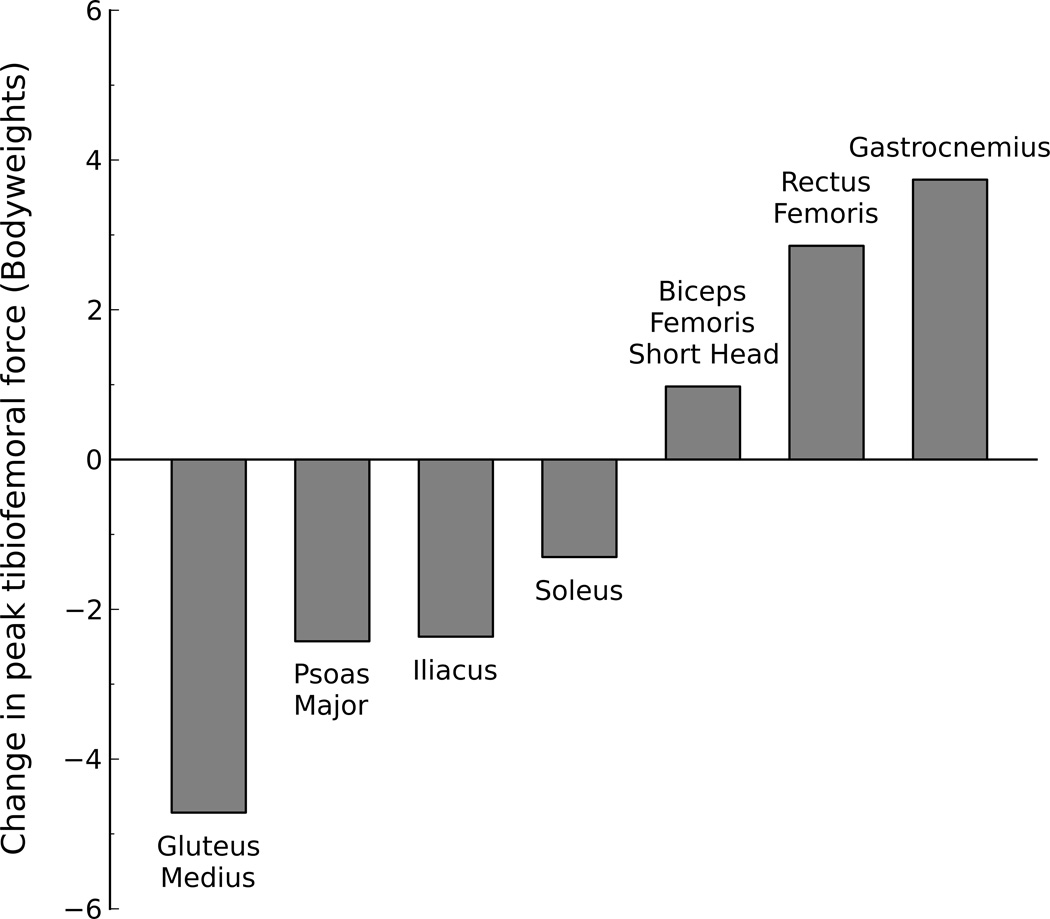
Promoting activation of the gluteus medius produced the largest decrease in peak tibiofemoral force during late stance (Fig. 4). Promoting activation of the psoas, iliacus, and soleus muscles also decreased peak tibiofemoral force during late stance. Promoting activation of the gastrocnemius produced the largest increase in peak tibiofemoral force during late stance. Promoting activation of the rectus femoris and biceps femoris short head also increased peak tibiofemoral force during late stance. The vasti remained inactive after 30% of gait and had little effect on peak tibiofemoral force during late stance. Changing the activation of other muscles of the lower limb had little effect on peak tibiofemoral force during late stance.
Figure 4.
Maximum change in peak tibiofemoral force due to activation of a muscle or muscle group during the late stance phase of walking. The maximum change was calculated as the difference between peak tibiofemoral forces obtained from the static optimizations with w = 0 (promote activity) and w = 100 (prohibit activity) for that muscle or muscle group. Note that increasing the activation of gluteus medius greatly decreased the tibiofemoral force, whereas increasing the activation of the gastrocnemius increased tibiofemoral force. The changes in peak tibiofemoral force during the late stance of walking were minimal for the muscles not shown.
DISCUSSION
Our results demonstrate that altering muscle activation patterns during walking can induce large changes in compressive forces at the tibiofemoral joint. Tibiofemoral forces were sensitive to activations of a small subset of lower limb muscles, including the gluteus medius, gastrocnemius, and rectus femoris, indicating that these muscles have the greatest potential to affect knee loading. This suggests that interventions aimed at retraining muscle coordination should target these muscles to reduce tibiofemoral loads.
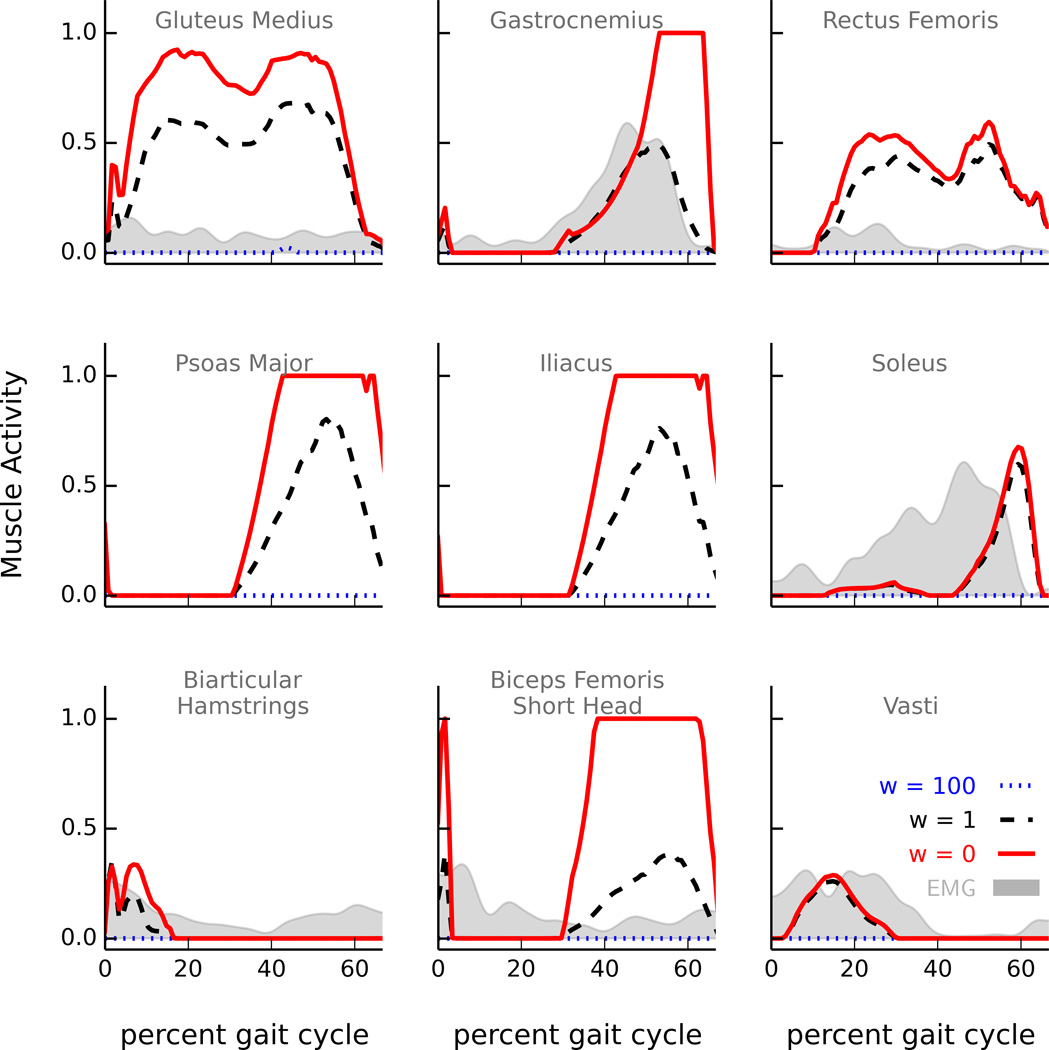
Our first goal was to evaluate whether a strategy minimizing the sum of muscle activations squared produced tibiofemoral forces that were consistent with in vivo measurements. When adopting this strategy, our model over-predicted tibiofemoral forces during the late stance phase. The discrepancy was due to over-activity of the rectus femoris and gastrocnemius, which were the largest contributors to the overpredicted tibiofemoral force. The model activated the rectus femoris during late stance, while electromyography (EMG) measured from the subject suggests that the rectus femoris may have been inactive at this time (Fig. 5). Similarly, the strategy minimizing muscle activations squared activated the gastrocnemius earlier and more than the soleus; however, EMG data show that the subject activated the gastrocnemius and soleus muscles equitably during late stance. Optimization objectives that penalized activity of the rectus femoris and gastrocnemius produced lower tibiofemoral forces that better matched in vivo measurements.
Figure 5.
Model-predicted activations of the nine most influential muscles produced by optimizations with varied muscle activity weights. For each muscle, activity ranged from 0 (no activity) to 1 (maximum activity). A muscle activity weight of w = 100 (dotted blue) prohibited the muscle from activating, while w = 0 (solid red) allowed the muscle to activate freely. The range of muscle activity used by the model (the area between the dotted blue and solid red lines) resulted in a corresponding variation in tibiofemoral forces (Fig. 3). Filtered electromyography (EMG) signals, measured from the subject during the same trial of normal walking, are provided for comparison. EMG was not measured from the psoas major or iliacus muscles.
Our second goal was to determine the potential for a subject to decrease tibiofemoral forces during walking by altering muscle coordination. Our model achieved tibiofemoral forces that were lower than in vivo measurements during late stance by adopting different muscle activation patterns compared to the TKR subject (Fig. 2). For example, the model minimized tibiofemoral forces by deactivating the gastrocnemius and hamstrings during late stance, whereas the TKR subject activated these muscles during late stance,1 resulting in higher tibiofemoral forces. Thus, the model demonstrated a lower bound for the subject’s tibiofemoral forces during late stance.
Increasing the activation of gluteus medius, a muscle crossing the hip, had the greatest potential to reduce tibiofemoral forces during walking. The gluteus medius produced the largest hip abduction moment throughout the stance phase of walking. In our simulations, increased activation of the gluteus medius resulted in a compensatory decrease in activation of the rectus femoris, tensor fasciae latae, and sartorius muscles to maintain the required hip abduction moment. The decrease in activations of the rectus femoris muscle in turn resulted in a compensatory decrease in activations of the gastrocnemius and biceps femoris short head muscles to maintain net knee moments. These decreased activations of the rectus femoris, gastrocnemius, and biceps femoris short head muscles resulted in decreased tibiofemoral forces. Conversely, decreasing activations of the gluteus medius muscle increased activations of the rectus femoris, gastrocnemius, and biceps femoris short head muscles, thereby increasing tibiofemoral forces. Thus, while the gluteus medius does not cross the knee, changes in activity or forces generated by this muscle produce substantial compensations from other muscles and have a potent effect on tibiofemoral forces. Our results may seem inconsistent with studies that have reported minimal contributions of the gluteus medius to tibiofemoral force7,16; however, these studies reported the contributions of individual muscles to tibiofemoral force based on a single muscle activation pattern and did not account for compensatory muscle activity. In contrast, we selectively changed activations of individual muscles and allowed other muscle activations to compensate to reproduce the walking motion.
Increasing the activation of the gastrocnemius and rectus femoris, two biarticular muscles crossing the knee, had the greatest potential to increase tibiofemoral forces during the late stance phase of walking. In addition to generating moments about the knee, the gastrocnemius and rectus femoris muscles produce ankle plantarflexion and hip flexion moments, respectively, in preparation for swinging the leg. During late stance, increased activation of the gastrocnemius generated a large knee flexion moment, causing compensatory coactivation of the rectus femoris to balance the net knee moment. Conversely, increased activation of the rectus femoris generated a knee extension moment, causing compensatory coactivation of the gastrocnemius and biceps femoris short head. Coactivation of the gastrocnemius, rectus femoris, and biceps femoris short head increased tibiofemoral forces. Previous studies have shown that the gastrocnemius and rectus femoris muscles contribute a higher proportion of the tibiofemoral force than the soleus and the uniarticular hip flexors.7,16 Sasaki and Neptune7 postulated that decreasing activations of the biarticular knee muscles may decrease tibiofemoral loading; our results support this idea. We also found that promoting activations of the soleus and uniarticular hip flexors could reduce tibiofemoral force. These results suggest that training to strengthen and activate the soleus and uniarticular hip flexors may decrease tibiofemoral forces and associated knee pain.
Tibiofemoral forces were most sensitive to muscle activations during the late stance phase of walking. During late stance, net knee flexion–extension moments are small compared to early stance.24,25 Low net knee flexion–extension moments during late stance allow a large range of muscle activations while still reproducing the measured walking motion. Since minimal muscle forces are required to generate the low net knee moments, the model can minimally activate muscles crossing the knee, especially the quadriceps (Fig. 5). However, the model can also co-activate the knee muscles, using a large portion of their force-generating capacity to generate co-contraction. This permits substantial freedom to vary muscle activations and tibiofemoral forces during late stance without altering the walking motion. In contrast, larger knee extension moments during early stance demand larger activations of the knee extensors. Therefore, muscle activations that reproduce walking are constrained to a narrow range, allowing only small variations in tibiofemoral forces during early stance.
A limitation of this study was that we used walking kinematics measured from one subject with bilateral TKR, and it is unclear if this dataset adequately represents a healthy or osteoarthritic population with intact knees. Subjects with TKR have been shown to walk with a straighter leg and reduced knee moments during stance,25,26 presumably to reduce quadriceps forces and tibiofemoral loading. In our case, the TKR subject displayed stance phase knee moments that are similar to pain free subjects. Peak knee moments of from 2% to 5% bodyweight times height are typically reported for pain free subjects walking at self-selected speed24,25,27; in comparison, our TKR subject generated peak knee moments of 4% bodyweight times height. The knee moments were similar across five walking trials; hence, our reported results from a single trial are representative of the remaining four normal walking trials. A second limitation of this study was that our simplified tibiofemoral joint did not permit knee abduction–adduction or internal–external rotation. Including these degrees of freedom would require the knee muscles to balance net moments in these directions. We speculate that producing these moments would increase muscle activations and tibiofemoral forces reported in this study. Tibiofemoral forces also depend on muscle geometry and strength; therefore, changes in the model’s muscle attachments and architecture would affect the reported results as well. A fourth limitation was that we permitted all muscles to activate independently. This may result in compensatory muscle coordination strategies that may be physiologically difficult for a patient to adopt. For example, a patient may have difficulty activating the soleus without activating the gastrocnemius. Finally, we calculated muscle activations that did not cause kinematic compensations (i.e., walking dynamics were unchanged when muscle activations were varied). Other studies have demonstrated that altered walking kinematics also decrease tibiofemoral loads.9,12,13 Permitting walking kinematics to change along with muscle activations will likely result in greater reductions in tibiofemoral forces than those reported here.
This study identified muscles that substantially affect tibiofemoral forces during walking. Interestingly, inactivity or weakness in the muscles crossing the hip and ankle joints can affect the loads of the knee joint. Increased activation and force in the gluteus medius, psoas major, iliacus, and soleus muscles may decrease tibiofemoral forces. Decreased activation of the gastrocnemius and rectus femoris muscles can also decrease tibiofemoral forces. Training programs targeting knee rehabilitation should include exercises that strengthen and activate the gluteus medius, psoas, and soleus muscles. It may be feasible to combine kinematic gait retraining with muscle coordination and strength training to design interventions that substantially decrease tibiofemoral forces during walking.
ACKNOWLEDGMENTS
We thank the contributors to the ASME Grand Challenge to Predict In Vivo Knee Loads for making unique and invaluable data available.
Grant sponsor: NIH; Grant numbers: U54 GM072970, R24 HD065690.
REFERENCES
- 1.Fregly BJ, Besier TF, Lloyd DG, et al. Grand challenge competition to predict in vivo knee loads. J Orthop Res. 2012;30:503–513. doi: 10.1002/jor.22023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kutzner I, Heinlein B, Graichen F, et al. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech. 2010;43:2164–2173. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Carter DR, Wong M. The role of mechanical loading histories in the development of diarthrodial joints. J Orthop Res. 1988;6:804–816. doi: 10.1002/jor.1100060604. [DOI] [PubMed] [Google Scholar]
- 4.Baliunas A. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 5.Sharma L, Hurwitz DE, Thonar EJ, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41:1233–1240. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Schnitzer TJ, Popovich JM, Andersson GB, et al. Effect of piroxicam on gait in patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36:1207–1213. doi: 10.1002/art.1780360905. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki K, Neptune RR. Individual muscle contributions to the axial knee joint contact force during normal walking. J Biomech. 2010;43:2780–2784. doi: 10.1016/j.jbiomech.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelburne KB, Torry MR, Pandy MG. Contributions of muscles, ligaments, and the ground-reaction force to tibiofemoral joint loading during normal gait. J Orthop Res. 2006;24:1983–1990. doi: 10.1002/jor.20255. [DOI] [PubMed] [Google Scholar]
- 9.Fregly BJ, D’Lima DD, Colwell CW. Effective gait patterns for offloading the medial compartment of the knee. J Orthop Res. 2009;27:1016–1021. doi: 10.1002/jor.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang A, Hurwitz D, Dunlop D, et al. The relationship between toe-out angle during gait and progression of medial tibiofemoral osteoarthritis. Ann Rheum Dis. 2007;66:1271–1275. doi: 10.1136/ard.2006.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo M, Axe MJ, Manal K. The influence of foot progression angle on the knee adduction moment during walking and stair climbing in pain free individuals with knee osteoarthritis. Gait Posture. 2007;26:436–441. doi: 10.1016/j.gaitpost.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Shull PB, Lurie KL, Cutkosky MR, et al. Training multi-parameter gaits to reduce the knee adduction moment with data-driven models and haptic feedback. J Biomech. 2011;44:1605–1609. doi: 10.1016/j.jbiomech.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Shull PB, Silder A, Shultz R, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. J Orthop Res. 2013;31:1020–1025. doi: 10.1002/jor.22340. [DOI] [PubMed] [Google Scholar]
- 14.Mündermann A, Asay JL, Mündermann L, et al. Implications of increased medio-lateral trunk sway for ambulatory mechanics. J Biomech. 2008;41:165–170. doi: 10.1016/j.jbiomech.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.D’Lima DD, Townsend CP, Arms SW, et al. An implantable telemetry device to measure intra-articular tibial forces. J Biomech. 2005;38:299–304. doi: 10.1016/j.jbiomech.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Sritharan P, Lin Y-C, Pandy MG. Muscles that do not cross the knee contribute to the knee adduction moment and tibiofemoral compartment loading during gait. J Orthop Res. 2012;30:1586–1595. doi: 10.1002/jor.22082. [DOI] [PubMed] [Google Scholar]
- 17.Steele KM, DeMers MS, Schwartz MH, et al. Compressive tibiofemoral force during crouch gait. Gait Posture. 2012;35:556–560. doi: 10.1016/j.gaitpost.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glitsch U, Baumann W. The three-dimensional determination of internal loads in the lower extremity. J Biomech. 1997;30:1123–1131. doi: 10.1016/s0021-9290(97)00089-4. [DOI] [PubMed] [Google Scholar]
- 19.Shelburne KB, Torry MR, Pandy MG. Muscle, ligament, and joint-contact forces at the knee during walking. Med Sci Sports Exerc. 2005;37:1948–1956. doi: 10.1249/01.mss.0000180404.86078.ff. [DOI] [PubMed] [Google Scholar]
- 20.Thelen DG, Anderson FC, Delp SL. Generating dynamic simulations of movement using computed muscle control. J Biomech. 2003;36:321–328. doi: 10.1016/s0021-9290(02)00432-3. [DOI] [PubMed] [Google Scholar]
- 21.Delp SL, Anderson FC, Arnold AS, et al. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54:1940–1950. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- 22.Delp SL, Loan JP, Hoy MG, et al. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- 23.Anderson FC, Pandy MG. Dynamic optimization of human walking. J Biomech Eng. 2001;123:381–390. doi: 10.1115/1.1392310. [DOI] [PubMed] [Google Scholar]
- 24.Liu MQ, Anderson FC, Schwartz MH, et al. Muscle contributions to support and progression over a range of walking speeds. J Biomech. 2008;41:3243–3252. doi: 10.1016/j.jbiomech.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClelland JA, Webster KE, Feller JA, et al. Knee kinetics during walking at different speeds in people who have undergone total knee replacement. Gait Posture. 2010;32:205–210. doi: 10.1016/j.gaitpost.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Bolanos AA, Colizza WA, McCann PD, et al. A comparison of isokinetic strength testing and gait analysis in patients with posterior cruciate-retaining and substituting knee arthroplasties. J Arthroplasty. 1998;13:906–915. doi: 10.1016/s0883-5403(98)90198-x. [DOI] [PubMed] [Google Scholar]
- 27.Pandy MG, Lin Y-C, Kim HJ. Muscle coordination of mediolateral balance in normal walking. J Biomech. 2010;43:2055–2064. doi: 10.1016/j.jbiomech.2010.04.010. [DOI] [PubMed] [Google Scholar]