Abstract
The respiratory epithelium are lung sentinel cells and are the first to contact inhaled inflammatory insults including air pollutants, smoke and microorganisms. To avoid damaging exuberant or chronic inflammation, the inflammatory process must be tightly controlled and terminated once the insult is mitigated. Inflammation-resolution is now known to be an active process involving a new genus of lipid mediators called “specialized pro-resolving lipid mediators” (SPMs) that includes resolvin D1 (RvD1). We and others have reported that RvD1 counteracts pro-inflammatory signaling and promotes resolution. A knowledge gap is that the specific cellular targets and mechanisms of action for RvD1 remain largely unknown. Here, we identified the mechanism whereby RvD1 disrupts inflammatory mediator production induced by the viral mimic poly(I:C) in primary human lung epithelial cells. RvD1 strongly suppressed the viral mimic poly(I:C)-induced IL-6 and IL-8 production and pro-inflammatory signaling involving MAP kinases and NF-κB. Most importantly, we found that RvD1 inhibited the phosphorylation of TAK1, a key upstream regulatory kinase common to both the MAP kinase and NF-κB pathways, by inhibiting the formation of a poly(I:C)-induced signaling complex composed of TAK1, TAB1 and TRAF6. We confirmed that ALX/FPR2 and GPR32, two RvD1 receptors, were expressed on hSAEC. Furthermore, blocking these receptors abrogated the inhibitory action of RvD1. Herein, we present the idea that RvD1 has the potential to be used as an anti-inflammatory and pro-resolving agent, possibly in the context of exuberant host responses to damaging respirable agents such as viruses.
Keywords: Pro-resolution, Lipid Mediators, RvD1, Airway Epithelium, Viral Infection, Inflammation
Introduction
The human respiratory tract acts as the front line of defense against inhaled hazards such as air pollution, viral and bacterial pathogens and smoky toxicants. Inhalation of dangerous insults ideally results in transient inflammatory responses that help neutralize the threat. Inflammation is a beneficial host response as long as it is well-controlled (1). However, failure to resolve inflammatory responses can lead to chronic inflammation characteristic of lung diseases, including chronic obstructive pulmonary disease (COPD), asthma and certain microbial infections (2). Resolution of inflammation was once thought to be a passive consequence of the removal of the initiating stimulus and the winding down of inflammatory mediator production (2, 3). A new paradigm is that resolution of inflammation is an active process mediated by a new genus of lipid mediators called “specialized pro-resolving lipid mediators” (SPMs) that actively counter-balance the inflammatory response (2). SPMs are mainly derived from dietary ω-3- and ω-6-polyunsaturated fatty acids (PUFAs) and are categorized as lipoxins, resolvins, protectins and maresins according to their specific chemical and structural assignments (2, 4). These SPMs are generated during inflammation and act as potent anti-inflammatory and pro-resolving agents by limiting neutrophil infiltration, enhancing macrophage uptake and clearance of apoptotic neutrophils and microbes (5), and stimulating mucosal anti-viral and anti-bacterial responses (6-8).
Earlier studies provided evidence that diets enriched in omega-3 PUFAs are beneficial to patients with chronic lung disease, by both relieving symptoms and improving lung function (9-11). Recently, we demonstrated that resolvin D1 (RvD1), a ω −3-PUFA-derived lipid molecule, reduced acute cigarette smoke-induced lung inflammation and actively promoted resolution of inflammation after smoking cessation (5). RvD1 has also been shown to attenuate lipopolysaccharide (LPS)-induced acute lung injury and ovalbumin (OVA)-initiated allergic airway inflammation in mice (12, 13).
While a growing body of evidence indicates that SPMs regulate lung homeostasis and exert anti-inflammatory effects, the cellular targets for SPMs and their mechanism of action remain a major knowledge gap. Here, we investigated the hypothesis that RvD1 dampens inflammatory signaling in primary human lung epithelial cells (hSAEC). Little or nothing is known about resolvins and effects on virally-induced inflammation. To begin to explore this, we used poly(I:C), a double stranded RNA analog of respiratory viruses such as respiratory syncytial virus, influenza A virus and rhinovirus (14), as a model stimulus, because its signaling pathways are well-described and these viruses are important in inciting human lung disease. We report that RvD1 inhibits poly(I:C)-induced pro-inflammatory signaling in hSAEC and we describe some of the key receptors and intracellular inflammatory signaling pathways involved.
Materials and Methods
Reagents and primary antibodies
Resolvin D1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid) was purchased from Cayman Chemical (Ann Arbor, MI). TAK1 inhibitor (5Z)-7-Oxozeaenol was purchased from Tocris (Minneapolis, MN) and was dissolved in 100% ethanol according to manufacturer’s recommendation. As RvD1 and (5Z)-7-Oxozeaenol (5Z-OX) were initially dissolved in 100% ethanol, control cultures were treated with the same final concentration of ethanol (less than 0.1%). ALX/FPR2 specific antagonist Boc-2 was purchased from Genscript (Piscataway, NJ). A GPR32 neutralizing antibody (GX71225) was purchased from GeneTex (Irvine, CA) and was used as described previously (15). Anti-FPR2 (ab26316), anti-β-actin (ab6046) and anti-GPR32 (ab79516) were purchased from Abcam (Cambridge, MA). Anti-p65 (sc-109), anti-pTAK (sc-130219), anti-TAK (sc-07162) and normal goat IgG (sc-2028) were purchased from Santa Cruz Biotechonology (Santa Cruz, CA). Anti-pERK (#9101), anti-total ERK (#4695), normal rabbit IgG (#9300) were purchased from Cell Signaling (Boston, MA).
Cell culture and the treatments
Two strains of primary human small airway epithelial cells (hSAEC) from different donors were purchased from Lonza Inc., (Allendale, NJ) and cultured in small airway epithelial cell growth medium (Lonza) supplemented as recommended by the supplier, and used for experiments at early passage (16). For the preparation of SAECs containing an NF-κB-luciferase reporter, hSAECs were infected with commercially available ready-to-transduce lentiviral particles that express the firefly luciferase gene under the control of a minimal CMV promoter and tandem repeats of the NF-κB transcriptional response element (SA Biosciences; Valencia CA). Typically, hSAECs were pre-incubated for 24 hours in basal medium (without supplements) to minimize the effect of medium components on inducing inflammatory signaling. Cells were then pretreated with RvD1 for 30 minutes, followed by 5μ/ml of high molecular weight (HMW) poly(I:C) (Invivogen, San Diego, CA) for the indicated time points.
ELISA
Human IL-6 and IL-8 were measured in cell culture media by commercially available ELISA kits according to the manufacturer’s instructions (R&D Systems; Minneapolis, MN).
Luciferase Assay
Cells were lysed with cell culture lysis reagent provided in the luciferase kit purchased from Promega (Madison, MI). The luciferase assay was carried out following the manufacturer’s protocol.
Immunofluorescent Staining
SAECs were seeded onto cover slides in a 12-well culture plate. Cells were fixed and permeabilized with Cytofix/Cytoperm solution (BD Bioscience, San Jose, CA) for 15 minutes and subsequently washed with Cytoperm/Wash buffer following the manufacturer’s protocol. Cells were then stained with anti-p65 antibody for 1 hour followed by staining with Alexa 568-conjugated donkey anti-rabbit antibody (Invitrogen, Carlsbad, CA) for another 30 minutes on ice. Samples were counterstained and mounted with ProLong® Gold anti-fade reagent that contains DAPI as a nuclear dye (Invitrogen).
RNA Isolation and Semi-quantitative PCR
Briefly, cells were lysed with lysis buffer provided by the manufacturer and RNA was isolated using a RNeasy mini total RNA isolation kit according to the manufacturer’s instructions (Qiagen; Valencia, CA). RNA (1 μ) was reverse-transcribed using iScript (BioRad; Hercules, CA) and the cDNA was the subjected to a PCR reaction, which was performed under the following conditions: 1 cycle at 95°C for 5 minutes, then 40 cycles at 95°C for 10 seconds, 55°C for 30 seconds and 72°C for 30 seconds, followed by inactivation at 95°C for 10 minutes. PCR products were then separated utilizing the E-Gel® system using a 2% pre-cast agarose gel supplied by Invitrogen (Grand Island, NY). Primer sequences are listed below:
18sRNA: 5′-GGTCGCTCGCTCCTCTCCCA-3′
18sRNA: 5′-AGGGGCTGACCGGGTTGGTT-3′
ALX/FPR2-F: 5′-AGTCTGCTGGCTACACTGTTC-3′
GPR2/ALX-R: 5′-AGCACCACCAATGGGAGGA-3′
GPR32-F: 5′-GTGATCGCTCTTGTTCCAGGA-3′
GPR32-R: 5′-GGACGCAGACAGGATAACCAC-3′
Flow Cytometric Analysis
Cells were lightly trypsinized, washed and then stained with rabbit polyclonal antibody against GPR32 or ALX/FPR2 independent aliquots for 30 minutes on ice. Cells were washed and stained with Alexa Fluor 568 goat anti-rabbit for a further 30 minutes. Samples were run on a FACSCanto II (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Forward and side-scatter corrections were used to exclude doublets.
Western Blotting
Cells were disrupted with lysis buffer with a supplement of protease (P-8340, Sigma-Aldrich, St Louis, MO) and phosphatase inhibitors (P0044, Sigma-Aldrich). Total protein in the lysates was determined using a bicinchoninic acid detection assay (Pierce, Rockford, IL). Protein (10ug) was separated via 12% SDS-PAGE, transferred onto an Immobilon-P membrane (Millipore) and blocked with 5% nonfat dry milk or BSA in 0.1% Tween 20 in TBS. Blots were then probed with the antibodies as indicated in legends.
Immunoprecipitation
Immunoprecipitation was performed as described (17). Briefly, hSAECs were washed once with ice-cold PBS and lysed in 300μl of RIPA lysis buffer (Cell Signaling) containing a final concentration of 1mM phenylmethylsulfonyl fluoride (PMSF). Cell extracts were incubated with TRAF6 or TAB1 antibodies or with a matching IgG controls overnight, followed by a 2-hour incubation with 50μl of washed protein A/G-Sepharose beads (Thermo Scientific; Rockford, IL). The immune complex was then washed with lysis buffer four times, then boiled in SDS loading buffer and analyzed by Western Blotting as described earlier. A horseradish peroxidase conjugated (HRP)-anti-rabbit secondary antibody was purchased from Rockland (Gilbertsville, PA) and was used to avoid the detection of pre-existing heavy and light chain IgG.
Statistical Analysis
All results are reported as the mean ± SD. One-way analysis of variance (ANOVA) with Bonferroni multiple comparisons were performed using GraphPad Prism version 5.0d for Mac (GraphPad Software, La Jolla California USA, www.graphpad.com). A P value <0.05 was considered significant.
Results
RvD1 attenuates poly(I:C)-induced pro-inflammatory signaling in hSAECs
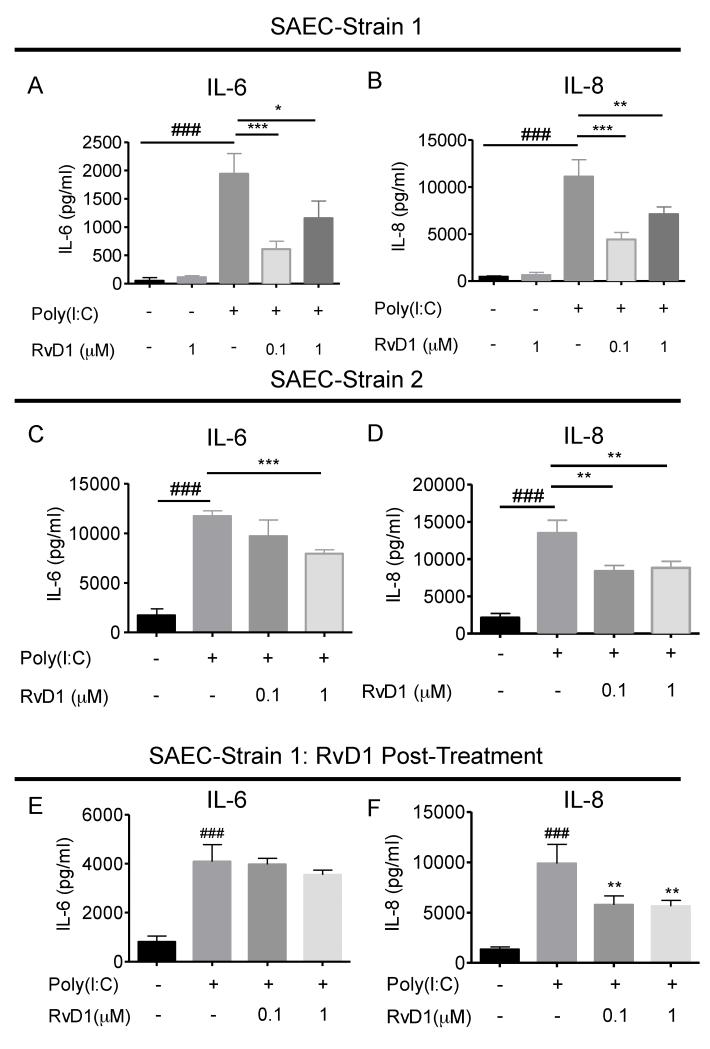
To assess whether or not RvD1 modulates poly(I:C)-induced inflammatory mediator production, we used two strains of human primary small airway epithelial cells (hSAEC) derived from different donors. These cells were pretreated with RvD1 for 30 minutes prior to the addition of poly(I:C). Supernatants were collected and the levels of IL-6 and IL-8 were determined. Poly(I:C) is a powerful inflammatory agonist, as proven by its ability to induce a high level of IL-6 and IL-8 production in hSAECs (Figure 1A-1D). Pre-treatment with RvD1 significantly reduced production of both pro-inflammatory mediators in both cell strains. (Figure 1A-1D). Previously, we and others showed that RvD1 promoted the resolution of inflammation in vivo when given after the insult (5, 13). Here, we next determined whether RvD1 is capable of limiting the inflammatory responses after activation by poly(I:C). RvD1 added 15 minutes after poly(I:C) significantly attenuated the production of IL-8 (Figure 1F), while there was a non-significant trend toward reduced IL-6 production (Figure 1E). Taken together, these results demonstrate that RvD1 attenuates production of pro-inflammatory mediators in hSAECs following stimulation with poly(I:C).
Figure 1. RvD1 attenuates Poly(I:C)-induced IL-6 and IL-8 production in primary human airway epithelial cells (SAECs).
Two strains of human primary SAECs were pre-treated with RvD1 for 30 minutes prior to the addition of poly(I:C) (5μ/ml) for 24 hours. Supernatants from the cultures were collected and subjected to ELISA to determine the levels of (A and C) IL-6 and (B and D) IL-8. (E and F) hSAECs were treated with poly(I:C) (5μg/ml) for 15 minutes prior to treatment with RvD1 (100nM) for a further 24 hours. Supernatants were collected and the levels of IL-6 and IL-8 were determined as described earlier. Data shown are mean ± SD of triplicate cultures, from one representative experiment of 3 performed. ###p<0.001 as compared to the non-treated control. *p<0.05; **p<0.01, ***p<0.001 compared to poly(I:C) stimulated culture, using one-way ANOVA with Bonferroni post-tests.
RvD1 attenuates Poly(I:C)-induced MAP kinase and NF-κB activation
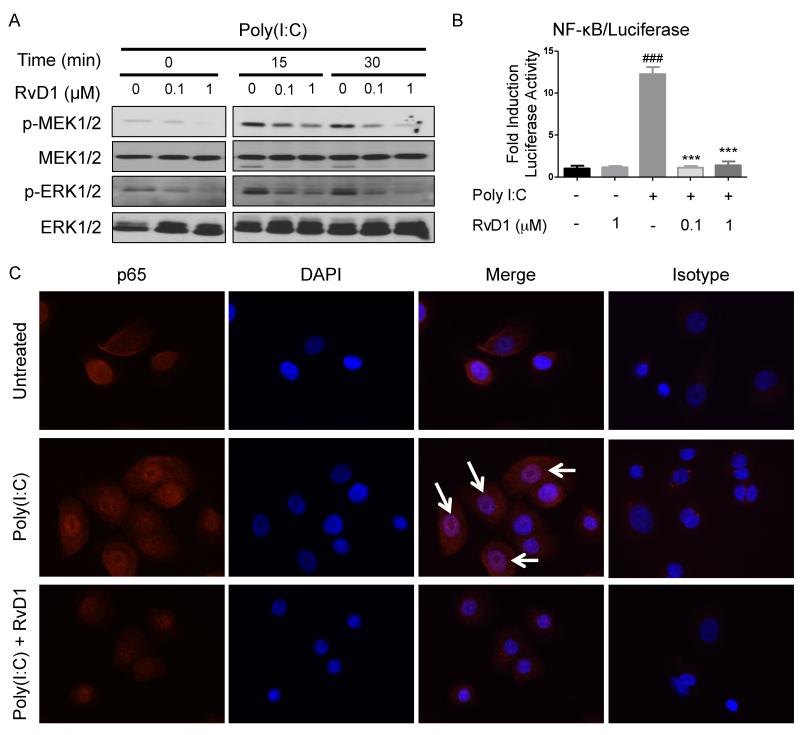
Poly(I:C) is a potent activator of MAPK and NF-κB pathways that are essential in upregulating the production of inflammatory mediators (16, 18). Given that RvD1 suppresses the production of poly(I:C)-induced IL-6 and IL-8, we investigated the effect of RvD1 on the NF-κB and MAPK pathways. Cells were pretreated with RvD1 for 30 minutes prior to the addition of poly(I:C), and harvested at key time points. As a potent MAPK activator, poly(I:C) activated ERK phosphorylation at 15 and 30 minutes (Figure 2A; panel p-ERK). RvD1 inhibited ERK activation in a dose-dependent manner (Figure 2A). We also examined MEK, the immediate upstream ERK kinase, and found that RvD1 also inhibited MEK phosphorylation in a dose-dependent manner. (Figure 2A).
Figure 2. RvD1 attenuates Poly(I:C)-induced MEK, ERK and NF-κB activation and translocation.
(A) SAECs were pretreated with the indicated concentration of RvD1, 30 minutes prior to the addition of poly(I:C) (5μg/ml) for the indicated times. Cell lysates were collected and subjected to Western blotting to visualize the phosphorylation of MEK (p-MEK) and ERK (p-ERK). Data shown are from same blot from one representative experiment out of three. (B) SAECs stably expressing a luciferase reporter were pretreated with the indicated concentration of RvD1prior to the addition of poly(I:C). Cell lysates were collected and NF-κB activation was determined by a luciferase assay. Data shown is mean ± SD of triplicate cultures, from one representative experiment of 3 performed. ###p<0.001 compared to a non-treated control culture. ***p<0.001 as compared to poly(I:C)-treated culture using one-way ANOVA with Bonferroni post-tests. (C) SAECs were pre-treated with RvD1 (100nM) for 30 minutes prior to stimulation with poly(I:C) (5μg/ml) for a further 1 hour. Samples were then stained with antibody against the NF-κB subunit p65. Cells were mounted and counterstained with DAPI. Arrows indicate co-localization of p65 and the DAPI nuclear stain. The experiment was performed twice with duplicate wells in each experiment and representative images are shown.
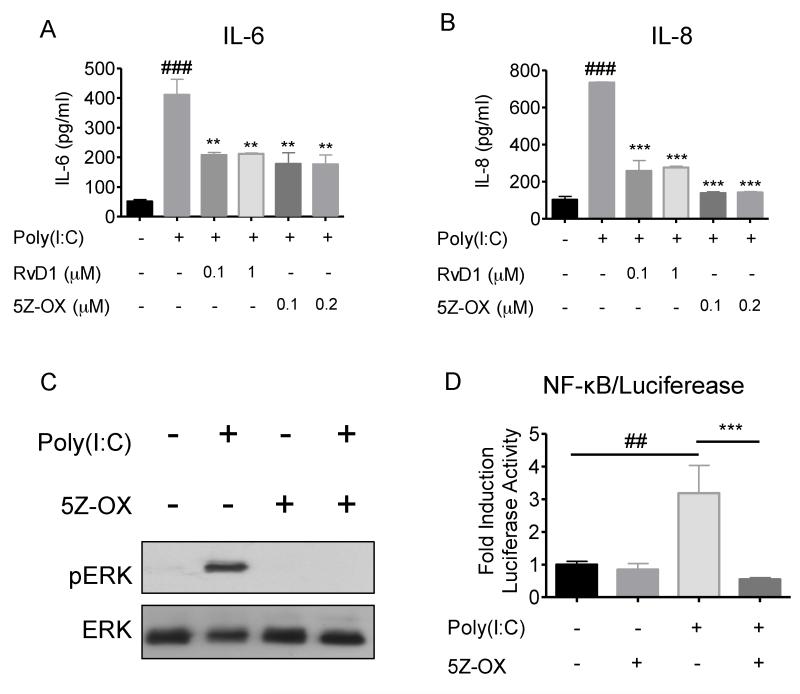
To evaluate NF-κB activity, we created a hSAEC strain that constitutively expresses a luciferase reporter under control of an NF-κB responsive element (hSAEC/NFκB-Luc). As a positive control, cells treated with poly(I:C) showed a significant increase in NF-κB transactivation. This activation was strongly inhibited by RvD1 (Figure 2B). We further probed NF-κB activation by assessing p65 nuclear translocation using immunocytochemistry. Poly(I:C) elicited the translocation of p65 into the nucleus at the 1 hour time point. Interestingly, RvD1 inhibited poly(I:C)-induced nuclear translocation of p65 (Figure 2C).
hSAECs express the RvD1 receptors GPR32 and ALX/FPR2
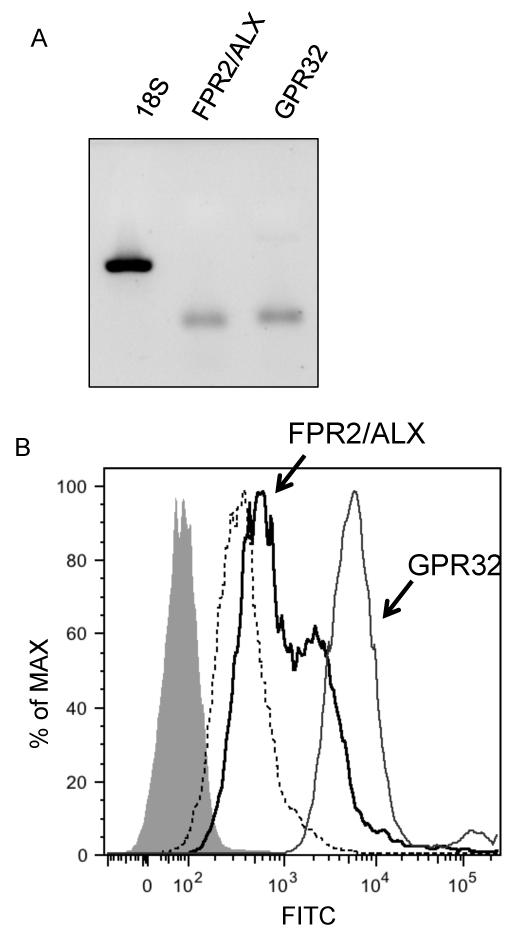
Two RvD1 receptors have been identified in human cells, ALX/FPR2 and GPR32 (15, 19). However, it is unknown whether or not these two receptors are expressed in SAECs and if so, whether they play roles in regulating inflammatory responses. To determine whether or not hSAECs express ALX/FPR2 and GPR32, we harvested RNA and analyzed the expression of these two receptors by semi-quantitative PCR. Flow cytometry was performed to evaluate cell surface expression. Human SAECs contain mRNA for both receptors (Figure 3A) and express both receptors on their cell surface (Figure 3B).
Figure 3. SAECs express the RvD1 receptors ALX/FPR2 and GPR32.
(A) Total RNA from the SAECs was isolated and subjected to a semi-quantitative PCR. PCR samples were analyzed on a 2% agarose gel. (B) Non-permeabilized SAECs were stained with antibodies against ALX/FPR2 (black line) or GPR32 (gray line) and analyzed by flow cytometry. Dotted line: a secondary antibody control; Solid, shaded area: a non-stained control.
The actions of RvD1 are mediated through the receptor ALX/FPR2 and GPR32
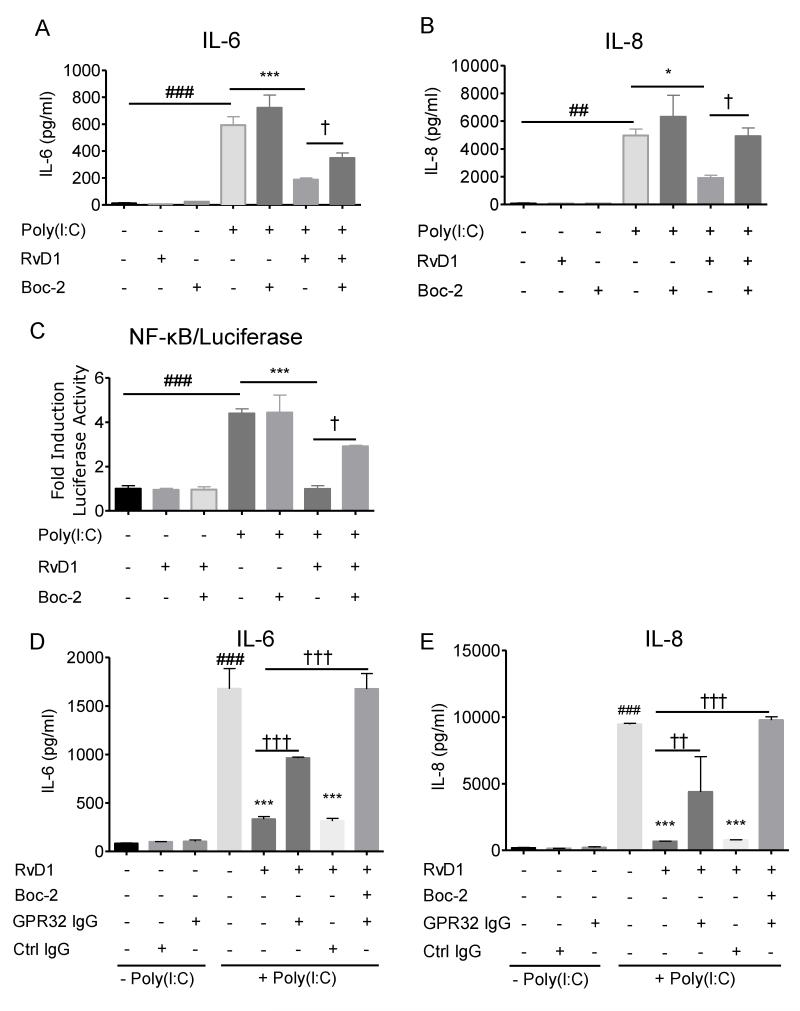
Pharmacological inhibition of ALX/FPR2 can be achieved using a receptor antagonist (20). To determine whether or not RvD1 acts on SAECs via this receptor, hSAECs were pretreated with Boc-2, an ALX/FPR2 specific antagonist, for 30 minutes (20). Cells were then treated with or without RvD1 for another 30 minutes prior to the addition of poly(I:C). Poly(I:C) induced strong production of IL-6 and IL-8 that was potently attenuated by RvD1. Of interest, Boc-2 alone neither triggered an inflammatory response (Figure 4) nor induced cell death (data not shown), while Boc-2 pretreatment partially neutralized the anti-inflammatory effect of RvD1 in hSAECs, implicating ALX/FPR2 as an essential component for RvD1 activation in airway epithelial cells (Figure 4A and 4B). Moreover, Boc-2 also partially reversed the inhibitory effect of RvD1 on NF-κB activation (Figure 4C). To further interrogate whether or not RvD1 also acts through GPR32, hSAECs were pre-incubated with a GPR32 neutralizing antibody (10 μg/ml), in the presence or absence of Boc-2 for 30 minutes, prior to treatment with RvD1. These cells were then stimulated with poly(I:C) for a further 24 hours to elicit the production of inflammatory mediators. Consistent with the above results, RvD1 inhibited the production of IL-6 and IL-8, and this inhibition was partially blunted by the GPR32 neutralizing antibody (Figure 4D and 4E). In the presence of both the GPR32 antibody and Boc-2, the inhibitory effect of RvD1 on production of IL-6 and IL-8 was fully reversed, indicating that the anti-inflammatory signaling by RvD1 is receptor-mediated.
Figure 4. The actions of RvD1 are receptor mediated.
SAECs were first treated with the receptor ALX/FPR2 antagonist Boc-2 (1μM) for 30 minutes prior to the addition of RvD1 (100nM) or vehicle for another 30 minutes. Cells were then treated with poly(I:C)(5 μg/ml) for 24 hours to activate inflammatory signaling. Supernatants were collected and analyzed to determine their levels of (A) IL-6 and (B) IL-8. (C) SAEC/NF-κB-Luc cells were treated with Boc-2 for 30 minutes, followed by a treatment of RvD1 (100nM) for another 30 minutes prior to the addition of poly(I:C). Cell lysates were collected and analyzed using a luciferase assay. (D and E) SAECs were treated with Boc-2 and/or a GPR32 neutralizing antibody for a total of 30 minutes prior to treatment with100nM RvD1. Cells were then treated with poly(I:C) (5 μg/ml) for 24 hours and (D) IL-6 and (E) IL-8 were determined as described previously. Data shown are the mean ± SD of triplicate cultures, from one representative experiment of 2 performed. ###p<0.001 as compared to untreated control. *p<0.05; ***p<0.001 comparing to poly(I:C)-treated culture, † <0.05; †† <0.01; †††p<0.001 as compared to RvD1-treated, poly(I:C)-exposed culture, by one-way ANOVA with Bonferroni post-tests.
TAK1 inhibition is sufficient to ablate poly(I:C)-induced inflammation in hSAECs
Given that RvD1 suppresses both ERK and NF-κB, we postulate that RvD1 might act on a molecule that controls both ERK and NF-κB. Based on the literature suggesting that TAK1 is responsible for phosphorylation of both MEK and NF-κB (p65/RelA) (21), we reasoned that TAK1 could be a target of RvD1 that accounts for RvD1’s dual activity. We first evaluated the role of TAK1 in poly(I:C)-induced inflammatory signaling. hSAECs were pretreated with the TAK1 specific inhibitor (5Z)-7-Oxozeaenol (5Z-OX) or RvD1 for 30 minutes, prior to the addition of poly(I:C). Inhibition of TAK1 was sufficient to terminate poly(I:C)-induced IL-6 and IL-8 production (Figure 5A and 5B). Moreover, 5Z-OX treatments also inhibited poly(I:C)-induced ERK phosphorylation and NF-κB activation (Figure 5C and 5D, respectively), suggesting that TAK1 is a main regulator of poly(I:C)-elicited inflammation in hSAECs.
Figure 5. Blockade of TAK1 is sufficient to dampen Poly(I:C)-induced inflammation in SAECs.
SAECs were pretreated with either the TAK1 inhibitor (5Z)-7-oxozeaenol (5Z-OX; 100 or 200nM) or RvD1 for 30 minutes prior to the addition of poly(I:C) (5μg/ml) for 24 hours. Supernatant from the culture was collected and the levels of (A) IL-6 and (B) IL-8 were analyzed by an ELISA assay following the manufacturer’s protocol. (C) SAECs were pretreated with 200nM of 5Z-OX or 100nM of RvD1 for 30 minutes prior to the addition of poly(I:C) for another 15 minutes. Cell lysates were collected and analyzed by Western blotting to visualize ERK phosphorylation. The membrane was stripped and re-probed, for total ERK as a loading control. Data shown are from the same blot from one representative experiment out of three. (D) SAEC/NF-κB-Luc cells were treated as previously stated. Cell lysates were collected and the level of luciferase was determined using a luciferase assay. Data shown is mean ± SD of triplicate cultures, from one representative experiment of 3 performed. ##p<0.01 as compared to untreated culture. **p<0.01, ***p<0.001 as compared to Poly(I:C)-treated culture, using one-way ANOVA with Bonferroni post-tests.
Resolvin D1 attenuates poly(I:C)-induced TAK1 activation
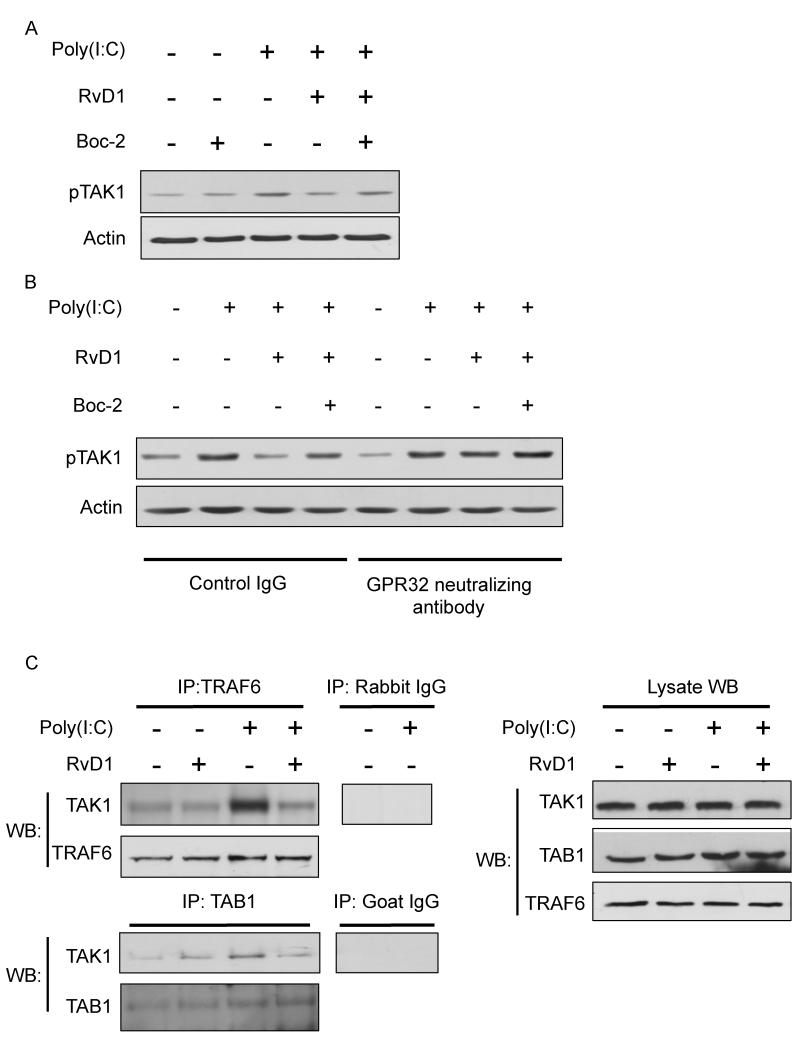
To assess whether or not RvD1 modulates TAK1 activation, hSAECs were treated with RvD1 for 30 minutes prior to stimulation for 10 minutes with poly(I:C). We found that poly(I:C) was able to activate TAK1 phosphorylation, and that this activation was suppressed by RvD1 (100nM) (Figure 6A). To determine if the inhibition of TAK1 phosphorylation by RvD1 was a receptor-mediated process, we pretreated the cells with or without Boc-2, and in the presence or absence of a GPR32 neutralizing antibody for 30 minutes, prior to addition of RvD1. Cells were then stimulated with poly(I:C) to activate inflammatory signaling. Again, in the presence of Boc-2, the inhibition TAK1 phosphorylation by RvD1 was partially reversed (Figure 6A, lane 5 and Figure 6B, lane 4). Pretreatment with GPR32 neutralizing antibody also partially reversed the effect of RvD1 (Figure 6B, lane 7). Finally, the combination of Boc-2 and GPR32 neutralizing antibody completely reverses the inhibitory effect of RvD1 (Figure 6B, lane 8).
Figure 6. Blockade of FPR2/ALX and GPR32 reverses the inhibitory effect of RvD1 on poly(I:C)-induced TAK1 activation.
(A) SAECs were pretreated with Boc-2 (1μM) for 30 minutes, followed by RvD1(100nM) for a further 30 minutes. Cells were then exposed to poly(I:C) (5μg/ml) for 10 minutes to activate TAK1. Cell lysates were collected and TAK1 phosphorylation (p-TAK1) was analyzed by Western Blotting. The same membrane was stripped and re-probed using total actin as a loading control. Data shown are from the same blot from one representative experiment out of three. (B) SAECs were pre-treated with GPR32 neutralizing antibody, with or without Boc-2, for 30 minutes. Cells were then treated RvD1 (100nM) for another 30 minutes, followed by poly(I:C) for 10 minutes. Cell lysates were collected and analyzed as described previously. Data shown are from the same blot from one representative experiment out of two. Total actin was used as a loading control. (C) Cells were pretreated with RvD1 (100nM) prior to the addition of poly(I:C) for a further 10 minutes. Poly(I:C)-induced signaling complex was immunoprecipitated with antibodies against TRAF6 or TAB1 followed by Western Blotting analyses with anti-TAK1 antibody. IgG control lanes were precipitated with non-specific rabbit or goat control antibody. Lysate indicates 1/10 input in each experiment. Levels of total TAK, TAB1 and TRAF6 in each sample were determined by Western Blotting.
RvD1 interferes with the association of the poly(I:C)-induced TRAF6/TAK1/TAB1 signaling complex
Upon stimulation with poly(I:C), TAK1 becomes activated through the formation of a signaling complex with TNF receptor-associated factor 6 (TRAF6) and TAK1 binding protein (TAB1). The formation of this signaling complex has proven to be essential for TAK1 activation, which in turn activates downstream signaling including MAP kinases and NF-κB (21, 22). Given that the specific inhibition of TAK1 blocks pro-inflammatory signaling and that RvD1 attenuates TAK1 activation, we hypothesized that RvD1 inhibits TAK1 activation by interfering with the formation of poly(I:C)-mediated signaling components. To evaluate this hypothesis, cells were pretreated with RvD1 (100nM) for 30 minutes prior to stimulation by poly(I:C) for a further 10 minutes. Cell lysates were collected and immunoprecipitated with TRAF6 or TAB1 antibodies, followed by Western Blot analyses using TAK1 antibody. In unstimulated cells, there is some association of TRAF6 and TAK1, consistent with previous reports in other cell types (Figure 6C). Poly(I:C) strongly increased the association of TAK1 with TRAF6 and TAB1, the first time this complex has been demonstrated in hSAECs. In cell cultures pre-treated with RvD1, the interaction of TAK1 to either TRAF6 or TAB1 was decreased, which suggests that RvD1 interferes with the formation of poly(I:C)-induced signaling complex (Figure 6C).
Discussion
The airway epithelium is the first line of contact for inhaled allergens, hazardous particles, and infectious agents such as viruses. Despite continuous exposure to these noxious agents, the overall homeostasis of the airway epithelium is remarkably stable due to tight coupling of pro-inflammatory and pro-resolving processes. Previously, resolution of inflammation was thought to be a passive process involving the deactivation of pro-inflammatory mediators and removal of the stimuli. Previous findings from our group and others support the concept that, in the lung, resolution is an active process controlled by a family of novel lipid-derived mediators termed SPMs (5, 12, 23, 24). Poly(I:C) is a potent stimuli that causes acute inflammatory responses in vitro and in vitro (16, 18, 25, 26) and resembles acute pathology in human lungs (26). In this study, we report the new findings that RvD1 suppresses the inflammatory response to poly(I:C) in airway epithelial cells through the inhibition of TAK1, leading to a subsequent reduction of both ERK and NF-κB activation. We further illustrate that RvD1 inhibits TAK1 activation, at least partly, via perturbing the association of a poly(I:C)-mediated complex involving TAK1, TAB1 and TRAF6. Overall, we provide compelling evidence to show that the anti-inflammatory actions of RvD1 are mediated by receptors ALX/FPR2 and GPR32
Activation of epithelial cells by viral infection results in an immediate host defense response resulting in the production of pro-inflammatory mediators such as IL-6 and IL-8 (14, 27). Here, we chose to use undifferentiated hSAECs as our in vitro model based on previous studies showing that hSAECs respond to various inflammatory stimuli and produce corresponding pro-inflammatory mediators similar to human airway pathophysiology (16, 28, 29). The production of these pro-inflammatory mediators can further amplify the inflammatory response by activating nearby cells including fibroblasts, macrophages and neutrophils (14). IL-6 and IL-8 are elevated during viral infection and during acute COPD exacerbations, and this increase is highly associated with persistent neutrophilic pathology in the human airway (30). As a potent activator and chemoattractant of neutrophils, IL-8 may be a potential therapeutic target to reduce neutrophilic inflammation.
In the present study we demonstrate that both ALX/FPR2 and GPR32 are expressed by hSAECs. We also demonstrate that by blocking these two receptors, RvD1 is no longer able to inhibit poly(I:C)-induced inflammatory responses. This finding supports the concept that both ALX/FPR2 and GPR32 are responsible for the inhibitory signaling mediated by RvD1(15). ALX/FPR2 is known to be a receptor for several SPMs including RvD1 and lipoxin A4 (31, 32). RvD1 attenuates allergic airway inflammation via ALX/FPR2 in a mouse model (24). Interestingly, ALX/FPR2 can deliver either pro-inflammatory or pro-resolving signals based on its conformational status (31). ALX/FPR2 is also elevated in the lungs of patients with COPD, although whether this indicates chronic pro-inflammatory signaling or an unsuccessful attempt to respond to pro-resolving signaling is unknown (33, 34). GPR32 was recently identified as a receptor for RvD1, RvD3 and RvD5 in human cell culture models (15, 19, 35), but its role in human disease states has not yet been determined. Given that both GPR32 and ALX/FPR2 signaling is active in airway epithelial cells, it will be of therapeutic interest to use SPMs and their derived analogs to treat inflammatory airway disease caused by inhaled stimuli such as microorganisms and cigarette smoke (1, 5, 36).
In the present study we identified transforming growth factor-β-activated kinase 1 (TAK1) as a master regulator of multiple pro-inflammatory pathways and as a physiological target of RvD1 in hSAECs (Figure 7). TAK1 was once thought to be activated by TGF- to promote cell growth and differentiation. However, more recent data shows that TAK1 is a key regulator of the immune response and inflammatory signaling that promotes tumorigenesis, fibrosis and multiple inflammatory disorders (37). Given that TAK1 is a common regulator of many important physiologically relevant pathways, the pharmacological targeting of TAK1 may have therapeutic benefits. In our current study, we show that RvD1 treatment largely blocked the TRAF6/TAK1/TAB1 association and TAK1 phosphorylation. It is worthwhile to note that docosahexaenoic acid (DHA), a precursor of RvD1, was shown to inhibit TAK1 activation via a GPR120-dependent signaling pathway. This inhibition involves the activation of-arrestin, which sequesters TAB1, leading to a reduction in TAK1 activation (38). Given that the RvD1 inhibits TAK1 activation and activates-arrestin, it is very likely that RvD1 also interferes with the complex formation via a similar mechanism (19, 38). It is also known that TAK1 can be negatively regulated by certain phosphatases (39, 40). As such, it would be interesting to examine whether or not RvD1 has a direct impact on phosphatase activity.
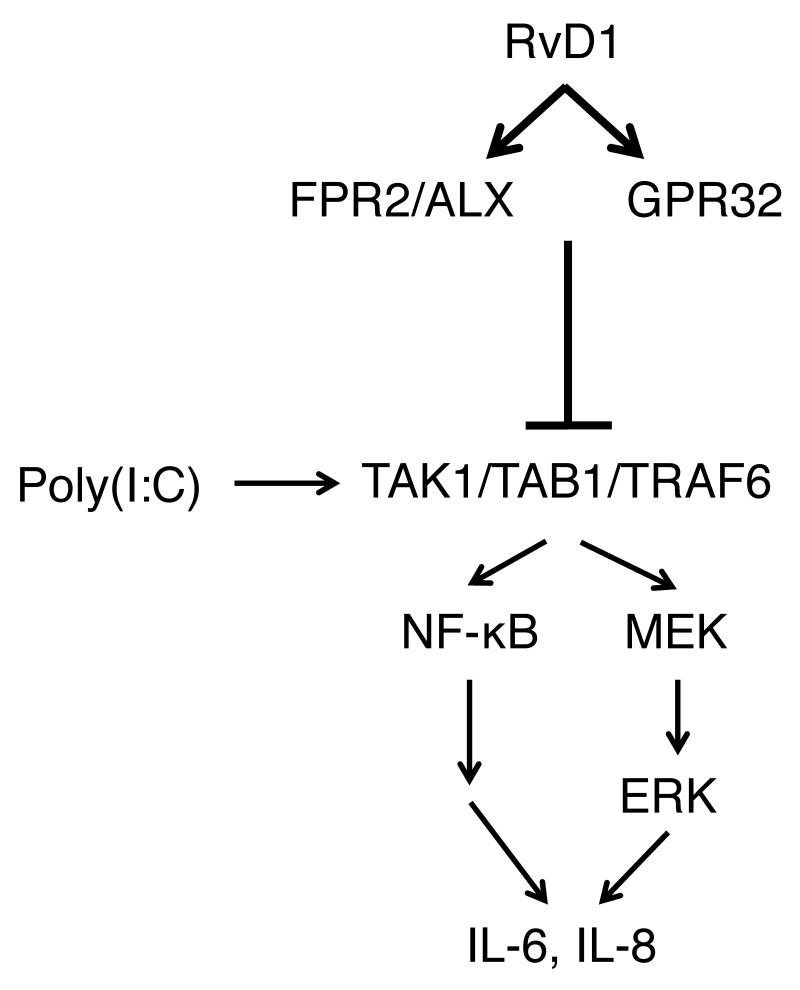
Figure 7. A diagram illustrating how RvD1 modulates poly(I:C)-induced inflammation in small airway epithelial cells.
In hSAEC, RvD1 potently inhibits pro-inflammatory signaling elicited by a viral mimetic ligand poly(I:C). RvD1 inhibits activation of TAK1, a common upstream regulatory protein of both MAP kinase and NF-κB pathways, via perturbing the formation of poly(I:C)-induced signaling complex composed of TAK1, TAB1 and TRAF6. By blocking RvD1’s receptors ALX/FPR2 and GPR32, the anti-inflammatory effect of RvD1 was abolished, suggesting that the actions of RvD1 are receptor-dependent.
One disease in which chronic inflammation is of critical clinical importance is chronic obstructive pulmonary disease (COPD, emphysema and chronic bronchitis). COPD patients have increased levels of pro-inflammatory cytokines and cells in their lungs, exhaled breath condensate and serum, long after smoking cessation (41). This chronic pro-inflammatory environment leaves them susceptible to viral and bacterial infections, resulting in rapid decline in lung function during acute exacerbations (42). Since patient mortality increases significantly with multiple exacerbations, finding therapies that can reverse the chronic inflammation and restore immune homeostasis is a critical clinical need. The currently recommended therapy for exacerbations of COPD includes treatments with corticosteroids, which have serious side-effects. It should also be noted that current therapies for lung inflammation, including lipoxygenase inhibitors and cycloxygenase inhibitors, block the production not only of pro-inflammatory leukotrienes and prostaglandins, but also of anti-inflammatory and pro-resolving lipoxins and resolvins (43-45). This highlights the significant therapeutic potential of endogenous pro-resolving mediators.
Here, we show that RvD1 attenuates inflammatory responses to poly(I:C), a double stranded RNA analog of important respiratory viruses implicated in chronic inflammatory conditions including COPD exacerbations. We propose that RvD1 will be effective in reducing excess inflammation in viral infections. Furthermore, because RvD1 and other SPMs are anti-inflammatory and pro-resolving, without being immunosuppressive, support the idea that they have significant clinical potential in COPD and other diseases involving chronic inflammation and viral infection (5, 24, 46).
Acknowledgements
We thank Wade Narrow and Steve Pollock for expert technical assistance.
Grant Support: This work was supported by National Institutes of Health Grants R01HL120908, R01HL088325, T32HL066988, and P30ES001247. The project described was supported by an Award (Number UL1TR000042) from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Footnotes
Author contribution: H.H., R.F., T.T., R.P., and P.S. conceived the study and designed the experiments. H.H., E. L., R.F. and K.O. performed experiments and collected data. H.H., T.T., E.L., R.F., R.P. and P.S. analyzed the data and wrote the manuscript.
Reference
- 1.Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Current opinion in pharmacology. 2013;13:632–640. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Scientific reports. 2013;3:1940. doi: 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henson PM. Resolution of inflammation. A perspective. Chest. 1991;99:2S–6S. [PubMed] [Google Scholar]
- 4.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? The American journal of pathology. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PloS one. 2013;8:e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 7.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. Journal of immunology. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 8.Canny G, Levy O, Furuta GT, Narravula-Alipati S, Sisson RB, Serhan CN, Colgan SP. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mickleborough TD. Dietary omega-3 polyunsaturated fatty acid supplementation and airway hyperresponsiveness in asthma. The Journal of asthma: official journal of the Association for the Care of Asthma. 2005;42:305–314. doi: 10.1081/JAS-62950. [DOI] [PubMed] [Google Scholar]
- 10.Arm JP, Horton CE, Spur BW, Mencia-Huerta JM, Lee TH. The effects of dietary supplementation with fish oil lipids on the airways response to inhaled allergen in bronchial asthma. The American review of respiratory disease. 1989;139:1395–1400. doi: 10.1164/ajrccm/139.6.1395. [DOI] [PubMed] [Google Scholar]
- 11.Shahar E, Folsom AR, Melnick SL, Tockman MS, Comstock GW, Gennaro V, Higgins MW, Sorlie PD, Ko WJ, Szklo M. Dietary n-3 polyunsaturated fatty acids and smoking-related chronic obstructive pulmonary disease. Atherosclerosis Risk in Communities Study Investigators. The New England journal of medicine. 1994;331:228–233. doi: 10.1056/NEJM199407283310403. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, Min S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulmonary pharmacology & therapeutics. 2011;24:434–441. doi: 10.1016/j.pupt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. Journal of immunology. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clinical microbiology reviews. 2011;24:210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritter M, Mennerich D, Weith A, Seither P. Characterization of Toll-like receptors in primary lung epithelial cells: strong impact of the TLR3 ligand poly(I:C) on the regulation of Toll-like receptors, adaptor proteins and inflammatory response. Journal of inflammation. 2005;2:16. doi: 10.1186/1476-9255-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaesu G, Ninomiya-Tsuji J, Kishida S, Li X, Stark GR, Matsumoto K. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Molecular and cellular biology. 2001;21:2475–2484. doi: 10.1128/MCB.21.7.2475-2484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rezaee F, Meednu N, Emo JA, Saatian B, Chapman TJ, Naydenov NG, De Benedetto A, Beck LA, Ivanov AI, Georas SN. Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. The Journal of allergy and clinical immunology. 2011;128:1216–1224 e1211. doi: 10.1016/j.jaci.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stenfeldt AL, Karlsson J, Wenneras C, Bylund J, Fu H, Dahlgren C. Cyclosporin H, Boc-MLF and Boc-FLFLF are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation. 2007;30:224–229. doi: 10.1007/s10753-007-9040-4. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. The Journal of biological chemistry. 2003;278:16713–16719. doi: 10.1074/jbc.M300562200. [DOI] [PubMed] [Google Scholar]
- 22.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 23.Xu MX, Tan BC, Zhou W, Wei T, Lai WH, Tan JW, Dong JH. Resolvin D1, an endogenous lipid mediator for inactivation of inflammation-related signaling pathways in microglial cells, prevents lipopolysaccharide-induced inflammatory responses. CNS neuroscience & therapeutics. 2013;19:235–243. doi: 10.1111/cns.12069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Levy BD. Resolvin D1 and Resolvin E1 Promote the Resolution of Allergic Airway Inflammation via Shared and Distinct Molecular Counter-Regulatory Pathways. Frontiers in immunology. 2012;3:390. doi: 10.3389/fimmu.2012.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster Marketon JI, Corry J. Poly I:C and respiratory syncytial virus (RSV) inhibit glucocorticoid receptor (GR)-mediated transactivation in lung epithelial, but not monocytic, cell lines. Virus research. 2013;176:303–306. doi: 10.1016/j.virusres.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Lindh HF, Lindsay HL, Mayberry BR, Forbes M. Polyinosinic-cytidylic acid complex (poly I:C) and viral infections in mice. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1969;132:83–87. doi: 10.3181/00379727-132-34153. [DOI] [PubMed] [Google Scholar]
- 27.Numata M, Chu HW, Dakhama A, Voelker DR. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus-induced inflammation and infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:320–325. doi: 10.1073/pnas.0909361107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dakhama A, Kraft M, Martin RJ, Gelfand EW. Induction of regulated upon activation, normal T cells expressed and secreted (RANTES) and transforming growth factor-beta 1 in airway epithelial cells by Mycoplasma pneumoniae. American journal of respiratory cell and molecular biology. 2003;29:344–351. doi: 10.1165/rcmb.2002-0291OC. [DOI] [PubMed] [Google Scholar]
- 29.Yadav UC, Ramana KV, Aguilera-Aguirre L, Boldogh I, Boulares HA, Srivastava SK. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PloS one. 2009;4:e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2004;1:115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 31.Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filep JG. Biasing the lipoxin A4/formyl peptide receptor 2 pushes inflammatory resolution. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18033–18034. doi: 10.1073/pnas.1317798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, Wark PA, Hutchinson A, Irving LB, Levy BD, Anderson GP. Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bozinovski S, Anthony D, Anderson GP, Irving LB, Levy BD, Vlahos R. Treating neutrophilic inflammation in COPD by targeting ALX/FPR2 resolution pathways. Pharmacology & therapeutics. 2013 doi: 10.1016/j.pharmthera.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends in pharmacological sciences. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kajino T, Ren H, Iemura S, Natsume T, Stefansson B, Brautigan DL, Matsumoto K, Ninomiya-Tsuji J. Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. The Journal of biological chemistry. 2006;281:39891–39896. doi: 10.1074/jbc.M608155200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng H, Li Q, Chen R, Zhang J, Ran Y, He X, Li S, Shu HB. The dual-specificity phosphatase DUSP14 negatively regulates tumor necrosis factor- and interleukin-1-induced nuclear factor-kappaB activation by dephosphorylating the protein kinase TAK1. The Journal of biological chemistry. 2013;288:819–825. doi: 10.1074/jbc.M112.412643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. American journal of respiratory cell and molecular biology. 2009;41:631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 42.Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respiratory care. 2003;48:1204–1213. discussion 1213-1205. [PubMed] [Google Scholar]
- 43.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature immunology. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 45.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. British journal of pharmacology. 2008;153(Suppl 1):S200–215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HN, Kundu JK, Cha YN, Surh YJ. Resolvin D1 stimulates efferocytosis through p50/p50-mediated suppression of tumor necrosis factor-alpha expression. Journal of cell science. 2013;126:4037–4047. doi: 10.1242/jcs.131003. [DOI] [PubMed] [Google Scholar]