Abstract
Direct interaction of α9βl integrin with nerve growth factor (NGF) has been previously reported to induce pro-proliferative and pro-survival activities of non-neuronal cells. We investigated participation of p75NTR in α9βl integrin-dependent cellular response to NGF stimulation. Using selective transfection of glioma cell lines with these receptors, we showed a strong, cation-independent association of α9 integrin subunit with p75NTR on the cellular membrane by selective immunoprecipitation experiments. Presence of the α9/p75NTR complex increases NGF-dependent cell adhesion, proliferation and migration. Other integrin subunits including β1 were not found in complex with p75NTR. FRET analysis indicated that p75NTR and α9 integrin subunit are not closely associated through their cytoplasmic domains, most probably because of the molecular interference with other cytoplasmic proteins such as paxillin. Interaction of α9βl integrin with another ligand, VCAM-1 was not modulated by the p75NTR. α9/p75NTR complex elevated NGF-dependent activation of MAPK Erk1/2 arty for integrin that may create active complexes with other types of receptors belonging to the TNF superfamily.
Keywords: integrin α9βl, p75NTR, receptor complex, cells adhesion, cell signaling
1. Introduction
Integrins are the major cell surface receptors responsible for tissue organization, as well as regulation of variety of cell activities. Their proper functioning is important for the development of mammalian organisms beginning at the embryonic stage and for further physiological processes. They require heterodimerization of α and β subunits to create an active conformation to bind a ligand. Currently, 18 α and 8 β subunits have been identified, which are associated with each other in a restricted manner to interact with specific ligands, creating 24 distinct α/β heterodimers [1]. In order to be activated many integrins require external or internal stimuli to gain the proper conformation for ligand binding [2]. This phenomenon is correlated with signaling, which may occur in a bidirectional manner: “outside-in” and “inside-out”. Many cytoplasmic proteins such as talin, kindlin, paxillin, which bind the integrin C-terminus, were characterized as effectors involved in integrin activation and signaling [3–6]. On the other hand, binding of extracellular ligands to integrins induces downstream intracellular signal transduction, which may induce specific responses including cell adhesion, migration and proliferation. The downstream activity of integrins often overlaps with signaling pathways induced by growth factor receptors (GFRs). Therefore, the concept of “cross-talk” between integrins and GFRs has been broadly investigated and discussed [7–10]. However, the mechanism of this cross-talk is poorly understood. The major hypothesis includes concomitant signaling, collaborative signaling, direct activation and amplification of signaling as a result of cooperation between integrins and GFRs on the plasma membrane [7]. To date all GFRs investigated in connection with integrins belong to the family of receptor tyrosine kinese (RTKs). In the presented work, we report a functional association of α9βl integrin with p75NTR, a common neurotrophin receptor of the tumor necrosis factor (TNF) family using transfection reconstruction experiemnts.
α9βl integrin is a receptor that interacts with a variety of extracellular matrix (ECM) proteins, adhesion molecules and certain growth factors. The α9 subunit is structurally related to the α4 integrin subunit that results in the sharing interaction with certain ligands, especially VCAM-1 [11]. However, α9βl integrin has also been reported as a receptor for several ECM proteins such as tensacin-C [12], osteopontin [13], thrombospondin-1 [14] and variety of members of the ADAMs family [15]. Moreover, α9βl directly interacts with two types of growth factors: VEGF [16,17] and NGF [18]. Both of these growth factors stimulate typical promigratory and pro-proliferative activities of cells expressing α9βl integrin, and induce phosphorylation of MAPK Erk1/2 as well as adaptor protein paxillin.
p75NTR is a cell surface receptor for neurotrophins and binds to NGF, BDNF, NT-3 and NT-4/5 with a similar affinity [19]. The biological role of p75NTR is controversial, because it may trigger both pro-survival and pro-apoptotic signals [20]. The pro-survival activity of p75NTR is attributed to a high-affinity complex formed with TrkA, an NGF receptor belonging to the RTK family. Physiologically, p75NTR/TrkA complex formation results in the convergence of signaling pathways that are induced independently by separated receptors into survival or differentiation pathways. Another important activity mediated by p75NTR is stimulation of internalization of the high-affinity complex, which may lead to its polyubiquitination [21].
In the present study, we documented formation of a complex between p75NTR and α9 integrin subunit. Similarly to the high affinity complex (TrkA/p75NTR), firm association of p75NTR with α9 results in an increase of pro-survival and pro-proliferative activities of the cell after stimulation with NGF. Cells expressing the α9/p75NTR complex showed significantly elevated MAPK Erk1/2 and AKT pathways activation in response to NGF.
2. Materials and methods
2.1 Antibodies and other reagents
Polyclonal serum against synthetic α9 subunit of integrin cytoplasmic domain was developed commercially in rabbit (Millipore, Billerica, MA, USA) and validated previously [14]. Other polyclonal sera against α1, α2, α4, α5, and β1 integrin subunits cytoplasmic tail, were purchased from Millipore. A monoclonal antibody (mab) against α9βl (clone Y9A2) was provided by Dr. D. Sheppard (University of California, San Francisco, CA). Anti-p75NTR (clone ME20.4) and anti-paxillin (clone 5H11) mabs were purchased from Millipore. Polyclonal antibodies against phospho and total cell signaling molecules (AKT, Erk, PTEN, paxillin, β-Actin) were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-GFP and anti-p75NTR polyclonal antibodies, and anti-p75NTR (clones H-6 and 192-IgG) mabs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-TrkA polyclonal serum was provided by Dr. L. Reichardt (University of California, San Francisco, CA, USA). β-NGF (mNGF 2.5S) was isolated from mouse submaxillary glands and kindly provided by Alomone Labs (Jerusalem, Israel). bFGF was purchased from Sigma Inc. (St Louis, MO, USA). VLO5 was purified from the venom of Vipera lebetina obtusa using two steps of reverse phase HPLC, as described previously [22]. LM-24, a derivative of caffeine was synthesized as described previously [23].
2.2 Cell lines and transfection
Human glioma cell lines LN18, LN229; human colon adenocarcinoma cell line SW480; human embryonic kidney 293 (HEK293T) cell line; and Chinese hamster ovary (CHO-K1) cell line were purchased from ATCC (Manassas, VA, USA).
2.2.1 LBC3 cell line development
The LBC3 cell line was developed from GBM tissue after surgical resection performed in Temple University Hospital, Department of Neuroscience. Isolation procedure was performed within one hour after GBM dissection. Tissue was cut into small pieces (approx. 10 mm3), digested with collagenease II and squeezed through a metal mesh. After washing by centrifugation with HBSS, cells were placed in tissue culture flasks in the presence of DMEM containing 20% FBS. Cells were allowing to growth and proliferate for about one week and then trypsynized. Cells were cloned and spontaneously immortalized after several passages. Cells were cultured using DMEM containing 10% FBS. Status of p53 in LBC3 cell line was evaluated by DNA exons sequencing, performed by GenScript USA, Inc. (Piscataway, NJ). Mutation was found in DNA-binding domain (Table S1).
2.2.2. Transfection of glioma cell lines
Transfection of LN18 and LBC3 cell lines with α9 integrin subunit was performed using the pcDNAIneo α9 expression plasmid and lipofectamine (Invitrogen, Carlsbad, CA, USA) method. Positive clones of LN18α9+ and GFP-LN18α9+, as well as LBC3α9+ cells were selected with G-418 (1 mg/ml). The same methodology for stable transfection and selection of positive clones was applied for generating the LBC3p75+ cell line, using the pcDNA™3.2/GW/D-TOPO expression vector (GenScript, Piscataway, NJ, USA). Generation of a double transfected LBC3 cell line containing α9β1 integrin and p75NTR (LBC3α9+/p75+) was performed starting from LBC3p75 cells. Since we used antibiotic (G-418) resistant genes in both constructs, we developed a new immuno-adhesion methodology for the selection of p75LBC3 cells that were also positive for α9β1 integrin (LBC3α9+/p75+). Briefly, after transfection with pcDNAIneoα9 plasmid LBC3p75 cells were propagated in media containing G-418. Cells were detached with 5 mM EDTA in HBSS and plated in a 6-well plate previously coated with anti-α9βl integrin antibody (clone Y9A2) and blocked with 1% BSA. The plate was incubated for 30 min at 37°C; unattached cells were removed by intensive washing (at lease five times) with DMEM. Attached cells were removed by scraping, transferred to new tissue culture dishes and grown in standard media (DMEM containing 10% FBS and 1 mg/ml G-418). The new colonies were separated by cloning rings. The presence of α9 and p75NTR receptors in the separated clones was assessed by flow cytometry and adhesion assay using intact cells, as well as in Western blot analysis of cell lysates.
2.2.3 Transfection of cells with fluorescein tag for FRET analysis
The following plasmids were obtained from Addgene and their identification numbers are included: p75-RFP (Addgene plasmid 24092, Moses Chao) and integrin alpha9 EGFP-N3 (Addgene plasmid 13600, Dean Sheppard). HEK293T and CHO-K1 cells were maintained according to ATCC protocols. Transient transfection was performed using TransIT®-LT1 (Mirus Bio LLC, Madison, WI, USA) according to the manufacturer’s protocol.
2.3. Immunoprecipitation and Western blot
Cells were lysed using Triton X-100 lysis buffer supplemented with inhibitors of proteases and phosphatases as described previously [14]. The concentration of cell lysate was adjusted to 1 mg/ml and then the respective antibody (6 μg/ml) was added. The reaction mixture was gently rocked overnight at 4°C. The immune complex was captured by mixing with slurry of Protein A agarose beads and further rocked at 4°C for 2 hours. Agarose beads were pelleted by centrifugation and washed in lysis buffer. Western blotting was performed as described earlier [14]. Separation of membrane fraction from other cell components was performed using Subcellular Protein Fractionation Kit (Thermo Sci.) according to the manufacturer instruction.
Tissue lysates from rat organs were prepared as described previously [18]. The same lysis protocol was applied from human GBM tissues obtained from four different surgical resections, performed in Temple University Hospital, Department of Neurosurgery.
2.4. ELISA assay
Integrin α9βl was purified from the lysates of LN229 or LBC3α9+/p75+ cell lines, using VLO5-agarose or Y9A2 mab immuno-affinity column chromatography as previously described [22]. Proteins retained on the column were eluted using 5 mM EDTA for VLO5-agarose and 100 mM glycine buffer, pH = 2.7 for Y9A2-agarose column, respectively, and analyzed by colorimetric ELISA assay.
2.5. Immunocytochemistry
Cells were grown on glass tissue culture chamber slides. After fixing (20 min) with 4% paraphormaldehyde, cells were permeabilized for 5 minutes on ice with 0.2% Triton X-100 in PBS. Chambers were blocked by incubation with 10% horse or/and goat serum in 1% BSA for 1 hour at room temperature and primary antibody or antibodies (for double staining) were added. Incubation was continued for another 1–2 hours. After three times washing with PBS, appropriate FITC (green) or Texas Red (red) conjugated secondary antibodies were added and incubated for 1 hour. Chambers were washed with PBS and coverslips were applied in mounting medium containing DAPI. Cells were observed under fluorescent microscope (Olympus IX81) with a 40x oil objective. MetaMorph digital imaging software was used for analysis.
The co-localization parameters were calculated using ImageJ and the JACoP plugin [24]. Photomicrographs of same size acquired in different channels were subjected to manual thresholding using ImageJ (v. 1.45S) and the Manders’ coefficient was calculated using the JACoP plugin [24]. The thresholding method was validated and used to estimate the Pearson’s coefficient (Fig. S4D) using the standard image set available from the Colocalization Benchmark Source (Image set CBS001RGM-CBS010RGM, www.colocalization-benchmark.com).
2.5.1. Confocal microscopy and FRET analysis
Cells were transferred to poly-D-lysine (Sigma-Aldrich Corporate, St. Louis, MO) precoated coverslips (12 mm) and maintained overnight at 37°C. In some experiments the cultures were pre-treated for 30 min with 50 ng/ml NGF before submission to FRET analyses. Fluorescent images were acquired on a FluoView FV1000 confocal system (Olympus) using a 63X/1.35 UPL-SAPO objective (Olympus). The acquired images were cropped and composed into figures with Adobe Photoshop. No image enhancement procedures were performed. FRET was measured by the donor-sensitized acceptor fluorescence technique as described previously [25,26]. Briefly, up to six images were acquired for each set of measurements: RFP excitation/RFP emission image (RFP channel), GFP excitation/GFP emission image (GFP channel), and GFP excitation/RFP emission image (FRET channel). A set of reference images was acquired from single-labeled GFP- or RFP-expressing cells for each set of acquisition parameters, and a calibration curve was derived to allow elimination of the non-FRET components from the FRET channel. The FRET efficiency (FRETeff) was calculated on a pixel-by-pixel basis using the following equation: FRETeff = FRETcorr/(FRETcorr + GFP) × 100%, where FRETcorr is the pixel intensity in the corrected FRET image, and GFP is the intensity of the corresponding pixel in the GFP channel image.
2.6. Immunohistochemistry
Tissues of GBM were obtained from surgical resection performed in Temple University Hospital, Department of Neurosurgery. Immunohistochemistry was performed with formalin fixed, paraffin-embedded tissue as previously described [27]. Briefly, formalin-fixed, paraffin-embedded tissue was sectioned at a 4 μm thickness, deparaffinized in xylene, hydrated through descending solutions of ethanol to water. For non-enzymatic antigen retrieval the slides were soaked in 0.01 M sodium citrate buffer (pH 6.0) and heated to 95°C for 40 min in a vacuum oven. After cooling, the slides were rinsed in PBS and incubated in methanol/3% H2O2 for 20 min to quench endogenous peroxidase activity. After blocking with 5% normal horse and goat serum, the slides were incubated overnight at room temperature in a humidified chamber with primary antibodies. Following washing with PBS the sections were incubated with biotinylated-labeled secondary antibodies, color was developed with a diaminobenzidine substrate, counterstained with Hematoxylin and mounted with Permount. For double labeling, the slides were incubated with FITC-streptavidin (green) and Texas Red-streptavidin (red). Digital images were captured using an Olympus A×70 microscope and with magnifications of 400×.
2.7. Cell adhesion assay
Cell adhesion was evaluated using cells labeled with CellTracker™ Green CMFDA (Invitrogen Inc.) as described previously [28].
2.8. Cell proliferation assays
2.8.1 Cell viability assay
Cell viability was determined by trypan blue exclusion. In brief, cells were grown in 6-well plates to 60–70% of confluence and serum-starved for 24 hours. NGF (100 ng/ml) was added in serum-free DMEM. Cells were trypsinized and counted every 24 hours, for a total 72 hours. Dead cells were distinguish by staining with trypan blue and not counted.
2.8.2. BrdUrd assay
Cell proliferation was assessed using a commercial 5-bromodeoxyuridine kit (BrdUrd, Roche, Mannheim, Germany) according to manufacturer instruction.
2.9. Wound healing in vitro assay
Cells were grown on 6-well plates to confluence and serum-starved for 24 hours in DMEM. The monolayers were gently scraped with a plastic tip to produce a wound area. The cultures were washed and new DMEM supplemented with 1% FBS was added in the presence or absence of NGF (100 ng/ml). Progression of wound closing was monitored by taking phase-contrast pictures at a magnification of 100× at times 0 and 24 hours. Images were analyzed using ImageJ software. The percentage of “wound closure” was calculated after 24 hours and normalized to the wound area at time 0.
2.10. Statistical analysis
Where appropriate the data were analyzed using 1-way ANOVA with all pairwise multiple comparison procedures (Holm-Sidak method) using SigmaStat (SPSS Inc.). Statistical significance was assigned for p< 0.05.
3. Results
3.1. p75NTR is associated with the α9 integrin subunit
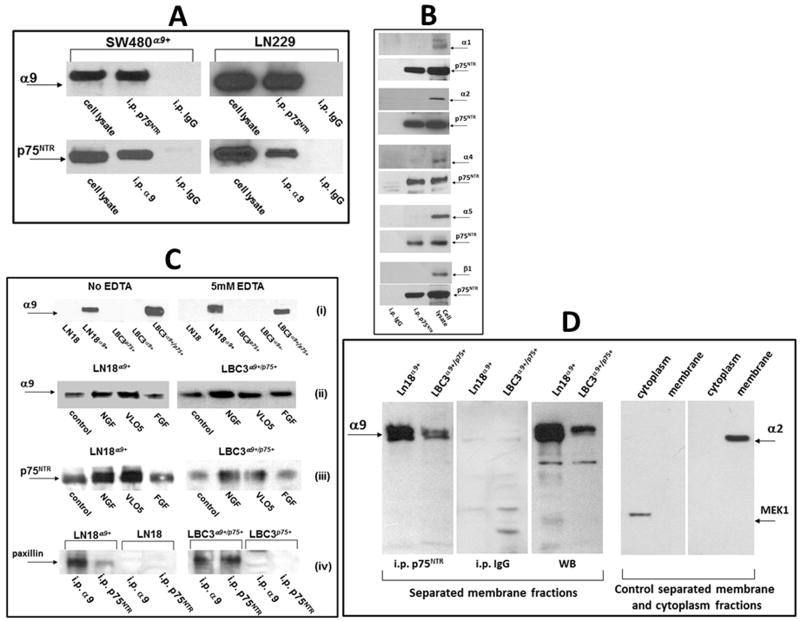
α9β1 integrin was previously characterized as a receptor for NGF responsible for the pro-survival and pro-proliferative activities of this growth factor [18]. p75NTR supports the activity of TrkA by forming a high affinity complex. Therefore, we hypothesized that a similar association on the cellular membrane of α9βl integrin with p75NTR may occur and enhance cell signaling and augment the pro-survival and pro-proliferative activities of NGF. To test this hypothesis we performed a series of immunoprecipitation experiments. The initial search was carried out in SW480 cells transfected with the α9 integrin subunit and in LN229 cells, which endogenously express α9βl integrin (Fig. 1). The presence of p75NTR in these cells was previously confirmed by Western blot and RT-PCR analysis [18]. Following immunoprecipitation with the anti-p75NTR mab and blotting with an anti-α9 antibody, the α9 integrin subunit was clearly observed in the α9SW480 and LN229 cells (Fig. 1A). The reverse procedure performed using anti-α9βl mab for immunoprecipitation also showed a positive band, when blotted with anti-p75NTR. Several other α subunits besides α9 are expressed on LN229 cells e.g. collagen receptor integrins subunits, α1 and α2 as well as a fibronectin receptor α5 integrin subunit and none of these α subunits were able to form immunoprecipitants with p75NTR (Fig. 1B). Particularly, it should be noted that α4 was not found in complex with p75NTR, since this integrin subunit is structurally homologous to α9. Furthermore, the β1 integrin subunit did also not complex with this neurotrophin receptor (Fig. 1B).
Fig. 1.
Detection of the α9/p75NTR complex in cellular lysates by immunoprecipitation. (A) Lysates of SW480α9+ and LN229 non-starved cells were immunoprecipitated with monoclonal antibodies against the indicated receptors. Detection of associated protein was performed by WB using polyclonal antibodies. Isotopic IgG for each of precipitation experiments was used as a control. (B) Lysates of LN229 cells were immunoprecipitated with anti-p75NTR mab and WB was performed with polyclonal antibodies against the indicated integrin subunits or against p75NTR. Whole lysates of LN229 cells were applied for the control detection of integrin subunits (right lines). (C) (i) Immunoprecipitation with anti-p75NTR mab from lysates of indicated cell lines in the presence or absence of EDTA; (ii) immunoprecipitation with anti-p75NTR mab or (iii) anti-α9βl mab from lysates of indicated cells previously treated or not for 60 min. with NGF (100 ng/ml), VLO5 (1μM) and bFGF (100 ng/ml), WB was performed with anti-α9 or anti-p75NTR polyclonal antibodies; (iv) immunoprecipitation with indicated mabs from cells expressing or not the α9/p75NTR complex, detection of associated paxillin was performed by WB using a polyclonal antibody. Graphic intensities of bands are presented on plots in Fig. S2. (D) Detection of the α9/p75NTR complex in separated membrane fractions by immunoprecipitation. Membrane fractions were separated from other cellular components using Subcellular Protein Fractionation Kit. Immunoprecipitation with anti-p75NTR mab or control isotopic IgG, as well as control Western blot (WB) with anti-α9 polyclonal antibody is presented in left panel. Efficiency of separation of membrane from cytoplasmic subcellular fractions was evaluated by Western blot detection of positive markers, MEK-1 for cytoplasm and α2 integrin subunit for membrane (right panels).
LN229 and SW480 cell lines were not suitable for testing the activity of the α9/p75NTR complex, since they both express TrkA, which induces similar cellular responses to NGF treatment as α9βl integrin. Therefore, we developed a TrkA-free system using other glioblastoma cell lines. LN18 cells do not express TrkA, but contain p75NTR (Fig. S1). Transfection of these cells with the α9 integrin subunit was useful for investigation of the activities of cells containing the α9/p75NTR complex vs. cells that expressed only the common neurotrophin receptor. Interestingly, the expression of p75NTR in the SW480 cells significantly increased after transfection with the α9 integrin subunit, although this trend was not observed for the LN18 glioma cell line (Fig. S1). Unfortunately, the LN18 cell system was not appropriate for the comparison of the activity of cells containing the α9/p75NTR complex and cells expressing only α9βl integrin. Therefore, we established a new cell line from human glioma tissue, which was intrinsically negative for all three NGF receptors (Fig. S1). This cell line, named LBC3, was first transfected with α9 (LBC3α9+) and p75NTR (LBC3p75+) separately, and then double transfected with both receptors (LBC3α9+/p75+). The presence of the α9/p75NTR complex in LN18 α9+ and LBC3 α9+/p75+ cells was ascertained through co-immunoprecipitation (Fig. 1C). Formation of this complex was cation-independent and the level of precipitant was similar despite of EDTA treatment. This complex was also formed in the absence of any α9βl integrin ligands, although presence of NGF appreciably increased the association ratio between α9 and p75NTR in LN18α9+ and LBC3 α9+/p75+ cells (Fig. 1C, Fig. S2). Snake venom MLD-disintegrin, VLO5 as a ligand for α9βl integrin, was also effective to increase α9/p75NTR complex level, in particular for LN18α9+ cells. FGF used as a factor, which is not a ligand for α9βl integrin, immunoprecipitated α9/p75NTR complex on the control level. Paxillin is an adaptor protein, which binds the cytoplasmic domain of the α9 integrin subunit [6]. Co-immunoprecipitation of this protein with α9 integrin subunit and p75NTR was observed for cells expressing both of these receptors, whereas in the case of cells expressing only p75NTR no paxillin was detectable in the precipitates (Fig. 1C, Fig. S2). Detection of α9/p75NTR complex was also confirmed in the membrane fractions. p75NTR co-immunoprecipitated with α9 integrin subunit in membrane lysates separated from of LN18α9+ and LBC3 α9+/p75+ cells (Fig. 1D). Two clones, ME20.4 and H-6 of anti p75NTR mabs were effective for immunoprecipitation of α9 integrin subunit. Control immunoprecipitation experiments were performed with isotopic rabbit or mouse IgG showing no respective bands on Western blot.
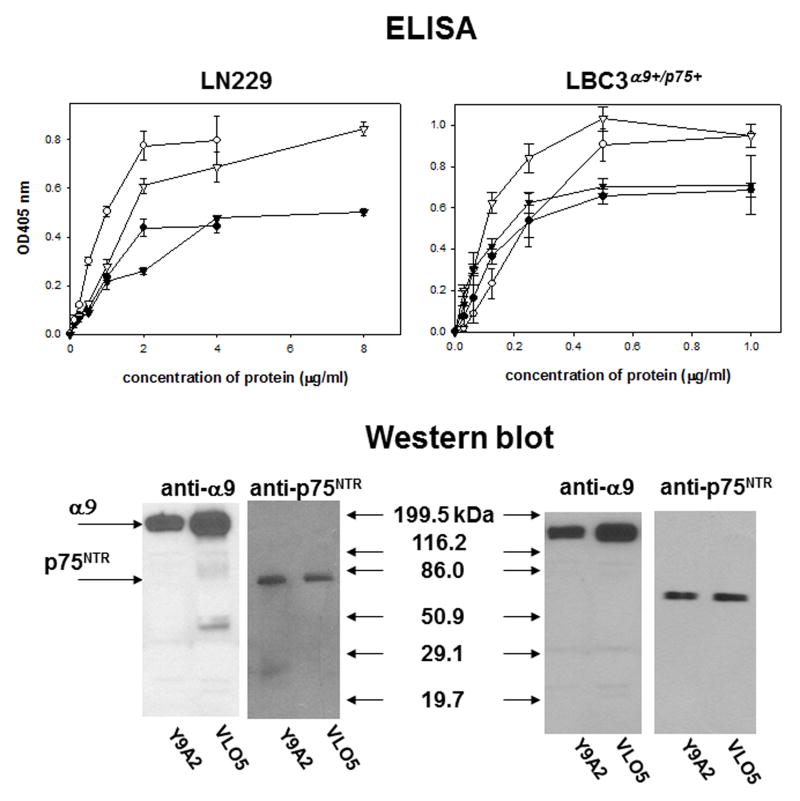
p75NTR and α9βl integrin form a stable complex, which is resistant to purification procedures on an affinity column. It is retained on columns with resin-coupled snake venom disintegrin VLO5 and Y9A2 mab. The presence of both α9βl integrin and p75NTR in the eluted preparations was detected in ELISA and Western blot (Fig. 2). These two receptors were co-purified from LN229 cells, which express them endogenously, as well as from LBC3α9+/p75+ cells, in which they are over-expressed after recombinant transfection. Bands with appropriate masses were also visible on the coomassie blue-stained SDS-PAGE (Fig. S3).
Fig. 2.
Detection of the α9/p75NTR complex in preparations obtained from affinity chromatography. Lysates of LN229 and LBC3α9+/p75+ cells were applied to affinity columns containing resins coupled with VLO5 or Y9A2. The retained proteins were eluted with 5 mM EDTA from the VLO5 column and low pH = 2.7 from the Y9A2 mab column. Identification of α9βl integrin and p75NTR was performed in ELISA with different concentrations of immobilized proteins obtained from the VLO5 column (circles) and Y9A2 column (triangles). Y9A2 mab was used for detection of the integrin (open symbols) and ME20.4 mab for detection of p75NTR (filled symbols). Error bars represent the standard deviation from three independent experiments. Western blot analysis of the purified proteins was performed using anti-α9 polyclonal antibody and anti-p75NTR mab.
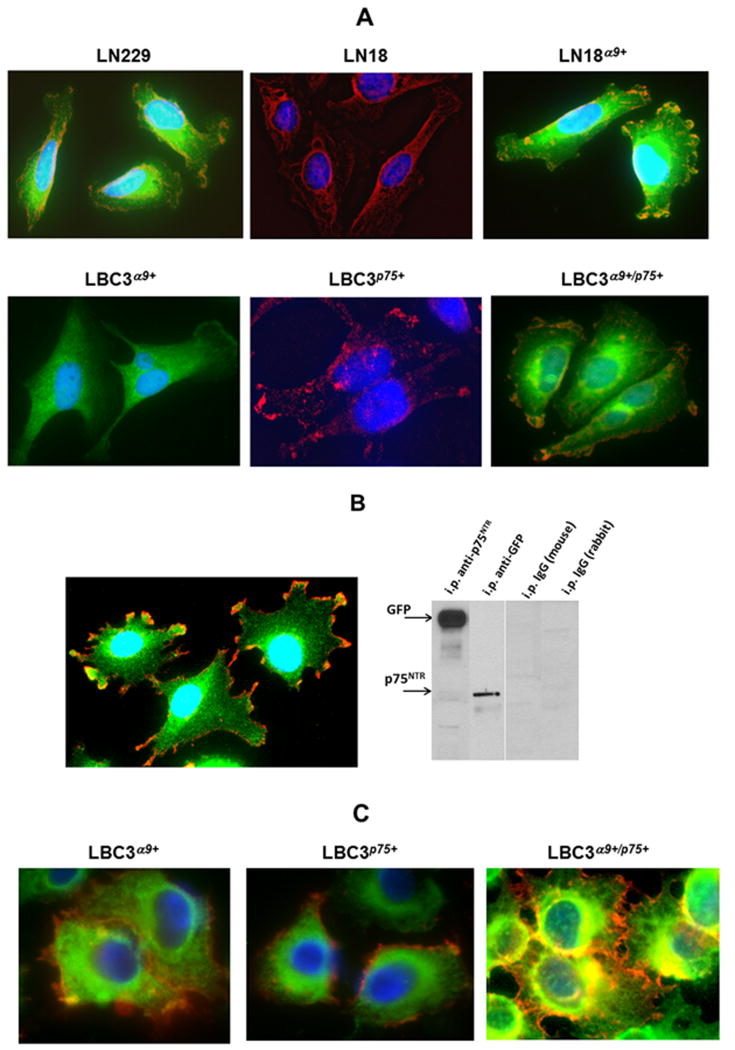
Co-localization of the α9 integrin subunit with p75NTR in the same regions of cells was also confirmed by an immunocytochemistry (Fig. 3A). LN229 cells endogenously expressing both receptors, showed areas of overlapping colors specifically stained with antibodies against the α9 and p75NTR under fluorescence microscope. Similar overlapping was also characteristic for LN18α9+ cells, which endogenously express p75NTR and for LBC3α9+/p75+ cells transfected with both cell surface receptors. Cells expressing only one of these receptors showed a single color. Co-localization of both receptors was observed in the cells transfected with GFP-tagged α9 integrin subunit (Fig. 3B). Overlapping areas of GFP with anti-p75NTR mab may facilitate formation of α9/p75NTR complex that was obtained by immunoprecipitation with anti-GFP polyclonal antibody. Immunocytochemistry also showed co-localization of paxillin with the α9 integrin subunit in LBC3α9+ cells (Fig. 3C). On the other hand, in the absence of the α9, paxillin does not co-localize with p75NTR. In LBC3α9+/p75+ cells there are also large overlapping areas of immune staining for paxillin and p75NTR. The overlapping of α9 and p75NTR in analyzed areas the cells was confirmed by calculation of Pearson’s coefficient (Fig. S4). This analysis revealed these two receptors overlap in 60–80%. These findings further support the hypothesis of a macromolecular complex composed of α9 and p75NTR transmembrane receptors, as well as cytoplasmic molecule, paxillin bound to the C-terminal domain of the integrin α9 subunit that was found by immunoprecipitation (Fig. 1C).
Fig. 3.
Immunocytochemical identification of the co-localization of α9 integrin subunit with p75NTR and paxillin. (A) Detection of co-localization of α9 and p75NTR. Cells were grown on a glass slide and fixed with 4% paraformaldehyde. Slides were blocked with 1% BSA supplemented with goat and horse serum and incubated with mixture of primary antibodies against α9 (polyclonal) and p75NTR (monoclonal). Slides were incubated with secondary FITC-goat anti-rabbit and Texas red-horse anti-mouse IgG and after washing analyzed using an Olympus IX81 fluorescent microscope with 400x magnification. (B) Detection of co-localization of GFP-tagged α9 integrin subunit with p75NTR by immunocytochemistry (left image). LN18GFP-α9+ cells were stained with polyclonal anti-GFP and monoclonal anti-p75NTR antibodies for fluorescence microscope imagining as described above. Immunoprecipitation of GFP-α9 with anti-p75NTR mab and p75NTR with anti-GFP polyclonal antibody (right image). Immunoprecipitations with isotopic control IgGs are indicated. (C) Detection of a co-localization of the paxillin with α9 integrin subunit. LBC3α9+ cells were stained with anti-α9 polyclonal antibody and anti-paxillin mab. LBC3p75+ and LBC3α9+/p75+ cells were stained with anti-paxillin polyclonal antibody and anti-p75NTR mab. In all images proteins stained with polyclonal antibodies are presented as green and mabs as red. Overlapping areas are yellow.
Presence of paxillin attached to the cytoplasmic tail of α9 integrin may explain the occurrence of very weak FRET between α9 integrin subunit and p75NTR (Fig. S5). HEK293T cells transfected with α9-GFP and p75-RFP exhibited very limited FRET. Cells treated (Fig. S5B) or not treated (Fig. S5A) with NGF showed similar FRET efficiency. Analogous results were obtained in other cell transfection system of CHO cells (data not shown). Positive FRET requires that the donor and acceptor molecules must be in very close proximity (<10 nm). Therefore, we can conclude that α9 and p75NTR are not in close distance in the complex, which contains other cytoplasmic elements such as paxillin.
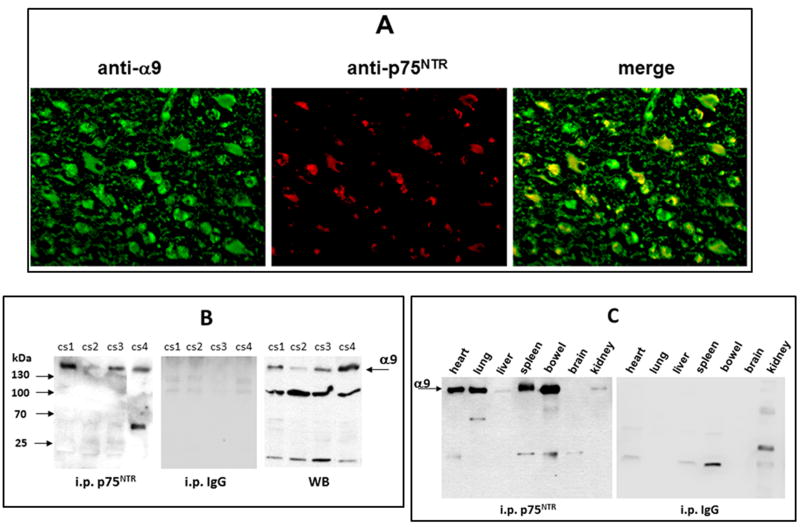
The expression of the α9 and p75NTR was also investigated in vivo in human brain cancer tissue and in normal rat tissue of different organs (Fig. 4). Paraffin sections of GBM stained with fluorescence-labeled antibodies revealed co-localization of α9 and p75NTR. Many cells in the tissue were positively stained for anti-α9 polyclonal antibody (green), whereas the staining for p75NTR (red) was significantly more restricted (Fig. 4A). Interestingly, the vast majority of cells/areas positive for p75NTR overlapped with those also expressing the α9 integrin subunit. Based on Pearson’s coefficient calculation expressed in vivo p75NTR overlaps with α9 integrin subunits in 100%. This indicates that majority of the p75NTR is co-localized with α9 integrin subunit to the same regions of the cells. This possibility was verified by immunoprecipitation from tissue lysates (Fig. 4B). Four different cases of GBM were investigated in these assays. Formation of α9/p75NTR complex was also confirmed in normal rat organs, by i.p. from tissue lysates (Fig. 4C). I.p. was negative only in the brain tissue, in which α9βl integrin is absent (Fig. S6).
Fig. 4.
Detection of the α9/p75NTR complex in vivo in GBM and normal tissues. (A) Representative images of immunohistochemical detection of co-localization of α9 and p75NTR in the paraffin sections of human GBM. The slides were incubated with polyclonal anti-α9 integrin subunit (green) and with anti-p75NTR mab (red). Co-localization areas of both receptors are in yellow. Pearson’s coefficient was calculated as approximately 1. (B) Immunoprecipitation of α9/p75NTR complex by p75NTR mab from human surgical GBM tissue lystes of four cases. Detection of α9 integrin subunit was performed in WB by anti-α9 polyclonal. I.p. by isotopic IgG was performed as a control. Presence of α9 integrin in tissue lysates before i.p. (direct Western blot) is presented in right panel. (C) Immunoprecipitation of α9/p75NTR complex by p75NTR mab from lysates of different rat organs. Isotopic IgG was used for control.
3.2. Formation of the α9/p75NTR complex is relevant for NGF-dependent cellular responses
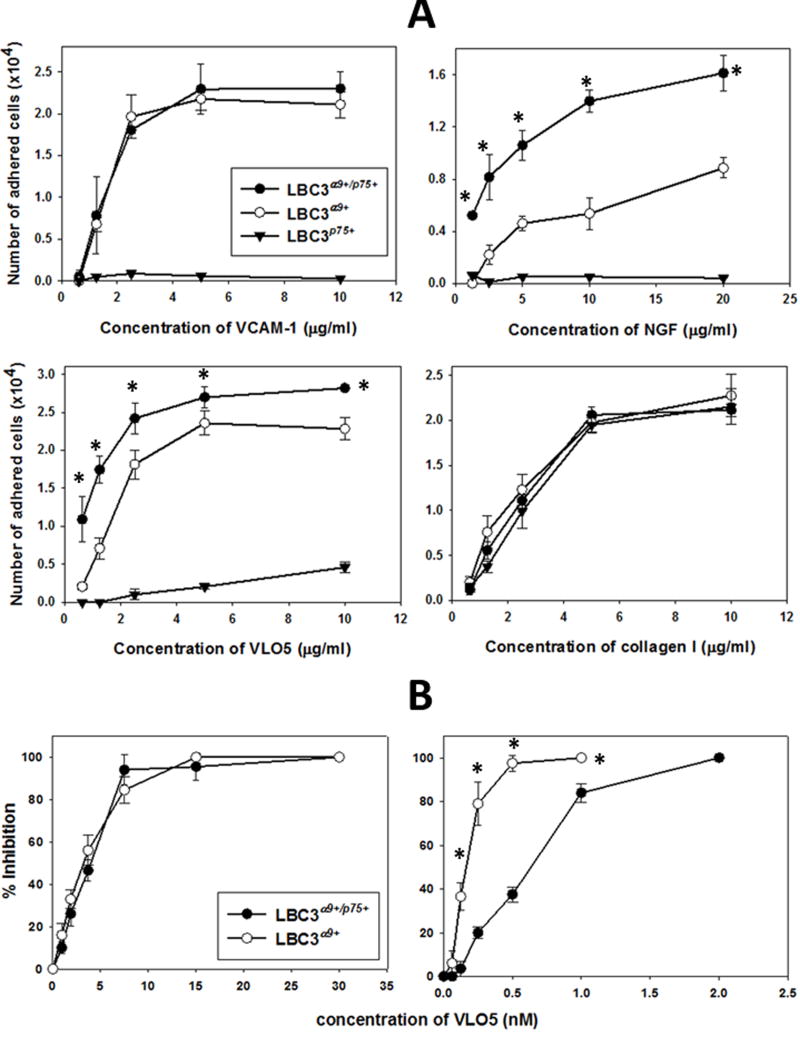
A series of in vitro experiments were performed to evaluate the effect of formation of the α9/p75NTR complex in response to stimulation of the receptors with their ligands. Pro-adhesive properties of cells were investigated using specific ligands for α9βl integrin (Fig. 5). LBC3α9+/p75+ cells adhered to VCAM-1 with the same potency as LBC3α9+ cells (Fig. 5A). On the other hand, LBC3α9+/p75+ cells adhered to immobilized NGF with a two-fold higher affinity than LBC3α9+ cells. A similar trend was also observed for the adhesion of these cells to the snake venom disintegrin VLO5, although the difference between both cell lines was significantly lower. LBC375+ did not adhere to nether VCAM-1 or NGF and only to very high concentrations of immobilized VLO5. This phenomenon is probably related to the ability of this snake venom disintegrin to interact with other integrins. Previously published studies showed a low cross-reactivity of VLO5 with α5βl integrin [22], which is present on LBC3 cells (data not shown). LBC3 cells also endogenously express the collagen receptor, α2βl integrin. Therefore, equal adhesion of all three types of LBC3 transfectants to collagen type I was selected as a positive control. The differential adhesion of α9βl integrin expressing cells to VCAM-1 and NGF was correlated with the ability of VLO5 to inhibit the adhesion of both cell types to either of these ligands (Fig. 5B). The inhibitory effect of the disintegrin to bind VCAM-1 was almost identical for LBC3α9+/p75+ and LBC3α9+ cells (IC50 = 4 nM). NGF as an immobilized ligand bound significantly stronger with LBC3α9+/p75+ cells (IC50 = 0.63 nM) than with LBC3α9+ cells (IC50 = 0.28 nM), suggesting the importance of the α9/p75NTR complex to increase the affinity of the cells toward this neurotrophin.
Fig. 5.
Interaction of different integrin ligands with cells expressing or not the α9/p75NTR complex in a cell adhesion assay. (A) Adhesion of the LBC3 cell line transfectants to immobilized VCAM-1, NGF, VLO5 and collagen I. Integrin ligands were immobilized on 96-well plate by overnight incubation in PBS at 4°C. Adhesion with CMFDA-labeled cells was performed as described in Material and Methods. (B) Inhibition of adhesion of LBC3α9+/p75+ and LBC3α9+ cell lines to immobilized VCAM-1 (3 μg/ml) and NGF (10 μg/ml) by VLO5. VLO5 was pre-incubated with CMFDA-labeled cells prior to addition to the wells with immobilized ligands. (*) p<0.001 for LBC3α9+/p75+ in comparison with LBC3α9+ cell lines. The error bars represent the standard deviation from three independent experiments.
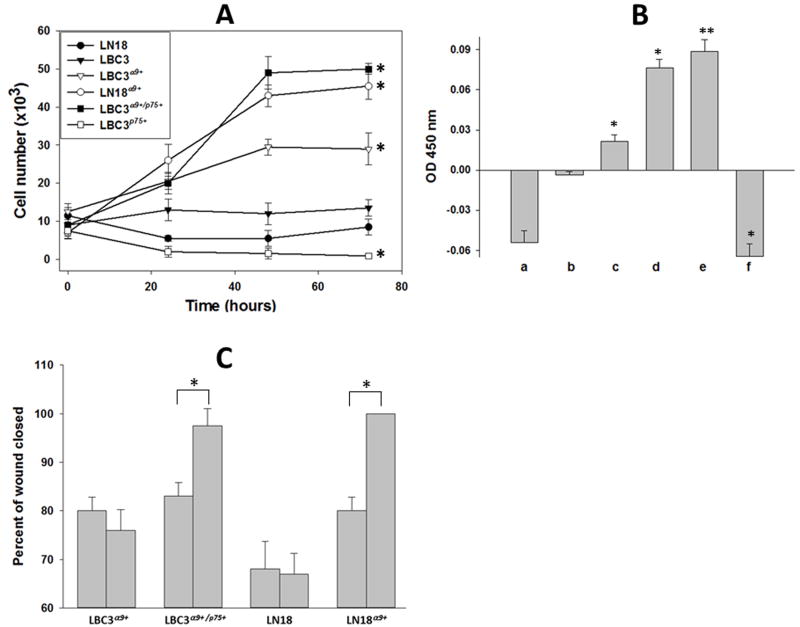
The presence of α9/p75NTR complex on the cell surface also stimulated cell proliferation induced by NGF. LBC3α9+/p75+ cells and LN18α9+ cells demonstrated increased proliferation ratio notably higher than any other tested cells observed in the cell viability assay (Fig. 6A), as well as in a BrdUrd assay (Fig. 6B). LBC3 cells, which lack both receptors, were not responsive to NGF stimulation, whereas cells expressing only α9βl integrin (LBC3α9+) had a slightly elevated proliferation rate. Proliferation of cells expressing p75NTR in the absence of any other NGF receptors was decreased in comparison to control non-treated cells.
Fig. 6.
Effect of NGF on proliferation and migration of cells expressing or not the α9/p75NTR complex. (A) Cell viability assay: Cells were seeded on a 6-well plate and allowed to grow up to 70% of confluence, starved for 24 hours and treated with NGF (100 ng/ml) (time 0). Viable cells were counted every 24 hours. (*) p<0.001 for all types of transfected cells if compare with cells non transfected. (B) Cell proliferation assay: LN18 (a), LBC3 (b), LBC3α9+ (c), LN18α9+ (d), LBC3α9+/p75+ (e) and LBC3p75+ (f) cells were grown on 96-well plate up to 70% confluence and then treated with NGF (100 ng/ml) as above. Cell proliferation was assessed using a colorimetric BrdUrd assay according to the manufacturer’s instruction. The values of absorbance indicate changes related to untreated cells. (*) p<0.01 for transfected LBC3 cells if compare with cell non-transfected; (**) p<0.001 LN18 cells if compare with cells non-transfected. (C) Wound healing assay: Cells were grown on a 6-well plate to reach confluence and serum-starved for 24 hours. Cells were gently scraped with a plastic tip to produce a wound area and treated or not with NGF (100 ng/ml). Progression of wound closing was monitored by taking microscope phase-contrast pictures under 100x magnification. Images after 24 hours were transferred to ImageJ software and percentages of closed areas were calculated. Left bars present non-treated cells, right bars cells treated with NGF. (*) p<0.001. Error bars indicate the standard deviation from three independent experiments.
The in vitro wound healing assay involves complex cellular events including proliferation and migration. Upon stimulation with NGF wound closure by cells expressing the α9/p75NTR complex was more efficient than by cells expressing only α9βl integrin or p75NTR (Fig. 6C). LBC3α9+ and LN18 cells were not responsive to NGF and no significant differences in wound closure between cells stimulated or not with NGF were observed. By the contrast, upon NGF treatment cells expressing both receptors completely filled the area of the wound within 24 hours.
3.3. Formation of the α9/p75NTR complex modulates cell signaling in response to NGF
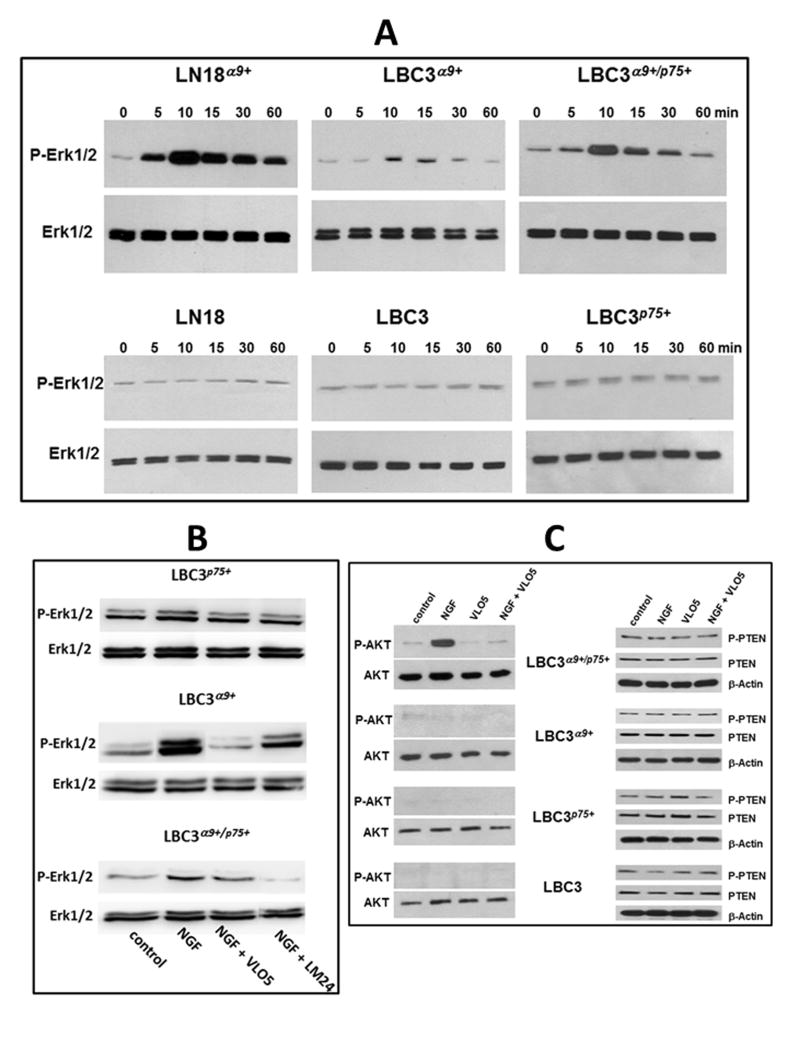
The effect of NGF on stimulation of pro-proliferative and pro-survival cellular pathways was investigated in cells with and without expression of α9βl integrin and p75NTR (Fig. 7, Fig. S8). Upon stimulation with NGF, MAPK Erk1/2 were efficiently and rapidly phosphorylated in LN18α9+ and LBC3α9+/p75+ cells, reaching a maximum after 10 minutes post stimulation (Fig. 7A, Fig. S8A). Cells expressing only α9βl integrin (LBC3α9+) upon NGF treatment showed a transient a very low increase in MAPK Erk1/2 phosphorylation. The absence of α9βl integrin completely abolished activation of this signaling pathway, as inferred from the lack of NGF-inducible of MAPK Erk1/2 phosphorylation via p75NTR. Specific inhibitors of α9βl integrin and p75NTR confirmed requirement of these receptors in NGF-induced activation of MAPK Erk1/2 (Fig. 7B, Fig. S8B). In the LBC3 cells transfection system, VLO5 showed potent inhibitory effect on NGF-stimulated MAPK Erk1/2 in both type of transfectants containing α9βl integrin. Interestingly, LM24 a low molecular weight inhibitor of the interaction of NGF with p75NTR, showed significantly higher inhibitory effect on MAPK Erk1/2 phosphorylation in the LBC3α9+/p75+ cells stimulated with NGF than VLO5, suggesting a leading role of p75NTR for cell signaling through α9/p75NTR complex.
Fig. 7.
The effect of NGF on induction of cell signaling in cells expressing or not α9/p75NTR complex. (A) The effect of NGF stimulation on phosphorylation of MAPK Erk1/2 in LBC3 and LN18 cell lines transfected or not with α9 and/or p75NTR. Serum-starved (24 hours) cells were treated or not with NGF (50 ng/ml) for the indicated time points. Cells were lysed, subjected to SDS-PAGE in 10% gel under reduced conditions and electro-transferred to PVDF membranes. Bands were visualized using anti-phospo-Erk1/2 antibody, and following stripping anti-Erk1/2 antibody as described in Materials and Methods. Graphic evaluation of phosphorylation ratio of cell signaling molecules is presented on Fig. S8A. (B) Effect of blocking of α9βl integrin by VLO5 and p75NTR by LM24 on Erk1/2 activation induced by NGF. Different types of LBC3 transfectants were stimulated with NGF (50 ng/ml) in the presence or absence of VLO5 (100 nM) or LM24 (10 μM) for 10 min. Western blot development and analysis was performed as described above. (C) The effect of NGF and VLO5 on phosphorylation of Akt and PTEN. Serum-starved (24 hours) cells were treated or not with NGF (50 ng/ml) and/or VLO5 (1 μM) for 60 min. The procedure of visualization of bands was performed as described above using antibodies against the indicated proteins.
Activation of the Akt (PKB) cascade specifies the ability of cell to enter and maintain the survival stage. NGF stimulated the phosphorylation of Akt, but only in the cells expressing the α9/p75NTR complex (Fig. 7C, Fig. S8C). In cells expressing individual receptors either α9βl integrin or p75NTR the Akt pathway was not activated upon NGF tratment. Similarly, no effect of NGF stimulation on Akt phosphorylation was seen in control LBC3 cells. VLO5 did not induce phosphorylation of Akt in LBC3α9+/p75+ cells. Moreover, this inhibitor of α9βl integrin antagonized the activity of NGF in this system. PTEN is a negative regulator of the Akt pathway. In majority of gliomas, PTEN is mutated or deleted at the protein level. All cell lines tested, including LBC3 cells exhibit similarly high endogenous levels of phosphorylation of PTEN, which was not altered by any of ligands for α9βl integrin (Fig. 7C).
Discussion
The ability of α9βl integrin to directly interact with growth factors renders this integrin unique amongst the members of this cellular membrane receptor family. The binding of VEGF and NGF to α9βl integrin induces cellular responses, which are similar to those when these growth factors bind to their “traditional” cognate receptors, VEGFRs and TrkA, respectively. Organization of TrkA on the cell surface during its interaction with NGF is unique, because of the engagement of p75NTR in the formation of a high affinity complex. Similar ability to associate with p75NTR was found for α9 integrin subunit. p75NTR forms a complex with the α9 integrin subunit, but not with other β1 integrin family members such as al, α2, α4, α5 (Fig. 1B). This specificity demonstrates that α9βl is exceptional among integrins as a direct receptor for NGF and indicates a supporting role of p75NTR as is also observed for TrkA. TrkA and other RTK neurotrophin receptors also co-precipitate with p75NTR and form stable complexes, which are resistant to detergent (1% Triton X-100) solubilization [29]. The α9/p75NTR complex is stable during purification on an affinity column, either with an immobilized mab or integrin ligand, VLO5 (Fig. 2). We previously reported that both subunits of α9βl integrin are required to bind NGF [18], although the β1 subunit is not directly associated with p75NTR (Fig. 1B). This suggests that the NGF molecule behaves as a typical integrin ligand, whereas the association of p75NTR with α9 integrin subunit may be a biochemically different.
The formation of the α9/p75NTR complex is most probably cation-independent, because it is resistant to EDTA treatment. By contrast the active form of the integrin heterodimer requires the presence of divalent cations, thus binding of p75NTR to the α9 integrin subunit is different than any typical integrin-ligand interaction. Moreover, it appears that the α9/p75NTR complex is constitutively present on the cell surface, although NGF significantly increases its expression (Fig. 1C). On the other hand, cooperation of α9βl integrin with VEGFRs during interaction with VEGF generates a different mechanism of receptor “cross-talk”. Although VEGFR enhanced α9βl integrin-dependent activation of cellular responses, association of these two receptors was only found in the presence of VEGF [17]. VEGFRs belong to RTK family, whereas p75NTR is a member of the TNF family. This difference may determine their diversity in association with α9βl integrin. It is very likely that the complexes TrkA/p75NTR and α9/p75NTR work in a complementary but not cooperative fashion in vivo [30], although formation of larger clusters of TrkA/p75NTR/α9/p75NTR cannot be excluded when all of these molecules are present on the non-neuronal cell surface.
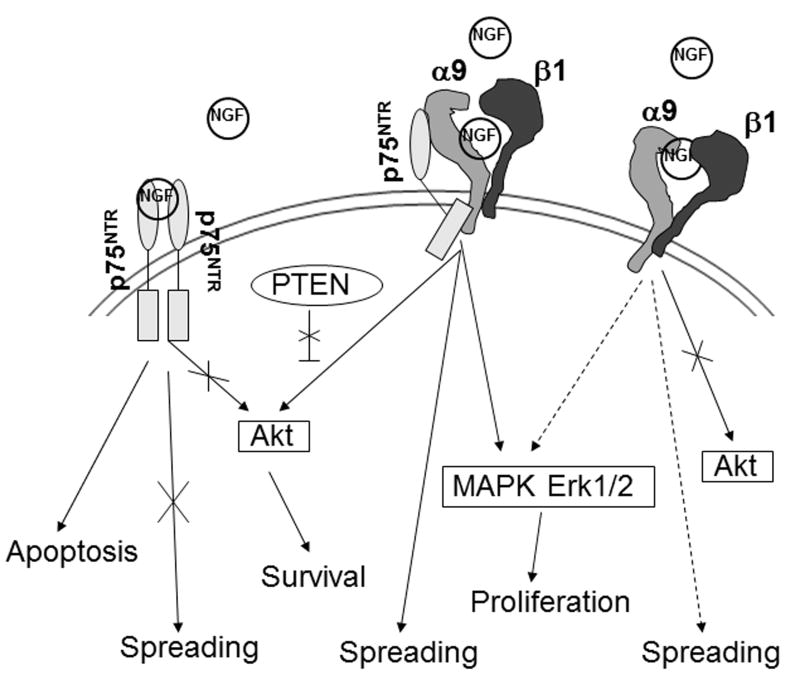
Complex formation between α9βl and p75NTR appears to be an important part of certain pathophysiologies; co-localization of both receptors was found in the GBM tissue by double immunostaining and in immunoprecipitation experiments (Fig. 4). However, the molecular organization of this complex requires further studies. FRET analysis indicates that most probably the distance between cytoplasmic domains of α9 and p75NTR in this complex is larger than 10 nm. That observation suggests that other cytoplasmic protein such as paxillin, participate in this macromolecular complex. This adaptor protein, co-immunoprecipitates with both receptors, although only in the presence of α9 integrin subunit (Fig. 1C). Paxillin was previously found in association with cytoplasmic domain of α9 integrin subunit as a negative regulator of cell spreading [6]. Therefore, it appears to be an important component associated with α9/p75NTR complex. Evaluation of detailed, mechanistic role of paxillin in this complex requires separate studies. However, we can predict that it may work as a bridge spinning cytoplasmic domains of both membrane receptors. On the other hand, lack of its binding to the p75NTR in the absence of α9 suggests that integrin subunit may change conformation of paxillin exposing a binding site for common neurotrophin receptor. Alternatively, paxillin may work as a spacer between cytoplasmic domains of both receptors when α9/p75NTR complex formation occurs through membrane or extracellular domains. Association through extracellular domain is very likely, because cell adhesion experiments showed that the presence of p75NTR is not important for α9βl integrin-dependent cell binding to VCAM-1, whereas it increased adhesion to NGF and VLO5 (Fig. 5). It may suggest that the presence of p75NTR in the closed distance to the α9 subunit of integrin in extracellular space generates conformational changes in the ligand-binding pocket of integrin. VLO5 and NGF have comparable low molecular weights and p75NTR may make this pocket more accessible for these specific ligands (Fig. 8).
Fig. 8.
Schematic summary of cell signaling pathways and cell activities occurring following binding of NGF to the α9/p75NTR complex. Dashed arrows indicate weak activity.
NGF-induced cell proliferation and cell migration were significantly enhanced when the α9/p75NTR complex was present on the cell surface. Expression of α9βl integrin in the absence of p75NTR resulted in a moderate increase of NGF-induced proliferation, whereas the presence of the α9/p75NTR complex strongly elevated the proliferation of LBC3 and LN18 cells. The opposite situation occurred, when p75NTR was expressed alone; cells decreased proliferation in response to NGF stimulation (Fig. 6). The published results from other laboratories indicated a p75NTR-dependent arrest of cell proliferation by NGF in breast cancer [31] and astrocytes [32]. Mechanistic studies revealed that the inhibition of proliferation of these cell types is related to arresting of cell cycle at the G0/G1 phase by decreasing the Rb phosphorylation and increasing p21waf1 level. Interestingly, NGF did not induce p75NTR-dependent apoptosis in these cells, although in other cell types e.g. in neurons and oligodendrocytes it generated a programmed cell death [33,34]. Therefore, we could predict that the inhibition of proliferation of LBC3 and LN18 cells that express p75NTR alone appears to be associated with cell cycle arresting, because both cell lines were derived from astrocytic tumors. Previously, we published that apoptosis may be induced in the LN229 glioma cell line in response to α9βl integrin-dependent binding of MLD-disintegrin, VLO5. NGF abolished the pro-apoptotic effect in this assay [18]. This suggests that the α9/p75NTR complex may transfer a death signal, when the ligand is in a soluble form. This phenomenon may be correlated with internalization of the α9 integrin subunit and translocation to the nucleus (unpublished data). The intracellular domain (ICD) of p75NTR is released by γ-secretase cleavage and translocated to the nucleus, where it modulates gene transcription [35]. Verification of the possibility that the ICD of p75NTR is associated with α9 integrin subunit after cleavage and that this complex is translocated to the nucleus may shed new light on the modulation of cell proliferation and survival by NGF and its receptors.
The effect of NGF on proliferation of cells expressing α9βl integrin in the presence or absence of p75NTR was correlated with activation of the MAPK Erk1/2 pathway. Efficient transient phosphorylation of this signaling molecule was observed only for cells expressing the α9/p75NTR complex, whereas NGF had very little effect on MAPK Erk1/2 phosphorylation in LBC3 cells expressing α9βl integrin alone (Fig. 7A). Specific blockers of two components of α9/p75NTR complex indicated that both of them are important in transferring NGF-induced signals. The high potency of p75NTR inhibitor underlined its importance in activation of this pro-proliferative pathway. This may be correlated with engagement of cytoplasmic domain of p75NTR in activation of up-stream components of MAPK Erk1/2. It is important observation in context of lack of ability of p75NTR alone to activate MAPK Erk1/2. The discussion above suggests supportive role of p75NTR in activating α9βl integrin by changing its conformation. Our cell signaling experiments indicate that integrin may also change conformation of C-terminal domain of p75NTR, which may start to interact with pro-proliferative molecules in the cytoplasm. Two other MAP kinases, p38 and SAPK/JNK were not activated in this assay (data not shown). Activation of the pro-survival PI3K/AKT pathway occurs following stimulation by a majority of growth factors, including NGF after binding to its high affinity complex [36]. Similarly, PI3K/AKT can also be activated through ligand binding to a variety of integrins [37–39], resulting in a cell migration. Our data demonstrate that NGF-induced cell migration requires the formation of the α9/p75NTR complex (Fig. 6), and that this effect correlates with an increase in AKT phosphorylation (Fig. 7C). Moreover, presented results in glioma cell lines confirmed previous observations from primary neurons and the PC12 cell line that lacking of p75NTR displays defects in neurotrophin-dependent Akt activation [40].
Glioblastoma cell lines are useful tools for investigating the cellular functions of PTEN, because 70–80% of this type of brain tumor is deficient in or express inactive mutants of this molecule. Over-expression of PTEN usually down-regulates cell proliferation by diminishing activation of MAPK Erk1/2 that may be stimulated either by NGF receptors [41] or integrins [37]. The LBC3 cells express PTEN, which is constitutively phosphorylated. NGF has no effect on the level of endogenous PTEN or its phosphorylation in LBC3 cells, regardless of the presence or absence of p75NTR or α9βl integrin. We suggest that PTEN does not participate in cell signaling pathways and cell activities induced by α9/p75NTR complex (Fig. 8). Interestingly, recent studies showed that a lack of association of α9βl integrin with its newly identified ligand, emilin, reduced PTEN and increased proliferation in fibroblasts and keratinocytes [42]. This clearly distinguishes the ability of NGF and ECM proteins to modulate α9βl integrin-dependent cell physiology.
In summary, we report a new paradigm of complex formation between an integrin α subunit and another cell surface protein, which belongs to the TNF receptor family. This complex increases the ability of cells to generate robust pro-proliferative and pro-survival signals in response to NGF. Further elucidation of interaction of these receptors might be useful for defining potential therapeutic targets in the efficient modulation of disease states that are affected by neurotrophins and integrins.
Supplementary Material
Highlights.
p75NTR and α9 integrin subunit are functionally associated on the plasma membrane
NGF induces α9/p75NTR complex-dependent cellular responses
α9/p75NTR complex is involved in generation of pro-survival cell signaling
Tissue-expression of α9/p75NTR complex indicates its physiological significance
Acknowledgments
We are grateful to Dr. Dean Sheppard for generously providing many of the reagents for investigating α9βl integrin and for his valuable comments during the preparation of the manuscript. This work was supported by National Institutes of Health National Cancer Institute [grant number R01CA133262 to CM.]; grant from The Louis & Bessie Stein Family Foundation (to P.I.L. and P.L.); David R. Bloom Center of Pharmacy and Dr. Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at The Hebrew University of Jerusalem, Israel (to P.L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Shattil SJ, Kim C, Ginsberg MH. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthis NJ, Campbell ID. Trends Biochem Sci. 2011;36:191–198. doi: 10.1016/j.tibs.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouaouina M, Calderwood DA. Curr Biol. 2011;21:R99–101. doi: 10.1016/j.cub.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Moser M, Legate KR, Zent R, Fässler R. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 6.Young BA, Taooka Y, Liu S, Askins KJ, Yokosaki Y, Thomas SM, Sheppard D. Mol Biol Cell. 2001;12:3214–3225. doi: 10.1091/mbc.12.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivaska J, Heino J. Annu Rev Cell Dev Biol. 2011;27:291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- 8.Streuli CH, Akhtar N. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- 9.Giancotti FG, Tarone G. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 10.Porter JC, Hogg N. Trends Cell Biol. 1998;8:390–396. doi: 10.1016/s0962-8924(98)01344-0. [DOI] [PubMed] [Google Scholar]
- 11.Taooka Y, Chen J, Yednock T, Sheppard D. J Cell Biol. 1999;145:413–420. doi: 10.1083/jcb.145.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokosaki Y, Matsuura N, Higashiyama S, Murakami I, Obara M, Yamakido M, Shigeto N, Chen J, Sheppard D. J Biol Chem. 1998;273:11423–11428. doi: 10.1074/jbc.273.19.11423. [DOI] [PubMed] [Google Scholar]
- 13.Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y, Sheppard D. J Biol Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 14.Staniszewska I, Zaveri S, Del Valle L, Oliva I, Rothman VL, Croul SE, Roberts DD, Mosher DF, Tuszynski GP, Marcinkiewicz C. Circ Res. 2007;100:1308–1316. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- 15.Eto K, Huet C, Tarui T, Kupriyanov S, Liu HZ, Puzon-McLaughlin W, Zhang XP, Sheppard D, Engvall E, Takada Y. J Biol Chem. 2002;277:17804–17810. doi: 10.1074/jbc.M200086200. [DOI] [PubMed] [Google Scholar]
- 16.Vlahakis NE, Young BA, Atakilit A, Sheppard D. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, Sheppard D. J Biol Chem. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 18.Staniszewska I, Sariyer IK, Lecht S, Brown MC, Tuszynski GP, Safak M, Lazarovici P, Marcinkiewicz C. J Cell Sci. 2008;121:504–513. doi: 10.1242/jcs.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao MV. J Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- 20.Nykjaer A, Willnow TE, Petersen CM. Curr Opin Neurobiol. 2005;15:49–57. doi: 10.1016/j.conb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Geetha T, Jiang J, Wooten MW. Mol Cell. 2005;20:301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Bazan-Socha S, Kisiel DG, Young B, Theakston RDG, Calvete JJ, Sheppard D, Marcinkiewicz C. Biochemistry. 2004;43:1639–1647. doi: 10.1021/bi035853t. [DOI] [PubMed] [Google Scholar]
- 23.Massa SM, Xie Y, Yang T, Harrington AW, Kim ML, Yoon SO, Kraemer R, Moore LA, Hempstead BL, Longo FM. J Neurosci. 2006;26:5288–5300. doi: 10.1523/JNEUROSCI.3547-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 25.Braiman A, Barda-Saad M, Sommers CL, Samelson LE. EMBO J. 2006;25:774–784. doi: 10.1038/sj.emboj.7600978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 27.Pina-Oviedo S, Urbanska K, Radhakrishnan S, Sweet T, Reiss K, Khalili K, Del Valle L. Am J Pathol. 2007;170:1291–1304. doi: 10.2353/ajpath.2007.060689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcinkiewicz C, Rosenthal LA, Mosser DM, Kunicki TJ, Niewiarowski S. Biochem J. 1996;317:817–852. doi: 10.1042/bj3170817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bibel M, Hoppe E, Barde YA. EMBO J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown MC, Staniszewska I, Lazarovici P, Tuszynski GP, Del Valle L, Marcinkiewicz C. Neuro-Oncol. 2008;10:968–980. doi: 10.1215/15228517-2008-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbeke S, Meignan S, Lagadec C, Germain E, Hondermarck H, Adriaenssens E, Bourhis XL. Cell Signal. 2010;22:1864–1873. doi: 10.1016/j.cellsig.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Cragnolini AB, Huang Y, Gokina P, Friedman WJ. Glia. 2009;57:1386–1392. doi: 10.1002/glia.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. J Neurosci. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parkhurst CN, Zampieri N, Chao MV. J Biol Chem. 2010;285:5361–5368. doi: 10.1074/jbc.M109.045054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yano H, Chao MV. Pharm Acta Helv. 2000;74:253–260. doi: 10.1016/s0031-6865(99)00036-9. [DOI] [PubMed] [Google Scholar]
- 37.Gu J, Tamura M, Yamada KM. J Cell Biol. 1998;143:1375–1383. doi: 10.1083/jcb.143.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dey N, Crosswell HE, De P, Parsons R, Peng Q, Dong Su J, Durden DL. Cancer Res. 2011;68:1862–1871. doi: 10.1158/0008-5472.CAN-07-1182. [DOI] [PubMed] [Google Scholar]
- 39.Defilles C, Montero MP, Lissitzky JC, Rome S, Siret C, Luis J, André F, Rigot V. J Biol Cell. 2011;103:519–529. doi: 10.1042/BC20100147. [DOI] [PubMed] [Google Scholar]
- 40.Ceni C, Kommaddi RP, Thomas R, Vereker E, Liu X, McPherson PS, Ritter B, Barker PA. J Cell Sci. 2010;123:2299–2307. doi: 10.1242/jcs.062612. [DOI] [PubMed] [Google Scholar]
- 41.Musatov S, Roberts J, Brooks AI, Pena J, Betchen S, Pfaff DW, Kaplitt MG. Proc Natl Acad Sci USA. 2004;101:3627–3631. doi: 10.1073/pnas.0308289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danussi C, Petrucco A, Wassermann B, Pivetta E, Modica TME, Del Bel Belluz L, Colombatti A, Spessotto P. J Cell Biol. 2011;195:131–145. doi: 10.1083/jcb.201008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.