Abstract
RAS-driven cancers exhibit variable dependency on autophagy for survival; however, it is not fully understood how. In this issue of the JCI, Cheong and colleagues demonstrate that RAS-dependent elevation of casein kinase 1α (CK1α) negatively regulates autophagy at the level of autophagy gene transcription. Moreover, combined inhibition of both CK1α and autophagy reduced proliferation of RAS-driven tumors. The results of this study provide insight into the connection between mutant RAS and autophagy, and suggest targeting CK1α as a potential therapeutic strategy to modulate autophagy in RAS-driven cancers.
The challenge of directly targeting RAS for cancer therapy
RAS is a canonical oncogenic driver, with RAS-activating mutations identified in 20%–30% of cancers. Constitutive RAS activation turns on many signaling pathways, including those that promote cell growth and survival. Despite the prevalence of RAS mutations in many forms of cancer, the development of drugs that directly target RAS has remained elusive. Small molecule RAS inhibitors have recently been discovered and shown to impair function in vitro (1); however, these will need to be further tested before clinical translation. Another focus for therapeutically targeting RAS-driven cancers has been the development of potent small molecule inhibitors against pathways downstream of mutant RAS, including MAPK and the PI3K/AKT/mTOR pathway (Figure 1). For instance, clinical trials of single agents, such as a MEK inhibitor (ClinicalTrials.gov, NCT01320085), and combination strategies that simultaneously target components of parallel pathways such as MEK and PI3K (NCT01363232) — or target the same pathway at two nodes, such as MEK and CDK4 (NCT01781572) — are being conducted to evaluate these strategies for use against melanoma. Unfortunately, these combinations can produce substantial toxicities; therefore, efficacy of targeted combination therapies has not been proven to be superior to single-agent therapy or standard-of-care chemotherapy in melanoma or any other RAS mutant cancer.
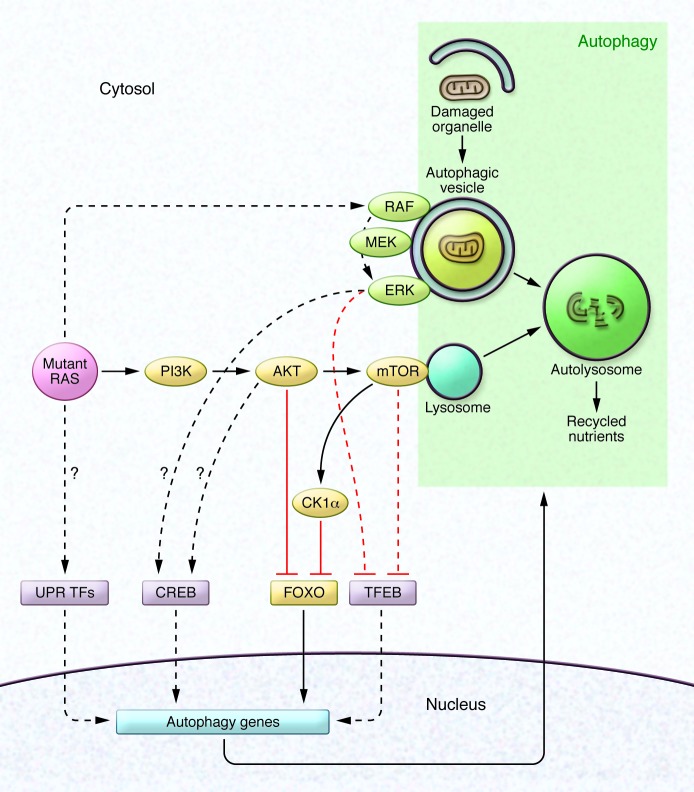
Figure 1. Transcriptional regulation of autophagy in RAS-driven cancers.
Mutant RAS activates several canonical growth factor signaling pathways, including the MAPK pathway (RAF/MEK/ERK) and the PI3K pathway (PI3K/AKT/mTOR). MAPK and PI3K signaling events take place in part on the surface of autophagic vesicles and lysosomes, respectively. Autophagy consists of the sequestration of damaged organelles within autophagic vesicles followed by fusion with the lysosome. A subset of known transcriptional regulators of autophagy genes are depicted, along with their regulation by growth factor kinase signaling pathways under the control of RAS. In this issue, Cheong et al. demonstrate that RAS-driven PI3K signaling increases levels of CK1α, which in turn phosphorylates and inhibits nuclear localization of FOXO3A, a transcription factor that positively regulates the expression of key autophagy genes (this pathway is denoted in yellow). Dashed lines indicate pathways described in other reports. Arrows indicate activation; lines ending in T indicate inhibition. UPR, unfolded protein response; TF, transcription factor.
Autophagy inhibition has potential
A number of studies have shown that autophagy is elevated in the setting of RAS transformation (2, 3), thereby providing another pathway — in addition to MAPK and PI3K — as a potential target for RAS mutant tumors. The intimacy between canonical growth factor kinase signaling pathways that are downstream of RAS and autophagy is underscored by the fact the MAPK signaling occurs on the surface of autophagic vesicles (4) and mTOR is physically attached to lysosomes (5). Autophagy as a therapeutic target is controversial, as autophagy can play different roles in early and late tumorigenesis (6). However, in the setting of advanced cancer, it is more and more appreciated that increased autophagy is due to oncogenic and metabolic stress, and is further increased in response to anticancer therapies (7). Moreover, drug-induced autophagy is cytoprotective in most animal models of cancer therapy. Because autophagy is a complex molecular pathway, numerous efforts are underway to develop small molecule inhibitors of canonical autophagy proteins.
While specific autophagy inhibitors have yet to be clinically evaluated, the chloroquine (CQ) derivatives, which inhibit autophagy by impairing lysosomal function (but may also inhibit cancer cells in other ways), have begun to be tested in clinical trials. For example, six recent publications report on different clinical trials that examined use of hydroxychloroquine (HCQ) combined with various anticancer agents (8–13). These trials demonstrated that HCQ does modulate autophagy in human tissues; however, the magnitude of this modulation was modest at best, even in those given the highest FDA-approved doses. No catastrophic toxicity was observed in HCQ combination regimens that involved temsirolimus, bortezomib, or vorinostat, though toxicity was observed when HCQ was administered with a specific temozolomide schedule. Taken together, these preliminary results suggest that more potent lysosomal autophagy inhibitors, combined with more effective chemotherapy or other targeted therapies, may yield better results. More potent CQ derivatives such as Lys05 (14) are now being evaluated for potential clinical development, and a second generation of HCQ clinical trials that pair HCQ with more potent cancer therapeutics are currently underway. However, a missing element in these efforts is a predictive biomarker that would identify patients likely to respond to autophagy inhibitor therapies.
Initially, studies suggested RAS mutation as a potential biomarker for patient selection. In animal models of mutant RAS–driven cancer, genetic inhibition of autophagy dramatically impaired tumor progression, leading to the notion that RAS mutant tumors are “addicted” to autophagy. For instance, a genetically engineered mouse model of KRAS-driven cancer and xenografts derived from patients with pancreatic cancer, which often harbor KRAS mutations, were strikingly susceptible to CQ derivatives (15); however, as the efficacy of autophagy inhibitors has been tested in more cell lines, it is has been shown that RAS mutation alone does not adequately predict susceptibility to autophagy inhibition. There is clear evidence that some RAS-driven cancers are autophagy dependent, while some are autophagy independent (16).
Linking RAS and autophagy provides an attractive target
In this issue, Cheong et al. identify a link between mutant RAS and autophagy, and demonstrate that CK1α is a key negative regulator of autophagy in RAS-driven tumors (17). CK1α is a constitutively active kinase that has been implicated in numerous signaling pathways (Figure 1), including a previous study that identified CK family members as important regulators of autophagy in an siRNA screen performed in breast cancer cells (18). Cheong and colleagues investigated whether members of CK1 could provide a mechanistic link between oncogenic RAS and autophagy by evaluating the effects of knockdown of CK1 family members on autophagy in RAS-transformed cells. Knockdown of CK1α, but not other CK1 isoforms, increased autophagic flux but only in the presence of oncogenic RAS. CK1α knockdown perturbed the transcription of a number of canonical autophagy genes, all of which are regulated by FOXO3A. Using a series of FOXO3A mutants, Cheong et al. demonstrated that CK1α phosphorylates FOXO3A on a specific serine residue that is distinct from the residue phosphorylated by AKT, and this CK1α-dependent phosphorylation impaired FOXO3A-dependent transcription of multiple autophagy genes, such as LC3B. Moreover, CK1α inhibition, via genetic means or with small molecules in RAS-transformed cells, resulted in nuclear localization of FOXO3A and induction of autophagy. Activation of PI3K, which is downstream of RAS, increased levels of CK1α, though this increase was not the result of elevated CK1A transcription. Cheong and colleagues combined CK1α inhibition with CQ to simultaneously induce autophagy and inhibit clearance of autophagosomes in RAS-mutant cancer cells. Compared with single-agent administration, this combination strategy impaired tumor cell proliferation in vitro and profoundly inhibited tumor growth in a xenograft model (17).
The study by Cheong et al. provides some important conceptual advances that link autophagy and cancer therapy, and identifies a feedback loop downstream of constitutively activated RAS that impacts the transcriptional regulation of autophagy genes. Importantly, the results of Cheong and colleagues provide a preclinical rationale for combining CK1α and autophagy inhibitors in RAS mutant cells; however, clinical translation is limited at this time by a lack of potent and specific CK1α inhibitors. CK1 family members have been difficult drug targets. Each isoform has been shown to perform different functions, depending on the cell context (19); therefore, the identification of selective inhibitors that are both potent and specific to a particular CK1 isoform has been challenging. While a number of groups have reported on specific inhibitors of other CK1 isoforms (20), no specific inhibitors of CK1α have been reported. This study by Cheong and colleagues, along with other reports that support a role for CK1α in promoting or limiting tumorigenesis in a subset of malignancies (19), provides a rationale for the focused development of such inhibitors.
Remaining questions and future directions
There are several questions that the work by Cheong and colleagues raises. First, does the augmented efficacy observed with combined inhibition of CK1α and autophagy depend on CK1α-dependent regulation of FOXO3A? Alternatively, could the benefit of combined therapy be due to one or more of the other pathways that CK1α regulates, such as β catenin/WNT, circadian rhythms, or p53 signaling? Second, how does PI3K signaling alter levels of CK1α? Finally, can CK1α levels be used to subclassify RAS-mutated tumors into autophagy dependent and autophagy independent categories to determine treatment options?
In a broader context (Figure 1), the CK1α-dependent transcriptional regulation of autophagy genes identified by Cheong et al. can be added to a growing list of PI3K pathway–dependent mechanisms that suppress autophagy. AKT-dependent phosphorylation results in cytoplasmic retention of FOXO transcription factors, preventing autophagy gene transcription (21). mTOR activation downstream of mutant RAS inactivates unc-like kinase 1 (ULK1) (22) and traps the master regulator of autophagy genes transcription factor EB (TFEB) in the cytoplasm (23). TFEB is also phosphorylated and sequestered in the cytoplasm by ERK (24). These negative regulatory events do not explain the observation that autophagy is elevated and required in some RAS-driven tumors. Potential RAS-dependent mediators for positive regulation of autophagy gene transcription include unfolded protein response transcription factors (25) and the cyclic AMP response element binding protein (CREB), which was recently identified as a master regulator of autophagy (26). CREB is an attractive candidate because it is phosphorylated and activated by p90RSK and AKT, which are downstream of the MAPK and PI3K pathways, respectively. While the CREB dependency of mutant RAS-driven autophagy has not been experimentally established, it is reasonable to speculate the presence of such a pathway. The identification of multiple positive and negative regulatory loops suggests that each aspect of autophagy may be quite dynamic in RAS-transformed cells, especially in the context of therapies that modulate PI3K and MAPK pathways. As highlighted by Cheong and colleagues, a better understanding of factors that regulate transcription of autophagy genes may point the way forward toward the ability to identify subsets of RAS-driven cancers that would be susceptible to therapeutic strategies that modulate autophagy.
Acknowledgments
This work was supported by NIH grant 1R01CA169134.
Footnotes
Conflict of interest: Ravi K. Amaravadi is a coinventor on a patent regarding Lys05 that was licensed to a biotechnology company by the University of Pennsylvania.
Reference information:J Clin Invest. 2015;125(4):1393–1395. doi:10.1172/JCI81504.
See the related article beginning on page 1401.
References
- 1.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang S, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25(7):717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo JY, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25(5):460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Lopez N, Athonvarangkul D, Mishall P, Sahu S, Singh R. Autophagy proteins regulate ERK phosphorylation. Nature Commun. 2013;4:2799. doi: 10.1038/ncomms3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334(6056):678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11(9):709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leone RD, Amaravadi RK. Autophagy: a targetable linchpin of cancer cell metabolism. Trends Endocrinol Metab. 2013;24(4):209–217. doi: 10.1016/j.tem.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenfeld MR, et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10(8):1359–1368. doi: 10.4161/auto.28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogl DT, et al. Combined autophagy and proteasome inhibition: A phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy. 2014;10(8):1380–1390. doi: 10.4161/auto.29264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rangwala R, et al. Combined MTOR and autophagy inhibition: Phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy. 2014;10(8):1391–1402. doi: 10.4161/auto.29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangwala R, et al. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy. 2014;10(8):1369–1379. doi: 10.4161/auto.29118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahalingam D, et al. Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy. 2014;10(8):1403–1414. doi: 10.4161/auto.29231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnard RA, Wittenburg LA, Amaravadi RK, Gustafson DL, Thorburn A, Thamm DH. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy. 2014;10(8):1415–1425. doi: 10.4161/auto.29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAfee Q, et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc Natl Acad Sci U S A. 2012;109(21):8253–8258. doi: 10.1073/pnas.1118193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang A, et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4(8):905–913. doi: 10.1158/2159-8290.CD-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan MJ, et al. Regulation of autophagy and chloroquine sensitivity by oncogenic RAS in vitro is context-dependent. Autophagy. 2014;10(10):1814–1826. doi: 10.4161/auto.32135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheong JK, Zhang F, Chua PJ, Bay BH, Thorburn A, Virshup DM. Casein kinase 1α–dependent feedback loop controls autophagy in RAS-driven cancers. J Clin Invest. 2015;125(4):1401–1418. doi: 10.1172/JCI78018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szyniarowski P, et al. A comprehensive siRNA screen for kinases that suppress macroautophagy in optimal growth conditions. Autophagy. 2011;7(8):892–903. doi: 10.4161/auto.7.8.15770. [DOI] [PubMed] [Google Scholar]
- 19.Schittek B, Sinnberg T. Biological functions of casein kinase 1 isoforms and putative roles in tumorigenesis. Mol Cancer. 2014;13:231. doi: 10.1186/1476-4598-13-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wager TT, et al. Casein kinase 1δ/ε inhibitor PF-5006739 attenuates opioid drug-seeking behavior. ACS Chem Neurosci. 2014;5(12):1253–1265. doi: 10.1021/cn500201x. [DOI] [PubMed] [Google Scholar]
- 21.Pietrocola F, et al. Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol. 2013;23(5):310–322. doi: 10.1016/j.semcancer.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Jung CH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20(7):1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roczniak-Ferguson A, et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal. 2012;5(228):ra42. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Settembre C, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332(6036):1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denoyelle C, et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol. 2006;8(10):1053–1063. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- 26.Seok S, et al. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature. 2014;516(7529):108–111. doi: 10.1038/nature13949. [DOI] [PMC free article] [PubMed] [Google Scholar]