Abstract
The mammalian blastocyst exhibits a high capacity for aerobic glycolysis, a metabolic characteristic of tumours. It has been considered that aerobic glycolysis is a means to ensure a high carbon flux to fulfil biosynthetic demands. Here, alternative explanations for this pattern of metabolism are considered. Lactate creates a microenvironment of low pH around the embryo to assist the disaggregation of uterine tissues to facilitate trophoblast invasion. Further it is proposed that lactate acts as a signalling molecule (especially at the reduced oxygen tension present at implantation) to elicit bioactive VEGF recruitment from uterine cells, to promote angiogenesis. Finally it is suggested that the region of high lactate/low pH created by the blastocyst modulates the activity of the local immune response, helping to create immune tolerance. Consequently, the mammalian blastocyst offers a model to study the role of microenvironments, and how metabolites and pH are used in signalling.
Keywords: implantation, lactate, microenvironment, signalling
Introduction: During the preimplantation period the mammalian embryo undergoes dramatic changes in its metabolism
The preimplantation mammalian embryo undergoes dramatic transformations in its physiology between fertilisation to implantation. The fertilised oocyte and cleavage stage embryo is dependent upon stable mRNA and proteins synthesized during oocyte maturation: embryonic genes are activated sequentially throughout the preimplantation period, but with major bouts of transcriptional activity around the 4- to 8-cell in the human, and a second round at the morula stage [1]. Of note, the early embryo during the first three cell cycles, similar to the unfertilised oocyte, cannot use glucose as the sole energy source, but rather requires pyruvate or sufficient aspartate and lactate to undergo the first cleavage division. The high ATP/ADP ratio of the early embryo [2], due to the low levels of energy requirements and biosynthesis during the first 48 hours of life, allosterically inhibits phosphofructokinase, thereby impairing glucose flux through the Embden-Meyeroff pathway (reviewed by [3]). A major change in physiology occurs around the 8- to 16-cell stage when the embryo creates the first transporting epithelium of the conceptus, through a process known as compaction facilitated by the formation of tight junctions, thereby enabling the creation of a specific internal environment for the embryo. As the embryonic genome becomes activated and biosynthesis increases, due to both cell proliferation and the formation of a blastocoel cavity (formed through the activity of basolateral ATPases of the trophectoderm) [4], glucose metabolism increases significantly so that, by the time the embryo forms the blastocyst, glucose has become the predominant nutrient. Of significance, viable blastocysts exhibit high glucose uptake [5,6]. Concomitantly, there is a parallel increase in oxygen consumption, as the blastocyst increases its capacity for respiration [7,8].
The blastocyst exhibits a rather idiosyncratic metabolism, converting much of the glucose consumed to lactate, even in the presence of oxygen
A high level of aerobic glycolysis is a common characteristic of rapidly dividing cells and tumours, with which the mammalian blastocyst shares several traits [9–11]. Aerobic glycolysis, the formation of lactate in the presence of oxygen, was first characterised by Warburg in 1956 [12], and was initially thought to be specific to certain cancers. However, it was subsequently revealed that aerobic glycolysis was a trait observed in other rapidly proliferating cells such as lymphocytes [13]. In the mouse and human blastocyst, >50% of the glucose consumed is not oxidized but is converted to lactate [5,14], in the presence of oxygen. In contrast to the cleavage stage embryo, which has mitochondria with poor cristae formation, the mammalian blastocyst possesses mitochondria with transverse cristae, and exhibits the highest oxygen consumption of the preimplantation stages [7]. Consequently, the phenomenon of high lactate production by blastocysts is not due to a deficiency or defect in mitochondria. Further, at reduced oxygen concentrations pyruvate oxidation may be restricted due to down-regulation of pyruvate dehydrogenase activity brought about by the increased activity of pyruvate dehydrogenase kinase induced by HIF-1 [15].
This poses the question as to why are such high levels of aerobic glycolysis required? As well as being used to generate energy for the energy-expensive process of blastocoel expansion and mitosis, high levels of glucose utilisation will be required for the synthesis of triacylglycerols and phospholipids for new membrane synthesis, and as a precursor for complex sugars of mucopolysaccharides and glycoproteins. Glucose metabolised by the pentose phosphate pathway (PPP) generates ribose moieties required for nucleic acid synthesis and the NADPH required for the biosynthesis of lipids and nucleotides [10,16,17]. NADPH is also required for the reduction of intracellular glutathione, an important antioxidant for the embryo [18].
Implantation in the mouse and human is characterised by the following four stages: apposition, adhesion, attachment and invasion [19]. During these processes the cells of the embryo and the endometrium of the uterus are in immediate proximity, and there is little free fluid within the uterus (measured to be just 150 nanolitres per uterine horn in the mouse) [20]. At the time of implantation, fluid is further removed from the uterine lumen [21]. The concentration of oxygen within the lumen of the uterus has been reported to be in the range of 1.5 to 5.3% depending upon species and time of the oestrus cycle [22], considerably less than atmospheric (∼20%). Furthermore, at the time of implantation itself, the levels of oxygen available to the embryo as it invades the endometrium (as is the case for humans and rodents for example), will be limited. It has been determined that the site of implantation will be relatively anoxic, because of the absence of maternal vasculature [23]. Of interest, oxygen is a powerful regulator of blastocyst function, where atmospheric oxygen induces aberrant changes in gene expression [24,25], the proteome [26] and metabolism of both amino acids and carbohydrates [27]. In the case of amino acid and carbohydrate metabolism it was found that glucose consumption by the blastocyst was inversely related to oxygen concentration. Therefore, lower oxygen concentrations are associated with up-regulation of glucose metabolism and high levels of lactate formation. As development proceeds, the implanting blastocyst moves to a more glycolysis-dependent metabolism, in which 90% of the consumed glucose forms lactate [28].
At the late blastocyst stage, and during the early phases of implantation, there is a switch in lactate dehydrogenase (LDH) isoforms from LDHB (which favours pyruvate formation) to LDHA (which favours lactate formation) [29], and is the isoform utilised by tumours to facilitate their metabolic phenotype and tissue invasion [30,31]. Monocarboxylate transporters (MCT) facilitate the movement of lactate in and out of cells [32]. Typically four isoforms are found: MCT 1, 2, 3 and 4. MCT4 has the lowest affinity for lactate of all the transporters, with a Km of >20mM, and is found in cells where there is a requirement for lactate export from cells [33,34]. The mouse blastocyst possesses Mct 1, 2, 3 and 4, whereas the human blastocyst expresses only MCT 1 mRNA, and MCT 4 has yet to be detected [35]. However, MCT 1 has an intermediate Km (3.5 to 10 mM) and can operate bidirectionally depending upon environmental conditions. For example in erythrocytes, proliferating lymphocytes and certain tumours, MCT 1 serves to export lactate [36–38]. Hence the mammalian blastocyst has at least one appropriate transporter to facilitate lactate export. The transport of lactate by an MCT across the plasma membrane is associated with the co-transport of a proton [39,40], which has the effect of reducing external pH (pHe). The pHe of most tumours is characterised by a significantly reduced pHe of around 6.7 to 7.1 [41–43].
Analysis of the metabolic activities of the mouse and human blastocyst reveals their considerable capacity to produce lactic acid into the surrounding fluids in the uterus, thereby altering their immediate environment. Around the time of implantation the mouse and human blastocyst will conservatively consume around 50 to 320 pmols glucose/embryo/h respectively. Over a 24 hour period in a uterine volume of 150 nl, and assuming a greater shift to lactate production, this equates to the capacity to produce around 16 mM lactic acid in the mouse and up to 100 mM lactic acid in the human. These values are in contrast to the 1–3 mM lactate found in blood and resting tissues [44]. As development proceeds the amount of lactate produced by the blastocyst will increase significantly [28] and the volumes of fluid surrounding the embryo will further diminish. Hence, the blastocyst has the capacity to significantly modify the external environment during implantation, which will subsequently be characterised by high extracellular lactate and concomitantly, low pHe.
For implantation to be successful, the blastocyst has not only to invade the surrounding endometrial tissue but has to remodel vasculature, while at the same time modulate the local immune system of the mother. It is hypothesised that the creation of a high lactate microenvironment by the blastocyst enables endometrial breakdown, angiogenesis and immunoregulation, thereby facilitating successful implantation.
Lactate as a facilitator of tissue disaggregation
A key aspect of implantation is the controlled degradation of the extracellular matrix (ECM) of the endometrium to facilitate trophoblast invasion. The ECM is dynamic and exists in a fine balance between the synthesis of matrix components and their subsequent breakdown. In the mouse and human, matrix metalloproteinases (MMPs) are the key proteinases underlying matrix degradation; the blastocyst expresses MMP-9, while during the peri-implantation period the late blastocyst also expresses MMP-1 and -2 [45]. In certain tumours, an acidic environment induces the expression of MMP-9 and increases the capacity of cells to degrade the ECM during in vitro assays [46–48]. Similarly, in glioma cells the production of lactate induces the expression of TGF-β2, which in turn results in an increased expression, secretion and release of MMP-2 [49]. T-reg cells accumulate in the decidual tissue and are known to secrete TGF-β [50]. Furthermore, TGF-β1 and 2 are present in the human [51] and rodent endometrium in the luminal and glandular epithelium around the time of implantation [52]. Consequently, lactate could enhance production of TGF-β from both endometrial and T-reg cells thereby increasing the production of blastocyst-derived MMPs.
Low external pH also creates a microenvironment that favours the activation of proteases such as the MMPs. For example, MMP-3 has an optimum range of 5.75 to 6.25, and a low pH can further increase total MMP activity by facilitating the proteolytic cascade that converts pro-MMPs to their active form. Of physiological significance, the activity of blastocyst-derived MMPs is tightly regulated by their inhibitors, tissue inhibitors of metalloproteinases (TIMPs) [53]. TIMP transcripts are readily detected in the decidual tissue next to the implanting blastocyst [54]. The presence of functional TIMPs therefore has the capacity to reduce ECM breakdown by decreasing blastocyst MMP-9 and MMP-2 activity. The relative activities of MMPs and TIMPs in endothelial cells appears to be inversely related under conditions of stress [55,56]. In the case of reduced pH, it has been demonstrated in nucleus pulposus cells that whereas the production of TIMPs was decreased by >90%, the production of MMPs remained unaffected [57]. Such an imbalance in the relative activities of these enzymes would consequently favour an increased breakdown of the ECM.
Another protease associated with extracellular digestion of the ECM by the mammalian blastocyst is cathepsin B [58]. Inhibition of cathepsin activity in utero on day 4.5 results in complete implantation failure [59]. Cathepsins are upregulated in a number of tumours and are associated with increased neoplastic activity [60]. Of note, it has been demonstrated that an acidic microenvironment around malignant cells induces enhanced secretion of active cathepsin B [61]. Consequently, it is realistic that a reduction in pH in the microenvironment of the blastocyst, induced by lactate production from aerobic glycolysis, will increase the release of both MMP-9 and cathepsin, and potentially other embryonic-derived proteases, to facilitate the breakdown of the ECM of the endometrial tissues, thereby promoting the implantation process.
Interestingly, lactate can stimulate fibroblasts and cancers to increase hyaluronan synthesis [62,63]. Hyaluronan has the capacity to make cells less adhesive, reducing their cell-cell contacts and thereby inducing them to separate from each other. Due to its vast ability to sequester water through hydration, which is several thousand times greater than the volume of the actual polymer, the secretion of hyaluronan has the ability to create spaces into which cells can move. An increase in hyaluronan in the surrounding endometrial tissues would therefore facilitate cell migration and implantation. Analysis of capillaries around the rodent blastocyst following implantation reveals an avascular area, determined to be in the primary decidual zone [64], plausibly due to the expansion induced by hyaluronan, stimulated by lactate. The increase of extracellular space from hyaluronan expansion combined with an absence of vascularity and may serve to facilitate invasion of the trophoblast cells. These events are summarised in Fig. 1.
Figure 1.
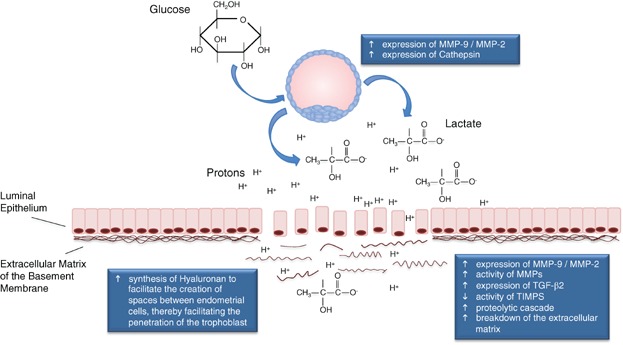
Blastocyst-derived lactate facilitates tissue disaggregation. The high lactate concentration, and resultant drop in extracellular pH, due to the co-transport of protons with lactate out of the blastocyst affects the breakdown of the extracellular matrix by increasing the expression and activity of MMPS, both in the blastocyst and endometrial tissue, whilst decreasing the activity of TIMPs, thereby inducing a proteolytic cascade. This microenvironment is able to stimulate the expression of cathepsin by the blastocyst and induce hyaluronan synthesis in endometrial tissue. The latter would facilitate the creation of spaces between endometrial cells. This, combined with the increased breakdown of the extracellular matrix, would facilitate the invasion of the trophectoderm.
Lactate as a signalling molecule to induce angiogenesis and increase vascular permeability
For embryo development post-implantation to be successful, it is paramount that a fetal-maternal blood supply is established, which in turn requires successful angiogenesis to proceed. It is proposed that lactate has a direct role to play in the successful establishment of a microcirculation to promote subsequent blastocyst development during implantation.
Wound healing is an example of a physiological process in which it is necessary to establish new microcirculation. It has been established that during wound healing tissues create a microenvironment characterised by a high lactate concentration (10 to 15 mM) as a consequence of microcirculation disruption and the need for tissue proliferation [65]. Hence, in the case of tissue damage and certain cancers, the surrounding tissues will be exposed to a high extracellular lactate concentration inducing the surrounding cells to take up lactate via MCTs, thereby increasing intracellular lactate. Lactate can then be converted to pyruvate by LDH with the concomitant consumption of NAD+ to form NADH, reducing the intracellular availability of NAD+ which is the only substrate for ADPribose. This decrease in NAD+ in turn reduces ADPribosylation. Hence the cells surrounding the blastocyst will perceive that they are in a state of hypoxia, and activate pathways that upregulate growth factor and cytokine signalling, even in the presence of molecular oxygen [44,66]. In a similar manner, the implanting blastocyst can release significant amounts of lactate into the surrounding extracellular space, inducing the uptake of lactate by the surrounding tissues.
Analysis of endometrial tissue in the area around the blastocyst at the time of implantation has revealed an accumulation of VEGF [67]. An extracellular microenvironment high in lactate will serve to reduce pH around the implantation site at a time when uterine VEGF expression increases. Lactate-induced acidosis has been shown to increase VEGF expression in tumour cells [68]. VEGF isoforms are involved in the majority of steps during angiogenesis, such as vasodilation, endothelial cell proliferation, their migration and finally tube formation. Furthermore, lactate, through its ability to enhance VEGF production, has been demonstrated to stimulate endothelial cell migration in a dose-dependent fashion in an in vitro migration assay [69]. Significantly, VEGF normally exists as a mixture of free (angiogenic) and ADP-ribosylated (poorly angiogenic) forms. As discussed, the suppression of intracellular NAD+ levels reduces the activity of ADP-ribosyl transferases, thereby affecting the levels of ADP-ribosylated VEGF. Consequently, blastocyst-derived lactate could not only be involved in the expression and production of VEGF, but also, through its ability to reduce NAD+ in the surrounding tissue, lactate could promote the release of active forms of VEGF to stimulate new vessel development [65].
Lactate activates several pathways in endothelial cells. In wounds, lactate has been shown to stabilise HIF-1α, through the inhibition of prolyl hydroxylase 2 [70], which in turn can also increase VEGF levels [44]. Specifically, lactate has the ability to enter endothelial cells resulting in increases in vascular permeability and transcription of VEGF. In primary human endothelial cells, lactate activates the PI3K/Akt pathway [71]. Lactate readily enters endothelial cells through MCT-1 and stimulates cell migration and tube formation through the action of nuclear factor kappaB (NFκB)[72]. NF-κB, which acts as a lactate-responsive transcription factor, is localised to the endometrial epithelium [73], and consequently will be readily upregulated by the presence of a metabolically active blastocyst. Indeed the highest activity of NFκB is detected in the mouse uterus 3.5 days after mating, i.e. when the blastocyst is present. Although the precise roles for NFκB in the uterus have yet to be fully elucidated, as NFκB can modulate the expression of cytokines, growth factors and MMPs, it is feasibly involved in signalling at implantation (Fig. 2).
Figure 2.
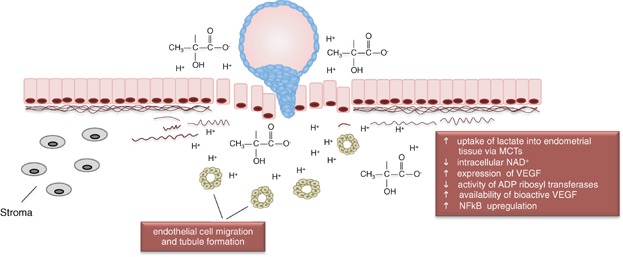
Blastocyst-derived lactate induces angiogenesis. The microenvironment of high lactate and reduced pH will facilitate angiogenesis. The now immediate proximity of the blastocyst to endometrial tissue means that adjacent tissues would readily take up lactate produced by the blastocyst. This will lead to changes in the intracellular physiology of the maternal tissues, culminating in a reduced NAD+ level, inducing changes to the levels of ADP ribosylation. This favours the formation of the bioactive from of VEGF (non ribosylated). The high concentration of lactate can also induce the migration of endothelial cells and promote tubule formation.
Finally, lactate may be considered as a component of a feedback loop whereby blastocyst-derived lactate promotes transcription of VEGF in the surrounding tissue, which in turn has a direct effect on both uterine remodelling, immune regulation and survival (via NFκB) and blastocyst function. For example, exogenous VEGF has now been shown to increase blastocyst adhesive capacity and subsequent development in utero [74,75]. It is, therefore, highly plausible that lactate is involved in the initial signalling to promote angiogenesis during the implantation process. Furthermore, it has been established in a primate model that there is significant up-regulation of endometrial factors, including TGF-β2 and IL-6 even before the blastocyst commences invasion, which is in response to embryo-derived signals [76]. Given its low molecular weight and high solubility, it is likely that lactate is one of the first signals from the blastocyst to reach the endometrium.
Lactate as a modulator of the local immune response
One of the biggest challenges to the embryo during implantation is to prevent the mother from rejecting it as foreign. It has been documented in several tumours that metabolically-derived lactate modulates the activity of the local immune response during growth and invasion [77]. Lactate, through its ability to reduce pH in the microenvironment, significantly decreases the growth and expression of T-cell receptors and production of cytokines by human cytotoxic T-cells [78–81]. Of note, activated T-cells are themselves dependent upon glycolysis for energy production. It is therefore feasible that the high production of lactate by the blastocyst could block lactate export by the T-cells inducing metabolic aberrations and subsequently leading to loss of their function or exclusion from the implantation site, thereby reducing the local immune response at the implantation site [82].
Significantly, T-cell regulatory cells are potent suppressors of the initiation and effector function of the inflammatory cell-mediated immune response, and are essential if implantation is to be successful [83]. A key regulator of T-cell function is the cytokine TGF-β, which exhibits immunosuppressive properties [84]. TGF-β has been linked to the induction of T-cell regulatory cells through the production of suppressor T-cells from naïve CD4+/CD25- T-cells [50,85]. Further, exogenous TGF-β administered vaginally has been shown to promote a regulatory T-cell response [86]. Of significance to this hypothesis, lactate has been shown to induce the expression of TGF-β in glioma cells [49,87], and can promote IL-17A production in macrophages and CD4+ T-cells [88]. The role of lactate in the induction of other interleukins has yet to be elucidated. Hence, lactate could act as a signal to up-regulate TGF-β expression, thereby modulating local immune function in the vicinity of the blastocyst (Fig. 3).
Figure 3.
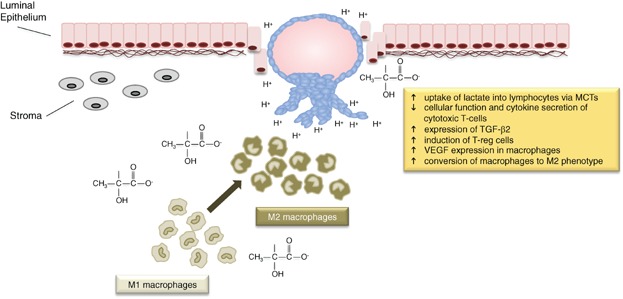
Lactate from the blastocyst modulates the immune response of the mother. Once the blastocyst has penetrated the epithelium of the endometrium and invasion of the stromal tissue commences, the lactate produced by the embryo serves to decrease the function of cytotoxic T-cells, and increase the production of the angiogenic factor VEGF by macrophages, which dominate the implantation site. Blastocyst-derived lactate would convert the phenotype of the macrophages from M1 (host defence) to M2 (healing), thereby creating an immune-safe environment. Rather than simply being perceived as an end product of metabolism, lactate should be considered as an integral part of the embryo's signalling system during implantation.
Characteristic of the implantation site itself is a high abundance of macrophages, which represent another source of cytokines and growth factors. It has therefore been suggested that such a high density of macrophages facilitates endometrial tissue remodelling required to accommodate proliferation of extra-embryonic tissue [89]. Consequently, macrophages could provide a microenvironment to promote blastocyst growth by reducing harmful local inflammatory immune reactions. In support of this, it has been revealed that tumour cells, through their ability to produce a microenvironment high in lactate, induce VEGF expression by macrophages [90]. The effect of lactate was found to induce, through HIF-1α, an M2-like phenotype in macrophages, the phenotype involved in down-regulation of the local immune response at the time of implantation.
The pH of the endometrium becomes acidic at implantation
This hypothesis has focused on the role of the blastocyst in regulating implantation, with reference to the role of its production lactate and its ability to reduce pHe. However, implantation is an exquisite dialogue between two tissue types, the blastocyst and the endometrium. An established dialogue facilitated by specific factors has been considered [19,91,92], but what of pH and the endometrium? Of potential significance an analysis of the pH within the lumen of the rodent uterus during pseudopregnancy demonstrated an increase in acidity to 7.14 (compared to 7.31 in oestrous) [93]. Further decreases in pH (below 7) are associated with decidualisation. This latter observation could be associated with the proliferation of the decidual cells themselves. More recently it has been demonstrated that acidification of the uterine tissue may be essential for implantation in the mouse. Prevention of uterine acidification using balifomycin A1, to inhibit V-ATPase which regulates the acidity of the environment, results in a dose-dependent disruption of implantation [94]. As balifomycin also inhibited decidualisation induced through the introduction of oil into the uterus, these data indicate that uterine acidification is also important for implantation to proceed successfully. This apparent reduction of uterine pH around the time of implantation could serve to increase the sensitivity of endometrial cells to the presence of blastocyst-derived lactate. Quantification of pHe around the blastocyst has not been undertaken, but given advances in imaging it may be possible to attain data in situ [42]. Given the calculated levels of lactate produced by the blastocyst during implantation, further reductions in the pHe in the immediate vicinity of the blastocyst are predicted.
Conclusions and outlook
Historically lactate has been perceived as an end point of glucose metabolism, yet a substantial body of evidence documents the utilisation of lactate to support a variety of cellular changes and functions. Here, direct roles for the lactate produced by the mammalian blastocyst have been proposed: in tissue breakdown to facilitate implantation, in signalling to facilitate angiogenesis, and in immune modulation to prevent maternal rejection. These are highlighted in Figs. 3. Consequently, impairment of continuous lactate release by the blastocyst could compromise the implantation process. By being able to either promote or inhibit the creation of a microenvironment characterised by high lactic acid around the blastocyst, one could theoretically either augment the implantation process, or develop a means of contraception, respectively. To date several extensive treatise exist on the complex dialogue that occurs between the blastocyst and endometrium during the implantation process [19,91,92], however none of them have considered the role of metabolites as a component or facilitator of the signalling processes involved. Rather than a metabolic by-product, lactate should be considered as a key embryo-derived signal to facilitate and/or augment several key events during implantation. Furthermore, the role of lactate in establishing an effective dialogue between the embryo and the mother will not cease after the initial phases of implantation. Rather, post-implantation the embryo becomes more dependent upon glycolysis [28], where lactate production will continue to characterise embryo metabolism, and will consequently maintain a specific microenvironment to facilitate events post-implantation. The concepts presented here could be verified in a number of ways though both in vitro models of implantation, and through analysis of endometrial tissue response to foci of high lactate and/or acidification.
Acknowledgments
I thank Alexandra Harvey for her most valuable comments on the manuscript and the ARC and NH&MRC of Australia for financial support.
Glossary
- ECM
extracellular matrix
- LDH
lactate dehydrogenase
- MCT
monocarboxylate transporter
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of metalloproteinases
References
- 1.Hamatani T, Ko MSH, Yamada M, Kuji N, et al. Global gene expression profiling of preimplantation embryos. Hum Cell. 2006;19:98–117. doi: 10.1111/j.1749-0774.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- 2.Leese HJ, Biggers JD, Mroz EA, Lechene C. Nucleotides in a single mammalian ovum or preimplantation embryo. Anal Biochem. 1984;140:443–8. doi: 10.1016/0003-2697(84)90191-x. [DOI] [PubMed] [Google Scholar]
- 3.Gardner DK, Wale PL. Analysis of metabolism to select viable human embryos for transfer. Fertil Steril. 2013;99:1062–72. doi: 10.1016/j.fertnstert.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Watson AJ, Natale DR, Barcroft LC. Molecular regulation of blastocyst formation. Anim Reprod Sci. 2004;82–83:583–92. doi: 10.1016/j.anireprosci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Gardner DK, Leese HJ. Assessment of embryo viability prior to transfer by the noninvasive measurement of glucose uptake. J Exp Zool. 1987;242:103–5. doi: 10.1002/jez.1402420115. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DK, Wale PL, Collins R, Lane M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum Reprod. 2011;26:1981–6. doi: 10.1093/humrep/der143. [DOI] [PubMed] [Google Scholar]
- 7.Houghton FD, Thompson JG, Kennedy CJ, Leese HJ. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev. 1996;44:476–85. doi: 10.1002/(SICI)1098-2795(199608)44:4<476::AID-MRD7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Trimarchi JR, Liu L, Porterfield DM, Smith PJ, et al. Oxidative phosphorylation-dependent and -independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod. 2000;62:1866–74. doi: 10.1095/biolreprod62.6.1866. [DOI] [PubMed] [Google Scholar]
- 9.Hume DA, Weidemann MJ. Role and regulation of glucose metabolism in proliferating cells. J Natl Cancer Inst. 1979;62:3–8. [PubMed] [Google Scholar]
- 10.Morgan MJ, Faik P. Carbohydrate metabolism in cultured animal cells. Biosci Rep. 1981;1:669–86. doi: 10.1007/BF01116465. [DOI] [PubMed] [Google Scholar]
- 11.Mandel LJ. Energy metabolism of cellular activation, growth, and transformation. Curr Top Memb Trans. 1986;27:261–91. [Google Scholar]
- 12.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 13.Wang T, Marquardt C, Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976;261:702–5. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]
- 14.Gott AL, Hardy K, Winston RM, Leese HJ. Non-invasive measurement of pyruvate and glucose uptake and lactate production by single human preimplantation embryos. Hum Reprod. 1990;5:104–8. doi: 10.1093/oxfordjournals.humrep.a137028. [DOI] [PubMed] [Google Scholar]
- 15.Jha MK, Suk K. Pyruvate dehydrogenase kinase as a potential therapeutic target for malignant gliomas. Brain Tumor Res Treat. 2013;1:57–63. doi: 10.14791/btrt.2013.1.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newsholme EA, Crabtree B, Ardawi MS. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985;5:393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- 17.Reitzer LJ, Wice BM, Kennell D. The pentose cycle. Control and essential function in HeLa cell nucleic acid synthesis. J Biol Chem. 1980;255:5616–26. [PubMed] [Google Scholar]
- 18.Rieger D. Relationships between energy metabolism and development of early mammalian embryos. Theriogenology. 1992;37:75–93. [Google Scholar]
- 19.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–67. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoversland RC, Weitlauf HM. The volume of uterine fluid in 'implanting' and 'delayed implanting' mice. J Reprod Fertil. 1981;62:105–9. doi: 10.1530/jrf.0.0620105. [DOI] [PubMed] [Google Scholar]
- 21.Salleh N, Baines DL, Naftalin RJ, Milligan SR. The hormonal control of uterine luminal fluid secretion and absorption. J Membr Biol. 2005;206:17–28. doi: 10.1007/s00232-005-0770-7. [DOI] [PubMed] [Google Scholar]
- 22.Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–9. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- 23.Rogers PAW, Murphy CR, Gannon BJ. Absense of capillaries in the endometrium surrounding the implanting rat blastocyst. Micron. 1982;13:373–4. [Google Scholar]
- 24.Rinaudo PF, Giritharan G, Talbi S, Dobson AT, et al. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86(4 Suppl):1252–65. doi: 10.1016/j.fertnstert.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, et al. Oxygen-regulated gene expression in bovine blastocysts. Biol Reprod. 2004;71:1108–19. doi: 10.1095/biolreprod.104.028639. [DOI] [PubMed] [Google Scholar]
- 26.Katz-Jaffe MG, Linck DW, Schoolcraft WB, Gardner DK. A proteomic analysis of mammalian preimplantation embryonic development. Reproduction. 2005;130:899–905. doi: 10.1530/rep.1.00854. [DOI] [PubMed] [Google Scholar]
- 27.Wale PL, Gardner DK. Oxygen regulates amino acid turnover and carbohydrate uptake during the preimplantation period of mouse embryo development. Biol Reprod. 2012;87:24. doi: 10.1095/biolreprod.112.100552. 1–8. [DOI] [PubMed] [Google Scholar]
- 28.Clough JR, Whittingham DG. Metabolism of [14C]glucose by postimplantation mouse embryos in vitro. J Embryol Exp Morphol. 1983;74:133–42. [PubMed] [Google Scholar]
- 29.Auerbach S, Brinster RL. Lactate dehydrogenase isozymes in the early mouse embryo. Exp Cell Res. 1967;46:89–92. doi: 10.1016/0014-4827(67)90411-9. [DOI] [PubMed] [Google Scholar]
- 30.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–34. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Yao F, Zhao T, Zhong C, Zhu J, et al. LDHA is necessary for the tumorigenicity of esophageal squamous cell carcinoma. Tumour Biol. 2013;34:25–31. doi: 10.1007/s13277-012-0506-0. [DOI] [PubMed] [Google Scholar]
- 32.Halestrap AP, Wilson MC. The monocarboxylate transporter family–role and regulation. IUBMB Life. 2012;64:109–19. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 33.Dimmer KS, Friedrich B, Lang F, Deitmer JW, et al. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350:219–27. [PMC free article] [PubMed] [Google Scholar]
- 34.Draoui N, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4:727–32. doi: 10.1242/dmm.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herubel F, El Mouatassim S, Guerin P, Frydman R, et al. Genetic expression of monocarboxylate transporters during human and murine oocyte maturation and early embryonic development. Zygote. 2002;10:175–81. doi: 10.1017/s096719940200223x. [DOI] [PubMed] [Google Scholar]
- 36.Deuticke B. Monocarboxylate transport in erythrocytes. J Membr Biol. 1982;70:89–103. doi: 10.1007/BF01870219. [DOI] [PubMed] [Google Scholar]
- 37.Murray CM, Hutchinson R, Bantick JR, Belfield GP, et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chem Biol. 2005;1:371–6. doi: 10.1038/nchembio744. [DOI] [PubMed] [Google Scholar]
- 38.Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, et al. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol. 2010;2010:427694. doi: 10.1155/2010/427694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer TL, Lehninger AL. L-lactate transport in Ehrlich ascites-tumour cells. Biochem J. 1976;154:405–14. doi: 10.1042/bj1540405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991;64:425–7. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillies RJ, Liu Z, Bhujwalla Z. 31P-MRS measurements of extracellular pH of tumors using 3-aminopropylphosphonate. Am J Physiol. 1994;267:C195–203. doi: 10.1152/ajpcell.1994.267.1.C195. [DOI] [PubMed] [Google Scholar]
- 42.Hashim AI, Zhang X, Wojtkowiak JW, Martinez GV, et al. Imaging pH and metastasis. NMR Biomed. 2011;24:582–91. doi: 10.1002/nbm.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–7. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 44.Hunt TK, Aslam RS, Beckert S, Wagner S, et al. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007;9:1115–24. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, Nakai M, Belton RJ, Jr, Nowak RA. Expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinases during mouse embryonic development. Reproduction. 2007;133:405–14. doi: 10.1530/rep.1.01020. [DOI] [PubMed] [Google Scholar]
- 46.Kato Y, Nakayama Y, Umeda M, Miyazaki K. Induction of 103-kDa gelatinase/type IV collagenase by acidic culture conditions in mouse metastatic melanoma cell lines. J Biol Chem. 1992;267:11424–30. [PubMed] [Google Scholar]
- 47.Kato Y, Lambert CA, Colige AC, Mineur P, et al. Acidic extracellular pH induces matrix metalloproteinase-9 expression in mouse metastatic melanoma cells through the phospholipase D-mitogen-activated protein kinase signaling. J Biol Chem. 2005;280:10938–44. doi: 10.1074/jbc.M411313200. [DOI] [PubMed] [Google Scholar]
- 48.Kato Y, Ozawa S, Tsukuda M, Kubota E, et al. Acidic extracellular pH increases calcium influx-triggered phospholipase D activity along with acidic sphingomyelinase activation to induce matrix metalloproteinase-9 expression in mouse metastatic melanoma. FEBS J. 2007;274:3171–83. doi: 10.1111/j.1742-4658.2007.05848.x. [DOI] [PubMed] [Google Scholar]
- 49.Baumann F, Leukel P, Doerfelt A, Beier CP, et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. 2009;11:368–80. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wahl SM, Swisher J, McCartney-Francis N, Chen W. TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol. 2004;76:15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- 51.Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol. 2011;210:5–14. doi: 10.1530/JOE-10-0461. [DOI] [PubMed] [Google Scholar]
- 52.Das SK, Flanders KC, Andrews GK, Dey SK. Expression of transforming growth factor-beta isoforms (beta 2 and beta 3) in the mouse uterus: analysis of the periimplantation period and effects of ovarian steroids. Endocrinology. 1992;130:3459–66. doi: 10.1210/endo.130.6.1375903. [DOI] [PubMed] [Google Scholar]
- 53.Harvey MB, Leco KJ, Arcellana-Panlilio MY, Zhang X, et al. Proteinase expression in early mouse embryos is regulated by leukaemia inhibitory factor and epidermal growth factor. Development. 1995;121:1005–14. doi: 10.1242/dev.121.4.1005. [DOI] [PubMed] [Google Scholar]
- 54.Whiteside EJ, Jackson MM, Herington AC, Edwards DR, et al. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 are key regulators of extracellular matrix degradation by mouse embryos. Biol Reprod. 2001;64:1331–7. doi: 10.1095/biolreprod64.5.1331. [DOI] [PubMed] [Google Scholar]
- 55.Ho FM, Liu SH, Lin WW, Liau CS. Opposite effects of high glucose on MMP-2 and TIMP-2 in human endothelial cells. J Cell Biochem. 2007;101:442–50. doi: 10.1002/jcb.21192. [DOI] [PubMed] [Google Scholar]
- 56.Reuter B, Rodemer C, Grudzenski S, Couraud PO, et al. Temporal profile of matrix metalloproteinases and their inhibitors in a human endothelial cell culture model of cerebral ischemia. Cerebrovasc Dis. 2013;35:514–20. doi: 10.1159/000350731. [DOI] [PubMed] [Google Scholar]
- 57.Razaq S, Wilkins RJ, Urban JP. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. Eur Spine J. 2003;12:341–9. doi: 10.1007/s00586-003-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afonso S, Romagnano L, Babiarz B. Expression of cathepsin proteinases by mouse trophoblast in vivo and in vitro. Dev Dyn. 1999;216:374–84. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<374::AID-DVDY6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 59.Afonso S, Romagnano L, Babiarz B. The expression and function of cystatin C and cathepsin B and cathepsin L during mouse embryo implantation and placentation. Development. 1997;124:3415–25. doi: 10.1242/dev.124.17.3415. [DOI] [PubMed] [Google Scholar]
- 60.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–75. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 61.Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994;54:6517–25. [PubMed] [Google Scholar]
- 62.Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res. 2002;276:24–31. doi: 10.1006/excr.2002.5508. [DOI] [PubMed] [Google Scholar]
- 63.Rudrabhatla SR, Mahaffey CL, Mummert ME. Tumor microenvironment modulates hyaluronan expression: the lactate effect. J Invest Dermatol. 2006;126:1378–87. doi: 10.1038/sj.jid.5700255. [DOI] [PubMed] [Google Scholar]
- 64.Rogers PA, Murphy CR, Rogers AW, Gannon BJ. Capillary patency and permeability in the endometrium surrounding the implanting rat blastocyst. Int J Microcirc Clin Exp. 1983;2:241–9. [PubMed] [Google Scholar]
- 65.Ghani QP, Wagner S, Becker HD, Hunt TK, et al. Regulatory role of lactate in wound repair. Methods Enzymol. 2004;381:565–75. doi: 10.1016/S0076-6879(04)81036-X. [DOI] [PubMed] [Google Scholar]
- 66.Trabold O, Wagner S, Wicke C, Scheuenstuhl H, et al. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 2003;11:504–9. doi: 10.1046/j.1524-475x.2003.11621.x. [DOI] [PubMed] [Google Scholar]
- 67.Halder JB, Zhao X, Soker S, Paria BC, et al. Differential expression of VEGF isoforms and VEGF(164)-specific receptor neuropilin-1 in the mouse uterus suggests a role for VEGF(164) in vascular permeability and angiogenesis during implantation. Genesis. 2000;26:213–24. [PubMed] [Google Scholar]
- 68.Shi Q, Le X, Wang B, Abbruzzese JL, et al. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20:3751–6. doi: 10.1038/sj.onc.1204500. [DOI] [PubMed] [Google Scholar]
- 69.Beckert S, Farrahi F, Aslam RS, Scheuenstuhl H, et al. Lactate stimulates endothelial cell migration. Wound Repair Regen. 2006;14:321–4. doi: 10.1111/j.1743-6109.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 70.Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. 2013;273:156–65. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- 71.Ruan GX, Kazlauskas A. Lactate engages receptor tyrosine kinases Axl, Tie2, and vascular endothelial growth factor receptor 2 to activate phosphoinositide 3-kinase/Akt and promote angiogenesis. J Biol Chem. 2013;288:21161–72. doi: 10.1074/jbc.M113.474619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vegran F, Boidot R, Michiels C, Sonveaux P, et al. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–60. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura H, Kimura T, Ogita K, Nakamura T, et al. NF-kappaB activation at implantation window of the mouse uterus. Am J Reprod Immunol. 2004;51:16–21. doi: 10.1046/j.8755-8920.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 74.Hannan NJ, Paiva P, Meehan KL, Rombauts LJ, et al. Analysis of fertility-related soluble mediators in human uterine fluid identifies VEGF as a key regulator of embryo implantation. Endocrinology. 2011;152:4948–56. doi: 10.1210/en.2011-1248. [DOI] [PubMed] [Google Scholar]
- 75.Binder NK, Evans J, Gardner DK, Salamonsen LA, et al. Endometrial signals improve embryo outcome: functional role of vascular endothelial growth factor isoforms on embryo development and implantation in mice. Hum Reprod. 2014;29:2278–86. doi: 10.1093/humrep/deu211. [DOI] [PubMed] [Google Scholar]
- 76.Nimbkar-Joshi S, Rosario G, Katkam RR, Manjramkar DD, et al. Embryo-induced alterations in the molecular phenotype of primate endometrium. J Reprod Immunol. 2009;83:65–71. doi: 10.1016/j.jri.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 77.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69:522–30. [PubMed] [Google Scholar]
- 78.Choi SY, Collins CC, Gout PW, Wang Y. Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? J Pathol. 2013;230:350–5. doi: 10.1002/path.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singer K, Gottfried E, Kreutz M, Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother. 2011;60:425–31. doi: 10.1007/s00262-010-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gottfried E, Kreutz M, Mackensen A. Tumor metabolism as modulator of immune response and tumor progression. Semin Cancer Biol. 2012;22:335–41. doi: 10.1016/j.semcancer.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 81.Calcinotto A, Filipazzi P, Grioni M, Iero M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–56. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 82.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–9. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 83.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 84.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Revi Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 85.Chen W, Jin W, Hardegen N, Lei KJ, et al. Conversion of peripheral CD4 + CD25- naive T cells to CD4 + CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clark DA, Fernandes J, Banwatt D. Prevention of spontaneous abortion in the CBA x DBA/2 mouse model by intravaginal TGF-beta and local recruitment of CD4+8+ FOXP3+ cells. Am J Reprod Immunol. 2008;59:525–34. doi: 10.1111/j.1600-0897.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- 87.Seliger C, Leukel P, Moeckel S, Jachnik B, et al. Lactate-modulated induction of THBS-1 activates transforming growth factor (TGF)-beta2 and migration of glioma cells in vitro. PLoS One. 2013;8:e78935. doi: 10.1371/journal.pone.0078935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yabu M, Shime H, Hara H, Saito T, et al. IL-23-dependent and -independent enhancement pathways of IL-17A production by lactic acid. Int Immunol. 2011;23:29–41. doi: 10.1093/intimm/dxq455. [DOI] [PubMed] [Google Scholar]
- 89.Hunt JS. Cytokine networks in the uteroplacental unit: macrophages as pivotal regulatory cells. J Reprod Immunol. 1989;16:1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 90.Colegio OR, Chu NQ, Szabo AL, Chu T, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang S, Lin H, Kong S, Wang S, et al. Physiological and molecular determinants of embryo implantation. Mol Aspects Med. 2013;34:939–80. doi: 10.1016/j.mam.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–99. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 93.Hall BV. Variations in acidity and oxidation-reduction potential of rodent uterine fluids. Phyiol Zool. 1936;9:471–97. [Google Scholar]
- 94.Xiao S, Ye X. 2013. Acidification of uterine luminal epithelium is critical for embryo implantation in mice. Biol Reprod The Annual Meeting of the Society for the Study of Reproduction, abstract 869.


