Abstract
The ability of Candida albicans to cause disease is associated with its capacity to undergo morphological transition between yeast and filamentous forms, but the role of morphology in colonization and dissemination from the gastrointestinal (GI) tract remains poorly defined. To explore this, we made use of wild-type and morphological mutants of C. albicans in an established model of GI tract colonization, induced following antibiotic treatment of mice. Our data reveal that GI tract colonization favours the yeast form of C. albicans, that there is constitutive low level systemic dissemination in colonized mice that occurs irrespective of fungal morphology, and that colonization is not controlled by Th17 immunity in otherwise immunocompetent animals. These data provide new insights into the mechanisms of pathogenesis and commensalism of C. albicans, and have implications for our understanding of human disease.
Introduction
Candida albicans is a polymorphic fungus which grows in both yeast and filamentous forms and resides as a commensal in humans, particularly within the gastrointestinal (GI) tract (Brown et al., 2012). However, C. albicans is also an opportunistic pathogen and one of the major aetiological agents of mucosal and systemic fungal infection (Brown et al., 2012). In susceptible individuals, systemic C. albicans infections are thought to arise from organisms in the GI tract; a hypothesis supported by data from both patients and animal models (Koh et al., 2008; Miranda et al., 2009). As filamentous forms predominate at sites of primary epithelial infection, morphogenic transition is thought to facilitate access of C. albicans to the bloodstream and subsequent systemic spread (Gow et al., 2012). Moreover, morphogenetic transition is essential for virulence of this pathogen as mutants locked in either the yeast (Lo et al., 1997) or filamentous (Murad et al., 2001) forms are highly attenuated in animal models of systemic disease.
Given the importance of mucosal barriers, considerable attention has been given to understanding the interactions between C. albicans and epithelial cells. These studies have generated evidence that hyphae, but not yeast, are responsible for damaging and triggering protective inflammatory responses in epithelial cells (Moyes et al., 2010; Wachtler et al., 2011). Moreover, hyphae can activate the inflammasome leading to IL-1β production and induction of the Th17 responses that are critical for protection at the mucosa (Hise et al., 2009; Joly et al., 2009; Cheng et al., 2011; Hernandez-Santos and Gaffen, 2012). Yet few studies have investigated the role of C. albicans morphology and host immunity during colonization of the GI tract in vivo (White et al., 2007; Pande et al., 2013), which is the focus of the studies presented herein.
Results and discussion
To study the role of C. albicans morphology and host immunity during colonization of the GI tract, we made use of an established model whereby antibiotic-treated mice were colonized with C. albicans, following infection via their drinking water (Supporting Information Fig. S1A) (Vautier et al., 2012). Using this model, mice were infected with wild-type strains of C. albicans (SC5314 and CAI4) as well as strains carrying mutations locking them into the yeast (efg1Δ/cph1Δ) or filamentous forms (nrg1Δ). Differences in GI colonization were characterized by measuring stool fungal burdens at 7 and 10 days following infection (Fig. 1A). Over this time frame, the level of GI tract colonization remained constant for each strain, similar to our previous observations (Vautier et al., 2012) (Fig. 1A). Colonization with SC5314 was equivalent to that of CAI4, the parental strain from which the mutant strains were derived (Supporting Information Fig. S1B). Notably, when compared with wild-type C. albicans, mice infected with the yeast-locked efg1Δ/cph1Δ strain had higher fungal burdens in the GI tract while the filamentous-locked nrg1Δ strain colonized at lower levels (Fig. 1A). Similar results were obtained with other morphologically locked mutants, including yeast-locked hgc1Δ and filamentous tup1Δ (Supporting Information Fig. S1C). The lower colonization rates of the hyphal-locked mutants were not due to differences in inoculum, as we have previously shown GI tract colonization is not affected by the inoculum level (Vautier et al., 2012).
Figure 1.
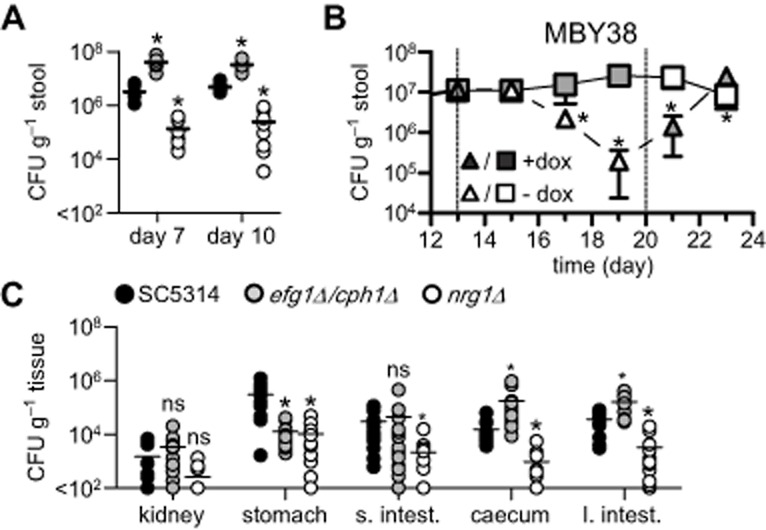
Candida albicans morphology influences colonization but not dissemination from the GI tract.A. Stool fungal burdens of 129Sv/Ev mice infected with wild-type (SC5314), yeast (efg1Δ/cph1Δ) and filamentous (nrg1Δ) C. albicans strains at day 7 and day 10 following infection (n = 10 per group).B. Stool fungal burdens of mice infected with MBY38 (n = 5 per group), following treatment with doxycycline, as indicated. The dotted lines indicate time points where doxycycline was administered or withdrawn.C. Tissue fungal burdens in the kidneys, stomach, small intestines (s. intest.), caecum and large intestines (l. intest.) at day 10 post infection with the fixed morphological mutants, as indicated (n = 14 per group). ns, not significant. *P < 0.05. See also Supporting Information Fig. S1.
To further confirm that the filamentous form of C. albicans is not favoured in the GI tract, we utilized an inducible filamentous strain (MBY38; tetO-UME6) in a modified model of oral infection (Supporting Information Fig. S1D). This strain is a conditional ume6 mutant, in which UME6 is expressed in the absence of doxycycline, driving filamentous growth (Carlisle et al., 2009). We also infected mice with wild-type Candida to monitor for any effects of doxycycline itself on colonization levels. In the presence of doxycycline, MBY38 achieved similar colonization levels to SC5314, as measured by stool burdens (Fig. 1B and Supporting Information Fig. S1E). However, removal of doxycycline and induction of filamentous growth on day 13 post infection led to a rapid decline in colonization of this strain from the GI tract (Fig. 1B). Reintroduction of doxycycline at day 20 restored colonization levels. In contrast, GI colonization by SC5314 was unaffected by the presence or absence of doxycycline Supporting Information Fig. S1E).
We next investigated the ability of these mutants to colonize tissues of the GI tract by analysing fungal burdens at various sites on day 10 post infection (Fig. 1C). All strains were detected throughout the GI tract and generally reflected the levels found in the stools, i.e. nrg1Δ-infected animals had lower tissue burdens while efg1Δ/cph1Δ-infected animals had similar or higher tissue burdens when compared with SC5314 or CAI4 (Fig. 1C and Supporting Information Fig. S1F). Similarly, animals infected with the filamentous form of MBY38 had significantly lower tissue fungal burdens (Supporting Information Fig. S1G). In the stomach, however, both morphologically locked strains were present at lower levels compared with SC5314, suggesting that the ability to transition between morphologies is important for colonization of this tissue (Fig. 1C).
Although our results for these C. albicans strains reflect disparate observations made by several other groups (Bendel et al., 2003; White et al., 2007; Koh et al., 2008; Pierce and Kumamoto, 2012), it was possible that morphotype-dependent levels of colonization were being influenced through confounding effects of alterations in co-regulated genes in these strains. Thus, to determine the morphological preference in the GI tract in an unmodified strain, we enumerated the presence of yeast and hyphal forms of wild-type C. albicans in the stools, stomach and caecal contents of infected animals (Supporting Information Fig. S1H). In all samples, C. albicans was predominantly found as yeast. Thus, taken together, these results strongly suggest that GI tract colonization primarily favours the yeast form of C. albicans. In support of this conclusion, C. albicans was recently found to induce a novel yeast-like GUT (gastrointestinally induced transition) morphotype, following colonization of the GI tract (Pande et al., 2013). Why the yeast form is favoured is unclear, but may reflect host immunity to the filamentous forms, interaction with the microbiota and/or direct physical influences under conditions of flow within the GI tract.
Notably, we also found that C. albicans disseminated at low levels to the kidneys following GI colonization (Fig. 1C). Such dissemination has been reported previously (Kennedy and Volz, 1985; Samonis et al., 1990) and also occurred at equivalent levels with the mutant strains, which were highly attenuated in systemic infection models (Supporting Information Fig. S1I), as expected (Lo et al., 1997; Murad et al., 2001). Thus, these results show that dissemination from the GI tract is not dependent on morphological transition in C. albicans. How this dissemination occurs is unclear, as the intestinal epithelium is covered by a thick layer of mucus preventing fungal contact (de Repentigny et al., 2000; Johansson et al., 2011; Iliev et al., 2012). Indeed, we could not detect C. albicans cells in close proximity of the intestinal epithelium by histology [data not shown (Iliev et al., 2012; Vautier et al., 2012)]. Thus, transepithelial transport of these organisms must be mediated by indirect mechanisms, such as by lumen sampling dendritic cells (Rescigno et al., 2001) or M cell transcytosis (Owen et al., 1986; Rochereau et al., 2013). Such mechanisms could explain how C. albicans can disseminate from the GI tract, even in the absence of mucosal damage (White et al., 2007; Koh et al., 2008; Vautier et al., 2012). Importantly, dissemination from the GI tract does not result in systemic disease in immunologically competent animals (data not shown).
We next determined if the various strains of C. albicans differentially influenced host immunity by analysing cytokine levels in various tissues at day 10 post infection. However, we only detected altered cytokine levels in the stomachs of infected animals with the morphologically locked strains (Fig. 2A), which correlated with their reduced tissue fungal burden (see Fig. 1C). Such differences were not observed in any other tissue (Supporting Information Fig. S2A). This is consistent with our previous observations demonstrating preferential infection of the stomach in this model (Vautier et al., 2012) and the fact that that colonization is restricted to the lumen elsewhere in the GI tract (discussed above). Notably, the stomachs of animals colonized with the morphologically locked mutants had reduced levels of IL-1β, IL-6 and IL-17; all of which are involved in mediating Th17 responses (Hernandez-Santos and Gaffen, 2012).
Figure 2.
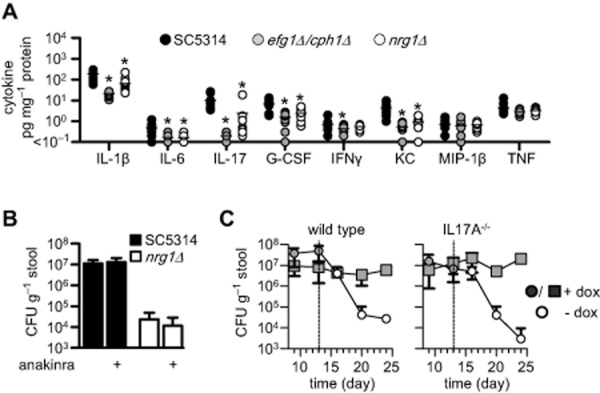
Immune responses during GI tract colonization.A. Selected cytokine levels in the stomachs of 129Sv/Ev mice at day 10 following infection with wild-type (SC5314), yeast-locked (efg1Δ/cph1Δ) and filamentous-locked (nrg1Δ) C. albicans strains (n = 10 per group).B. Day 6 stool fungal burdens of mice infected with wild-type (SC5314) and filamentous-locked (nrg1Δ) C. albicans strains, following daily treatment with IL-1RA (Anakinra), as indicated (n = 5 per group).C. Stool fungal burdens of C57BL/6 wild-type (n = 4 per group) or Il17A−/− (n = 5 per group) mice infected with MBY38 in the presence or absence of doxycycline, as indicated. The dotted lines indicate time points where doxycycline was withdrawn. *P < 0.05. See also Supporting Information Fig. S2.
Th17 immunity is required for controlling C. albicans infections at the mucosa, although the exact role of this response at different mucosal sites, especially in the GI tract, is controversial and poorly understood (Hernandez-Santos and Gaffen, 2012). We therefore explored the possibility that interfering with Th17 responses would alter GI tract colonization, particularly of filamentous forms. As IL-1β is essential for controlling systemic candidiasis (Vonk et al., 2006) and driving Th17 immunity (Hernandez-Santos and Gaffen, 2012), we first blocked the effects of IL-1β by daily administration of an IL-1 receptor antagonist (Anakinra) to mice and monitoring stool fungal burdens (Fig. 2B and data not shown). The i.p. administration of Anakinra has physiological effects in the intestines at the doses used in our experiments (Meinzer et al., 2012). Despite its importance in systemic models (Vonk et al., 2006), blocking the IL-1 receptor had no effect on colonization levels of either SC5314 or nrg1Δ. This suggests that IL-1 receptor signalling is not involved in controlling GI tract colonization.
We then looked at the role of IL-17-related responses, monitoring C. albicans colonization in the GI tract of both IL17A−/− and IL17RA−/− mice (Fig. 2C and Supporting Information Fig. S2B). For these experiments, we made use of our inducible strain (MBY38) to examine the effects on colonization by the filamentous morphotypes. Unexpectedly, we found no differences in C. albicans colonization in either mouse knockout strain. As before, induction of filamentous growth by withdrawal of doxycycline led to rapid reduction in the fungal burdens in the GI tract, but this was not significantly affected in the knockout mice. Thus, although we cannot exclude a role for IL-22 (De Luca et al., 2010), Th17 immunity does not appear to regulate GI colonization by C. albicans.
In conclusion, our data show that in immunocompetent animals the yeast form of C. albicans is favoured during colonization of the GI tract, from which there is a constitutive low level of systemic dissemination which is not dependent on morphological transition. Moreover, we show that colonization of the GI tract is not influenced by Th17 immunity, indicating that other mechanisms of control are occurring in these tissues. These data provide new insights into the mechanisms of pathogenesis and commensalism of C. albicans, and have implications for our understanding of human disease.
Experimental procedures
Mice and ethics statement
Eight to twelve week old female 129Sv/Ev, C57BL/6, Il17a−/− and Il17ra−/− mice on C57BL/6 background were bred and maintained at specific pathogen-free facilities at the University of Aberdeen and University of Pittsburgh. Mice were randomly allocated to groups and housed in individually ventilated cages, and provided with food and water ad libitum. All experimentation conformed to the terms and conditions of United Kingdom Home Office licenses for research on animals and the University of Aberdeen and University of Pittsburgh ethical review committees.
Candida albicans strains, culture media and growth conditions
Candida albicans strains used in this study included SC5314 (wild type), CAI4 (wild type), MBY38 [tetO-UME6 (Carlisle et al., 2009)], efg1Δ/cphΔ1 (Lo et al., 1997), nrg1Δ (Murad et al., 2001), hgc1Δ (Zheng and Wang, 2004) and tup1Δ (Braun and Johnson, 1997). Strains were routinely grown and maintained on YPD agar (Sigma-Aldrich). For the MBY38 strain, plates contained 40 μg ml−1 of doxycycline. For inoculum preparation, a single colony was grown in Sabouraud broth (Oxoid) at 30°C for 24 h with shaking. Cells were washed twice in sterile phosphate-buffered saline (PBS) and counted using a haemocytometer. Inoculums of the filamentous strains, nrg1Δ and tup1Δ, were standardized to 1 × 107 CFU ml−1 of SC5314 by quantifying protein concentration (Meyers et al., 1998).
GI model
The GI model was performed essentially as described previously (see Supporting Information Fig. S1) (Vautier et al., 2012). To reduce commensal flora, mice were provided with sterile antibiotic water containing 2 mg ml−1 of streptomycin (Invitrogen), 2000 U ml−1 of penicillin (Invitrogen), 0.25 mg ml−1 of fluconazole (Enzo) for 3 days and then switched to water containing the same concentrations of streptomycin and penicillin for a further 24 h. Mice were then provided with sterile water containing 1 × 107 CFU ml−1 of C. albicans, 2 mg ml−1 of streptomycin and 2000 U ml−1 of penicillin for 5 days. After C. albicans exposure, mice were maintained on sterile water containing 2 mg ml−1 of streptomycin, 2000 U ml−1 of penicillin and 0.2 mg ml−1 of gentamicin (Invitrogen). For strain MBY38, 2 mg ml−1 of doxycycline was added in addition to the drinking water as detailed in Supporting Information Fig. S1. To monitor colonization, stools were collected from individual mice and homogenized in 1 ml of PBS, serially diluted, and 25 μl of each dilution plated on YPD agar containing 0.01 mg ml−1 of vancomycin (Sigma) and 0.1 mg ml−1 of gentamicin. Plates were incubated overnight at 37°C under aerobic conditions and fungal levels determined by viable cell count. Mice were killed at the indicated time points post exposure to C. albicans. Kidney, stomach, small intestine, caecum and large intestine samples (harvested in that order) were washed three times with 1 ml of sterile PBS to remove gut contents. Tissue weights were determined, and samples were transferred into tubes containing 0.5 ml of PBS, 0.05% (v/v) Triton X-100 and complete mini EDTA-free protease inhibitor cocktail (Roche). The tissues were then homogenized, serially diluted and plated on YPD as above. Cell debris was removed from the remaining tissue homogenates by centrifugation at 15871 g for 15 min at 4°C and stored at −80°C for subsequent cytokine analysis.
IL-1R blocking
The GI model was performed essentially as above, except mice were injected i.p. daily throughout the experiment with 50 mg kg−1 Kineret (Anakinra, UDG) (Sgroi et al., 2011) or PBS starting 2 days prior to infection.
Cytokine analysis
Cytokine levels were measured using the Bio-Rad, Bio-Plex Pro™ Mouse 23-Plex kit and analysed on the Bio-Plex system using Bio-Plex Manager™ software as per manufacturer's instructions. Stored tissue sample supernatants were defrosted and centrifuged for 15 min at 15871 g at 4°C to remove debris. For each test, 50 μl of undiluted sample was used and cytokine concentrations were normalized to sample protein concentrations (BCA kit, Pierce).
Yeast/hyphae counts
Ten microlitres of samples from homogenized stools, stomach and caecal contents were added to slides, coverslips applied and Candida cells counted under a microscope at 40× magnification. Four fields of view were counted per sample. The percentage of yeast/hyphae was then represented as a ratio of the total number of cells counted (between 50 and 100 for every field of view).
Statistical analysis
The two-tailed student's t-test was used to compare two groups, while multiple groups analyses were performed using two-way analysis of variance (ANOVA). All data were analysed with GraphPad Prism software version 5.04.
Acknowledgments
This work was supported by the Wellcome Trust (086558, 080088, 102705), a Wellcome Trust Strategic Award (097377) and a studentship from the University of Aberdeen. D.K. was supported by grant 5R01AI083344 from the National Institute of Allergy and Infectious Diseases and by a Voelcker Young Investigator Award from the Max and Minnie Tomerlin Voelcker Fund. The authors declare no competing financial interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Candida albicans morphology influences colonization but not dissemination from the GI tract. (A) Cartoon representation of the systemic experimental GI tract colonization model with sampling points (underlined). (B) Stool fungal burdens of 129Sv/Ev mice infected with C. albicans SC5314 and CAI4 at day 7 and day 10 following infection (n = 4 per group). (C) Stool fungal burdens of 129Sv/Ev mice infected with wild-type (SC5314), yeast (hgc1Δ) and filamentous (tup1Δ) C. albicans strains at day 9 following infection (n = 6 per group). (D) Cartoon representation of the modified GI tract colonization model with doxycycline (dox) and C. albicans MBY38. (E) Stool fungal burdens of 129Sv/Ev mice infected with SC5314, following treatment with doxycycline, as indicated (n = 5 per group). The dotted lines indicate the time intervals where doxycycline was administered or withdrawn. (F) Tissue fungal burdens in the kidneys, stomach, small intestines (s. intest.), caecum and large intestines (l. intest.) at day 10 post infection with the wild strains, as indicated (n = 4 per group). (G) Tissue fungal burdens in the kidneys, stomach, small intestines (s. intest.), caecum and large intestines (l. intest.) at day 20 post infection with SC5314 or MBY38, as indicated (n = 5 per group). (H) Percentage yeast morphology of C. albicans SC5314 and CAI4 in stools and in stomach and caecum contents at day 10 post infection. (I) Survival of mice following intravenous infection with (1 × 105) wild-type (SC5314), yeast (efg1Δ/cph1Δ) or filamentous (nrg1Δ) C. albicans strains (n = 6 per group). *P < 0.05.
Immune responses during GI tract colonization. (A) Selected cytokine levels in the caecum and large intestines of 129Sv/Ev mice at day 10 following infection with wild-type (SC5314), yeast-locked (efg1Δ/cph1Δ) and filamentous-locked (nrg1Δ) C. albicans strains, as indicated (n = 10 per group). (B) Stool fungal burdens of wild-type C57BL/6 (n = 6) or Il17ra−/− mice (n = 9) infected with MBY38, following treatment with or without doxycycline, as indicated.
References
- Bendel CM, Hess DJ, Garni RM, Henry-Stanley M. Wells CL. Comparative virulence of Candida albicans yeast and filamentous forms in orally and intravenously inoculated mice. Crit Care Med. 2003;31:501–507. doi: 10.1097/01.CCM.0000049954.48239.A1. [DOI] [PubMed] [Google Scholar]
- Braun BR. Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NAR, Netea MG. White T. Human fungal infections: the Hidden Killers. Sci Transl Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, Lopez-Ribot JL. Kadosh D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci USA. 2009;106:599–604. doi: 10.1073/pnas.0804061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol. 2011;90:357–366. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A, et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010;3:361–373. doi: 10.1038/mi.2010.22. [DOI] [PubMed] [Google Scholar]
- Gow NAR, van de Veerdonk FL, Brown AJ. Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2012;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Santos N. Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11:425–435. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD. Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Larsson JM. Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108(Suppl. 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL. Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ. Volz PA. Effect of various antibiotics on gastrointestinal colonization and dissemination by Candida albicans. Sabouraudia. 1985;23:265–273. doi: 10.1080/00362178585380391. [DOI] [PubMed] [Google Scholar]
- Koh AY, Kohler JR, Coggshall KT, Van Rooijen N. Pier GB. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 2008;4:e35. doi: 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A. Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Meinzer U, Barreau F, Esmiol-Welterlin S, Jung C, Villard C, Leger T, et al. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe. 2012;11:337–351. doi: 10.1016/j.chom.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Meyers PR, Bourn WR, Steyn LM, van Helden PD, Beyers AD. Brown GD. Novel method for rapid measurement of growth of mycobacteria in detergent-free media. J Clin Microbiol. 1998;36:2752–2754. doi: 10.1128/jcm.36.9.2752-2754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda LN, van der Heijden IM, Costa SF, Sousa AP, Sienra RA, Gobara S, et al. Candida colonisation as a source for candidaemia. J Hosp Infect. 2009;72:9–16. doi: 10.1016/j.jhin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad AM, Leng P, Straffon M, Wishart J, Macaskill S, MacCallum D, et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001;20:4742–4752. doi: 10.1093/emboj/20.17.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen RL, Pierce NF, Apple RT. Cray WC., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986;153:1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Pande K, Chen C. Noble SM. Passage through the mammalian gut triggers a phenotypic switch that promotes Candida albicans commensalism. Nat Genet. 2013;45:1088–1091. doi: 10.1038/ng.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JV. Kumamoto CA. Variation in Candida albicans EFG1 expression enables host-dependent changes in colonizing fungal populations. MBio. 2012;3:e117–e112. doi: 10.1128/mBio.00117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Repentigny L, Aumont F, Bernard K. Belhumeur P. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect Immun. 2000;68:3172–3179. doi: 10.1128/iai.68.6.3172-3179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Rochereau N, Drocourt D, Perouzel E, Pavot V, Redelinghuys P, Brown GD, et al. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 2013;11:e1001658. doi: 10.1371/journal.pbio.1001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonis G, Anaissie EJ, Rosenbaum B. Bodey GP. A model of sustained gastrointestinal colonization by Candida albicans in healthy adult mice. Infect Immun. 1990;58:1514–1517. doi: 10.1128/iai.58.6.1514-1517.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgroi A, Gonelle-Gispert C, Morel P, Baertschiger RM, Niclauss N, Mentha G, et al. Interleukin-1 receptor antagonist modulates the early phase of liver regeneration after partial hepatectomy in mice. PLoS ONE. 2011;6:e25442. doi: 10.1371/journal.pone.0025442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautier S, Drummond RA, Redelinghuys P, Murray GI, MacCallum DM. Brown GD. Dectin-1 is not required for controlling Candida albicans colonization of the gastrointestinal tract. Infect Immun. 2012;80:4216–4222. doi: 10.1128/IAI.00559-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW. Kullberg BJ. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- Wachtler B, Wilson D, Haedicke K, Dalle F. Hube B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS ONE. 2011;6:e17046. doi: 10.1371/journal.pone.0017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SJ, Rosenbach A, Lephart P, Nguyen D, Benjamin A, Tzipori S, et al. Self-regulation of Candida albicans population size during GI colonization. PLoS Pathog. 2007;3:e184. doi: 10.1371/journal.ppat.0030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Candida albicans morphology influences colonization but not dissemination from the GI tract. (A) Cartoon representation of the systemic experimental GI tract colonization model with sampling points (underlined). (B) Stool fungal burdens of 129Sv/Ev mice infected with C. albicans SC5314 and CAI4 at day 7 and day 10 following infection (n = 4 per group). (C) Stool fungal burdens of 129Sv/Ev mice infected with wild-type (SC5314), yeast (hgc1Δ) and filamentous (tup1Δ) C. albicans strains at day 9 following infection (n = 6 per group). (D) Cartoon representation of the modified GI tract colonization model with doxycycline (dox) and C. albicans MBY38. (E) Stool fungal burdens of 129Sv/Ev mice infected with SC5314, following treatment with doxycycline, as indicated (n = 5 per group). The dotted lines indicate the time intervals where doxycycline was administered or withdrawn. (F) Tissue fungal burdens in the kidneys, stomach, small intestines (s. intest.), caecum and large intestines (l. intest.) at day 10 post infection with the wild strains, as indicated (n = 4 per group). (G) Tissue fungal burdens in the kidneys, stomach, small intestines (s. intest.), caecum and large intestines (l. intest.) at day 20 post infection with SC5314 or MBY38, as indicated (n = 5 per group). (H) Percentage yeast morphology of C. albicans SC5314 and CAI4 in stools and in stomach and caecum contents at day 10 post infection. (I) Survival of mice following intravenous infection with (1 × 105) wild-type (SC5314), yeast (efg1Δ/cph1Δ) or filamentous (nrg1Δ) C. albicans strains (n = 6 per group). *P < 0.05.
Immune responses during GI tract colonization. (A) Selected cytokine levels in the caecum and large intestines of 129Sv/Ev mice at day 10 following infection with wild-type (SC5314), yeast-locked (efg1Δ/cph1Δ) and filamentous-locked (nrg1Δ) C. albicans strains, as indicated (n = 10 per group). (B) Stool fungal burdens of wild-type C57BL/6 (n = 6) or Il17ra−/− mice (n = 9) infected with MBY38, following treatment with or without doxycycline, as indicated.


