Abstract
Transient high-frequency oscillations (150-600 Hz) in local field potential generated by human hippocampal and parahippocampal areas have been related to both physiological and pathological processes. The cellular basis and effects of normal and abnormal forms of high-frequency oscillations (HFO) has been controversial. Here, we searched for HFOs in slices of the subiculum prepared from human hippocampal tissue resected for treatment of pharmacoresistant epilepsy. HFOs occurred spontaneously in extracellular field potentials during interictal discharges (IID) and also during pharmacologically induced preictal discharges (PID) preceding ictal-like events. While most of these events might be considered pathological since they invaded the fast ripple band (>250 Hz), others were spectrally similar to physiological ripples (150-250 Hz). Do similar cellular mechanisms underly IID-ripples and PID-ripples? Are ripple-like oscillations a valid proxy of epileptogenesis in human TLE? With combined intra- or juxta-cellular and extracellular recordings, we showed that, despite overlapping spectral components, ripple-like IID and PID oscillations were associated with different cellular and synaptic mechanisms. IID-ripples were associated with rhythmic GABAergic and glutamatergic synaptic potentials with moderate neuronal firing. In contrast, PID-ripples were associated with depolarizing synaptic inputs frequently reaching the threshold for bursting in most cells. Thus ripple-like oscillations (100-250 Hz) in the human epileptic hippocampus are associated with different mechanisms for synchrony reflecting distinct dynamic changes in inhibition and excitation during interictal and pre-ictal states.
Introduction
Ripple oscillations (~200 Hz) are observed in hippocampal-entorhinal networks of rodents, monkeys and humans during quiet wakefulness and slow-wave sleep (Buzsaki et al., 1992; Skaggs et al., 2007; Le Van Quyen et al., 2008). They commonly co-occur with large amplitude sharp-waves that originate from the synchronized firing of CA3 cells and spread along the CA1-subicular-entorhinal axis (Chrobak and Buzsaki, 1996). Co-activation of hippocampal and neocortical pathways during sharp-wave ripples may be crucial for memory consolidation (Buzsaki, 1989; Lee and Wilson, 1992; Wilson and McNaughton, 1994; Girardeau et al., 2009; Diekelmann and Born, 2010). Cellular evidence suggests ripples reflect rhythmic perisomatic inhibitory potentials in pyramidal cells (Ylinen et al. 1995; Csicsvari et al., 1999; Klausberger et al., 2003, 2004; Maier et al., 2003) together with rhythmic excitatory potentials (Maier et al. 2010) and phase-locked firing (Csicsvari et al. 1999, 2000). Inhibitory interneurons would then secure an orderly recruitment of pyramidal cells (Klausberger and Somogyi 2008) together perhaps with contributions to synchrony from gap junctions (Draguhn et al., 1998; Traub and Bibbig, 2000) and the ephaptic entrainment of neurons by large sharp-wave fields (Anastassiou et al., 2010).
High-frequency oscillations (HFOs, 150-500 Hz) have been linked to epilepsy and have a frequency range that overlaps partially with physiological ripples (Le Van Quyen et al., 2012). HFOs are strongly associated with epileptogenic regions in the human (Bragin et al., 1999; Staba et al., 2004; Jirsch et al, 2006; Crépon et al., 2010), in slices of human epileptic neocortex (Roopun et al., 2010) and in animal models of epilepsy (Bragin et al., 2002; Grenier at al., 2003; Foffani et al., 2007). They sometimes precede seizure onset (Jirsch et al, 2006) and may also co-occur with electroencephalographic (EEG) epileptic interictal discharges between seizures (De Curtis and Avanzini, 2001). Strongly expressed in hippocampus and parahippocampal regions of patients with mesial temporal lobe (MTL) epilepsy, HFOs have been viewed as a pathological variant of physiological ripples (Foffani et al., 2007; Aivar et al. 2014). Nevertheless, even though spectral frequencies overlaps, it is unclear whether HFOs associated with interictal discharges and physiological ripples share similar cellular correlates (Engel et al., 2009). Specifically pathological HFOs are suggested to result from population spike fields due to synchrony in clusters of abnormal synchronously bursting neurons. In physiological ripples, HFO are assumed to derive in part from summed IPSPs (Bragin et al., 2002; Engel et al., 2009; but see also Maier et al. 2010). The involvement of clusters of hyperexcitable neurons in epileptiform HFOs is consistent with an impaired inhibitory function in epilepsy. The efficacy of inhibitory signaling may be reduced by a loss of some interneuronal types (Esclapez and Houser, 1999), differential changes in dendritic and somatic inhibitory potentials (Cossart et al., 2001), defects of GABA release (Hirsch et al., 1999) and perturbation of chloride homeostasis in some pyramidal cells with low levels of KCC2 and high levels of NKCC1 (Cohen et al. 2002; Huberfeld et al. 2007). Reduced inhibitory signals together with changes in several potassium currents and the cationic Ih current (Bernard et al, 2004) would tend to enhance pyramidal cell excitability and favor disorganized burst firing (Chen et al., 2011; Ibarz et al. 2010; Simeone et al. 2013). Nevertheless, it remains unclear how these processes give rise to epileptic forms of ripples (Engel et al., 2009).
In the present study, we asked whether HFOs are associated with two distinct epileptiform activities, interictal (IID) and preictal discharges (PID), generated in the subiculum of patients with MTL epilepsy. Field records of both IID and PID, revealed oscillations at 150-250 Hz, very similar to physiological ripples of normal mammals. We then combined intra- or juxta-cellular and extracellular recordings to examine the underlying cellular and synaptic correlates of these oscillations. We conclude that, despite overlap in their spectral components, HFOs associated with IIDs and PIDs are generated by different mechanisms.
Methods
Human epileptic tissue
Temporal lobe tissue blocks containing the hippocampus, subiculum and part of the entorhinal cortex were obtained after operations on 26 people suffering from pharmacoresistant MTL epilepsies associated with hippocampal sclerosis (age 18–52 years, seizures for 3–35 years). Post-surgical tissue was transported in a cold, oxygenated solution containing 248 mM d-sucrose, 26 mM NaHCO3, 1 mM KCl, 1 mM CaCl2, 10 mM MgCl2 and 10 mM d-glucose, equilibrated with 5% CO2 in 95% O2. Hippocampal-subicular-entorhinal cortex slices or isolated subicular slices (400 μm thick) were cut with a vibratome (HM650V, Microm). They were maintained at 37 °C, equilibrated with 5% CO2 in 95% O2, in an interface chamber perfused with a solution containing: 124 mM NaCl, 26 mM NaHCO3, 4 mM KCl, 2 mM MgCl2, 2 mM CaCl2 and 10 mM d-glucose. The tissue generated interictal-like discharges (IIDs), spontaneously (Cohen et al., 2002). Ictal-like activity preceded by pre-ictal discharges (PIDs) was induced by pro-convulsant solutions (Huberfeld et al. 2011) consisting of either an increase of extracellular K+, and a reduction of extracellular Ca2+ and Mg2+ or an alkalinization coupled with an increase of extracellular K+ or a decrease in Mg++. Ictal discharges preceded by PIDs emerged from a subicular region of ~2 × 8 mm and were never observed in the dentate gyrus, CA3 or entorhinal cortex (Huberfeld et al. 2011).
Recordings
Local field potentials were recorded with etched, tungsten electrodes. Signals were amplified 1000 times and analogically filtered between 0.1 Hz to 10 kHz (AM systems, 1700). Intracellular records were made with glass microelectrodes containing 2 M potassium acetate and beveled to a resistance of 50–100 MΩ. Signals were amplified with an Axoclamp 2B amplifier in current-clamp mode. Juxtacellular records were made with glass micro-electrodes of resistance 3–5 MΩ filled with the perfusion solution. Intracellular and extracellular signals were digitized at 10 kHz with a 12–bit and 16-channel A-D converter (Digidata 1200A, Axon Instruments).
Automatic detection of epileptic discharges (IID and PID)
Local field potentials (LFP) were filtered between 3 – 30 Hz, with infinite impulse response (IIR) filters of order 8 using a forward-backward filtering strategy to avoid phase shift. The amplitude distribution of sharp-wave peaks was measured after filtering. Criteria for analyzed events included peaks above the 80th percentile, duration less than 200 ms, and inter-event interval longer than 400 ms. Artifactual false positive events were rejected by visual inspection.
Estimation of the power spectral density (PSD)
A time-frequency representation of detected, wide-band individual events (400 ms epochs) was first obtained with the Gabor wavelet transform (Gaussian modulated window, 80-600 Hz, 5 cycles). The power spectral density (PSD) was then extracted from the wavelet transform as the mean within a time window of ± 50 ms around the peak of the discharge. More traditional methods for PSD estimation were not used due to an unacceptably high dependency on parameters.
Quantification of high frequency oscillations in the ripple band
HFOs associated to detected epileptic discharges, and classified as IID or PID, were further quantified using a ripple index (from 0 to 1) defined from the PSD as the proportion of total power in the ripple band (180-250Hz) (Ibarz et al. 2010). Due to spectral leak associated to multi-unit activity, we constrained this quantification to the 80-450 Hz band.
Spike-field coherence and intra-field coherence
Spike-field coherence was estimated from power spectra of cross-correlations between spikes and the filtered LFP. For single cells, the time epochs corresponding to all detected events were concatenated. Spike time series correspond to a binary vector with logic ‘1’ at the spike times. Field time series correspond to a filtered version of the raw signal in the 80 – 500 Hz frequency band. The power spectrum was estimated using the Welch periodogram. A further global index of spike-field synchronization was computed as the decimal logarithmic of the area below the spectrum (Foffani et al. 2007). A similar procedure using the concatenated intracellular membrane potential rather than spikes provided a measure of intra-field coherence.
Statistical Inference
The significance of differences between spectral distributions was examined with a permutation test using alpha = 0.01 (Butar, 2008). This non-parametric statistical test avoided assumptions of normality. Data is represented as mean ± SD. Pairwise comparisons were tested using non-parametric tests.
Results
IID and PID associated ripple-like oscillations in the local field potential
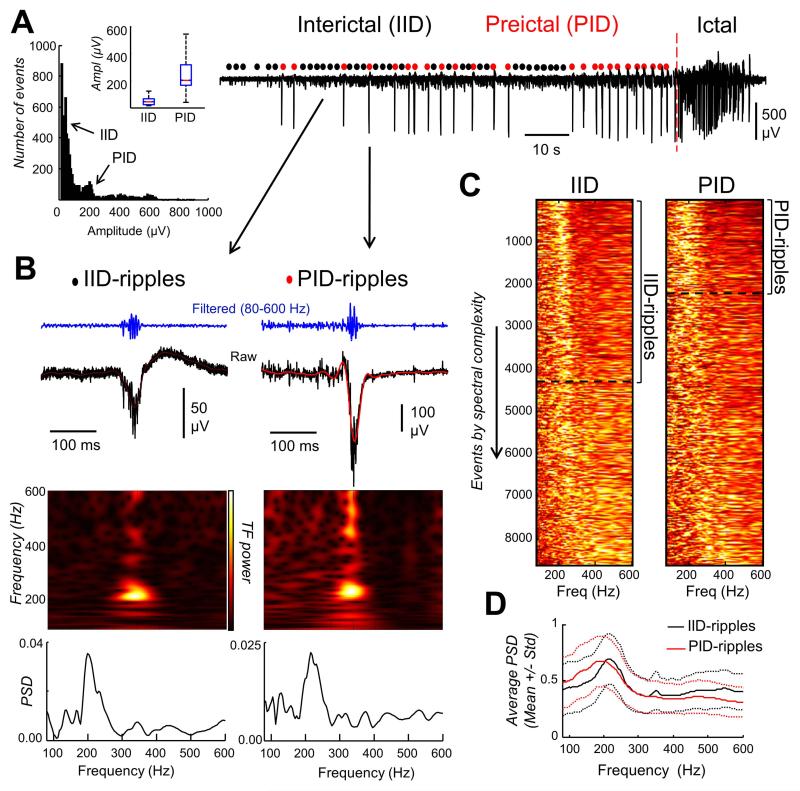
Extracellular field potential recorded in 83 slices from 26 patients revealed spontaneous IIDs were invariably generated in the subiculum (Cohen et al., 2002). Their mean frequency was 1.2 ± 0.6 Hz and duration from onset to peak positivity was 33 ± 11 ms, similar to values for interictal spikes recorded in situ (Fabo et al. 2008; Huberfeld et al. 2011). Perfusion of a pro-convulsant solution induced ictal-like events. They were consistently preceded by recurring PID of larger amplitude than IID (Huberfeld et al., 2011; Fig. 1A). These larger amplitude PID events could coexist with IID events. It is suggested that different circuits underlie PIDs and IIDs (Huberfeld et al., 2011). From our dataset, 8866 IID events and 7752 PID events were automatically detected and classified from amplitude criteria (Fig. 1A, left).
Figure 1.
A. Extracellular recording from a representative human subicular slice where ictal-like activity was induced by convulsants. Spontaneous interictal (IID) and preictal discharges (PID) could be differentiated by their amplitudes (left plot for data shown in traces; inset shows mean group comparisons). B. Examples of interictal and preictal discharges associated with ripple-like oscillations (IID-ripples and PID-ripples, respectively). Both time-frequency plots and the power spectral densities show a narrow band component around 200 Hz. Note also spectral bleeding of multi-unit activity at >400 Hz C. Power spectrum of all individual events from 83 slices/26 patients. Events are organized by the ripple index, and only those with a well-defined narrow peak at 100-250 Hz were selected. D. Averaged power spectra over all events show similar distributions of frequency components for both IID-ripples and PID-ripples.
We found that high-frequency oscillations (> 100 Hz) were associated with both IIDs and PIDs, either superimposed on their rising phase or their peak. Time-frequency plots and power spectral density (PSD) analyses revealed a narrow band spectral peak between 150 and 250 Hz (Fig. 1B). LFP oscillations were also identified at higher frequencies (300-500 Hz, fast ripple band) in some events as isolated spectral peaks (not shown). However, frequency components of some of these oscillatory activities could overlap with those more specific to multi-unit activity. This spectral leakage of spiking activity is of concern in studies on LFP oscillations above 100 Hz (Scheffer-Teixeira et al., 2013).
We attempted to reduce the influence of spike contamination by analyzing only oscillations limited to a narrow frequency band, selected according to the proportion of spectral power in the 180-250 Hz band (Ibarz et al., 2010). Ripple-like oscillations selected in this way appeared more frequently during IID (4313 events, ~50%) than during PID (1983 events, ~25%) in the whole database (Fig.1C). When analyzed from each electrode in a given slice we found a similar trend, suggesting that ripple-like oscillations represented a proportion of all detected events. Excluded, non-ripple-like, events possessed spectral components which spread into the fast ripple range (>250 Hz) suggestive of pathological activities and contaminated by multi-unit activities. In spite of differences in the amplitude of IID and PID events, the average power spectra of ripple-like oscillations were remarkably similar (Fig. 1D). The mean frequency of IID-ripples was 191 ± 53 Hz and that of PID-ripples was 173 ± 47 Hz while the duration of IID-ripples was 139 ± 40 ms and that for PID-ripples was 101 ± 45 ms.
Single-cell firing dynamics during IID-ripples and PID-ripples
Do similar cellular mechanisms underly IID-ripples and PID-ripples? Are ripple-like oscillations a valid proxy of epileptogenesis in human TLE? We pursued these questions by analyzing single cell firing dynamics during IID-ripples and PID-ripples from intra- and juxta-cellular recordings. We focused on pyramidal cells in view of their major contribution to local field potentials (Linden et al. 2011; Reimann et al. 2013).
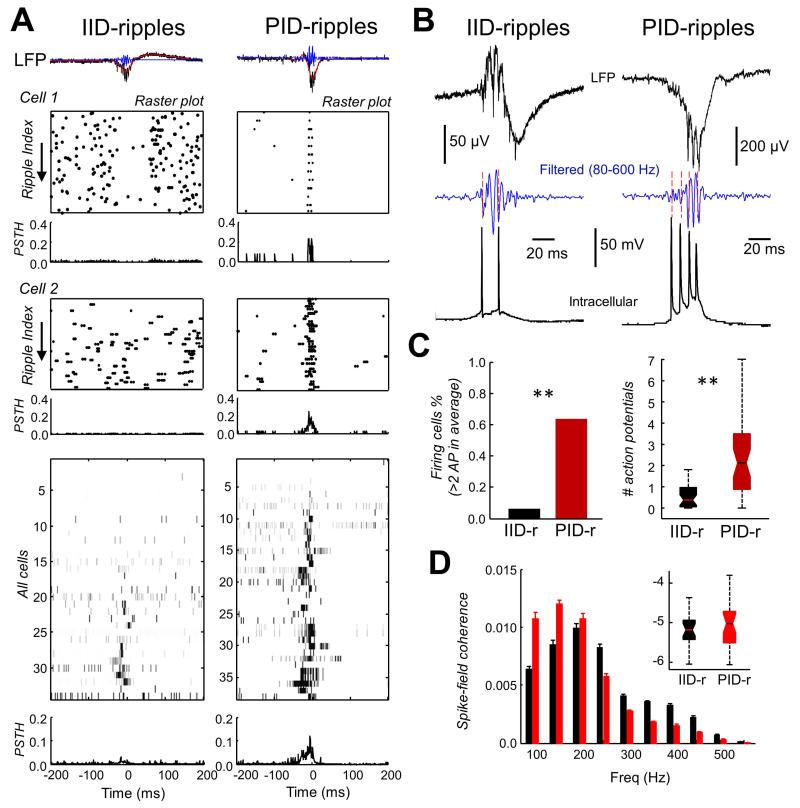
During IID-ripples, firing changed significantly for about 83% of recorded cells (34 cells; firing reduced in about half and increased in the other neurons) while firing of the remaining cells did not change (Fig. 2A, left). Firing neurons discharged one or at most two action potentials during IID-ripples (Fig. 2B, left). In contrast, most cells (73% of 38 cells) fired characteristic bursts during PID-ripples (Fig. 2A,B right). There were significant differences in firing probability and frequency between IID-ripples and PID-ripples (Fig. 2C). These differences were even more evident in a subset of cells (n=7) where firing during both IID-ripples and PID-ripples was analyzed (cells 1 and 2 in Fig.2A), and confirmed in the mean PSTH from all cells examined (Fig.2A, bottom).
Figure 2.
A. Raster plots of spike responses from two different cells during IID-ripples and PID-ripples with corresponding peri-event histograms (PSTH, bin size, 10 ms). They demonstrate two different firing dynamics underlying oscillations of the same frequency (~200 Hz). During IID-ripples, firing of Cell 1 is suppressed while that of Cell 2 is unchanged. In contrast, PID-ripples are associated with bursting activity in both cells. Firing probabilities (gray-scale maps and average PSTH at the bottom) show PID-ripples are often associated with bursts, whereas IID-ripples could be associated with either decreased or increased neuronal firing. B. Representative traces show cell spike responses phase-locked to one or more cycles of field oscillations. C. The proportion of firing cells (left) and mean firing rate (right, p<0.0001) are significantly different during IID-ripples and PID-ripples. D. Spike-field coherence shows a major modulation of spikes by the field at 100-250 Hz for both IID-ripples and PID-ripples. Inset shows grouped data on spike-field coherence at 100-250Hz expressed at a log-scale.
For those cells that fired more than two spikes during more than 10 individual events, we analyzed the coherence between the inter-spike intervals and the LFP filtered at 80 – 600 Hz. For both IID and PID events, cell firing and the associated LFP were more coherent at frequencies from 100 to 250 Hz (Fig 2D). This reflected close relations between the timing of cell firing and HFO cycles (Fig. 2B, red bars). There was no statistical difference in spike-field coherence in this band between IID- and PID- ripple events (randomization test; Fig.2D, inset). Therefore, rhythmic pyramidal cell firing contributes to both IID and PID ripple-like oscillations in the 100-250 Hz frequency band.
Membrane potential oscillations associated to IID-ripples and PID-ripples
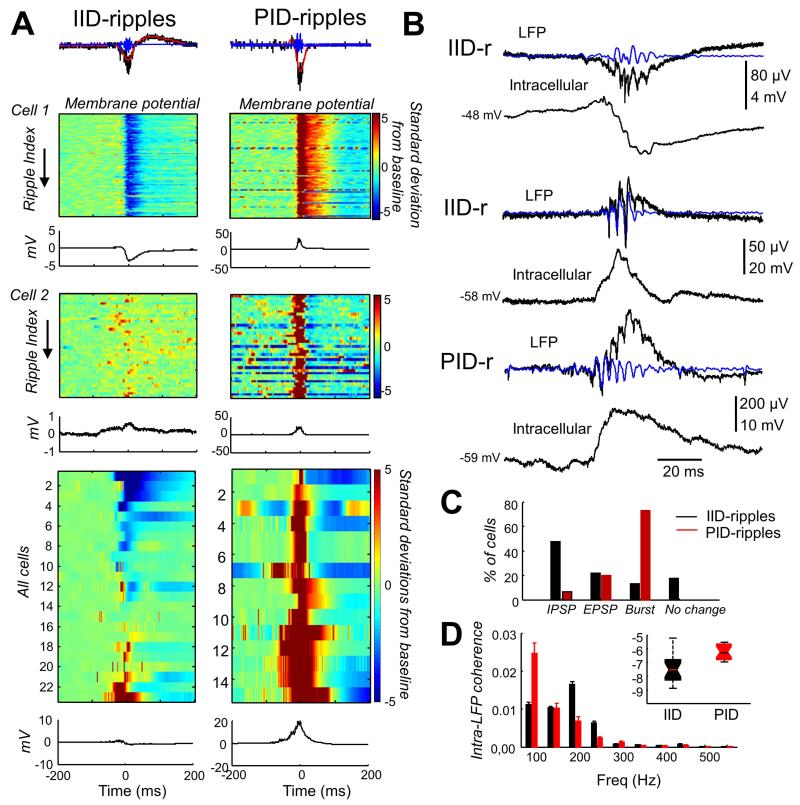
We next investigated post-synaptic potentials associated with ripple-like events during IIDs and PIDs (Fig. 3A). During IID-ripples, many cells displayed rhythmic IPSPs (48% of 23 cells; Vrev = −73 ± 7 mV; n=4: Fig.3A, right and Fig.3B, top traces), while a minority exhibited superimposed excitatory potentials (22%; Fig. 3B, middle traces). The remaining 30% cells showed no clear or monophasic synaptic events. Depolarizing potentials associated with IIDs may have included depolarizing GABAergic events due to a depolarised chloride equilibrium potential (Cohen et al. 2002; Huberfeld et al. 2007). In contrast, PID-ripples were typically associated with large, mostly suprathreshold depolarizations (93% of 15 cells; Fig.3A, right) and presumably glutamatergic EPSPs (Vrev= −15 ± 6 mV; n=3) were evident in some recordings (Fig. 3B, bottom traces). This was even more obvious in a subset of cells with coexisting IID- and PID-ripple like oscillations permitting direct comparisons (Fig.3A, cell 1 and 2). Cell 1 was predominantly hyperpolarized during IID-ripples and mainly depolarized during PID-ripples (Fig.3A), consistent with firing rate data in Fig.2A. While a similar percentage of cells exhibited subthreshold EPSPs associated with IID-ripples and PID-ripples, IPSPs were more often present during IID-ripples (Fig.3C). EPSPs that triggered burst firing were more closely associated with PID-ripples (Fig. 3C).
Figure 3.
A. Membrane potential behaviors of two different cells during IID-ripples and PID-ripples. Potential changes over a range of ±5 mV are shown in color. During IID-ripples, Cell 1 tended to recieve IPSPs, whereas Cell 2 tended to exhibit EPSPs. During PID-ripples both cells recieved EPSPs. The average membrane potential from all cells (color maps and average peri-event traces, bottom) shows a diversity of synaptic activity associated with IID-ripples. In contrast, during PID-ripples membrane potentials mostly reflect depolarization. B. Different examples of simultaneous intracellular and field recordings show IPSP (top) or EPSP (middle and bottom) sequences in intracellular potentials were synchronous with field oscillations. C. The percentage of synaptic events (IPSPs, subthreshold EPSPs, EPSPs leading to burst or no change) shows IPSPs are more expressed during IID-ripples and EPSPs leading to bursts are dominant for PID-ripples. D. Intracellular-field coherence shows that subthreshold membrane potentials and LFPs synchronize below 250 Hz, with significant tuning for IID-ripples at about 200 Hz. Inset shows group data on spike-field coherence at 100-250Hz expressed in log
We studied relations between rhythmic post-synaptic potentials and LFP oscillations by measuring intracellular-field coherence in the 80-600 Hz range. Contamination of spike signals was avoided by choosing sweeps with post-synaptic potentials but no firing. Coherence between intracellular and LFP signals was constrained to the 100-250 Hz band for both IID and PID (Fig. 3D). No differences were found in the intra-LFP coherence when IID-associated rhythmic IPSPs and EPSPs were analyzed separately (not shown). Intracellular deflections during IID were more tuned at 200 Hz and PID exhibited less oscillatory components, though there was no difference in the 100-250 Hz band (Fig.3D, inset). This suggests that while both IID-ripples and PID-ripples could reflect rhythmic synaptic components and population spikes, IID-ripples were more likely to be associated with rhythmic synaptic activity and moderate phase-locked firing. In contrast, PID-ripples were more typically associated with neuronal burst firing.
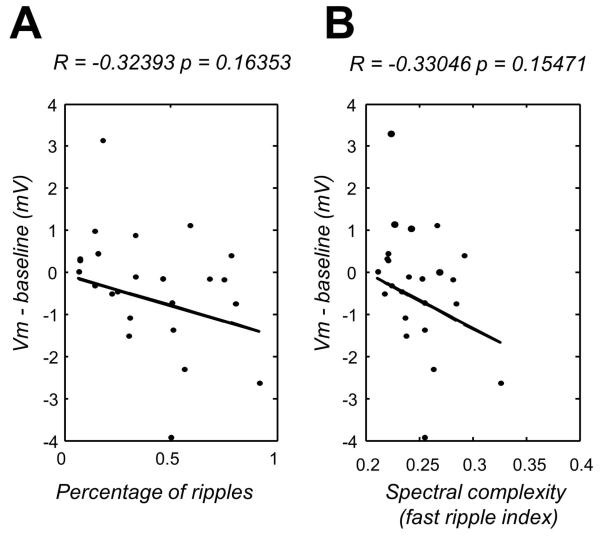
Finally, we correlated the detection of ripple-like oscillations near to a given cell with the dominant membrane potential deflection. We reasoned that if either IID- or PID-associated hyperpolarizing or depolarizing synaptic events were more closely associated with HFOs in either the ripple or fast ripple band, then an interaction might be evident between intracellular and extracellular potentials. However, there was no clear correlation between the average membrane potential deflection and the proportion of ripple-like oscillations in a given electrode (Fig.4A). Nor were interactions evident between membrane potential changes and the spectral complexity of HFOs, an index reflecting spectral disorganization typical of fast ripple components (Ibarz et al. 2010). Thus excitatory and inhibitory synaptic activities appear to contribute to both IID and PID ripple-like oscillations and these high frequency local field potentials do not separate underlying cellular processes.
Figure 4.
A. Correlation between mean subthreshold membrane potential for each cell and the proportion of ripple-like oscillations recorded in the local field potential. B. Correlation between mean membrane potential deflections and spectral complexity.
Discussion
Hippocampal tissue obtained during surgery from epileptic patients provides a unique opportunity to explore pathological cellular and network properties of human hippocampus. This study focused on high-frequency oscillations associated with IID and PID generated by the subiculum., We report that the human epileptic subiculum generates HFOs in a wide frequency band (150-600 Hz) associated with both IIDs and PIDs. For both discharge types a proportion of events (20-50 %) are confined to the 150-250 Hz band, spectrally similar to ripples seen in vivo in non-epileptogenic regions of humans (Staba et al., 2002), monkeys (Skaags et al. 2007) and rodents (Buzsaki et al. 1983). These IID and PID ripple-like oscillations were associated with distinct cellular and synaptic processes. IID-ripples depend on a heterogeneous firing pattern typically associated with rhythmic inhibitory potentials and moderate phase-locking firing. In contrast, PID-ripples were largely associated with single-cell bursting and correlated with depolarizing potentials that trigger rhythmic firing. Thus, ripple-like oscillations in the human TLE subicular circuits appear to be generated by different forms of synchronizing mechanisms.
We found that IID-ripples depend on cellular interactions including a strong inhibition of pyramidal cells, confirmed by predominant IPSPs. This pronounced inhibition may result from high frequency discharges of inhibitory interneurons which can follow field oscillations (Ylinen et al. 1995; Csicsvari et al. 1999), consistent with high-frequency phasic inflections evident in pyramidal cell potentials (Fig.3B). Surprisingly, this pattern closely resembles physiological ripples in the rodent hippocampus in vivo (Ylinen et al. 1995; Maier et al., 2010). While firm conclusions cannot be drawn from these data, the epileptic subicular cortex may be generating normal ripple oscillations perhaps due to an enhanced connectivity that would encourage network synchrony. Such ripple-like rhythmicity of postsynaptic potentials has been detected both in epileptic human and normal monkey hippocampus in vitro (Schwartzkroin and Haglund; 1986; Köhling et al. 1998). Hence, IID-ripples are reminiscent of physiological ripples, even if they reflect multiple processes including IPSPs, EPSPs and depolarizing GABA signaling. Therefore, as previously suggested (Staba et al. 2002; Le Van Quyen et al, 2008), LFP ripples of human hippocampus may be detected in both epileptogenic and non-epileptogenic areas associated with both IID and PID events.
PID-ripples differ from IID-ripples in that they are linked to glutamatergic signals and more strongly associated with coherent cellular bursting. This is consistent with in vivo data from animal models of mesial temporal lobe epilepsy (Ibarz et al. 2010; Bragin et al. 2011), work on epileptic foci in animals (Matsumoto 1964) and slice models of epileptiform events (Gutnick et al. 1982; Traub et al. 1989; Perreaul and Avoli 1999; Gnatkovsky et al. 2008). In particular, in kainate treated rats, epileptic HFOs are evident in a stable spatial zone which is enlarged by the application of bicuculline, the GABAa-receptor antagonist (Bragin et al., 2002). Possibly, inhibition normally controls the spread of interictal events in these epileptic animals (Sabolek et al, 2012; Menendez de la Prida and Pozo 2002). However, in territories generating epileptic HFOs, the high-frequency component survives, and may be enhanced by blocking GABAa receptors (Nimmrich et al., 2005). Epileptogenic regions that generate HFOs may be disinhibited, either due to interneuron loss or to a functional collapse of GABA release (Aivar et al. 2014; Karlocai et al. 2014). PID-ripples may then reflect epileptic activity due to an impaired inhibition and mediated by a pathological synchronization of bursting principal cells (Traub et al. 1999; Dzhala and Staley, 2004; Menendez de la Prida and Gal, 2004).
Recent research has asked whether epileptic HFOs are a variant of normal ripples, or reflect instead distinct pathologic neuronal interactions. It is difficult to distinguish normal ripples from HFOs on the basis of frequency content alone, especially in clinical records with intracranial macroelectrodes made from patients with refractory epilepsies (Le Van Quyen, 2012; Worrell et al. 2008). The dominant oscillatory frequency clearly does not suffice to separate normal ripples from epileptic HFOs (Engel et al., 2009). This data on IID- and PID-ripples, together with recent results from rodent slices (Aivar et al. 2014; Karlocai et al. 2014), suggests that ripples can result from several mechanisms. With a balanced excitation and inhibition, neuronal activity is constrained by rhythmic IPSPs to phasic coherent firing in a relatively low proportion of cells (Csicsvari et al. 2000, Maier et al. 2011, Schombourg et al. 2012; Aivar et al. 2014). In the interictal state, even if depolarizing GABAergic signals excite some cells (Cohen et al. 2002; Huberfeld et al. 2007), the level of inhibition may suffice to control expression of physiological-like ripple oscillations. In contrast, during a pre-ictal state induced by enhanced excitability, a failure of GABAergic signaling (Huberfeld et al. 2011) underlies the glutamatergic recruitment of neuronal burst firing that now contributes to pathological ripples (Foffani et al. 2007; Aivar et al. 2014, Karlocai et al. 2014). Therefore LFP ripples do not distinguish between the underlying neuronal processes (Menendez de la Prida and Trevelyan 2011; Aivar et al. 2014). When neuronal firing disorganizes due to intense synaptic activity, activity clustering, loss of spike-timing reliability and patchy synaptic reorganization, the ripple spectrum destabilizes and HFOs become evident (Foffani et al. 2007; Sabolek et al. 2012; Jefferys et al. 2012; Simeone et al. 2013). In these human data, HFOs invaded the fast ripple band for a high proportion of events (Fig.1C). Multiple forms of HFO synchrony can thus co-exist in an epileptic hippocampus as previously suggested (Staba et al. 2002).
PID- ripples may prove useful in seizure prediction since they reliably preceed ictal events at seizure initiation sites,. Supporting this idea, HFOs increase before seizures both in vitro (Khosravani et al. 2005) and HFO events increase tens of seconds before seizure onset in human records in situ (Zijlmans et al., 2011). However, another investigation analyzing fifteen minutes preceding seizures found no clear systematic trends across patients and seizures (Jacobs et al. 2009). Since we found PID events associated with HFOs in the fast ripple band, spectral variability may be a typical hallmark (Jefferys et al. 2012). Most of these analyses have examined the occurrence and spectral content of HFOs recorded extracellularly with macroelectrodes. This work suggests data on single cell firing and associated synaptic inputs represent additional information needed to fully characterize changes that precede a seizure.
Acknowledgements and funding
This work was supported by grants from the Institut National de la Santé Et de la Recherche Médicale (INSERM), the Centre National pour la Recherche Scientifique (CNRS), the Assistance Publique – Hopitaux de Paris (APHP), the European Research Council (322721), the Fédération pour la Recherche sur le Cerveau (FRC), the University Pierre & Marie Curie (UPMC), the ENP Foundation, the Spanish Ministry of Innovation and Science (BFU2009-07989 and BFU2012-37156-C03-01) and Programa Salvador de Madariaga of the Spanish Ministry of Education, Culture and Sports (PR2011-0226).
References
- Aivar P, Valero M, Bellistri E, Menendez de la Prida Extracellular calcium controls the expression of two different forms of ripple-like hippocampal oscillations. J Neurosci. 2014 doi: 10.1523/JNEUROSCI.2826-13.2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiou CA, Montgomery SM, Barahona M, Buzsáki G, Koch C. The Effect of Spatially Inhomogeneous Extracellular Electric Fields on Neurons. The Journal of Neuroscience. 2010;30:1925–1936. doi: 10.1523/JNEUROSCI.3635-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8:1560–1567. doi: 10.1038/nn1571. [DOI] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired Dendritic Channelopathy in Temporal Lobe Epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson C, Engel J. Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Benassi SK, Kheiri F, Engel J., Jr. Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011 Jan;52(1):45–52. doi: 10.1111/j.1528-1167.2010.02896.x. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butar F, Park J. Permutation Tests for Comparing Two Populations. Journal of Mathematical Sciences & Mathematics Education. 2008;3(2):19–30. [Google Scholar]
- Buzsáki G. Two-stage model of memory-trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Chen S, Su HL, Yue CY, Remy S, Royeck M, Sochivko D, Opitz T, Beck H, Yaari Y. An Increase in Persistent Sodium Current Contributes to Intrinsic Neuronal Bursting After Status Epilepticus. Journal of Neurophysiology. 2011;105:117–129. doi: 10.1152/jn.00184.2010. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–21. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nature Neuroscience. 2001 doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Crépon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, Adam C, Le Van Quyen M. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133:33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsáki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave associated population events. Neuron. 2000;28:585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–67. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Schmitz D, Jefferys JG. Electrical coupling underlies highfrequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- Dzhala V, Staley K. Mechanisms of fast ripples in the hippocampus. J Neurosci. 2004;24:8896–8906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Houser CR. Up-regulation of GAD65 and GAD67 in remaining hippocampal GABA neurons in a model of temporal lobe epilepsy. J Comp Neurol. 1999;412(3):488–505. [PubMed] [Google Scholar]
- Fabó D, Maglóczky Z, Wittner L, Pék A, Eross L, Czirják S, Vajda J, Sólyom A, Rásonyi G, Szucs A, Kelemen A, Juhos V, Grand L, Dombovári B, Halász P, Freund TF, Halgren E, Karmos G, Ulbert I. Properties of in vivo interictal spike generation in the human subiculum. Brain. 2008 Feb;131(Pt 2):485–99. doi: 10.1093/brain/awm297. [DOI] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:990–941. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol. 2008 Dec;64(6):674–86. doi: 10.1002/ana.21519. 2008. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80– 200 Hz) during seizures: Intracellular correlates. J Neurophysiol. 2003;89:841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- Gutnick MJ, Connors BW, Prince DA. Mechanisms of neocortical epileptogenesis in vitro. J Neurophysiol. 1982 Dec;48(6):1321–35. doi: 10.1152/jn.1982.48.6.1321. 1982. [DOI] [PubMed] [Google Scholar]
- Hirsch JC, Agassandian C, Merchan-Perez A, Ben Ari Y, DeFelipe J, Esclapez M, Bernard C. Deficit of quantal release of GABA in experimental models of temporal lobe epilepsy. Nat Neurosci. 1999;2:499–500. doi: 10.1038/9142. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;2737:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. Glutamatergic preictal discharges emerge at the transition to seizure in the human epileptic temporal lobe. Nature Neurosci. 2011;14:627–34. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- Ibarz JM, Foffani G, Cid E, Inostroza M, Menendez de la Prida L. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neuroscience. 2010;30:16249–16261. doi: 10.1523/JNEUROSCI.3357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zelmann R, Jirsch J, Chander R, Dubeau CE, Gotman J. High frequency oscillations (80-500 Hz) in the preictal period in patients with focal seizures. Epilepsia. 2009;50:1780–1792. doi: 10.1111/j.1528-1167.2009.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012 Sep;98(3):250–64. doi: 10.1016/j.pneurobio.2012.02.005. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Karlócai MR, Kohus Z, Káli S, Ulbert I, Szabó G, Máté Z, Freund TF, Gulyás AI. Physiological sharp wave-ripples and interictal events in vitro: what’s the difference? Brain. 2014 doi: 10.1093/brain/awt348. 10.1093/brain/awt348. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal Diversity and Temporal Dynamics: The Unity of Hippocampal Circuit Operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravani H, Pinnegar CR, Mitchell JR, Bardakjian BL, Federico P, Carlen PL. Increased high-frequency oscillations precede in vitro low-Mg seizures. Epilepsia. 2005;46:1188–1197. doi: 10.1111/j.1528-1167.2005.65604.x. [DOI] [PubMed] [Google Scholar]
- Köhling R, Lücke A, Straub H, Speckmann EJ, Tuxhorn I, Wolf P, Pannek H, Oppel F. Spontaneous sharp waves in human neocortical slices excised from epileptic patients. Brain. 1998 Jun;121(Pt 6):1073–87. doi: 10.1093/brain/121.6.1073. 1998. [DOI] [PubMed] [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A, Staba R, Crépon B, Wilson CL, Engel J. Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci. 2008;28:6104–6110. doi: 10.1523/JNEUROSCI.0437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M. Editorial “Special issue on High-frequency oscillations in cognition and epilepsy”. Prog. Neurobiol. 2012;98:239–318. [Google Scholar]
- Lindén H, Tetzlaff T, Potjans TC, Pettersen KH, Grün S, Diesmann M, Einevoll GT. Modeling the spatial reach of the LFP. Neuron. 2011 Dec 8;72(5):859–72. doi: 10.1016/j.neuron.2011.11.006. 2011. [DOI] [PubMed] [Google Scholar]
- Maier N, Nimmrich V, Draguhn A. Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices. J. Physiol. 2003;550:873–887. doi: 10.1113/jphysiol.2003.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N, Tejero-Cantero A, Dorrn AL, Winterer J, Beed PS, Morris G, Kempter R, Poulet JF, Leibold C, Schmitz D. Coherent phasic excitation during hippocampal ripples. Neuron. 2011;72(1):137–52. doi: 10.1016/j.neuron.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Matsumoto H. Intracellular events during the activation of cortical epileptiform discharges. Electroencephalogr Clin Neurophysiol. 1964 Sep;17:294–307. doi: 10.1016/0013-4694(64)90130-0. 1964. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Gal B. Synaptic contributions to focal and widespread spatiotemporal dynamics in the isolated rat subiculum in vitro. J Neurosci. 2004;24(24):5525–36. doi: 10.1523/JNEUROSCI.0309-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez de la Prida L, Pozo MA. Excitatory and inhibitory control of epileptiform discharges in combined hippocampal/entorhinal cortical slices. Brain Research. 2002;940:27–35. doi: 10.1016/s0006-8993(02)02564-7. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Trevelyan A. Cellular mechanisms of high frequency oscillations in epilepsy: on the diverse sources of pathological actitivies. Epilepsy Research. 2011;97:308–317. doi: 10.1016/j.eplepsyres.2011.02.009. 2011. [DOI] [PubMed] [Google Scholar]
- Nimmrich V, Maier N, Schmitz D, Draguhn A. Induced sharp wave-ripple complexes in the absence of synaptic inhibition in mouse hippocampal slices. J Physiol. 2005;563:663–670. doi: 10.1113/jphysiol.2004.079558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991 Apr;65(4):771–85. doi: 10.1152/jn.1991.65.4.771. 1991. [DOI] [PubMed] [Google Scholar]
- Reimann MW, Anastassiou CA, Perin R, Hill SL, Markram H, Koch C. A biophysically detailed model of neocortical local field potentials predicts the critical role of active membrane currents. Neuron. 2013 Jul 24;79(2):375–90. doi: 10.1016/j.neuron.2013.05.023. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun AK, Simonotto JD, Jenkins A, Nicholson C, Schofield IS, Whittaker RG, Kaiser M, Whittington MA, Traub RD, Cunningham MO. A nonsynaptic mechanism underlying interictal discharges in human epileptic neocortex. PNAS. 2012;107:338–43. doi: 10.1073/pnas.0912652107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabolek HR, Swiercz WB, Lillis KP, Cash SS, Huberfeld G, Zhao G, Ste Marie L, Clemenceau S, Barsh G, Miles R, Staley KJ. A candidate mechanism underlying the variance of interictal spike propagation. J Neurosci. 2012 Feb 29;32(9):3009–21. doi: 10.1523/JNEUROSCI.5853-11.2012. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg EW, Anastassiou CA, Buzsáki G, Koch C. The spiking component of oscillatory extracellular potentials in the rat hippocampus. J Neurosci. 2012 Aug 22;32(34):11798–811. doi: 10.1523/JNEUROSCI.0656-12.2012. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Haglund MM. Spontaneous rhythmic synchronous activity in epileptic human and normal monkey temporal lobe. Epilepsia. 1986;27:523–33. doi: 10.1111/j.1528-1157.1986.tb03578.x. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Simeone KA, Samson KK, Kim do Y, Rho JM. Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices. Neurobiol Dis. 2013 Jun;54:68–81. doi: 10.1016/j.nbd.2013.02.009. 2013. doi: 10.1016/j.nbd.2013.02.009. Epub 2013 Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Permenter M, Archibeque M, Vogt J, Amaral DG, Barnes CA. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol. 2007;98:898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J. High frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Traub RD, Miles R, Wong RK. Model of the origin of rhythmic population oscillations in the hippocampal slice. Science. 1989 Mar 10;243(4896):1319–25. doi: 10.1126/science.2646715. 1989. [DOI] [PubMed] [Google Scholar]
- Scheffer-Teixeira R, Belchior H, Leão RN, Ribeiro S, Tort A. On High-Frequency Field Oscillations (>100 Hz) and the Spectral Leakage of Spiking Activity. The Journal of Neuroscience. 2013;33:1535–1539. doi: 10.1523/JNEUROSCI.4217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Bibbig A. A model of high-frequency ripples in the hippocampus based on synaptic coupling plus axon-axon gap junctions between pyramidal neurons. J. Neurosci. 2000;20:2086–2093. doi: 10.1523/JNEUROSCI.20-06-02086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008 Apr;131(Pt 4):928–37. doi: 10.1093/brain/awn006. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Kahn YU, Zelmann R, Dubeau F, Gotman J. Ictal and interictal high frequency oscillations in patients with focal epilepsy. Clin Neurophysiol. 2011;122:664–671. doi: 10.1016/j.clinph.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]