Abstract
Surgical removal of the epileptogenic zone provides an effective therapy for several epileptic syndromes. This surgery offers the opportunity to study pathological activity in living human tissue for pharmacoresistant partial epilepsy syndromes including (1) temporal lobe epilepsies with hippocampal sclerosis, (2) cortical dysplasias, (3) epilepsies associated with tumors and (4) developmental malformations. Slices of tissue from patient with these syndromes retain functional neuronal networks and may generate epileptic activities. The properties of cells in this tissue may not be greatly changed, but excitatory synaptic transmission is often enhanced and GABAergic inhibition is preserved. Typically epileptic activity is not generated spontaneously by the neocortex, whether dysplastic or not, but can be induced by convulsants. The initiation of ictal discharges in neocortex depends on both GABAergic signaling and increased extracellular potassium. In contrast, a spontaneous interictal-like activity is generated by tissues from patients with temporal lobe epilepsies associated with hippocampal sclerosis. This activity is initiated, not in the hippocampus but in the subiculum an output region which projects to the entorhinal cortex. Interictal events seem to be triggered by GABAergic cells which paradoxically excite about 20% of subicular pyramidal cells while simultaneously inhibiting the majority. Interictal discharges thus depend on both GABAergic and glutamatergic signaling. The depolarizing effects of GABA depend on a pathological elevation in levels of chloride in some subicular cells, similar to those of developmentally immature cells. Such defect is caused by a perturbed expression of the cotransporters regulating intracellular chloride concentration, the importer NKCC1 and the extruder KCC2. Blockade of NKCC1 actions by the diuretic bumetanide, restores intracellular chloride and thus hyperpolarizing GABAergic actions so suppressing interictal activity.
Keywords: epilepsy, human, subiculum, in vitro, GABA-A receptors
Introduction
Epilepsy is an emblematic brain disease. Epileptic activities depend on a mode of neuronal discharge, the burst of action potentials as well as a mode of collective activity, neuronal synchrony (Varela et al., 2001), both of which are physiological. Epileptic discharges therefore replay an excessive, dysregulated brain function. Explaining epileptic activities requires understanding neuronal functions at levels from single ion channels and neurotransmitter receptors, through the cellular and synaptic properties of single neurons to the collective activities of groups of interconnected neurons modulated by glial cells. Epileptic syndromes are thus diseases of the entire nervous system as it interacts with the environment and electrophysiology is a key discipline to understand epileptic activities and their relations and boundaries with physiological function.
The epilepsies are a diverse and distributed group of diseases. Numerous natural or engineered models have been used to reproduce epileptic features in animals. Clinically human epilepsies are typically first explored by electrophysiological recordings. ElectroEncephaloGraphy (EEG) records field potentials produced by the synchronization of synaptic potentials in large cortical areas (several cm3) and transmission can vary between different brain regions (Cooper et al., 1965, Ray et al., 2007, Tao et al., 2007). Intracranial recordings improve the quality and spatial precision of the data, and permit sampling of higher frequency oscillations but usually cannot resolve the mechanisms of epileptic activities. Recent use of intracranial records to detect discharges of single neurons in situ may help understanding the genesis of epileptic synchrony (Buzsáki et al., 2012).
Animal models have been used to study epileptogenic alterations of brain tissues that lead to seizures, but less attention has been paid to ictogenesis, or mechanisms controlling the emergence of seizures from the remodeled brain. In vivo models, even if they do not replicate all aspects of a disease, have provided much of our data on epilepsy. Finally, in vitro work, especially from rodent hippocampal slices, has advanced understanding on potential mechanisms of neuronal synchrony. Neural networks are maintained intact and epileptiform activities are induced by blocking GABAA receptors or by increasing neuronal excitability. These models can provide precise data on ionic conductances, synaptic events and neuronal firing even if they are a compromise between levels of integration in a slice versus a whole brain and a healthy animal tissue rather than a diseased human brain.
In vitro studies on human epileptic tissue provided a privileged window onto human epileptic activities (Kohling & Avoli 2006). They permit studies on the behaviors of single cells and neuronal ensembles and can be combined with imaging and genetic explorations. Finally, tissue slice microenvironment can be modified to mimic changes in brain extra-cellular medium to induce specific activities or for pharmacological investigation.
Here we review data from electrophysiological studies on human mesial temporal epileptogenic tissue in vitro. Mesial temporal lobe epilepsies associated with hippocampal sclerosis (mTLE-HS) are relatively frequent and homogeneous (Van Paesschen et al., 1998). Slices with intact connections between the hippocampus, subiculum and entorhinal cortex, can be prepared from surgically removed tissue (Wieser, 2004). There are, however, two limitations (Avoli et al., 2005) Connections to and from other brain regions are cut and no satisfactory control tissue is available. With critical functional roles, non-pathologic mesial temporal tissue is never resected, so data is compared, at best, to healthy tissue from animals or internal controls can sometimes be devised. This review describes initial work on human temporal epileptic tissue and examines more recent suggestions of a causative role for a defect in inhibitory GABAergic signaling in generating interictal activity.
Neuronal properties of the epileptogenic hippocampus
Studies on human epileptic tissue show that single neurons are physiologically quite similar to those of healthy rodents (Avoli et al., 1994a, Avoli and Olivier, 1989 Schwartzkroin and Prince, 1976 Tasker et al., 1996). Some neurons discharge bursts of action potentials, a potentially ‘epileptic’ property (Prince, 1978) but not in greater proportions than in healthy rodent tissue (Avoli and Olivier, 1989, Koch et al., 2005). Afferent fiber stimulation evoked similar responses in cortical neurons of epileptic patients and in epileptic animals: a large, long lasting ‘depolarization shift’ was initiated at long, variable latencies in an all-or-nothing threshold behavior (Prince and Wong, 1981). Some differences may exist. Atypical, persistent sodium conductances which might account for burst discharges, have been detected in epileptic subicular neurons (Vreugdenhil et al., 2004). Equally the hyperpolarization-activated cation current I(h) seems to be upregulated by 80% in dentate granule cells in hippocampi with sclerosis compared to cases without (Surges et al., 2012).
Synapses made in slices of human epileptic tissue have been studied by stimulating afferent fibers. At low intensities mono-synaptic EPSPs of varying amplitude are evoked in pyramidal cells. Beyond a threshold, possibly of population firing, ‘all or nothing’ bursts of post synaptic potentials with an NMDA receptor component and variable latency were recruited in tissue from epileptic temporal or frontal neocortex (Avoli and Olivier, 1989). In the hippocampus, prolonged responses with after-discharges are initiated in the dentate gyrus by perforant path stimuli. In tissue from healthy animals such responses occur only when GABAA receptors are partly blocked, pointing to defects in epileptogenic human tissue (Masukawa et al., 1989). Stimulation of granule cells via the perforant path generates prolonged excitatory postsynaptic potential, depending on polysynaptic circuits and NMDA receptors (Isokawa et al., 1997 Isokawa and Levesque, 1991). Such amplified responses may depend on an upregulation of certain ionotropic glutamate receptors (NMDA R2, GluR3 AMPA) (Mathern et al., 1997), and kainate receptors (Perret et al., 2014), in the epileptic dentate gyrus of children (Represa et al., 1998). The control of release due to mGluRs is reduced at synapses terminating on epileptic dentate granule cells (Dietrich et al., 1999).
High frequency stimulation of the perforant path generates GABAergic inhibition in human dentate cells that may be less effective in tissue from mTLE patients with a sclerosis than those without HS (Isokawa and Fried, 1996, Swanson et al., 1998), supporting the idea that deficient inhibition contributes to hyperexcitability. Reduced GABAergic currents in dentate granule cells of patients with HS (Williamson et al., 1999), are prolonged in duration perhaps due to a reduced GABA reuptake (Williamson et al., 1995). Finally, Menendez de la Pridahas shown that interneurons of epileptic human neocortex, retain a ‘regular spiking’ discharge pattern and glutamatergic synaptic inputs (Menendez of Prida et al., 2002).
Some properties of hippocampal cells may also be related to functional cognitive defects. The dentate gyrus can generate new neurons throughout life. The ability of human postoperative dentate gyrus to display neurogenic properties has been correlated to presurgical memory performances. Patients with low proliferation capacities had poor memories while those with many proliferative cells did not have memory defects (Coras et al., 2010). Memory impairment be mediated via a reduced long-term plasticity at dentate synapses excited by the perforant path (Beck et al., 2000)
Induced population activities in the epileptic hippocampus
Post-operative tissues from cortex or hippocampus very rarely generate spontaneous synchronous activity. Almost all work, on temporal neocortex, rather than the hippocampal formation, has concerned pharmacologically induced epileptic activities
‘Epileptiform’ activities in human tissue have frequently been induced by blocking GABAA receptors as in rodent studies. Suppressing fast inhibition generates interictal-like population bursts (Avoli and Olivier, 1989, Franck et al., 1995, Hwa et al., 1991, McCormick, 1989 Tasker et al., 1992) associated with an intracellular ‘Paroxysmal Depolarization Shift’. Bicuculline doses needed to induce this form of synchrony in the dentate gyrus were lower in tissue associated with a HS, possibly reflecting a hyperexcitability of this region resulting from aberrant t=recurrent connexions due to mossy fiber sprouting (Franck et al., 1995). Presumably the need to block GABAergic transmission signaling indicates that synaptic inhibition normally controls recurrent excitation in epileptic tissue.
Application of ‘zero’ Mg2+ induces prolonged ictal events as well as interictal bursts both in neocortical slices (Avoli et al., 1999, Avoli et al., 1991, Kohling et al., 1998 Kohling et al., 1999) and hippocampus (Remy et al., 2003). This action may result from a relief of NMDA receptor blockade (Whittington et al., 1995) an increase in neuronal excitability due to a membrane surface charge effect (Mangan and Kapur, 2004; Kohling and Avoli, 2006) as well as a suppression of inhibition Postsynaptic GABAA potentials are recorded during the interictal phase, but are reduced and disappear at the transition to ictal discharges (Avoli et al., 1995). Ictal events are sustained by a NMDA signaling.
4-aminopyridine (4-AP), which blocks some K+ channels so enhancing release of excitatory and inhibitory neurotransmitters, also induces both ictal and interictal discharges in human epileptic temporal neocortex (Avoli et al., 1994b). In dysplastic cortical tissue, ictal discharges depend not only on NMDA and AMPA excitatory transmission but also on signals mediated by GABA (Avoli et al., 1999). GABAergic preictal potentials, which precede seizure onset, are associated with increases in external K+ levels from 3.2 to 4.5 mM (D’Antuono et al., 2004). At the transition to ictal discharges, the increase reaches 6.4 mM, suggesting external K+ may contribute to seizure initiation. Such a role is emphasized by several studies. Using barium to block delayed rectifier K-channels, may impair K+ buffering by astrocytes which express these channels at high levels. Elevations of external K+ in response to fiber stimulation are much enhanced (Kivi et al., 2000). K+ buffering seems to be depressed in reactive astrocytes from temporal lobe epilepsy tissues due to a down regulation of these inwardly rectifying K+ channels (Bordey and Sontheimer, 1998 Hinterkeuser et al., 2000 Schroder et al., 2000). However, GABAergic signaling remains fundamental to the generation of epileptiform activities since the μ opioid receptor agonist DAGO, which hyperpolarizes interneurons and so reduces GABA release, transforms ictal events into shorter interictal-like events (D’Antuono et al., 2004). GABAB signals play a protective role since baclofen blocks seizures, which are then re-induced by a GABAB antagonist. Epileptiform discharges induced by 4-AP are also suppressed by blocking gap junctions (Gigout et al., 2006).
The human epileptic dentate gyrus also generates little spontaneous activity. Increasing extracellular K+ concentration to 10-12 mM evokes ictal-like discharges (Gabriel et al., 2004 Jandova et al., 2006) which depend on AMPA receptors but remain unchanged when NMDA-mediated signals are suppressed.
Neocortical tissues obtained after mTLE surgery generate theta and gamma rhythms in vitro in response to kainate and carbachol application (Florez et al., 2013). These activities are very similar to those recorded in vivo, suggesting that they are locally generated and do not depend on afferent connections with the other brain structures.
Spontaneous epileptiform population activities in human hippocampus
Post-operative tissue rarely generates epileptiform activity. However, a year after finding no activity (Schwartzkroin et al., 1983), Schwartzkroin and colleagues described interesting spontaneously occurring synaptic events in tissue from mesial and lateral temporal cortex of epileptic patients (Schwartzkroin and Knowles, 1984). It consisted of complex synaptic events, with depolarizing and hyperpolarizing components and sometimes firing, that was detected in pairs of pyramidal cells. Such activity suggested that groups of pyramidal cells and interneurons were firing synchronously without external stimuli. These complex events recurred at about 2 Hz, and were recorded from cells in a range of 500-700 μm. While the reversal potential of these composite synaptic events was not measured at first, later work (Schwartzkroin and Haglund, 1986) confirmed that the events included a GABAergic component since they were blocked by bicuculline. Intracellular records showed that interneurons fired both before and during synchronous synaptic events.
Field potentials associated with spontaneous activity generated by temporal neocortex from epileptic patients in vitro were first described by Kohling in the late 90s (Kohling et al., 1998). These events, which permit direct comparisons with in situ EEG traces, recurred at about 1 Hz, lasted 20-200 msec and had an amplitude of 20-300 μV (Figure 1). They were synchronized over areas of 100s of μm and several autonomous foci could coexist in the same slice. Intracellular record described depolarizing (37%), hyperpolarizing (44%) or mixed (19%) postsynaptic potentials associated with extracellular fields. Field events were suppressed by antagonists at both AMPA and GABAA receptors. The authors noted that blocking GABAA receptors could initiate synchrony in slices that were originally silent, while bicuculline blocked spontaneously occurring fields. The authors termed this activity ‘sharp waves’, and suggested they might ‘facilitate the initiation or propagation of seizures. Similar ‘sharp waves’ are recorded in neocortical tissue from mTLE-HS patients (Florez et al., 2013)
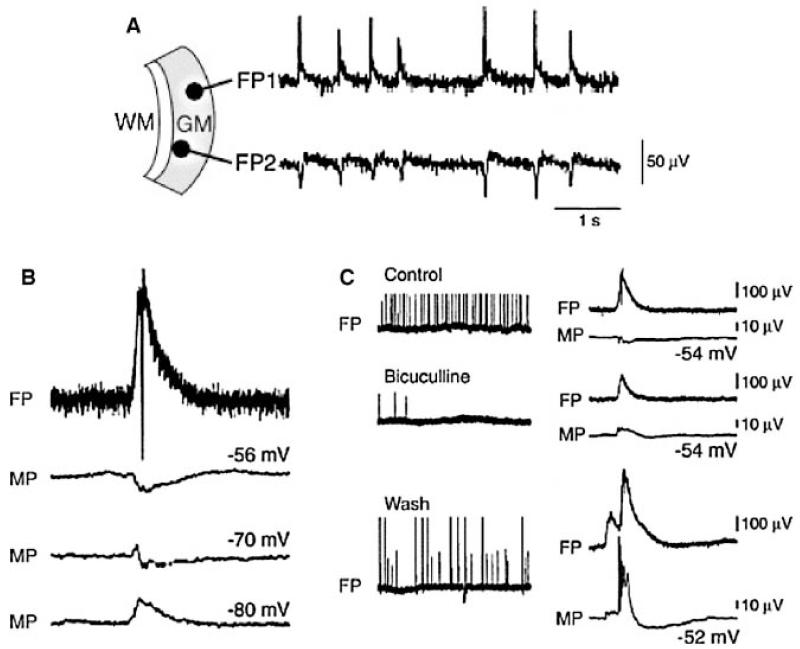
Figure 1.
Spontaneous Interictal activity recorded in the temporal neocortex (based on Kohling et al., 1998).
Synchronous spontaneous interictal field potentials (FP) (A) are associated with a postsynaptic potential which reverses at a membrane potential (MB) near −70 mV (B) and blocked by the GABA receptor antagonist bicuculline (C).
Interictal activities generated in the human epileptic hippocampus
Tissue slices containing the hippocampus, subiculum and entorhinal cortex, obtained after surgery on patients with defined mTLE-SH have provided data on human epileptiform activities (Cohen et al., 2002; Huberfeld et al., 2011). Field potentials of duration 20-50 msec, recurring at 0.5-2 Hz are generated, without stimulation, by a region of 3-6 × 1-3 mm in the subiculum (Figure 2). These events are comparable to interictal discharges recorded in situ with intracerebral electrodes and were termed interictal-like activity. They were generated locally in isolated slices of the subiculum. Similar activity was not detected in the dentate gyrus, CA3, CA1 or entorhinal cortex. Subicular interictal fields were suppressed by antagonists at glutamatergic AMPA and also GABAA receptors. The role of GABAergic circuits was highlighted by the observation that interneurons fired just before and during interictal-like discharges. Most (~80%) subicular pyramidal cells were hyperpolarized during interictal events, however a minority (~20%) received depolarizing synaptic events and could sometimes discharge. Measurements of reversal potentials for isolated GABAergic synaptic events confirmed they were sometimes depolarizing from pyramidal cell resting potential. Cells hyperpolarized during interictal discharges had a GABAergic reversal potential of 72±7 mV (rest potential: −62±8 mV), while those associated with a depolarizing response reversed at −55±12 mV (rest: 67±10 mV). These data were interpreted as resulting from a disturbed Cl− homeostasis in a minority of subicular pyramidal cells. It was suggested that the depolarizing GABAergic response might be linked to a deafferentation due to neuronal loss in the sclerotic CA1 region. Possibly, it was argued, the loss of innervation from CA1 might cause post-synaptic cells to regress to earlier stages of development when GABAergic reversal potentials are relatively depolarized (Cohen et al., 2003).
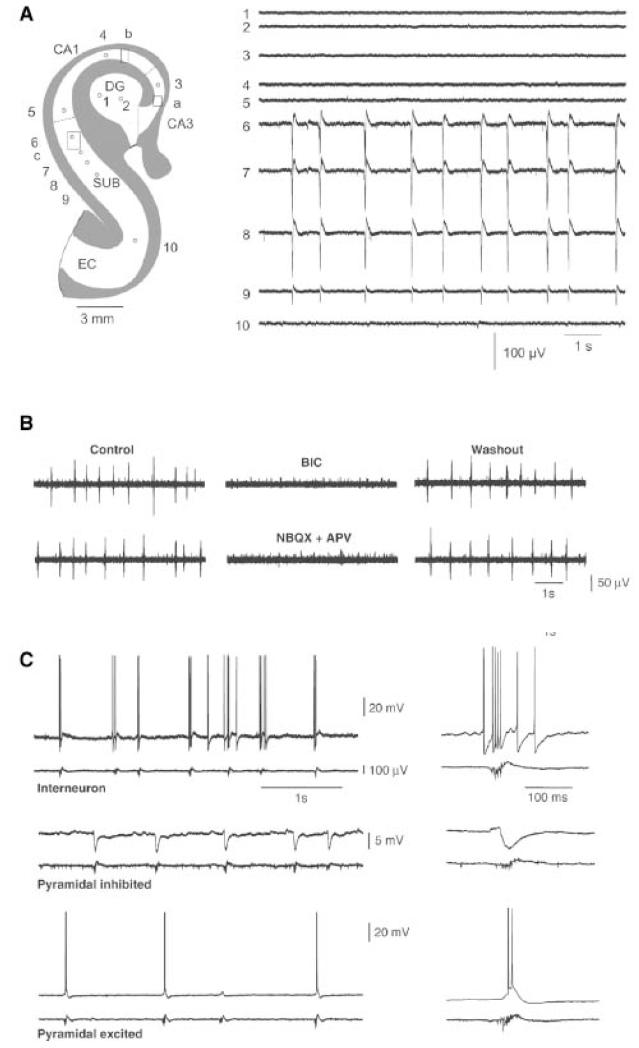
Figure 2.
Interictal activity generated in vitro by the subiculum obtained from mTLE-HS patients (from Cohen et al., 2002).
A. Multiple extracellular recordings showing spontaneous synchronous population activity within the subiculum.
B. Pharmacological characterization of interictal activities: suppression by the GABAA receptor antagonists (bicuculline) and glutamate AMPA and NMDA receptors antagonists (NBQX + APV).
C. Behavior of different cell types during interictal activities. The top trace is an intracellular recording, the lower one an extracellular recording. Interneurons (top trace) discharge before and during the first phase of the burst. Pyramidal cells are hyperpolarized in the usually hot (middle trace) but can also be depolarized (bottom traces).
This activity differs drastically from that of temporal cortex where no interictal-like activity is observed and where GABAA antagonists induce (Avoli & Olivier 1989; Hwa et al., 1991, McCormick 1989) epileptiform activity rather than block it as in human subiculum.
These data from Parisian patients were confirmed in patients suffering from mTLE treated in Berlin. Wozny and colleagues showed the subiculum generated a spontaneous interictal activity (Wozny et al., 2003; Wozny et al., 2005) both in patients with and those without hippocampal sclerosis. This data challenges the notion that deafferentation contributes to events in the subiculum. However the category ‘without sclerosis’ was defined to include patients with 0-50% neuronal loss in CA1. In tissue studied in Berlin, interictal-like events were also blocked by GABAA receptor antagonists. Subicular cell excitability was found to be enhanced with reduced after-hyperpolarizations detected in cells that fired with interictal-like events. Most cells recorded in the epileptogenic focus discharged regularly, about 25% fired in bursts.
After in vitro data pointed to the subiculum, multi-electrode records were made from this zone in situ. Several forms of interictal spikes were detected, with distinct sites of origin and propagation patterns confirming a role in epileptogenesis for this site (Fabo et al., 2008).
The cornu ammonis 2 (CA2) region of the human hippocampus is resistant to the cell loss associated with mTLE-HS. It also generates spontaneous interictal-like discharges in slices, which are completely dissociated from those generated in subiculum (Wittner et al., 2009). However cellular behavior differs. Most pyramidal cells fire during interictal-events in CA2, and the efficacy of GABAergic inhibition, mediated by parvalbumin containing cells, seems to be reduced.
Subsequent studies found no clear anatomical differences between pyramidal cells with perturbed and those with normal Cl− homeostasis (Huberfeld et al., 2007). The morphology of cells that were depolarized and those hyperpolarized during interictal bursts was comparable (Figure 3). Neuronal physiology was not dramatically different either in terms of resting potential, input resistance, firing pattern or the existence of an Ih current.
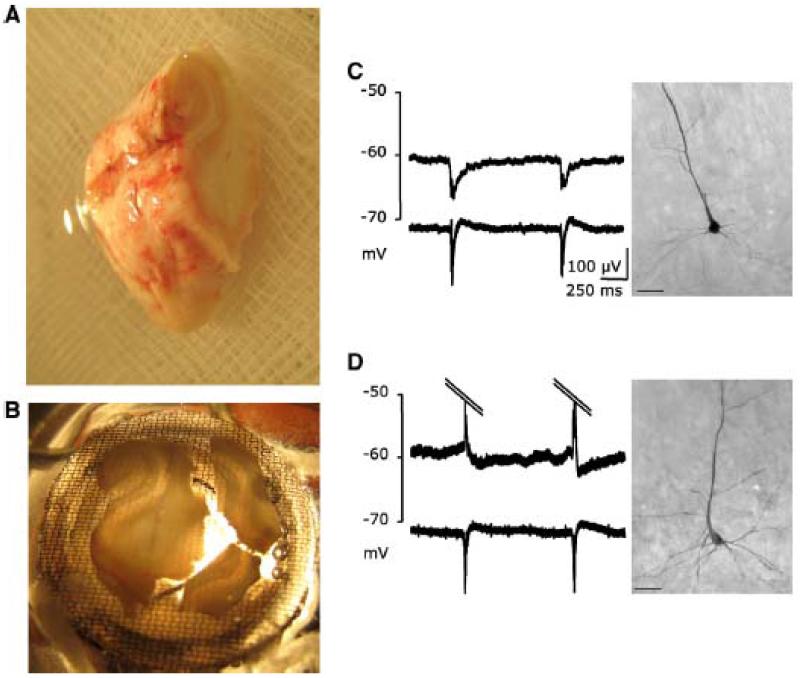
Figure 3.
Interictal epileptiform activity recorded in vitro from the human subiculum obtained from surgical specimens of patients with mTLE-HS (from Huberfeld et al., 2007).
A: A photograph of the block of postoperative tissue.
B: Tissue slices including the subiculum in the interface recording chamber
C: Records of intracellular (top) and extracellular (bottom) of a pyramidal cell hyperpolarized during interictal discharges, rebuilt after biocytin filling.
D: same as C for a depolarized cell.
A detailed study examined the state of Cl− homeostasis in the epileptic subiculum. When GABAA receptors are activated, an anion-permeable channel opens with effects that vary according to the electrical and ionic environment. GABA channels are mainly permeable to Cl− ions. The direction and strength of Cl− movement depends in part on concentrations on both sides of a membrane. Since extracellular Cl−-concentration ([Cl−]e) is higher than intracellular ([Cl−]i), Cl− anions tend to enter the cell, provoking a hyperpolarization. A second force controlling Cl− ion movements is the transmembrane electrical gradient. Negatively charged Cl− anions are repelled by the negatively polarized cell. Charge and concentration combine to generate an electrochemical gradient for Cl− movement. For given concentrations of internal and external Cl−, there is a reversal potential (EINV) where charge and concentration gradients are equal and opposed. Then at any membrane potential, Vm > EINV Cl− flows inward and the cell is hyperpolarized. Inversely, if Vm < EINV, Cl− moves outward to depolarize the cell. Depolarizations beyond neuronal threshold could even cause cell firing.
In human, epileptic pyramidal cells sampled from the subiculum, the reversal potential of GABAA responses formed a distribution centered around −70 mV. But, it extended asymmetrically to values as depolarized as −45 mV: a skewed continuum of reversal potentials rather than two distinct cell populations. About 20% of pyramidal cells were depolarized by GABA; that is the reversal potential for GABA was more depolarized than the resting membrane potential. A similar proportion to that of the cells depolarized during interictal discharges. In neocortical tissues, interictal-like discharges were associated with synaptic inputs reversing at a mean −62±2mV also suggesting depolarizing GABA signaling may be involved (Florez et al., 2013).
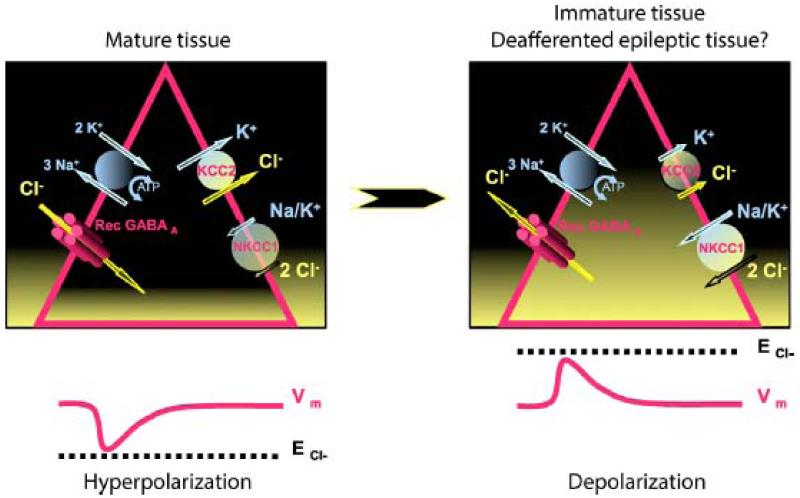
Regulation of [Cl−]i depends on multiple molecules including the Na-K-Cl (NKCC1) and K-Cl (KCC2) cotransporters (Payne et al., 2003). NKCC1 loads the cell with Cl−, using the electrochemical gradient of Na+ ions. KCC2 extrudes Cl− from the cell, with energy derived from the chemical gradient of K+ ions. In normal and mature tissues, KCC2 is highly expressed while NKCC1 is expression may be repressed (Dzhala et al., 2005). [Cl−]i is very low, ~4 mmol/L, compared to [Cl−]e, close to 147 mmol/L, resulting in a reversal potential of −72 mV. Adult neuron resting potentials are more depolarized than the reversal potential for GABA which thus hyperpolarizes and inhibits. In contrast during early development, KCC2 is not expressed and NKCC1 acts to charge immature neurons with Cl− (Rivera et al., 1999). The reversal potential for GABA is more depolarized than resting potential, so GABA depolarizes (Ben-Ari, 2002).
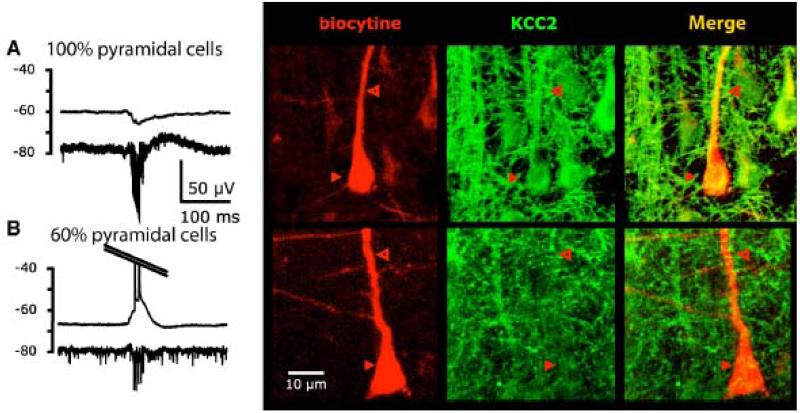
The hypothesis of a loss of expression of KCC2 within deafferented cells of the subiculum (Coull et al., 2003, Vale and Sanes, 2000) was tested using in situ hybridization to evaluate expression of mRNA for KCC2 (Figure 4). Nearly all cells in the CA2 region and the dentate gyrus expressed message for KCC2. These cells served as non-deafferented controls. In contrast only 60-80% of subicular pyramidal cells expressed KCC2 message. As for the KCC2 protein, immunohistochemistry showed that KCC2 was expressed by ~90% of subicular pyramidal cells compared to 97% in dentate gyrus and almost 100% in the subiculum of control monkeys.
Figure 4.
Regulation of the intracellular Cl− concentration by the cotransporters KCC2 & NKCC1 and effects on GABAergic responses in mature (left) and immature and pathological tissues (right). Within pyramidal cells (triangles), Cl− concentration is represented by the height of the filling in yellow. Vm: membrane potential, ECl−: reversal potential of Cl− currents.
Single neuron expression of KCC2 was compared to the hyper- or depolarizing nature of potential changes during interictal discharges (Figure 5). All hyperpolarized pyramidal cells, expressed KCC2. However, not all cells that were depolarized, presumably with higher levels of internal Cl−, lacked KCC2 expression. Possibly immuno-labelled KCC2 may not be functional due to posttranslational regulation(Blaesse et al., 2006, Los Heros et al., 2006, and Khirug al., 2005). Alternatively distinct Cl− homeostatic pathways could be involved. NKCC1, the transporter which tends to import Cl−, was functional in that the antagonist bumetanide, hyperpolarized GABA-mediated responses and suppressed interictal activity.
Figure 5.
Correlation between the behavior of a pyramidal cell during interictal discharges (hyperpolarization in a A vs depolarization in B) and the expression of KCC2 (based on Huberfeld et al., 2007). Electrophysiological trace is shown on the left (top: intracellular recording – bottom: extracellular). The recorded cell is then filled with biocytin and KCC2 is labeled by immunohistochemistry.
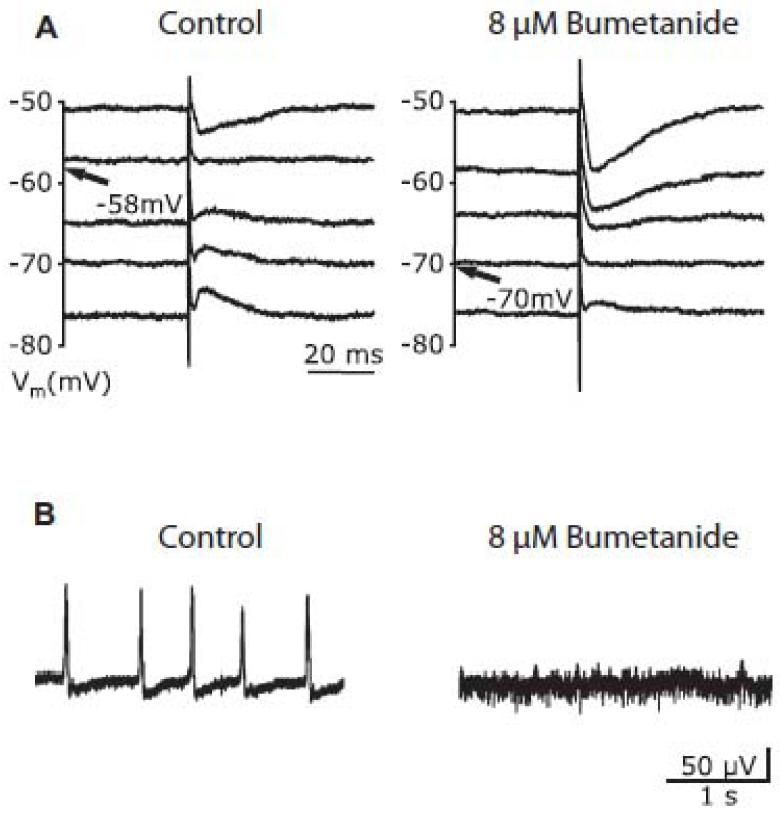
Selective blockade of NKCC1 by low doses of loop diuretic bumetanide hyperpolarized the GABAergic reversal potential by about 10 mV and blocked epileptiform activity over 10s of minutes, kinetics consistent with a reduced internal Cl− (Figure 6). Work on animal models confirms the antiepileptic potential of this diuretic (Dzhala et al., 2005).
Figure 6.
The diuretic Bumetanide blocks interictal activity (based on Huberfeld et al., 2007).
A: Measurements of the GABAergic reversal potential in a pyramidal cell, before (−58 mV) and after applying Bumetanide (−70 mV).
B: Bumetanide blocks interictal activity (extracellular recording)
These studies have validated a role of an impaired Cl− homeostasis in epileptiform activity of the human subiculum. They identified a continuum of depolarizing responses to GABA associated with a disturbed expression and / or function of the co-transporters KCC2 and NKCC1. Identification of these defects opens new therapeutic avenues by restoring Cl− homeostasis. Possibly depolarizing responses to GABA are a common feature to epileptogenic tissue. A recent study of human neocortex surrounding epileptic brain gliomas shows this tissue also generates interictal-like discharges. In the same way, interneurons initiate population events which depend on depolarizing responses to GABA. Cl− homeostasis in a majority of pyramidal cells is perturbed due to both a down regulation of KCC2 and an up regulation of NKKC1 (Pallud et al., 2014).
What causes these disturbances in Cl− homeostasis in human epileptic tissue? In the subiculum of sclerotic hippocampus, it may result from deafferentation due to CA1 pyramidal cell loss in the CA1. Deafferentation depolarizes GABAA responses by repressing KCC2 expression or function in several animal models (Sanes and Vale, 2000; Nabekura et al., 2002; Coull et al., 2003). After a delay, cortical transection can lead to ictal activities in deafferented cortex of the cat (Topolnik et al., 2003). Perturbed Cl− homeostasis after deafferentation in the spinal cord may be mediated via BDNF release from activated microglia (Coull et al., 2005). BDNF affects post-synaptic KCC2 via trkB receptors on neurons (Rivera et al. 2002). Epileptic activity itself may prolong disturbances in Cl− homeostasis (Rivera et al., 2004). It remains puzzling though that Cl− homeostasis is perturbed in only about 20% of subicular pyramidal cells in spite of severe neuronal loss and gliosis in the CA1 region. A limited cell loss in layer III of the entorhinal cortex (Du et al., 1995) may reduce afferents from this region (Witter and Amaral, 1991) to the subiculum. These changes in afferent connectivity may reorganize recurrent synaptic circuits between subicular cells (Finch et al., 1988, Gigg et al., 2000, Harris and Stewart, 2001). Enhanced recurrent loops would tend to generate epileptic activity and provoke cognitive disturbances in patients.
Changes in Cl− homeostasis after deafferentation, recall early stages of neuronal development. During development, GABAergic precedes glutamatergic signaling, at a stage of neuronal migration, synaptogenesis and circuit formation (Ben-Ari 2004). Possibly interictal discharges in epileptic subiculum should be compared to the synchronous Giant Depolarizing Potentials (Ben-Ari et al., 1989), emblematic of this period which also depend on depolarizing GABAergic signals. Synaptogenesis in the human deafferented subiculum, could favor the genesis of synchronous activities. Neuronal density in the subiculum increases by ~50% during CA1 sclerosis (Andrioli et al., 2007). However changes in recurrent excitatory connections have not been demonstrated and subicular axonal sprouting seems to be absent in animal models of epilepsy associated with CA1 sclerosis (Knopp et al., 2005).
Further work is needed to define connectivity and post-synaptic actions for interneurons of human epileptic subiculum. It is not clear which type of interneuron discharges before interictal events. Possibly chandelier cells which make divergent contacts with proximal pyramidal cell axons, a site devoid of KCC2 (Szabadics et al., 2006), but connectivity of these cells seem to be unchanged in human tissue (Arellano et al., 2004). In contrast, parvalbumin-positive basket interneurons contacts with proximal pyramidal cells in the subiculum apparently involve large numbers of contacts (Munoz et al., 2007). Numbers of these cells are reduced in epileptic subiculum with or without HS (Andrioli et al., 2007) but no data is yet available on their discharge pattern during interictal-like activities.
In addition to a role in generating interictal events, GABAergic signaling affects their spread. Antagonists suppress variability in propagation pathways (Saboleck etal., 2010)
Genesis of ictal activities within the human epileptogenic subiculum
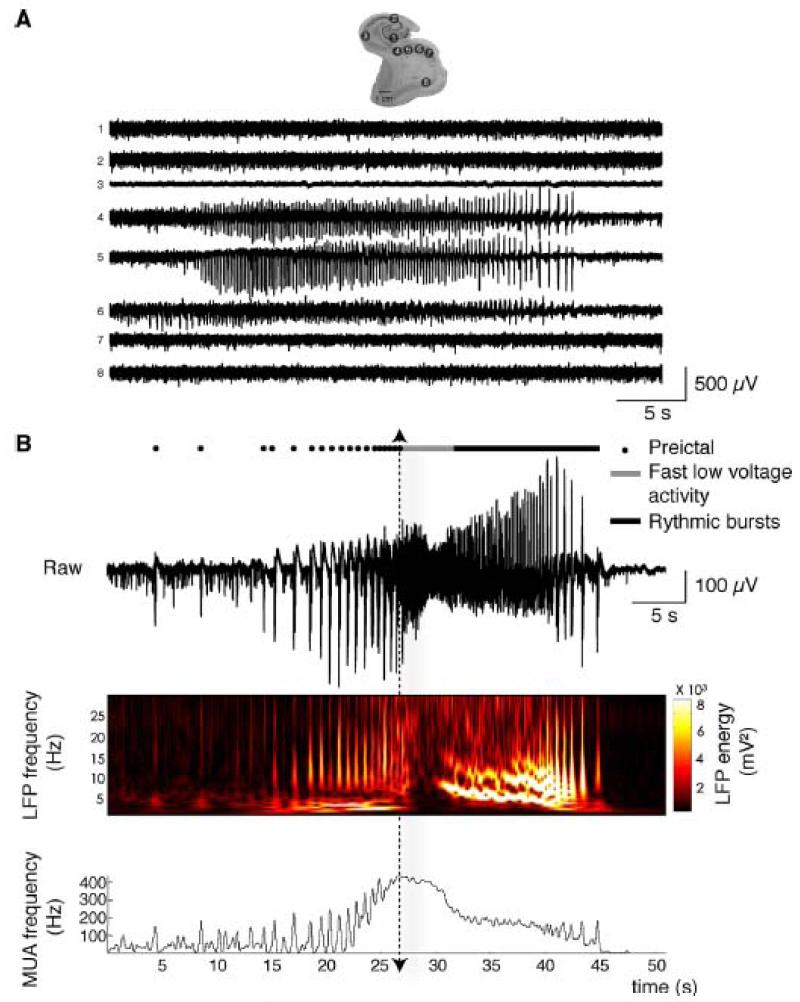
In vitro, human postoperative tissues generate interictal activity with no stimulation. Ictal like events must be induced. Effective stimuli have combined an increase in cellular excitability (increased external K+ or reduced Ca2+ and Mg2+) with an alkalinization, 4-AP or a reduced external Mg2+ (Huberfeld et al., 2011). Ictal-like events generated by the subiculum typically comprise low-voltage fast activities followed by larger rhythmic oscillations (Figure 7). Ictogenesis mechanisms can be studied with these pro-ictal stimuli. In vitro the transition from interictal to ictal-like events is accompanied by the emergence of larger, preictal recurrent population bursts.
Figure 7.
Ictal-like discharges induced in vitro in the epileptic human subiculum (from Huberfeld et al. 2011).
A: Multiple extracellular recordings of an ictal event in a slice containing the hippocampus, subiculum and entorhinal cortex. Electrode locations: 1, dentate gyrus; 2, CA2; 3, CA1; 4–5–6, subiculum; 7, presubiculum; 8, entorhinal cortex.
B: Ictal event structure. Top, pre-ictal discharges (larger events, filled circles) recurred before a fast low-voltage activity (gray line) at seizure onset followed by rythmic bursts (black line). Seizure onset is indicated by the dotted arrow. Middle, time frequency representation of the local field potential (LFP). Bottom, multi-unit activity (MUA) revealed recurring PIDs of short duration followed by the rapid unpatterned action potential discharges at seizure onset and then by oscillatory bursts.
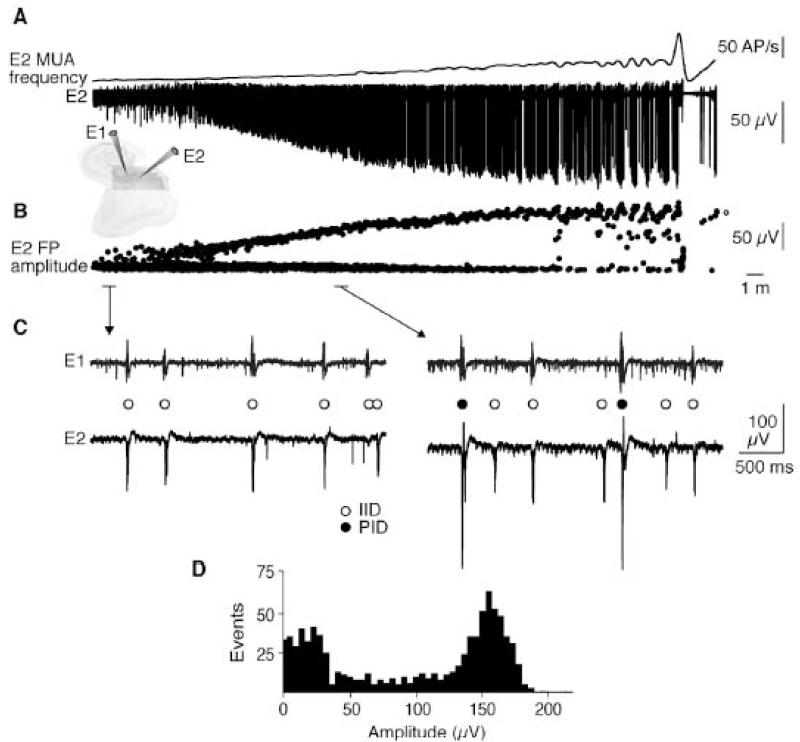
Preictal discharges arise progressively during a long lasting transition period from the interictal state to the first seizure and coexist with interictal discharges (Figure 8).
Figure 8.
Preictal discharges progressively emerge during the transition to the seizure.
A: Extracellular recordings of the transition to seizure-like activity. E1 and E2 are recordings from two subicular electrodes. MUA frequency (upper trace) and the extracellular signal from E2 (lower trace) are shown.
B: Amplitude measurements for all field potentials recorded by electrode E2 during the transition show the emergence of larger PIDs, whereas the amplitude of inter-ictal events did not change.
C: Dual extracellular recordings showing IIDs (open circles, left) before convulsant application and coexpression of PIDs (filled circles, right) with IIDs during the transition.
D: Amplitude distribution for all field potentials during the 35-min transition period showed IIDs of amplitude 10–50 μV and PIDs of amplitude 125–175 μV.
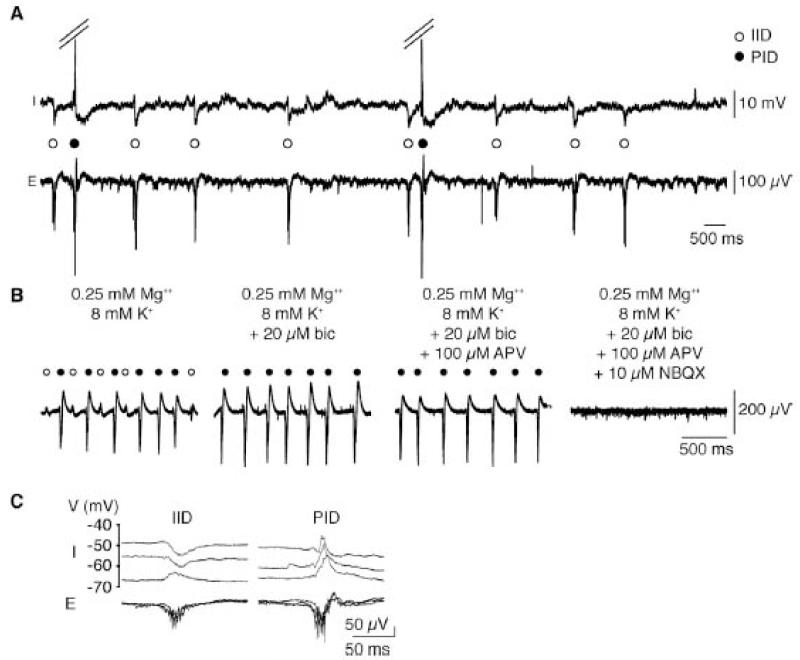
Preictal discharges seem to depend on exclusively glutamatergic signals in contrast to the implication of both GABAergic and glutamatergic mechanisms in interictal events. Multiple observations support this point. In pyramidal cells, synaptic events associated with preictal bursts are exclusively depolarizing, with no trace of the hyperpolarization recorded from most pyramidal cells during interictal bursts (Figure 9a). Pre-ictal bursts are blocked by AMPA receptor antagonists, but unchanged by blockers at GABAA receptors (Figure 9b). The reversal potential of synaptic events associated with preictal discharges is −10 to −20 mV, while that for synaptic events associated with interictal discharges is more negative at −50 to −80 mV, compatible with mixed glutamate and GABA signals (Figure 9c).
Figure 9.
Synaptic characteristics of interictal (IID) and preictal discharges (PID).
A: Intracellular recording from a subicular pyramidal cell (I) with a local extracellular recording (E). This cell received hyperpolarizing synaptic inputs during IIDs (open circle) both before and after convulsants were added. In contrast, it was depolarized during PIDs (filled circle) in the presence of convulsants.
B: IIDs and PIDs coexisted during the transition to ictal discharges (left trace). Bicuculline (bic) blocked IIDs, but not PIDs (middle left). The NMDA receptor blocker D,L-AP5 (100 μM) did not change PIDs (middle right), but the AMPA receptor antagonist NBQX (10 μM) suppressed them (right).
C: Distinct reversal potentials for synaptic events associated with IIDs and PIDs.
Juxtacellular recordings confirm that preictal bursts were preceded, and presumably initiated, by firing in a subset of pyramidal cells, while interictal discharges were preceded by interneuron firing. The buildup of preictal discharges was associated with an increased cellular excitability mediated by NMDA-dependent processes of plasticity.
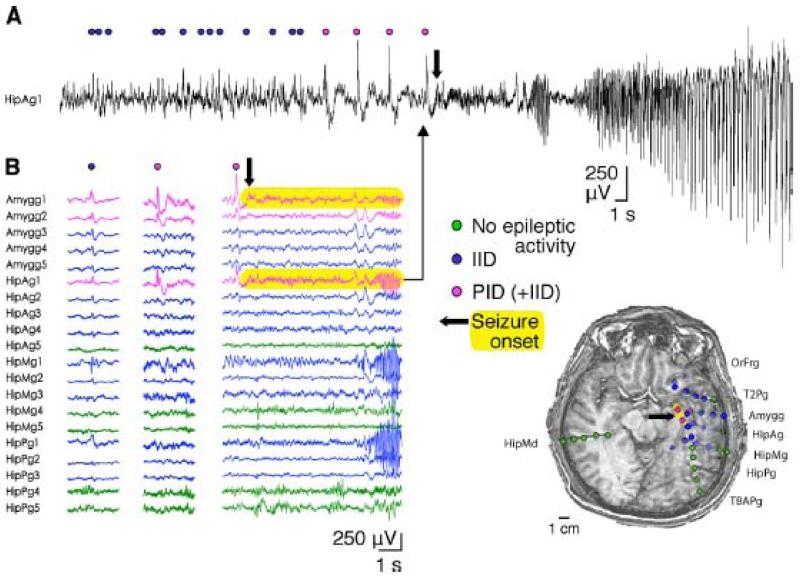
Finally, preictal discharges have been recorded from patients with mTLE-HS with in situ stereoelectroencephalography (Figure 10). As in vitro, they precede seizure onset, their amplitude clearly differs from that of interictal discharges. In vivo their sites of generation are restricted to areas of seizure initiation.
Figure 10.
In vivo StereoElectroEncephaloGraphic (SEEG) recording of preictal discharges (PID).
A: Stereo EEG recording (electrode HipAg1) showing activity of the subiculum and head of the hippocampus at seizure onset. The seizure was preceded by recurring IIDs (blue circles) and PIDs (pink circles). It began (arrow) with fast low-voltage activity and continued with oscillatory rhythmic bursts.
B: Recordings from multiple electrode contacts on a referential montage. Traces from contacts with no epileptic activity are shown in green, those with isolated IIDs in blue, and those recording both IIDs and PIDs in pink. Left, IID sample (blue circle). Middle, PID sample (pink circle). Right, seizure onset (thick arrow). Seizure onset is highlighted in yellow. Electrodes are identified according to the recorded area.
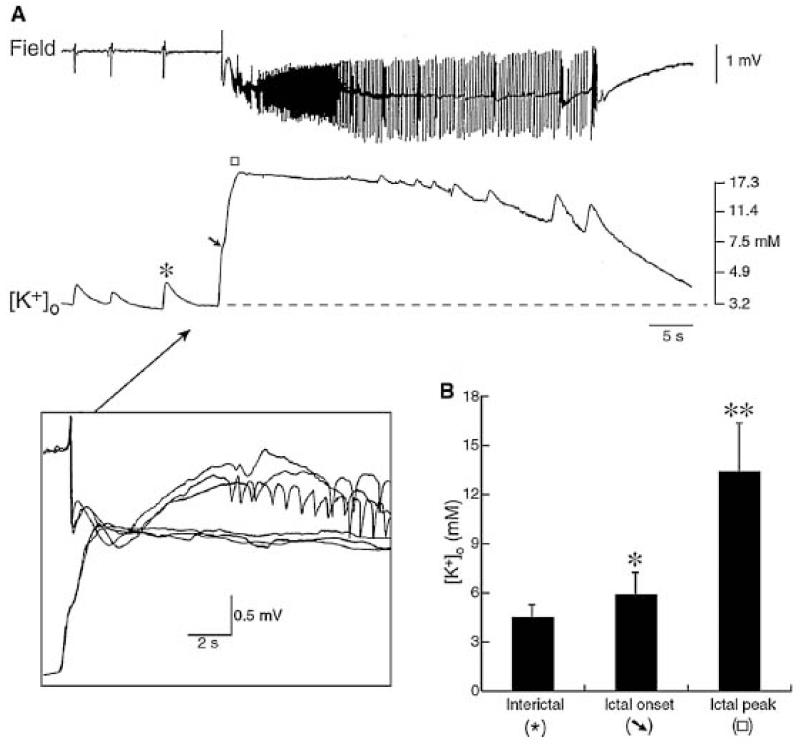
Ictal discharges have been induced in several ways. Zero Mg2+ media, which increase local excitability and enhance NMDA mediated excitation, induce seizure like events in neocortical temporal tissue treated by (Kostopoulos et al., 1989, Avoli et al., 1991, Kohling et al., 1998) and in hippocampal slices (Remy et al., 2003). 4-aminopyridine (4-AP), a K+ channel blocker which increases neuronal excitability and transmitter release, induces ictal events in neocortical slices (Avoli et al., 1999, D’Antuono et al., 2004). Equally, increasing extracellular potassium (to 8-12 mM), which increases excitability and probably affect Cl− regulation, induces seizure-like events in hippocampal tissue (Gabriel et al., 2004). Similar effects of these diverse stimuli highlight phenomena that may participate in seizures: activation of NMDA receptors (Avoli et al., 1995), release of adenosine (Kostopoulos et al., 1989) which controls synaptic liberation of glutamate, reductions in external Ca2+ (Avoli et al., 1995), defects in GABAB control of inhibitory synapses (D’Antuono et al., 2004). However, two major ictogenic mechanisms predominate. During preictal discharges, extracellular K+ increases transiently, then increases more dramatically to a plateau at seizure onset (D’Antuono et al., 2004) (Figure 11). It is not clear if this increase is a cause or a consequence of neuronal firing at seizure onset. The second key mechanism is GABAergic signaling (D’antuono et al., 2004, Huberfeld et al., 2011). Interneurons fire at high frequency at seizure onset, leading to a dynamic depolarizing switch of GABAergic signaling, dependent on Cl− dysregulation in pyramidal cells.
Figure 11.
A: Extracellular K+ concentration ([K+]o) dynamics during epileptic activities (From D’Antuono et al., 2004).
[K+]o increases up to 4.5 mM accompany the isolated negative field events (asterisk), while an elevation up to 6.4 mM characterizes the field potential leading to the ictal discharge onset (arrow). The insert, which represents three graphically superimposed ictal onsets at faster time base, demonstrates that the initial increase in [K+]o occurs at the same time as the initial positive–negative field event, while the slower secondary negative field event follows the peak of the elevation in [K+]o.
B: Quantitative summary of the maximal elevations in [K+]o associated with the isolated negative-going events, those occurring during the negative-going events preceding ictal discharge and during the ongoing ictal activity (identified as ictal peak).
Conclusion: practical benefits for the care of patients
The cellular basis of human epileptic activities may be better understood from work on slices of surgical specimens since they permit the use of recording techniques and pharmacological manipulations that are not possible in situ. This approach has revealed a role for depolarizing GABA responses in epileptiform activities in mTLE-HS and peritumoral tisue that could underly, at least in part, a poor seizure control with GABAergic drugs. They assign a significant role to the subiculum which has been shown to generate both interictal and ictal epileptic activities. Slice data points to new therapeutic options to restore inhibitory of effects of GABA and suppress epileptic activities by targeting Cl− regulators.
Human postoperative tissue may also help address the problem of pharmacoresistance. Multidrug transporter proteins effectively block antiepileptic drug access to the epileptic focus (Kovacs et al., 2011) while Na+ channels targeted by specific drugs may be modified consequently reducing drug efficacy (Jandova et al., 2006). Human tissue is evidently useful to test anti-epileptic drugs and verify new therapeutic targets. Distinctions must be made between interictal and ictal discharges, since in rodents most current drugs block seizures but not interictal events (D’Antuono et al., 2010). Recently a microRNA (small non-coding RNA exerting a post-transcriptional regulation of coding mRNAs), miR-487a, was shown to be down regulated in TLE tissues with dentate gyrus pathology (Zucchini et al., 2014), which may affect the expression of the gene ANTXR1, a promoter of cell migration.
Work on slices also has limitations. Tissue is certainly damaged by surgery and slicing (Verwer et al., 2014). Axons must frequently be cut, leading to a possible dys-regulation of cellular Cl− and thus GABAergic signaling (Dzhala et al 2012). Long-term organotypic culture of human epileptic slices (Eugene et al., 2014) could assist recovery from these traumas, and also permit long-term manipulations such as transfection. However the activity after several weeks differs from that of acute slices. In addition to subicular events, interictal-like bursts are also generated in the dentate gyrus. As in acute slices temporal neocortex in culture did not generate interictal events, although ictal-like firing was induced by GABAA receptors antagonists or 4-AP, indicating distinct features of mesial and lateral temporal areas.
Several novel techniques are being applied to human tissue slices. Microelectrode arrays (Hsiao et al., 2014; Dossi et al., 2014) permit records from up to 100s of sites at high spatial and temporal resolution. Fluorescent dye techniques permit the activity of many cells to be combined with electrical records at cellular and population scale paving the way for exciting multimodal investigations (Kerekes et al., 2014).
References
- Andrioli A, Alonso-Nanclares L, Arellano JI, Defelipe J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–43. doi: 10.1016/j.neuroscience.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Pumain R, Köhling R. Cellular and molecular mechanisms of epilepsy in the human brain. Prog Neurobiol. 2005 Oct;77(3):166–200. doi: 10.1016/j.pneurobio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Arellano JI, Munoz A, Ballesteros-Yanez I, Sola RG, DeFelipe J. Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain. 2004;127:45–64. doi: 10.1093/brain/awh004. [DOI] [PubMed] [Google Scholar]
- Avoli M, Bernasconi A, Mattia D, Olivier A, Hwa GG. Epileptiform discharges in the human dysplastic neocortex: in vitro physiology and pharmacology. Ann Neurol. 1999;46:816–26. doi: 10.1002/1531-8249(199912)46:6<816::aid-ana3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Avoli M, Drapeau C, Louvel J, Pumain R, Olivier A, Villemure JG. Epileptiform activity induced by low extracellular magnesium in the human cortex maintained in vitro. Ann Neurol. 1991;30:589–96. doi: 10.1002/ana.410300412. [DOI] [PubMed] [Google Scholar]
- Avoli M, Hwa GG, Lacaille JC, Olivier A, Villemure JG. Electrophysiological and repetitive firing properties of neurons in the superficial/middle layers of the human neocortex maintained in vitro. Exp Brain Res. 1994a;98:135–44. doi: 10.1007/BF00229118. [DOI] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Drapeau C, Pumain R, Kurcewicz I. GABAA-mediated inhibition and in vitro epileptogenesis in the human neocortex. J Neurophysiol. 1995;73:468–84. doi: 10.1152/jn.1995.73.2.468. [DOI] [PubMed] [Google Scholar]
- Avoli M, Louvel J, Pumain R, Kohling R. Cellular and molecular mechanisms of epilepsy in the human brain. Prog Neurobiol. 2005;77:166–200. doi: 10.1016/j.pneurobio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Avoli M, Mattia D, Siniscalchi A, Perreault P, Tomaiuolo F. Pharmacology and electrophysiology of a synchronous GABA-mediated potential in the human neocortex. Neuroscience. 1994;62:655–66. doi: 10.1016/0306-4522(94)90467-7. [DOI] [PubMed] [Google Scholar]
- Avoli M, Olivier A. Electrophysiological properties and synaptic responses in the deep layers of the human epileptogenic neocortex in vitro. J Neurophysiol. 1989;61:589–606. doi: 10.1152/jn.1989.61.3.589. [DOI] [PubMed] [Google Scholar]
- Beck H, Goussakov IV, Lie A, Helmstaedter C, Elger CE. Synaptic plasticity in the human dentate gyrus. J Neurosci. 2000 Sep 15;20(18):7080–6. doi: 10.1523/JNEUROSCI.20-18-07080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–25. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr C, D’Antuono M, Hamidi S, Herrington R, Lévesque M, Salami P, Shiri Z, Köhling R, Avoli M. Limbic networks and epileptiform synchronization: the view from the experimental side. Int Rev Neurobiol. 2014;114:63–87. doi: 10.1016/B978-0-12-418693-4.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Guillemin I, Schindler J, Schweizer M, Delpire E, Khiroug L, et al. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J Neurosci. 2006;26:10407–19. doi: 10.1523/JNEUROSCI.3257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. doi: 10.1016/s0920-1211(98)00059-x. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–21. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Le Duigou C, Miles R. Mesial temporal lobe epilepsy: a pathological replay of developmental mechanisms? Biol Cell. 2003;95:329–33. doi: 10.1016/s0248-4900(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Cooper R, Winter AL, Crow HJ, Walter WG. Comparison of Subcortical, Cortical and Scalp Activity Using Chronically Indwelling Electrodes in Man. Electroencephalogr Clin Neurophysiol. 1965;18:217–28. doi: 10.1016/0013-4694(65)90088-x. [DOI] [PubMed] [Google Scholar]
- Coras R, Siebzehnrubl FA, Pauli E, Huttner HB, Njunting M, Kobow K, Villmann C, Hahnen E, Neuhuber W, Weigel D, Buchfelder M, Stefan H, Beck H, Steindler DA, Blümcke I. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain. 2010 Nov;133(11):3359–72. doi: 10.1093/brain/awq215. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–42. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, Louvel J, Kohling R, Mattia D, Bernasconi A, Olivier A, et al. GABAA receptor-dependent synchronization leads to ictogenesis in the human dysplastic cortex. Brain. 2004;127:1626–40. doi: 10.1093/brain/awh181. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, Köhling R, Ricalzone S, Gotman J, Biagini G, Avoli M. Antiepileptic drugs abolish ictal but not interictal epileptiform discharges in vitro. Epilepsia. 2010 Mar;51(3):423–31. doi: 10.1111/j.1528-1167.2009.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Los Heros P, Kahle KT, Rinehart J, Bobadilla NA, Vazquez N, San Cristobal P, et al. WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci U S A. 2006;103:1976–81. doi: 10.1073/pnas.0510947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich D, Kral T, Clusmann H, Friedl M, Schramm J. Reduced function of L-AP4-sensitive metabotropic glutamate receptors in human epileptic sclerotic hippocampus. Eur J Neurosci. 1999;11:1109–13. doi: 10.1046/j.1460-9568.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- Dossi E, Blauwblomme T, Nabbout R, Huberfeld G, Rouach N. Multi-electrode array recordings of human epileptic postoperative cortical tissue. Journal Of Visual Experiments. doi: 10.3791/51870. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Schwarcz R, Tamminga CA. Entorhinal cortex in temporal lobe epilepsy. Am J Psychiatry. 1995;152:826. doi: 10.1176/ajp.152.6.826. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–13. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Dzhala V, Valeeva G, Glykys J, Khazipov R, Staley K. Traumatic alterations in GABA signaling disrupt hippocampal network activity in the developing brain. J Neurosci. 2012 Mar 21;32(12):4017–31. doi: 10.1523/JNEUROSCI.5139-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugène E, Cluzeaud F, Cifuentes-Diaz C, Fricker D, Le Duigou C, Clemenceau S, Baulac M, Poncer JC, Miles R. An organotypic brain slice preparation from adult patients with temporal lobe epilepsy. J Neurosci Methods. 2014 Sep 30;235:234–44. doi: 10.1016/j.jneumeth.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabó D, Maglóczky Z, Wittner L, Pék A, Eross L, Czirják S, Vajda J, Sólyom A, Rásonyi G, Szucs A, Kelemen A, Juhos V, Grand L, Dombovári B, Halász P, Freund TF, Halgren E, Karmos G, Ulbert I. Properties of in vivo interictal spike generation in the human subiculum. Brain. 2008 Feb;131(Pt 2):485–99. doi: 10.1093/brain/awm297. [DOI] [PubMed] [Google Scholar]
- Finch DM, Tan AM, Isokawa-Akesson M. Feedforward inhibition of the rat entorhinal cortex and subicular complex. J Neurosci. 1988;8:2213–26. doi: 10.1523/JNEUROSCI.08-07-02213.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez CM, McGinn RJ, Lukankin V, Marwa I, Sugumar S, Dian J, Hazrati LN, Carlen PL, Zhang L, Valiante TA. In Vitro Recordings of Human Neocortical Oscillations. Cereb Cortex. 2013 Sep 17; doi: 10.1093/cercor/bht235. [DOI] [PubMed] [Google Scholar]
- Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA. Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia. 1995;36:543–58. doi: 10.1111/j.1528-1157.1995.tb02566.x. [DOI] [PubMed] [Google Scholar]
- Gabriel S, Njunting M, Pomper JK, Merschhemke M, Sanabria ER, Eilers A, et al. Stimulus and potassium-induced epileptiform activity in the human dentate gyrus from patients with and without hippocampal sclerosis. J Neurosci. 2004;24:10416–30. doi: 10.1523/JNEUROSCI.2074-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigg J, Finch DM, O’Mara SM. Responses of rat subicular neurons to convergent stimulation of lateral entorhinal cortex and CA1 in vivo. Brain Res. 2000;884:35–50. doi: 10.1016/s0006-8993(00)02878-x. [DOI] [PubMed] [Google Scholar]
- Gigout S, Louvel J, Kawasaki H, D’Antuono M, Armand V, Kurcewicz I, et al. Effects of gap junction blockers on human neocortical synchronization. Neurobiol Dis. 2006;22:496–508. doi: 10.1016/j.nbd.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Harris E, Stewart M. Intrinsic connectivity of the rat subiculum: II. Properties of synchronous spontaneous activity and a demonstration of multiple generator regions. J Comp Neurol. 2001;435:506–18. doi: 10.1002/cne.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterkeuser S, Schroder W, Hager G, Seifert G, Blumcke I, Elger CE, et al. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci. 2000;12:2087–96. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- Hsiao MC, Yu PN, Song D, Liu CY, Heck CN, Millett D, Berger TW. An in vitro seizure model from human hippocampal slices using multi-electrode arrays. J Neurosci Methods. 2014 Sep 22; doi: 10.1016/j.jneumeth.2014.09.010. pii: S0165-0270(14)00335-5. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–73. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, Menendez of, Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. Glutamatergic pre-ictal Discharges emerge at the transition to seizure in the human epilepsy by Nature. Neuroscience. 2011;14(5):627–34. doi: 10.1038/nn.2790. [DOI] [PubMed] [Google Scholar]
- Hwa GG, Avoli M, Oliver A, Villemure JG. Bicuculline-induced epileptogenesis in the human neocortex maintained in vitro. Exp Brain Res. 1991;83:329–39. doi: 10.1007/BF00231156. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Fried I. Extracellular slow negative transient in the dentate gyrus of human epileptic hippocampus in vitro. Neuroscience. 1996;72:31–7. doi: 10.1016/0306-4522(95)00544-7. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Levesque M, Fried I, Engel J., Jr. Glutamate currents in morphologically identified human dentate granule cells in temporal lobe epilepsy. J Neurophysiol. 1997;77:3355–69. doi: 10.1152/jn.1997.77.6.3355. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Levesque MF. Increased NMDA responses and dendritic degeneration in human epileptic hippocampal neurons in slices. Neurosci Lett. 1991;132:212–6. doi: 10.1016/0304-3940(91)90304-c. [DOI] [PubMed] [Google Scholar]
- Jandova K, Pasler D, Antonio LL, Raue C, Ji S, Njunting M, et al. Carbamazepine-resistance in the epileptic dentate gyrus of human hippocampal slices. Brain. 2006;129:3290–306. doi: 10.1093/brain/awl218. [DOI] [PubMed] [Google Scholar]
- Kerekes BP, Tóth K, Kaszás A, Chiovini B, Szadai Z, Szalay G, Pálfi D, Bagó A, Spitzer K, Rózsa B, Ulbert I, Wittner L. Combined two-photon imaging, electrophysiological, and anatomical investigation of the human neocortex in vitro. Neurophoton. 2014;1(1):011013. doi: 10.1117/1.NPh.1.1.011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khirug S, Huttu K, Ludwig A, Smirnov S, Voipio J, Rivera C, et al. Distinct properties of functional KCC2 expression in immature mouse hippocampal neurons in culture and in acute slices. Eur J Neurosci. 2005;21:899–904. doi: 10.1111/j.1460-9568.2005.03886.x. [DOI] [PubMed] [Google Scholar]
- Kivi A, Lehmann TN, Kovacs R, Eilers A, Jauch R, Meencke HJ, et al. Effects of barium on stimulus-induced rises of [K+]o in human epileptic non-sclerotic and sclerotic hippocampal area CA1. Eur J Neurosci. 2000;12:2039–48. doi: 10.1046/j.1460-9568.2000.00103.x. [DOI] [PubMed] [Google Scholar]
- Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2005;483:476–88. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- Koch UR, Musshoff U, Pannek HW, Ebner A, Wolf P, Speckmann EJ, et al. Intrinsic excitability, synaptic potentials, and short-term plasticity in human epileptic neocortex. J Neurosci Res. 2005;80:715–26. doi: 10.1002/jnr.20498. [DOI] [PubMed] [Google Scholar]
- Kohling R, Avoli M. Methodological approaches to exploring epileptic disorders in the human brain in vitro. J Neurosci Methods. 2006;155:1–19. doi: 10.1016/j.jneumeth.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Kohling R, Lucke A, Straub H, Speckmann EJ, Tuxhorn I, Wolf P, et al. Spontaneous sharp waves in human neocortical slices excised from epileptic patients. Brain. 1998;121(Pt 6):1073–87. doi: 10.1093/brain/121.6.1073. [DOI] [PubMed] [Google Scholar]
- Kohling R, Qu M, Zilles K, Speckmann EJ. Current-source-density profiles associated with sharp waves in human epileptic neocortical tissue. Neuroscience. 1999;94:1039–50. doi: 10.1016/s0306-4522(99)00327-9. [DOI] [PubMed] [Google Scholar]
- Kostopoulos G, Drapeau C, Avoli M, Olivier A, Villemeure JG. Endogenous adenosine can reduce epileptiform activity in the human epileptogenic cortex maintained in vitro. Neurosci Lett. 1989 Nov 20;106(1-2):119–24. doi: 10.1016/0304-3940(89)90212-7. [DOI] [PubMed] [Google Scholar]
- Kovács R, Raue C, Gabriel S, Heinemann U. Functional test of multidrug transporter activity in hippocampal-neocortical brain slices from epileptic patients. J Neurosci Methods. 2011 Sep 15;200(2):164–72. doi: 10.1016/j.jneumeth.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Kapur J. Factors underlying bursting behavior in a network of cultured hippocampal neurons exposed to zero magnesium. J Neurophysiol. 2004;91:946–57. doi: 10.1152/jn.00547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa LM, Higashima M, Kim JH, Spencer DD. Epileptiform discharges evoked in hippocampal brain slices from epileptic patients. Brain Res. 1989;493:168–74. doi: 10.1016/0006-8993(89)91012-3. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Kornblum HI, Mendoza D, Lozada A, Leite JP, et al. Human hippocampal AMPA and NMDA mRNA levels in temporal lobe epilepsy patients. Brain. 1997;120(Pt 11):1937–59. doi: 10.1093/brain/120.11.1937. [DOI] [PubMed] [Google Scholar]
- McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989;62:1018–27. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida L, Benavides-Piccione R, Sola R, Pozo MA. Electrophysiological properties of interneurons from intraoperative spiking areas of epileptic human temporal neocortex. Neuroreport. 2002;13:1421–5. doi: 10.1097/00001756-200208070-00015. [DOI] [PubMed] [Google Scholar]
- Munoz A, Mendez P, DeFelipe J, Alvarez-Leefmans FJ. Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia. 2007;48:663–73. doi: 10.1111/j.1528-1167.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- Nabekura J, Ueno T, Okabe A, Furuta A, Iwaki T, Shimizu-Okabe C, et al. Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J Neurosci. 2002;22:4412–7. doi: 10.1523/JNEUROSCI.22-11-04412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallud J, Le Van Quyen M, Bielle F, Pellegrino C, Varlet P, Labussiere M, Cresto N, Dieme MJ, Baulac M, Duyckaerts C, Kourdougli N, Chazal G, Devaux B, Rivera C, Miles R, Capelle L, Huberfeld G. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci Transl Med. 2014 Jul 9;6(244):244ra89. doi: 10.1126/scitranslmed.3008065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Peret A, Christie LA, Ouedraogo DW, Gorlewicz A, Epsztein J, Mulle C, Crépel V. Contribution of aberrant GluK2-containing kainate receptors to chronic seizures in temporal lobe epilepsy. Cell Rep. 2014 Jul 24;8(2):347–54. doi: 10.1016/j.celrep.2014.06.032. [DOI] [PubMed] [Google Scholar]
- Prince DA. Neurophysiology of epilepsy. Annu Rev Neurosci. 1978;1:395–415. doi: 10.1146/annurev.ne.01.030178.002143. [DOI] [PubMed] [Google Scholar]
- Prince DA, Wong RK. Human epileptic neurons studied in vitro. Brain Res. 1981;210:323–33. doi: 10.1016/0006-8993(81)90905-7. [DOI] [PubMed] [Google Scholar]
- Ray A, Tao JX, Hawes-Ebersole SM, Ebersole JS. Localizing value of scalp EEG spikes: a simultaneous scalp and intracranial study. Clin Neurophysiol. 2007;118:69–79. doi: 10.1016/j.clinph.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Remy S, Gabriel S, Urban BW, Dietrich D, Lehmann TN, Elger CE, et al. A novel mechanism underlying drug resistance in chronic epilepsy. Ann Neurol. 2003;53:469–79. doi: 10.1002/ana.10473. [DOI] [PubMed] [Google Scholar]
- Represa A, Robain O, Tremblay E, Ben-Ari Y. Hippocampal plasticity in childhood epilepsy. Neurosci. Lett. 1989;99:351–355. doi: 10.1016/0304-3940(89)90472-2. [DOI] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, et al. BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol. 2002;159:747–52. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, et al. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–5. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, et al. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci. 2004;24:4683–91. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabolek HR, Swiercz WB, Lillis KP, Cash SS, Huberfeld G, Zhao G, Ste Marie L, Clemenceau S, Barsh G, Miles R, Staley KJ. A candidate mechanism underlying the variance of interictal spike propagation. J Neurosci. 2012 Feb 29;32(9):3009–21. doi: 10.1523/JNEUROSCI.5853-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder W, Hinterkeuser S, Seifert G, Schramm J, Jabs R, Wilkin GP, et al. Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia. 2000;41(Suppl 6):S181–4. doi: 10.1111/j.1528-1157.2000.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Haglund MM. Spontaneous rhythmic synchronous activity in epileptic human and normal monkey temporal lobe. Epilepsia. 1986;27:523–33. doi: 10.1111/j.1528-1157.1986.tb03578.x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Knowles WD. Intracellular study of human epileptic cortex: in vitro maintenance of epileptiform activity? Science. 1984;223:709–12. doi: 10.1126/science.6695179. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Epileptogenesis in mammalian cortex studied in vitro. Trans Am Neurol Assoc. 1976;101:288–91. [PubMed] [Google Scholar]
- Schwartzkroin PA, Turner DA, Knowles WD, Wyler AR. Studies of human and monkey “epileptic” neocortex in the in vitro slice preparation. Ann Neurol. 1983;13:249–57. doi: 10.1002/ana.410130305. [DOI] [PubMed] [Google Scholar]
- Surges R, Kukley M, Brewster A, Rüschenschmidt C, Schramm J, Baram TZ, Beck H, Dietrich D. Hyperpolarization-activated cation current Ih of dentate gyrus granule cells is upregulated in human and rat temporal lobe epilepsy. Biochem Biophys Res Commun. 2012 Mar 30;420(1):156–60. doi: 10.1016/j.bbrc.2012.02.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer RW, Sluiter AA, Balesar RA, Baaijen JC, de Witt Hamer PC, Speijer D, Li Y, Swaab DF. Injury response of resected human brain tissue in vitro. Brain Pathol. 2014 Aug 20; doi: 10.1111/bpa.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson TH, Sperling MR, O’Connor MJ. Strong paired pulse depression of dentate granule cells in slices from patients with temporal lobe epilepsy. J Neural Transm. 1998;105:613–25. doi: 10.1007/s007020050083. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–5. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tao JX, Baldwin M, Hawes-Ebersole S, Ebersole JS. Cortical substrates of scalp EEG epileptiform discharges. J Clin Neurophysiol. 2007;24:96–100. doi: 10.1097/WNP.0b013e31803ecdaf. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Hoffman NW, Kim YI, Fisher RS, Peacock WJ, Dudek FE. Electrical properties of neocortical neurons in slices from children with intractable epilepsy. J Neurophysiol. 1996;75:931–9. doi: 10.1152/jn.1996.75.2.931. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Peacock WJ, Dudek FE. Local synaptic circuits and epileptiform activity in slices of neocortex from children with intractable epilepsy. J Neurophysiol. 1992;67:496–507. doi: 10.1152/jn.1992.67.3.496. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Steriade M, Timofeev I. Hyperexcitability of intact neurons underlies acute development of trauma-related electrographic seizures in cats in vivo. Eur J Neurosci. 2003;18:486–96. doi: 10.1046/j.1460-9568.2003.02742.x. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J Neurosci. 2000;20:1912–21. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Paesschen W, Duncan JS, Stevens JM, Connelly A. Longitudinal quantitative hippocampal magnetic resonance imaging study of adults with newly diagnosed partial seizures: one-year follow-up results. Epilepsia. 1998;39:633–9. doi: 10.1111/j.1528-1157.1998.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Hoogland G, van Veelen CW, Wadman WJ. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci. 2004;19:2769–78. doi: 10.1111/j.1460-9568.2004.03400.x. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Erosion of inhibition contributes to the progression of low magnesium bursts in rat hippocampal slices. J Physiol. 1995;486(Pt 3):723–34. doi: 10.1113/jphysiol.1995.sp020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser HG. ILAE Commission Report. Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- Williamson A, Patrylo PR, Spencer DD. Decrease in inhibition in dentate granule cells from patients with medial temporal lobe epilepsy. Ann Neurol. 1999;45:92–9. doi: 10.1002/1531-8249(199901)45:1<92::aid-art15>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Williamson A, Telfeian AE, Spencer DD. Prolonged GABA responses in dentate granule cells in slices isolated from patients with temporal lobe sclerosis. J Neurophysiol. 1995;74:378–87. doi: 10.1152/jn.1995.74.1.378. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol. 1991;307:437–59. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- Wittner L, Huberfeld G, Clémenceau S, Eross L, Dezamis E, Entz L, Ulbert I, Baulac M, Freund TF, Maglóczky Z, Miles R. The epileptic human hippocampal cornu ammonis 2 region generates spontaneous interictal-like activity in vitro. Brain. 2009 Nov;132(Pt 11):3032–46. doi: 10.1093/brain/awp238. [DOI] [PubMed] [Google Scholar]
- Wozny C, Kivi A, Lehmann TN, Dehnicke C, Heinemann U, Behr J. Comment on “On the origin of interictal activity in human temporal lobe epilepsy in vitro”. Science. 2003;301:463. doi: 10.1126/science.1084237. author reply 463. [DOI] [PubMed] [Google Scholar]
- Wozny C, Knopp A, Lehmann TN, Heinemann U, Behr J. The subiculum: a potential site of ictogenesis in human temporal lobe epilepsy. Epilepsia. 2005;46(Suppl 5):17–21. doi: 10.1111/j.1528-1167.2005.01066.x. [DOI] [PubMed] [Google Scholar]
- Zucchini S, Marucci G, Paradiso B, Lanza G, Roncon P, Cifelli P, Ferracin M, Giulioni M, Michelucci R, Rubboli G, Simonato M. Identification of miRNAs differentially expressed in human epilepsy with or without granule cell pathology. PLoS One. 2014 Aug 22;9(8):e105521. doi: 10.1371/journal.pone.0105521. [DOI] [PMC free article] [PubMed] [Google Scholar]