Abstract
Bacteriophage-mediated horizontal gene transfer is one of the primary driving forces of bacterial evolution. The pac-type phages are generally thought to facilitate most of the phage-mediated gene transfer between closely related bacteria, including that of mobile genetic elements-encoded virulence genes. In this study, we report that staphylococcal cos-type phages transferred the Staphylococcus aureus pathogenicity island SaPIbov5 to non-aureus staphylococcal species and also to different genera. Our results describe the first intra- and intergeneric transfer of a pathogenicity island by a cos phage, and highlight a gene transfer mechanism that may have important implications for pathogen evolution.
Classically, transducing phages use the pac site-headful system for DNA packaging. Packaging is initiated on concatemeric post-replicative DNA by terminase cleavage at the sequence-specific pac site, a genome slightly longer than unit length is packaged, and packaging is completed by non-sequence-specific cleavage (reviewed in Rao and Feiss, 2008). Generalized transduction results from the initiation of packaging at pac site homologs in host chromosomal or plasmid DNA, and typically represents ∼1% of the total number of phage particles. In the alternative cos site mechanism packaging is also initiated on concatemeric post-replicative DNA by terminase cleavage at a sequence-specific (cos) site. Here, however, packaging is completed by terminase cleavage at the next cos site, generating a precise monomer with the cohesive termini used for subsequent circularization (Rao and Feiss, 2008). Although cos site homologs may exist in host DNA, it is exceedingly rare that two such sites would be appropriately spaced. Consequently, cos phages, of which lambda is the prototype, do not engage in generalized transduction. For this reason, cos-site phages have been preferred for possible phage therapy, since they would not introduce adventitious host DNA into target organisms.
The Staphylococcus aureus pathogenicity islands (SaPIs) are the best-characterized members of the phage-inducible chromosomal island family of mobile genetic elements (MGEs; Novick et al., 2010). SaPIs are ∼15 kb mobile elements that encode virulence factors and are parasitic on specific temperate (helper) phages. Helper phage proteins are required to lift their repression (Tormo-Más et al., 2010, 2013), thereby initiating their excision, circularization and replication. Phage-induced lysis releases vast numbers of infectious SaPI particles, resulting in high frequencies of transfer. Most SaPI helper phages identified to date are pac phages, and many well-studied SaPIs are packaged by the headful mechanism (Ruzin et al., 2001; Ubeda et al., 2007). Recently, we have reported that some SaPIs, of which the prototype is SaPIbov5 (Viana et al., 2010), carry phage cos sequences in their genomes, and can be efficiently packaged and transferred by cos phages to S. aureus strains at high frequencies (Quiles-Puchalt et al., 2014). Here we show that this transfer extends to non-aureus staphylococci and to Listeria monocytogenes.
Since the pac phages transfer SaPIs to non-aureus staphylococci and to the Gram-positive pathogen Listeria monocytogenes (Maiques et al., 2007; Chen and Novick, 2009), we reasoned that cos phages might also be capable of intra- and intergeneric transfer. We tested this with SaPIbov5, into which we had previously inserted a tetracycline resistance (tetM) marker to enable selection, and with lysogens of two helper cos phages, φ12 and φSLT, carrying SaPIbov5 (strains JP11010 and JP11194, respectively; Supplementary Table 1). The prophages in these strains were induced with mitomycin C, and the resulting lysates were adjusted to 1 μg ml−1 DNase I and RNase A, filter sterilized (0.2 μm pore), and tested for SaPI transfer with tetracycline selection, as previously described (Ubeda et al., 2008). To test for trans-specific or trans-generic transduction, coagulase-negative staphylococci species and L. monocytogenes strains were used as recipients for SaPIbov5 transfer, respectively, as previously described (Maiques et al., 2007; Chen and Novick, 2009). As shown in Table 1, SaPIbov5 was transferred to S. xylosus, S. epidermidis and L. monocytogenes strains at frequencies only slightly lower than to S. aureus. PCR analysis demonstrated that the complete island was transferred to the recipient strains and integrated at the cognate attB site in the host chromosome (Figure 1 and Supplementary Table 2). In contrast, deletion of the SaPIbov5 cos site (strains JP11229 and JP11230) did not affect SaPI replication (Supplementary Figure 1), but completely eliminated SaPIbov5 transfer (Table 1). To rule out the possibility that other mechanisms of gene transfer were involved in this process, we generated a φ12 phage mutant in the small terminase (terS) gene (strain JP11012), using plasmid pJP1511 (Supplementary Table 2). The TerS protein is essential for φ12 and SaPIbov5 DNA packaging, but not for phage-mediated lysis (Quiles-Puchalt et al., 2014). As expected, this mutation abolished SaPIbov5 transfer (Table 1). Taken together, these results show that intra- and intergeneric transfer of the island was cos phage mediated. Furthermore, SaPI proteins, such as integrase (Int) and potentially toxins, can be expressed and functional in non-aureus strains.
Table 1. Intra- and intergeneric SaPIbov5 transfera.
|
Donor strain |
|||
|---|---|---|---|
| Phage | SaPI | Recipient strain | SaPI titreb |
| φ12 | SaPIbov5 | S. aureus JP4226 | 8.3 × 104 |
| S. epidermidis JP829 | 2.4 × 104 | ||
| S. epidermidis JP830 | 4.7 × 104 | ||
| L. monocytogenes SK1351 | 6.6 × 103 | ||
| L. monocytogenes EGDe | 2.1 × 104 | ||
| S. xylosus C2a | 7.1 × 104 | ||
| φ12 | SaPIbov5 Δcos | S. aureus JP4226 | <10 |
| S. epidermidis JP829 | <10 | ||
| S. epidermidis JP830 | <10 | ||
| L. monocytogenes SK1351 | <10 | ||
| L. monocytogenes EGDe | <10 | ||
| S. xylosus C2a | <10 | ||
| φ12 ΔterS | SaPIbov5 | S. aureus JP4226 | <10 |
| S. epidermidis JP829 | <10 | ||
| S. epidermidis JP830 | <10 | ||
| L. monocytogenes SK1351 | <10 | ||
| L. monocytogenes EGDe | <10 | ||
| S. xylosus C2a | <10 | ||
| φSLT | SaPIbov5 | S. aureus JP4226 | 4.1 × 103 |
| S. epidermidis JP829 | 1.1 × 103 | ||
| S. epidermidis JP830 | 2.1 × 103 | ||
| L. monocytogenes SK1351 | 3.6 × 102 | ||
| L. monocytogenes EGDe | 3.1 × 103 | ||
| S. xylosus C2a | 4.0 × 103 | ||
| φSLT | SaPIbov5 Δcos | S. aureus JP4226 | <10 |
| S. epidermidis JP829 | <10 | ||
| S. epidermidis JP830 | <10 | ||
| L. monocytogenes SK1351 | <10 | ||
| L. monocytogenes EGDe | <10 | ||
| S. xylosus C2a | <10 | ||
Abbreviation: SAPI, Staphylococcus aureus pathogenicity island.
The means of results from three independent experiments are shown. Variation was within ±5% in all cases.
No. of transductants per ml induced culture.
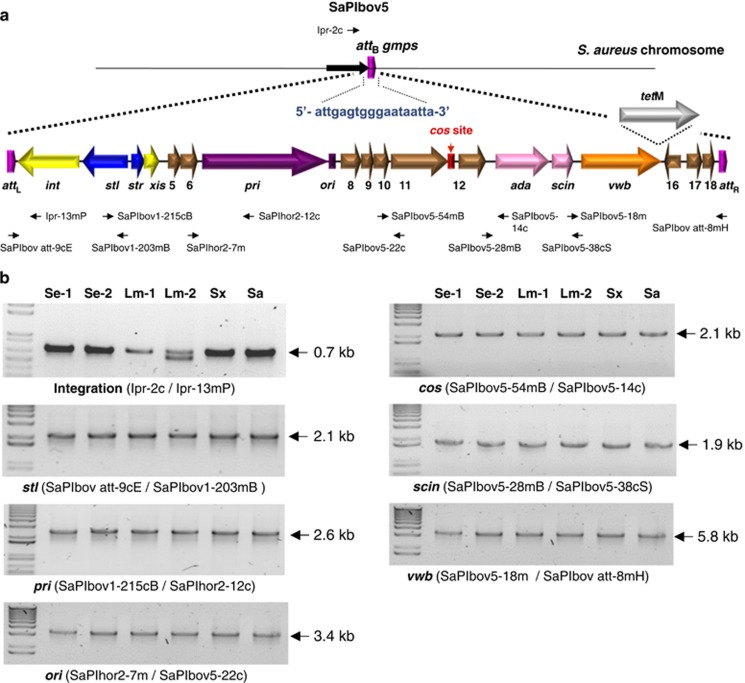
Figure 1.
(a) Map of SaPIbov5. Arrows represent the localization and orientation of ORFs greater than 50 amino acids in length. Rectangles represent the position of the ori (in purple) or cos (in red) sites. Positions of different primers described in the text are shown. (b) Amplimers generated for detection of SaPIbov5 in the different recipient strains. Supplementary Table 2 lists the sequence of the different primers used. The element was detected in S. epidermidis JP829 (Se-1), S. epidermidis JP830 (Se-2), L. monocytogenes SK1351 (Lm-1), L. monocytogenes EGDe (Lm-2), S. xylosus C2a (Sx) and S. aureus JP4226 (Sa).
Because plaque formation is commonly used to determine phage host range, we next determined the ability of phages φ12 and φSLT to parasitize and form plaques on S. xylosus, S. epidermidis and L. monocytogenes strains. As shown in Supplementary Figure 2, phages φ12 and φSLT can parasitize and form plaques on their normal S. aureus hosts, but are completely unable to lyse the non-aureus strains. Therefore, as previously observed with pac phages (Chen and Novick, 2009), these results indicate that the overall host range of a cos phage may also be much wider if it includes infection without plaque formation.
Previous studies have demonstrated pac phage-mediated transfer of MGEs between S. aureus and other bacterial species (Maiques et al., 2007; Chen and Novick, 2009; Uchiyama et al., 2014); however, no previous studies have described the natural intra- or intergeneric transfer of pathogenicity islands by cos phages. As bacterial pathogens become increasingly antibiotic resistant, lytic and poorly transducing phages, such as cos phages, have been proposed for phage therapy, on the grounds that they would not introduce adventitious host DNA into target organisms and that the phages are so restricted in host range that the resulting progeny are harmless and will not result in dysbiosis of human bacterial flora. Because plaque formation was once thought to determine the host range of a phage, the evolutionary impact of phages on bacterial strains they can transduce, but are unable to parasitize, has remained an unrecognized aspect of phage biology and pathogen evolution. Our results add to the recently recognized concept of ‘silent transfer' of pathogenicity factors carried by MGEs (Maiques et al., 2007; Chen and Novick, 2009) by phages that cannot grow on the target organism. They extend this capability to cos phages, which have hitherto been unrecognized as mediators of natural genetic transfer.
The potential for gene transfer of MGEs by this mechanism is limited by the ability of cos phages to adsorb and inject DNA into recipient strains, and also by the presence of suitable attachment sites in recipient genomes. However, since different bacterial genera express wall teichoic acid with similar structures, which can act as bacteriophage receptors governing the routes of horizontal gene transfer between major bacterial pathogens, horizontal gene transfer even across long phylogenetic distances is possible (Winstel et al., 2013). In addition, our previous results also demonstrated that the SaPI integrases have much lower sequence specificity than other typical integrases, and SaPIs readily integrate into alternative sites in the absence of the cognate attC site, such that any bacterium that can adsorb SaPI helper phage is a potential recipient (Chen and Novick, 2009). Thus, we anticipate that cos phages can have an important role in spreading MGEs carrying virulence and resistance genes. We also predict that cos sites will be found on many other MGEs, enabling cos phage-mediated transfer of any such element that can generate post-replicative concatemeric DNA.
Acknowledgments
We thank R Calendar for gifts of strains and phages. This work was supported by grants Consolider-Ingenio CSD2009-00006, BIO2011-30503-C02-01 and Eranet-pathogenomics PIM2010EPA-00606 to JRP, from the Ministerio de Ciencia e Innovación (MICINN, Spain), and grant R01AI022159 to RPN and JRP, from the National Institutes of Health.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Chen J, Novick RP. Phage-mediated intergeneric transfer of toxin genes. Science. 2009;323:139–141. doi: 10.1126/science.1164783. [DOI] [PubMed] [Google Scholar]
- Maiques E, Ubeda C, Tormo MA, Ferrer MD, Lasa I, Novick RP, et al. Role of staphylococcal phage and SaPI integrase in intra- and interspecies SaPI transfer. J Bacteriol. 2007;189:5608–5616. doi: 10.1128/JB.00619-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8:541–551. doi: 10.1038/nrmicro2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiles-Puchalt N, Carpena N, Alonso JC, Novick RP, Marina A, Penadés JR. Staphylococcal pathogenicity island DNA packaging system involving cos-site packaging and phage-encoded HNH endonucleases. Proc Natl Acad Sci USA. 2014;111:6016–6021. doi: 10.1073/pnas.1320538111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VB, Feiss M. The bacteriophage DNA packaging motor. Annu Rev Genet. 2008;42:647–681. doi: 10.1146/annurev.genet.42.110807.091545. [DOI] [PubMed] [Google Scholar]
- Ruzin A, Lindsay J, Novick RP. Molecular genetics of SaPI1—a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol. 2001;41:365–377. doi: 10.1046/j.1365-2958.2001.02488.x. [DOI] [PubMed] [Google Scholar]
- Tormo-Más MÁ, Donderis J, García-Caballer M, Alt A, Mir-Sanchis I, Marina A, et al. Phage dUTPases control transfer of virulence genes by a proto-oncogenic G protein-like mechanism. Mol Cell. 2013;49:947–958. doi: 10.1016/j.molcel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Tormo-Más MÁ, Mir I, Shrestha A, Tallent SM, Campoy S, Lasa I, et al. Moonlighting bacteriophage proteins derepress staphylococcal pathogenicity islands. Nature. 2010;465:779–782. doi: 10.1038/nature09065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C, Maiques E, Barry P, Matthews A, Tormo MA, Lasa I, et al. SaPI mutations affecting replication and transfer and enabling autonomous replication in the absence of helper phage. Mol Microbiol. 2008;67:493–503. doi: 10.1111/j.1365-2958.2007.06027.x. [DOI] [PubMed] [Google Scholar]
- Ubeda C, Maiques E, Tormo MA, Campoy S, Lasa I, Barbé J, et al. SaPI operon I is required for SaPI packaging and is controlled by LexA. Mol Microbiol. 2007;65:41–50. doi: 10.1111/j.1365-2958.2007.05758.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama J, Takemura-Uchiyama I, Sakaguchi Y, Gamoh K, Kato S-I, Daibata M, et al. Intragenus generalized transduction in Staphylococcus spp. by a novel giant phage. ISME J. 2014;8:1949–1952. doi: 10.1038/ismej.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana D, Blanco J, Tormo-Más MÁ, Selva L, Guinane CM, Baselga R, et al. Adaptation of Staphylococcus aureus to ruminant and equine hosts involves SaPI-carried variants of von Willebrand factor-binding protein. Mol Microbiol. 2010;77:1583–1594. doi: 10.1111/j.1365-2958.2010.07312.x. [DOI] [PubMed] [Google Scholar]
- Winstel V, Liang C, Sanchez-Carballo P, Steglich M, Munar M, Bröker BM, et al. Wall teichoic acid structure governs horizontal gene transfer between major bacterial pathogens. Nat Commun. 2013;4:2345. doi: 10.1038/ncomms3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.