Background: Cocaine-activated p38 MAPK-mediated norepinephrine transporter (NET) threonine 30 phosphorylation up-regulates NET.
Results: p38 MAPK inhibition and TAT-NET-Thr30 peptide blocks cocaine-mediated NET up-regulation and cocaine-induced locomotor sensitization and conditioned place preference (CPP).
Conclusion: Blockade of p38 MAPK-mediated Thr30-dependent NET regulation attenuates cocaine-induced locomotor sensitization, CPP, and CPP reinstatement.
Significance: Regulation of NET plays a mechanistic role in cocaine-mediated behaviors.
Keywords: Catecholamine, Cell Signaling, Membrane Protein, Membrane Trafficking, Phosphorylation, Drug Abuse, psychostimulants
Abstract
The noradrenergic and p38 mitogen-activated protein kinase (p38 MAPK) systems are implicated in cocaine-elicited behaviors. Previously, we demonstrated a role for p38 MAPK-mediated norepinephrine transporter (NET) Thr30 phosphorylation in cocaine-induced NET up-regulation (Mannangatti, P., Arapulisamy, O., Shippenberg, T. S., Ramamoorthy, S., and Jayanthi, L. D. (2011) J. Biol. Chem. 286, 20239–20250). The present study explored the functional interaction between p38 MAPK-mediated NET regulation and cocaine-induced behaviors. In vitro cocaine treatment of mouse prefrontal cortex synaptosomes resulted in enhanced NET function, surface expression, and phosphorylation. Pretreatment with PD169316, a p38 MAPK inhibitor, completely blocked cocaine-mediated NET up-regulation and phosphorylation. In mice, in vivo administration of p38 MAPK inhibitor SB203580 completely blocked cocaine-induced NET up-regulation and p38 MAPK activation in the prefrontal cortex and nucleus accumbens. When tested for cocaine-induced locomotor sensitization and conditioned place preference (CPP), mice receiving SB203580 on cocaine challenge day or on postconditioning test day exhibited significantly reduced cocaine sensitization and CPP. A transactivator of transcription (TAT) peptide strategy was utilized to test the involvement of the NET-Thr30 motif. In vitro treatment of synaptosomes with TAT-NET-Thr30 (wild-type peptide) completely blocked cocaine-mediated NET up-regulation and phosphorylation. In vivo administration of TAT-NET-Thr30 peptide but not TAT-NET-T30A (mutant peptide) completely blocked cocaine-mediated NET up-regulation and phosphorylation. In the cocaine CPP paradigm, mice receiving TAT-NET-Thr30 but not TAT-NET-T30A on postconditioning test day exhibited significantly reduced cocaine CPP. Following extinction, TAT-NET-Thr30 when given prior to cocaine challenge significantly reduced reinstatement of cocaine CPP. These results demonstrate that the direct inhibition of p38 MAPK or the manipulation of NET-Thr30 motif/phosphorylation via a TAT peptide strategy prevents cocaine-induced NET up-regulation, locomotor sensitization, and CPP, suggesting a role for Thr30-linked NET regulation in cocaine-elicited behaviors.
Introduction
Efforts with NET3 knock-out (KO) mice provided unequivocal evidence that NET is important for synaptic NE clearance. More importantly, elevated locomotor activity after psychostimulants and enhanced cocaine reward phenotypes in NET KO mice underscores the importance of NET in drug addiction (2). The rewarding and powerfully addictive effects of psychostimulants are attributed to dopamine (DA) signaling in the mesolimbic system. However, DA clearance in the prefrontal cortex (PFC) is largely controlled by the NET (3–7), and DA-related behavior is linked to NET function (8). It is possible that cocaine effects on NET and hence on NE/DA signaling may represent one of many neuroadaptations that occur in the development of drug addiction.
NE clearance is a highly orchestrated process involving regulation of NET function via phosphorylation by second messenger-linked signaling pathways downstream of receptor activation (9–11). NET contains multiple consensus sites for several kinases including PKCs and MAPKs. Although PKCϵ is implicated in cardiovascular diseases and alcohol addiction (12–14), other PKC isoforms and p38 MAPKs are implicated in several brain disorders such as psychostimulant addiction and depression (15, 16). We have documented that phosphorylation of NET may be a common mechanism dictating NET expression and thus NE transport function in maintaining NE homeostasis (1, 17, 18). In particular, our recent in vitro and in vivo studies documented that cocaine increases NET function and surface expression in rodent PFC and NAc via p38 MAPK-mediated NET phosphorylation (1). Thus, there exists a significant association between cocaine up-regulation of NET and p38 MAPK signaling. This raises the possibility that manipulation of p38 MAPK-dependent NET regulation in animals could alter the behavioral response to cocaine.
Cocaine and other psychostimulants induce MAPK signaling in the brain (19–22). Acute in vivo cocaine administration results in NET up-regulation as well as p38 MAPK activation in rat PFC (1). Using PFC synaptosomes and human placental trophoblast cells expressing hNET (HTR-hNET), our studies demonstrated that in vitro application of cocaine stimulates NET function, surface expression, and phosphorylation in a manner sensitive to p38 MAPK inhibition. Cocaine reduces NET endocytosis contributing to transporter up-regulation (1). Furthermore, our studies provided evidence for cocaine-induced p38 MAPK-dependent phosphorylation of NET-Thr30 dictating NET endocytosis and hence NE transport. These published results (1) demonstrated a novel molecular mechanism by which cocaine controls NE transport.
Here we extended this investigation to in vivo manipulation of p38 MAPK to determine whether there is a link between p38 MAPK-mediated NET regulation and cocaine-induced behaviors. To explore the effects of p38 MAPK inhibition on behavioral responses to the psychostimulant drug cocaine, we tested cocaine-induced locomotor sensitization and conditioned place preference (CPP) in C57BL/6J mice. Initial in vitro experiments showed cocaine-mediated NET up-regulation and enhanced NET phosphorylation in mouse PFC synaptosomes. These effects were completely blocked by prior treatment with either the p38 MAPK inhibitor PD169316 or TAT-NET-Thr30. Similar to in vitro treatment, in vivo cocaine administration to mice resulted in NET up-regulation in the PFC and NAc regions in a manner that is sensitive to in vivo administration of the p38 MAPK inhibitor SB203580 and TAT-NET-Thr30 peptide. In vivo administration of the p38 MAPK inhibitor SB203580 or TAT-NET-Thr30 peptide significantly attenuated cocaine-induced locomotor sensitization and CPP. These results suggested that p38 MAPK-mediated NET up-regulation is linked to cocaine-induced behaviors and that this regulation may have implications in the neuronal adaptations underlying cocaine addiction.
EXPERIMENTAL PROCEDURES
Animals
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) of 8–9 weeks of age and weighing around 25 g were used for the experiments. Mice were housed in groups of four to five in polypropylene cages with corn cob bedding and had free access to food (Harlan Teklad) and tap water. They were maintained on a 12-h light/12-h dark cycle at an ambient temperature of 22 °C and 42% humidity. All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocols of this study were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Materials
Cocaine hydrochloride (cocaine) and SB203580 (4-[4-fluorophenyl]-2-[4-methylsulfinylphenyl]-5-[4-pyridyl]-1H-imidazole) were purchased from Sigma-Aldrich. [3H]NE was from PerkinElmer Life Sciences. Norepinephrine transporter antibody (NET05-2) was from MAb Technologies (Stone Mountain, GA). Dopamine transporter (DAT) polyclonal antibody was from Santa Cruz Biotechnology Inc. (Dallas, TX). Polyclonal antibodies to NET and serotonin transporter (SERT) were generated and characterized in the laboratory (1, 23). Antibody to p38 MAPK was from Santa Cruz Biotechnology Inc. Phospho-p38 MAPK antibody was purchased from Cell Signal Technology (Danvers, MA). Antibody to tyrosine hydroxylase (TH) was from EMD Millipore (Billerica, MA). All other chemicals were from Sigma or Fisher Scientific unless otherwise indicated. The sequence LPEQPLRPCKTADLLVVKERN (amino acids 20–40) was selected encompassing Thr30 (underlined) in NET. Both NET-Thr30 and NET-T30A peptides with added TAT sequence YGRKKRRQRRR were synthesized by Thermo Scientific/Pierce and were of more than 95% purity. The sequences are YGRKKRRQRRRLPEQPLRPCKTADLLVVKR N for TAT-NET-Thr30 and YGRKKRRQRRRLPEQPLRPCKAADLLVVKERN for TAT-NET-T30A.
In Vivo Drug Administrations
Cocaine was dissolved in injectable grade isotonic saline solution (0.9% NaCl). Saline or cocaine (15, 20, or 30 mg/kg) was administered intraperitoneally in a volume of 10 μl/g of body weight. SB203580 was dissolved in DMSO and diluted with saline so that the final DMSO concentration was 0.002% when injected. Vehicle control contained 0.002% DMSO. Vehicle or SB203580 (50 μg/kg intraperitoneally) was administered in a volume of 10 μl/g of body weight. Vehicle (saline) or membrane-permeable TAT-NET-Thr30 or TAT-NET-T30A (12 mg/kg in saline) was administered intravenously in a volume of 1 μl/g of body weight via the tail vein. This accounts for 300 μg of peptide per mouse of 25-g weight.
Synaptosome Preparations
For in vitro experiments, mice were decapitated, and the brains were collected in ice-cooled dishes for synaptosome preparation. To examine the in vivo effect of p38 MAPK inhibition on cocaine-mediated NET regulation, mice received intraperitoneal injections of SB203580 or the vehicle 15 min prior to saline or cocaine administration. To examine the effect of TAT-NET peptides, mice received tail intravenous injections of either TAT-NET-Thr30, TAT-NET-T30A, or the vehicle 15 min prior to saline or cocaine administration. Following 60 min of drug administrations, mice were decapitated, and the brains were collected in ice-cooled dishes. Brain tissues from PFC and NAc were dissected and collected in 10 volumes (w/v) of cold 0.32 m sucrose. The tissue was immediately homogenized using a Teflon-glass homogenizer and centrifuged at 1000 × g for 10 min at 4 °C. The resulting supernatant was centrifuged at 12,000 × g for 20 min, and the pellet was washed by resuspending in 0.32 m sucrose (24). Protein concentration was determined by DC protein assay (Bio-Rad) using bovine serum albumin as the standard.
In Vitro Drug Treatments
Synaptosomes (300 μg) were preincubated with vehicle or 20 μm PD169316 in some experiments and with vehicle or 10 μm TAT-NET-Thr30 peptide in others at 37 °C for 15 min. The incubations were continued in the presence or absence of 50 μm cocaine for 60 min in a total volume of 1 ml. Following in vitro treatments, the reaction mixture was centrifuged at 15,000 × g for 20 min, and the pellet was resuspended in 300 μl of Krebs-Ringer HEPES buffer. The resuspended synaptosomes were washed three times using 1 ml of Krebs-Ringer HEPES buffer followed by centrifugations at 15,000 × g for 5 min, each time transferring the resuspended synaptosomes into fresh tubes. The pellet from the final wash was suspended in 300 μl of Krebs-Ringer HEPES buffer, and the uptake assay was performed as described previously (24) using [3H]NE.
NE and DA Uptake Measurements in Synaptosomes
The NE uptake assay was performed as described previously (1, 25) using 40 nm [3H]NE (35.0 Ci/mmol l-[7,8-3H]norepinephrine) for 5 min. Synaptosomes from either PFC or NAc were preincubated with the NET inhibitor desipramine (100 μm) at 37 °C for 5 min followed by the addition of [3H]NE to determine the nonspecific NE uptake. Nonspecific uptake was defined as uptake in the presence of 100 μm desipramine and subtracted from total accumulation to yield specific NET-mediated NE uptake. DAT-mediated DA uptake by NAc synaptosomes was measured using 10 nm [3H]DA (78 Ci/mmol [2,5,6,7,8-3H]dihydroxyphenylethylamine, PerkinElmer Life Sciences) at 37 °C for 5 min as described earlier (26, 27). DA uptake was measured in the presence of 100 nm nisoxetine (a NET-specific blocker to isolate total DAT-mediated DA uptake). DA uptake was also measured in the presence of 100 μm cocaine to isolate nonspecific DA uptake. Specific DAT-mediated DA uptake was calculated by subtracting nonspecific DA uptake (measured in the presence of cocaine) from total DA uptake (measured in the presence of nisoxetine). This allowed us to isolate only DAT-mediated DA uptake. Uptake was terminated by addition of 1 ml of ice-cold Krebs-Ringer HEPES buffer followed by rapid filtration over 0.3% polyethylenimine-coated GF-B filters on a Brandel cell harvester (Gaithersburg, MD). Filters were washed rapidly with 15 ml of cold PBS, and radioactivity bound to filters was quantified by liquid scintillation counting using a MicroBeta2 LumiJet (PerkinElmer Life Sciences). Mean values of specific uptake ±S.E. of at least three separate experiments were determined.
Surface Biotinylation of Synaptosomes
Synaptosomes (300 μg) treated as above or obtained from drug-administered animals were subjected to surface biotinylation and isolation of avidin-bound and -unbound fractions as described previously (1, 24, 25). Aliquots from total extracts (50 μl) and entire eluted fractions were separated by SDS-PAGE (10%), transferred to membrane, and probed with mouse NET antibody or DAT antibody. The rodent NET-specific monoclonal antibody has been characterized for its suitability to identify rat and mouse NET proteins by Western blotting, immunoprecipitations, and immunocytochemistry (28). Blots were stripped and reprobed with anti-TH antibody. NET or DAT proteins were visualized using ECL or ECL Plus reagent followed by exposure to Hyperfilm ECL. Multiple exposures of immunoblots were taken to ensure that the band development on the film was within the linear range. Band densities were quantified by scanning and analyzed using NIH ImageJ (v1.62) software. Anti-TH antibody was used to validate the surface biotinylation of plasma membrane proteins. NET band densities from total and biotinylated (representing the surface pool) fractions were normalized using levels of TH in the total extract.
Phosphorylation of NET
Synaptosomes (300 μg) were incubated with 5.0 mCi of 32PO4 carrier-free orthophosphate/mg of protein for 30 min before the addition of modulators. Following metabolic labeling with 32P, synaptosomes were treated with drugs as described above (under “In Vitro Drug Treatments”). At the end of the incubation, samples were centrifuged, and the pellet was resuspended in radioimmune precipitation assay buffer containing protease inhibitors (1 μm pepstatin A, 250 μm PMSF, 1 mg/ml leupeptin, and 1 μg/ml aprotinin) and phosphatase inhibitors (10 mm NaF, 50 mm sodium pyrophosphate, 1 mm sodium orthovanadate, and 1 m okadaic acid) by passing 10 times through a 25-gauge needle and solubilized by gently shaking on a nutator for 1 h at 4 °C. The clear supernatant obtained after centrifuging the solubilized synaptosomes at 45,000 × g for 40 min at 4 °C was subjected to immunoprecipitation with NET-82 antibody and autoradiography as described earlier (1). To test whether NET protein was completely immunoprecipitated by our polyclonal NET antibody following various drug treatments, synaptosomes that were not metabolically labeled were subjected to immunoprecipitation followed by immunoblotting with NET-specific monoclonal antibody.
Immunoprecipitations Using Synaptosomes
Synaptosomes were suspended in radioimmune precipitation assay buffer containing protease inhibitors (1 μm pepstatin A, 250 μm PMSF, 1 mg/ml leupeptin, and 1 μg/ml aprotinin) (29) by passing 10 times through a 25-gauge needle and solubilized by gentle shaking on a nutator for 1 h at 4 °C. The clear supernatant (200 μg of protein) obtained after centrifuging the solubilized synaptosomes at 25,000 × g for 30 min at 4 °C was subjected to (a) pulldown experiment with phospho-p38 MAPK beads or (b) immunoprecipitation with NET-specific antibody as indicated below.
Pulldown Experiment
Sepharose beads that were conjugated with monoclonal antibody that binds to the phosphorylated (Thr180/Tyr182) form of p38 MAPK were used to pull down the proteins associated with the active/phosphorylated form of p38 MAPK. The supernatants were incubated with the 20 μl of immobilized phospho-p38 MAPK antibody at 4 °C overnight with continuous shaking, and the bound proteins were eluted following thorough washings. The eluates were subjected to SDS-PAGE (10%), and the proteins were detected by immunoblotting with antibodies specific to NET as well as dopamine and serotonin transporters.
NET Immunoprecipitation
The supernatants were first precleared using Protein G-Sepharose (50 μl). NET protein was immunoprecipitated overnight at 4 °C by the addition of NET-specific antibody (NET05-2) and end-over-end continuous mixing followed by 2-h incubation with Protein G-Sepharose at 22 °C (room temperature). The immunoadsorbents captured by Protein G-Sepharose beads were washed with radioimmune precipitation assay buffer and eluted by adding 50 μl of urea-based sample buffer. The eluates were subjected to SDS-PAGE (10%), and the proteins were detected by immunoblotting with antibodies specific to rat/mouse NET (NET05-2), p38 MAPK, and phospho-p38 MAPK. Band densities were measured on multiple exposures to ensure quantitation within the linear range of the film using NIH Image software.
Open Field Activity Monitoring
The locomotor activity of the mice was monitored in open field activity chambers (Med Associates, St. Albans, VT; Model ENV-510; 10.75 × 10.75 inches). The movements of the mice were tracked using 16 evenly spaced infrared sources and sensors juxtaposed around the periphery of the four sides of the chamber. Each chamber is enclosed in a sound-attenuating shell with artificial ventilation. We adapted a short term cocaine sensitization protocol as described previously (30). Briefly, following a 1-day habituation period (day 0), mice were injected with 15 mg/kg cocaine on day 1 followed by 30 mg/kg cocaine on days 2, 3, and 4. Locomotor activity was measured on days 1, 2, and 4 for 60 min. Mice were kept in home cages for 4 days without any further drug injections. On day 9, mice received a 15 mg/kg cocaine challenge, and the locomotor activity was measured for 60 min. A single intraperitoneal injection of SB203580 at 50 μg/kg was given on challenge day prior to cocaine or saline injection to examine its effect on cocaine sensitization. This concentration was chosen based on our initial finding that when tested at 10, 50, and 100 μg/kg we found that although 10 μg/kg of SB203580 failed to attenuate cocaine sensitization 100 μg/kg produced a locomotor activating effect by itself. In addition, a similar dose has been used in a published study demonstrating the validity of detecting the behavioral effects in rodents without altering amine transport (31). We maintained two groups of mice. One group received saline and the other received cocaine from days 1 to 4 and no drug from days 5 to 8. On day 9, half of the mice from each group (saline and cocaine) received vehicle, and the other half received 50 μg/kg SB203580 15 min prior to cocaine challenge. Thus, we had four groups of mice to compare: vehicle/saline (n = 9), SB203580/saline (n = 9), vehicle/cocaine (n = 9), and SB203580/cocaine (n = 9). The locomotor activity of each mouse was recorded for 60 min in a computer with DIG-729 software provided by the manufacturer. Data were plotted as total distance traveled against time. Immediately following locomotor activity measurement on sensitization day, mice were sacrificed, and NE uptake was measured as described earlier using both PFC and NAc synaptosomes.
Conditioned Place Preference
An unbiased mouse CPP paradigm was utilized as described by several investigators (32–36). In brief, mice were placed in an enriched environment and handled for 3 days prior to initiation of CPP testing. The CPP apparatus (Med Associates, ENV-3013) consisted of white and black chambers (20 × 20 × 20 cm each), which differed in floor texture (white mesh and black rod; Med Associates, ENV-3013WM and ENV-3013BR) to help the mice further differentiate between the two environments. Place conditioning chambers were separated by a smaller intermediate compartment with a smooth PVC floor and partitions that allowed access to the black and white chambers. On day 1, mice were introduced into the chamber, and their baseline preference for each chamber was measured for 15 min. After testing for initial chamber preference on day 1, mice received saline in one chamber in the a.m. and saline (control group; n = 24) or 20 mg/kg intraperitoneal cocaine (n = 24) in the opposite chamber in the p.m. once a day for 3 days (chambers were counterbalanced across treatments). On day 5, CPP was tested at a time point midway between the a.m. and p.m. sessions. On this day of postconditioning test, 15 min prior to CPP testing, half of the mice from each of the saline or cocaine group received vehicle, and the other half received SB203580 (50 μg/kg intraperitoneally). Thus, we had four groups of mice to compare: saline/vehicle (n = 12), saline/SB203580 (n = 12), cocaine/vehicle (n = 12), and cocaine/SB203580 (n = 12). To examine whether SB203580 alone induces CPP, mice were conditioned with SB203580 (50 μg/kg intraperitoneally) for 3 days and tested for CPP on postconditioning day. To examine the specificity of SB203580 on cocaine CPP, mice were conditioned with morphine (5 mg/kg subcutaneously) for 3 days and on postconditioning day, half of the mice from the morphine group received vehicle (morphine/vehicle), and the other half received 50 μg/kg SB203580 intraperitoneally (morphine/SB203580) 15 min prior to CPP testing.
An identical protocol was followed for testing the effect of TAT-NET peptides. On the day of the postconditioning test, 15 min prior to CPP testing, ⅓ of the mice from each of the saline or cocaine group received vehicle, another ⅓ received TAT-NET-Thr30, and the rest received TAT-NET-T30A. Thus, we had six groups of mice: saline/vehicle (n = 9), saline/TAT-NET-Thr30 (n = 9), saline/TAT-NET-T30A (n = 9), cocaine/vehicle (n = 9), cocaine/TAT-NET-Thr30 (n = 9), and cocaine/TAT-NET-T30A (n = 9). The concentration of TAT-NET peptides was fixed at 12 mg/kg (300 μg per mouse of ∼25-g body weight) based on previous studies (37, 38) and following our initial testing with varying concentrations of TAT-NET-Thr30 peptide. Preference scores measured in seconds reflect the time the mice spent in the drug-paired side during postconditioning day subtracted from the time spent in the drug-paired side preconditioning when baseline scores were taken. A positive number indicated a preference for the drug-paired side, whereas a negative number implied an aversion to the drug-paired side. A number of zero or near zero indicated no preference for either side. The distance traveled was also recorded simultaneously for further analysis of the ambulatory counts.
Cocaine-induced Reinstatement of CPP
Mice were first tested for cocaine CPP as described above. Following the CPP test, mice were repeatedly exposed to the drug-paired chambers for 3 weeks without any further injections (3 days per week) and tested for CPP at the end of every week until CPP was extinguished. Although several methods have been used to extinguish CPP (39, 40), in our experiments the above described method was more effective in extinguishing cocaine CPP than repeated exposure to the drug-paired chambers following saline injections. Mice exhibiting a CPP score close to the baseline score or significantly below the initial cocaine CPP score are considered extinguished. Reinstatement was carried out by a cocaine priming injection (10 mg/kg cocaine given intraperitoneally) and testing for place preference by measuring the CPP score. To test the effect of TAT-NET-Thr30 on cocaine reinstatement, half of the mice from the extinguished group received vehicle, and the other half received TAT-NET-Thr30 (12 mg/kg intravenously via the tail) prior to saline or cocaine injection. Thus, we had four groups to compare: vehicle/saline (n = 10), vehicle/cocaine (n = 7), TAT-NET-Thr30/saline (n = 7), and TAT-NET-Thr30/cocaine (n = 6).
Statistical Analyses
Statistical analyses of the data were performed using GraphPad Prism software (La Jolla, CA).
RESULTS
Cocaine-induced NET Up-regulation and Phosphorylation Are p38 MAPK-dependent in the Mouse PFC
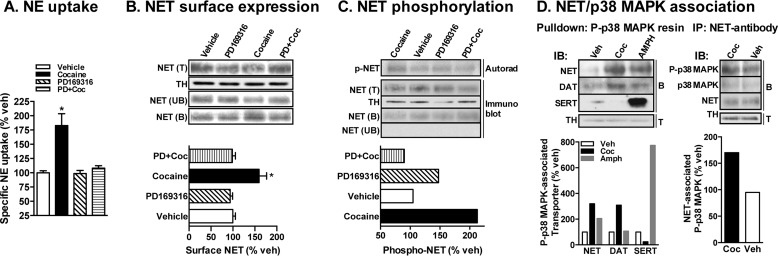
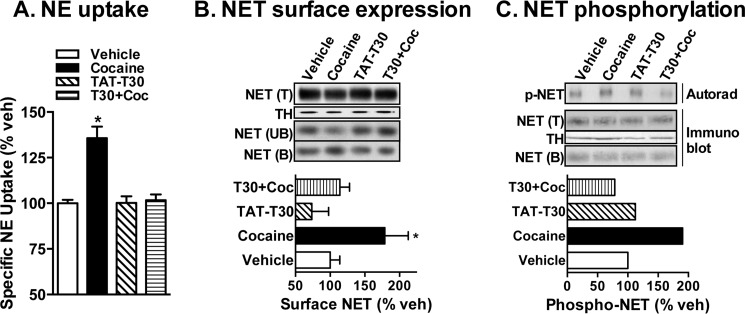
Our previous studies identified the p38 MAPK pathway in cocaine-induced NET up-regulation in heterologous system and in rat brain synaptosomes (1). Similar to the observations made from our previous study (1), incubation of mouse PFC synaptosomes with 50 μm cocaine for 1 h produced a significant increase in NE transport capacity compared with vehicle controls (Fig. 1A). Surface biotinylation experiments also revealed a significant increase in surface NET levels (Fig. 1B). In addition, cocaine treatment resulted in an ∼2.0-fold increase in basal NET phosphorylation (Fig. 1C). Furthermore, pretreatment of PFC synaptosomes with PD169316 (20 μm; 15 min) abolished the stimulatory effect of cocaine on NE transport (Fig. 1A), surface NET expression (Fig. 1B), and NET phosphorylation (Fig. 1C). Importantly, treatment with PD169316 alone did not affect NET phosphorylation (Fig. 1C). PD169316 not only blocked cocaine-induced NET phosphorylation but also decreased basal phosphorylation of mouse brain NET (Fig. 1C). Parallel immunoprecipitations using unlabeled synaptosomes showed no changes in the amount of NET protein pulled down following different treatments (Fig. 1C). Fig. 1C also shows no NET protein in the unbound fractions from protein A-Sepharose, indicating complete pulldown of NET by our NET antibody. In addition, we found that the NET exists in association with p38 MAPK and that NET-associated p38 MAPK was activated following cocaine administration (Fig. 1D). Pulldown experiments were conducted using phospho-p38 MAPK antibody-immobilized beads and synaptosomal extracts from the PFC. There was an increase in the level of NET bound to the beads (associated with phospho-p38 MAPK) when the synaptosomes were treated with cocaine (50 μm; 60 min) compared with vehicle treatment (Fig. 1D). This effect was both drug-specific and transporter-specific. Although we found an increased level of NET that was associated with phospho-p38 MAPK following cocaine or amphetamine (10 μm; 60 min) treatment, a slightly increased level of DAT was found following cocaine treatment (Fig. 1D). Conversely, an increased level of SERT associated with phospho-p38 MAPK was found following amphetamine treatment, and no change was found following cocaine treatment (Fig. 1D). Fig. 1D also shows co-immunoprecipitation of p38 MAPK with NET, which was increased following cocaine treatment. In addition, there was also an increase in phospho-p38 MAPK immunoreactivity in NET immunoprecipitates isolated from the synaptosomes treated with cocaine (Fig. 1D). These results suggest that p38 MAPK and NET exist in a physical complex and that cocaine enhances this association and activates NET-associated p38 MAPK.
FIGURE 1.
Cocaine-induced NET up-regulation and phosphorylation in the mouse PFC are p38 MAPK-dependent. Mouse PFC synaptosomes were preincubated with vehicle (Veh) or 20 μm PD169316 (PD) at 37 °C for 15 min. Incubations were continued in the presence or absence of 50 μm cocaine (Coc) for 60 min. A, NE uptake. Data derived from three separate experiments, each in triplicate, are given as mean ± S.E. (error bars). * indicates a significant change in NE transport (one-way analysis of variance; Dunnett's test: F(4,44) = 5.31, p < 0.05). B, NET surface biotinylation. Synaptosomes treated with drugs as above were biotinylated, and biotinylated NETs were isolated by avidin binding. Equal aliquots from total (T) and avidin-unbound fractions (UB) and entire eluates from avidin beads representing bound fractions (B) were loaded onto gels, and the blots were probed with commercially available mouse NET monoclonal antibody as described under “Experimental Procedures.” Representative blots show a NET-specific band at ∼85 kDa. Increased avidin-bound NET in cocaine-treated synaptosomes compared with vehicle-, PD169316-, or PD169316 + cocaine-treated synaptosomes is shown. Quantified surface NET densities (normalized to TH levels in the total blot) from three separate experiments are given in the bar graph as mean ± S.E. (error bars). * indicates a significant change from vehicle control (one-way analysis of variance; Dunnett's test: F(4,8) = 4.16, p < 0.05). A TH-reprobed immunoblot shows relative equivalent loading of total protein. C, NET phosphorylation. Following metabolic labeling with 32P, synaptosomes were treated with drugs as described above and solubilized. The synaptosomal extract was subjected to immunoprecipitation with NET-82 polyclonal antibody and autoradiography as described under “Experimental Procedures.” A representative autoradiogram shows enhanced density of phospho-NET following cocaine treatment compared with vehicle, PD169316, or PD169316 + cocaine treatments. Parallel immunoprecipitations (IP) using unlabeled synaptosomes show pulldown of an equal amount of NET following drug treatments as well as no NET protein in the unbound fractions (shown in the immunoblots (IB) below the autoradiogram). Mean values of quantified phospho-NET (p-NET) density (normalized to NET pulldowns from unlabeled synaptosomes) from two experiments are given below in the bar graph. D, NET-p38 MAPK interaction. Synaptosomes treated as indicated were solubilized, and equal aliquots of synaptosome extracts were subjected to pulldown experiment using phospho-p38 MAPK (p-p38MAPK) antibody-immobilized beads or immunoprecipitation with NET antibody followed by SDS-PAGE and immunoblotted with NET, DAT, SERT, or phospho- or total p38 MAPK antibodies. Representative NET-, DAT-, SERT-, and total or phospho-p38 MAPK-immunoreactive bands from two independent experiments are shown. Total extracts subjected to SDS-PAGE and immunoblotting with TH antibody show input levels, and bar graphs below the immunoblots show mean values of quantified phospho-p38 MAPK-associated NET, DAT, and SERT densities (normalized to TH input) or NET-associated phospho-p38 MAPK densities (normalized to TH input) from two experiments. Amph, amphetamine.
In Vivo p38 MAPK Inhibition Blocks Acute Cocaine-induced NET Up-regulation and p38 MAPK Activation in Mice
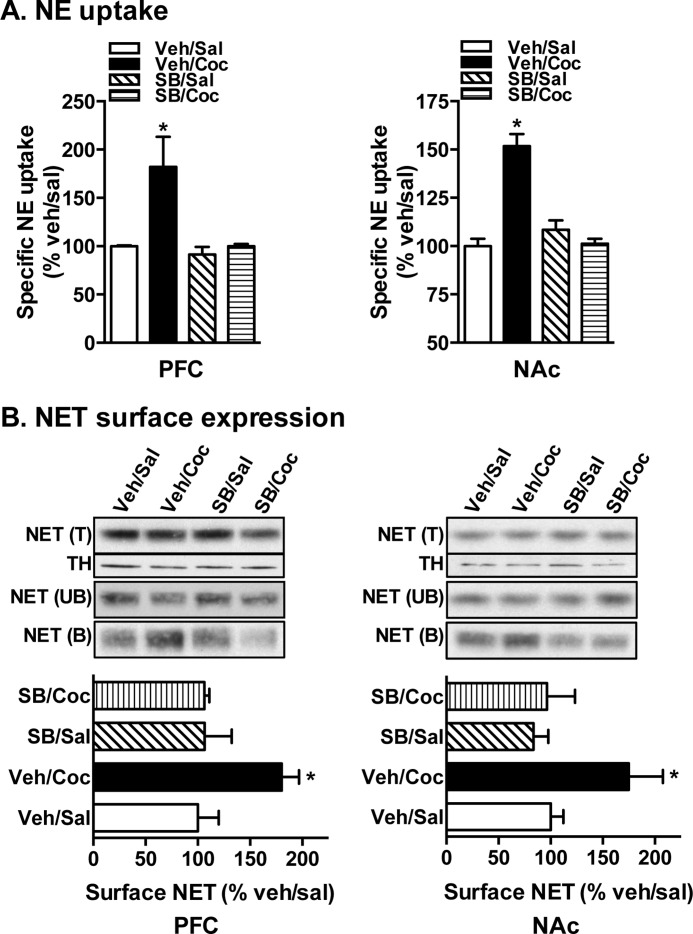
Having found that in vitro treatment with p38 MAPK inhibitor completely blocked cocaine-induced NET up-regulation in PFC synaptosomes, we next tested whether in vivo administration of p38 MAPK inhibitor blocks cocaine-induced NET up-regulation. Similar to the observations made from our previous study (1), NE uptake by both PFC and NAc synaptosomes from acute cocaine (30 mg/kg; 60 min)-administered mice (vehicle/cocaine) was significantly higher compared with that from all other groups (vehicle/saline, SB203580/saline, and SB203580/cocaine) (Fig. 2A). Results from surface biotinylation show that SB203580 injection 15 min prior to cocaine administration blocked the cocaine-induced increase in NET surface expression (Fig. 2B, NET (B)). TH levels show equal loading and use of total protein (Fig. 2B). SB203580 alone had no significant effect on NET function and surface expression (Fig. 2, A and B). Together, these results suggest that cocaine up-regulation of NE transport in the mice is sensitive to in vivo p38 MAPK inhibition and that inhibiting in vivo p38 MAPK blocks cocaine-mediated NET up-regulation.
FIGURE 2.
Acute in vivo p38 MAPK inhibition blocks cocaine-induced NE transport and NET surface expression in the mouse PFC and NAc. Mice were injected with vehicle (Veh) or SB203580 (SB) (50 μg/kg intraperitoneally) 15 min prior to saline (Sal) or cocaine (Coc) (30 mg/kg intraperitoneally) injections. Brains were collected 1 h postinjection for PFC and NAc synaptosomal preparations. A, NE uptake. PFC and NAc synaptosomes were used for NE uptake assays as described under “Experimental Procedures.” NE uptake data derived from three separate experiments, each in triplicate, are given as mean ± S.E. (error bars). * indicates a significant change in NE transport (one-way analysis of variance; Dunnett's test: F(4,44) = 3.09 for PFC, p < 0.05 and F(4,44) = 5.82 for NAc, p < 0.05). B, NET surface biotinylation. Synaptosomes obtained from drug-administered mice were biotinylated, and biotinylated NETs were isolated by avidin binding. Equal aliquots from total (T) and avidin-unbound fractions (UB) and entire eluates from avidin beads representing bound fractions (B) were loaded onto gels, and the blots were probed with commercially available mouse NET monoclonal antibody as described under “Experimental Procedures.” Representative blots show a NET-specific band at ∼85 kDa. Quantified surface NET densities (normalized to TH levels in the total blot) from three separate experiments are given in bar graphs as mean ± S.E. (error bars). * indicates a significant change from vehicle control (one-way analysis of variance; Dunnett's test: F(4,8) = 5.34 for PFC and F(4,8) = 3.96 for NAc, p < 0.05). TH immunoblots corresponding to total are shown for equal protein loading.
In Vivo p38 MAPK Inhibition Attenuates Cocaine-induced Locomotor Sensitization
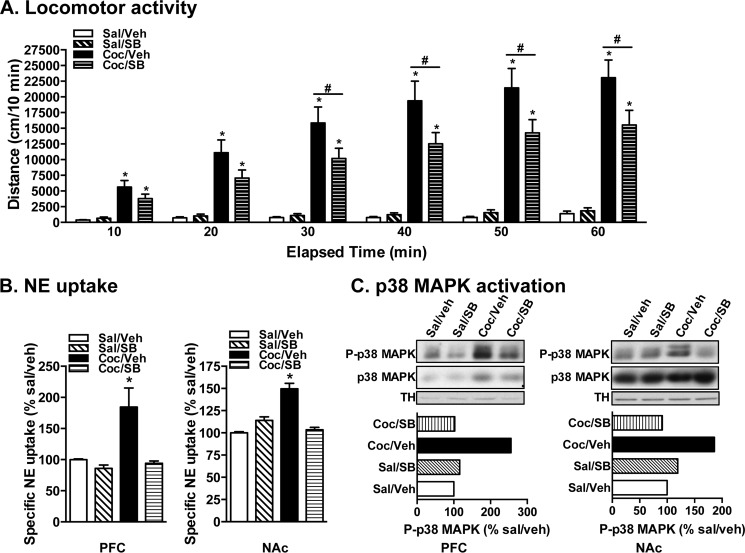
NET is a cocaine target, and because cocaine-induced NET regulation is sensitive to p38 MAPK inhibition, we asked whether cocaine-induced behaviors such as locomotor sensitization and CPP are affected by p38 MAPK inhibition. Fig. 3A shows the locomotor activity of mice injected with saline or cocaine from days 1 to 4 and challenged with 15 mg/kg cocaine on day 9. In this protocol, two groups of mice, one receiving saline on days 1, 2, 3, and 4 and the other receiving cocaine (15 mg/kg on day 1 and 30 mg/kg on days 2, 3, and 4), were used. Locomotor activity was measured on days 1, 2, and 4. On day 9, half of the mice from each group (saline and cocaine) received vehicle or SB203580 prior to cocaine challenge with 15 mg/kg, and the locomotor activity measured on challenge day over a 60-min time period in 10-min bins is given as the distance traveled against time in Fig. 3A. The locomotor activity was higher in the cocaine group compared with the saline group (Fig. 3A), indicating a psychostimulant effect of cocaine. In the saline group, there was no significant difference in the total distance traveled between vehicle (vehicle/saline) and SB203580/saline groups (Fig. 3A). In the cocaine group, cocaine challenge produced significant locomotor sensitization in mice that were injected with vehicle prior to cocaine challenge (Fig. 3A). However, mice that were injected with SB203580 prior to cocaine challenge exhibited significantly lower locomotor sensitization (Fig. 3A). SB203580 alone did not have any significant effect when given prior to cocaine challenge (Fig. 3A). These results suggest that a single injection of SB203580 prior to cocaine challenge can significantly attenuate the locomotor sensitizing effects of cocaine. Immediately following measurement of locomotor sensitization, NE uptake measured using PFC and NAc synaptosomes showed effective blockade of cocaine-mediated NET up-regulation by SB203580 (Fig. 3B). In addition, phospho-p38 MAPK immunoreactivity was higher in the vehicle/cocaine group compared with that in the vehicle/saline group (Fig. 3C). No differences were observed in phospho-p38 MAPK immunoreactivity levels in the SB203580/saline group or the SB203580/cocaine group compared with the vehicle/saline control group (Fig. 3C). In another experiment, mice received SB203580 prior to every cocaine injection from days 1 to 4 as well as prior to cocaine challenge on day 9. In this protocol, SB203580 pretreatment enhanced cocaine-induced locomotor activity on day 1 but produced a blocking effect on days 2, 4, and 9 that was not significant (data not shown). These results demonstrate that cocaine sensitization and NET up-regulation are sensitive to p38 MAPK inhibition by SB203580 and indicate the involvement of cocaine-activated p38 MAPK-dependent NET regulation in cocaine sensitization.
FIGURE 3.
In vivo p38 MAPK inhibition attenuates cocaine-induced locomotor sensitization, NET up-regulation, and p38 MAPK activation in mice. A, locomotor activity. Locomotor activity measured over a 1-h period on challenge day given as 10-min bin data. 50 μg/kg intraperitoneal SB203580 (SB) given prior to cocaine (Coc) challenge significantly blocked cocaine sensitization. * indicates significant changes in locomotor activity (*, p < 0.001 for drug; #, p < 0.001 for time; two-way analysis of variance; Bonferroni's test: F(4,192) = 1086 for drug, p = 0.0001; F(6,192) = 113.4 for time, p = 0.0001; F(16,192) = 32.61 for interaction, p = 0.0001). B, NE uptake. PFC and NAc synaptosomes were used for NE uptake assays as described under “Experimental Procedures.” NE uptake data derived from three separate experiments, each in triplicate, are given as mean ± S.E. (error bars). * indicates a significant change in NE transport (one-way analysis of variance; Dunnett's test: F(4,44) = 3.2 for PFC, p < 0.05; F(4,47) = 5.86 for NAc, p < 0.05). C, p38 MAPK activation. PFC and NAc synaptosomes were solubilized, and equal aliquots of synaptosome extracts were subjected to SDS-PAGE and sequential immunoblotting with phospho-p38 MAPK (p-p38MAPK), total p38 MAPK, or TH antibodies. Representative blots of phospho (P)-p38 MAPK, total (T) p38 MAPK, and TH from two independent experiments are shown. Bar graphs below the immunoblots show mean values of quantified phospho-p38 MAPK densities (normalized to TH input) from two experiments. Veh, vehicle; Sal, saline.
In Vivo p38 MAPK Inhibition Attenuates Cocaine CPP in Mice
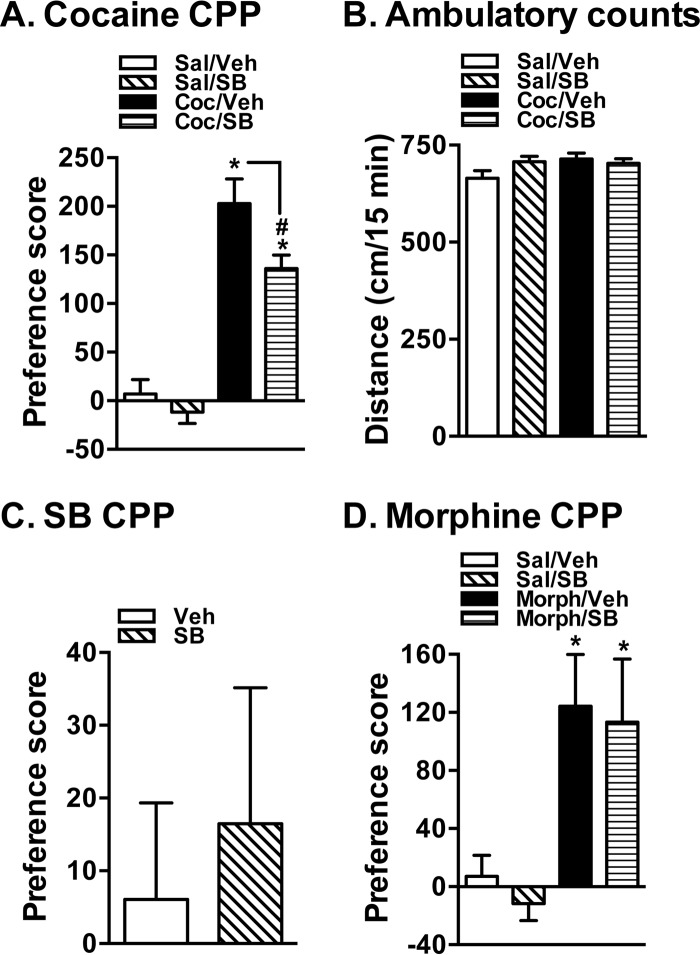
As reported in the literature (41), cocaine produced a significant CPP compared with the saline control group (cocaine/vehicle compared with saline/vehicle) (Fig. 4A). Interestingly, this cocaine CPP was significantly blocked by SB203580 when injected on the postconditioning test day (Fig. 4A). Mice that received SB203580 alone did not exhibit preference to the SB203580-injected side (Fig. 4A). These results suggest that although SB203580 alone exhibits no significant CPP it can significantly attenuate cocaine-induced CPP. Fig. 4B shows that there are no significant changes in the total distance traveled by the mice from all four groups, suggesting that the blockade of cocaine CPP by SB203580 is not due to effects on ambulatory movements in general. Fig. 4C shows that mice receiving SB203580 did not show either enhanced or decreased preference to the drug-injected side compared with vehicle group. Mice that received morphine showed enhanced preference to the drug-injected side compared with saline-injected mice (Fig. 4D). In addition, unlike cocaine CPP, morphine CPP was not affected/blocked by SB203580 (Fig. 4D). These results suggest that although SB203580 by itself does not induce CPP it specifically blocks cocaine CPP.
FIGURE 4.
In vivo p38 MAPK inhibition attenuates cocaine CPP in mice. A, cocaine CPP. Mice received 20 mg/kg intraperitoneal cocaine (Coc) for conditioning and were tested for CPP on day 4 following vehicle (Veh) or SB203580 (SB) injections. The CPP score is given as mean ± S.E. (error bars). * indicates a significant difference in cocaine CPP (one-way analysis of variance; Dunnett's test: F(4,44) = 5.31, p < 0.05). SB203580 (50 μg/kg intraperitoneally) given on test day produced significant blockade of cocaine CPP. # indicates a significant decrease in cocaine CPP following SB203580 injection (Student's t test: t = 2.30, df = 22, p = 0.03). B, ambulatory counts. Ambulatory counts measured over a 15-min CPP test period showed no differences among all four groups. C, SB203580 CPP. Mice received 50 μg/kg intraperitoneal SB203580 for conditioning and were tested for CPP on day 4. The CPP score given as mean ± S.E. (error bars) shows no significant effect (Student's t test: t = 0.46, df = 26, p = 0.65). D, morphine CPP. Mice received 5 mg/kg subcutaneous morphine (Morph) for conditioning and were tested for CPP on day 4 following vehicle or SB203580 injections. The CPP score is given as mean ± S.E. (error bars). * indicates a significant difference in morphine CPP (one-way analysis of variance; Dunnett's test: F(4,43) = 5.97, p < 0.05). SB203580 (50 μg/kg intraperitoneally) given on test day produced no significant blockade of morphine CPP. Sal, saline.
In Vitro Treatment of Mouse PFC Synaptosomes with TAT-NET-Thr30 Peptide Blocks Cocaine-induced NET Up-regulation and Phosphorylation
Our previous study demonstrated that p38 MAPK activation mediates cocaine up-regulation of NET via NET-Thr30 phosphorylation (1). Here we tested the effect of interrupting the Thr30 motif using a TAT peptide strategy. Fig. 5A shows that TAT-NET-Thr30 peptide treatment of mouse PFC synaptosomes 15 min prior to cocaine treatment completely blocked the cocaine-induced increase in NE uptake. A parallel surface biotinylation experiment showed an increase in NET surface expression following cocaine treatment that was also blocked by TAT-NET-Thr30 pretreatment (Fig. 5B). Similarly, cocaine-induced NET phosphorylation was also blocked by pretreatment with TAT-NET-Thr30 peptide (Fig. 5C). TAT-NET-Thr30 peptide alone exhibited either no effect or a slightly inhibitory effect on NE uptake or NET surface expression or phosphorylation (Fig. 5, A, B, and C). The results collectively suggest that TAT-NET-Thr30 peptide interferes with NET-Thr30 phosphorylation and thereby blocks cocaine-induced NET up-regulation.
FIGURE 5.
In vitro treatment of mouse PFC synaptosomes with TAT-NET-Thr30 peptide blocks cocaine-induced NET up-regulation and phosphorylation. Mouse PFC synaptosomes were preincubated with vehicle (Veh) or 10 μm TAT-NET-Thr30 or TAT-NET-T30A at 37 °C for 15 min. Incubations were continued in the presence or absence of 50 μm cocaine (Coc) for 60 min. A, NE uptake. Data derived from three separate experiments, each in triplicate, are given as mean ± S.E. (error bars). * indicates a significant change in NE transport (one-way analysis of variance; Dunnett's test: F(4,32) = 6.16, p < 0.05). B, NET surface biotinylation. Synaptosomes treated as above were biotinylated, and biotinylated NETs were isolated by avidin binding. Equal aliquots from total (T) and avidin-unbound fractions (UB) and entire eluates from avidin beads representing bound fractions (B) were loaded onto gels, and the blots were probed with commercially available mouse NET monoclonal antibody as described under “Experimental Procedures.” Representative blots show a NET-specific band at ∼85 kDa. Quantified surface NET densities (normalized to TH levels in the total blot) from three separate experiments are given in bar graphs as mean ± S.E. (error bars). * indicates a significant change from vehicle control (one-way analysis of variance; Dunnett's test: F(4,8) = 4.21, p < 0.05). TH immunoblots corresponding to total are shown for equal protein loading. C, NET phosphorylation. Following metabolic labeling with 32P, synaptosomes were treated with drugs as described above and solubilized. The synaptosomal extract was subjected to immunoprecipitation with NET-82 polyclonal antibody and autoradiography as described as described under “Experimental Procedures.” A representative autoradiogram (Autorad) from two independent experiments shows phospho-NET (p-NET) bands and enhanced density of phospho-NET following cocaine treatment compared with vehicle, TAT-NET-Thr30, or TAT-NET-Thr30 + cocaine. Parallel immunoprecipitations using unlabeled synaptosomes show pulldown of an equal amount of NET following drug treatments (shown in the immunoblot below the autoradiogram). Mean values of quantified phospho-NET density (normalized to NET pulldowns from unlabeled synaptosomes) from two experiments are given below in the bar graph.
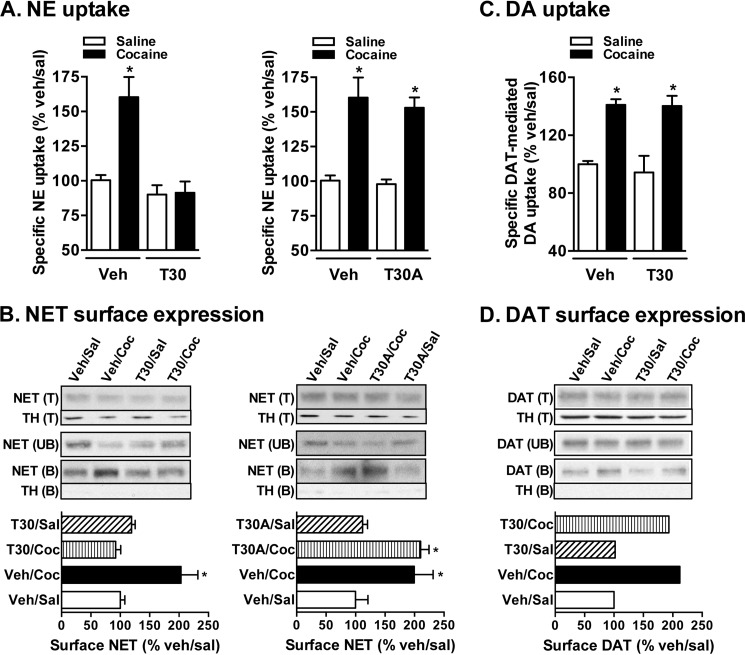
In Vivo TAT-NET-Thr30 Peptide Administration Attenuates Cocaine-induced NET Up-regulation but Not DAT Up-regulation
Based on the above results from the in vitro experiment, it is clear that cocaine up-regulation of NET is dependent on NET-Thr30 phosphorylation. Therefore, next we tested the effect of interrupting the Thr30 site in vivo using a TAT-NET-Thr30 peptide strategy. TAT-conjugated peptides cross the blood-brain barrier and have been shown to accumulate in brain tissues following systemic administration (42). Fig. 6 shows that NE uptake (A) and NET surface expression (B) from cocaine-administered mice (vehicle/cocaine group) were significantly higher compared with those from the vehicle/saline group. These cocaine-induced increases in NE uptake and NET surface expression were not evident in mice that received TAT-NET-Thr30 prior to cocaine (Fig. 6, A and B). Moreover, cocaine-induced NET up-regulation was unaffected in mice that received TAT-NET-T30A prior to cocaine (Fig. 6, A and B). TAT-NET-Thr30 or TAT-NET-T30A alone had no significant effect on NET function or surface expression (Fig. 6, A and B). Corresponding changes in the avidin-unbound fractions show intracellular NET in Fig. 6B. There was a reduction in unbound or intracellular NET protein in vehicle/cocaine and TAT-NET-T30A/cocaine groups (Fig. 6B) compared with the vehicle/saline or vehicle/TAT-NET-Thr30-WT group. The results from both in vitro and in vivo experiments suggest that in vivo manipulation of the NET-Thr30 motif by the TAT-NET-Thr30 peptide strategy can effectively block cocaine-mediated NET up-regulation.
FIGURE 6.
In vivo TAT-NET-Thr30 peptide administration attenuates NET up-regulation by cocaine. Mice were injected with vehicle (Veh), TAT-NET-Thr30, or TAT-NET-T30A (12 mg/kg intravenously via the tail) 15 min prior to saline (Sal) or cocaine (Coc) (30 mg/kg intraperitoneally) injections. Brains were collected 1 h postinjection for PFC synaptosomal preparations. A, NE uptake. NE uptake was measured as described under “Experimental Procedures.” NE uptake data derived from three separate experiments, each in triplicate, are given as mean ± S.E. (error bars). * indicates a significant change in NE transport (one-way analysis of variance; Dunnett's test: F(4,20) = 13.36 for TAT-NET-Thr30 and F(4,20) = 15.31 for TAT-NET-T30A, p < 0.01). B, NET surface biotinylation. PFC synaptosomes obtained from drug-administered mice were biotinylated, and biotinylated NETs were isolated by avidin binding. Equal aliquots from total (T) and avidin-unbound fractions (UB) and entire eluates from avidin beads representing bound fractions (B) were loaded onto gels, and the blots were probed with commercially available mouse NET monoclonal antibody as described under “Experimental Procedures.” Representative blots show a NET-specific band at ∼85 kDa. Quantified surface NET densities (normalized to TH levels in the total blot) from three separate experiments are given in bar graphs as mean ± S.E. (error bars). * indicates a significant change from vehicle control (one-way analysis of variance; Dunnett's test: F(4,8) = 31.67 for TAT-NET-Thr30 and F(4,8) = 23.00 for TAT-NET-T30A, p < 0.05). TH immunoblots corresponding to total are shown for equal protein loading. C, DAT-mediated DA uptake. DAT-mediated DA uptake was measured as described under “Experimental Procedures.” Uptake data derived from three separate experiments, each in triplicate, are given as mean ± S.E. (error bars). * indicates a significant change in DA transport (one-way analysis of variance; Dunnett's test: F(4,28) = 12.65, p < 0.01). D, DAT surface biotinylation. DATs from NAc synaptosomes obtained from drug-administered mice were biotinylated as described above for NET, and avidin-bound (B), total (T), and unbound (UB) fractions were subjected to SDS-PAGE and immunoblotting with DAT antibody. Representative blots show a DAT-specific band at ∼75 kDa. Mean values of quantified surface DAT band densities (normalized to TH levels in the total blot) from two separate experiments are given in the bar graph below.
Because cocaine also up-regulates DAT, we examined the specificity of TAT-NET-Thr30 peptide on cocaine-mediated DAT regulation. DAT-mediated DA uptake and DAT surface expression were measured in NAc synaptosomes from cocaine-administered mice. Similar to previous studies, DAT-mediated DA uptake (Fig. 6C) and DAT surface expression (Fig. 6D) were significantly elevated in the vehicle/cocaine group compared with the vehicle/saline group. These cocaine-induced increases in DA uptake and DAT surface expression remained elevated in mice that received TAT-NET-Thr30 prior to cocaine (Fig. 6, C and D). These results indicate that TAT-NET-Thr30 specifically blocks cocaine-induced NET up-regulation but not DAT up-regulation.
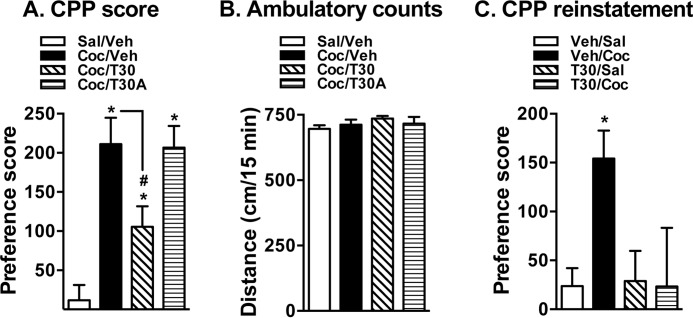
In Vivo TAT-NET-Thr30 Peptide Administration Attenuates Cocaine CPP and Reinstatement
The above results using the TAT-NET peptide strategy suggested a role for NET-Thr30 phosphorylation in cocaine-induced NET up-regulation. Therefore, next we asked whether cocaine-induced behavior such as CPP and reinstatement are also sensitive to manipulation of NET-Thr30 motif. Similar to SB203580 administration, TAT-NET-Thr30 significantly blocked cocaine CPP when injected 15 min prior to the CPP test on postconditioning day (Fig. 7A). Administration of TAT-NET-T30A had no significant effect on cocaine CPP (Fig. 7A). Mice that received TAT-NET-Thr30 or TAT-NET-T30A alone did not show a preference to the peptide-injected side (data not shown). These results suggest that TAT-NET-Thr30 but not the TAT-NET-T30A is able to attenuate cocaine CPP. In addition, Fig. 7B shows that there were no significant changes in the total distance traveled by the mice from all four groups, suggesting that the blockade of cocaine CPP by TAT-NET-Thr30 is not due to effects on ambulatory movements in general. Fig. 7C shows that although saline injection produced no reinstatement cocaine challenge injection produced reinstatement of cocaine CPP. In addition, TAT-NET-Thr30 but not the vehicle injections 15 min prior to cocaine challenge significantly blocked reinstatement of cocaine CPP (Fig. 7C). TAT-NET-Thr30 alone did not elicit CPP reinstatement (Fig. 7C). These results demonstrate the efficacy of TAT-NET-Thr30 in blocking not only cocaine CPP but also CPP reinstatement by cocaine.
FIGURE 7.
In vivo TAT-NET-Thr30 peptide administration attenuates cocaine CPP and reinstatement. A, cocaine CPP. Mice received 20 mg/kg intraperitoneal cocaine (Coc) for conditioning and were tested for CPP on day 4 following vehicle (Veh), TAT-NET-Thr30, or TAT-NET-T30A (12 mg/kg intravenously via the tail) injections. The CPP score is given as mean ± S.E. (error bars). * indicates a significant difference in cocaine CPP (one-way analysis of variance; Dunnett's test: F(4,32) = 5.10–5.18, p < 0.05). # indicates a significant decrease in cocaine CPP following TAT-NET-Thr30 injection (Student's t test: t = 2.66, df = 16, p = 0.02). B, ambulatory counts. Ambulatory counts measured over a 15-min CPP test period showed no differences among all four groups. C, reinstatement. Mice were first tested for cocaine CPP, and following CPP extinction, mice received either vehicle or TAT-NET-Thr30 (12 mg/kg intravenously via the tail) 15 min prior to saline (Sal) or cocaine priming injection (10 mg/kg intraperitoneally) and were tested for preference. The CPP score is given as mean ± S.E. (error bars). * indicates a significant difference in cocaine-induced reinstatement of place preference (one-way analysis of variance; Dunnett's test: F(4,26) = 3.64, p < 0.05).
DISCUSSION
Psychostimulants enhance monoaminergic signaling by interfering with monoamine transporter function. Studies in nonhuman primates support a significant role for NE in the discriminative stimulus effects of cocaine (43). Although earlier studies indicated little evidence for NE playing a primary role in the reinforcing properties of psychomotor stimulants (44–46), more recent studies have documented that NET inhibition can play a significant role in cocaine-induced reinstatement (43). Norepinephrine in the locus coeruleus plays a critical role in arousal (47), attention, memory, mood, and cognition (48). Noradrenergic neurotransmission is terminated by the transport of released NE by the plasma membrane NET (49). Altered cell surface expression of monoamine transporters is a critical mechanism for regulating amine transport, and trafficking of monoamine transporters including that of NET has been shown to occur under basal conditions and following treatment with transporter substrates and inhibitors (51). Given the fact that cocaine is a potent inhibitor of NET, the finding that cocaine up-regulates NET via p38 MAPK-mediated Thr30 phosphorylation suggests that NET up-regulation can affect amine signaling and behavior. The results from the current study provide the first evidence that cocaine-activated p38 MAPK plays an important role in some of the cocaine-induced behaviors by linking NET up-regulation to cocaine sensitization and CPP via a common molecular mechanism, namely NET-Thr30 phosphorylation.
The results from our in vitro experiments demonstrate that cocaine-induced NET up-regulation and phosphorylation are sensitive to p38 MAPK inhibition and NET-Thr30 intervention in the mouse PFC and NAc. All three monoamine transporters are regulated by p38 MAPK (1, 23, 52). In support of this, we found evidence for the association of phospho-p38 MAPK with NET, SERT, and DAT. However, increased NET association following cocaine suggests that cocaine-activated p38 MAPK might be involved in NET-Thr30 phosphorylation either directly or indirectly. However, further studies are warranted to understand the role of NET association with phospho-p38 MAPK. Using in vivo manipulations, we further demonstrated that inhibition of p38 MAPK abolished cocaine-induced NET up-regulation in mice. In vitro, the wild-type TAT-NET-Thr30 peptide but not the phospho-mutant peptide TAT-NET-T30A prevented cocaine-mediated NET up-regulation and phosphorylation. In vivo administration of TAT-NET-Thr30 peptide targeting the Thr30 motif also completely blocked cocaine-induced NET up-regulation. We confirmed the involvement of NET-Thr30 in cocaine-mediated NET up-regulation by testing the specificity of the TAT-NET-Thr30 peptide strategy. DAT-mediated DA uptake was measured in the presence of 100 nm nisoxetine to exclude NET-mediated DA uptake. In vivo TAT-NET-Thr30 administration did not block the cocaine-induced increase in DAT-mediated DA uptake or DAT surface expression in the NAc. The sequence LPEQPLRPCKTADLLVVKERN selected around Thr30 in mouse NET to synthesize TAT-NET-Thr30 has very little homology with the corresponding sequence AKEPNAVGPREVELILVKEQN in mouse DAT. Although both NET and DAT share a high degree of homology, the sequences at the amino and carboxyl termini differ significantly (53, 54). This further supports the idea that mechanisms underlying cocaine-mediated NET up-regulation are transporter- and motif-specific and that Thr30 is a site for the actions of cocaine on NET.
Our results from in vivo experiments show that cocaine-induced behaviors such as locomotor sensitization and CPP were sensitive to inhibition of p38 MAPK, a stress-induced kinase. Cocaine is known to produce stress signals and MAPK activation in cardiomyocytes (55), and it is possible such mechanisms may exist in the brain, contributing to behavioral plasticity. A single injection of SB203580 prior to cocaine significantly blocked cocaine-evoked locomotor sensitization when given prior to cocaine challenge. Importantly, parallel experiments showed that cocaine-mediated NET up-regulation and p38 MAPK activation were sensitive to p38 MAPK inhibition by SB203580. These results suggest centrally mediated effects of SB203580 following systemic administration. Minocycline, an indirect inhibitor of p38 MAPK, is known to attenuate cocaine-induced locomotor sensitization (56) and methamphetamine-induced CPP (57). We found that minocycline (40 mg/kg intraperitoneally) attenuated cocaine CPP (data not shown). These data further support our findings with SB203085 on cocaine behaviors. Cocaine CPP was also sensitive to manipulations of the NET-Thr30 motif by the TAT-NET-Thr30 peptide strategy. TAT-conjugated DAT carboxyl-terminal peptide, which disrupts DAT-Ca2+/calmodulin-dependent protein kinase IIα interaction, attenuates amphetamine-stimulated locomotor activity in mice when administered systemically (38). These studies indicate that TAT peptides effectively reach brain regions following systemic administration and elicit their effects on psychostimulant-mediated behaviors. It is well known that cocaine produces locomotor activation and sensitization in rodents. Behavioral sensitization to cocaine reflects neuroadaptive changes that intensify drug effects. Repeated use of addictive drugs produces incremental neuroadaptations in the limbic neural system, rendering it increasingly hypersensitive to drugs. Because NET is one of the known targets for cocaine, our observations suggest that Thr30-dependent NET regulation plays a role in cocaine-induced behaviors.
Enhanced cocaine CPP in NET KO mice (58) suggests a role for NET in cocaine reward and perhaps in the NAc. The NAc plays a crucial role in cocaine reward and reinforcement (59, 60), and the PFC is considered to be a critical area for the development and maintenance of CPP (61, 62). A specific lesion of the prelimbic medial PFC was sufficient to block cocaine-induced CPP (62, 63). Furthermore, noradrenergic depletion of PFC attenuates cocaine CPP (64). At the transporter level, NET mediates DA clearance in the PFC (3–8), underscoring the importance of NET function in controlling DA signaling. Thus, our finding that p38 MAPK inhibition or NET-Thr30 intervention can attenuate cocaine CPP indicates a role for NET regulation in the PFC and in cocaine-reinforcing effects. It is also known that PFC plays a critical role in cue-elicited and cocaine-primed reinstatement of extinguished cocaine-seeking behavior in rodents. In this regard, TAT-NET-Thr30 when given prior to cocaine priming completely abolished reinstatement of cocaine CPP, further supporting a role for NET regulation in cocaine-induced behaviors.
Our observation that inhibition of p38 MAPK or intervention of NET-Thr30 phosphorylation attenuates cocaine sensitization and CPP suggests that NET up-regulation represents one of the neurobiological mechanisms contributing to the altered NE or DA signaling seen in cocaine-reinforcing effects. It is known that cocaine binds to monoamine transporters and blocks reuptake of monoamines, resulting in increased synaptic amines. Increased DA tone is an established theory behind many models of drug addiction (65–67). However, a recent study highlights the key role of noradrenergic autoreceptor signaling in the persistent modifications induced by repeated drug administration (68). NE terminals can release DA, perhaps under conditions where dopamine β-hydroxylase located in vesicles of NE terminals is saturated (69). A recent study showed that either a decrease in tyrosine hydroxylase expression in the locus ceruleus or blockade of NET prevents the DA-mediated response, indicating that locus ceruleus terminals can release both NE and DA and the importance of functional NET (70). Thus, the cocaine-mediated NET up-regulation has implications considering the evidence that psychostimulants induce locomotor hyperactivity not only because they increase DA transmission but also because they release NE.
Cocaine is known to up-regulate both NET and DAT (1, 71–73). Although the signals or the mechanisms involved in DAT up-regulation have not been identified, our earlier study demonstrated that cocaine-activated p38 MAPK and Thr30 phosphorylation play a role in cocaine-mediated NET up-regulation (1). Together, the present study furthers our understanding of the role of this cocaine-mediated NET regulation in cocaine behavior. Dopamine signaling in the mesolimbic system is a known player in drug addiction (65–67) and is largely controlled by the DA transporter (74, 75). Abolished cocaine reward in mice constitutively expressing cocaine-insensitive DAT has been reported (41). Several neuronal substrates in the mesolimbic reward pathway have been identified, and their involvement and/or requirement have been demonstrated in cocaine- and other psychostimulant-associated addictive behaviors (76, 77). It is possible that although cocaine binding to DAT may be necessary to initiate cocaine reward subsequent alterations in DA signals may lead to modulation of other neuronal substrates including NET regulation, which in collaboration may evoke cocaine-associated behaviors. In addition, it is now clear that multiple DA brain regions are implicated in producing different aspects of cocaine reward, which may be attributed by the differences in DAT expression levels in different brain regions (78, 79). NET expression is localized to noradrenergic terminals where it facilitates transport of NE and DA (80, 81). Importantly, DA is a better substrate for NET, and NET plays a critical role in DA clearance in brain regions where DAT levels are low. In addition, NET also plays a role in striatal DA clearance in the absence of DA innervation (50, 82). Cocaine up-regulation of NET is evident in both the PFC and NAc, which are important brain regions in the mesolimbic reward pathway. This suggests that cocaine-induced NET-Thr30 phosphorylation and regulation in these areas may serve as one of the neural substrates in mediating cocaine behavioral effects. Thus, it is possible that multiple brain regions and circuits are involved in a phenomenon as complex as cocaine reward and reinstatement and that the neuroadaptations including NET up-regulation by cocaine may be one of the mechanisms contributing to the altered NE/DA signaling seen in psychostimulant abuse. In this regard, future investigations using intracranial infusions of p38 MAPK inhibitors and TAT-NET peptides are warranted to delineate brain region-specific involvement of NET regulation in cocaine-induced behaviors.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-GM081054 (to L. D. J.) and R01-MH083928 (to S. R.). This work was also supported by start-up funds from Virginia Commonwealth University (to L. D. J. and S. R.).
- NET
- norepinephrine transporter
- CPP
- conditioned place preference
- PFC
- prefrontal cortex
- NAc
- nucleus accumbens
- NE
- norepinephrine
- DA
- dopamine
- DAT
- dopamine transporter
- SERT
- serotonin transporter
- TH
- tyrosine hydroxylase
- TAT
- transactivator of transcription.
REFERENCES
- 1. Mannangatti P., Arapulisamy O., Shippenberg T. S., Ramamoorthy S., Jayanthi L. D. (2011) Cocaine up-regulation of the norepinephrine transporter requires threonine 30 phosphorylation by p38 mitogen-activated protein kinase. J. Biol. Chem. 286, 20239–20250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu F., Gainetdinov R. R., Wetsel W. C., Jones S. R., Bohn L. M., Miller G. W., Wang Y. M., Caron M. G. (2000) Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat. Neurosci. 3, 465–471 [DOI] [PubMed] [Google Scholar]
- 3. Carboni E., Tanda G. L., Frau R., Di Chiara G. (1990) Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J. Neurochem. 55, 1067–1070 [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto B. K., Novotney S. (1998) Regulation of extracellular dopamine by the norepinephrine transporter. J. Neurochem. 71, 274–280 [DOI] [PubMed] [Google Scholar]
- 5. Morón J. A., Brockington A., Wise R. A., Rocha B. A., Hope B. T. (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J. Neurosci. 22, 389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valentini V., Frau R., Di Chiara G. (2004) Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: differences with mianserin and clozapine. J. Neurochem. 88, 917–927 [DOI] [PubMed] [Google Scholar]
- 7. Carboni E., Silvagni A., Vacca C., Di Chiara G. (2006) Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J. Neurochem. 96, 473–481 [DOI] [PubMed] [Google Scholar]
- 8. Siuta M. A., Robertson S. D., Kocalis H., Saunders C., Gresch P. J., Khatri V., Shiota C., Kennedy J. P., Lindsley C. W., Daws L. C., Polley D. B., Veenstra-Vanderweele J., Stanwood G. D., Magnuson M. A., Niswender K. D., Galli A. (2010) Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS Biol. 8, e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barker E. L., Blakely R. D. (1995) in Psychopharmacology: the Fourth Generation of Progress (Bloom F. E., Kupfer D. J., eds) pp. 321–333, Raven Press, New York [Google Scholar]
- 10. Kaye D. M., Wiviott S. D., Kobzik L., Kelly R. A., Smith T. W. (1997) S-Nitrosothiols inhibit neuronal norepinephrine transport. Am. J. Physiol. Heart Circ. Physiol. 272, H875–H883 [DOI] [PubMed] [Google Scholar]
- 11. Apparsundaram S., Galli A., DeFelice L. J., Hartzell H. C., Blakely R. D. (1998) Acute regulation of norepinephrine transport: I. PKC-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J. Pharmacol. Exp. Ther. 287, 733–743 [PubMed] [Google Scholar]
- 12. Malhotra A., Begley R., Kang B. P., Rana I., Liu J., Yang G., Mochly-Rosen D., Meggs L. G. (2005) PKC-ϵ-dependent survival signals in diabetic hearts. Am. J. Physiol. Heart Circ. Physiol. 289, H1343–H1350 [DOI] [PubMed] [Google Scholar]
- 13. Murphy S., Frishman W. H. (2005) Protein kinase C in cardiac disease and as a potential therapeutic target. Cardiol. Rev. 13, 3–12 [DOI] [PubMed] [Google Scholar]
- 14. Olive M. F., McGeehan A. J., Kinder J. R., McMahon T., Hodge C. W., Janak P. H., Messing R. O. (2005) The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase Cϵ-dependent mechanism. Mol. Pharmacol. 67, 349–355 [DOI] [PubMed] [Google Scholar]
- 15. Olive M. F., Messing R. O. (2004) Protein kinase C isozymes and addiction. Mol. Neurobiol. 29, 139–154 [DOI] [PubMed] [Google Scholar]
- 16. Gurguis G. N., Vo S. P., Griffith J. M., Rush A. J. (1999) Platelet α2A-adrenoceptor function in major depression: Gi coupling, effects of imipramine and relationship to treatment outcome. Psychiatry Res. 89, 73–95 [DOI] [PubMed] [Google Scholar]
- 17. Jayanthi L. D., Samuvel D. J., Ramamoorthy S. (2004) Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J. Biol. Chem. 279, 19315–19326 [DOI] [PubMed] [Google Scholar]
- 18. Jayanthi L. D., Annamalai B., Samuvel D. J., Gether U., Ramamoorthy S. (2006) Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J. Biol. Chem. 281, 23326–23340 [DOI] [PubMed] [Google Scholar]
- 19. Valjent E., Herve D., Girault J. A. (2005) Drugs of abuse, protein phosphatases, and ERK pathway. Med. Sci. 21, 453–454 [DOI] [PubMed] [Google Scholar]
- 20. Nestler E. J., Berhow M. T., Brodkin E. S. (1996) Molecular mechanisms of drug addiction: adaptations in signal transduction pathways. Mol. Psychiatry 1, 190–199 [PubMed] [Google Scholar]
- 21. Li G., Xiao Y., Zhang L. (2005) Cocaine induces apoptosis in fetal rat myocardial cells through the p38 mitogen-activated protein kinase and mitochondrial/cytochrome c pathways. J. Pharmacol. Exp. Ther. 312, 112–119 [DOI] [PubMed] [Google Scholar]
- 22. Choe E. S., Wang J. Q. (2002) CaMKII regulates amphetamine-induced ERK1/2 phosphorylation in striatal neurons. Neuroreport 13, 1013–1016 [DOI] [PubMed] [Google Scholar]
- 23. Samuvel D. J., Jayanthi L. D., Bhat N. R., Ramamoorthy S. (2005) A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J. Neurosci. 25, 29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samuvel D. J., Jayanthi L. D., Manohar S., Kaliyaperumal K., See R. E., Ramamoorthy S. (2008) Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: evidence for differential regulation in caudate putamen and nucleus accumbens. J. Pharmacol. Exp. Ther. 325, 293–301 [DOI] [PubMed] [Google Scholar]
- 25. Arapulisamy O., Mannangatti P., Jayanthi L. D. (2013) Regulated norepinephrine transporter interaction with the neurokinin-1 receptor establishes transporter subcellular localization. J. Biol. Chem. 288, 28599–28610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tejeda H. A., Counotte D. S., Oh E., Ramamoorthy S., Schultz-Kuszak K. N., Bäckman C. M., Chefer V., O'Donnell P., Shippenberg T. S. (2013) Prefrontal cortical κ-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38, 1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kivell B., Uzelac Z., Sundaramurthy S., Rajamanickam J., Ewald A., Chefer V., Jaligam V., Bolan E., Simonson B., Annamalai B., Mannangatti P., Prisinzano T. E., Gomes I., Devi L. A., Jayanthi L. D., Sitte H. H., Ramamoorthy S., Shippenberg T. S. (2014) Salvinorin A regulates dopamine transporter function via a κ opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology 86, 228–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matthies H. J., Han Q., Shields A., Wright J., Moore J. L., Winder D. G., Galli A., Blakely R. D. (2009) Subcellular localization of the antidepressant-sensitive norepinephrine transporter. BMC Neurosci. 10, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramamoorthy S., Giovanetti E., Qian Y., Blakely R. D. (1998) Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J. Biol. Chem. 273, 2458–2466 [DOI] [PubMed] [Google Scholar]
- 30. Marquez P., Hamid A., Lutfy K. (2013) The role of NOP receptors in psychomotor stimulation and locomotor sensitization induced by cocaine and amphetamine in mice. Eur. J. Pharmacol. 707, 41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu C. B., Lindler K. M., Owens A. W., Daws L. C., Blakely R. D., Hewlett W. A. (2010) Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35, 2510–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kota D., Martin B. R., Robinson S. E., Damaj M. I. (2007) Nicotine dependence and reward differ between adolescent and adult male mice. J. Pharmacol. Exp. Ther. 322, 399–407 [DOI] [PubMed] [Google Scholar]
- 33. Jackson K. J., Sanjakdar S. S., Chen X., Damaj M. I. (2012) Nicotine reward and affective nicotine withdrawal signs are attenuated in calcium/calmodulin-dependent protein kinase IV knockout mice. PLoS One 7, e51154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jackson K. J., Wang J. B., Barbier E., Chen X., Damaj M. I. (2012) Acute behavioral effects of nicotine in male and female HINT1 knockout mice. Genes Brain Behav. 11, 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ignatowska-Jankowska B. M., Muldoon P. P., Lichtman A. H., Damaj M. I. (2013) The cannabinoid CB2 receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology 229, 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jackson K. J., McLaughlin J. P., Carroll F. I., Damaj M. I. (2013) Effects of the κ opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology 226, 763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fawell S., Seery J., Daikh Y., Moore C., Chen L. L., Pepinsky B., Barsoum J. (1994) Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. U.S.A. 91, 664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rickhag M., Owens W. A., Winkler M. T., Strandfelt K. N., Rathje M., Sørensen G., Andresen B., Madsen K. L., Jørgensen T. N., Wörtwein G., Woldbye D. P., Sitte H., Daws L. C., Gether U. (2013) Membrane-permeable C-terminal dopamine transporter peptides attenuate amphetamine-evoked dopamine release. J. Biol. Chem. 288, 27534–27544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Itzhak Y., Anderson K. L. (2012) Changes in the magnitude of drug-unconditioned stimulus during conditioning modulate cocaine-induced place preference in mice. Addict. Biol. 17, 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Conrad K. L., Louderback K. M., Milano E. J., Winder D. G. (2013) Assessment of the impact of pattern of cocaine dosing schedule during conditioning and reconditioning on magnitude of cocaine CPP, extinction, and reinstatement. Psychopharmacology 227, 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen R., Tilley M. R., Wei H., Zhou F., Zhou F. M., Ching S., Quan N., Stephens R. L., Hill E. R., Nottoli T., Han D. D., Gu H. H. (2006) Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc. Natl. Acad. Sci. U.S.A. 103, 9333–9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brebner K., Wong T. P., Liu L., Liu Y., Campsall P., Gray S., Phelps L., Phillips A. G., Wang Y. T. (2005) Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310, 1340–1343 [DOI] [PubMed] [Google Scholar]
- 43. Platt D. M., Rowlett J. K., Spealman R. D. (2007) Noradrenergic mechanisms in cocaine-induced reinstatement of drug seeking in squirrel monkeys. J. Pharmacol. Exp. Ther. 322, 894–902 [DOI] [PubMed] [Google Scholar]
- 44. Kleven M. S., Woolverton W. L. (1990) Effects of bromocriptine and desipramine on behavior maintained by cocaine or food presentation in rhesus monkeys. Psychopharmacology 101, 208–213 [DOI] [PubMed] [Google Scholar]
- 45. Tella S. R. (1995) Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol. Biochem. Behav. 51, 687–692 [DOI] [PubMed] [Google Scholar]
- 46. Mello N. K., Lukas S. E., Bree M. P., Mendelson J. H. (1990) Desipramine effects on cocaine self-administration by rhesus monkeys. Drug Alcohol Depend. 26, 103–116 [DOI] [PubMed] [Google Scholar]
- 47. Astier B., Van Bockstaele E. J., Aston-Jones G., Pieribone V. A. (1990) Anatomical evidence for multiple pathways leading from the rostral ventrolateral medulla (nucleus paragigantocellularis) to the locus coeruleus in rat. Neurosci. Lett. 118, 141–146 [DOI] [PubMed] [Google Scholar]
- 48. Aston-Jones G., Rajkowski J., Cohen J. (1999) Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 46, 1309–1320 [DOI] [PubMed] [Google Scholar]
- 49. Axelrod J. (1971) Noradrenaline: fate and control of its biosynthesis. Science 173, 598–606 [DOI] [PubMed] [Google Scholar]
- 50. Jeannotte A. M., McCarthy J. G., Sidhu A. (2009) Desipramine induced changes in the norepinephrine transporter, α- and γ-synuclein in the hippocampus, amygdala and striatum. Neurosci. Lett. 467, 86–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramamoorthy S., Shippenberg T. S., Jayanthi L. D. (2011) Regulation of monoamine transporters: role of transporter phosphorylation. Pharmacol. Ther. 129, 220–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morón J. A., Zakharova I., Ferrer J. V., Merrill G. A., Hope B., Lafer E. M., Lin Z. C., Wang J. B., Javitch J. A., Galli A., Shippenberg T. S. (2003) Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J. Neurosci. 23, 8480–8488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fritz J. D., Jayanthi L. D., Thoreson M. A., Blakely R. D. (1998) Cloning and chromosomal mapping of the murine norepinephrine transporter. J. Neurochem. 70, 2241–2251 [DOI] [PubMed] [Google Scholar]
- 54. Brüss M., Wieland A., Bönisch H. (1999) Molecular cloning and functional expression of the mouse dopamine transporter. J. Neural Transm. 106, 657–662 [DOI] [PubMed] [Google Scholar]
- 55. Fan L., Sawbridge D., George V., Teng L., Bailey A., Kitchen I., Li J. M. (2009) Chronic cocaine-induced cardiac oxidative stress and mitogen-activated protein kinase activation: the role of Nox2 oxidase. J. Pharmacol. Exp. Ther. 328, 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen H., Uz T., Manev H. (2009) Minocycline affects cocaine sensitization in mice. Neurosci. Lett. 452, 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fujita Y., Kunitachi S., Iyo M., Hashimoto K. (2012) The antibiotic minocycline prevents methamphetamine-induced rewarding effects in mice. Pharmacol. Biochem. Behav. 101, 303–306 [DOI] [PubMed] [Google Scholar]
- 58. Hall F. S., Li X. F., Sora I., Xu F., Caron M., Lesch K. P., Murphy D. L., Uhl G. R. (2002) Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience 115, 153–161 [DOI] [PubMed] [Google Scholar]
- 59. Cornish J. L., Kalivas P. W. (2001) Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J. Addict. Dis. 20, 43–54 [DOI] [PubMed] [Google Scholar]
- 60. Smith R. J., Lobo M. K., Spencer S., Kalivas P. W. (2013) Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr. Opin. Neurobiol. 23, 546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sorg B. A., Davidson D. L., Kalivas P. W., Prasad B. M. (1997) Repeated daily cocaine alters subsequent cocaine-induced increase of extracellular dopamine in the medial prefrontal cortex. J. Pharmacol. Exp. Ther. 281, 54–61 [PubMed] [Google Scholar]
- 62. Tzschentke T. M., Schmidt W. J. (1998) Discrete quinolinic acid lesions of the rat prelimbic medial prefrontal cortex affect cocaine- and MK-801-, but not morphine- and amphetamine-induced reward and psychomotor activation as measured with the place preference conditioning paradigm. Behav. Brain Res. 97, 115–127 [DOI] [PubMed] [Google Scholar]
- 63. Tzschentke T. M., Schmidt W. J. (1999) Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur. J. Neurosci. 11, 4099–4109 [DOI] [PubMed] [Google Scholar]
- 64. Pum M. E., Carey R. J., Huston J. P., Müller C. P. (2008) Role of medial prefrontal, entorhinal, and occipital 5-HT in cocaine-induced place preference and hyperlocomotion: evidence for multiple dissociations. Psychopharmacology 201, 391–403 [DOI] [PubMed] [Google Scholar]
- 65. White F. J., Kalivas P. W. (1998) Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 51, 141–153 [DOI] [PubMed] [Google Scholar]
- 66. Volkow N. D., Wang G. J., Telang F., Fowler J. S., Logan J., Childress A. R., Jayne M., Ma Y., Wong C. (2006) Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 26, 6583–6588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kalivas P. W. (2007) Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am. J. Addict. 16, 71–78 [DOI] [PubMed] [Google Scholar]
- 68. Doucet E. L., Bobadilla A. C., Houades V., Lanteri C., Godeheu G., Lanfumey L., Sara S. J., Tassin J. P. (2013) Sustained impairment of α2A-adrenergic autoreceptor signaling mediates neurochemical and behavioral sensitization to amphetamine. Biol. Psychiatry 74, 90–98 [DOI] [PubMed] [Google Scholar]
- 69. Devoto P., Flore G., Saba P., Fà M., Gessa G. L. (2005) Co-release of noradrenaline and dopamine in the cerebral cortex elicited by single train and repeated train stimulation of the locus coeruleus. BMC Neurosci. 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Smith J. S., Schindler A. G., Martinelli E., Gustin R. M., Bruchas M. R., Chavkin C. (2012) Stress-induced activation of the dynorphin/κ-opioid receptor system in the amygdala potentiates nicotine conditioned place preference. J. Neurosci. 32, 1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ding Y. S., Singhal T., Planeta-Wilson B., Gallezot J. D., Nabulsi N., Labaree D., Ropchan J., Henry S., Williams W., Carson R. E., Neumeister A., Malison R. T. (2010) PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S,S)-[11C]O-methylreboxetine and HRRT. Synapse 64, 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang L., Elmer L. W., Little K. Y. (1998) Expression and regulation of the human dopamine transporter in a neuronal cell line. Mol. Brain Res. 59, 66–73 [DOI] [PubMed] [Google Scholar]
- 73. Daws L. C., Callaghan P. D., Moron J. A., Kahlig K. M., Shippenberg T. S., Javitch J. A., Galli A. (2002) Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem. Biophys. Res. Commun. 290, 1545–1550 [DOI] [PubMed] [Google Scholar]
- 74. Cao C. J., Shamoo A. E., Eldefrawi M. E. (1990) Cocaine-sensitive, ATP-dependent dopamine uptake into striatal synaptosomes. Biochem. Pharmacol. 39, R9–R14 [DOI] [PubMed] [Google Scholar]
- 75. Shimada S., Kitayama S., Lin C. L., Patel A., Nanthakumar E., Gregor P., Kuhar M., Uhl G. (1991) Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science 254, 576–578 [DOI] [PubMed] [Google Scholar]
- 76. Kalivas P. W., Volkow N. D. (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol. Psychiatry 16, 974–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Volkow N. D., Wang G. J., Fowler J. S., Tomasi D., Telang F. (2011) Addiction: beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. U.S.A. 108, 15037–15042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Steinkellner T., Mus L., Eisenrauch B., Constantinescu A., Leo D., Konrad L., Rickhag M., Sørensen G., Efimova E. V., Kong E., Willeit M., Sotnikova T. D., Kudlacek O., Gether U., Freissmuth M., Pollak D. D., Gainetdinov R. R., Sitte H. H. (2014) In vivo amphetamine action is contingent on αCaMKII. Neuropsychopharmacology 39, 2681–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. O'Neill B., Tilley M. R., Han D. D., Thirtamara-Rajamani K., Hill E. R., Bishop G. A., Zhou F. M., During M. J., Gu H. H. (2014) Behavior of knock-in mice with a cocaine-insensitive dopamine transporter after virogenetic restoration of cocaine sensitivity in the striatum. Neuropharmacology 79, 626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Amara S. G., Kuhar M. J. (1993) Neurotransmitter transporters: recent progress. Annu. Rev. Neurosci. 16, 73–93 [DOI] [PubMed] [Google Scholar]
- 81. Borgkvist A., Mrejeru A., Sulzer D. (2011) Multiple personalities in the ventral tegmental area. Neuron 70, 803–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Arai A., Tomiyama M., Kannari K., Kimura T., Suzuki C., Watanabe M., Kawarabayashi T., Shen H., Shoji M. (2008) Reuptake of L-DOPA-derived extracellular DA in the striatum of a rodent model of Parkinson's disease via norepinephrine transporter. Synapse 62, 632–635 [DOI] [PubMed] [Google Scholar]