Abstract
Lipoprotein (a) [Lp(a)] is a highly atherogenic lipid particle. Although earlier reports suggested that Lp(a) levels are mostly determined by genetic factors, several recent studies have revealed that Lp(a) induction is also caused by chronic inflammation. Therefore, we aimed to examine whether cytokine blockade by monoclonal antibodies may inhibit Lp(a) metabolism. We found that interleukin 6 (IL-6) blockade by tocilizumab (TCZ) reduced Lp(a) while TNF-α-inhibition by adalimumab in humans had no effect. The specificity of IL-6 in regulating Lp(a) was further demonstrated by serological measurements of human subjects (n = 1,153) revealing that Lp(a) levels are increased in individuals with elevated serum IL-6. Transcriptomic analysis of human liver biopsies (n = 57) revealed typical IL-6 response genes being correlated with the LPA gene expression in vivo. On a molecular level, we found that TCZ inhibited IL-6-induced LPA mRNA and protein expression in human hepatocytes. Furthermore, examination of IL-6-responsive signal transducer and activator of transcription 3 binding sites within the LPA promoter by reporter gene assays, promoter deletion experiments, and electrophoretic mobility shift assay analysis showed that the Lp(a)-lowering effect of TCZ is specifically mediated via a responsive element at −46 to −40. Therefore, IL-6 blockade might be a potential therapeutic option to treat elevated Lp(a) serum concentrations in humans and might be a noninvasive alternative to lipid apheresis in the future.
Keywords: interleukin, acute phase response, inflammation, therapy of elevated Lp(a), lipoprotein metabolism
Lipoprotein (a) [Lp(a)], first discovered by Kåre Berg in 1963 (1), is a unique lipoprotein being formed in the liver through the extracellular association of an apoB-100-containing lipoprotein to a highly glycosylated apo(a) (official gene symbol: LPA) (2). Apo(a) displays a high homology to plasminogen and has been shown to inhibit fibrinolysis (3). Thus, Lp(a) combines atherosclerosis and local thrombosis, and therefore elevated serum levels are considered a severe and independent risk factor for the development of CVD (4).
Studies have demonstrated apo(a) to be genetically polymorphic (2) with apo(a) isoproteins ranging in approximate size from 200 to 800 kDa (5). These different apo(a) phenotypes are thought to importantly determine the rates of hepatic synthesis of apo(a) and thus Lp(a) serum concentrations with an inverse correlation between the two parameters (6–8).
While earlier studies suggested that Lp(a) serum levels are mostly determined by genetic factors (7, 8), several recent reports indicate that Lp(a) is also induced by mediators of the innate immune system (9–11). In that respect, it is important to mention that in humans several chronic inflammatory diseases (CIDs) such as rheumatoid arthritis (RA) and Crohn’s disease are associated with elevated Lp(a) serum levels (9, 10), which might be responsible for the increased cardiovascular risk found in such subjects (10). Also, sepsis as a model for acute activation of the innate immune system is associated with alterations of Lp(a) metabolism in humans (12). Moreover, there is evidence that Lp(a) levels also increase with other conditions such as surgery or myocardial infarction (13), all possibly being associated with induction of the innate immune system.
In 2009, a monoclonal antibody against the interleukin 6 (IL-6) receptor, referred to as tocilizumab (TCZ), was approved for the treatment of RA in Europe. In a recent clinical study of our group, we have shown that TCZ lowers Lp(a) serum levels in RA patients by up to 50% (14). This was confirmed recently in an independent cohort (15). Due to this finding, one may consider modern anti-cytokine therapies as a new promising approach for lowering Lp(a) levels in affected patients as an alternative to the much more invasive lipid apheresis. The aim of the present study was to examine whether the Lp(a)-lowering effect is specific to TCZ or whether the much more commonly used anti-TNF-α antibodies [e.g., adalimumab (ADB)] exert comparable effects. Furthermore, we aimed to identify the molecular mechanisms by which an anti-cytokine therapy influences serum Lp(a) concentrations in order to provide a comprehensive pathophysiological concept for potential future clinical trials on monoclonal anticytokine antibodies as treatment options for human subjects with elevated Lp(a) serum concentrations and progressive CVD.
MATERIALS AND METHODS
The study was approved by the local ethics committees before the commencement of the study. All patients provided written, informed consent.
Study populations
TNF-α inhibition study population.
Sera of 12 RA patients were taken before and 3 months after anti-TNF-α therapy in order to study the influence of this intervention on serum Lp(a) levels. This study design is comparable to our TCZ study published in 2010 (14). Mean weight, BMI, age, and gender distribution of the treated subjects included in the study population were 78.3 ± 4.7 kg, 25.1 ± 1.3 kg/m2, 52.1 ± 4.4 years, and 52.9% male subjects, respectively.
Liver biopsy study population.
The liver biopsy cohort was described recently in a different research project (16). The subjects did not suffer from any CIDs but had a BMI ≥28 kg/m2.
FoCus cohort study population.
As part of the Food Chain Plus (FoCus) project (http://www.focus.uni-kiel.de), human subjects (n = 1,153) were examined in order to relate Lp(a) serum levels to several inflammatory and metabolic parameters. Of the human subjects (n = 1,153), 500 were enrolled from the obesity outpatient clinic of the Department of Internal Medicine I of the University of Kiel. The remaining 653 subjects were recruited from the regional registration offices as cross-sectional controls. Basic characteristics of the study cohort (mean + SEM) were as follows: BMI, 31.96 + 0.31 kg/m2; HOMA-IR, 5.28 + 0.31; CRP, 5.02 + 0.25 mg/l; IL-6, 4.65 + 0.24 pg/ml; Lp(a), 224.00 + 6.83 mg/l; TG, 132.60 + 2.92 mg/dl; gender distribution, 34.69% male; and mean age, 51.82 + 0.43 years.
In the statistical analysis, IL-6 cutoff (6 pg/ml) was chosen according to the reference ranges being established by the Institute of Clinical Chemistry of the University Medical Center in Kiel. C-reactive protein (CRP) cutoff was set at 3.0 mg/l according to defined risk groups of CRP by the American Heart Association and the US Centers for Disease Control and Prevention.
Reagents for in vitro experiments
TCZ (RoActemra®, Roche Group; 20 mg/ml) and ADB (Humira®, Abbott Laboratories; 50 mg/ml) stock solutions were applied at final concentrations of 100 µg/ml and 100 ng/ml, respectively, in all in vitro experiments. Concentrations were chosen according to preliminary experiments in which both agents have been shown not to be toxic to our cells and sufficient to suppress their target (TNF-α, IL-6) effects.
Cell culture
For luciferase reporter gene assays, human hepato cellular carcinoma cells (HepG2) cells were maintained in RPMI1640 medium supplemented with 10% FCS, 2 mM l-glutamine, and 1% penicillin/streptomycin at 5% CO2 and 37°C. For quantitative real-time RT-PCR, electrophoretic mobility shift assay (EMSA), and Western blotting experiments, human hepato cellular carcinoma cells (Huh7), were maintained in DMEM with high glucose (4.5 g/l), supplemented with 2 mM l-glutamine, 10% FCS, and 1% penicillin/streptomycin (all from PAA) at 5% CO2 and 37°C.
RNA isolation and quantitative real-time RT-PCR
RNA of Huh7 cells was isolated using peqGOLD Total RNA Kit (12-6834-01, PEQLAB Biotechnologie GmbH) following the manufacturer’s instructions. cDNA synthesis was further generated using Maxima First Strand cDNA Synthesis Kit for RT-qPCR (K1641, Thermo Fisher Scientific) according to the manufacturer’s instructions. Expression levels of LPA and housekeeping gene β-actin (supplementary Table 1) were determined using specific primers and Maxima SYBR Green/Fluorescein qPCR Master Mix (K0241, Thermo Fisher Scientific). Primer sequences were designed with Primer3 standard software (version 0.4.0; http://frodo.wi.mit.edu/primer3/), NCBI BLAST, and NCBI PrimerBLAST. Primer pairs were obtained from MWG Biotech AG.
Western blotting
For Western blotting analyses of LPA protein, cells were washed with PBS and scraped into RIPA lysis buffer. After centrifugation at 4°C at 14,000 rpm for 30 min, 30 μg of protein per sample was added to 4× loading buffer (bromophenol blue and Laemmli buffer), heated to 95°C for 5 min, and separated on a NuPAGE 3–8% Tris-Acetate gel (Life Technologies GmbH) for 2 h at 150 V. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Carl Roth GmbH) for 1.5 h at 30 V. The following antibodies were used according to the instructions of the manufacturer: apo(a) (5402-1, Epitomics) and HSP90α/β (sc-7947, Santa Cruz Biotechnology). All secondary antibodies were purchased from Cell Signaling Technology.
For Western blotting analyses of signal transducer and activator of transcription 3 (STAT3) protein, cells were washed with PBS and scraped into Passive Lysis Buffer (Promega). Cells were lysed for 15 min at room temperature while rocking. After centrifugation at 4°C at 14,000 rpm for 5 min, 20 µg of protein per sample was added to 4× loading buffer, heated to 95°C for 5 min, and separated on a 12% SDS-PAGE for 1.5 h at 120 V. Proteins were transferred to PVDF membranes for 45 min at 70 mA. The following antibodies were used according to the instructions of the manufacturer: STAT3 (H-190) (sc-7179, Santa Cruz Biotechnology) and GAPDH (#2118, Cell Signaling Technology). All secondary antibodies were purchased from Cell Signaling Technology.
Cloning of LPA promoter and luciferase reporter gene assay
To investigate whether both the human apo(a) (LPA) and FAS are specifically activated by the cytokine IL-6, the LPA promoter was first amplified by PCR from human genomic DNA. Therefore, a specific sense primer flanking −1,293 to −1,270 [according to the published sequence in Ref. (17)] and an antisense primer flanking +153 to +131 of the LPA promoter were used to amplify the full-length promoter region. Because primers were created including restriction sites for SacI and BglII, PCR products were directly cloned into the multiple cloning site of pGL3 basic luciferase vector once digestion of both vector and insert with appropriate restriction enzymes had been performed. Integrity was confirmed by sequencing. The resulting plasmid is further referred to as pGL3-LPA. For transient transfection using lipofection agent Roti®-Fect (Carl Roth GmbH), HepG2 cells were grown in 24-well plates to 60–70% confluency. Then 0.5 µg of pGL3-LPA or pGL2-FAS was applied per well. To correct for transfection efficiency, 12.5 ng/well phRL-TK (Promega) were cotransfected. For overexpression experiments, 1.5 µg of the pcEP4-mSTAT3 construct, kindly provided by Dr. Christoph Garbers (Department of Biochemistry, University of Kiel), was applied in addition to 0.5 µg of pGL3-LPA and 12.5 ng phRL-TK. Cells were subsequently incubated with transfection medium (Opti-MEM®, 10% FCS, Roti®-Fect) for 24 h followed by a 12 h incubation in serum-reduced full medium (1% FCS). Twenty-four hours after stimulation with 10 ng/ml IL-6 as well as 100 µg/ml TCZ or 100 ng/ml ADB (both antibodies 1 h prior to IL-6 stimulation), cells were lysed by applying Passive Lysis Buffer (Promega), and luciferase activity was detected in a luminometer (Berthold Mithras LB 940) using the dual luciferase reporter assay kit (Promega). In the case of STAT3 pathway inhibition experiments, 0.5 µM of WP1066, a cell permeable inhibitor of STAT3 and Janus kinase 2 (JAK-2), a protein tyrosine kinase, was applied 1 h prior to IL-6 stimulation.
LPA promoter deletion experiments
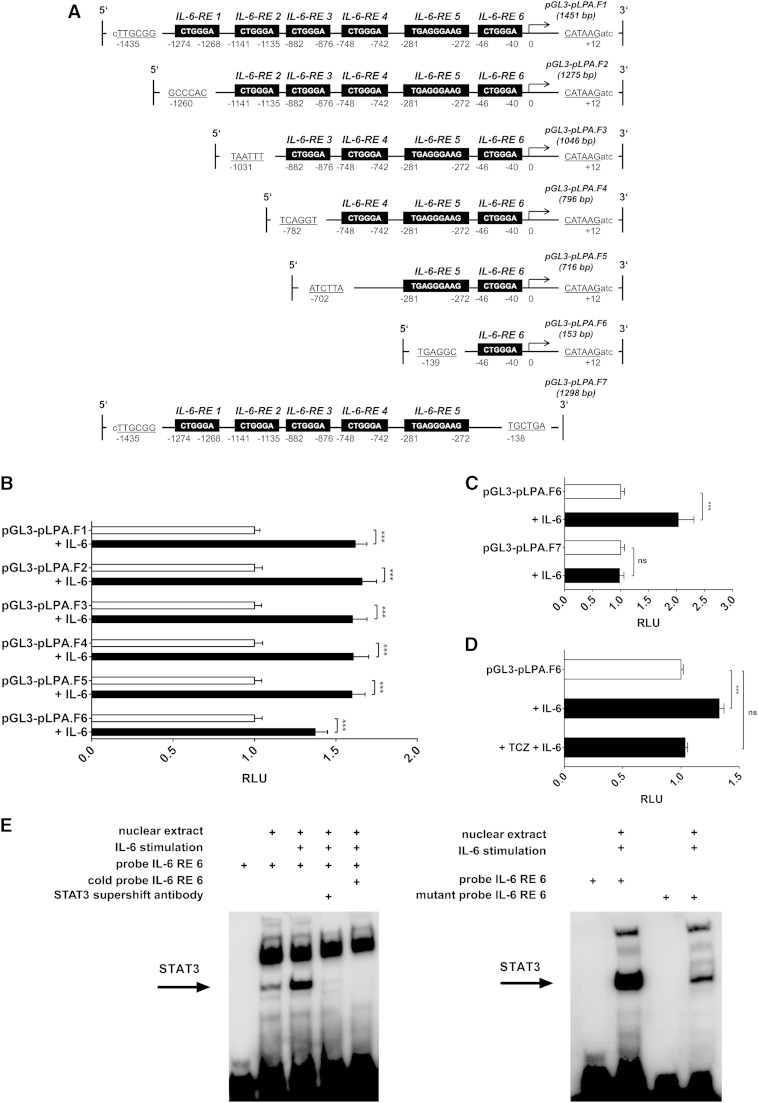
For examining the six putative IL-6 binding sites (17) within the LPA promoter, the cloned LPA promoter was truncated using existing restriction sites within the LPA promoter sequence. pGL3-LPA (1,452 bp) served as fragment 1 containing all IL-6 responsive elements (REs). For fragment 2 (1,275 bp) containing IL-6-REs 2 to 6 (see Fig. 6A), pGL3-LPA was restricted using NlaIV and BglII, blunted, and subsequently cloned into an SmaI-restricted pGL3 basic vector. AseI and BglII were used to obtain fragment 3 (1,046 bp) containing IL-6-REs 3 to 6; Bsu36I and BglII, for fragment 4 (796 bp) containing IL-6-REs 4 to 6; EcoRV and BglII, for fragment 5 (716 bp) containing IL-6-REs 5 to 6; BlpI and BglII, for fragment 6 (153 bp) containing only IL-6-RE 6; and SacI and BlpI, for fragment 7 (1,298 bp), followed by the procedure described above. Sense directed inserts were selected using PCR primers flanking the region of pGL3 basic vector and promoter fragment linkage (supplementary Table 1) and further confirmed by control digestion. Established promoter constructs were analyzed using luciferase reporter gene assays according to the previous section.
Fig. 6.
IL-6-RE 6 of LPA promoter confers major transcriptional activity following IL-6-dependent binding of transcription factor STAT3. Shown are IL-6-induced LPA promoter activities (RLU) (B, C) of truncated LPA promoter fragments (A) in human hepatocytes. D: Inhibition of IL-6-induced promoter activity of truncated LPA promoter containing IL-6-RE 6 by TCZ. Data are expressed in x-fold of the respective control (white bar) and are given as means + SEM of at least n = 4 independent experiments, each performed in duplicate. Statistical significance was tested using Student’s t-test or ANOVA. *** P < 0.001; ns, not significant. E: One representative EMSA out of at least n = 3 independent experiments using oligonucleotides being complementary to IL-6-RE 6 of the LPA promoter (left panel) and using oligonucleotides in which the specific STAT3 binding sequence (CTGGGA) of IL-6-RE 6 is mutated (right panel). The STAT3 supershift antibody used specifically blocks the DNA binding domain resulting in disappearance of the STAT3 shift [also see (36)].
EMSA
For EMSA experiments, nuclear extracts of Huh7 cells were generated by scraping washed cells into extraction buffer A [10 mM HEPES, 10 mM KCl, 0.2 mM EDTA, pH 7.9, 1 mM DTT, PhosSTOP (Roche), protease inhibitor cocktail (Sigma)] to separate the cytosolic fraction from the nuclear fraction, subsequently centrifuged for 1 min at 13,000 rpm and 4°C. The remaining pellet (nuclear fraction) was resuspended in extraction buffer B [20 mM HEPES, 0.4 M NaCl, 0.2 mM EDTA, pH 7.9, 1 mM DTT, PhosSTOP (Roche), protease inhibitor cocktail (Sigma)], vortexed for 20 min at 4°C, and afterward centrifuged for 5 min at 13,000 rpm and 4°C. Supernatant (nuclear extract) was collected and stored at −80°C. Five micrograms of nuclear protein extract was then incubated with 1 pmol 3′-end biotin-labeled double-stranded oligonucleotides being complementary to IL-6-RE 6 of the LPA promoter (17) or 1 pmol 3′-end biotin-labeled double-stranded oligonulceotides in which the specific STAT3 binding motif of IL-6-RE 6 is mutated (for sequences, see supplementary Table 1) and 1 µg poly deoxyinosinic-deoxycytidylic in supplied binding buffer (supplemented with 5% glycerine and 1 mM EDTA) for 30 min at room temperature. Competitive reaction was performed under identical conditions adding 200-fold of the amount of the respective unlabeled oligonucleotide. In supershift assays, 4 µg STAT3 supershift antibody [STAT3 (C20): sc-482×, Santa Cruz Biotechnology] was incubated with 5 µg of nuclear extracts and 1 µg poly dI·dC in binding buffer for 30 min at 15°C. Afterward, 1 pmol 3′-end biotin-labeled double-stranded oligonucleotide was added and again incubated for 30 min at 20°C. Samples were separated on a 5% PAGE, and final analysis was performed according to the LightShift® Chemiluminescent EMSA Kit (20148, Pierce Thermo Scientific).
Oligonucleotide liver microarray hybridization
For homogenization of 5 to 10 mg frozen tissue and subsequent nucleic acid isolation, tubes with 1.4 mm ceramic beads (Precellys) and the AllPrep DNA/RNA Mini Kit (Qiagen) were used. Hybridization of the HumanMethylation450k Bead Chip (Illumina) permitting combined analysis of global methylation status and gene expression levels, subsequent scanning (iScan, Illumina), and mRNA expression analysis using the HuGene 1.1 ST gene (Affymetrix) were performed according to the manufacturers’ protocols.
Correlation analysis as to which genes are correlated with the LPA gene was initially performed. Next, annotation of gene symbols being positively and significantly (r ≥ 0, P < 0.05) correlated with the LPA gene was performed using the GeneID Conversion Tool of the DAVID Bioinformatics Resources 6.7 software (National Institute of Allergy and Infectious Diseases, National Institutes of Health; http://david.abcc.ncifcrf.gov/). The converted gene list was further analyzed using the Functional Annotation Clustering Tool in order to select only genes being related to “acute phase response,” “acute inflammatory response,” “inflammatory response,” “activation of plasma proteins involved in acute inflammatory response,” “regulation of inflammatory response,” “regulation of acute inflammatory response,” and “positive regulation of inflammatory response.”
Statistical analysis
Statistical analysis was performed using SPSS Statistics Software Version 22 (IBM) and GraphPad Prism Software Version 5.04. If the assumption of normal distribution of the data was not violated (tested using Shapiro-Wilk), Student’s t-test or ANOVA was used. In the case of data that were not normally distributed, the Mann-Whitney U-test (Wilcoxon sign-rank test for paired observations) or Kruskal-Wallis test was applied. The significance level was set at 5%, and multiple comparisons were performed using Bonferroni or Dunn’s posttest.
RESULTS
Lowering of Lp(a) serum levels in humans is specific for IL-6 inhibition
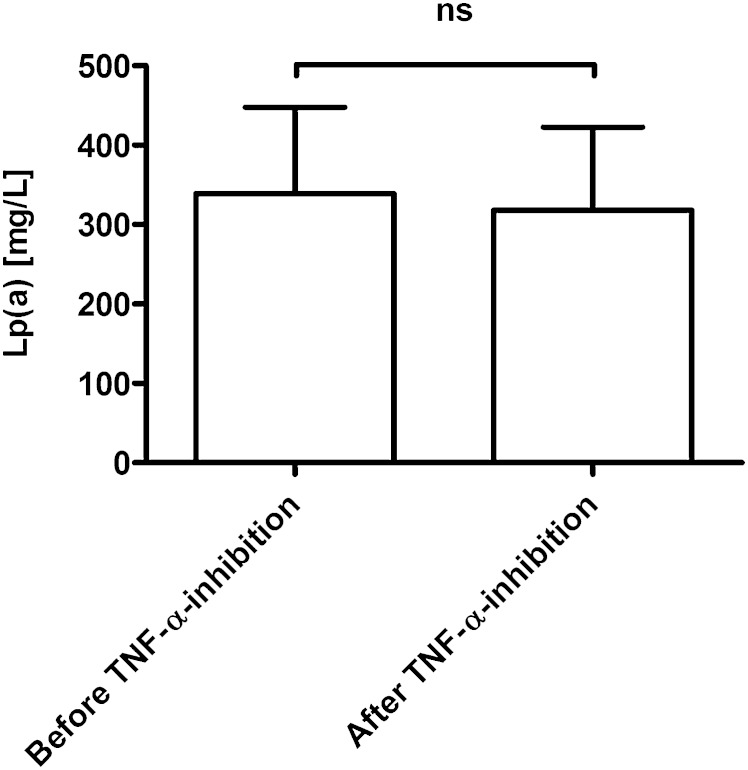
In a recent clinical study from Schultz et al. (14), we have shown that IL-6 inhibition due to the monoclonal antibody TCZ results in a reduction of Lp(a) serum levels to a greater extent than the drug niacin, which for a long period of time has been suggested to be the standard therapy for that metabolic abnormality (18). In order to investigate whether the Lp(a)-lowering effect is specific for IL-6 inhibition, we measured Lp(a) in the serum of 12 human RA subjects before and after 3 months of anti-TNF-α therapy with ADB, which is a study design comparable to our first report (14). This analysis showed no significant change of Lp(a) serum levels in response to anti-TNF-α therapy (Fig. 1). This finding suggests that the effect of the innate immune system on serum Lp(a) levels in humans is not of a common unspecific nature but is highly specific for the cytokine IL-6.
Fig. 1.
TNF-α-antibody treatment does not decrease Lp(a) serum levels in RA patients. Data are given as means + SEM of patients (n = 12) with RA treated with TNF-α-antibody ADB for a time period of n = 3 months. Statistical significance was tested using Wilcoxon sign-rank test for paired observations; ns, not significant.
Elevated IL-6 serum levels are associated with an increase of Lp(a) in humans in vivo
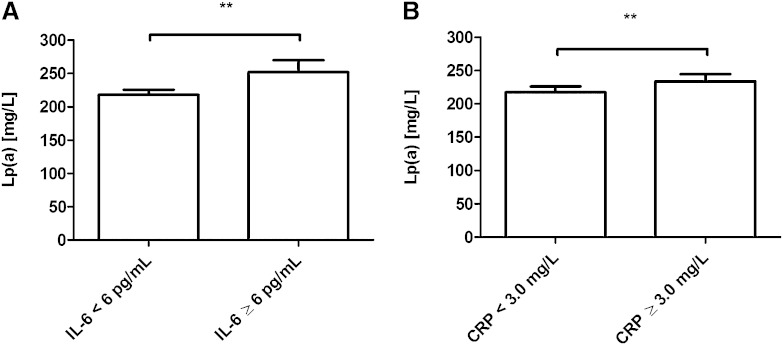
To further analyze the association of elevated Lp(a) serum levels and IL-6 in humans, we measured both factors in a population-based cohort including human subjects (n = 1,153) from the north of Germany. In that analysis, we compared Lp(a) serum levels between individuals exhibiting normal IL-6 serum levels (<6 pg/ml) and individuals exhibiting elevated IL-6 serum levels (>6 pg/ml). This comparison revealed significantly higher Lp(a) concentrations in subjects with elevated IL-6 compared with individuals with normal IL-6 serum levels (Fig. 2). Strikingly, as shown in Tables 1, 2, further analysis revealed that Lp(a) serum concentrations are more correlated to IL-6 (rs = 0.103, P = 0.0005, n = 1,153) compared with metabolic parameters (BMI, HOMA-IR, TG), suggesting that Lp(a) serum concentrations are not only genetically determined but are also influenced by IL-6. To further support the finding that Lp(a) and IL-6 are indeed biologically related, we also performed a subgroup analysis, which showed that the correlation between Lp(a) and IL-6 still remained significant in a nonobese group with normal CRP (rs = 0.104, P = 0.009, n = 635). In addition, the association of Lp(a) and IL-6 found in that population-based cohort suggests that IL-6 is important in Lp(a) metabolism in humans in vivo, not only in the context of RA but also in the general population.
Fig. 2.
Elevated IL-6 serum levels and those of IL-6 response protein CRP are significantly associated with an increase of Lp(a) in humans in vivo. This association analysis represents significant differences of Lp(a) serum levels in groups with normal (n = 955) and elevated (n = 198) IL-6 serum levels (P = 0.0015) (A). In addition, significant differences of Lp(a) serum levels in groups with normal (n = 680) and elevated (n = 473) CRP serum levels (P = 0.0018) were found (B). CRP is an IL-6-induced acute phase response protein, which suggests a major role of IL-6 regarding the relationship to Lp(a). Data are given as means + SEM, and statistical significance was tested using Mann-Whitney U-test. ** P < 0.01.
TABLE 1.
Correlation analysis between BMI and different inflammatory and metabolic parameters in the FoCus cohort
| BMI (kg/m2) | ||
| rs | P | |
| HOMA-IR | 0.6170 | <0.0001 |
| CRP (mg/l) | 0.6138 | <0.0001 |
| IL-6 (pg/ml) | 0.4448 | <0.0001 |
| TG (mg/dl) | 0.4345 | <0.0001 |
Shown are the Spearman correlation coefficient (rs) and the corresponding P value of each correlation. Correlation analysis between BMI and different inflammatory and metabolic parameters is shown to demonstrate that the FoCus cohort (n = 1,153), collected in the north of Germany and designed to examine the impact of inflammatory parameters in the pathogenesis of metabolic and nutrition-associated diseases, is a reliable cohort in terms of known associations described in other cohorts.
TABLE 2.
Correlations between Lp(a) and different inflammatory and metabolic parameters in the FoCus cohort
| Lp(a) (mg/l) | ||
| rs | P | |
| CRP (mg/l) | 0.1074 | 0.0003 |
| IL-6 (pg/ml) | 0.1025 | 0.0005 |
| BMI (kg/m2) | 0.0935 | 0.0015 |
| HOMA-IR | 0.0732 | 0.0129 |
| TG (mg/dl) | 0.0707 | 0.0164 |
Shown are the Spearman correlation coefficient (rs) and the corresponding P value of each correlation.
Expression of typical IL-6 response genes correlates with LPA expression in human liver in vivo
Because serum levels of Lp(a) might also be influenced by serological factors other than cytokines [e.g., estrogens (19)], we next aimed to further examine the impact of IL-6 signaling on LPA gene expression in human liver in vivo. Therefore, we performed reanalysis of a previous microarray study (16) in which liver biopsies were obtained during elective surgical procedures. In this clinical study, human subjects without any CID were included. By correlation analysis of hepatic LPA gene expression with the expression of known inflammatory response-associated genes, we found significant associations with several typical IL-6 acute phase response genes (20), for example, complement C9 (r = 0.61677), C4b binding protein (r = 0.60312), and three serpin peptidase inhibitors (supplementary Table 2). These data clearly indicate a relationship of the IL-6 activity and LPA expression in human liver in vivo even in the absence of a classical CID such as RA.
TCZ inhibits IL-6-induced expression of LPA in human hepatocytes
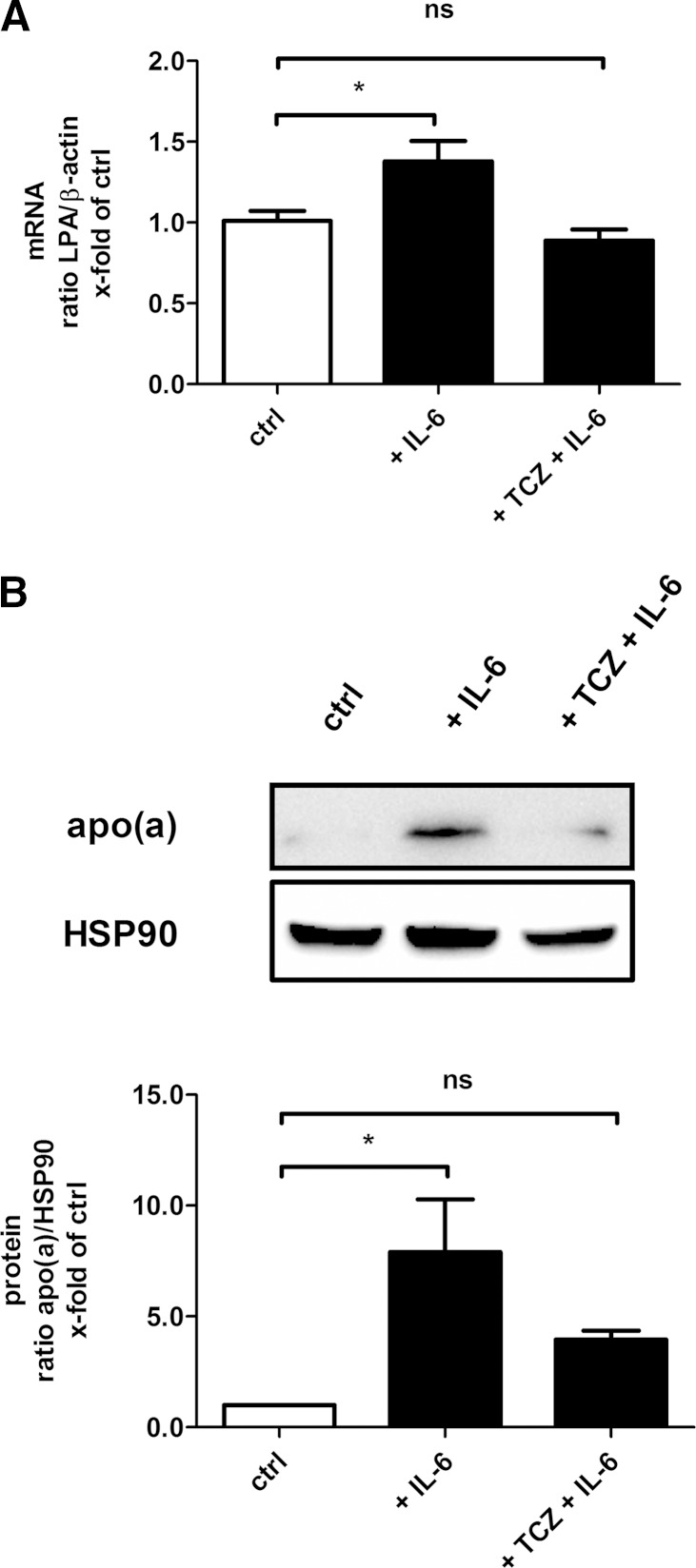
Because our clinical studies suggested that there may be a possible link between IL-6 and LPA expression in humans in vivo, we next aimed to further analyze that relationship on a molecular level. The exposure of human hepatocytes to IL-6 for 12 and 48 h resulted in a significant upregulation of hepatic LPA at mRNA and protein levels, respectively (Fig. 3). Next, we coincubated the cells with IL-6 and the IL-6-receptor antibody TCZ. This indeed led to a significant inversion of the effect caused by IL-6 stimulation on LPA mRNA and protein expression (Fig. 3), indicating also on a molecular level that an IL-6-blockade may be a promising tool for the treatment of elevated Lp(a) in humans.
Fig. 3.
TCZ inhibits IL-6-induced expression of LPA in human hepatocytes. Shown are LPA mRNA (A) and protein (B) expression following IL-6 and TCZ exposure for 12 and 48 h in whole cell extracts of human hepatocytes, respectively. A: β-actin expression served as the housekeeping gene in real-time RT-PCR. Data are expressed in ratios of LPA/β-actin and are given as means + SEM of n = 3 independent experiments in duplicate. B: Detection of heat shock protein 90 (HSP90) expression served as loading control. The shown immunoblot is one representative out of n = 3 independent experiments. Densitometry graph data in this figure are shown as means + SEM. Statistical significances were tested using ANOVA. * P < 0.05; ns, not significant, compared with control (ctrl).
TCZ specifically inhibits LPA promoter activity in human hepatocytes
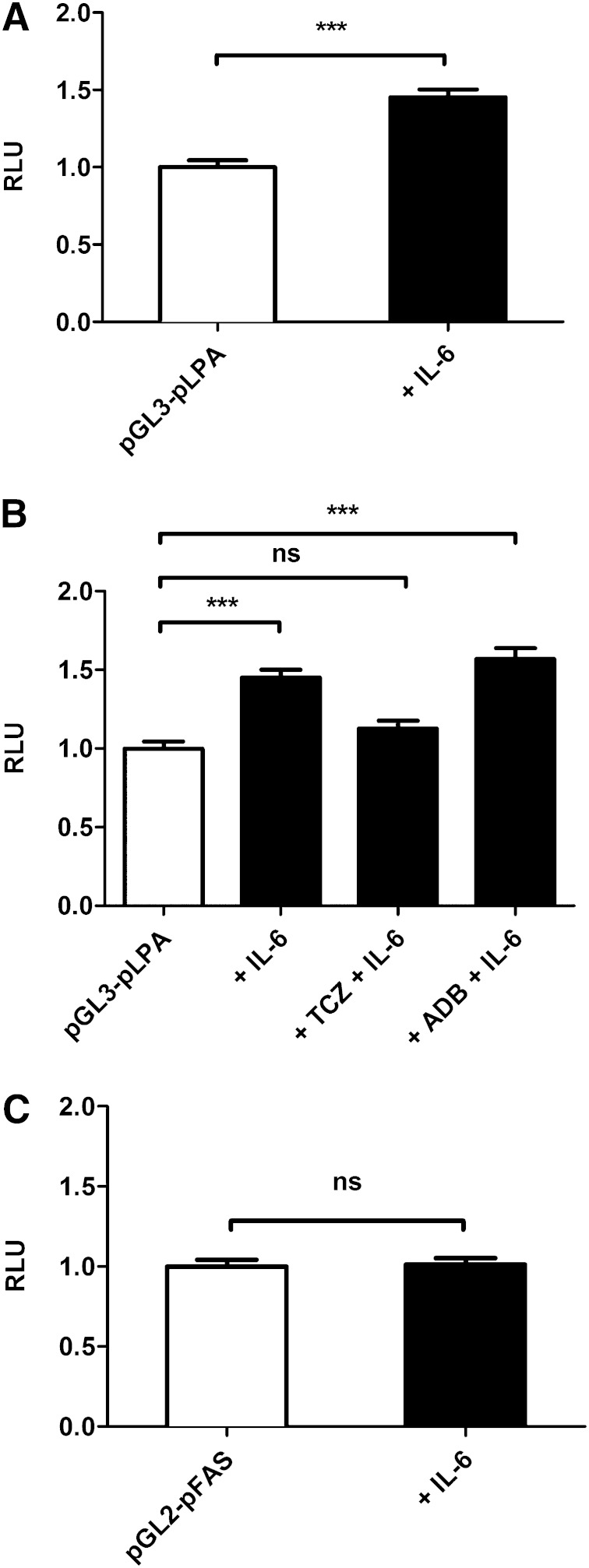
In order to further characterize the effects of IL-6 on LPA promoter activity, we cloned the full-length promoter of LPA into the pGL3 plasmid and subsequently performed luciferase reporter gene assays in human hepatocytes. In this experiment, we found that IL-6 is able to activate the LPA promoter (Fig. 4A). When coincubated with TCZ and IL-6, IL6-induced LPA promoter activity decreased down to the baseline (without stimulation) (Fig. 4B). However, having costimulated the cells with the anti-TNF-α antibody ADB, LPA gene promoter activity did not change (Fig. 4B), indicating the effect to be specific for TCZ. Next, we examined whether the promoter of FAS as an additional gene of the lipid metabolism may also be activated by IL-6 in order to exclude unspecific metabolic effects of hepatocytes in that in vitro experiment. However, this control experiment revealed that IL-6 does not activate the FAS promoter (Fig. 4C), indicating specificity of the TCZ effect on the LPA promoter.
Fig. 4.
TCZ specifically inhibits LPA promoter activity in human hepatocytes. Shown is LPA (A, B) and FAS (C) promoter activity (RLU) stimulated with IL-6 (A, C) and TCZ or ADB (B) in human hepatocytes. Data are expressed in x-fold of pGL3-pLPA or pGL2-pFAS and are given as means + SEM of n = 5 independent experiments, each performed in duplicate. Statistical significance was tested using Student’s t-test, Mann-Whitney U-test, or Kruskal-Wallis test. *** P < 0.001; ns, not significant, compared with pGL3-pLPA or pGL2-pFAS.
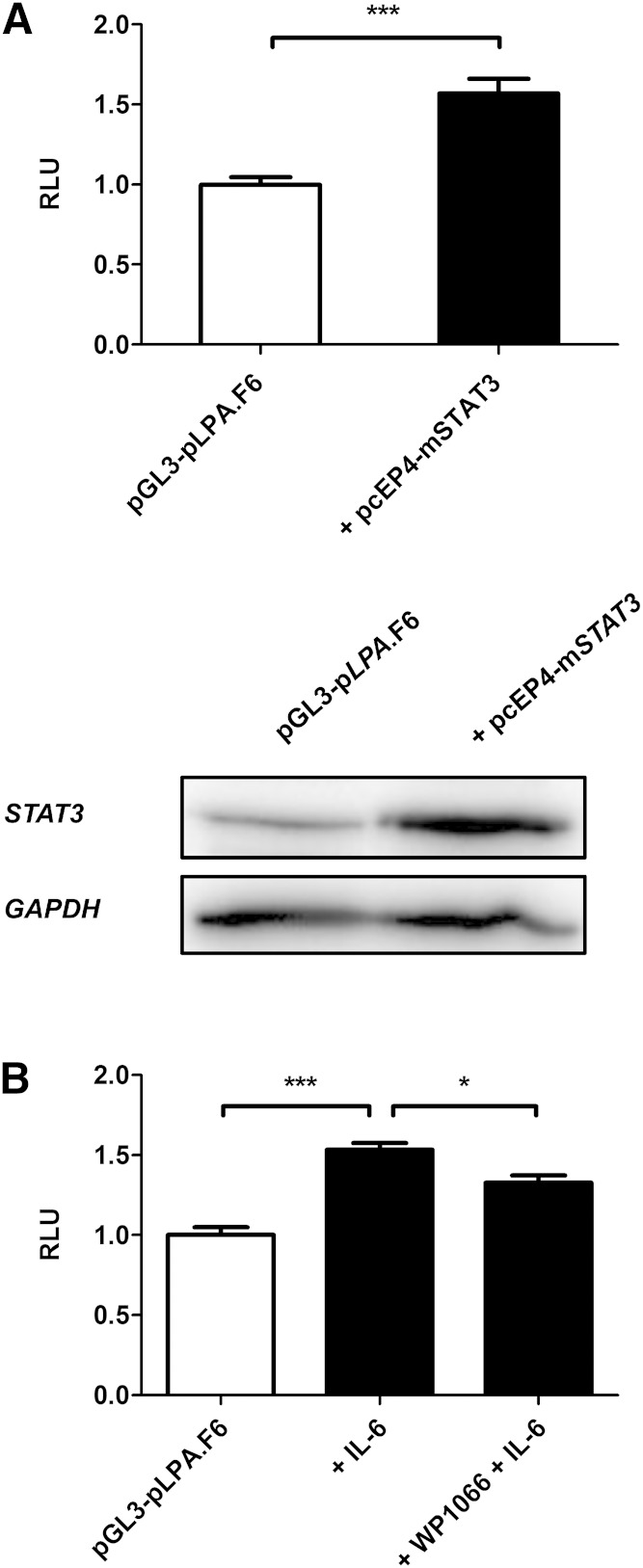
STAT3 overexpression and inhibition regulates LPA promoter activity in human hepatocytes
We additionally present evidence that STAT3 overexpression led to a significant upregulation of LPA promoter activity (Fig. 5A, upper panel) in human hepatocytes. A Western blot for STAT3 confirmed a successful overexpression of the STAT3 protein (Fig. 5A, lower panel). Furthermore, we examined whether the STAT3/JAK2 inhibitor WP1066 might downregulate LPA promoter activity. Indeed, this experiment revealed that WP1066 is able to significantly diminish IL-6-induced LPA promoter activity (Fig. 5B).
Fig. 5.
STAT3 overexpression and inhibition regulates LPA promoter activity in human hepatocytes. Shown is LPA promoter activity (RLU) (upper panel) and STAT3 protein expression (lower panel) when STAT3 is overexpressed by introducing a STAT3 overexpression plasmid containing STAT3 cDNA (pcEP4-mSTAT3) in human hepatocytes (A). B: LPA promoter activity in human hepatocytes stimulated with IL-6 and with IL-6 in the presence of STAT3/JAK-2 inhibitor WP1066. All data are expressed in x-fold of pGL3-pLPA.F6 and are given as means + SEM of n = 3 independent experiments, each performed in duplicate. Statistical significance was tested using Student’s t-test and ANOVA. * P < 0.05, *** P < 0.001, compared with pGL3-pLPA.F6.
TCZ regulates LPA promoter activity via an RE at −46 to −40
The LPA promoter contains six established IL-6-REs (also STAT3 binding sites), which is why we have truncated the full-length LPA promoter and generated seven constructs, the first containing the fragment including all six IL-6-REs, the sixth only containing IL-6-RE 6, and the seventh containing all IL-6-REs except for IL-6-RE 6 (Fig. 6A). We then performed luciferase reporter gene assays by incubating transfected human hepatocytes with IL-6 for 24 h in order to examine which IL6-REs are responsible for the transcriptional activity. Our results indicated that IL-6-REs 1–5 may not be important for the expression of the LPA gene, whereas IL-6-RE 6 at −46 to −40 seems to be the major regulatory element when bound to transcription factor STAT3 (Fig. 6B, C). Because we have shown that most likely only IL-6-RE 6 regulates the transcription, we further costimulated the cells transfected with the promoter construct containing IL-6-RE 6 (designated as pGL3-pLPA.F6; Fig. 6A) with TCZ and IL-6 showing that the effect of IL-6 is indeed inhibited by TCZ (Fig. 6D). To further analyze TCZ effects on LPA, we additionally carried out EMSAs for IL-6-RE 6. This analysis showed that IL-6 specifically activates STAT3 consequently binding to IL-6-RE 6 (Fig. 6E). To confirm specificity of the binding of STAT3 to IL-6-RE 6, a STAT3 antibody was used. As shown in Fig. 6E, preincubation with that antibody blocked the binding of STAT3 to the IL-6-RE 6, proving specificity of the effect. A mutant version of the oligonucleotide being complementary to IL-6-RE 6 of the LPA promoter was used to further verify a targeted binding of transcription factor STAT3 to IL-6-RE 6 of the LPA promoter. Indeed, the mutation of STAT3 binding motif (CTGGGA) resulted in a substantial attenuation of the STAT3 band (Fig. 6E).
DISCUSSION
It was demonstrated that Lp(a) levels are only influenced by a limited number of physiological factors [such as estrogens (19)], disease conditions [such as renal failure (21–23)], or environmental agents [such as alcohol (24)]. Circulating levels of Lp(a) are further remarkably resistant to common lipid-lowering therapies (25–27). One exception to effectively lower Lp(a) levels is niacin when given in high doses (2–3 g/day) (28, 29). These doses of niacin, however, can be associated with headaches, flushing, and liver toxicity and finally noncompliance. Also, due to unfavorable side effects, a special slow-release formation of niacin (Tredaptive™) was recently withdrawn from the market. Aspirin at low doses was further shown to decrease Lp(a) serum levels slightly (30) indicating rather minor importance. Thus, there are currently no robust pharmaceutical treatments available for the reduction of Lp(a). Therefore, at present, lipid apheresis, which is invasive, costly, and labor intensive, is the only therapeutic option for subjects with CVD progression and highly elevated Lp(a) levels (31, 32). For this reason, alternative well-tolerated and more convenient therapeutic approaches are required to decrease elevated Lp(a) serum level.
Our present study and our two previous reports on that subject (14, 33) present several lines of evidence that IL-6 is a promising drug target for the treatment of elevated Lp(a) levels in humans: 1) Lp(a) serum levels are elevated in human subjects with increased IL-6 levels in vivo (Fig. 2); 2) Lp(a) serum levels are elevated in human subjects carrying the −174G/C polymorphism in the human IL-6 promoter (33); 3) LPA expression in human liver is related to IL-6 signaling in vivo (supplementary Table 2); 4) treatment of human subjects with TCZ lowers Lp(a) serum levels to a greater extent than niacin (14, 15); 5) TCZ inhibits IL-6-induced expression of LPA in human hepatocytes on mRNA and protein level (Fig. 3); 6) TCZ inhibits IL-6-mediated LPA promoter activity on a molecular level (Fig. 4); 7) STAT3 overexpression and inhibition significantly regulates LPA promoter activity (Fig. 5); 8) the TCZ effect is mediated by a specific STAT3 binding site within the human LPA promoter (Fig. 6); and 9) anti-TNF-α antibody treatment does not significantly influence Lp(a) levels in a time period of 3 months (Fig. 1) while IL-6 inhibition by TCZ reduced Lp(a) serum levels within a few weeks (14), clearly suggesting a direct and specific effect.
However, besides that strong evidence, until now the concept of using IL-6 as a drug target for Lp(a) therapy has had two limitations. First, the effect of TCZ on Lp(a) levels so far has only been shown in patients with a treatment indication for TCZ (e.g., RA). Although in the present report we provide evidence for a link between IL-6 and Lp(a) also in the general population, a future clinical study will be necessary including patients with elevated Lp(a) levels not suffering from RA, demonstrating that the metabolic effect of TCZ does not depend on the presence of a CID.
Second, several reports in the past have shown that TCZ increases LDL-cholesterol levels in RA patients (15). Indeed, in collaboration with the group of P. P. Tak from the University of Amsterdam, we have recently shown that LDL-cholesterol elevation is the result of a specific inhibition of TCZ on LDL-receptor expression in hepatocytes (34). However, it has to be taken into account that LDL-cholesterol levels in RA patients are known to be lower compared with the general population (35). Thus, the slight increase found during TCZ therapy of RA patients might just indicate an increase of the LDL-cholesterol into the individual “normal” range. Also, it is important to mention that the MEASURE study (15) recently showed that the increase of LDL-cholesterol in TCZ-treated RA subjects is mostly due to an increase in high- and normal-density LDL particles, whereas small dense LDL particles, being most important for atherosclerosis progression, are not affected by IL-6 inhibition due to TCZ (15). Finally, it has to be mentioned that most of the patients with elevated Lp(a) levels and progressive CVD are on statin therapy, which potentially compensates the unfavorable LDL effect of TCZ. Hence, at least from our point of view, the slight elevation of LDL-cholesterol in TCZ-treated RA patients is not a major factor arguing against a future clinical trial for TCZ as a treatment option for elevated Lp(a) levels in humans with progressive CVD.
In summary, the data obtained in the present study suggest that IL-6 blockade by monoclonal antibodies might be a promising future therapeutic approach to treat elevated serum Lp(a) concentrations and to reduced CVD risk in affected patients and might therefore be a convenient alternative to lipid apheresis.
Supplementary Material
Acknowledgments
The authors would like to thank all subjects who participated in the study.
Footnotes
Abbreviations:
- ADB
- adalimumab
- CID
- chronic inflammatory disease
- CRP
- C-reactive protein
- EMSA
- electrophoretic mobility shift assay
- FoCus
- Food Chain Plus
- IL-6
- interleukin 6
- JAK-2
- Janus kinase 2
- Lp(a)
- lipoprotein (a)
- RA
- rheumatoid arthritis
- RE
- responsive elements
- STAT3
- signal transducer and activator of transcription 3
- TCZ
- tocilizumab
The work was supported by intramural funding from the University of Kiel. M.L. has received lecturer fees from Chugai/Roche. R.Z. has received a travel grant to EULAR 2011 from Chugai/Roche. All other authors have nothing to declare.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Berg K. 1963. A new serum type system in man—the Lp system. Acta Pathol. Microbiol. Scand. 59: 369–382. [DOI] [PubMed] [Google Scholar]
- 2.Utermann G. 1989. The mysteries of lipoprotein(a). Science. 246: 904–910. [DOI] [PubMed] [Google Scholar]
- 3.McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., Lawn R. M. 1987. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 330: 132–137. [DOI] [PubMed] [Google Scholar]
- 4.Steinmetz A., Utermann G. 1992. [Lipoprotein(a) as a risk factor for arteriosclerosis]. Internist (Berl.). 33: 24–31. German. [PubMed] [Google Scholar]
- 5.Utermann G., Weber W. 1983. Protein composition of Lp(a) lipoprotein from human plasma. FEBS Lett. 154: 357–361. [DOI] [PubMed] [Google Scholar]
- 6.Utermann G., Menzel H. J., Kraft H. G., Duba H. C., Kemmler H. G., Seitz C. 1987. Lp(a) glycoprotein phenotypes. Inheritance and relation to Lp(a)-lipoprotein concentrations in plasma. J. Clin. Invest. 80: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boerwinkle E., Leffert C. C., Lin J., Lackner C., Chiesa G., Hobbs H. H. 1992. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J. Clin. Invest. 90: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mooser V., Scheer D., Marcovina S. M., Wang J., Guerra R., Cohen J., Hobbs H. H. 1997. The Apo(a) gene is the major determinant of variation in plasma Lp(a) levels in African Americans. Am. J. Hum. Genet. 61: 402–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koutroubakis I. E., Malliaraki N., Vardas E., Ganotakis E., Margioris A. N., Manousos O. N., Kouroumalis E. A. 2001. Increased levels of lipoprotein (a) in Crohn’s disease: a relation to thrombosis? Eur. J. Gastroenterol. Hepatol. 13: 1415–1419. [DOI] [PubMed] [Google Scholar]
- 10.Missala I., Kassner U., Steinhagen-Thiessen E. 2012. A systematic literature review of the association of lipoprotein(a) and autoimmune diseases and atherosclerosis. Int. J. Rheumatol. 2012: 480784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asanuma Y., Kawai S., Aoshima H., Kaburaki J., Mizushima Y. 1999. Serum lipoprotein(a) and apolipoprotein(a) phenotypes in patients with rheumatoid arthritis. Arthritis Rheum. 42: 443–447. [DOI] [PubMed] [Google Scholar]
- 12.Mooser V., Berger M. M., Tappy L., Cayeux C., Marcovina S. M., Darioli R., Nicod P., Chiolero R. 2000. Major reduction in plasma Lp(a) levels during sepsis and burns. Arterioscler. Thromb. Vasc. Biol. 20: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 13.Maeda S., Abe A., Seishima M., Makino K., Noma A., Kawade M. 1989. Transient changes of serum lipoprotein(a) as an acute phase protein. Atherosclerosis. 78: 145–150. [DOI] [PubMed] [Google Scholar]
- 14.Schultz O., Oberhauser F., Saech J., Rubbert-Roth A., Hahn M., Krone W., Laudes M. 2010. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS ONE. 5: e14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McInnes I. B., Thompson L., Giles J. T., Bathon J. M., Salmon J. E., Beaulieu A. D., Codding C. E., Carlson T. H., Delles C., Lee J. S., et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis. Epub ahead of print. December 24, 2013; 10.1136/annrheumdis-2013-204345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahrens M., Ammerpohl O., von Schonfels W., Kolarova J., Bens S., Itzel T., Teufel A., Herrmann A., Brosch M., Hinrichsen H., et al. 2013. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 18: 296–302. [DOI] [PubMed] [Google Scholar]
- 17.Wade D. P., Clarke J. G., Lindahl G. E., Liu A. C., Zysow B. R., Meer K., Schwartz K., Lawn R. M. 1993. 5′ control regions of the apolipoprotein(a) gene and members of the related plasminogen gene family. Proc. Natl. Acad. Sci. USA. 90: 1369–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seed M., O’Connor B., Perombelon N., O’Donnell M., Reaveley D., Knight B. L. 1993. The effect of nicotinic acid and acipimox on lipoprotein(a) concentration and turnover. Atherosclerosis. 101: 61–68. [DOI] [PubMed] [Google Scholar]
- 19.Espeland M. A., Marcovina S. M., Miller V., Wood P. D., Wasilauskas C., Sherwin R., Schrott H., Bush T. L.; for the PEPI Investigators. 1998. Effect of postmenopausal hormone therapy on lipoprotein(a) concentration. Circulation. 97: 979–986. [DOI] [PubMed] [Google Scholar]
- 20.Bode J. G., Albrecht U., Haussinger D., Heinrich P. C., Schaper F. 2012. Hepatic acute phase proteins – regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur. J. Cell Biol. 91: 496–505. [DOI] [PubMed] [Google Scholar]
- 21.Thillet J., Doucet C., Issad B., Allouache M., Chapman J. M., Jacobs C. 1994. Elevated Lp(a) levels in patients with end-stage renal disease. Am. J. Kidney Dis. 23: 620–621. [DOI] [PubMed] [Google Scholar]
- 22.Kronenberg F., Konig P., Neyer U., Auinger M., Pribasnig A., Lang U., Reitinger J., Pinter G., Utermann G., Dieplinger H. 1995. Multicenter study of lipoprotein(a) and apolipoprotein(a) phenotypes in patients with end-stage renal disease treated by hemodialysis or continuous ambulatory peritoneal dialysis. J. Am. Soc. Nephrol. 6: 110–120. [DOI] [PubMed] [Google Scholar]
- 23.Mooser V., Marcovina S. M., Wang J., Hobbs H. H. 1997. High plasma levels of apo(a) fragments in Caucasians and African-Americans with end-stage renal disease: implications for plasma Lp(a) assay. Clin. Genet. 52: 387–392. [DOI] [PubMed] [Google Scholar]
- 24.Fontana P., Mooser V., Bovet P., Shamlaye C., Burnand B., Lenain V., Marcovina S. M., Riesen W., Darioli R. 1999. Dose-dependent inverse relationship between alcohol consumption and serum Lp(a) levels in black African males. Arterioscler. Thromb. Vasc. Biol. 19: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 25.McCormick S. P. 2004. Lipoprotein(a): biology and clinical importance. Clin. Biochem. Rev. 25: 69–80. [PMC free article] [PubMed] [Google Scholar]
- 26.Lippi G., Targher G. 2012. Optimal therapy for reduction of lipoprotein(a). J. Clin. Pharm. Ther. 37: 1–3. [DOI] [PubMed] [Google Scholar]
- 27.Berthold H. K., Gouni-Berthold I. 2013. Hyperlipoproteinemia(a): clinical significance and treatment options. Atheroscler. Suppl. 14: 1–5. [DOI] [PubMed] [Google Scholar]
- 28.Crouse J. R., III 1996. New developments in the use of niacin for treatment of hyperlipidemia: new considerations in the use of an old drug. Coron. Artery Dis. 7: 321–326. [DOI] [PubMed] [Google Scholar]
- 29.Gouni-Berthold I., Berthold H. K. 2013. The role of niacin in lipid-lowering treatment: are we aiming too high? Curr. Pharm. Des. 19: 3094–3106. [DOI] [PubMed] [Google Scholar]
- 30.Akaike M., Azuma H., Kagawa A., Matsumoto K., Hayashi I., Tamura K., Nishiuchi T., Iuchi T., Takamori N., Aihara K., et al. 2002. Effect of aspirin treatment on serum concentrations of lipoprotein(a) in patients with atherosclerotic diseases. Clin. Chem. 48: 1454–1459. [PubMed] [Google Scholar]
- 31.Borberg H. 2006. Quo vadis haemapheresis. Current developments in haemapheresis. Transfus. Apher. Sci. 34: 51–73. [DOI] [PubMed] [Google Scholar]
- 32.Sakata S., Komaki T., Kojima N., Matsuda M., Maeda S., Kawade M., Miura K. 1987. Dynamics of plasma lipoproteins and lipids during double filtration plasmapheresis (DEP). Jpn. J. Med. 26: 176–179. [DOI] [PubMed] [Google Scholar]
- 33.Berthold H. K., Laudes M., Krone W., Gouni-Berthold I. 2011. Association between the interleukin-6 promoter polymorphism -174G/C and serum lipoprotein(a) concentrations in humans. PLoS ONE. 6: e24719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strang A. C., Bisoendial R. J., Kootte R. S., Schulte D. M., Dallinga-Thie G. M., Levels J. H., Kok M., Vos K., Tas S. W., Tietge U. J., et al. 2013. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis. 229: 174–181. [DOI] [PubMed] [Google Scholar]
- 35.Liao K. P., Diogo D., Cui J., Cai T., Okada Y., Gainer V. S., Murphy S. N., Gupta N., Mirel D., Ananthakrishnan A. N., et al. 2014. Association between low density lipoprotein and rheumatoid arthritis genetic factors with low density lipoprotein levels in rheumatoid arthritis and non-rheumatoid arthritis controls. Ann. Rheum. Dis. 73: 1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joe Y., Kim H. J., Kim S., Chung J., Ko M. S., Lee W. H., Chang K. C., Park J. W., Chung H. T. 2011. Tristetraprolin mediates anti-inflammatory effects of nicotine in lipopolysaccharide-stimulated macrophages. J. Biol. Chem. 286: 24735–24742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.