Abstract
The benefits of dietary phytosterols (PhySs) and long-chain n-3 PUFA (ω3) have been linked to their effects as cholesterol- and triglyceride (TGL)-lowering agents. However, it remains unknown whether these compounds have further metabolic effects on LDL lipid composition. Here, we studied the effects of PhyS- or ω3-supplemented low-fat milk (milk) on the LDL-lipidome. Overweight and moderately hypercholesterolemic subjects (n = 32) were enrolled in a two-arm longitudinal crossover study. Milk (250 ml/day), enriched with either 1.57 g PhyS or 375 mg ω3 (EPA + DHA), was given to the participants during two sequential 28 day intervention periods. Compared with baseline, PhyS-milk induced a higher reduction in the LDL cholesterol (LDLc) level than ω3-milk. LDL resistance to oxidation was significantly increased after intervention with PhyS-milk. Changes in TGL and VLDL cholesterol were only evident after ω3-milk intake. Lipidomic analysis revealed a differential effect of the PhyS- and ω3-milk interventions on the LDL lipid metabolite pattern. Content in LDL-glycerophospholipids was reduced after PhyS-milk intake, with major changes in phosphatidylcholine (PC) and phosphatidylserine subclasses, whereas ω3-milk induced significant changes in the long-chain polyunsaturated cholesteryl esters and in the ratio PC36:5/lysoPC16:0, associated to a reduced inflammatory activity. In conclusion, daily intake of milk products containing PhyS or ω3 supplements induce changes in the LDL-lipidome that indicate reduced inflammatory and atherogenic effects, beyond their LDLc- and TGL-lowering effects.
Keywords: atherosclerosis, cholesterol, triglycerides, diet and dietary lipids, phospholipids, mass spectrometry, glycerophospholipids, low density lipoprotein-lipid metabolites
The prevalence of overweight and obesity is increasing and represents a primary health concern due to the relationship between obesity and a number of diseases and comorbidities including hyperlipidemia, a major risk factor for atherosclerosis and CVD. Dyslipidemias, typically characterized by high serum LDL cholesterol (LDLc), or low levels of HDL cholesterol (HDLc), and/or elevated triglyceride (TGL) levels, are common among patients with established CVD, type 2 diabetes mellitus, and the metabolic syndrome (1).
Because cholesterol lowering is a major target for reducing CVD risk (2), dietary interventions to reduce LDLc levels in individuals with borderline dyslipidemia and obesity without overall cardiovascular risk are becoming mandatory. Cholesterol concentrations within the circulatory pool are products of input from gut absorption and endogenous synthesis relative to clearance through hepatic and extrahepatic tissue pathways (3). Thus, subjects with overweight and mild dyslipidemia are usually advised to limit their intake of saturated fat and dietary cholesterol, in particular, those obtained from dairy products, in order to reduce LDLc concentration and, therefore, reduce the risk of cardiovascular complications (4). There is a wealth of evidence from randomized controlled trials that a number of foods and food components can significantly turn the blood lipid profile into a more antiatherogenic pattern (5). Daily intake of plant sterols and stanols, their saturated counterparts, has been reported as a nutritional strategy to reduce serum total cholesterol (TC) and LDLc concentrations (4, 6). These plant components, which closely resemble cholesterol in their molecular structure, are believed to exert their hypocholesterolemic effect primarily through competitive inhibition of cholesterol micellar solubilization, therefore reducing its intestinal absorption (7). Thus, a daily intake of phytosterols (PhySs) in the range of 1–3 g/day is now recommended for reducing plasma cholesterol (8, 9). Recent studies support the view that dietary PhyS interventions might also induce plasma TGL reduction, especially in subjects with overt hypertriglyceridemia and metabolic syndrome (10–12). However, hypotriglyceridemic effects of natural compounds are mainly related to the intake of long-chain n-3 PUFA (ω3) (13–16). Thus, the beneficial influence on cardiovascular risk of dietary supplementation with EPA and DHA has been largely related to their effects in lowering fasting and postprandial serum TGLs (17, 18). Contrarily, studies based on PUFA-enriched dairy food have controversial results when plasma LDLc levels are considered (19, 20).
During the last years, we have witnessed an extraordinary increase in the number of functional foods and especially as supplements of low-fat enriched foods due to their efficacy as lipid-lowering agents. Up to now, however, it is not clear whether these low-fat enriched foods also lead to metabolic effects at the level of specific plasma lipids and lipoproteins. Global mapping of metabolites by LC/MS/MS-based lipidomics has provided a powerful and reliable analytical tool to identify changes in lipid composition in biological fluids (21–23).
The purpose of the present study was to compare the effects of low-fat milk supplemented with PhyS or with ω3 FAs on the lipid profile of overweight subjects, either normolipemic or with mild dyslipidemia, in a double-blind randomized longitudinal crossover exploratory study. We report that the dietary interventions with low-fat milk supplemented with PhyS and ω3 induce significant changes in the LDL-lipidome beyond their effects on LDLc and TGL levels.
METHODS
Subjects
Healthy adult males and females between the ages of 25 and 70 years (n = 32), attending regular medical controls, were eligible for participation if they had overweight or grade 1 obesity (BMI 25–35 kg/m2). Subjects were excluded if they reported existing chronic illnesses including cancer, overt hyperlipidemia, diabetes mellitus, hypertension, or heart, liver, or kidney disease. Other exclusion criteria included use of lipid-lowering drugs, β-blockers, or diuretics, history of CVD, lactose intolerance, or being in a weight-loss program. To confirm health status, all subjects underwent a complete physical examination conducted by the study physician. Those consuming a PhyS-enriched spread and/or fish oil supplements or with strong aversion to milk-derived products were also excluded. The study was approved by the Human Ethical Review Committee of the Hospital Sant Pau in Barcelona. Informed written consent was obtained from all participants.
Study design
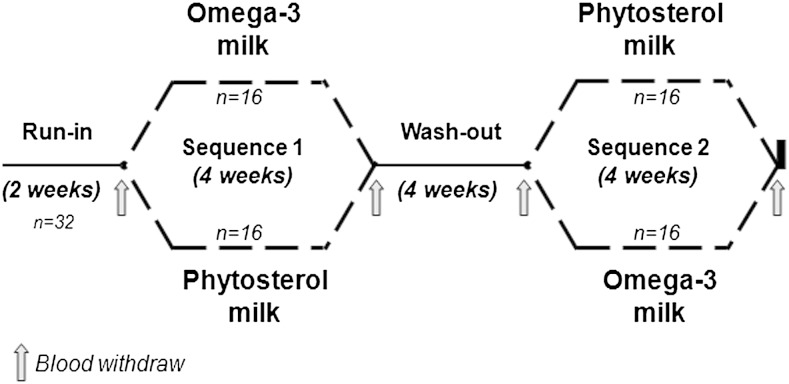
The trial consisted of a double-blinded randomized two-arm longitudinal crossover study with 28 day intervention periods (Fig. 1). Thus, all subjects were submitted to two 28 day treatment sequences, separated by a 4 week wash-out period. Before the initiation of the intervention, individuals were submitted to a 2 week run-in period. During the run-in and wash-out periods, participants received a commercially available plain low-fat milk (without PhyS or ω-3), with the same composition to that used for preparing the PhyS- and ω-3-enriched milks. As shown in Fig. 1, at the end of the run-in period, subjects were randomly allocated to receive one of the two treatment sequences (study arm 1, PhyS-enriched milk in the first intervention period and ω-3-enriched milk in the second intervention period; study arm 2, ω-3-enriched milk in the first intervention period and PhyS-enriched milk in the second intervention period). Participants were instructed to consume a pack of milk (250 ml) per day, distributed in one or more portions according to their normal habits, replacing their habitual milk product consumption.
Fig. 1.
Flow diagram describing the study design.
Subjects were requested to maintain their habitual diet at levels consistent with maintenance of a stable body weight and to continue their normal pattern of physical activity throughout the study period. Dietary habits, determined by using food frequency questionnaires, were recorded prior to each visit, and rare changes in diet habits were reported.
The plant sterol-enriched milk (PhyS-enriched milk) used in this study refers to a commercially available product, whereas the ω3-enriched milk was specifically prepared for the study. Both products were simultaneously produced under factory-controlled conditions by CAPSA Food (Spain) and portions packed in 250 ml plain containers only identified by a code were provided to the study participants. The nutrient composition is shown in Table 1. Lipid content varied from 0.4 g per 100 ml milk in the control low-fat milk to 0.8 g in the ω3-enriched milk and 0.9 g in the PhyS-enriched milk. In the ω3-enriched milk, 20.6% of the lipid content was ω3 FAs, mainly consisting of EPA and DHA (150 mg EPA + DHA/100 ml milk) that represented the 90.8% of the total ω3 FA content. In addition to lipid content, every 100 ml of PhyS-enriched milk contained 0.64 g plant sterols (expressed as free PhyS). According to the provider’s information, the supplemented vegetable oil-based sterols (VEGAPURE® 95E) were mainly found as sterol esters (>94%) with 68% PUFA (C 18:2), 19% monounsaturated FA (18:1), and 13% saturated FA (16:0, 18:0). The PhyS mix mainly consisted of sitosterol, campesterol, and stigmasterol (>77%), with sitostanol and campestanol as the more abundant stanols. The content in other sterols/stanols did not exceed the 3%.
TABLE 1.
Nutrient composition of the study products
| Low-Fat Milk (250 ml) | |||
| Control | PhyS Supplemented | ω3 Supplemented | |
| Energy | |||
| Kilojoules | 395 | 440 | 430 |
| Kilocalories | 92.50 | 104.0 | 102.5 |
| Proteins (g) | 9.25 | 9.25 | 9.25 |
| Carbohydrates (g) | 11.75 | 11.75 | 11.75 |
| Lipidsa | 1.00 | 2.25 | 2.00 |
| Saturated (g) | 0.675 | 0.880 | 0.975 |
| Monounsaturated (g) | 0.300 | 0.570 | 0.600 |
| Polyunsaturated (g)b | 0.025 | 0.800 | 0.425 |
| Fiber | — | — | — |
| Sodium | 0.15 | 0.15 | 0.15 |
| Calcium (g) | 0.325 (40%)c | 0.325 (40%)c | 0.325 (40%)c |
| Vitamin B1 (g) | — | 0.0008 (50%)c | — |
| Supplements | |||
| PhyS (g) | — | 1.60 | — |
| ω3 [EPA + DHA] (g) | — | — | 0.375 |
Excluding PhySs.
The ω3-supplemented milk includes the amount of added EPA + DHA (0.375 g/250 ml milk). PhyS-supplemented milk includes the content of PUFAs in the sterol product (VEGAPURE® 95E, BASF), according to the information given by the providers.
Percent of the daily required consumption.
Compliance was monitored by regular telephone contact with participants and interviewing them at the end of each intervention period. Participants also recorded whether they had consumed the milk product on a diary card each day.
Blood sampling
Twelve hour fasting blood samples were collected on days 1 and 28 (baseline and endpoint first treatment period) and on days 56 and 84 (baseline and endpoint second treatment period). Blood samples were collected without anticoagulant or in EDTA-containing Vacutainer tubes for serum and plasma preparation, respectively. Serum and plasma fractions were separated by centrifugation at 3,000 g for 20 min and stored at −80°C until analysis.
LDL sample preparation and purity control
LDLs (density 1.019–1.063 g/ml) were prepared from 2 ml plasma-EDTA samples by sequential ultracentrifugation between the densities of 1.019 and 1.063 g/ml, according to the method originally described by Havel, Eder, and Bragdon (24). Briefly, plasma was adjusted to a density of 1.019 g/ml with a concentrated salt solution (potassium bromide) and centrifuged at 225,000 g for 18 h in a Beckman Optima L-100 XP preparative ultracentrifuge with a fixed-angle type 50.4 Ti rotor. After removal of the top layer, containing very low and intermediate density lipoproteins (VLDL and IDL), the density of the infranatant was adjusted to 1.063 g/ml, followed by centrifugation for 20 h at 225,000 g. LDLs were collected from the top of the tube and were dialyzed against buffer [150 mmol/l NaCl, 20 mmol/l Tris-HCl, and 1 mmol/l EDTA (pH 7.4)] and against PBS. After dialysis, the LDL fraction (mean value 450 ± 32 μl) was adjusted to 500 μl with PBS. Mean LDL-protein content was 2.59 ± 1.3 mg/100 ml, as determined by the colorimetric assay BCA (Pierce).
LDL purity was routinely analyzed by electrophoresis (2 μl sample) in agarose gels using a commercial assay (SAS-MX Lipo 10 kit, Helena Biosciences), as described by the providers. In addition, LDL purity was checked by analyzing the LDL profile in pooled samples (four different extractions) of randomly selected subjects (1 subject/ultracentrifugation batch, n = 7). Chromatography analysis was performed by microgel filtration using a Superose 6 PC 3.2/30 column and an Agilent 1200 HPLC system, as described by Garber, Kulkarni, and Anantharamaiah (25), with slight modifications. Briefly, 10 μl of undiluted LDL sample fraction were loaded in the system and run with a constant flow of 100 μl/min. Retention time for the LDL fraction was compared with those for VLDL and HDL.
Anthropometric data, blood pressure, serum lipid profile, and other biochemical measurements
Anthropometrical measurements, except height (cm), were determined at the initial visit and along the study at baseline and postintervention (days 1 and 28 of each intervention period). BMI was calculated as weight (kilograms)/height (square meters). Waist circumference (WC) was measured between the lowest rib and the iliac crest with the participant standing. Waist-to-height ratio (WHtR) was calculated as WC in centimeters divided by height in centimeters.
Serum biochemical measurements were performed using routine commercially available methods, including those for glucose, hepatic and renal markers, and standard serum lipid profile [TGLs, TC, and HDLc (Roche Diagnostics, Basel, Switzerland)]. As there were no cases of hypertriglyceridemia, LDLc was calculated using the Friedewald equation.
FA and lipidomic pattern analysis
FA and lipidomic pattern were determined in plasma and LDL samples, respectively, after lipid extraction in methanol/chloroform according to the Bligh and Dyer method (26), with some modifications. Dry samples were reconstituted in chloroform:methanol:isopropanol (1:2:4) containing ammonium acetate and analyzed by LC/MS/MS using a HPLC system (Agilent 1200) coupled to a AB Sciex 3200 Qtrap triple quadrupole mass spectrometer.
Analysis of the FA profile by LC/MS/MS was performed after derivatization of the samples to convert FAs in trimethylaminoethyl ester iodide derivatives. The mass spectrometer instrument was equipped with a turbo spray source operated in positive ionization mode. The source was operated at 400°C with ion voltage of 5,500 V, and nitrogen as curtain and nebulizer gas. For analysis of FA profiles, a neutral loss scan (NL) of 59.0 was used. Triple quadruple mass spectrometry (QqQ-MS) data were analyzed with LipidView version 1.1 software (AB Sciex). Lipid identification was based on their characteristic head groups and corresponding FAs against the LipidView library, which contains over 50 lipid classes, 25,000 lipid species, and more than 600 characteristic lipid fragments. Lipid quantification was referred to defined concentrations of the internal standards added in the derivatization step [myristic-d27 acid (3,3 M), palmitic acid-1 13C (0.3 M), and stearic-2-2-d2 acid (1.3 M)].
For accurate plasma level quantification of specific targeted FAs, a multiple reaction monitoring approach was performed, using the MS instrument on ionspray and positive ion operating mode at 400°C. Quantification was made on the basis of an internal standard mixture added to the samples prior to the derivatization procedure (3.1 M myristic-d27 acid, 0.3 M palmitic acid 1-13C, 1.3 M stearic-2-2-d2 acid from Sigma Aldrich, and 0.3 M arachidonic acid-d8 and 0.3 M linoleic acid-d4 from Cayman Chemical).
The LDL lipidomic profile was analyzed in a mixture of 30 μl LDL and 20 μl of lipid internal standard mixture [16.1 μM phosphatidylserine (PS) 17:0/17:0 (16.1), 23.5 μM phosphatidylethanolamine (PE) 17:0/17:0, 59.3 μM cholesteryl ester (CE) 17:0, 41.4 μM phosphatidylcholine (PC) 17:0/17:0, and 13.7 μM lysoPC (LPC) 19:0; Avanti Polar Lipids). Lipids were separated using a LC system with a Zorbax Eclipse XDB-C18 column (4.6 × 50 mm, 1.8 μ particle size) and binary gradient program. The binary solvent consisted of 10 mM ammonium acetate in 40:60 acetonitrile:water (mobile phase A), and 10 mM ammonium acetate in 10:90 acetonitrile:isopropanol (mobile phase B). The MS analysis was performed by ESI-MS/MS operating in positive ionization mode, using a method described by Hu et al. (27), with minor changes. The declustering potential was 70 V, and the collision energy was 32 V. A specific scan mode was created for each lipid class (head group) with the following precursor ion (PI): PI m/z 369.3 for CEs, PI m/z 184.1 for PCs and SMs, NL m/z 141.0 for PEs, NL m/z 185.0 for PSs, and FA scans for ammonium adducts of FAs to determine the lipid FA composition. For all specific scan modes, the following conditions were used: unit resolution for Q1 and Q3, scan range from m/z 350 to m/z 1,200, and sample acquisition through 20 min. Lipid names and abbreviations were assigned according to LIPID MAPS nomenclature. The analytical data were processed with LipidView V1.1 (AB Sciex) software. Total lipid composition was determined as number of carbon atoms and double bonds without specifying the location of double bonds or the stereochemistry of the acyl chains. Peak areas for each lipid species were normalized with the corresponding lipid internal standard according to the phospholipid subclasses measured (phopholipids with an odd number of carbon atoms were used as internal standard due to their extremely low presence or absence in human plasma).
LDL particle size measurement
LDL size was directly measured in plasma-EDTA (500 μl) by NMR (Biosfer Teslab, Reus, Spain), as described by Mallol et al (28). Briefly, particle concentration and the diffusion coefficients were obtained from the measured amplitudes and attenuation of their spectroscopically distinct lipid methyl group NMR signals using the 2D diffusion-ordered 1H NMR spectroscopy (DSTE) pulse. The methyl signal was surface fitted with nine Lorentzian functions associated with each lipoprotein subtype: large, medium, and small of the LDL. The area of each Lorentzian function was related to the lipid concentration of each lipoprotein subtype, and the size of each subtype was calculated from their diffusion coefficient. The particle numbers of each lipoprotein subtype were calculated by dividing the lipid volume by the particle volume of a given class. The lipid volumes were determined by using common conversion factors to convert concentration units into volume units (29).
LDL susceptibility to oxidation
The resistance of LDL to form peroxides was assessed by measuring thiobarbituric acid-reactive substances (TBARSs) after incubation with Cu2+. Briefly, LDLs (1 μg/ml adjusted in PBS) were oxidized in the presence of 5 mM of Cu2SO4 for 6 h at 37°C. At the end of the incubation period, LDL oxidation was stopped by cooling the samples to 4°C and adding 5 μl of 5 mM EDTA. Lipid peroxidation of LDL was assessed by TBARSs formation, according to Ohkawa, Ohishi, and Yagi (30), with slight modifications. To this aim, samples were incubated with 0.5 ml 20% trichloroacetic acid and 0.5 ml of 1% thiobarbituric acid. After incubation at 100°C for 15 min, samples were cooled on ice and the absorbance was measured at 532 nm. A calibration curve was prepared with malondialdehyde (MDA) as standard. Results were expressed as nanomoles of MDA per milligram of LDL protein. All samples gave results which were within the linear portion of the MDA standard curve (1.25–20.0 nM).
Statistical analysis
Statistical analyses were conducted using Statview and SPSS software, except when indicated. Data are expressed by the number of cases (qualitative variable) and as mean ± SEM or median [interquartile range] for the quantitative variable. Levels of serum lipids, FAs, LDL lipid components, body weight, BMI, and WHtR at the end of the run-in and the wash-out periods were considered as the baseline value for the following intervention period. Differences in the baseline characteristics of the groups and in the percentage of change between intervention diets were analyzed by unpaired Student’s t-test for parametric variables and chi-square test for nonparametric variables. Treatment order and gender were entered in the models as an additional factor, but they were not significantly different and were not considered further. Effects of the 4 week interventions were evaluated using a paired Student’s t-test (baseline and postintervention values) or an ANOVA test introducing the different obesity and lipid-related variables as covariable when required. Principal component analysis (PCA) was performed to evaluate the global variation of the plasma FA profiles with R statistical software. Correlation between continuous variables was assessed by means of Pearson’s correlation. All reported P values are two-sided, and a P value of 0.05 or less was considered to indicate statistical significance.
Due to the exploratory character of the LDL-lipidome studies, adjustments for multiple testing (LDL lipid components) have been performed by the false discovery rate (FDR) using the graphically sharpened method described by Benjamin and Hochberg (31). The new calculated adjusted q values (FDR adjusted) indicate the probability of false positives for variables considered to be significant. In the present study it refers to q values <0.10, which represents more than 90% truly positive for differentially expressed LDL lipid species.
RESULTS
Clinical and biochemical characteristics before and after intervention
Thirty-two subjects initially recruited for the study (13 males and 19 females) completed both experimental phases and were included in the final analysis. There were not drop-outs. Table 2 shows the characteristics of the study population at baseline, after the run-in period, and at the time of starting each experimental period. All subjects included in the study had overweight or type 1 obesity (BMI values in the range 25.0–34.0 kg/m2). BMI was similar between males and females, with a median BMI of 28.0[4.4] kg/m2 in males and 27.4[2.9] kg/m2 in females (Mann-Whitney test, P = 0.126). Baseline values of all variables were not statistically different between dietary interventions (Phys-milk vs. ω3-milk; Table 2). As shown in supplementary Table 1, after the four week intervention with PhyS-enriched milk, the study group did not show any significant change in body weight, BMI, WC, or WHtR. A minimal, but significant, decrease in body weight and BMI was observed after treatment with ω3-milk.
TABLE 2.
Characteristics of the study population at baseline after the run-in period and at the time of starting each interventional food
| After Run-in Period | Before PhyS Dietary Phase | Before ω3 Dietary Phase | Pa | |
| Male/female | 19/13 | — | — | — |
| Age (years) | 50.5 ± 1.6 | — | — | — |
| Body weight (kg) | 75.4 ± 2.0 | 75.3 ± 2.0 | 75.4 ± 2.0 | NS |
| BMI (kg/m2) | 27.8 ± 0.4 | 27.8 ± 0.4 | 27.9 ± 0.4 | NS |
| Waist (cm) | ||||
| Females | 92.4 ± 1.6 | 92.4 ± 1.6 | 92.4 ± 1.6 | NS |
| Males | 100.4 ± 2.2 | 100.4 ± 2.2 | 100.4 ± 2.2 | NS |
| WHtR (cm/cm) | 0.58 ± 0.01 | 0.58 ± 0.01 | 0.59 ± 0.01 | NS |
| Glucose (mM) | 4.8 ± 0.1 | 4.7 ± 0.1 | 4.8 ± 0.1 | NS |
| Urea (mM) | 6.0 ± 0.2 | 5.8 ± 0.2 | 6.0 ± 0.2 | NS |
| Uric Acid (μM) | 296.4 ± 12.1 | 299.0 ± 13.7 | 300.9 ± 12.4 | NS |
| Creatinine (μM) | 66.1 ± 1.8 | 69.2 ± 2.2 | 68.0 ± 2.0 | NS |
| GGT (U/l) | 29.2 ± 4.7 | 29.4 ± 4.5 | 28.8 ± 4.0 | NS |
| ALT (U/l) | 23.1 ± 2.2 | 22.3 ± 2.1 | 25.5 ± 2.6 | NS |
| AST (U/l) | 21.1 ± 1.2 | 20.7 ± 1.1 | 22.2 ± 1.4 | NS |
| TC (mg/dl) | 215.0 ± 4.2 | 213.8 ± 6.0 | 216.2 ± 6.1 | NS |
| HDLc (mg/dl) | 55.3 ± 2.1 | 54.5 ± 3.1 | 56.5 ± 2.9 | NS |
| Non-HDLc (mg/dl) | 159.7 ± 4.1 | 159.3 ± 5.8 | 159.7 ± 6.0 | NS |
| LDLc (mg/dl) | 137.3 ± 3.5 | 137.7 ± 4.9 | 136.9 ± 5.0 | NS |
| VLDLc (mg/dl) | 22.4 ± 1.7 | 22.0 ± 2.0 | 22.8 ± 4.7 | NS |
| TGL (mg/dl) | 112.5 ± 8.8 | 109.8 ± 10.3 | 115.1 ± 14.3 | NS |
Difference in baseline values between dietary phases (plant sterol-enriched milk and ω3-enriched milk) were assessed by Student’s t-test.
Fifty-six percent of the participants had baseline LDLc levels in the borderline-high range (130–159 mg/dl) or in the high range (160–189 mg/dl), according the ATPIII guidelines (32), and were defined as the High-LDL group in comparison with those subjects with baseline LDLc <130 mg/dl (Low-LDL group) (supplementary Table 2A). The two subgroups, with mean serum LDLc levels of 113.3 ± 4.2 mg/dl and 160.4 ± 4.7 mg/dl, respectively, significantly differed in non-HDLc, but they did not show any difference in HDLc, VLDL cholesterol (VLDLc), or TGL levels. In addition to these groups, a group of subjects with TGL levels above 150 mg/dl (range 150–290 mg/dl) and low HDLc levels (males <40 mg/dl, females <50 mg/dl) was classified as the atherogenic-dyslipidemia group (AT-DYSL) (supplementary Table 2B). None of the participants were under lipid lowering therapy during the duration of the study.
Consumption of all milk products was well-tolerated and no adverse effects were reported. Compliance was judged as 100% in each diet period according to the participant’s response to the weekly telephonic controls and the returned filled cards, based on the consumption registry, at the end of each intervention period.
Effect of dietary treatments on serum lipid profile
Mean serum lipid concentration at baseline and after each intervention period are presented in Table 3. Mean serum concentrations of TC, non-HDLc, and LDLc were significantly reduced after intervention with milk supplemented with PhyS, whereas they did not show any significant change after milk supplemented with ω3. In contrast, VLDLc and TGL levels were significantly reduced after intervention with ω3-enriched milk. HDLc plasma levels did not differ significantly from baseline either after ω3- or PhyS-enriched milk intake.
TABLE 3.
Serum lipid levels before and after 4 week dietary intervention with low-fat milk supplemented with PhySs or ω3
| Serum Lipids | PhyS-Supplemented Milk Intervention | ω3-Supplemented Milk Intervention | ||||
| Before | After | Pa | Before | After | Pa | |
| TC (mg/dl) | 216 ± 6 | 204 ± 6 | 0.001 | 216 ± 6 | 214 ± 6 | 0.193 |
| Variation (%)a | (−4.2 ± 1.2) | (−1.1 ± 1.0) | ||||
| HDLc (mg/dl) | 54 ± 3 | 54 ± 3 | 0.999 | 56 ± 3 | 56 ± 3 | 0.317 |
| Variation (%)a | (1.5 ± 3.0) | (−0.8 ± 1.5) | ||||
| Non-HDLc (mg/dl) | 159 ± 6 | 150 ± 6 | 0.001 | 160 ± 6 | 156 ± 6 | 0.263 |
| Variation (%)a | (−5.8 ± 1.8) | (−1.2 ± 1.1) | ||||
| LDLc (mg/dl) | 138 ± 5 | 127 ± 5 | 0.000 | 140 ± 5 | 138 ± 5 | 0.676 |
| Variation (%)a | (−7.3 ± 1.8) | (1.1 ± 1.7) | ||||
| VLDLc (mg/dl) | 22 ± 2 | 23 ± 3 | 0.647 | 23 ± 5 | 20 ± 2 | 0.045 |
| Variation (%)a | (3.2 ± 3.9) | (−6.3 ± 4.0) | ||||
| TGL (mg/dl) | 110 ± 10 | 115 ± 15 | 0.649 | 115 ± 14 | 99 ± 9 | 0.050 |
| Variation (%)a | (3.1 ± 3.9) | (−5.9 ± 4.1) | ||||
Lipid levels are expressed in milligrams per deciliter before and after intervention and changes relative to baseline (%) are given between brackets. Data are given as mean ± SEM. Values before and after the 4 week interventions were analyzed by paired Student’s t-test. n = 32. P ≤ 0.05 indicates significance.
Percentage of changes in plasma lipids levels after 4 week intervention with PhyS- and ω3-supplemented milk.
As shown in Table 3, changes relative to baseline for serum TC, non-HDLc, and LDLc were significantly different between PhyS- and ω3-supplemented milk intakes. Thus, a mean 7.3% decrease in the LDLc level was found with the PhyS intervention, while no beneficial effect was seen in LDLc after consumption of ω3-milk (P = 0.002). In addition, the intervention with the PhyS-milk had a lower number of nonresponders (5 out of 32 did not show an LDLc decrease) than the intervention with ω3-milk (15 out of 32) (chi-square for differences P = 0.007). On the contrary, there was a significant reduction (absolute values) in serum TGL and VLDLc after ω3-milk intake that was not seen after PhyS-milk intake (Table 3). Differences in the response of LDLc and TGL to PhyS- and ω3-milk interventions were also found when BMI or WHtR was included as a covariable in the ANOVA analysis (changes in LDLc, P < 0.005 for BMI or WHtR; changes in TGL, P < 0.05 for BMI or WHtR).
The response to the ω3 and PhyS interventions was analyzed in subjects with plasma LDLc levels below and above the cut-off level of 130 mg/dl (Low-LDLc and High-LDLc groups, see supplementary Table 2). As shown in Table 4, intervention with the PhyS-supplemented milk significantly reduced serum TC, non-HDLc, and LDLc levels in the High-LDLc subgroup, while effects were of less magnitude in the Low-LDLc subgroup. In contrast, intervention with ω3-supplemented milk did not induce any significant change when the groups with LDLc below and above the borderline cut-off were analyzed separately (Table 4). A similar trend was observed when the effects of milk interventions were expressed as percent of change from baseline levels (supplementary Fig. 1A, B).
TABLE 4.
Changes induced by intake of PhyS- and ω3-enriched milk in serum lipid levels of individuals with LDL below and above the borderline-high cut-off value (130 mg/dl) according ATPIII guidelines at baseline (absolute values)
| Serum Lipids | PhyS-Supplemented Milk Intervention | ω3-Supplemented Milk Intervention | ||
| Low-LDL Group (n = 14) | High-LDL Group (n = 18) | Low-LDL Group (n = 14) | High-LDL Group (n = 18) | |
| TC | −7.2 ± 3.7a | −10.0 ± 3.4a | −3.2 ± 2.5 | −2.6 ± 3.4 |
| HDLc | 1.8 ± 2.4 | −1.3 ± 1.7 | −0.8 ± 1.4 | −0.9 ± 1.0 |
| Non-HDLc | −9.0 ± 4.9a | −8.7 ± 2.8b | −2.4 ± 1.6 | −1.8 ± 3.1 |
| LDLc | −7.5 ± 4.3 | −10.8 ± 2.7c | −0.7 ± 2.3 | 2.5 ± 4.1 |
| VLDLc | −1.4 ± 1.3 | 2.2 ± 1.0 | −1.8 ± 1.4 | −4.3 ± 2.5 |
| TGL | −6.8 ± 6.2 | 10.7 ± 4.9 | −8.7 ± 6.9 | −21.2 ± 12.6 |
Low LDL and High-LDL groups refer to subjects with baseline LDLc level below and above 130 mg/dl, defined as a borderline value for high LDL levels according the ATPIII guidelines (27). Data are expressed in milligrams per deciliter and given as mean ± SEM. Statistical significance in changes after intervention was determined by Student’s t-test.
P ≤ 0.05 for differences from baseline.
P < 0.01 for differences from baseline.
P < 0.001 for differences from baseline.
Plasma TGL reduction after intervention with ω3-milk was especially evident in subjects with low HDLc levels (<40 mg/dl in males and <50 mg/dl in females) and TGL levels above 150 mg/dl (atherogenic dyslipidemia group) (Table 5, supplementary Fig. 1D). The subgroup with a ratio TGL/HDLc above the median level of the study population (median ratio, 1.87) showed a 15% reduction in TGL levels (P = 0.02 vs. baseline) following the ω3-milk, whereas no significant changes were found in the subgroup with TGL/HDLc ratios below the median value (difference between groups, P = 0.03; supplementary Fig. 1F). The Phys-milk intervention reduced LDLc levels independently of the absence/presence of atherosclerotic dyslipidemia (supplementary Fig. 1C) or the TGL/HDLc ratio (supplementary Fig. 1E).
TABLE 5.
Impact of baseline TGL and HDLc in the response of serum lipid levels to PhyS- and ω3- supplemented milk intake
| Serum Lipids | PhyS-Supplemented Milk Intervention | ω3-Supplemented Milk Intervention | ||
| Non-AT-DYSLP Group (n = 27) | AT-DYSLP Group (n = 5) | Non-AT-DYSLP Group (n = 27) | AT-DYSLP Group (n = 5) | |
| TC | −8.0 ± 2.7 | −15.7 ± 5.8 | −4.3 ± 2.2 | 4.9 ± 6.8 |
| HDLc | −0.3 ± 1.6 | +0.3 ± 1.0 | −1.2 ± 0.9 | 1.5 ± 1.0 |
| Non-HDLc | −8.0 ± 2.8 | −15.9 ± 6.8 | −3.1 ± 1.9 | 3.4 ± 6.2 |
| LDLc | −8.5 ± 2.4 | −18.1 ± 9.6 | −2.6 ± 2.1 | 20.8 ± 5.4a |
| VLDLc | 0.5 ± 0.8 | 2.3 ± 5.6 | −0.6 ± 0.8 | −17.4 ± 6.0a |
| TGL | 2.3 ± 3.8 | 10.9 ± 27.5.5 | −2.5 ± 3.9 | −87.1 ± 29.7a |
AT-DYSLP, atherosclerotic dyslipidemia was defined by TGL levels >150 mg/ml and HDLc levels <40 mg/dl in males or <50 mg/dl in females. Data are expressed in milligrams per deciliter and given as mean ± SEM. Statistical significance in changes after intervention were determined by Student’s t-test.
P < 0.001 for differences from baseline.
Effect of ω3- and PhyS-enriched milk interventions on the LDL resistance to oxidation
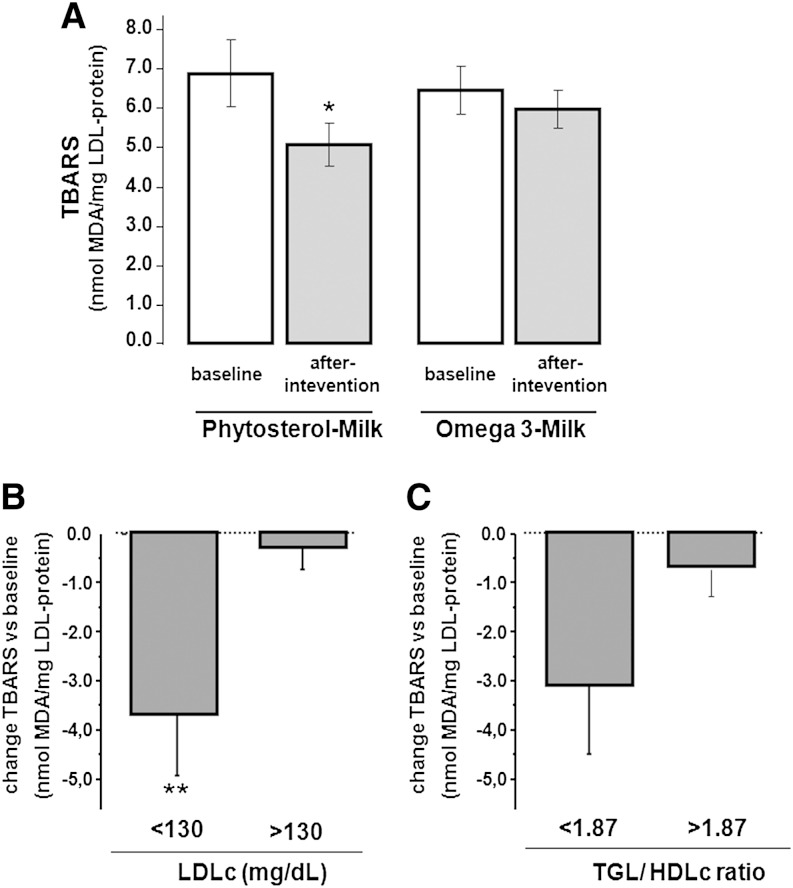
LDL susceptibility to oxidation was determined by TBARSs at baseline and after interventions with ω3- and PhyS-enriched milk. The study was performed in the subgroup of 18 patients included in the LDL-lipidome analysis. As shown in Fig. 2A, susceptibility of LDL to copper ion-induced oxidation was significantly reduced after intervention with PhyS-enriched milk (26% decrease, P = 0.045), whereas the effect was not significant with ω3-enriched-milk intake (7.3% decrease, P = NS). The PhyS-induced decrease of LDL susceptibility to oxidation was significantly more evident in the subgroup with LDLc values below 130 mg/dl (Fig. 2B). A trend to a lower susceptibility to LDL oxidation was also found in the subgroup with a low TGL/HDLc ratio (Fig. 2C).
Fig. 2.
Level of TBARSs for in vitro oxidized LDL at baseline and after PhyS- and ω3-milk interventions. A: Bar diagram refers to the level of TBARSs expressed as nanomoles of MDA obtained from LDL (1 μg/ml) incubated with 5 μM Cu2+. B: Changes in TBARSs (from baseline) in the Low- and High-LDL subgroups (plasma LDLc <130 mg/dl and >130 mg/dl, respectively). C: Changes in TBARSs (from baseline) in the subgroups with TGL/HDL ratios below/above the median cut-off value (1.87). *P < 0.05, **P < 0.001 compared with baseline.
Plasma FA profile
The plasma FA profile is shown in supplementary Fig. 2. Plasma levels of FAs did not differ significantly after run-in and wash-out periods (supplementary Table 3). Analysis by PCA was performed to investigate the global variation of the plasma FA profile after interventions. PCA results showed that the two principal components (PC1 and PC2) of the established PCA model explained 62 and 58%, respectively, of the total variance of the plasma FA data set. ANCOVA test of the principal components (PC1–PC5, >75% variability) revealed that global differences in plasma FA patterns were negligible. Thus, the plasma FA profile after 4 weeks of intervention with either ω3- or PhyS-supplemented milk did not differ significantly from baseline. Similarly, nonsignificant differences were detected when total levels of polyunsaturated, monounsaturated, or saturated FAs were compared with baseline after the intervention periods (data not shown).
Plasma levels of DHA and EPA (long-chain n-3 FAs supplemented into the ω3-enriched milk), the two major plasma FAs [palmitic acid (27% plasma) and stearic acid (22%)], linoleic acid and arachidonic acid (ω-6 FAs), oleic acid (ω-9 FA), as well as α- and γ-linolenic FAs (α- and γ-linolenic acid) were further quantified by multiple reaction monitoring. PhyS-milk did not induce any change relative to baseline in the total plasma levels of these FAs. However, after intervention with ω3-enriched milk, palmitic acid was significantly reduced (8% vs. baseline, P = 0.03) and a trend to lower oleic acid (8% reduction, P = 0.07) and higher DHA levels (12%, P = 0.07) were observed.
Effect of ω3- and PhyS-milk on the LDL-lipidome
The LDL fraction obtained by ultracentrifugation resulted in a single band by electrophoresis in agarose gels and it was found as a single peak with elution time at 130 min (VLDL at 128 min, HDL at 134 minutes), when analyzed by LC.
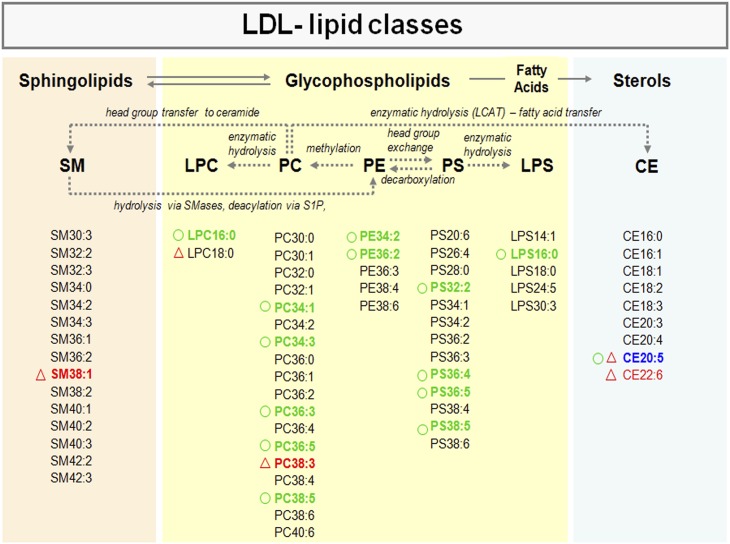
Analysis of the LDL-lipidome by LC-ESI-MS/MS led to detection of different lipid classes: CEs, sphingophospholipids (SM), and glycerophospholipids (GPLs) including PC, LPC, PEs, PS, and lysoPS (LPS) (Fig. 3, supplementary Fig. 3). CE was the most abundant lipid class in the LDL, representing 75% of the total LDL lipid content at baseline, whereas GPL and SM contents were 20% and 5%, respectively. Among GPL subclasses, PC represented 95% of the total GPL content and PS 4.9%. Only traces of PE metabolites (0.1%) were detected in the LDL, either at baseline or after the interventions.
Fig. 3.
Scheme of interrelation pathways between lipid classes contained in human LDL. Lipid metabolites consistently detected by LC/MS/MS (see Materials and Methods section) for each lipid subclass are listed. LDL metabolites reduced (green) or increased (red) after interventions are indicated (P < 0.05 with ANOVA for repeated measures). Blue refers to metabolites with the opposite trend after both interventions The circles refer to the PhyS-milk intervention and the triangles to the ω3-intervention. The scheme refers to a global overview on relationships among major lipids. It is to note that: 1) LCAT mediates a two-step reaction in which a FA is cleaved from a PC and transesterified to the 3-β-hydroxyl group on cholesterol to form CE. 2) SM derives in PE through hydrolysis by SMases and deacylation via sphingosine 1-phosphate (S1P). 3) SM is generated by the transfer of phosphocholine from phosphatidylcholine (PC) to a ceramide with the generation of diacylglycerol (DAG) through SM synthase (SMS).
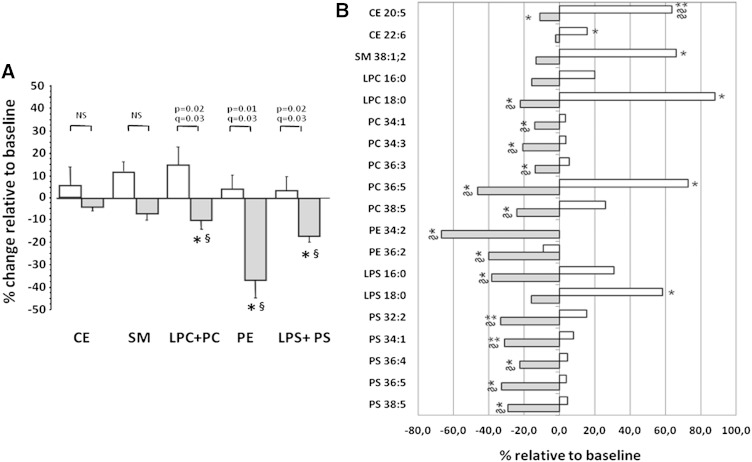
As shown in Table 6, intervention with Phys-milk induced a significant reduction in the content of the different GPL lipid groups (P < 0.05; FDR adjustment: q value = 0.075), whereas nonsignificant changes were induced by the ω3-milk. Changes relative to baseline were significantly different between both interventions for all GPL groups (Fig. 4A; P < 0.05; FDR adjustment: q value = 0.03). Neither total CE content nor SM content was modified after intervention with Phys- or ω3-milk (Table 6). Although there were significant decreases in the GPL families after the intervention with PhyS-supplemented milk compared with baseline, no significant changes were observed when the ratios LDL-GPL/protein (0.159 ± 0.15 at baseline vs. 0.176 ± 0.35 after PhyS intervention; P = 0.142) or LDL-GPL/SM (2.21 ± 0.08 at baseline vs. 2.06 ± 0.09 after PhyS intervention; P = 0.087) were calculated. Similarly, no significant changes were found after intervention with ω3-milk.
TABLE 6.
Total level of lipid classes in plasma LDL before and after 4 weeks dietary intervention with low-fat milk supplemented with PhySs or ω3
| Lipid Classes | PhyS-Supplemented Milk Intervention | ω3-Supplemented Milk Intervention | ||||
| Before | After | Pa | Before | After | Pa | |
| CE | 339.7 ± 22.5 | 336.1 ± 23.0 | 0.857 | 337.9 ± 22.8 | 344.5 ± 24.6 | 0.745 |
| SM | 17.8 ± 1.2 | 16.3 ± 1.1 | 0.213 | 18.4 ± 1.9 | 20.2 ± 3.1 | 0.559 |
| PC + LPC | 37.3 ± 2.35 | 32.6 ± 2.38 | 0.050b | 37.0 ± 3.7 | 40.0 ± 6.6 | 0.655 |
| PE | 0.05 ± 0.01 | 0.03 ± 0.00 | 0.049b | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.862 |
| PS + LPS | 1.64 ±0.25 | 1.34 ± 0.19 | 0.023b | 1.46 ± 0.20 | 1.54 ± 0.20 | 0.606 |
Data are shown as mean ± SEM of LDL lipid specimens given as milligrams per 100 ml plasma for the CE species and micrograms per 100 ml plasma for SM, LPC, PC, PE, LPS, and PS species. n = 19 per intervention group.
P value obtained by paired Student’s t-test between values before and after intervention for each group.
The q values after FDR adjustment = 0.075.
Fig. 4.
LDL lipid composition after dietary intervention with (grey bars) and ω3- (empty bars)-supplemented milk. A: Percentage of change relative to baseline for accumulative intensities in each lipid class: CE, SM, LPC, PC, PE, LPS, and PS. B: Bars refer to mean percentage of change relative to baseline for specific lipid species. Only lipid species showing a statistically significant change either after the PhyS- or ω3-milk interventions are displayed. P values obtained by paired Student’s t-test and q values by FDR adjustment (n = 19 subjects). *P < 0.05 and **P < 0.001 for difference relative to baseline. §The q value <0.08 and §§q value <0.001 after FDR adjustment.
From the three lipid classes analyzed by LC-ESI-MS/MS in LDL, 64 lipid species were consistently detected (Fig. 3). These refer to 9 CE, 17 SM, and 38 GPL (18 PC, 2 LPC, 13 PS, and 5 LPS). In addition, traces of five PE species (<1 μg/dl plasma) were also detected. Mean values for each individual lipid species (baseline and after the 4 week interventions) are shown in supplementary Table 4 and those giving a significant change by the univariate ANOVA analysis for repeated measures (P < 0.05), after 4 week dietary intervention, are shown in Fig. 3 and Fig. 4B. Compared with baseline, CE20:5 and CE22:6 were increased 65 and 15%, respectively (ANOVA, P < 0.05), after intervention with ω3-milk, whereas CE20:5 level was 11% reduced (ANOVA P = 0.05) by the PhyS intervention. However, FDR adjustment gave q values >0.10, except for changes induced by ω3-milk in CE20:5 (q value <0.001), pointing to this CE metabolite as the most consistently affected by the milk interventions. In the LDL, none of the SM-containing lipids were modified by the PhyS-milk or the ω3-milk intervention. Among the 43 GPL species, 14 were significantly reduced by PhyS-milk according to the ANOVA analysis for repeated measures (P < 0.05) with q values ≤0.085 after FDR adjustment, which indicates a probability below 1.2 of false positives among the 14 GPL metabolites. In contrast, three GPL species showed an increasing trend after the ω3 intervention (P < 0.05 by ANOVA) with q values >0.1 after FDR adjustment. Changes induced by the PhyS-milk referred to five PCs (PC34:1, PC34:3, PC36.3, PC36:5, PC38:5), five PSs (PS32:2, PS34:1, PS36:4, PS36:5, PS38:5), the lysoforms LPC16:0 and LPS16:0, and two PEs (PE34:2 and PE36:2). The changes induced by PhyS-milk in the LDL lipid species remained statistically significant (P < 0.05, q = 0.03) after including plasma LDLc or TGL levels as covariable in a multivariate ANOVA analysis (supplementary Table 5).
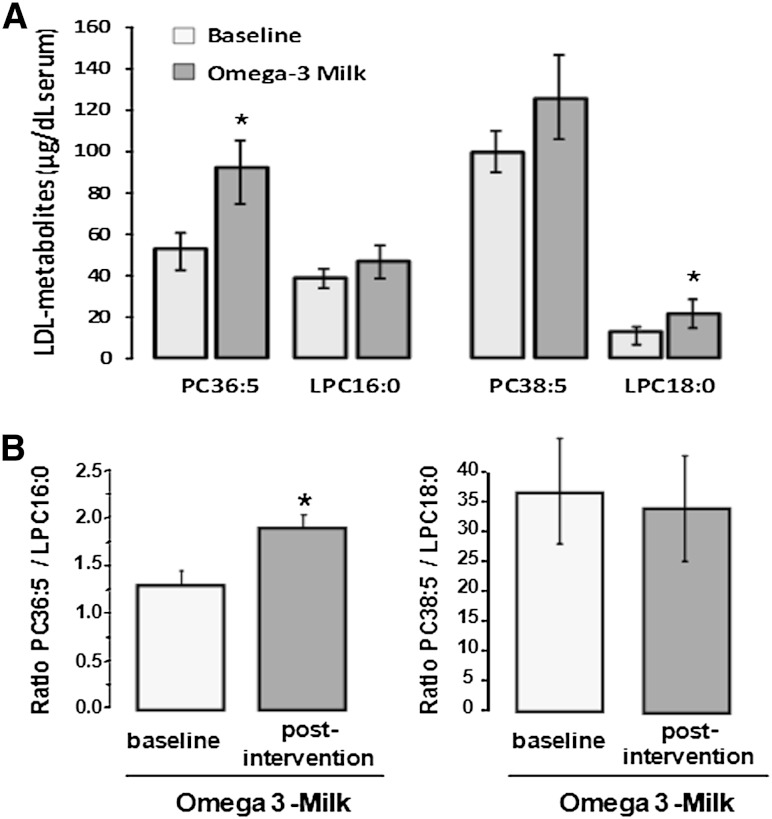
Four week intervention with ω3-milk induced a 75–80% increase in the LDL metabolites PC36:5 and LPC18:0 (ANOVA, P < 0.05), and a positive trend (20% increase), although not statistically significant, in the metabolites PC38:5 and LPC16:0 (Fig. 5A, supplementary Table 5). PC36:5 might be derived from different FA combinations including 20:5/16:0 and 18:5/18:0. However, FA18:5 was not detected in plasma in our study and to our knowledge, it mainly refers to a component of the lipids in dinoflagellates (33). Therefore, we considered 20:5/16:0 as the most plausible combination forming PC36:5 and we selected this combination for calculating the ratio PC/LPC. As shown in Fig. 5B, the ratio PC36:5/LPC16:0 was significantly increased after ω3 intervention (P = 0.001). In contrast, the ratio PC38:5/LPC18:0 did not show any statistically significant change at the end of the ω3 intervention period when compared with baseline.
Fig. 5.
PC/LPC levels after dietary intervention with ω3-supplemented milk. A: Bar-diagram for levels of the LDL-metabolites PC36:5, LPC16:00, PC38:5, and LPC18:0 at baseline and after dietary intervention with ω3-supplemented milk. B: Bar-diagram showing ratios PC36:5/LPC16:0 and PC38:5/LPC18:0 at baseline and after dietary intervention with ω3-supplemented milk. It is of note the increase in PC36:5/LPC16:0 at the end of the intervention periods. A, B: Values are given as mean ± SEM. *P < 0.05 for difference relative to baseline; P values obtained by paired Student’s t-test (n = 19 subjects).
Effect of ω3- and PhyS-milk on the LDL particle size
To better explore the effects of PhyS and ω3 interventions on LDL structure and its relationship with the lipid composition, LDL particle size was determined by NMR in a subset of samples obtained before (baseline) and after Phys and ω3 interventions from five independent subjects. The mean value for LDL diameter size in our study was of 20.7 ± 0.18 nm. The amount of the small size LDL (sLDL) fraction was approximately 60% of the total LDL particles in plasma. LDL size negatively correlated with the LDL-GPL/SM ratio (r = −0.672, P < 0.001), LDL-GPL/protein ratio (r = −0.489, P < 0.027), and total TGL plasma level (r = −0.881, P < 0.001). In contrast, LDL size positively correlated with the LDL-CE content (r = 0.458, P < 0.041). Among these variables, only LDL-CE content and plasma TGL levels remained significantly correlated with LDL size in a multiple variable correlation analysis (P < 0.0001 for each one). Four weeks of intervention with PhyS- or ω3-supplemented milk did not induce any significant change in the LDL diameter size or the percent of sLDL (Table 7).
TABLE 7.
Changes induced by intake of PhyS- and ω3-enriched milk on LDL particle size
| PhyS-Supplemented Milk Intervention | ω3-Supplemented Milk Intervention | |||||
| Before | After | Pa | Before | After | Pa | |
| LDL size (diameter, nm) | 20.7 ± 0.39 | 20.5 ± 0.49 | 0.387 | 20.6 ± 0.32 | 20.8 ± 0.36 | 0.300 |
| sLDL-P (% total) | 485.8 ± 96.4 | 484.2 ± 87.9 | 0.453 | 512.0 ± 93.7 | 477.4 ± 89.4 | 0.198 |
| sLDL-P (nm/l) | 59.7 ± 8.7 | 63.3 ± 10.2 | 0.981 | 62.7 ± 7.3 | 57.5 ± 7.7 | 0.331 |
Data are shown as mean ± SEM. sLDL-P (% total), small LDL particles expressed as percentage of the total LDL particles in plasma.
P value obtained by paired Student’s t-test between values before and after intervention for each group.
Correlation networks of specific LDL lipid metabolites as response to ω3- and PhyS-milk interventions
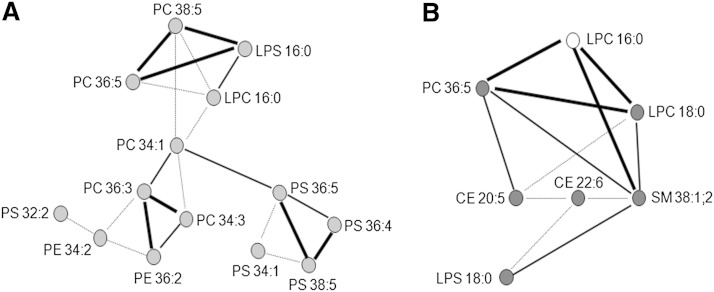
To better understand changes in LDL lipid composition related to dietary interventions with PhyS- and ω3-milk, we studied the correlation networks of lipid species that showed noteworthy changes according to the P values obtained by univariate ANOVA analysis for repeated measures after intervention with milk supplemented with PhyS or ω3 (Fig. 6). Specific lipid metabolites were linked when the Pearson correlation coefficient for changes relative to baseline had a P value <0.05. As shown in Fig. 6A, LDL metabolites with a significant change after the PhyS intervention were distributed in a single network with three separated clusters linked by PC34:1, one of the most abundant phosphatidylcholines in LDL. The network clustering revealed that changes in lipid species belonging to the same class were more likely to be linked [e.g., PS36:5, PS38:5, PS36:4, PS34:1]. In addition, change in LPS16:0 strongly correlated with those in PC36:5 and PC38:5, as it did with LPC16:0. After the ω3 intervention, LDL lipid metabolites with changes showing P < 0.05 by univariate ANOVA analysis for repeated measures, were closely linked between them (Fig. 6B). In addition, changes in LPC16:0 were highly related to those in PC36:5, SM38:1:2, and LPC18:0.
Fig. 6.
Correlation networks for specific lipid species with positive response to dietary intervention with PhyS- or ω3-supplemented milk. Lipid metabolites were associated based on their Pearson correlation coefficient. Thick lines represent a statistical significance for the correlation coefficient <0.0001; thin lines indicate P < 0.001 and pointed lines indicate P < 0.05. A: Network constructed using data of intervention with PhyS-supplemented milk. Light gray nodes refer to significantly decreased lipid species compared with baseline. B: Network constructed using data of intervention with ω3-supplemented milk. Dark gray nodes refer to significantly increased lipid species compared with baseline. White node represents an increased tendency without significant change compared with baseline.
DISCUSSION
Normalization of the plasma lipid profile and lipoprotein molecular composition is required for providing cardioprotection in patients with dyslipidemia and metabolic risk. A number of clinical trials have tested the efficacy of plant sterols/stanols and long-chain n-3 PUFAs incorporated into low-fat foods, including low-fat milk or low-fat yogurt as cholesterol- or TGL-lowering agents (34, 35). Up to now, however, the relationship between dietary PhySs or long-chain PUFAs and LDL lipid composition has scarcely been addressed. Here, we performed a double-blind randomized longitudinal crossover exploratory study to search for lipidomic changes induced by PhySs and ω3 in plasma and in the LDL, beyond their lowering effect in total plasma cholesterol or TGLs. Our results provide unambiguous evidence that daily intake of PhySs or ω3 fatty acids, within ranges of international recommendations, derives in differential lipidomic composition of LDL particles. To our knowledge, a differential LDL lipid molecular pattern as a result of ω3 or PhyS dietary supplementation had not been reported before.
Four weeks of intervention with the PhyS-supplemented milk appreciably reduced plasma cholesterol levels, particularly LDLc. In contrast, the effects of ω3-supplemented milk (250 ml/day) relate to plasma TGL and VLDLc, both changing in a similar range, without affecting plasma LDLc levels. The lowering of 9.8% in plasma LDLc, induced by the PhyS-milk, is in the range reported in previous plant sterol supplementation trials with once-a-day intake design (36, 37). It is to be noted that the effect of the PhyS-supplemented milk in our study was observed with a daily dose of 1.57 g/day, which was not consumed with the main meals. In addition, LDL particles were less prone to oxidation after 4 weeks of intervention with PhyS-milk, an effect that was not found with the ω3-milk, although both types of supplemented milks had a similar quantity of PUFAs, which are considered to be prone to oxidation (38). A recent study in primary cultured hippocampal cells describes that β-sitosterol treatment protects against glucose-oxidase-induced oxidative stress and lipid peroxidation (39).
TGL reductions have been described in metabolic syndrome patients consuming sterol/stanol-enriched foods (11, 40), and a recent pooled analysis of 12 different studies has suggested a modest TGL-lowering effect of plant sterols, which is dependent on baseline concentrations (12). This effect was not observed in the present study, based in a group population with baseline plasma TGL levels within the normal range. Major changes after PhyS and ω3 interventions were observed in subjects with LDLc levels above 130 mg/dl and those with a higher atherogenic dyslipidemic risk, respectively. Therefore, on an individual basis, the results of the present study indicate that subjects with moderate hypercholesterolemia could significantly benefit from plant sterol consumption while subjects with atherogenic dyslipidemia would benefit from a dietary management including ω3-supplemented milk for controlling cardiovascular risk.
Recent studies have pointed to a variety of lipid species, not only LDLc levels, as novel biomarkers and targets in atherosclerosis (41). Therefore, here we have used a top-down lipidomic approach to gain new insights into the lipid biochemical changes induced in LDL by the dietary use of PhyS- and ω3-supplemented milks. In this exploratory lipidomic study, using a LC-ESI-MS/MS strategy, we consistently identified 69 different lipid species from three different lipid classes in the LDL, and we have unambiguously shown that regular intake of PhyS- and ω3-milk resulted in differential LDL lipid metabolite patterns, specific for each dietary intervention. Interestingly, changes in the identified LDL lipid metabolites, as response to PhyS- or ω3-milk interventions, remained significant after adjustment for LDLc or TGL levels. The lipid classes accounting for major differences between ω3 and PhyS interventions were CE and GPL, with a probability below 8% of false positives, after FDR adjustment for multiple testing, for the LDL lipid metabolites considered to be significantly affected by the dietary use of PhyS- and ω3-supplemented milks.
LDL consisted primarily of CE, with cholesteryl linoleate (CE18:2) being the most abundant metabolite. In the present study, the level of CE18:2 in LDL remained unchanged after the ω3- or PhyS-milk intervention, suggesting the change reported in serum CE18:2 after 4 weeks of intervention with PhyS-enriched yogurt (42) has a different origin than LDL because they reported on unfractionated plasma and not LDL-lipidomics. Indeed, CE18:2 is also the major CE in the vessel wall, both in healthy vessels and in unstable plaques (43). It is noteworthy that the intervention with PhyS-milk, although significantly reducing the plasma LDLc level, did not induce any significant change in the LDL CE profile, suggesting a major effect of dietary PhySs on the free-cholesterol fraction of LDL. On the contrary, the fact that CEs containing EPA (CE20:5) and DHA (CE22:6) were increased in LDL after intervention with ω3-milk suggests that regular intake of ω3-supplemented milk facilitates incorporation of these highly polyunsaturated FAs in CEs of the LDL core, which may confer a lower pro-inflammatory potential to the LDL (44).
Besides CEs, phospholipids are a relevant lipid component of the LDL, with a key role in LDL membrane structure and function (45). Consistent with other studies (27, 46), major phospholipid components in the LDL were SM and GPLs. PC was the most abundant GPL subclass, whereas other GPL subtypes, such as PEs, PS, and LPS, were identified at a remarkably lower abundance.
A relevant finding of our study has been the significant reduction in the content of LDL-GPLs after 4 weeks of PhyS-milk intake. Despite this reduction, neither the ratio GPL to SM nor the ratio PC to SM (which has been related with susceptibility to form aggregated LDLs that induce higher atherogenicity) were significantly changed after the PhyS intervention. Plasma LDLs circulate in several apparently discrete sizes, varying in their relative composition (47). Here, we provide evidence that the increase in LDL size is accompanied by a decrease in the ratio GPL/SM, as well as in the ratio GPL/total protein.
VLDL-PE has been shown to mediate activation of the contact coagulation system through stimulation of FXII activity (48), and targeting of contact system proteins is suggested as a new approach for safe anticoagulation associated with minimal bleeding (49). Therefore, the decrease in LDL-associated PE metabolites, as a result of a regular intake of PhyS-milk, highlights a novel protective effect of plant sterols. In addition, LDL-GPL, after 4 weeks of PhyS-milk intake, was further characterized by a significant consistent decrease in PS content. PSs are negatively charged metabolites that may affect the pH at the lipid surface, thereby regulating electrostatic interactions of apolipoproteins with polar head groups of phospholipids (50). The importance of electrostatic interactions in lipoprotein metabolism is well-established, and the presence of plasma LDL with increased electronegative charge on the particle surface has been reported in patients with diabetes mellitus (51).
Five PC species were positively associated with cardiovascular mortality in the recently published LURIC study (52). One of these metabolites was the monounsaturated PC34:1, a highly abundant PC in LDL that has been significantly reduced by the PhyS-milk intervention. The species that showed the strongest positive mortality association in the LURIC analysis, PC32:0, has also shown a decrease after 4 week’s intake of PhyS-milk. The strongest coordinated changes after PhyS-milk intervention were observed between PC metabolites differing by two carbon atoms in their FA side chain, which might result from products of elongation processes occurring in the LDL.
The susceptibility of LDLs to oxidation has been linked to their content in SMs, lipid components reported as physiological inhibitors of lipoprotein oxidation due to their decreasing effects in LDL surface fluidity and propagation of the oxidation reaction in the PC monolayer (53). In our study, baseline LDL depicted a SM/PC ratio within the range of 0.4–0.5, associated to protection against PC oxidation (54). As described above, the SM/PC ratios did not significantly change after the intervention periods, suggesting that the observed effect of PhySs on LDL susceptibility to oxidation is not related to LDL surface fluidity. LPC is a product of PC hydrolysis, mainly generated by activated phospholipase-A during LDL oxidation (55–57). In the present study, we found lower LPC16:0 levels in LDL following intake of PhyS-supplemented milk, which directly correlated with the reduced susceptibility of LDL to be oxidized by cupric sulfate (Spearman correlation 0.470; P = 0.05). With respect to this, Kim et al. (58) have recently described, in middle-aged men, a positive correlation between age-related changes in LPC16:0 and 8-epi-PGF2α, a reliable marker of oxidative stress. In addition, different studies have reported an increase in plasma concentration of saturated LPC in disease conditions such as obesity, diabetes, and rheumatoid arthritis (59). Levels of LPC containing C18:0 and C16:0 saturated FAs showed an increasing trend after intervention with ω3. However, increased levels of EPA-containing PC, rather than activation of the enzyme phospholipase-A, seems to account for the LPC levels after the ω3 intervention. Indeed, the positive trend in the ratio PC36:5/LPC16:0 after intervention with ω3-milk and the strong correlation between the intervention-related changes between PC and LPC metabolites strongly support this view. It is of note that LPC levels represent less than 2% of total PC content both before and after ω3 intervention, which agrees with the reported content for nonoxidized LDL (58).
In summary, the regular intake of PhyS- and long-chain PUFA-enriched milks elicit distinct and complementary lipid effects beyond lowering plasma LDLc and TGL levels, respectively. Despite the small sample size and the exploratory character of this study, we found important results. As such, the significant reduction in LDL-GPLs, primarily of the PC and LPC subclasses, and the decrease in LDL susceptibility to oxidation may account for the cardiovascular risk protection associated with regular intake of sterol/stanol-rich food products. Additionally, long-chain n-3 PUFA-enriched milk increases the content of long-chain n-3 PUFAs in LDL, mainly as CEs and phosphatidylcholine. The synergistic and complementary cholesterol- and TGL-lowering effects of ω3 and PhyS supplementation, along with the distinct changes induced in LDL lipid composition by both dietary interventions, support the view that a product, such as milk, containing the two active components in appropriate dose levels might ever further increase their individual beneficial effects.
Supplementary Material
Acknowledgments
The authors are indebted with Montse Gomez-Pardo and Maria-José Bartolome for assistance during the study, to Joaquim Gordo for his support in the lipidomic analysis, and to Dr. Sandra Camino in the statistical analysis. The authors thank the participants in the present study for their outstanding collaboration and CAPSA Food S.A. for providing the milk products used in the study.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- FDR
- false discovery rate
- GPL
- glycerophospholipid
- HDLc
- HDL cholesterol
- LDLc
- LDL cholesterol
- LPC
- lysophosphatidylcholine
- LPS
- lysophosphatidylserine
- MDA
- malondialdehyde
- NL
- neutral loss scan
- PC
- phosphatidylcholine
- PCA
- principal component analysis
- PE
- phosphatidylethanolamine
- PhyS
- phytosterols
- PI
- precursor ion
- PS
- phosphatidylserine
- sLDL
- small size LDL
- TBARS
- thiobarbituric acid-reactive substance
- TC
- total cholesterol
- TGL
- triglyceride
- VLDLc
- VLDL cholesterol
- WC
- waist circumference
- WHtR
- waist-to-height ratio
- ω3
- long-chain n-3 PUFA
This work was supported by CEN-20101016 (HENUFOOD) given by CDTI-Spanish Ministry of Competitivity and Economy (MINECO) to L.B., the Ministry of Economy and Competitiveness (SAF2013-42962-R to L.B. and SAF2012-40208 to G.V.), Institute of Health Carlos III (ISCIII) (FIS PI13/02850 to T.P. and “Red de Investigación Cardiovascular” RIC-RD12/0042/0027 to L.B.), and FEDER “Una Manera de Hacer Europa.” Continuous support has been provided by Fundación Jesus Serra, Barcelona. G.V. is a recipient of a contract from the Innovation and Science Spanish Ministry (RyC-2009-5495, MICINN, Spain). M.H. is an employee of CAPSA Food, Nutrition Department.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures and five tables.
REFERENCES
- 1.Klop B., Elte J. W. F., Castro Cabezas M. 2013. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 5: 1218–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C., Blackwell L., Emberson J., Holland L. E., Reith C., Bhala N., Peto R., Barnes E. H., Keech A., Simes J., et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration. 2010. Efficacy and safety of more ntensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 376: 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthan N. R., Lichtenstein A. H. 2004. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 174: 197–205. [DOI] [PubMed] [Google Scholar]
- 4.Demonty I., Ras R. T., van der Knaap H. C., Duchateau G. S., Meijer L., Zock P. L., Geleijnse J. M., Trautwein E. A. 2009. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J. Nutr. 139: 271–284. [DOI] [PubMed] [Google Scholar]
- 5.Badimon L., Vilahur G., Padro T. 2010. Nutraceuticals and atherosclerosis: human trials. Cardiovasc. Ther. 28: 202–215. [DOI] [PubMed] [Google Scholar]
- 6.Abumweis S. S., Barake R., Jones P. J. 2008. Plant sterols/stanols as cholesterol lowering agents: a meta-analysis of randomized controlled trials. Food Nutr. Res. 52 10.3402/fnr.v52i0.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong A., Plat J., Mensink R. P. 2003. Metabolic effects of plant sterols and stanols. J. Nutr. Biochem. 14: 362–369. [DOI] [PubMed] [Google Scholar]
- 8.Gonçalves S., Maria A. V., Silva A. S., Martins-Silva J., Saldanha C. 2006. Phytosterols in milk as a depressor of plasma cholesterol levels: experimental evidence with hypercholesterolemic Portuguese subjects. Clin. Hemorheol. Microcirc. 35: 251–255. [PubMed] [Google Scholar]
- 9.Patch C. S., Tapsell L. C., Williams P. G. 2005. Plant sterol/stanol prescription is an effective treatment strategy for managing hypercholesterolemia in outpatient clinical practice. J. Am. Diet. Assoc. 105: 46–52. [DOI] [PubMed] [Google Scholar]
- 10.Theuwissen E., Plat J., van der Kallen C. J., van Greevenbroek M. M., Mensink R. P. 2009. Plant stanol supplementation decreases serum triacylglycerols in subjects with overt hypertriglyceridemia. Lipids. 44: 1131–1140. [DOI] [PubMed] [Google Scholar]
- 11.Plat J., Brufau G., Dallinga-Thie G. M., Dasselaar M., Mensink R. P. 2009. A plant stanol yogurt drink alone or combined with a low-dose statin lowers serum triacylglycerol and non-HDL cholesterol in metabolic syndrome patients. J. Nutr. 139: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 12.Demonty I., Ras R. T., Knaap H. C. M., Meijer L., Zock P. L., Geleijnse J. M., Trautwein E. A. 2013. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: a pooled analysis of 12 randomised controlled trials. Eur. J. Nutr. 52: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balk E. M., Lichtenstein A. H., Chung M., Kupelnick B., Chew P., Lau J. 2006. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk, a systematic review. Atherosclerosis. 189: 19–30. [DOI] [PubMed] [Google Scholar]
- 14.Robinson J. G., Stone N. J. 2006. Anti-atherosclerotic and antithrombotic effects of omega-3 fatty acids. Am. J. Cardiol. 98: 39i–49i. [DOI] [PubMed] [Google Scholar]
- 15.Moore C. S., Bryant S. P., Mishra G. G., Krebs J. D., Browning L. M., Miller G. J., Jebb S. A. 2006. Oily fish reduces plasma triacylglycerols: a primary prevention study in overweight men and women. Nutrition. 22: 1012–1024. [DOI] [PubMed] [Google Scholar]
- 16.Rizos E. C., Ntzani E. E., Bika E., Kostapanos M. S., Elisaf M. S. 2012. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 308: 1024–1033. [DOI] [PubMed] [Google Scholar]
- 17.Roche H. M., Gibney M. J. 2000. Effect of long-chain n-3 polyunsaturated fatty acids on fasting and postprandial triacylglycerol metabolism. Am. J. Clin. Nutr. 71: 232S–237S. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub M. S., Zechner R., Brown A., Eisenberg S., Breslow J. L. 1988. Dietary polyunsaturated fats of the W-6 andW-3 series reduce postprandial lipoprotein levels. Chronic and acute effects of fat saturation on postprandial lipoprotein metabolism. J. Clin. Invest. 82: 1884–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baró L., Fonollá J., Peña J. L., Martinez-Ferez A., Lucena A., Jiménez J., Boza J. J., López-Huertas E. 2003. n-3 Fatty acids plus oleic acid supplemented milk reduces total and LDL cholesterol, homocysteine and levels of endothelial adhesion molecules in healthy humans. Clin. Nutr. 22: 175–182. [DOI] [PubMed] [Google Scholar]
- 20.Carrero J. J., Baró L., Fonollá J., González-Santiago M., Martínez-Férez A., Castillo R., Jiménez J., Boza J. J., López-Huertas E. 2004. Cardiovascular effects of milk enriched with omega-3 polyunsaturated fatty acids, oleic acid, folic acid, and vitamins E and B6 in volunteers with mild hyperlipidemia. Nutrition. 20: 521–527. [DOI] [PubMed] [Google Scholar]
- 21.Smilowitz J. T., Zivkovic A. M., Wan Y. J., Watkins S. M., Nording M. L., Hammock B. D., German J. B. 2013. Nutritional lipidomics: molecular metabolism, analytics, and diagnostics. Mol. Nutr. Food Res. 57: 1319–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontush A., Chapman M. J. 2010. Lipidomics as a tool for the study of lipoprotein metabolism. Curr. Atheroscler. Rep. 12: 194–201. [DOI] [PubMed] [Google Scholar]
- 23.Wiesner P., Leidl K., Boettcher A., Schmitz G., Liebisch G. 2009. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 50: 574–585. [DOI] [PubMed] [Google Scholar]
- 24.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garber D. W., Kulkarni K. R., Anantharamaiah G. M. 2000. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. J. Lipid Res. 41: 1020–1026. [PubMed] [Google Scholar]
- 26.Bligh E. G., Dyer W. J. 1959. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 27.Hu C., van Dommelen J., van der Heijden R., Spijksma G., Reijmers T. H., Wang M., Slee E., Lu X., Xu G., van der Greef J., et al. 2008. RRLC-ion-trap-FTMS method for lipid profiling of plasma: method validation and application to p53 mutant mouse model. J. Proteome Res. 7: 4982–4991. [DOI] [PubMed] [Google Scholar]
- 28.Mallol R., Rodríguez M. A., Heras M., Vinaixa M., Cañellas N., Brezmes J., Plana N., Masana L., Correig X. 2011. Surface fitting of 2D diffusion-edited 1H NMR spectroscopy data for the characterization of human plasma lipoproteins. Metabolomics. 7: 572–582. [Google Scholar]
- 29.Jeyarajah E. J., Cromwell W. C., Otvos J. D. 2006. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 26: 847–870. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawa H., Ohishi N., Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95: 351–358. [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y., Hochberg Y. 2000. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J. Educ. Behav. Stat. 25: 60–83. [Google Scholar]
- 32.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2002. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106: 3143–3421. [PubMed] [Google Scholar]
- 33.Ghioni C., Porter A. E. A., Sadler I. H., Tocher D. R., Sargent J. R. 2001. Cultured fish cells metabolize octadecapentaenoic acid (all-cis delta3,6,9,12,15-18:5) to octadecatetraenoic acid (all-cis delta6,9,12,15-18:4) via its 2-trans intermediate (trans delta2, all-cis δ6,9,12,15-18:5). Lipids. 36: 145–152. [DOI] [PubMed] [Google Scholar]
- 34.Castro I. A., Barraso L. P., Sinnecker P. 2005. Functional foods for coronary heart disease risk reduction: a meta-analysis using a multivariate approach. Am. J. Clin. Nutr. 82: 32–40. [DOI] [PubMed] [Google Scholar]
- 35.Garg M. L., Blake R. J., Clayton E., Munro I. A., Macdonald-Wicks L., Singh H., Moughan P. J. 2007. Consumption of an n-3 polyunsaturated fatty acid-enriched dip modulates plasma lipid profile in subjects with diabetes type II. Eur. J. Clin. Nutr. 61: 1312–1317. [DOI] [PubMed] [Google Scholar]
- 36.Seppo L., Jauhiainen T., Nevala R., Poussa T., Korpela R. 2007. Plant stanol esters in low-fat milk products lower serum total and LDL cholesterol. Eur. J. Nutr. 46: 111–117. [DOI] [PubMed] [Google Scholar]
- 37.Hyun Y. J., Kim O. Y., Kang J. B., Lee J. H., Jang Y., Loponkoski L., Salo P. 2005. Plant stanol esters in low-fat yogurt reduces total and low-density lipoprotein cholesterol and low-density lipoprotein oxidation in normocholesterolemic and mildly hypercholesterolemic subjects. Nutr. Res. 25: 743–753. [Google Scholar]
- 38.Albert B. B., Cameron-Smith D., Hofman P. L., Cutfield W. S. 2013. Oxidation of marine omega-3 supplements and human health. BioMed Res Int. 2013: 10.1155/2013/464921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi C., Wu F., Zhu X. C., Xu J. 2013. Incorporation of beta-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor-mediated PI3K/GSK3beta signaling. Biochim. Biophys. Acta. 1830: 2538–2544. [DOI] [PubMed] [Google Scholar]
- 40.Sialvera T. E., Pounis G. D., Koutelidakis A. E., Richter D. J., Yfanti G., Kapsokefalou M., Goumas G., Chiotinis N., Diamantopoulos E., Zampelas A. 2012. Phytosterols supplementation decreases plasma small and dense LDL levels in metabolic syndrome patients on a westernized type diet. Nutr. Metab. Cardiovasc. Dis. 22: 843–848. [DOI] [PubMed] [Google Scholar]
- 41.Ekroos K., Jänis M., Tarasov K., Hurme R., Laaksonen R. 2010. Lipidomics: a tool for studies of atherosclerosis. Curr. Atheroscler. Rep. 12: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szymańska E., van Dorsten F. A., Troost J., Paliukhovich I., van Velzen E. J., Hendriks M. M., Trautwein E. A., van Duynhoven J. P., Vreeken R. J., Smilde A. K. 2012. A lipidomic analysis approach to evaluate the response to cholesterol-lowering food intake. Metabolomics. 8: 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stegemann C., Drozdov I., Shalhoub J., Humphries J., Ladroue C., Didangelos A., Baumert M., Allen M., Davies A. H., Monaco C., et al. 2011. Comparative lipidomics profiling of human atherosclerotic plaques. Circ Cardiovasc Genet. 4: 232–242. [DOI] [PubMed] [Google Scholar]
- 44.Serhan C. N., Chiang N., Van Dyke T. E. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibdah J. A., Lund-Katz S., Phillips M. C. 1989. Molecular packing of high-density and low-density lipoprotein surface lipids and apolipoprotein A-I binding. Biochemistry. 28: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 46.Dashti M., Kulik W., Hoek F., Veerman E. C., Peppelenbosch M. P., Rezaee F. 2011. A phospholipidomic analysis of all defined human plasma lipoproteins. Sci. Rep. 1: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teerlink T., Scheffer P. G., Bakker S. J., Heine R. J. 2004. Combined data from LDL composition and size measurement are compatible with a discoid particle shape. J. Lipid Res. 45: 954–966. [DOI] [PubMed] [Google Scholar]
- 48.Klein S., Spannagl M., Engelmann B. 2001. Phosphatidylethanolamine participates in the stimulation of the contact system of coagulation by very-low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 21: 1695–1700. [PubMed] [Google Scholar]
- 49.Müller F., Renné T. 2008. Novel roles for factor XII-driven plasma contact activation system. Curr. Opin. Hematol. 15: 516–521. [DOI] [PubMed] [Google Scholar]
- 50.Fukuda M., Nakano M., Miyazaki M., Tanaka M., Saito H., Kobayashi S., Ueno M., Handa T. 2008. Conformational change of apolipoprotein A-I and HDL formation from model membranes under intracellular acidic conditions. J. Lipid Res. 49: 2419–2426. [DOI] [PubMed] [Google Scholar]
- 51.Yano M., Inoue M., Maehata E., Shiba T., Yamakado M., Hirabayashi Y., Taniyama M., Suzuki S. 2004. Increased electronegative charge of serum low-density lipoprotein in patients with diabetes mellitus. Clin. Chim. Acta. 340: 93–98. [DOI] [PubMed] [Google Scholar]
- 52.Sigruener A., Kleber M. E., Heimerl S., Liebisch G., Schmitz G., Maerz W. 2014. Glycerophospholipid and sphingolipid species and mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS ONE. 9: e85724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subbaiah P. V., Subramanian V. S., Wang K. 1999. Novel physiological function of sphingomyelin in plasma. Inhibition of lipid peroxidation in low density lipoproteins. J. Biol. Chem. 274: 36409–36414. [DOI] [PubMed] [Google Scholar]
- 54.Liu M., Krul E. S., Subbaiah P. V. 1992. Effect of apoprotein B conformation on the activation of lysolecithin acyltransferase and lecithin: cholesterol acyltransferase. Studies with subfractions of low density lipoproteins. J. Biol. Chem. 267: 5139–5147. [PubMed] [Google Scholar]
- 55.Matsumoto T., Kobayashi T., Kamata K. 2007. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 14: 3209–3220. [DOI] [PubMed] [Google Scholar]
- 56.Steinbrecher U. P., Pritchard P. H. 1989. Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J. Lipid Res. 30: 305–315. [PubMed] [Google Scholar]
- 57.Aiyar N., Disa J., Ao Z., Ju H., Nerurkar S., Willette R. N., Macphee C. H., Johns D. G., Douglas S. A. 2007. Lysophosphatidylcholine induces inflammatory activation of human coronary artery smooth muscle cells. Mol. Cell. Biochem. 295: 113–120. [DOI] [PubMed] [Google Scholar]
- 58.Kim J. Y., Kim O. Y., Paik J. K., Kwon D. Y., Kim H. J., Lee J. H. 2013. Association of age-related changes in circulating intermediary lipid metabolites, inflammatory and oxidative stress markers, and arterial stiffness in middle-aged men. Age (Dordr.). 35: 1507–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuchs B., Schiller J., Wagner U., Häntzschel H., Arnold K. 2005. The phosphatidylcholine/ lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: investigations by 31P NMR and MALDI-TOF MS. Clin. Biochem. 38: 925–933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.