Abstract
The intestine must challenge the profuse daily flux of dietary fat that serves as a vital source of energy and as an essential component of cell membranes. The fat absorption process takes place in a series of orderly and interrelated steps, including the uptake and translocation of lipolytic products from the brush border membrane to the endoplasmic reticulum, lipid esterification, Apo synthesis, and ultimately the packaging of lipid and Apo components into chylomicrons (CMs). Deciphering inherited disorders of intracellular CM elaboration afforded new insight into the key functions of crucial intracellular proteins, such as Apo B, microsomal TG transfer protein, and Sar1b GTPase, the defects of which lead to hypobetalipoproteinemia, abetalipoproteinemia, and CM retention disease, respectively. These “experiments of nature” are characterized by fat malabsorption, steatorrhea, failure to thrive, low plasma levels of TGs and cholesterol, and deficiency of liposoluble vitamins and essential FAs. After summarizing and discussing the functions and regulation of these proteins for reader’s comprehension, the current review focuses on their specific roles in malabsorptions and dyslipidemia-related intestinal fat hyperabsorption while dissecting the spectrum of clinical manifestations and managements. The influence of newly discovered proteins (proprotein convertase subtilisin/kexin type 9 and angiopoietin-like 3 protein) on fat absorption has also been provided. Finally, it is stressed how the overexpression or polymorphism status of the critical intracellular proteins promotes dyslipidemia and cardiometabolic disorders.
Keywords: hypobetalipoproteinemia, abetalipoproteinemia, chylomicron retention disease, intestinal fat malabsorption, apolipoprotein B-48, microsomal triglyceride transfer protein, Sar1b GTPase, lipoprotein production, dietary lipids, intestine
BRIEF OVERVIEW OF INTESTINAL FAT ABSORPTION
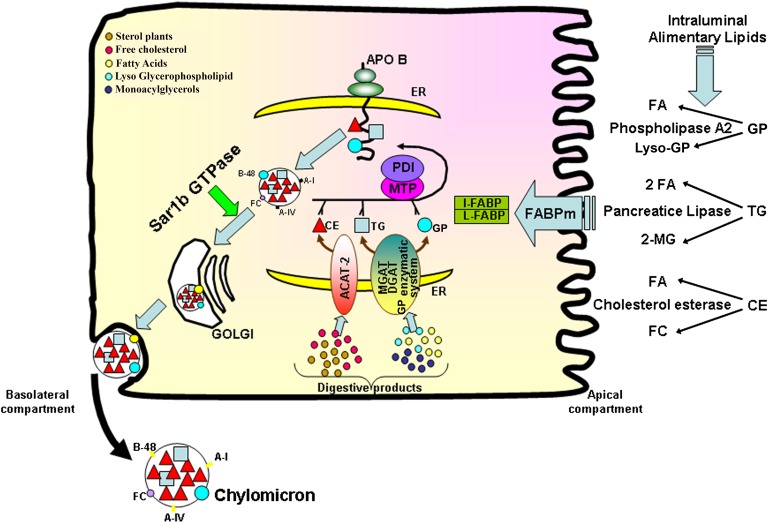
The small intestine is the major site for the transport of alimentary fat, the most calorically dense nutrient, in the form of lipoproteins. The process of lipid absorption may schematically be divided in three sequential phases: intraluminal, intestinal, and secretory events (Fig. 1). The intraluminal phase includes chemical hydrolysis of lipids [mainly TGs, glycerophospholipids (GPs), and cholesteryl esters (CEs)] by lipolytic enzymes (pancreatic lipase, phospholipase A2, and cholesterol esterase, respectively), the micellar solubilization of lipolytic products by bile acids (1–13), and the shuttle of micelles through the unstirred water layer to the surface of the microvillus membrane (14–19). The resistance of the unstirred water layer is influenced by the chain length and saturation of FAs, as well as lipid species (14, 16, 20). The intestinal phase comprises passive diffusion and protein-mediated facilitated transport of hydrolyzed products (FAs, monoacylglycerols, free cholesterol, and lyso-GPs) across the microvillous membrane of the enterocyte involving several transporters [e.g., cluster of differentiation 36, FA binding protein (FABP)4, and plasma membrane FABP] (21–27). However, it is important to specify that the transfer of long-chain FAs, in particular, is a highly controversial question that has attracted the interest of several reviews (28–34). Once in the cytoplasm, the lipolytic products are bound by cytosolic FABPs (intestinal- and liver-FABP) (35–43) before being targeted to the endoplasmic reticulum (ER) for the reesterification of TG (essentially by monoacylglycerol acyltransferase and diacylglycerol acyltransferase), CE (with the involvement of ACAT-2), and GP (via combination of diacylglycerol with choline and ethanolamine), and finally the formation of lipid-carrying lipoproteins in the secretory pathway (44–46). The delivery phase involves the exocytosis of chylomicrons (CMs) from the absorptive cells and their subsequent delivery into the systemic circulation via the intestinal lymph. Experimental and clinical approaches have significantly advanced our understanding of the intra-enterocyte step that brings together the large amphipathic apolipoprotein (Apo) B-48 polypeptide and newly esterified lipids in a fixed temporal sequence (47). Once the translation and translocation of Apo B-48 into the lumen of the ER are initiated, microsomal TG transfer protein (MTP) shuttles lipids from the ER membrane to the growing Apo B-48 chain in the ER, allowing the protein to translocate completely into the lumen (47, 48). However, deprivation of lipid substrate causes degradation of Apo B-48 and inhibition of nascent CM assembly (47, 49). The preCM is transported via a preCM transport vesicle to the Golgi apparatus (50), where the coat is removed and a mature CM is formed through acquisition of more neutral lipids (51). More specifically, the maturation of CMs in the Golgi apparatus is accompanied by further alterations in Apo B-48 glycosylation (52, 53), Apo A-I accretion (54), lipid composition (53), and size (54). The transfer process is highly dependent on the small Sar1b GTPase that initiates the vesicular coat protein complex II (COPII)-dependent transport of cargo from the ER to the Golgi apparatus (55) (Fig. 1).
Fig. 1.
Schematic intracellular network required for lipid metabolism, Apo B biogenesis, and CM assembly in the enterocyte. Digestion takes place in the intestinal lumen where TGs, CEs, and GPs are hydrolyzed by pancreatic lipase, cholesterol esterase, and phospolipase A2, respectively. The lipolytic products (FA, 2-MG, FC, lyso-GP) are emulsified into mixed micelles by bile salts before their diffusion through the unstirred water layer. At the apical surface, they enter the enterocyte via passive diffusion when they are in high concentrations. However, protein transporters (e.g., FABPm) are required at weaker concentrations. Once inside the enterocyte, the lipolytic products are bound to cytoplasmic FABPs (I- and L-FABP) in order to migrate to the ER where they are reesterified by various enzymes: monoacylglycerol acyltransferase (MGAT) and diacylglycerol acyltransferase (DGAT) for the conversion of 2-MG to TG, ACAT-2 for the esterification of FC into CE, and an enzymatic system (glycerophosphate acyltransferase, phosphatidate phosphodiesterase, lyso-PC acyltransferase) for the production of GP. The synthesis of Apo B-48 and its interaction with MTP, a heterodimeric complex with the chaperone protein, protein disulfide isomerase (PDI), through the NH2-terminal domain, is essential for preCM assembly. This packaging process starts when the growing Apo B-48 polypeptide is cotranslationally lipidated by MTP in the lumen of the ER: the association of Apo B-48 with lipids is required not only to ensure proper initiation of folding, but also to prevent its destruction by the proteosomal degradation pathway. The formation of preCM transport vesicles is critical for the transport of the nascent lipoproteins to the Golgi, a process mediated by Sar1b GTPase. The maturation of preCMs is completed in the Golgi by addition of lipids and glycosylation of Apos, yielding a mature CM particle characterized by TG and CE in its core and FC, GP, and Apo B on its surface. Finally, CMs are released by the enterocyte into the intestinal lymph through exocytosis. MG, monoacylglycerol; FC, free cholesterol; I-FABP, intestinal-FABP; L-FABP, liver-FABP; PC, phosphatidylcholine.
This article is aimed at summarizing insights gained in the last two decades on the important pathways modulating key intracellular proteins in CM formation and intestinal fat absorption. One of the major goals for this article is to familiarize readers with this expanding and evolving understanding related to the regulation, ontogeny, and functions of the crucial proteins: Apo B-48, MTP, and Sar1b GTPase. The impact of their genetic defects on enterocyte lipid transport will also be summarized, while pointing out clinical disorders and management.
ABETALIPOPROTEINEMIA
Introduction
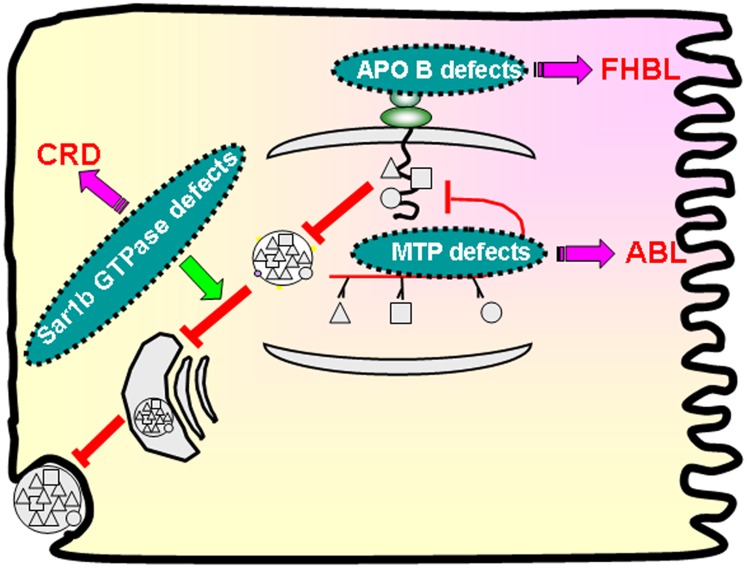
Abetalipoproteinemia (ABL; OMIM#200100) is an autosomal recessive disorder that is usually detected during infancy due to failure to thrive, severe diarrhea, and fat malabsorption. The defective gene was discovered 40 years after the first description of the disease (56). The primary cause is due to the defects in MTP (48) (Fig. 2), leading to abnormal assembly of intestinal and hepatic Apo B-containing lipoproteins (57–61), thereby explaining the virtual absence of plasma Apo B-100 and Apo B-48, as well as the low plasma concentrations of TG and cholesterol (62).
Fig. 2.
Congenital disorders of intestinal lipid absorption. Defects in the key proteins, Apo B-48, MTP, and Sar1b GTPase, result in the genetic disorders characterized by FHBL, ABL, and CRD, respectively.
MTP properties
MTP is located within the lumen of microsomes of the liver and intestine (63, 64). It appears as a heterodimer with two subunits of apparent molecular mass 58,000 and 88,000 kDa (65). The small subunit has been identified as the multifunctional protein disulfide isomerase (63), while the unique large subunit confers the catalytic property to the protein complex (65). It catalyzes the transfer of TG, CE, and phosphatidylcholine between GP surfaces. In fact, by transporting lipids by a shuttle mechanism (Ping Pong Bi Bi kinetics), MTP acts as a carrier of lipids from their site of synthesis to nascent lipoproteins within the ER (66). Additional evidence for the critical role of MTP in CM and VLDL production in the enterocyte and hepatocyte, respectively, has been provided with pharmacological approaches utilizing specific MTP inhibitors that simultaneously inhibit lipid transfer activity in situ while blocking Apo B secretion (67–70). Treatment with MTP inhibitors results in a dose-dependent decrease in Apo B secretion, suggesting that MTP is rate-limiting for TG-rich lipoprotein secretion (67). Investigators have rapidly exploited the discovery of ABL molecular basis to elaborate on the concept that pharmacologic MTP inhibition may constitute a powerful strategy to reduce Apo B-containing lipoproteins (CMs, VLDLs, and LDLs) in dyslipidemia, with a considerable impact on the prevention of cardiovascular diseases. Given gastrointestinal and hepatic adverse effects, the development of most of MTP inhibitors was halted except lomitapide that has succeeded to cross phase 2 clinical trial (71, 72) and exhibited efficacy and safety in a recent phase 3 trial focusing on homozygous familial hypercholesterolemia (73). Importantly, MTP interacts with Apo B through its N-terminal β-barrel domain (residues 22–297), while the middle α-helical domain (residues 298–603) mediates the interaction with protein disulfide isomerase, and the C terminal mediates the lipid-binding and transfer catalytic activity of MTP (74–76). It is now well-established that MTP elicits lipidation of the growing Apo B polypeptide chain, thus favoring the assembly and secretion of lipoproteins (77, 78). In addition to achieving net transfer of lipid to Apo B during its translation, MTP can also activate the trafficking of TG droplets from the cytosol to the ER lumen and catalyze the fusion of nascent Apo B-containing particles with TG droplets, thereby contributing to CM expansion (79, 80). If MTP is absent, as seen in the condition of ABL, Apo B does not fold properly because of the defect of adequate lipidation in the ER, which irremediably leads to its proteosomal degradation (81). Therefore, the transient physical interaction of MTP with Apo B is crucial at early stages of lipoprotein synthesis (78, 82–84) (Fig. 1). The absence of MTP is triggered by mutations in the gene for the large MTP subunit, which contains 18 exons and spans approximately 55–60 kb in chromosome 4 (4q22-q24) (85). Interestingly, one of the quantitative trait loci for LDL is located in a region of chromosome 4 that contains MTP that constitutes a clearly strong positional candidate gene for association for the variation effects on LDL size fractions (86, 87). Furthermore, a systemic exploration of the chromosome 4 linkage has identified the MTP gene as a modifier of lifespan in a cohort of long-lived individuals (88). Because the description of MTP as the cause of the rare inherited ABL disease (86, 89), over 30 mutations in MTP have been identified in more than 50 ABL patients described so far: 21.3% missense, 7% nonsense, 12% small indel, 3% gross indel, and 27.7% splicing variants (90–93). The functional characterization of various mutations found in ABL has not only disclosed their effects on the expression, subcellular location, and interaction of MTP with protein disulfide isomerase, but has also emphasized requirements for the transfer of both TGs and GPs to support Apo B secretion (94).
MTP expression in different tissues and during human development
The large subunit expression of MTP, along with its transfer activity, is predominantly present in differentiated epithelial cells of the small intestinal regions (duodenum, proximal jejunum), but absent in crypt and colonic cells (95–97). Interestingly, MTP was identified in villus and crypt epithelial cells as well in different regions of the human fetal intestine, including the colon (95). Staining was detected as early as the 13th week of gestation in all gut segments and was almost entirely confined to the columnar epithelial cells of the jejunum and colon (95). Also, a trend toward increasing MTP activity was noticed at 20–22 weeks of gestation (95). These observations combined with previous reports demonstrate the small intestine’s ability to synthesize and secrete Apo B-containing lipoproteins during development (95, 98–101). The liver also represents a major organ of MTP expression, but hepatocytes constitute the unique cell population containing MTP protein with an expression gradient: a decrease toward the periphery of the lobule opposing the portal triad and an increase in cells proximal to the central vein (96).
Interesting findings support the presence of MTP in the kidney and heart, which allows the secretion of their content of Apo B-containing particles, thereby protecting proximal tubules and myocardium, respectively, against accumulation of toxic lipids (102–104). MTP mRNA and protein were also detected in antigen-presenting cells, including monocytes, splenocytes, B cells, and T cells (105), with an involvement of CD1d-restricted lipid antigen presentation (106).
Functional consequences of MTP absence: ABL clinical spectrum
Mice homozygous for an MTP gene disruption died at ∼E10.5, thereby underscoring the essential role of MTP, and the importance of the synthesis and secretion of Apo B-containing lipoproteins during early stages for lipid delivery to embryos (107, 108). Additionally, half-normal levels of the MTP mRNA, protein, and TG transfer activity in tissues of heterozygous mice are insufficient for normal levels of lipoprotein secretion and developmental functions, emphasizing the lack of physiological compensation for reduced levels of MTP gene expression in Mttp+/− (107). In contrast, obligate human heterozygotes exhibited normal plasma lipid and lipoprotein levels, and adult human subjects survive with a near-total absence of Apo B-containing lipoproteins (109), which clearly implies that both alleles of the MTP 97 kDa gene need to be defective in order to observe the major decline in plasma lipids and intestinal absorption, and the recessive character of ABL disease. So far, we do not know if MTP is present in excess under normal circumstances, as losing approximately 50% of its expression is not enough to affect lipoprotein assembly. Because no one has ever measured the levels of MTP in the intestine or liver from human heterozygotes, we also do not know whether the explanation for the flagrant paradox (e.g., humans vs. mice) stems from a potential compensatory upregulation of MTP expression from the normal allele or is a result of a reduction in MTP mRNA or protein turnover. This could also be due to different embryonic growth rates or maternal-fetal transport methods. Although a number of different in vitro model systems have been employed to address these issues, extrapolation to in vivo physiology might be hazardous. In this context, it is also worth recalling that the placental circulation is established very early during human gestation, and the transport mechanisms for lipids in the placenta and lipid requirements may be quite different from those in the mouse yolk sac.
Consequent to MTP abnormalities in ABL, various multi-system manifestations are noted in Table 1. Most of the reported young patients have diarrhea, steatorrhea, acanthocytosis, low serum cholesterol, and Apo B deficiency, which are accompanied with failure to thrive and essential FA (EFA) deficiency. In view of the severe fat malabsorption, transport of fat-soluble vitamins is flawed and even worsened with fat consumption (57).
TABLE 1.
Clinical phenotypes of the main congenital malabsorption disorders
| Disorder | CRD | ABL | HBL Homozygous | HBL Heterozygous |
| Retinopathy | + | +++ | +++ | − |
| Myopathy | + | +++ | +++ | +/− |
| Neuropathy | + | +++ | +++ | +/− |
| Cardiomyopathy | +/− | ++ | ++ | − |
| Hepatic steatosis | + | ++ | ++ | ++ |
| Steatorrhea | ++ | +++ | +++ | +/− |
| Acanthocytosis | − | + | + | +/− |
−, none; +/−, possible; +, low intensity; ++, moderate intensity; +++, high intensity.
Elevated serum transaminases with hepatomegaly due to hepatic steatosis have frequently been reported (110–112). Few patients presented with cirrhosis. Apparently, with post transplantation, the profile of the liver and lipoproteins was normal, but intestinal fat absorption persisted as the mutant MTP remains expressed in the intestine (113).
One of the most serious clinical manifestations is at the level of both central and peripheral nervous systems. In fact, a progressive ataxic neuropathic disease and retinopathy develop in later childhood and are probably due to vitamin E deficiency (111, 114, 115). However, one can observe absent tendon reflexes as early clinical signs, which are followed by deep sensory loss in the lower limbs and then a cerebella syndrome with an ataxic gait, dysmetria, and dysarthria (109). Pes cavus, pes equinovarus, and kyphoscoliosis are frequently encountered. Upper motor neuron signs, including Babinski sign or weakness of legs, can be observed in ABL patients. Nevertheless, the primary driving pathology is demyelination (111). Intriguingly, some patients escaped serious affliction until much later in life (111).
Among the wide range of ophthalmic symptoms and manifestations, the most prominent abnormality is pigmentary retinal degeneration (116). Early in the course of disease, patients have loss of night vision and some of them also exhibit loss of color vision. The retinopathy often produces slowly enlarging annular scotomas with macular sparing, such that patients are relatively unaware of the progression of the disease. Complete loss of vision can ultimately occur (116). Fundoscopic examination reveals an atypical pigmentation of the retina characterized by small irregularly distributed white spots. Electroretinogram and fluorescein angiography investigations have shown the retina to be affected in asymptomatic ABL patients (116).
Profound muscle weakness has been described in ABL. Striated and smooth muscles are affected and may represent the cause of premature death among a few ABL patients (117–119). The etiology of myopathy remains unclear, although myositis appears to be related to ceroid pigment deposition and muscle weakness to vitamin E deficiency and neuropathy. Death related to cardiomyopathy has been described for some patients (117, 118).
Acanthocytosis is among the characteristic hematologic manifestations of ABL. Circulating erythrocytes present abnormally shaped structures that inhibit rouleaux formation and culminate in extremely low erythrocyte sedimentation rates. Anemia has been found in some cases of ABL, probably as a consequence of deficiencies of iron, folate, and other nutrients secondary to fat malabsorption (110, 117).
Treatment
As highlighted in a very recent review, early diagnoses, combined with appropriate supplements, help prevent the severe sequelae of ABL (120). Fat intake should be reduced to 5–20 g/day, which will decrease steatorrhea while favoring marked clinical improvement and growth acceleration. Nevertheless, the diet has to be supplemented with EFAs (e.g., 5 g corn oil or safflower oil/day) to avoid EFA deficiency. Medium-chain TGs are often recommended to subjects with ABL as a caloric substitute for long-chain FAs, but under high precautions given their secondary effects such as hepatic fibrosis. The classical treatment also includes supplements of fat-soluble vitamins (E, A, D, and K). Noteworthy, oral α-tocopherol supplementation has to be initiated as early as possible to prevent neurological and retinal disability and halt/abrogate progression of the neuromuscular and myocardiopathy complications associated with this disease (109, 121, 122). If short-term efficacy of high-dose oral vitamin supplements has been proven (123–126), longer-term management in ABL has been little reported. Nevertheless, some studies stressed that combined vitamin E and A therapy initiated before age 2 leads to attenuation of retinal degeneration 10 years later (127).
HYPOBETALIPOPROTEINEMIA
Introduction
Familial hypobetalipoproteinemia (FHBL; OMIM 107730), an autosomal codominant disorder, is characterized by molecular defects in the APOB gene (Fig. 2) on chromosome locus 2p23-24, which interfere with the translation of the full-length of Apo B mRNA (128–130). Consequently, the formation of truncated Apo B of various sizes prevents the active export of TGs from the intestine by CMs and from the liver by VLDLs, resulting in intestinal and hepatic TG accumulation. Therefore, abnormally low TG-rich lipoproteins and LDLs are observed in blood circulation. As a function of the genetic status, the clinical manifestations may vary from none to neurological, endocrine, hematological, and liver dysfunctions.
Apo B properties
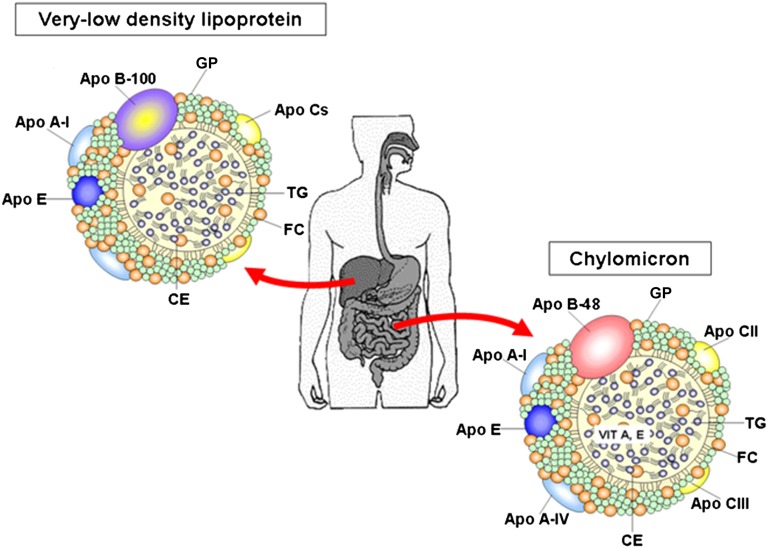
The majority of bulk lipid transport is achieved by Apo B-containing lipoproteins (Fig. 3). Apo B constitutes the largest glycoprotein that plays a central role in human TG-rich lipoprotein metabolism. The greatest form of Apo B is also the dominant protein in LDL and the ligand for the LDL receptor. Its structure is characterized by a globular amphipathic NH2 domain spanning the first 15–20% of the polypeptide followed by an extended hydrophobic β-sheet domain from about 20 to 48%, while the rest of the polypeptide is comprised of an α-helical amphipathic region, another long β-sheet domain, and another α-helical domain (131–133). Contrary to the other Apos that are exchangeable among circulating lipoproteins, Apo B is not transposable because it remains bound to the nascent lipoprotein until its recognition by specific receptors and uptake of the whole Apo B-containing particle in several tissues. Another unique feature is that only one single Apo B molecule is detectable in Apo B-containing lipoproteins. However, this single Apo B molecule is fully sufficient to provide the structural framework for the assembly of TG-rich lipoproteins in the liver and the small intestine (134) (Fig. 3). The synthesis of Apo B is not regulated at the transcriptional level, but it rather requires complex posttranslational processing, including lipidation and glycosylation, for proper folding and secretion (135). In fact, degradation by the ubiquitin proteasome system represents the major mechanism for regulation of Apo B secretion, whereas the availability of newly synthesized lipids protects Apo B from destruction and serves as the critical factor in targeting Apo B for secretion (136–139). Mechanistically, the translocation arrest provokes a prolonged association of Apo B with the Sec61β translocon and ribosomes, thereby resulting in impairment in the elongation stage of Apo B (140–143). In contrast, efficient ongoing lipid synthesis (during cotranslational translocation across the ER) prevents Apo B membrane from slowing or immobilizing, which favors its elongation. Of particular interest, a high physiologic concentration of lipids, provided over longer periods, induces ER stress that decreases the secretion of Apo B-100 (144, 145). It is recognized that the assembly of TG-rich lipoprotein occurs in two steps, one cotranslational (the first step) and another posttranslational (the second step) where the larger amount of TG is added, likely via fusion of a primordial Apo B lipoprotein particle with an Apo B-free TG droplet in the secretory pathway (146) (Fig. 1).
Fig. 3.
Schematic structure of plasmatic CM and VLDL. CMs, secreted by the intestine, and VLDLs, delivered by the liver, appear as spherical particles in the plasma. They are composed of a hydrophobic center (TGs and CEs) with an external monolayer of lipids [GPs and unesterified cholesterol (FC)] along with different Apos. The main form of Apo Bs in CMs and VLDLs is Apo B-48 and Apo B-100, respectively.
The human Apo B gene covers 43 kb of chromosome 2p and the coding portion of the gene extends over 43 kb and contains 29 exons and 28 introns. It produces two forms of circulating Apo B, namely Apo B-48 (2,152 amino acids) and Apo B-100 (4,536 amino acids), by a unique mRNA editing process (147–149). This RNA editing mechanism converts a codon (CAA) in the human intestine to a translation stop codon (UAA) at 48% of the full-length coding sequence. The resulting Apo B-48 protein is identical to the N-terminal 48% of Apo B-100 and is obligatory for CMs produced by the small intestine, whereas full-length Apo B-100 is structurally essential for VLDL synthesized by the liver. The remarkable posttranscriptional modification of Apo B mRNA is performed by a multicomponent enzyme complex, termed the C-to-U editosome (150), that contains APOBEC-1, a single catalytic subunit, and other proteins known as auxiliary or complementation factors (150, 151). The editosome has been suggested to favor more efficient absorption (152) and, conversely, low or no Apo B mRNA editing activity may result in relatively higher levels of VLDL and/or LDL, as noted in the liver of animals (153). These Apo B-100-containing lipoproteins are more atherogenic than Apo B-48-containing CMs. It is noteworthy that there is a small amount of Apo B mRNA that escapes editing, resulting in a low level of Apo B-100 expression by the intestine (109).
Apo B expression in different tissues and during human development
If previously the belief was that only the small intestine and liver have the capability to assemble and secrete Apo B-containing lipoproteins (Fig. 3), it now turns out that various organs are able enough to do it. Proximal tubule cells of mammalian kidney produce Apo B-containing lipoproteins and, conversely, inhibition of Apo B expression increases fasting-induced lipid accumulation in the kidney cortex (64, 102, 128). Apparently, the size and density of kidney-derived Apo B-containing lipoproteins depend on lipid availability (154). The human heart also expresses the Apo B gene in addition to the MTP gene (103). Likely, the mandatory presence of Apo B and MTP in cardiac myocytes allows the output of Apo B-containing lipoproteins in order to prevent pathological TG accumulation in the heart (103, 104, 155, 156). The placenta elaborates Apo B-100-containing particles that participate in lipid transport between mother and fetus (157, 158). The importance of this process is underscored by the increased lethality following the disruption of Apo B and MTP in the yolk sac of mice (107, 159, 160), which probably assures the early delivery of fat-soluble nutrients from the yolk sac to the embryo by Apo B-100-containing particles packaging and discharge. Ocular Apo B synthesis was also noted (161). Lipid overload in retinal pigmented epithelium possibly triggers the local machinery to produce Apo-B-containing lipoproteins to avoid apoptosis or degeneration of the tissue (162).
The human fetal intestine was demonstrated to possess an efficient lipoprotein-lipid transport system during development (163, 164). The assembly and secretion of TG-rich lipoproteins were active in the jejunum and colon of the human fetus (165, 166). For this task, it produces Apo B-100 early, but the switch in dominance from Apo B-100 to Apo B-48 mRNA takes place later during development (99, 100, 167–170). These processes are highly regulated by many hormones (167, 171, 172).
Taken together, there is now clear evidence that the intracellular assembly process is driven by Apo B. Thus, accidents of nature limiting Apo B production can have adverse effects on the export of lipids with potentially toxic fat accumulation in the different tissues and with a significant impact on TG-consuming organs. As expected from the central role of the Apo B-containing lipoproteins in delivering lipids, antioxidant vitamins, and fuel to cells, mutations of Apo B may profoundly affect development.
Functional consequences of Apo B absence: FHBL clinical spectrum
To date, more than 45 truncations in the Apo B gene have been reported and most of them are frequently due to mutations, exon deletions, and splicing variations (130, 173). The Apo B truncations have traditionally been named according to a centile system, with a wide range from Apo B-2 to Apo B-90 relative to normal Apo B-100 (174-197). The various truncations give rise to different sizes, densities, functions, and metabolism of lipoprotein fractions (198). In particular, they are characterized by a lower production rate and higher clearance rate, thereby contributing to abnormally reduced concentrations of circulating Apo B (179, 199–202), while fragments of Apo B <27.6 are undetectable (199). Even in heterozygous subjects with FHBL, the concentrations of Apo B do not exceed 30% of the normal values (203, 204).
FHBL heterozygotes may be asymptomatic, whereas homozygous FHBL patients present with steatorrhea, intestinal fat malabsorption, deficient absorption of EFA and lipid-soluble vitamins (A, D, E and K), hypocholesterolemia, and Apo B deficiency along with neurological, ocular, endocrine, and hematological abnormalities (205–207) (Table 1). Affected individuals may exhibit red cell acanthocytosis and retinitis pigmentosa. Sometimes fatty liver is accompanied by mild elevation of serum liver enzymes. In fact, sophisticated techniques could detect nonalcoholic fatty liver disease in FHBL subjects (187, 208–213).
Treatment
Although homozygous FHBL and ABL have a different genetic basis, they share similar signs, symptoms, and laboratory findings. Likewise, the clinical follow-up and management are comparable for the two disorders. As for ABL, it is mandatory to pay special attention to growth monitoring and prevention of complications in pediatrics by offering specialized dietary advice and fat-soluble vitamin treatments.
CM RETENTION DISEASE
Introduction
CM retention disease (CRD, OMIM 246700), or Anderson disease, is an autosomal-recessive condition caused by mutations in the SARA2 gene encoding the Sar1b protein. Contrary to ABL and FHBL, the synthesis of preCMs occurs in the ER, but without the possibility to reach the Golgi apparatus (Fig. 2). Lipids accumulate in the intestine and liver, while there is a selective absence of postprandial Apo B-48 and CMs. Thus, the young patients experience fat malabsorption, failure to thrive, and steatorrhea. Almost 50 cases have been reported so far in the literature.
Sar1b properties
Trafficking of CM-containing vesicles through the early secretory pathway is mediated by coat protein (COPII), a process requiring the small Sar1b GTPase for the exchange of GDP for GTP (214). Activated Sar1b initiates vesicle formation by recruiting first the inner COPII coat components (Sec23 and Sec24) and subsequently the components of the outer flexible coat (Sec13/Sec31) able to accommodate various sizes of vesicles (215). The mature coated vesicles bud from the ER and reach the Golgi apparatus where CMs transit prior to their discharge into the intercellular space and their transfer to the lymphatics before flowing into the blood circulation. Genetic defects in Sar1b GTPase inhibit the step of preCM trafficking to the Golgi (Fig. 2). The obligatory role of Sar1b GTPase is evidenced by the presence of its paralog Sar1a GTPase (216–218) that is 90% identical, differing by only 20 amino acid residues, but does not compensate for the lack of the Sar1b protein in CRD (219). Of note, if Sar1a could not compensate for loss of Sar1b in the gut, only limited data are, however, available regarding its contributions in the liver despite the fact that VLDL secretion was not substantially affected in CRD (220). Nevertheless, a recent study has provided strong evidence that Sar1b promotes the secretion of TG-rich Apo B-containing lipoproteins from the liver, which would neatly explain the counter-intuitive observation that some CRD children develop hepatic steatosis, despite severe intestinal fat malabsorption (221). Furthermore, SAR1b has been shown to be the predominantly expressed isoform in human jejunum and liver. Although Sar1a antagonizes the lipoprotein secretion-promoting activity of Sar1b, both isoforms were noted to modulate the expression of genes encoding cholesterol biosynthetic enzymes and the synthesis of cholesterol de novo (221). It is noteworthy that Sar1b GTPase is not only central for the COPII responsible for the biosynthetic transport of proteins from the ER to the Golgi apparatus, but also for fusion of the specific CM transport vesicle, the preCM transport vesicle, with the Golgi (50, 54, 222, 223).
The 3D structure of Sar1b protein is formed by six α-helices and six parallel β-strands that lead to a hydrophobic β-sheet sandwiched between three α-helices (224–226). The NH2-terminus segment comprises the site interacting with Sec12 and the two GTP binding and hydrolysis sites (224, 226–228), while the C-terminus segment includes the α-6 helix and the regulation loop (224, 225). Importantly, the N-terminus part allows the anchorage of Sar1b-GTP complex on the ER membrane, GTP binding, and hydrolysis, whereas the C terminus regulates interactions of Sar1b with the membrane. All the mutations affecting the C or N terminus provoke failure to secrete CMs, albeit with diverse phenotypes among CRD patients (229). Additional studies are necessary to examine the functionality of the mutated proteins, thereby providing further insights into this disease, as well as on the normal pathway for the export of CM.
Sar1b expression in different tissues and during human development
Despite growing knowledge on Sar1b GTPase, little is known about its tissue distribution and developmental regulation. Evaluation of Sar1b mRNA revealed skeletal muscle as the tissue with the highest Sar1b expression, followed by the heart and liver, the organs composing the digestive tract, the brain, and finally the lung and the adipose tissue (230). Sar1b protein expression levels follow a similar pattern among the organs, except for its higher expression in the heart. The abundant expression of Sar1b in skeletal muscle and heart suggests the highly specialized role of Sar1b in these particular tissues, including the regulation of calcium trafficking among multiple calcium storage organelles, for instance, the sarcoplasmic reticulum and the ER as reported previously (231–234). Accordingly, it was reported that patients with CRD suffer from myolysis, cardiac abnormalities, and elevated creatine kinase levels (235).
The proteins from the Sar1 family have not previously been reported as being involved in morphogenesis and development. Intuitively, the importance of the small GTPase classes in this process is predicted given their crucial functions in vesicular trafficking between the membranes of the ER and the Golgi apparatus, as noted in the initial stage of root hair (236) and axon (237) development, the membrane recruitment of cargo-sorting coat proteins, the modulation of membrane lipid composition, and the interaction with regulators of other G proteins. Evidently, additional efforts must be invested to support the possibility that the modulation of COPII-like trafficking machinery by Sar1b is active during development.
Functional consequences of Sar1b absence: CRD clinical spectrum
To date, about 20 gene defects have been described in SARA2, including missense or nonsense mutations, which affect splice acceptor and donor sites or correspond to insertions, deletions, and duplications, thereby disrupting coding sequences. Heterozygous carriers remain asymptomatic, whereas homozygous carriers present within the first few months or years of life with failure to thrive and diarrhea (Table 1). Furthermore, the young children exhibit white coating mucosa because of fat-laden villous enterocytes. Epithelial cells also show marked accumulation of large lipid droplets in the cytoplasm along with lipoprotein-sized structures in membrane bound compartments. The postprandial state is characterized by absence of TG elevation, Apo B-48, and CMs in response to a fat meal test, confirming the typical steatorrhea in CRD patients. These abnormalities are in line with closed juxtaposition of the intercellular spaces in the mucosa and are accompanied with severe hypocholesterolemia and low concentrations of total lipids, GPs, lipid soluble vitamins, EFAs, LDLs and HDLs, and Apos (B and AI) in the plasma. Clinically, electromyographic irregularities and diminished osteotendinous reflexes were common, but areflexia was rarely observed. Ophthalmological manifestations of vitamin A and E deficiencies were limited to electrophysiological anomalies detected by electroretinograms and evoked potentials. Hepatic steatosis was detected in only a few patients. Common at the time of diagnosis, the aforementioned anomalies proved largely reversible with treatment. Although the investigation of the Canadian subjects with the allele 409G>A reveals a more severe degree of hypocholesterolemia and few clinical parameters, no genotype-phenotype correlation has been evidenced (238).
Treatment
As per ABL and FHBL, CRD patients have a significant amelioration when treated with a low fat diet and supplementation with fat-soluble vitamins. To distinguish between the biochemical and clinical phenotypes, as well as management of the three congenital malabsorptions, a summary is presented in Table 2.
TABLE 2.
Comparative pathophysiology, clinical manifestations, and management of the main congenital malabsorption disorders
| Disorder | Gene | Biochemical Phenotype | Clinical Phenotype | Treatment |
| ABL | MTP | Ø LDL cholesterol | Failure to thrive | Low fat (<30% of total calories) with reduced LCFAs |
| Ø CMs | Abdominal distension | PUFAs | ||
| Ø VLDL | Lipid malabsorption | Vitamin E, 100–300 IU/kg/day | ||
| ↓ TGs | Spinocerebellar degeneration | Vitamin A, 100–400 IU/kg/day | ||
| ↓ Vitamins (A, D, E, K) | Night blindness | Vitamin D, 800–1,200 IU/day | ||
| Ø Apo B | Coagulopathy | Vitamin K, 5–35 mg/week | ||
| ↓ Other Apos | ||||
| Altered lipoprotein composition | ||||
| HBL | APOB | Heterozygous | Heterozygotes | Low fat (<30% of total calories) with reduced LCFAs |
| ↓ LDL cholesterol (less than 30% of levels normal for age and sex | Asymptomatic | PUFAs | ||
| Homozygous | Loose stools | Vitamin E, 100–300 IU/kg/day | ||
| Ø or ↓ LDL cholesterol | Mild fat malabsorption | Vitamin A, 100–400 IU/kg/day | ||
| ↓ TGs | Gallstones | Vitamin D, 800–1200 IU/day | ||
| Ø Apo B | Homozygous | Vitamin K, 5–35 mg/week | ||
| ↓ Vitamins (A, D, E, K) | Insulin sensitivity | |||
| ↓ Other Apos | Hepatic cirrhosis | |||
| Hepatocarcinoma | ||||
| CRD | SARA2 | Ø CMs | Anthropometry (failure-to-thrive) | Low fat diet |
| ↓ LDL cholesterol (less than 50% of levels normal for age and sex) | Digestive (diarrhea, vomiting, abdominal distension) | Enriched in EFA (vegetable oils, fish, …) | ||
| Anemia | Hepatomegaly | ± Enriched in medium-chain TGs | ||
| ↓ Total cholesterol | Neurological abnormalities (areflexia, ↓ deep proprioception) | Vitamin E, 50 IU/kg/day | ||
| ↓ HDL | Malnutrition (accumulation of lipid droplets in enterocytes) | Vitamin A, 15,000 IU/day (adjust according to plasma levels) | ||
| EFA deficiency | Anemia | |||
| ↓ Vitamins | Vitamin D, 800–1,200 IU/kg/day | |||
| Vitamin K, 15 mg/week (adjust according to INR and plasma levels) | ||||
| One perfusion | ||||
| FAs, intralipid 20% 2 g/kg/month | ||||
| Vitamin E, 4 to 6 mg/kg/month | ||||
| Vitamin A, 500 IU/kg/month |
INR, international normalized ratio; LCFA, long-chain FA.
CONGENITAL HYPOCHOLESTEROLEMIA IDENTIFIED IN PATIENTS WITH LOSS-OF-FUNCTION MUTATIONS OR PCSK9 VARIANTS
Proprotein convertase subtilisin/kexin type 9 (PCSK9), a serine protease expressed mainly in liver and intestine, is strongly involved in LDL metabolism (239). Its gene is localized on human chromosome 1p32 and encodes a 692-amino acid proteinase K-like serine protease that has a central role in regulation of cholesterol homeostasis, essentially by targeting the receptor to degradation, leading to reduced LDL-cholesterol clearance from the circulation (240). If the dominant gain-of-function mutations in the PCSK9 gene cause a phenotype similar to autosomal dominant familial hypercholesterolemia (241), loss-of-function variants are associated with hypocholesterolemia and protection against coronary artery disease (242–244). For example, R46L is a loss-of-function PCSK9 mutation because R46L-PCSK9 undergoes nearly a 16% increase in cell surface LDL receptors and a 35% increase in internalized LDL compared with wild-type PCSK9, suggesting that R46L causes hypocholesterolemia through a decreased ability to degrade LDL receptors (245). Other loss-of-function mutations may have drastic effects on cholesterolemia because they lower circulating LDL-cholesterol levels below ≈0.4 mmol/l (244, 246). So far, humans can survive and stay healthy as also confirmed in Pcsk9-knockout mice (247, 248). However, this does not seem to be the case in some lower vertebrates where knockdown of PCSK9 mRNA in zebrafish leads to disorganization of the nervous system and lethality.
CONGENITAL HYPOCHOLESTEROLEMIA IDENTIFIED IN PATIENTS WITH ANGPTL3
A form of familial combined hypolipidemia is due to defects in the angiopoietin-like 3 protein (ANGPTL3) gene in a family with primary hypocholesterolemia (249). ANGPTL3 is located on 1p31.3, exhibiting a signal peptide, an N-terminal coiled-coil domain, and a C-terminal fibrinogen-like domain. The mode of inheritance and the clinical implications of familial combined hypolipidemia are not well-defined. ANGPTL3 is located primarily in the liver and regulates lipid metabolism (250). Through its N-terminal region, it acts as a dual inhibitor of lipoprotein lipase and endothelial lipase and increases plasma HDL-cholesterol (251, 252). Patients with the loss-of-function mutation in ANGPTL3 have extremely lower plasma TG and LDL- and HDL-cholesterol levels than individuals with no mutation (249, 253). Accordingly, mice with the loss of ANGPTL3 expression display lower levels of TGs and HDL-cholesterol (250, 254, 255), whereas ANGPTL3 injection or overexpression increases circulating lipid levels (250, 251). Diabetes and cardiovascular disease were absent in homozygotes, raising the possibility that absence of ANGPTL3 is protective for these conditions. The prevalence of ANGPTL3 mutations giving rise to a combined hypolipidemia phenotype in subjects with severe primary hypobetalipoproteinemia (HBL) is about 10% (256). Of subjects with a total cholesterol concentration below the second percentile, those with HDL-cholesterol concentration below the second decile may be carrying ANGPTL3 mutations, whereas those with higher HDL-cholesterol concentrations may be carrying APOB mutations (256).
POLYMORPHISMS OF Apo B AND MTP AND LIPID CHANGES
To date, several variants on Apo B and MTP genes have been detected and studied due to their plausible role in the modulation of lipid/lipoprotein profiles and postprandial lipemia. One must be aware about this phenomenon because it may explain the low and high postprandial responses in relation with intestinally or hepatically derived TG-rich lipoproteins and the risk of myocardial infarction/premature coronary heart diseases.
Numerous polymorphisms of the Apo B gene have been described with an effect of the insertion (ins)/deletion (del) polymorphisms on lipid levels (257–259), and the kinetics of the secretion of VLDLs has already been found (260). It is noteworthy that the XbaI polymorphism was related to the inter-individual variability observed during postprandial lipemia, showing a significantly augmented or reduced postprandial response (261, 262). Particularly, a variable number of tandem repeats polymorphism, which is located 75 bp downstream of the second polyadenylation signal at the 3′ end of the Apo B gene (2p24-p23), has been found to be common in some ethnic groups (263–266). They were associated with modifications of lipid concentrations (267–271) and the risk of coronary heart diseases (270–272). Nevertheless, not one of these associations has been consistently observed in a large number of studies (273, 274). Discrepancies may be related to differences of ethnic groups and environmental factors.
As mentioned before, mutations in the MTP gene have been established in cases of ABL (90–93). Several of these genetic abnormalities (premature stop codons, mutations in canonical splice sites, or frameshift mutations) have been informative as to MTP function status. However, in many cases, predictions were difficult to establish (275, 276) despite the cosegregation of the genetic defects with the clinical phenotypes. With additional determinations of MTP activity (48, 92) and by scrutinizing the repercussions of intronic mutations (277) on intestinal or hepatic biopsies, as was performed by various groups, a clear picture of the genotype-phenotype relationship will certainly be obtained.
The most studied promoter polymorphism at the MTTP locus (−493G/T, located 493 bp upstream from the transcriptional start site) lowers the expression of MTP while reducing the formation and secretion of CMs and VLDLs (278–281). There is also evidence that the MTP −493G/T polymorphism modulates postprandial Apo B-48 and Apo B-100 of TG-rich lipoproteins (282). Various groups reported an association between the MTP −493T allele and low levels of serum TG, total cholesterol, LDL-cholesterol, and Apo B (58, 283–286). However, other studies revealed the opposite (287–289) or detected no relationship between this polymorphism and any lipid phenotype (290), demonstrating a huge gap across races on MTP −493T, which might be attributed to profoundly different evolutionary pressures at this locus.
Little is known as to the role of Sar1b polymorphisms on the inter-individual variability of the postprandial response, although studies have shown potential genotype-phenotype links (229) and elevated output of TG secretion in response to Sar1b overexpression (291, 292).
INTESTINAL LIPID FLUX, LIPID HOMEOSTASIS AND CARDIOMETABOLIC DISORDERS
It is now well-established that the small intestine is a significant determinant of postprandial dyslipidemia, cardiometabolic disorders, and atherogenesis. Various groups have pointed out the direct role of intestinally derived TG-rich lipoproteins in the progression of atherosclerosis, particularly during insulin resistance and type 2 diabetes mellitus (293, 294). Clearly, these disorders favor the increased basal rate of Apo B-48-containing lipoprotein secretion in fed and fasting states (295, 296). Demonstration has been obtained in various animal models (297–299), as well as in humans (300, 301). The contribution of the intestinal Apo B 48-containing lipoproteins to the promotion of atherosclerosis is increasingly being recognized as: i) Apo B-48 is correlated with postprandial lipemia (302), carotid intima-media thickness (303), and arterial disease (304, 305); and ii) CM remnants have access to and accumulate in the sub-endothelial space, thereby triggering the formation of atherosclerotic lesions (306–309). Therefore, one should be aware of the danger of overproduction of intestinal lipoprotein particles in cardiometabolic states such as obesity, insulin resistance, and diabetes
CONCLUSIONS
We have emphasized that mutations in Apo B-48, MTP, and SARA2 genes result in low or absent lipid, LDL-cholesterol, and Apo B levels. They cause intestinal fat malabsorption along with deficiency of EFA and liposoluble vitamins, thereby triggering various clinical disorders. Genetic defects in PCSK9 and ANGPTL3 also lead to familial HBL with an evident impact on metabolic and biochemical disorders. An update on management strategies has been presented. The postabsorptive concentrations of plasma TGs in response to particularly common polymorphisms of Apo B and MTP have also been documented. Definitely, the intestine exerts important influences not only on fat malabsorptions, but also on disease-related abnormalities of postprandial lipoprotein metabolism, including insulin resistance, type 2 diabetes, and atherosclerosis. Molecular testing should be available to rapidly define molecular aberrations and prevent complications. Hopefully, new nutritional and genetic developments will offer opportunities in the near future to develop strategies to target the intestine in order to enhance fat absorption in congenital malabsorptions on the one hand, and reduce postprandial lipemia and prevent atherosclerosis on the other hand.
Acknowledgments
The author thanks Mrs. Schohraya Spahis for her technical assistance.
Footnotes
Abbreviations:
- ABL
- abetalipoproteinemia
- ANGPTL3
- angiopoietin-like 3 protein
- CE
- cholesteryl ester
- CM
- chylomicron
- COPII
- coat protein complex II
- CRD
- chylomicron retention disease
- EFA
- essential FA
- ER
- endoplasmic reticulum
- FABP
- FA binding protein
- FHBL
- familial hypobetalipoproteinemia
- GP
- glycerophospholipid
- HBL
- hypobetalipoproteinemia
- MTP
- microsomal TG transfer protein
- PCSK9
- proprotein convertase subtilisin/kexin type 9
This work was supported by grants from Canadian Institutes of Health Research (MOP 10584) and the J. A. deSève Research Chair in Nutrition.
REFERENCES
- 1.Winkler F. K., D’Arcy A., Hunziker W. 1990. Structure of human pancreatic lipase. Nature. 343: 771–774. [DOI] [PubMed] [Google Scholar]
- 2.Carey M. C., Small D. M. 1970. The characteristics of mixed micellar solutions with particular reference to bile. Am. J. Med. 49: 590–608. [DOI] [PubMed] [Google Scholar]
- 3.Carey M. C., Small D. M. 1972. Micelle formation by bile salts. Physical-chemical and thermodynamic considerations. Arch. Intern. Med. 130: 506–527. [PubMed] [Google Scholar]
- 4.Borgström B. 1980. Importance of phospholipids, pancreatic phospholipase A2, and fatty acid for the digestion of dietary fat: in vitro experiments with the porcine enzymes. Gastroenterology. 78: 954–962. [PubMed] [Google Scholar]
- 5.Lombardo D., Guy O. 1980. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. II. Action on cholesterol esters and lipid-soluble vitamin esters. Biochim. Biophys. Acta. 611: 147–155. [DOI] [PubMed] [Google Scholar]
- 6.Mu H., Hoy C. E. 2004. The digestion of dietary triacylglycerols. Prog. Lipid Res. 43: 105–133. [DOI] [PubMed] [Google Scholar]
- 7.Phan C. T., Tso P. 2001. Intestinal lipid absorption and transport. Front. Biosci. 6: D299–D319. [DOI] [PubMed] [Google Scholar]
- 8.Lowe M. E. 1997. Structure and function of pancreatic lipase and colipase. Annu. Rev. Nutr. 17: 141–158. [DOI] [PubMed] [Google Scholar]
- 9.Thomson A. B., Schoeller C., Keelan M., Smith L., Clandinin M. T. 1993. Lipid absorption: passing through the unstirred layers, brush-border membrane, and beyond. Can. J. Physiol. Pharmacol. 71: 531–555. [DOI] [PubMed] [Google Scholar]
- 10.Hui D. Y., Howles P. N. 2002. Carboxyl ester lipase: structure-function relationship and physiological role in lipoprotein metabolism and atherosclerosis. J. Lipid Res. 43: 2017–2030. [DOI] [PubMed] [Google Scholar]
- 11.Cash J. G., Kuhel D. G., Goodin C., Hui D. Y. 2011. Pancreatic acinar cell-specific overexpression of group 1B phospholipase A2 exacerbates diet-induced obesity and insulin resistance in mice. Int. J. Obes. (Lond). 35: 877–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollie N. I., Hui D. Y. 2011. Group 1B phospholipase A(2) deficiency protects against diet-induced hyperlipidemia in mice. J. Lipid Res. 52: 2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugawara T., Kushiro M., Zhang H., Nara E., Ono H., Nagao A. 2001. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 131: 2921–2927. [DOI] [PubMed] [Google Scholar]
- 14.Thomson A. B., McIntyre Y., MacLeod J., Keelan M. 1986. Dietary fat content influences uptake of hexoses and lipids into rabbit jejunum following ileal resection. Digestion. 35: 78–88. [DOI] [PubMed] [Google Scholar]
- 15.Shiau Y. F. 1981. Mechanisms of intestinal fat absorption. Am. J. Physiol. 240: G1–G9. [DOI] [PubMed] [Google Scholar]
- 16.Westergaard H., Dietschy J. M. 1974. Delineation of the dimensions and permeability characteristics of the two major diffusion barriers to passive mucosal uptake in the rabbit intestine. J. Clin. Invest. 54: 718–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson F. A., Dietschy J. M. 1972. Characterization of bile acid absorption across the unstirred water layer and brush border of the rat jejunum. J. Clin. Invest. 51: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy C. C., Weber A. M., Lepage G., Smith L., Levy E. 1988. Digestive and absorptive phase anomalies associated with the exocrine pancreatic insufficiency of cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 7(Suppl 1): S1–S7. [DOI] [PubMed] [Google Scholar]
- 19.Levy E. 1992. The 1991 Borden Award Lecture. Selected aspects of intraluminal and intracellular phases of intestinal fat absorption. Can. J. Physiol. Pharmacol. 70: 413–419. [DOI] [PubMed] [Google Scholar]
- 20.Thomson A. B. 1982. Influence of dietary modifications on uptake of cholesterol, glucose, fatty acids, and alcohols into rabbit intestine. Am. J. Clin. Nutr. 35: 556–565. [DOI] [PubMed] [Google Scholar]
- 21.Stremmel W., Lotz G., Strohmeyer G., Berk P. D. 1985. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J. Clin. Invest. 75: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stremmel W. 1988. Uptake of fatty acids by jejunal mucosal cells is mediated by a fatty acid binding membrane protein. J. Clin. Invest. 82: 2001–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaffer J. E., Lodish H. F. 1994. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 79: 427–436. [DOI] [PubMed] [Google Scholar]
- 24.Poirier H., Degrace P., Niot I., Bernard A., Besnard P. 1996. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP). Eur. J. Biochem. 238: 368–373. [DOI] [PubMed] [Google Scholar]
- 25.Gimeno R. E., Hirsch D. J., Punreddy S., Sun Y., Ortegon A. M., Wu H., Daniels T., Stricker-Krongrad A., Lodish H. F., Stahl A. 2003. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J. Biol. Chem. 278: 49512–49516. [DOI] [PubMed] [Google Scholar]
- 26.Trigatti B. L., Anderson R. G., Gerber G. E. 1999. Identification of caveolin-1 as a fatty acid binding protein. Biochem. Biophys. Res. Commun. 255: 34–39. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqi S., Sheth A., Patel F., Barnes M., Mansbach C. M. 2013. Intestinal caveolin-1 is important for dietary fatty acid absorption. Biochim. Biophys. Acta. 1831: 1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berk P. D., Stump D. D. 1999. Mechanisms of cellular uptake of long chain free fatty acids. Mol. Cell. Biochem. 192: 17–31. [PubMed] [Google Scholar]
- 29.McArthur M. J., Atshaves B. P., Frolov A., Foxworth W. D., Kier A. B., Schroeder F. 1999. Cellular uptake and intracellular trafficking of long chain fatty acids. J. Lipid Res. 40: 1371–1383. [PubMed] [Google Scholar]
- 30.Hajri T., Abumrad N. A. 2002. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu. Rev. Nutr. 22: 383–415. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton J. A., Guo W., Kamp F. 2002. Mechanism of cellular uptake of long-chain fatty acids: Do we need cellular proteins? Mol. Cell. Biochem. 239: 17–23. [PubMed] [Google Scholar]
- 32.Pohl J., Ring A., Stremmel W. 2002. Uptake of long-chain fatty acids in HepG2 cells involves caveolae: analysis of a novel pathway. J. Lipid Res. 43: 1390–1399. [DOI] [PubMed] [Google Scholar]
- 33.Mashek D. G., Coleman R. A. 2006. Cellular fatty acid uptake: the contribution of metabolism. Curr. Opin. Lipidol. 17: 274–278. [DOI] [PubMed] [Google Scholar]
- 34.Kampf J. P., Kleinfeld A. M. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? An unknown protein mediates free fatty acid transport across the adipocyte plasma membrane. Physiology (Bethesda). 22: 7–14. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton J. A. 2004. Fatty acid interactions with proteins: what X-ray crystal and NMR solution structures tell us. Prog. Lipid Res. 43: 177–199. [DOI] [PubMed] [Google Scholar]
- 36.Storch J., Thumser A. E. 2010. Tissue-specific functions in the fatty acid-binding protein family. J. Biol. Chem. 285: 32679–32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelsers M. M., Namiot Z., Kisielewski W., Namiot A., Januszkiewicz M., Hermens W. T., Glatz J. F. 2003. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin. Biochem. 36: 529–535. [DOI] [PubMed] [Google Scholar]
- 38.Richieri G. V., Ogata R. T., Kleinfeld A. M. 1994. Equilibrium constants for the binding of fatty acids with fatty acid-binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J. Biol. Chem. 269: 23918–23930. [PubMed] [Google Scholar]
- 39.Storch J., Corsico B. 2008. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 28: 73–95. [DOI] [PubMed] [Google Scholar]
- 40.Storch J., Thumser A. E. 2000. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta. 1486: 28–44. [DOI] [PubMed] [Google Scholar]
- 41.Storch J. 1993. Diversity of fatty acid-binding protein structure and function: studies with fluorescent ligands. Mol. Cell. Biochem. 123: 45–53. [DOI] [PubMed] [Google Scholar]
- 42.Thumser A. E., Storch J. 2000. Liver and intestinal fatty acid-binding proteins obtain fatty acids from phospholipid membranes by different mechanisms. J. Lipid Res. 41: 647–656. [PubMed] [Google Scholar]
- 43.Lagakos W. S., Guan X., Ho S. Y., Sawicki L. R., Corsico B., Kodukula S., Murota K., Stark R. E., Storch J. 2013. Liver fatty acid-binding protein binds monoacylglycerol in vitro and in mouse liver cytosol. J. Biol. Chem. 288: 19805–19815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tso P., Balint J. A. 1986. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am. J. Physiol. 250: G715–G726. [DOI] [PubMed] [Google Scholar]
- 45.Black D. D. 1995. Intestinal lipoprotein metabolism. J. Pediatr. Gastroenterol. Nutr. 20: 125–147. [DOI] [PubMed] [Google Scholar]
- 46.Davidson N. O., Shelness G. S. 2000. Apolipoprotein B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu. Rev. Nutr. 20: 169–193. [DOI] [PubMed] [Google Scholar]
- 47.Borén J., Rustaeus S., Olofsson S. O. 1994. Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. J. Biol. Chem. 269: 25879–25888. [PubMed] [Google Scholar]
- 48.Wetterau J. R., Aggerbeck L. P., Bouma M. E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., Gregg R. E. 1992. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 258: 999–1001. [DOI] [PubMed] [Google Scholar]
- 49.Adeli K. 1994. Regulated intracellular degradation of apolipoprotein B in semipermeable HepG2 cells. J. Biol. Chem. 269: 9166–9175. [PubMed] [Google Scholar]
- 50.Siddiqi S., Saleem U., Abumrad N. A., Davidson N. O., Storch J., Siddiqi S. A., Mansbach C. M. 2010. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J. Lipid Res. 51: 1918–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levy E., Stan S., Delvin E., Menard D., Shoulders C., Garofalo C., Slight I., Seidman E., Mayer G., Bendayan M. 2002. Localization of microsomal triglyceride transfer protein in the Golgi: possible role in the assembly of chylomicrons. J. Biol. Chem. 277: 16470–16477. [DOI] [PubMed] [Google Scholar]
- 52.Berriot-Varoqueaux N., Dannoura A. H., Moreau A., Verthier N., Sassolas A., Cadiot G., Lachaux A., Munck A., Schmitz J., Aggerbeck L. P., et al. 2001. Apolipoprotein B48 glycosylation in abetalipoproteinemia and Anderson’s disease. Gastroenterology. 121: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 53.Olofsson S. O., Bjursell G., Bostrom K., Carlsson P., Elovson J., Protter A. A., Reuben M. A., Bondjers G. 1987. Apolipoprotein B: structure, biosynthesis and role in the lipoprotein assembly process. Atherosclerosis. 68: 1–17. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqi S. A., Gorelick F. S., Mahan J. T., Mansbach C. M. 2003. COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the pre-chylomicron transport vesicle. J. Cell Sci. 116: 415–427. [DOI] [PubMed] [Google Scholar]
- 55.Siddiqi S., Mansbach C. M. 2012. Phosphorylation of Sar1b protein releases liver fatty acid-binding protein from multiprotein complex in intestinal cytosol enabling it to bind to endoplasmic reticulum (ER) and bud the pre-chylomicron transport vesicle. J. Biol. Chem. 287: 10178–10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bassen F. A., Kornzweig A. L. 1950. Malformation of the erythrocytes in a case of atypical retinitis pigmentosa. Blood. 5: 381–387. [PubMed] [Google Scholar]
- 57.Berriot-Varoqueaux N., Aggerbeck L. P., Samson-Bouma M., Wetterau J. R. 2000. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 20: 663–697. [DOI] [PubMed] [Google Scholar]
- 58.Gordon D. A., Jamil H. 2000. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein-B lipoprotein assembly. Biochim. Biophys. Acta. 1486: 72–83. [DOI] [PubMed] [Google Scholar]
- 59.Hussain M. M. 2014. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 25: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abumrad N. A., Davidson N. O. 2012. Role of the gut in lipid homeostasis. Physiol. Rev. 92: 1061–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shoulders C. C., Shelness G. S. 2005. Current biology of MTP: implications for selective inhibition. Curr. Top. Med. Chem. 5: 283–300. [DOI] [PubMed] [Google Scholar]
- 62.Levy E., Marcel Y. L., Milne R. W., Grey V. L., Roy C. C. 1987. Absence of intestinal synthesis of apolipoprotein B-48 in two cases of abetalipoproteinemia. Gastroenterology. 93: 1119–1126. [DOI] [PubMed] [Google Scholar]
- 63.Wetterau J. R., Combs K. A., Spinner S. N., Joiner B. J. 1990. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. J. Biol. Chem. 265: 9800–9807. [PubMed] [Google Scholar]
- 64.Wetterau J. R., Zilversmit D. B. 1986. Localization of intracellular triacylglycerol and cholesteryl ester transfer activity in rat tissues. Biochim. Biophys. Acta. 875: 610–617. [DOI] [PubMed] [Google Scholar]
- 65.Wetterau J. R., Aggerbeck L. P., Laplaud P. M., McLean L. R. 1991. Structural properties of the microsomal triglyceride-transfer protein complex. Biochemistry. 30: 4406–4412. [DOI] [PubMed] [Google Scholar]
- 66.Atzel A., Wetterau J. R. 1993. Mechanism of microsomal triglyceride transfer protein catalyzed lipid transport. Biochemistry. 32: 10444–10450. [DOI] [PubMed] [Google Scholar]
- 67.Jamil H., Chu C. H., Dickson J. K., Jr, Chen Y., Yan M., Biller S. A., Gregg R. E., Wetterau J. R., Gordon D. A. 1998. Evidence that microsomal triglyceride transfer protein is limiting in the production of apolipoprotein B-containing lipoproteins in hepatic cells. J. Lipid Res. 39: 1448–1454. [PubMed] [Google Scholar]
- 68.Jamil H., Gordon D. A., Eustice D. C., Brooks C. M., Dickson J. K., Jr, Chen Y., Ricci B., Chu C. H., Harrity T. W., Ciosek C. P., Jr, et al. 1996. An inhibitor of the microsomal triglyceride transfer protein inhibits apoB secretion from HepG2 cells. Proc. Natl. Acad. Sci. USA. 93: 11991–11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haghpassand M., Wilder D., Moberly J. B. 1996. Inhibition of apolipoprotein B and triglyceride secretion in human hepatoma cells (HepG2). J. Lipid Res. 37: 1468–1480. [PubMed] [Google Scholar]
- 70.Benoist F., Nicodeme E., Grand-Perret T. 1996. Microsomal triacylglycerol transfer protein prevents presecretory degradation of apolipoprotein B-100. A dithiothreitol-sensitive protease is involved. Eur. J. Biochem. 240: 713–720. [DOI] [PubMed] [Google Scholar]
- 71.Samaha F. F., McKenney J., Bloedon L. T., Sasiela W. J., Rader D. J. 2008. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 5: 497–505. [DOI] [PubMed] [Google Scholar]
- 72.Cuchel M., Bloedon L. T., Szapary P. O., Kolansky D. M., Wolfe M. L., Sarkis A., Millar J. S., Ikewaki K., Siegelman E. S., Gregg R. E., et al. 2007. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 356: 148–156. [DOI] [PubMed] [Google Scholar]
- 73.Cuchel M., Meagher E. A., du Toit T. H., Blom D. J., Marais A. D., Hegele R. A., Averna M. R., Sirtori C. R., Shah P. K., Gaudet D., et al. 2013. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 381: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hussain M. M., Shi J., Dreizen P. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44: 22–32. [DOI] [PubMed] [Google Scholar]
- 75.Mann C. J., Anderson T. A., Read J., Chester S. A., Harrison G. B., Kochl S., Ritchie P. J., Bradbury P., Hussain F. S., Amey J., et al. 1999. The structure of vitellogenin provides a molecular model for the assembly and secretion of atherogenic lipoproteins. J. Mol. Biol. 285: 391–408. [DOI] [PubMed] [Google Scholar]
- 76.Bradbury P., Mann C. J., Kochl S., Anderson T. A., Chester S. A., Hancock J. M., Ritchie P. J., Amey J., Harrison G. B., Levitt D. G., et al. 1999. A common binding site on the microsomal triglyceride transfer protein for apolipoprotein B and protein disulfide isomerase. J. Biol. Chem. 274: 3159–3164. [DOI] [PubMed] [Google Scholar]
- 77.Gordon D. A., Jamil H., Sharp D., Mullaney D., Yao Z., Gregg R. E., Wetterau J. 1994. Secretion of apolipoprotein B-containing lipoproteins from HeLa cells is dependent on expression of the microsomal triglyceride transfer protein and is regulated by lipid availability. Proc. Natl. Acad. Sci. USA. 91: 7628–7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leiper J. M., Bayliss J. D., Pease R. J., Brett D. J., Scott J., Shoulders C. C. 1994. Microsomal triglyceride transfer protein, the abetalipoproteinemia gene product, mediates the secretion of apolipoprotein B-containing lipoproteins from heterologous cells. J. Biol. Chem. 269: 21951–21954. [PubMed] [Google Scholar]
- 79.Gordon D. A., Jamil H., Gregg R. E., Olofsson S. O., Boren J. 1996. Inhibition of the microsomal triglyceride transfer protein blocks the first step of apolipoprotein B lipoprotein assembly but not the addition of bulk core lipids in the second step. J. Biol. Chem. 271: 33047–33053. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., McLeod R. S., Yao Z. 1997. Normal activity of microsomal triglyceride transfer protein is required for the oleate-induced secretion of very low density lipoproteins containing apolipoprotein B from McA-RH7777 cells. J. Biol. Chem. 272: 12272–12278. [DOI] [PubMed] [Google Scholar]
- 81.Ginsberg H. N., Fisher E. A. 2009. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J. Lipid Res. 50(Suppl): S162–S166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dashti N., Gandhi M., Liu X., Lin X., Segrest J. P. 2002. The N-terminal 1000 residues of apolipoprotein B associate with microsomal triglyceride transfer protein to create a lipid transfer pocket required for lipoprotein assembly. Biochemistry. 41: 6978–6987. [DOI] [PubMed] [Google Scholar]
- 83.Hussain M. M., Bakillah A., Nayak N., Shelness G. S. 1998. Amino acids 430-570 in apolipoprotein B are critical for its binding to microsomal triglyceride transfer protein. J. Biol. Chem. 273: 25612–25615. [DOI] [PubMed] [Google Scholar]
- 84.Patel S. B., Grundy S. M. 1996. Interactions between microsomal triglyceride transfer protein and apolipoprotein B within the endoplasmic reticulum in a heterologous expression system. J. Biol. Chem. 271: 18686–18694. [DOI] [PubMed] [Google Scholar]
- 85.Narcisi T. M., Shoulders C. C., Chester S. A., Read J., Brett D. J., Harrison G. B., Grantham T. T., Fox M. F., Povey S., de Bruin T. W. 1995. Mutations of the microsomal triglyceride-transfer-protein gene in abetalipoproteinemia. Am. J. Hum. Genet. 57: 1298–1310. [PMC free article] [PubMed] [Google Scholar]
- 86.Sharp D., Blinderman L., Combs K. A., Kienzle B., Ricci B., Wager-Smith K., Gil C. M., Turck C. W., Bouma M. E., Rader D. J. 1993. Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia. Nature. 365: 65–69. [DOI] [PubMed] [Google Scholar]
- 87.Sharp D., Ricci B., Kienzle B., Lin M. C., Wetterau J. R. 1994. Human microsomal triglyceride transfer protein large subunit gene structure. Biochemistry. 33: 9057–9061. [DOI] [PubMed] [Google Scholar]
- 88.Geesaman B. J., Benson E., Brewster S. J., Kunkel L. M., Blanche H., Thomas G., Perls T. T., Daly M. J., Puca A. A. 2003. Haplotype-based identification of a microsomal transfer protein marker associated with the human lifespan. Proc. Natl. Acad. Sci. USA. 100: 14115–14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shoulders C. C., Brett D. J., Bayliss J. D., Narcisi T. M., Jarmuz A., Grantham T. T., Leoni P. R., Bhattacharya S., Pease R. J., Cullen P. M. 1993. Abetalipoproteinemia is caused by defects of the gene encoding the 97 kDa subunit of a microsomal triglyceride transfer protein. Hum. Mol. Genet. 2: 2109–2116. [DOI] [PubMed] [Google Scholar]
- 90.Fu J., Kwok S., Sinai L., Abdel-Razek O., Babula J., Chen D., Farago E., Fernandopulle N., Leith S., Loyzer M., et al. 2013. Western Database of Lipid Variants (WDLV): a catalogue of genetic variants in monogenic dyslipidemias. Can. J. Cardiol. 29: 934–939. [DOI] [PubMed] [Google Scholar]
- 91.Burnett J. R., Bell D. A., Hooper A. J., Hegele R. A. 2012. Clinical utility gene card for: Abetalipoproteinaemia. Eur. J. Hum. Genet. 20 Epub February 29, 2012. 10.1038/ejhg.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pons V., Rolland C., Nauze M., Danjoux M., Gaibelet G., Durandy A., Sassolas A., Levy E., Terce F., Collet X., et al. 2011. A severe form of abetalipoproteinemia caused by new splicing mutations of microsomal triglyceride transfer protein (MTTP). Hum. Mutat. 32: 751–759. [DOI] [PubMed] [Google Scholar]
- 93.Benayoun L., Granot E., Rizel L., Allon-Shalev S., Behar D. M., Ben-Yosef T. 2007. Abetalipoproteinemia in Israel: evidence for a founder mutation in the Ashkenazi Jewish population and a contiguous gene deletion in an Arab patient. Mol. Genet. Metab. 90: 453–457. [DOI] [PubMed] [Google Scholar]
- 94.Khatun I., Walsh M. T., Hussain M. M. 2013. Loss of both phospholipid and triglyceride transfer activities of microsomal triglyceride transfer protein in abetalipoproteinemia. J. Lipid Res. 54: 1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levy E., Stan S., Garofalo C., Delvin E. E., Seidman E. G., Menard D. 2001. Immunolocalization, ontogeny, and regulation of microsomal triglyceride transfer protein in human fetal intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 280: G563–G571. [DOI] [PubMed] [Google Scholar]
- 96.Swift L. L., Jovanovska A., Kakkad B., Ong D. E. 2005. Microsomal triglyceride transfer protein expression in mouse intestine. Histochem. Cell Biol. 123: 475–482. [DOI] [PubMed] [Google Scholar]
- 97.Dai K., Khatun I., Hussain M. M. 2010. NR2F1 and IRE1beta suppress microsomal triglyceride transfer protein expression and lipoprotein assembly in undifferentiated intestinal epithelial cells. Arterioscler. Thromb. Vasc. Biol. 30: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Farese R. V., Jr, Cases S., Ruland S. L., Kayden H. J., Wong J. S., Young S. G., Hamilton R. L. 1996. A novel function for apolipoprotein B: lipoprotein synthesis in the yolk sac is critical for maternal-fetal lipid transport in mice. J. Lipid Res. 37: 347–360. [PubMed] [Google Scholar]
- 99.Giannoni F., Chou S. C., Skarosi S. F., Verp M. S., Field F. J., Coleman R. A., Davidson N. O. 1995. Developmental regulation of the catalytic subunit of the apolipoprotein B mRNA editing enzyme (APOBEC-1) in human small intestine. J. Lipid Res. 36: 1664–1675. [PubMed] [Google Scholar]
- 100.Patterson A. P., Tennyson G. E., Hoeg J. M., Sviridov D. D., Brewer H. B., Jr 1992. Ontogenetic regulation of apolipoprotein B mRNA editing during human and rat development in vivo. Arterioscler. Thromb. 12: 468–473. [DOI] [PubMed] [Google Scholar]
- 101.Hopkins B., Brice A. L., Schofield P. N., Baralle F. E., Graham C. F. 1987. Identity of cells containing apolipoprotein B messenger RNA, in 6- to 12-week postfertilization human embryos. Development. 100: 83–93. [DOI] [PubMed] [Google Scholar]
- 102.Krzystanek M., Pedersen T. X., Bartels E. D., Kjaehr J., Straarup E. M., Nielsen L. B. 2010. Expression of apolipoprotein B in the kidney attenuates renal lipid accumulation. J. Biol. Chem. 285: 10583–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nielsen L. B., Veniant M., Boren J., Raabe M., Wong J. S., Tam C., Flynn L., Vanni-Reyes T., Gunn M. D., Goldberg I. J., et al. 1998. Genes for apolipoprotein B and microsomal triglyceride transfer protein are expressed in the heart: evidence that the heart has the capacity to synthesize and secrete lipoproteins. Circulation. 98: 13–16. [DOI] [PubMed] [Google Scholar]
- 104.Bjorkegren J., Veniant M., Kim S. K., Withycombe S. K., Wood P. A., Hellerstein M. K., Neese R. A., Young S. G. 2001. Lipoprotein secretion and triglyceride stores in the heart. J. Biol. Chem. 276: 38511–38517. [DOI] [PubMed] [Google Scholar]
- 105.Dougan S. K., Salas A., Rava P., Agyemang A., Kaser A., Morrison J., Khurana A., Kronenberg M., Johnson C., Exley M., et al. 2005. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J. Exp. Med. 202: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brozovic S., Nagaishi T., Yoshida M., Betz S., Salas A., Chen D., Kaser A., Glickman J., Kuo T., Little A., et al. 2004. CD1d function is regulated by microsomal triglyceride transfer protein. Nat. Med. 10: 535–539. [DOI] [PubMed] [Google Scholar]
- 107.Raabe M., Flynn L. M., Zlot C. H., Wong J. S., Veniant M. M., Hamilton R. L., Young S. G. 1998. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. USA. 95: 8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang B. H., Liao W., Li L., Nakamuta M., Mack D., Chan L. 1999. Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein/low density lipoprotein production in a viable conditional knockout mouse. J. Biol. Chem. 274: 6051–6055. [DOI] [PubMed] [Google Scholar]
- 109.Kane J. P., Havel R. J. 2001. Disorders of the biogenesis and secretion of lipoproteins containing the B apolipoproteins. In The Metabolic and Molecular Bases of Inherited Disease. D. Valle, A. L. Beaudet, B. Vogelstein, et al., editors. McGraw Hill, New York. 2717–2752. [Google Scholar]
- 110.Collins J. C., Scheinberg I. H., Giblin D. R., Sternlieb I. 1989. Hepatic peroxisomal abnormalities in abetalipoproteinemia. Gastroenterology. 97: 766–770. [DOI] [PubMed] [Google Scholar]
- 111.Kudo A., Tanaka N., Oogaki S., Niimura T., Kanehisa T. 1977. Hypobetalipoproteinemia with abnormal prebetalipoprotein. J. Neurol. Sci. 31: 411–419. [DOI] [PubMed] [Google Scholar]
- 112.Gharib H., Fairbanks V. F., Bartholomew L. G. 1969. Hepatic failure with acanthocytosis: association with hemolytic anemia and deficiency of erythrocyte glutathione peroxidase. Mayo Clin. Proc. 44: 96–101. [PubMed] [Google Scholar]
- 113.Braegger C. P., Belli D. C., Mentha G., Steinmann B. 1998. Persistence of the intestinal defect in abetalipoproteinaemia after liver transplantation. Eur. J. Pediatr. 157: 576–578. [DOI] [PubMed] [Google Scholar]
- 114.Kayden H. J. 1972. Abetalipoproteinemia. Annu. Rev. Med. 23: 285–296. [DOI] [PubMed] [Google Scholar]
- 115.Muller D. P., Lloyd J. K., Bird A. C. 1977. Long-term management of abetalipoproteinaemia. Possible role for vitamin E. Arch. Dis. Child. 52: 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Segal S., Sharma S. 2005. Ophthaproblem. Vitamin A and vitamin E. Can. Fam. Physician. 51: 1079, 1085–1086. [PMC free article] [PubMed] [Google Scholar]
- 117.Sobrevilla L. A., Goodman M. L., Kane C. A. 1964. Demyelinating central nervous system disease, macular atrophy and acanthocytosis (Bassen-Kornzweig syndrome). Am. J. Med. 37: 821–828. [DOI] [PubMed] [Google Scholar]
- 118.Dische M. R., Porro R. S. 1970. The cardiac lesions in Bassen-Kornzweig syndrome. Report of a case, with autopsy findings. Am. J. Med. 49: 568–571. [DOI] [PubMed] [Google Scholar]
- 119.Kott E., Delpre G., Kadish U., Dziatelovsky M., Sandbank U. 1977. Abetalipoproteinemia (Bassen-Kornzweig syndrome). Muscle involvement. Acta Neuropathol. 37: 255–258. [DOI] [PubMed] [Google Scholar]
- 120.Welty F. K. 2014. Hypobetalipoproteinemia and abetalipoproteinemia. Curr. Opin. Lipidol. 25: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Granot E., Deckelbaum R. J. 1989. Hypocholesterolemia in childhood. J. Pediatr. 115: 171–185. [DOI] [PubMed] [Google Scholar]
- 122.Runge P., Muller D. P., McAllister J., Calver D., Lloyd J. K., Taylor D. 1986. Oral vitamin E supplements can prevent the retinopathy of abetalipoproteinaemia. Br. J. Ophthalmol. 70: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hegele R. A., Angel A. 1985. Arrest of neuropathy and myopathy in abetalipoproteinemia with high-dose vitamin E therapy. Can. Med. Assoc. J. 132: 41–44. [PMC free article] [PubMed] [Google Scholar]
- 124.Muller D. P., Lloyd J. K., Wolff O. H. 1985. The role of vitamin E in the treatment of the neurological features of abetalipoproteinaemia and other disorders of fat absorption. J. Inherit. Metab. Dis. 8(Suppl 1): 88–92. [DOI] [PubMed] [Google Scholar]
- 125.Muller D. P., Lloyd J. K. 1982. Effect of large oral doses of vitamin E on the neurological sequelae of patients with abetalipoproteinemia. Ann. N. Y. Acad. Sci. 393: 133–144. [DOI] [PubMed] [Google Scholar]
- 126.Bishara S., Merin S., Cooper M., Azizi E., Delpre G., Deckelbaum R. J. 1982. Combined vitamin A and E therapy prevents retinal electrophysiological deterioration in abetalipoproteinaemia. Br. J. Ophthalmol. 66: 767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chowers I., Banin E., Merin S., Cooper M., Granot E. 2001. Long-term assessment of combined vitamin A and E treatment for the prevention of retinal degeneration in abetalipoproteinaemia and hypobetalipoproteinaemia patients. Eye (Lond.). 15: 525–530. [DOI] [PubMed] [Google Scholar]
- 128.Tarugi P., Averna M., Di L. E., Cefalu A. B., Noto D., Magnolo L., Cattin L., Bertolini S., Calandra S. 2007. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis. 195: e19–e27. [DOI] [PubMed] [Google Scholar]
- 129.Burnett J. R., Zhong S., Jiang Z. G., Hooper A. J., Fisher E. A., McLeod R. S., Zhao Y., Barrett P. H., Hegele R. A., van Bockxmeer F. M., et al. 2007. Missense mutations in APOB within the betaalpha1 domain of human APOB-100 result in impaired secretion of ApoB and ApoB-containing lipoproteins in familial hypobetalipoproteinemia. J. Biol. Chem. 282: 24270–24283. [DOI] [PubMed] [Google Scholar]
- 130.Zhong S., Magnolo A. L., Sundaram M., Zhou H., Yao E. F., Di L. E., Loria P., Wang S., Bamji-Mirza M., Wang L., et al. 2010. Nonsynonymous mutations within APOB in human familial hypobetalipoproteinemia: evidence for feedback inhibition of lipogenesis and postendoplasmic reticulum degradation of apolipoprotein B. J. Biol. Chem. 285: 6453–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Segrest J. P., Jones M. K., De L. H., Dashti N. 2001. Structure of apolipoprotein B-100 in low density lipoproteins. J. Lipid Res. 42: 1346–1367. [PubMed] [Google Scholar]
- 132.Jiang Z. G., Gantz D., Bullitt E., McKnight C. J. 2006. Defining lipid-interacting domains in the N-terminal region of apolipoprotein B. Biochemistry. 45: 11799–11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fisher E. A., Ginsberg H. N. 2002. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 277: 17377–17380. [DOI] [PubMed] [Google Scholar]
- 134.Xiao C., Hsieh J., Adeli K., Lewis G. F. 2011. Gut-liver interaction in triglyceride-rich lipoprotein metabolism. Am. J. Physiol. Endocrinol. Metab. 301: E429–E446. [DOI] [PubMed] [Google Scholar]
- 135.Rutledge A. C., Su Q., Adeli K. 2010. Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly. Biochem. Cell Biol. 88: 251–267. [DOI] [PubMed] [Google Scholar]
- 136.Yeung S. J., Chen S. H., Chan L. 1996. Ubiquitin-proteasome pathway mediates intracellular degradation of apolipoprotein B. Biochemistry. 35: 13843–13848. [DOI] [PubMed] [Google Scholar]
- 137.Fisher E. A., Zhou M., Mitchell D. M., Wu X., Omura S., Wang H., Goldberg A. L., Ginsberg H. N. 1997. The degradation of apolipoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. J. Biol. Chem. 272: 20427–20434. [DOI] [PubMed] [Google Scholar]
- 138.Gusarova V., Caplan A. J., Brodsky J. L., Fisher E. A. 2001. Apoprotein B degradation is promoted by the molecular chaperones hsp90 and hsp70. J. Biol. Chem. 276: 24891–24900. [DOI] [PubMed] [Google Scholar]
- 139.Qiu W., Kohen-Avramoglu R., Mhapsekar S., Tsai J., Austin R. C., Adeli K. 2005. Glucosamine-induced endoplasmic reticulum stress promotes ApoB100 degradation: evidence for Grp78-mediated targeting to proteasomal degradation. Arterioscler. Thromb. Vasc. Biol. 25: 571–577. [DOI] [PubMed] [Google Scholar]
- 140.Mitchell D. M., Zhou M., Pariyarath R., Wang H., Aitchison J. D., Ginsberg H. N., Fisher E. A. 1998. Apoprotein B100 has a prolonged interaction with the translocon during which its lipidation and translocation change from dependence on the microsomal triglyceride transfer protein to independence. Proc. Natl. Acad. Sci. USA. 95: 14733–14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pan M., Liang J., Fisher E. A., Ginsberg H. N. 2000. Inhibition of translocation of nascent apolipoprotein B across the endoplasmic reticulum membrane is associated with selective inhibition of the synthesis of apolipoprotein B. J. Biol. Chem. 275: 27399–27405. [DOI] [PubMed] [Google Scholar]
- 142.Pariyarath R., Wang H., Aitchison J. D., Ginsberg H. N., Welch W. J., Johnson A. E., Fisher E. A. 2001. Co-translational interactions of apoprotein B with the ribosome and translocon during lipoprotein assembly or targeting to the proteasome. J. Biol. Chem. 276: 541–550. [DOI] [PubMed] [Google Scholar]
- 143.Chen Y., Le C. F., Chuck S. L. 1998. Calnexin and other factors that alter translocation affect the rapid binding of ubiquitin to apoB in the Sec61 complex. J. Biol. Chem. 273: 11887–11894. [DOI] [PubMed] [Google Scholar]
- 144.Ota T., Gayet C., Ginsberg H. N. 2008. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J. Clin. Invest. 118: 316–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Caviglia J. M., Gayet C., Ota T., Hernandez-Ono A., Conlon D. M., Jiang H., Fisher E. A., Ginsberg H. N. 2011. Different fatty acids inhibit apoB100 secretion by different pathways: unique roles for ER stress, ceramide, and autophagy. J. Lipid Res. 52: 1636–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ledford A. S., Cook V. A., Shelness G. S., Weinberg R. B. 2009. Structural and dynamic interfacial properties of the lipoprotein initiating domain of apolipoprotein B. J. Lipid Res. 50: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koito A., Ikeda T. 2012. Apolipoprotein B mRNA-editing, catalytic polypeptide cytidine deaminases and retroviral restriction. Wiley Interdiscip. Rev. RNA. 3: 529–541. [DOI] [PubMed] [Google Scholar]
- 148.Chan L., Boerwinkle E. 1992. Structure, function, molecular genetics, and epidemiology of apolipoprotein B. Semin. Liver Dis. 12: 311–320. [DOI] [PubMed] [Google Scholar]
- 149.Mitsche M. A., Packer L. E., Brown J. W., Jiang Z. G., Small D. M., McKnight C. J. 2014. Surface tensiometry of apolipoprotein B domains at lipid interfaces suggests a new model for the initial steps in triglyceride-rich lipoprotein assembly. J. Biol. Chem. 289: 9000–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Navaratnam N., Fujino T., Bayliss J., Jarmuz A., How A., Richardson N., Somasekaram A., Bhattacharya S., Carter C., Scott J. 1998. Escherichia coli cytidine deaminase provides a molecular model for ApoB RNA editing and a mechanism for RNA substrate recognition. J. Mol. Biol. 275: 695–714. [DOI] [PubMed] [Google Scholar]
- 151.Teng B., Burant C. F., Davidson N. O. 1993. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 260: 1816–1819. [DOI] [PubMed] [Google Scholar]
- 152.Kendrick J. S., Chan L., Higgins J. A. 2001. Superior role of apolipoprotein B48 over apolipoprotein B100 in chylomicron assembly and fat absorption: an investigation of apobec-1 knock-out and wild-type mice. Biochem. J. 356: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Greeve J., Altkemper I., Dieterich J. H., Greten H., Windler E. 1993. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J. Lipid Res. 34: 1367–1383. [PubMed] [Google Scholar]
- 154.Walzem R. L., Hansen R. J., Williams D. L., Hamilton R. L. 1999. Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 129: 467S–472S. [DOI] [PubMed] [Google Scholar]
- 155.Borén J., Veniant M. M., Young S. G. 1998. Apo B100-containing lipoproteins are secreted by the heart. J. Clin. Invest. 101: 1197–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yokoyama M., Yagyu H., Hu Y., Seo T., Hirata K., Homma S., Goldberg I. J. 2004. Apolipoprotein B production reduces lipotoxic cardiomyopathy: studies in heart-specific lipoprotein lipase transgenic mouse. J. Biol. Chem. 279: 4204–4211. [DOI] [PubMed] [Google Scholar]
- 157.Demmer L. A., Levin M. S., Elovson J., Reuben M. A., Lusis A. J., Gordon J. I. 1986. Tissue-specific expression and developmental regulation of the rat apolipoprotein B gene. Proc. Natl. Acad. Sci. USA. 83: 8102–8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Madsen E. M., Lindegaard M. L., Andersen C. B., Damm P., Nielsen L. B. 2004. Human placenta secretes apolipoprotein B-100-containing lipoproteins. J. Biol. Chem. 279: 55271–55276. [DOI] [PubMed] [Google Scholar]
- 159.Homanics G. E., Smith T. J., Zhang S. H., Lee D., Young S. G., Maeda N. 1993. Targeted modification of the apolipoprotein B gene results in hypobetalipoproteinemia and developmental abnormalities in mice. Proc. Natl. Acad. Sci. USA. 90: 2389–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Farese R. V., Jr, Ruland S. L., Flynn L. M., Stokowski R. P., Young S. G. 1995. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc. Natl. Acad. Sci. USA. 92: 1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Malek G., Li C. M., Guidry C., Medeiros N. E., Curcio C. A. 2003. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am. J. Pathol. 162: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fujihara M., Bartels E., Nielsen L. B., Handa J. T. 2009. A human apoB100 transgenic mouse expresses human apoB100 in the RPE and develops features of early AMD. Exp. Eye Res. 88: 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thibault L., Menard D., Loirdighi N., Levy E. 1992. Ontogeny of intestinal lipid and lipoprotein synthesis. Biol. Neonate. 62: 100–107. [DOI] [PubMed] [Google Scholar]
- 164.Levy E., Menard D. 2000. Developmental aspects of lipid and lipoprotein synthesis and secretion in human gut. Microsc. Res. Tech. 49: 363–373. [DOI] [PubMed] [Google Scholar]
- 165.Levy E., Thibault L., Menard D. 1992. Intestinal lipids and lipoproteins in the human fetus: modulation by epidermal growth factor. J. Lipid Res. 33: 1607–1617. [PubMed] [Google Scholar]
- 166.Levy E., Loirdighi N., Thibault L., Nguyen T. D., Labuda D., Delvin E., Menard D. 1996. Lipid processing and lipoprotein synthesis by the developing human fetal colon. Am. J. Physiol. 270: G813–G820. [DOI] [PubMed] [Google Scholar]
- 167.Levy E., Sinnett D., Thibault L., Nguyen T. D., Delvin E., Menard D. 1996. Insulin modulation of newly synthesized apolipoproteins B-100 and B-48 in human fetal intestine: gene expression and mRNA editing are not involved. FEBS Lett. 393: 253–258. [DOI] [PubMed] [Google Scholar]
- 168.Henderson J. O., Blanc V., Davidson N. O. 2001. Isolation, characterization and developmental regulation of the human apobec-1 complementation factor (ACF) gene. Biochim. Biophys. Acta. 1522: 22–30. [DOI] [PubMed] [Google Scholar]
- 169.Teng B., Verp M., Salomon J., Davidson N. O. 1990. Apolipoprotein B messenger RNA editing is developmentally regulated and widely expressed in human tissues. J. Biol. Chem. 265: 20616–20620. [PubMed] [Google Scholar]
- 170.Funahashi T., Giannoni F., DePaoli A. M., Skarosi S. F., Davidson N. O. 1995. Tissue-specific, developmental and nutritional regulation of the gene encoding the catalytic subunit of the rat apolipoprotein B mRNA editing enzyme: functional role in the modulation of apoB mRNA editing. J. Lipid Res. 36: 414–428. [PubMed] [Google Scholar]
- 171.Loirdighi N., Menard D., Delvin D., Levy E. 1997. Selective effects of hydrocortisone on intestinal lipoprotein and apolipoprotein synthesis in the human fetus. J. Cell. Biochem. 66: 65–76. [DOI] [PubMed] [Google Scholar]
- 172.Levy E., Thibault L., Delvin E., Menard D. 1994. Apolipoprotein synthesis in human fetal intestine: regulation by epidermal growth factor. Biochem. Biophys. Res. Commun. 204: 1340–1345. [DOI] [PubMed] [Google Scholar]
- 173.Srivastava N., Noto D., Averna M., Pulai J., Srivastava R. A., Cole T. G., Latour M. A., Patterson B. W., Schonfeld G. 1996. A new apolipoprotein B truncation (apo B-43.7) in familial hypobetalipoproteinemia: genetic and metabolic studies. Metabolism. 45: 1296–1304. [DOI] [PubMed] [Google Scholar]
- 174.Young S. G., Hubl S. T., Chappell D. A., Smith R. S., Claiborne F., Snyder S. M., Terdiman J. F. 1989. Familial hypobetalipoproteinemia associated with a mutant species of apolipoprotein B (B-46). N. Engl. J. Med. 320: 1604–1610. [DOI] [PubMed] [Google Scholar]
- 175.Young S. G., Bertics S. J., Curtiss L. K., Dubois B. W., Witztum J. L. 1987. Genetic analysis of a kindred with familial hypobetalipoproteinemia. Evidence for two separate gene defects: one associated with an abnormal apolipoprotein B species, apolipoprotein B-37; and a second associated with low plasma concentrations of apolipoprotein B-100. J. Clin. Invest. 79: 1842–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Linton M. F., Pierotti V., Young S. G. 1992. Reading-frame restoration with an apolipoprotein B gene frameshift mutation. Proc. Natl. Acad. Sci. USA. 89: 11431–11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Collins D. R., Knott T. J., Pease R. J., Powell L. M., Wallis S. C., Robertson S., Pullinger C. R., Milne R. W., Marcel Y. L., Humphries S. E. 1988. Truncated variants of apolipoprotein B cause hypobetalipoproteinaemia. Nucleic Acids Res. 16: 8361–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Krul E. S., Kinoshita M., Talmud P., Humphries S. E., Turner S., Goldberg A. C., Cook K., Boerwinkle E., Schonfeld G. 1989. Two distinct truncated apolipoprotein B species in a kindred with hypobetalipoproteinemia. Arteriosclerosis. 9: 856–868. [DOI] [PubMed] [Google Scholar]
- 179.Parhofer K. G., Barrett P. H., Bier D. M., Schonfeld G. 1992. Lipoproteins containing the truncated apolipoprotein, Apo B-89, are cleared from human plasma more rapidly than Apo B-100-containing lipoproteins in vivo. J. Clin. Invest. 89: 1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Young S. G., Hubl S. T., Smith R. S., Snyder S. M., Terdiman J. F. 1990. Familial hypobetalipoproteinemia caused by a mutation in the apolipoprotein B gene that results in a truncated species of apolipoprotein B (B-31). A unique mutation that helps to define the portion of the apolipoprotein B molecule required for the formation of buoyant, triglyceride-rich lipoproteins. J. Clin. Invest. 85: 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Huang L. S., Ripps M. E., Korman S. H., Deckelbaum R. J., Breslow J. L. 1989. Hypobetalipoproteinemia due to an apolipoprotein B gene exon 21 deletion derived by Alu-Alu recombination. J. Biol. Chem. 264: 11394–11400. [PubMed] [Google Scholar]
- 182.Welty F. K., Hubl S. T., Pierotti V. R., Young S. G. 1991. A truncated species of apolipoprotein B (B67) in a kindred with familial hypobetalipoproteinemia. J. Clin. Invest. 87: 1748–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McCormick S. P., Fellowes A. P., Walmsley T. A., George P. M. 1992. Apolipoprotein B-32: a new truncated mutant of human apolipoprotein B capable of forming particles in the low density lipoprotein range. Biochim. Biophys. Acta. 1138: 290–296. [DOI] [PubMed] [Google Scholar]
- 184.Talmud P. J., Converse C., Krul E., Huq L., McIlwaine G. G., Series J. J., Boyd P., Schonfeld G., Dunning A., Humphries S. 1992. A novel truncated apolipoprotein B (apo B55) in a patient with familial hypobetalipoproteinemia and atypical retinitis pigmentosa. Clin. Genet. 42: 62–70. [DOI] [PubMed] [Google Scholar]
- 185.Yue P., Yuan B., Gerhard D. S., Neuman R. J., Isley W. L., Harris W. S., Schonfeld G. 2002. Novel mutations of APOB cause ApoB truncations undetectable in plasma and familial hypobetalipoproteinemia. Hum. Mutat. 20: 110–116. [DOI] [PubMed] [Google Scholar]
- 186.Hegele R. A., Miskie B. A. 2002. Acanthocytosis in a patient with homozygous familial hypobetalipoproteinemia due to a novel APOB splice site mutation. Clin. Genet. 61: 101–103. [DOI] [PubMed] [Google Scholar]
- 187.Tarugi P., Lonardo A., Ballarini G., Grisendi A., Pulvirenti M., Bagni A., Calandra S. 1996. Fatty liver in heterozygous hypobetalipoproteinemia caused by a novel truncated form of apolipoprotein B. Gastroenterology. 111: 1125–1133. [DOI] [PubMed] [Google Scholar]
- 188.Tarugi P., Lonardo A., Gabelli C., Sala F., Ballarini G., Cortella I., Previato L., Bertolini S., Cordera R., Calandra S. 2001. Phenotypic expression of familial hypobetalipoproteinemia in three kindreds with mutations of apolipoprotein B gene. J. Lipid Res. 42: 1552–1561. [PubMed] [Google Scholar]
- 189.Pulai J. I., Zakeri H., Kwok P. Y., Kim J. H., Wu J., Schonfeld G. 1998. Donor splice mutation (665 + 1 G_T) in familial hypobetalipoproteinemia with no detectable apoB truncation. Am. J. Med. Genet. 80: 218–220. [PubMed] [Google Scholar]
- 190.Talmud P., King-Underwood L., Krul E., Schonfeld G., Humphries S. 1989. The molecular basis of truncated forms of apolipoprotein B in a kindred with compound heterozygous hypobetalipoproteinemia. J. Lipid Res. 30: 1773–1779. [PubMed] [Google Scholar]
- 191.Welty F. K., Guida K. A., Andersen J. J. 2001. Donor splice-site mutation (210+1G_C) in the ApoB gene causes a very low level of ApoB-100 and LDL cholesterol. Arterioscler. Thromb. Vasc. Biol. 21: 1864–1865. [PubMed] [Google Scholar]
- 192.Welty F. K., Ordovas J., Schaefer E. J., Wilson P. W., Young S. G. 1995. Identification and molecular analysis of two apoB gene mutations causing low plasma cholesterol levels. Circulation. 92: 2036–2040. [DOI] [PubMed] [Google Scholar]
- 193.Pullinger C. R., Hillas E., Hardman D. A., Chen G. C., Naya-Vigne J. M., Iwasa J. A., Hamilton R. L., Lalouel J. M., Williams R. R., Kane J. P. 1992. Two apolipoprotein B gene defects in a kindred with hypobetalipoproteinemia, one of which results in a truncated variant, apoB-61, in VLDL and LDL. J. Lipid Res. 33: 699–710. [PubMed] [Google Scholar]
- 194.Hardman D. A., Pullinger C. R., Hamilton R. L., Kane J. P., Malloy M. J. 1991. Molecular and metabolic basis for the metabolic disorder normotriglyceridemic abetalipoproteinemia. J. Clin. Invest. 88: 1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Young S. G., Pullinger C. R., Zysow B. R., Hofmann-Radvani H., Linton M. F., Farese R. V., Jr, Terdiman J. F., Snyder S. M., Grundy S. M., Vega G. L. 1993. Four new mutations in the apolipoprotein B gene causing hypobetalipoproteinemia, including two different frameshift mutations that yield truncated apolipoprotein B proteins of identical length. J. Lipid Res. 34: 501–507. [PubMed] [Google Scholar]
- 196.Talmud P. J., Krul E. S., Pessah M., Gay G., Schonfeld G., Humphries S. E., Infante R. 1994. Donor splice mutation generates a lipid-associated apolipoprotein B-27.6 in a patient with homozygous hypobetalipoproteinemia. J. Lipid Res. 35: 468–477. [PubMed] [Google Scholar]
- 197.Tarugi P., Averna M. 2011. Hypobetalipoproteinemia: genetics, biochemistry, and clinical spectrum. Adv. Clin. Chem. 54: 81–107. [PubMed] [Google Scholar]
- 198.Groenewegen W. A., Averna M. R., Pulai J., Krul E. S., Schonfeld G. 1994. Apolipoprotein B-38.9 does not associate with apo[a] and forms two distinct HDL density particle populations that are larger than HDL. J. Lipid Res. 35: 1012–1025. [PubMed] [Google Scholar]
- 199.Zhu X. F., Noto D., Seip R., Shaish A., Schonfeld G. 1997. Organ loci of catabolism of short truncations of apoB. Arterioscler. Thromb. Vasc. Biol. 17: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 200.Chen Z., Saffitz J. E., Latour M. A., Schonfeld G. 1999. Truncated apo B-70.5-containing lipoproteins bind to megalin but not the LDL receptor. J. Clin. Invest. 103: 1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Parhofer K. G., Daugherty A., Kinoshita M., Schonfeld G. 1990. Enhanced clearance from plasma of low density lipoproteins containing a truncated apolipoprotein, apoB-89. J. Lipid Res. 31: 2001–2007. [PubMed] [Google Scholar]
- 202.Lam M. C., Singham J., Hegele R. A., Riazy M., Hiob M. A., Francis G., Steinbrecher U. P. 2012. Familial hypobetalipoproteinemia-induced nonalcoholic steatohepatitis. Case Rep. Gastroenterol. 6: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Linton M. F., Farese R. V., Jr, Young S. G. 1993. Familial hypobetalipoproteinemia. J. Lipid Res. 34: 521–541. [PubMed] [Google Scholar]
- 204.Chen Z., Fitzgerald R. L., Li G., Davidson N. O., Schonfeld G. 2004. Hepatic secretion of apoB-100 is impaired in hypobetalipoproteinemic mice with an apoB-38.9-specifying allele. J. Lipid Res. 45: 155–163. [DOI] [PubMed] [Google Scholar]
- 205.Lee J., Hegele R. A. 2014. Abetalipoproteinemia and homozygous hypobetalipoproteinemia: a framework for diagnosis and management. J. Inherit. Metab. Dis. 37: 333–339. [DOI] [PubMed] [Google Scholar]
- 206.Seckeler M. D., Linden J. 2008. Maternal abetalipoproteinemia resulting in multiple fetal anomalies. Neonatology. 94: 310–313. [DOI] [PubMed] [Google Scholar]
- 207.Matsuo M., Nomura S., Hara T., Kinoshita M., Yamamoto K., Kuno T., Maeda Y., Miyazaki S. 1994. A variant form of hypobetalipoproteinaemia associated with ataxia, hearing loss and retinitis pigmentosa. Dev. Med. Child Neurol. 36: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 208.Gutiérrez-Cirlos C., Ordonez-Sanchez M. L., Tusie-Luna M. T., Patterson B. W., Schonfeld G., Aguilar-Salinas C. A. 2011. Familial hypobetalipoproteinemia in a hospital survey: genetics, metabolism and non-alcoholic fatty liver disease. Ann. Hepatol. 10: 155–164. [PubMed] [Google Scholar]
- 209.Della Corte C., Fintini D., Giordano U., Cappa M., Brufani C., Majo F., Mennini C., Nobili V. 2013. Fatty liver and insulin resistance in children with hypobetalipoproteinemia: the importance of aetiology. Clin. Endocrinol. (Oxf.). 79: 49–54. [DOI] [PubMed] [Google Scholar]
- 210.Tarugi P., Lonardo A. 1997. Heterozygous familial hypobetalipoproteinemia associated with fatty liver. Am. J. Gastroenterol. 92: 1400–1402. [PubMed] [Google Scholar]
- 211.Lonardo A., Tarugi P., Ballarini G., Bagni A. 1998. Familial heterozygous hypobetalipoproteinemia, extrahepatic primary malignancy, and hepatocellular carcinoma. Dig. Dis. Sci. 43: 2489–2492. [DOI] [PubMed] [Google Scholar]
- 212.Schonfeld G., Patterson B. W., Yablonskiy D. A., Tanoli T. S., Averna M., Elias N., Yue P., Ackerman J. 2003. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J. Lipid Res. 44: 470–478. [DOI] [PubMed] [Google Scholar]
- 213.Whitfield A. J., Barrett P. H., Robertson K., Havlat M. F., van Bockxmeer F. M., Burnett J. R. 2005. Liver dysfunction and steatosis in familial hypobetalipoproteinemia. Clin. Chem. 51: 266–269. [DOI] [PubMed] [Google Scholar]
- 214.Kuge O., Dascher C., Orci L., Rowe T., Amherdt M., Plutner H., Ravazzola M., Tanigawa G., Rothman J. E., Balch W. E. 1994. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J. Cell Biol. 125: 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M. F., Ravazzola M., Amherdt M., Schekman R. 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 77: 895–907. [DOI] [PubMed] [Google Scholar]
- 216.Jones B., Jones E. L., Bonney S. A., Patel H. N., Mensenkamp A. R., Eichenbaum-Voline S., Rudling M., Myrdal U., Annesi G., Naik S., et al. 2003. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat. Genet. 34: 29–31. [DOI] [PubMed] [Google Scholar]
- 217.Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G., et al. 2004. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA. 101: 6062–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gurkan C., Lapp H., Alory C., Su A. I., Hogenesch J. B., Balch W. E. 2005. Large-scale profiling of Rab GTPase trafficking networks: the membrome. Mol. Biol. Cell. 16: 3847–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Georges A., Bonneau J., Bonnefont-Rousselot D., Champigneulle J., Rabes J. P., Abifadel M., Aparicio T., Guenedet J. C., Bruckert E., Boileau C., et al. 2011. Molecular analysis and intestinal expression of SAR1 genes and proteins in Anderson’s disease (chylomicron retention disease). Orphanet J. Rare Dis. 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Levy E., Marcel Y., Deckelbaum R. J., Milne R., Lepage G., Seidman E., Bendayan M., Roy C. C. 1987. Intestinal apoB synthesis, lipids, and lipoproteins in chylomicron retention disease. J. Lipid Res. 28: 1263–1274. [PubMed] [Google Scholar]
- 221.Fryer L. G., Jones B., Duncan E. J., Hutchison C. E., Ozkan T., Williams P. A., Alder O., Nieuwdorp M., Townley A. K., Mensenkamp A. R., et al. 2014. The endoplasmic reticulum coat protein II transport machinery coordinates cellular lipid secretion and cholesterol biosynthesis. J. Biol. Chem. 289: 4244–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Siddiqi S., Siddiqi S. A., Mansbach C. M. 2010. Sec24C is required for docking the prechylomicron transport vesicle with the Golgi. J. Lipid Res. 51: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mansbach C. M., Siddiqi S. A. 2010. The biogenesis of chylomicrons. Annu. Rev. Physiol. 72: 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huang M., Weissman J. T., Beraud-Dufour S., Luan P., Wang C., Chen W., Aridor M., Wilson I. A., Balch W. E. 2001. Crystal structure of Sar1-GDP at 1.7 A resolution and the role of the NH2 terminus in ER export. J. Cell Biol. 155: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bi X., Corpina R. A., Goldberg J. 2002. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 419: 271–277. [DOI] [PubMed] [Google Scholar]
- 226.Lee M. C., Orci L., Hamamoto S., Futai E., Ravazzola M., Schekman R. 2005. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 122: 605–617. [DOI] [PubMed] [Google Scholar]
- 227.Bielli A., Haney C. J., Gabreski G., Watkins S. C., Bannykh S. I., Aridor M. 2005. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J. Cell Biol. 171: 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nakańo A., Muramatsu M. 1989. A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J. Cell Biol. 109: 2677–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Charcosset M., Sassolas A., Peretti N., Roy C. C., Deslandres C., Sinnett D., Levy E., Lachaux A. 2008. Anderson or chylomicron retention disease: molecular impact of five mutations in the SAR1B gene on the structure and the functionality of Sar1b protein. Mol. Genet. Metab. 93: 74–84. [DOI] [PubMed] [Google Scholar]
- 230.Marcil V., Seidman E., Sinnett D., Sanchez R., Spahis S., Sane A. T., Levy E. 2014. Tissue distribution and regulation of the small Sar1b GTPase in mice. Cell. Physiol. Biochem. 33: 1815–1826. [DOI] [PubMed] [Google Scholar]
- 231.Nori A., Bortoloso E., Frasson F., Valle G., Volpe P. 2004. Vesicle budding from endoplasmic reticulum is involved in calsequestrin routing to sarcoplasmic reticulum of skeletal muscles. Biochem. J. 379: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yamasaki A., Tani K., Yamamoto A., Kitamura N., Komada M. 2006. The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol. Biol. Cell. 17: 4876–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Higazi D. R., Fearnley C. J., Drawnel F. M., Talasila A., Corps E. M., Ritter O., McDonald F., Mikoshiba K., Bootman M. D., Roderick H. L. 2009. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol. Cell. 33: 472–482. [DOI] [PubMed] [Google Scholar]
- 234.Guo A., Cala S. E., Song L. S. 2012. Calsequestrin accumulation in rough endoplasmic reticulum promotes perinuclear Ca2+ release. J. Biol. Chem. 287: 16670–16680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Silvain M., Bligny D., Aparicio T., Laforet P., Grodet A., Peretti N., Menard D., Djouadi F., Jardel C., Begue J. M., et al. 2008. Anderson’s disease (chylomicron retention disease): a new mutation in the SARA2 gene associated with muscular and cardiac abnormalities. Clin. Genet. 74: 546–552. [DOI] [PubMed] [Google Scholar]
- 236.Janiak A., Piorko S., Matros A., Mock H. P., Kwasniewski M., Chwialkowska K., Chmielewska B., Szarejko I. 2012. A comparative analysis of proteins that accumulate during the initial stage of root hair development in barley root hair mutants and their parent varieties. J. Appl. Genet. 53: 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aridor M., Fish K. N. 2009. Selective targeting of ER exit sites supports axon development. Traffic. 10: 1669–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Peretti N., Roy C. C., Sassolas A., Deslandres C., Drouin E., Rasquin A., Seidman E., Brochu P., Vohl M. C., Labarge S., et al. 2009. Chylomicron retention disease: a long term study of two cohorts. Mol. Genet. Metab. 97: 136–142. [DOI] [PubMed] [Google Scholar]
- 239.Seidah N. G., Benjannet S., Wickham L., Marcinkiewicz J., Jasmin S. B., Stifani S., Basak A., Prat A., Chretien M. 2003. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA. 100: 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lambert G., Sjouke B., Choque B., Kastelein J. J., Hovingh G. K. 2012. The PCSK9 decade. J. Lipid Res. 53: 2515–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Abifadel M., Varret M., Rabes J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. 2003. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34: 154–156. [DOI] [PubMed] [Google Scholar]
- 242.Fasano T., Cefalu A. B., Di L. E., Noto D., Pollaccia D., Bocchi L., Valenti V., Bonardi R., Guardamagna O., Averna M., et al. 2007. A novel loss of function mutation of PCSK9 gene in white subjects with low-plasma low-density lipoprotein cholesterol. Arterioscler. Thromb. Vasc. Biol. 27: 677–681. [DOI] [PubMed] [Google Scholar]
- 243.Cohen J. C., Boerwinkle E., Mosley T. H., Jr, Hobbs H. H. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354: 1264–1272. [DOI] [PubMed] [Google Scholar]
- 244.Zhao Z., Tuakli-Wosornu Y., Lagace T. A., Kinch L., Grishin N. V., Horton J. D., Cohen J. C., Hobbs H. H. 2006. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 79: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cameron J., Holla O. L., Ranheim T., Kulseth M. A., Berge K. E., Leren T. P. 2006. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum. Mol. Genet. 15: 1551–1558. [DOI] [PubMed] [Google Scholar]
- 246.Hooper A. J., Marais A. D., Tanyanyiwa D. M., Burnett J. R. 2007. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 193: 445–448. [DOI] [PubMed] [Google Scholar]
- 247.Rashid S., Curtis D. E., Garuti R., Anderson N. N., Bashmakov Y., Ho Y. K., Hammer R. E., Moon Y. A., Horton J. D. 2005. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA. 102: 5374–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zaid A., Roubtsova A., Essalmani R., Marcinkiewicz J., Chamberland A., Hamelin J., Tremblay M., Jacques H., Jin W., Davignon J., et al. 2008. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 48: 646–654. [DOI] [PubMed] [Google Scholar]
- 249.Musunuru K., Pirruccello J. P., Do R., Peloso G. M., Guiducci C., Sougnez C., Garimella K. V., Fisher S., Abreu J., Barry A. J., et al. 2010. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 363: 2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shimamura M., Matsuda M., Yasumo H., Okazaki M., Fujimoto K., Kono K., Shimizugawa T., Ando Y., Koishi R., Kohama T., et al. 2007. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 27: 366–372. [DOI] [PubMed] [Google Scholar]
- 251.Ono M., Shimizugawa T., Shimamura M., Yoshida K., Noji-Sakikawa C., Ando Y., Koishi R., Furukawa H. 2003. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 278: 41804–41809. [DOI] [PubMed] [Google Scholar]
- 252.Lee E. C., Desai U., Gololobov G., Hong S., Feng X., Yu X. C., Gay J., Wilganowski N., Gao C., Du L. L., et al. 2009. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J. Biol. Chem. 284: 13735–13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Martín-Campos J. M., Roig R., Mayoral C., Martinez S., Marti G., Arroyo J. A., Julve J., Blanco-Vaca F. 2012. Identification of a novel mutation in the ANGPTL3 gene in two families diagnosed of familial hypobetalipoproteinemia without APOB mutation. Clin. Chim. Acta. 413: 552–555. [DOI] [PubMed] [Google Scholar]
- 254.Köster A., Chao Y. B., Mosior M., Ford A., Gonzalez-DeWhitt P. A., Hale J. E., Li D., Qiu Y., Fraser C. C., Yang D. D., et al. 2005. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 146: 4943–4950. [DOI] [PubMed] [Google Scholar]
- 255.Fujimoto K., Koishi R., Shimizugawa T., Ando Y. 2006. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp. Anim. 55: 27–34. [DOI] [PubMed] [Google Scholar]
- 256.Noto D., Cefalu A. B., Valenti V., Fayer F., Pinotti E., Ditta M., Spina R., Vigna G., Yue P., Kathiresan S., et al. 2012. Prevalence of ANGPTL3 and APOB gene mutations in subjects with combined hypolipidemia. Arterioscler. Thromb. Vasc. Biol. 32: 805–809. [DOI] [PubMed] [Google Scholar]
- 257.Hansen P. S., Gerdes L. U., Klausen I. C., Gregersen N., Faergeman O. 1993. Polymorphisms in the apolipoprotein B-100 gene contributes to normal variation in plasma lipids in 464 Danish men born in 1948. Hum. Genet. 91: 45–50. [DOI] [PubMed] [Google Scholar]
- 258.Choong M. L., Koay E. S., Khaw M. C., Aw T. C. 1999. Apolipoprotein B 5′-Ins/Del and 3′-VNTR polymorphisms in Chinese, Malay and Indian Singaporeans. Hum. Hered. 49: 31–40. [DOI] [PubMed] [Google Scholar]
- 259.Boekholdt S. M., Peters R. J., Fountoulaki K., Kastelein J. J., Sijbrands E. J. 2003. Molecular variation at the apolipoprotein B gene locus in relation to lipids and cardiovascular disease: a systematic meta-analysis. Hum. Genet. 113: 417–425. [DOI] [PubMed] [Google Scholar]
- 260.Riches F. M., Watts G. F., van Bockxmeer F. M., Hua J., Song S., Humphries S. E., Talmud P. J. 1998. Apolipoprotein B signal peptide and apolipoprotein E genotypes as determinants of the hepatic secretion of VLDL apoB in obese men. J. Lipid Res. 39: 1752–1758. [PubMed] [Google Scholar]
- 261.Lopez-Miranda J., Ordovas J. M., Ostos M. A., Marin C., Jansen S., Salas J., Blanco-Molina A., Jimenez-Pereperez J. A., Lopez-Segura F., Perez-Jimenez F. 1997. Dietary fat clearance in normal subjects is modulated by genetic variation at the apolipoprotein B gene locus. Arterioscler. Thromb. Vasc. Biol. 17: 1765–1773. [DOI] [PubMed] [Google Scholar]
- 262.Boerwinkle E., Chan L. 1989. A three codon insertion/deletion polymorphism in the signal peptide region of the human apolipoprotein B (APOB) gene directly typed by the polymerase chain reaction. Nucleic Acids Res. 17: 4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jaramillo-Correa J. P., Keyeux G., Ruiz-Garcia M., Rodas C., Bernal J. 2001. Population genetic analysis of the genes APOE, APOB(3′VNTR) and ACE in some black and Amerindian communities from Colombia. Hum. Hered. 52: 14–33. [DOI] [PubMed] [Google Scholar]
- 264.Verbenko D. A., Pogoda T. V., Spitsyn V. A., Mikulich A. I., Bets L. V., Bebyakova N. A., Ivanov V. P., Abolmasov N. N., Pocheshkhova E. A., Balanovskaya E. V., et al. 2003. Apolipoprotein B 3′-VNTR polymorphism in Eastern European populations. Eur. J. Hum. Genet. 11: 444–451. [DOI] [PubMed] [Google Scholar]
- 265.Horvath A., Chorbov V., Zaharova B., Ganev V. 2003. Five polymorphisms of the apolipoprotein B gene in healthy Bulgarians. Hum. Biol. 75: 69–80. [DOI] [PubMed] [Google Scholar]
- 266.Verbenko D. A., Knjazev A. N., Mikulich A. I., Khusnutdinova E. K., Bebyakova N. A., Limborska S. A. 2005. Variability of the 3′APOB minisatellite locus in Eastern Slavonic populations. Hum. Hered. 60: 10–18. [DOI] [PubMed] [Google Scholar]
- 267.Alavantić D., Glisic S., Kandic I. 1998. APO B 3′ HVR polymorphism in healthy population: relationships to serum lipid levels. Genet. Epidemiol. 15: 113–122. [DOI] [PubMed] [Google Scholar]
- 268.Jemaa R., El-Asmi M., Mebazaa A. 2002. VNTR3ʹ polymorphism of apoliproprotein B gene in obese people [article in French]. Ann. Biol. Clin. (Paris). 60: 559–564. [PubMed] [Google Scholar]
- 269.Garasto S., Berardelli M., DeRango F., Mari V., Feraco E., De B. G. 2004. A study of the average effect of the 3′APOB-VNTR polymorphism on lipidemic parameters could explain why the short alleles (<35 repeats) are rare in centenarians. BMC Med. Genet. 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Friedl W., Ludwig E. H., Paulweber B., Sandhofer F., McCarthy B. J. 1990. Hypervariability in a minisatellite 3′ of the apolipoprotein B gene in patients with coronary heart disease compared with normal controls. J. Lipid Res. 31: 659–665. [PubMed] [Google Scholar]
- 271.Pan J. P., Chiang A. N., Chou C. Y., Chan W. L., Tai J. J. 1998. Polymorphisms of the apolipoprotein B 3′ variable number of tandem repeats region associated with coronary artery disease in Taiwanese. J. Formos. Med. Assoc. 97: 233–238. [PubMed] [Google Scholar]
- 272.Ye P., Chen B., Wang S. 1995. Association of polymorphisms of the apolipoprotein B gene with coronary heart disease in Han Chinese. Atherosclerosis. 117: 43–50. [DOI] [PubMed] [Google Scholar]
- 273.Tikkanen M. J., Helio T. 1992. Genetic variants of apolipoprotein B: relation to serum lipid levels and coronary artery disease among the Finns. Ann. Med. 24: 357–361. [DOI] [PubMed] [Google Scholar]
- 274.Heliö T., Palotie A., Totterman K. J., Ott J., Kauppinen-Makelin R., Tikkanen M. J. 1992. Lack of association between the apolipoprotein B gene 3′ hypervariable region alleles and coronary artery disease in Finnish patients with angiographically documented coronary artery disease. J. Intern. Med. 231: 49–57. [DOI] [PubMed] [Google Scholar]
- 275.Wang J., Hegele R. A. 2000. Microsomal triglyceride transfer protein (MTP) gene mutations in Canadian subjects with abetalipoproteinemia. Hum. Mutat. 15: 294–295. [DOI] [PubMed] [Google Scholar]
- 276.Ohashi K., Ishibashi S., Osuga J., Tozawa R., Harada K., Yahagi N., Shionoiri F., Iizuka Y., Tamura Y., Nagai R., et al. 2000. Novel mutations in the microsomal triglyceride transfer protein gene causing abetalipoproteinemia. J. Lipid Res. 41: 1199–1204. [PubMed] [Google Scholar]
- 277.Najah M., Di L. E., Awatef J., Magnolo L., Imene J., Pinotti E., Bahri M., Barsaoui S., Brini I., Fekih M., et al. 2009. Identification of patients with abetalipoproteinemia and homozygous familial hypobetalipoproteinemia in Tunisia. Clin. Chim. Acta. 401: 51–56. [DOI] [PubMed] [Google Scholar]
- 278.Di Filippo M., Crehalet H., Samson-Bouma M. E., Bonnet V., Aggerbeck L. P., Rabes J. P., Gottrand F., Luc G., Bozon D., Sassolas A. 2012. Molecular and functional analysis of two new MTTP gene mutations in an atypical case of abetalipoproteinemia. J. Lipid Res. 53: 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Okumura K., Imamura A., Murakami R., Takahashi R., Cheng X. W., Numaguchi Y., Murohara T. 2009. Microsomal triglyceride transfer protein gene polymorphism strongly influences circulating malondialdehyde-modified low-density lipoprotein. Metabolism. 58: 1306–1311. [DOI] [PubMed] [Google Scholar]
- 280.Ledmyr H., McMahon A. D., Ehrenborg E., Nielsen L. B., Neville M., Lithell H., MacFarlane P. W., Packard C. J., Karpe F. 2004. The microsomal triglyceride transfer protein gene-493T variant lowers cholesterol but increases the risk of coronary heart disease. Circulation. 109: 2279–2284. [DOI] [PubMed] [Google Scholar]
- 281.Rubin D., Schneider-Muntau A., Klapper M., Nitz I., Helwig U., Folsch U. R., Schrezenmeir J., Doring F. 2008. Functional analysis of promoter variants in the microsomal triglyceride transfer protein (MTTP) gene. Hum. Mutat. 29: 123–129. [DOI] [PubMed] [Google Scholar]
- 282.Klop B., Verseyden C., Ribalta J., Salazar J., Masana L., Cabezas M. C. 2014. MTP gene polymorphisms and postprandial lipemia in familial combined hyperlipidemia: effects of treatment with atorvastatin. Clin. Investig. Arterioscler. 26: 49–57. [DOI] [PubMed] [Google Scholar]
- 283.Karpe F., Lundahl B., Ehrenborg E., Eriksson P., Hamsten A. 1998. A common functional polymorphism in the promoter region of the microsomal triglyceride transfer protein gene influences plasma LDL levels. Arterioscler. Thromb. Vasc. Biol. 18: 756–761. [DOI] [PubMed] [Google Scholar]
- 284.Chen S. P., Tan K. C., Lam K. S. 2003. Effect of the microsomal triglyceride transfer protein -493 G/T polymorphism and type 2 diabetes mellitus on LDL subfractions. Atherosclerosis. 167: 287–292. [DOI] [PubMed] [Google Scholar]
- 285.Ledmyr H., Karpe F., Lundahl B., McKinnon M., Skoglund-Andersson C., Ehrenborg E. 2002. Variants of the microsomal triglyceride transfer protein gene are associated with plasma cholesterol levels and body mass index. J. Lipid Res. 43: 51–58. [PubMed] [Google Scholar]
- 286.Lundahl B., Skoglund-Andersson C., Caslake M., Bedford D., Stewart P., Hamsten A., Packard C. J., Karpe F. 2006. Microsomal triglyceride transfer protein -493T variant reduces IDL plus LDL apoB production and the plasma concentration of large LDL particles. Am. J. Physiol. Endocrinol. Metab. 290: E739–E745. [DOI] [PubMed] [Google Scholar]
- 287.Juo S. H., Han Z., Smith J. D., Colangelo L., Liu K. 2000. Common polymorphism in promoter of microsomal triglyceride transfer protein gene influences cholesterol, ApoB, and triglyceride levels in young african american men: results from the coronary artery risk development in young adults (CARDIA) study. Arterioscler. Thromb. Vasc. Biol. 20: 1316–1322. [DOI] [PubMed] [Google Scholar]
- 288.Schgoer W., Eller P., Mueller T., Tancevski I., Wehinger A., Ulmer H., Sandhofer A., Ritsch A., Haltmayer M., Patsch J. R. 2008. The MTP -493TT genotype is associated with peripheral arterial disease: results from the Linz Peripheral Arterial Disease (LIPAD) Study. Clin. Biochem. 41: 712–716. [DOI] [PubMed] [Google Scholar]
- 289.Zák A., Jachymova M., Tvrzicka E., Vecka M., Duffkova L., Zeman M., Slaby A., Stankova B. 2008. The influence of polymorphism of -493G/T MTP gene promoter and metabolic syndrome on lipids, fatty acids and oxidative stress. J. Nutr. Biochem. 19: 634–641. [DOI] [PubMed] [Google Scholar]
- 290.Couture P., Otvos J. D., Cupples L. A., Wilson P. W., Schaefer E. J., Ordovas J. M. 2000. Absence of association between genetic variation in the promoter of the microsomal triglyceride transfer protein gene and plasma lipoproteins in the Framingham Offspring Study. Atherosclerosis. 148: 337–343. [DOI] [PubMed] [Google Scholar]
- 291.Levy E., Spahis S., Garofalo C., Marcil V., Montoudis A., Sinnet D., Sanchez R., Peretti N., Beaulieu J. F., Sane A. 2014. Sar1b transgenic male mice are more susceptible to high-fat diet-induced obesity, insulin insensitivity and intestinal chylomicron overproduction. J. Nutr. Biochem. 25: 540–548. [DOI] [PubMed] [Google Scholar]
- 292.Levy E., Harmel E., Laville M., Sanchez R., Emonnot L., Sinnett D., Ziv E., Delvin E., Couture P., Marcil V., et al. 2011. Expression of Sar1b enhances chylomicron assembly and key components of the coat protein complex II system driving vesicle budding. Arterioscler. Thromb. Vasc. Biol. 31: 2692–2699. [DOI] [PubMed] [Google Scholar]
- 293.Taskinen M. R. 2003. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 46: 733–749. [DOI] [PubMed] [Google Scholar]
- 294.Rivellese A. A., De N. C., Di M. L., Patti L., Iovine C., Coppola S., Del P. S., Riccardi G., Annuzzi G. 2004. Exogenous and endogenous postprandial lipid abnormalities in type 2 diabetic patients with optimal blood glucose control and optimal fasting triglyceride levels. J. Clin. Endocrinol. Metab. 89: 2153–2159. [DOI] [PubMed] [Google Scholar]
- 295.Guo Q., Avramoglu R. K., Adeli K. 2005. Intestinal assembly and secretion of highly dense/lipid-poor apolipoprotein B48-containing lipoprotein particles in the fasting state: evidence for induction by insulin resistance and exogenous fatty acids. Metabolism. 54: 689–697. [DOI] [PubMed] [Google Scholar]
- 296.Curtin A., Deegan P., Owens D., Collins P., Johnson A., Tomkin G. H. 1996. Elevated triglyceride-rich lipoproteins in diabetes. A study of apolipoprotein B-48. Acta Diabetol. 33: 205–210. [DOI] [PubMed] [Google Scholar]
- 297.Haidari M., Leung N., Mahbub F., Uffelman K. D., Kohen-Avramoglu R., Lewis G. F., Adeli K. 2002. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J. Biol. Chem. 277: 31646–31655. [DOI] [PubMed] [Google Scholar]
- 298.Zoltowska M., Ziv E., Delvin E., Sinnett D., Kalman R., Garofalo C., Seidman E., Levy E. 2003. Cellular aspects of intestinal lipoprotein assembly in Psammomys obesus: a model of insulin resistance and type 2 diabetes. Diabetes. 52: 2539–2545. [DOI] [PubMed] [Google Scholar]
- 299.Vine D. F., Takechi R., Russell J. C., Proctor S. D. 2007. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 190: 282–290. [DOI] [PubMed] [Google Scholar]
- 300.Duez H., Lamarche B., Uffelman K. D., Valero R., Cohn J. S., Lewis G. F. 2006. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler. Thromb. Vasc. Biol. 26: 1357–1363. [DOI] [PubMed] [Google Scholar]
- 301.Veilleux A., Grenier E., Marceau P., Carpentier A. C., Richard D., Levy E. 2014. Intestinal lipid handling: evidence and implication of insulin signaling abnormalities in human obese subjects. Arterioscler. Thromb. Vasc. Biol. 34: 644–653. [DOI] [PubMed] [Google Scholar]
- 302.Masuda D., Sakai N., Sugimoto T., Kitazume-Taneike R., Yamashita T., Kawase R., Nakaoka H., Inagaki M., Nakatani K., Yuasa-Kawase M., et al. 2011. Fasting serum apolipoprotein B-48 can be a marker of postprandial hyperlipidemia. J. Atheroscler. Thromb. 18: 1062–1070. [DOI] [PubMed] [Google Scholar]
- 303.Nakatani K., Sugimoto T., Masuda D., Okano R., Oya T., Monden Y., Yamashita T., Kawase R., Nakaoka H., Inagaki M., et al. 2011. Serum apolipoprotein B-48 levels are correlated with carotid intima-media thickness in subjects with normal serum triglyceride levels. Atherosclerosis. 218: 226–232. [DOI] [PubMed] [Google Scholar]
- 304.Lapice E., Cipriano P., Patti L., Romano G., Vaccaro O., Rivellese A. A. 2012. Fasting apolipoprotein B48 is associated with asymptomatic peripheral arterial disease in type 2 diabetic subjects: a case-control study. Atherosclerosis. 223: 504–506. [DOI] [PubMed] [Google Scholar]
- 305.Masuda D., Sugimoto T., Tsujii K., Inagaki M., Nakatani K., Yuasa-Kawase M., Tsubakio-Yamamoto K., Ohama T., Nishida M., Ishigami M., et al. 2012. Correlation of fasting serum apolipoprotein B-48 with coronary artery disease prevalence. Eur. J. Clin. Invest. 42: 992–999. [DOI] [PubMed] [Google Scholar]
- 306.Nordestgaard B. G., Tybjaerg-Hansen A. 1992. IDL, VLDL, chylomicrons and atherosclerosi. Eur. J. Epidemiol. 8(Suppl 1): 92–98. [DOI] [PubMed] [Google Scholar]
- 307.Proctor S. D., Mamo J. C. 2003. Intimal retention of cholesterol derived from apolipoprotein. Arterioscler. Thromb. Vasc. Biol. 23: 1595–1600. [DOI] [PubMed] [Google Scholar]
- 308.Doi H., Kugiyama K., Ohgushi M., Sugiyama S., Matsumura T., Ohta Y., Nakano T., Nakajima K., Yasue H. 1998. Remnants of chylomicron and very low density lipoprotein impair endothelium-dependent vasorelaxation. Atherosclerosis. 137: 341–349. [DOI] [PubMed] [Google Scholar]
- 309.Twickler T. B., Dallinga-Thie G. M., Cohn J. S., Chapman M. J. 2004. Elevated remnant-like particle cholesterol concentration: a characteristic feature of the atherogenic lipoprotein phenotype. Circulation. 109: 1918–1925. [DOI] [PubMed] [Google Scholar]