Abstract
Cholesterol homeostasis is strictly regulated through the modulation of HMG-CoA reductase (HMGCR), the rate-limiting enzyme of cholesterol synthesis. Phosphorylation of HMGCR inactivates it and dephosphorylation activates it. AMP-activated protein kinase (AMPK) is the major kinase phosphorylating the enzyme. Our previous study found that thyroid-stimulating hormone (TSH) increased the hepatocytic HMGCR expression, but it was still unclear whether TSH affected hepatic HMGCR phosphorylation associated with AMPK. We used bovine TSH (bTSH) to treat the primary mouse hepatocytes and HepG2 cells with or without constitutively active (CA)-AMPK plasmid or protein kinase A inhibitor (H89), and set up the TSH receptor (Tshr)-KO mouse models. The p-HMGCR, p-AMPK, and related molecular expression were tested. The ratios of p-HMGCR/HMGCR and p-AMPK/AMPK decreased in the hepatocytes in a dose-dependent manner following bTSH stimulation. The changes above were inversed when the cells were treated with CA-AMPK plasmid or H89. In Tshr-KO mice, the ratios of liver p-HMGCR/HMGCR and p-AMPK/AMPK were increased relative to the littermate wild-type mice. Consistently, the phosphorylation of acetyl-CoA carboxylase, a downstream target molecule of AMPK, increased. All results suggested that TSH could regulate the phosphorylation of HMGCR via AMPK, which established a potential mechanism for hypercholesterolemia involved in a direct action of the TSH in the liver.
Keywords: adenosine 5′-monophosphate-activated protein kinase, cholesterol, hydroxy-methylglutaryl coenzyme A reductase
The liver plays a vital role in regulating cholesterol homeostasis in the body. HMG-CoA reductase (HMGCR) is the rate-limiting enzyme in cholesterol biosynthesis (1), so its activity is instrumental in controlling de novo cholesterol synthesis. To maintain cholesterol homeostasis, HMGCR could be regulated by multiple mechanisms such as transcription, translation (2), enzyme degradation rate (3), phosphorylation-dephosphorylation (4), and feedback inhibition (5). Hormones could regulate the expression of HMGCR by acting at different levels. For example, glucocorticoids act at a posttranslational level (1), whereas insulin reportedly affects both the transcriptional and posttranslational processes (6). Recently, Wu et al. (7) found that the changes of the phosphorylated HMGCR play an important role in regulation of the hepatic cholesterol biosynthesis process. The site of phosphorylation on HMGCR has been identified as serine 871 in rodents (8) and serine 872 in humans (9). The HMGCR is physiologically present in the cell in unphosphorylated active form and phosphorylated inactive form. In general, phosphorylation of HMGCR leads to inactivation of the enzyme, while dephosphorylation activates it. The ratio of the phosphorylated form to total form indicates an inactivation state of HMGCR.
Subclinical hypothyroidism is a type of thyroid function abnormality; its characteristics are elevated serum thyroid-stimulating hormone (TSH) and normal serum thyroid hormone levels, as well as an increased serum cholesterol level (10). Our study and other clinical studies have addressed a positive correlation between the high serum TSH and increased cholesterol levels (11, 12). Additionally, our laboratory data have shown that the TSH receptor (TSHR) was expressed in hepatocytes and the TSH could enhance the expression of HMGCR by activating the cAMP/PKA pathway, leading to an elevated cholesterol level via the TSHR in the hepatocytes (13, 14).
AMP-activated protein kinase (AMPK) is activated by increased intracellular AMP concentrations and is generally described as a “metabolite-sensing kinase.” Henin et al. (15) indicated that an AMPK stimulator, such as the AICAR, could decrease the synthesis of cholesterol in the liver. Several studies reported that the HMGCR can be phosphorylated in vitro by several protein kinases, such as AMPK, PKC, and Ca2+ calmodulin-dependent kinase (16, 17). AMPK seems to be the major kinase that targets HMGCR in the liver (18). It is still unclear whether TSH, as a hormone, can affect AMPK and HMGCR phosphorylation. Djouder et al. (19) found that PKA associates with and phosphorylates AMPKa1 at Ser173 to impede threonine (Thr172) phosphorylation and thus activation of AMPK. Our previous study had shown that TSH could increase the cAMP level and then activate PKA. Based on these reports, we hypothesized that the TSH regulated HMGCR phosphorylation through PKA/AMPK in the liver.
In this study, our results showed that TSH could decrease the phosphorylated HMGCR expression via AMPK in the liver and lead to increased HMGCR activity, which may help to understand the extra-thyroid action of the TSH in the liver and the mechanism of hypercholesterolemia in subclinical hypothyroidism.
MATERIALS AND METHODS
Animal experiments
Genetically Tshr-deficient mice (B6;129S1-Tshrtm1Rmar/J,004858) were obtained from the Jackson Laboratory (Bar Harbor, ME). The wild-type (Tshr+/+) mice and Tshr-KO (Tshr−/−) mice were housed at 23°C in a 12 h light-dark cycle and humidity-controlled (60%) environment. All procedures were carried out with the approval of the Institutional Animal Care Committee, and were in compliance with the Guide for the Care and Use of Laboratory Animals. Heterozygote mice were bred separately to generate the Tshr-KO and wild-type mice used in this study. Genotyping was tested using tail DNA, as previously described (20). When fed the nonsupplemented diet, Tshr-KO mice exhibited decreased serum T4/T3 levels and an elevated TSH level (20). Therefore, after weaning at the end of the third week, all Tshr-KO (homozygotic type) mice were fed a diet containing 100 ppm thyroid powder (Sigma-Aldrich, USA) to maintain thyroid hormone levels that were the same as wild-type mice (21).
Male adult (aged 8 weeks) Tshr-KO mice and their respective wild-type littermates were used in the experiments. At 10:00 to 12:00 PM, after fasting for 8 h, mice were euthanized to harvest blood samples. The serum total thyroxine (T4), free thyroxine (FT4), and insulin concentrations were measured using radioimmunoassay (Jiuding, Tianjin Biomedical Engineering Company Limited, and HTA Co., Ltd., China). The serum TSH level was assayed using an ELISA kit (Uscn Life Science, Inc.). The blood glucose level was detected with a OneTouch Ultra glucometer (Johnson and Johnson, USA). One part of the liver tissues was immediately frozen in liquid nitrogen for protein analysis. The other part was fixed in buffered 4% paraformaldehyde for immunohistochemistry.
Genotyping
Genomic DNA was isolated from the tail of each mouse using Direct Lysis reagent (Qiagen DP304). Genotypes of mice were confirmed by PCR using the following primer sets: a) 5′-CAG GGT GGA GAC GCA CAC TC-3′ and 5′-AGA GAG TCC CAC AAC AGT C-3′, which amplifies a 590 bp fragment from the wild-type allele; and b) 5′-AAG TTC ATC TGC ACC ACC G-3′ and 5′-TCC TTG AAG AAG ATG GTG CG-3′, which amplifies a 173 bp fragment from the Tshr-KO allele. The PCR cycle profile used for genotyping was as follows: 94°C 3 min, 94°C 30 s/69°C 1 min/72°C 1 min for 35 cycles.
Isolation and culture of primary mouse hepatocytes
Hepatocytes were isolated from adult male wild-type mice by retrograde two-step collagenase perfusion of the mouse liver (22). The liver was perfused after cannulation of the hepatic portal vein. The organ was washed with Hank’s calcium- and magnesium-free buffer for 3 min. After the liver had been freed of blood, the calcium-free buffer was replaced by a collagenase buffer (0.5 mg/ml) for 7–10 min. A perfusion rate of 7 ml/min and a temperature around 39°C were maintained for both perfusates during the entire procedure. After the perfusion had been terminated, the liver was rapidly excised from the body cavity and transferred to a sterile Petri dish. The gall bladder and remnants of the diaphragm were removed, and cells were released by disrupting the liver capsule mechanically and by shaking the cells into attachment medium. The cells were separated from undigested tissue with a sterile 50 μm mesh nylon filter. After washing by low-speed centrifugation at 50 g for 5 min several times, the cells were used only if cell viability, as determined by trypan blue exclusion, was >80%. Cells were seeded onto plastic Petri dishes in William’s E medium supplemented with 10% FBS, 50 U/ml of penicillin, 50 μg/ml of streptomycin, 100 nM insulin, and 1 μM hydrocortisone, at a density of 5E 6 cells per dish. After 24 h, medium was replaced by fresh serum-free medium for the indicated times. Cultures were kept with the different conditions in a 5% CO2 atmosphere at 37°C.
Cell culture and treatments
The HepG2 cells were cultured in Eagle’s minimal essential medium (Gibco, Life Technologies Corporation, USA) and supplemented with 10% FBS and 100 U/ml penicillin-streptomycin. When the HepG2 cells reached 70–80% confluence, different doses of bovine TSH (bTSH) (Sigma-Aldrich) stimulated for 48 h in the presence or absence of constitutively active (CA)-AMPK plasmid or H89 (10 μM, PKA inhibitor, Sigma-Aldrich) in serum-free EMEM medium.
Hepatocellular microsome HMGCR activity assay
Hepatocellular microsomes for assay of HMGCR activity were prepared as described by Honda et al. (23). The HepG2 cells stimulated by different concentrations of bTSH (0, 1, and 4 μM) were homogenized with a loose-fitting Teflon pestle in 4 vol of 3 mM Tris-HCl buffer (pH 7.4) containing 0.25 M sucrose, 0.1 mM EDTA, and either 50 mM sodium chloride or 50 mM sodium fluoride. The homogenates were centrifuged by differential ultracentrifugation, and the pellet (microsomal fraction) was suspended in storage buffer containing 100 mM potassium phosphate buffer [pH 7.4, 1 mM EDTA, 5 mM DTT, 50 mM KCl, and 20% glycerol (v/v)].
The conventional method for the measurement of microsomal HMGCR activity was based on the methods of Brown, Goldstein, and Dietschy (24) with some modification. Microsomes (100 μg of protein) were incubated for 30 min at 37°C in a total 150 μl volume including 100 mM potassium phosphate buffer (pH 7.4) containing a NADPH generating system and 30 nmol [14C]HMG-CoA (diluted with unlabeled HMG-CoA to give a specific activity of 30 dpm/pmol, PerkinElmer, USA). The [14C]mevalonate formed was converted into lactone, isolated by thin layer chromatography (25), and counted using an internal standard of [3H]mevalonate (PerkinElmer) to correct for incomplete recovery (26). HMGCR activity is expressed as the picomoles of [14C]mevalonate formed per minute per milligram of microsomal protein.
Phosphorylation of acetyl-CoA carboxylase assay
The acetyl-CoA carboxylase (ACC) was prepared as described by Neil B. Madsen with some modification (27). The AMPK was obtained by immunoprecipitation. The ACC and AMPK were incubated together at 30°C for 20 min in a reaction buffer (pH 7.4), containing 50 mM Tris-HCl, 0.25 mM EGTA, 5.0 mM MgCl2, 1.0 mM DTT, and 0.5 mM ATP. To measure incorporation of phosphate into ACC, the reaction was started by adding [γ-32P]ATP (100–150 cpm/pmol ATP), and the incorporation of phosphate into ACC was measured by the filter disc method using P81 paper (28). Radioactivity was determined using standard liquid scintillation procedures.
Liver total cholesterol content assay
The liver total cholesterol (TC) content was measured using a cholesterol assay kit (Applygen Technologies, Beijing, China) according to the manufacturer’s instructions. Briefly, the liver tissues were extracted with lysis buffer, the mixture was then centrifuged (2,000 g, 5 min), and 10 μl of the supernatant was added to glass tube containing working liquid for the cholesterol assay. After incubating for 20 min at 37°C, the absorbance was measured at 550 nm. Protein concentrations were also quantified. The cholesterol content was expressed as micrograms per milligram of protein.
Transfection of CA-AMPK plasmid
HepG2 cells in exponential growth were seeded into 6-well plates at a concentration of 1 × 105/ml. After 24 h, liposomes mixed with 3 μg of active AMPK (CA-AMPK, a generous gift from Prof. Dave Carling, Imperial College London) were used to transfect the HepG2 cells. The culture medium was replaced after 6 h of incubation, and the cells were cultured with or without 4 μM bTSH for 48 h.
Western blotting
An equal amount of protein from each sample was resolved by 10% SDS-PAGE and electrotransferred to nitrocellulose membranes (Millipore). All membranes were incubated overnight at 4°C with commercial anti-HMGCR (1:1,000, Upstate), anti-p-HMGCR (Ser872) (1:1,000, Upstate), anti-p-AMPK (T172) (1:2,000, Cell Signaling Technology), anti-AMPK (1:1,000, Cell Signaling Technology), anti-P-ACC (Ser79) (1:1,000, Cell Signaling Technology). After incubation with corresponding secondary antibody (Zsbio, Ltd., China), immune complexes were detected using the enhanced chemiluminescence plus detection system (Amersham). Immunoreactive bands were quantified using Alphaimager 2200. The same membrane was reincubated with anti-β-actin (Abcam, 1:10,000) as an internal correction and the relative target protein levels were normalized to β-actin.
Immunochemistry staining
The liver tissue specimens were immersed in 4% paraformaldehyde for 12 h and dehydrated in 50–100% ethanol, cleared in xylene, and embedded in paraffin wax. The tissue was sectioned with 3 μm thickness. Sections were stained using a two-step method. The sections were incubated with 3% H2O2 to block the activity of endogenous peroxidase. After washing three times in PBS for 5 min each time, the sections were incubated for 15 min with 10% normal rabbit serum, and then incubated with primary antibody [rabbit anti-p-HMGCR (S872), 1:100 dilution] overnight in a humid chamber at 4°C. After washing in PBS for 5 min each time, the sections were incubated at room temperature with polymer peroxidase anti-rabbit serum for 30 min. After washing three times in PBS for 5 min each time, the peroxidase was revealed by a 3,3-diaminobenzidine tetrahydrochloride substrate kit (ZSGB-BIO). Negative controls were performed in PBS, instead of the primary antibody. Moreover, the obtained sections above were also performed with hematoxylin and eosin staining and examined under an optical microscope.
Statistical analysis
The data were analyzed using SPSS17.0 software and are expressed as the mean ± SD. Statistical significance was assessed by unpaired Student’s t-tests for differences between two experimental groups and one-way ANOVA was used for multiple groups. P < 0.05 was considered statistically significant.
RESULTS
TSH declines the hepatic HMGCR phosphorylation levels
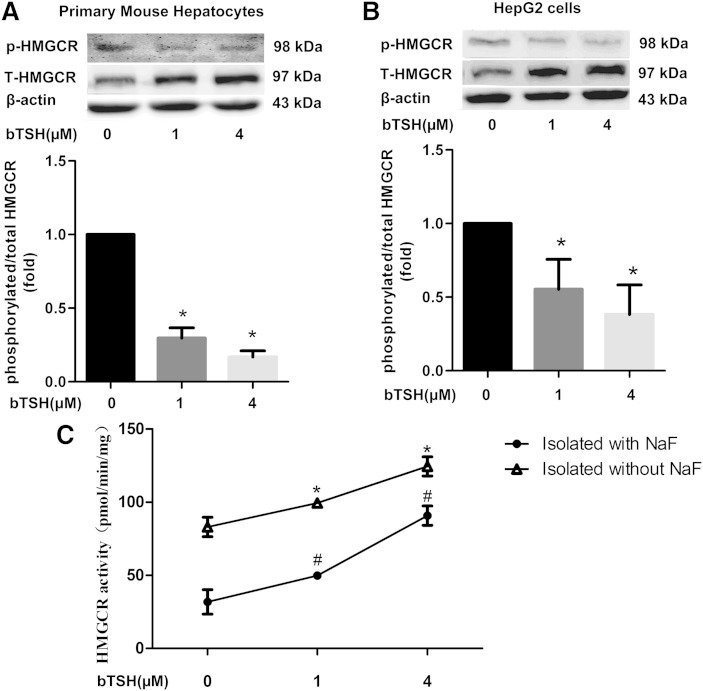
To identify whether the TSH could regulate the phosphorylation of the HMGCR, we chose the primary mouse hepatocytes and treated the cells with different TSH concentrations (0, 1, and 4 μM). As shown in Fig. 1A, the expression of the phosphorylated HMGCR decreased and the total HMGCR increased in a TSH concentration-dependent manner (P < 0.05). The ratio of the phosphorylated form to total form of HMGCR, demonstrating an inactivation state of the HMGCR, showed a decline of up to 75% at the large dose of TSH, suggesting that TSH could directly decrease the phosphorylated HMGCR. Similar results were also found in HepG2 cells (Fig. 1B).
Fig. 1.
TSH decreases the hepatic HMGCR phosphorylation levels in vitro. The primary mouse hepatocytes (A) and HepG2 cells (B) were cultured in serum-free medium with or without different concentrations of bTSH (0, 1, and 4 μM) for 48 h. The protein levels of phosphorylated and total HMGCR were determined by Western immunoblotting, respectively. The inactive status of HMGCR was assessed as a ratio of phosphorylated HMGCR to total HMGCR by semiquantitative analysis. Data are representative of three independent experiments. C: The HMGCR activity in HepG2 cells with different concentrations of bTSH (0, 1, and 4 μM) stimulation for 48 h. The microsomes were isolated with or without NaF and then the HMGCR activity was tested by the hepatocellular microsome HMGCR activity assay described in the Materials and Methods. *P < 0.05 versus 0 μM TSH stimulation isolated without NaF. #P < 0.05 versus 0 μM TSH stimulation isolated with NaF.
We also tested the HMGCR activity in HepG2 cells with or without bTSH stimulation, and found, when the microsomes were isolated and measured in the presence of NaF, which preserves the phosphorylated state seen in vivo, the HMGCR activity increased in a dose-dependent manner in the TSH-stimulated HepG2 cells (Fig. 1C). To obtain information with regard to the phosphorylation of the HMGCR, the microsomes were also isolated in the absence of NaF. Under these conditions, an increase in the HMGCR activity was seen in the HepG2 cells isolated in the absence of NaF relative to those isolated with NaF. This is thought to be due to a dephosphorylation and activation of HMGCR during isolation (24). The smaller increase in HMGCR activity was observed in HepG2 cells stimulated by bTSH (Fig. 1C).
Inhibition of HMGCR phosphorylation by TSH via decreased AMPK activity
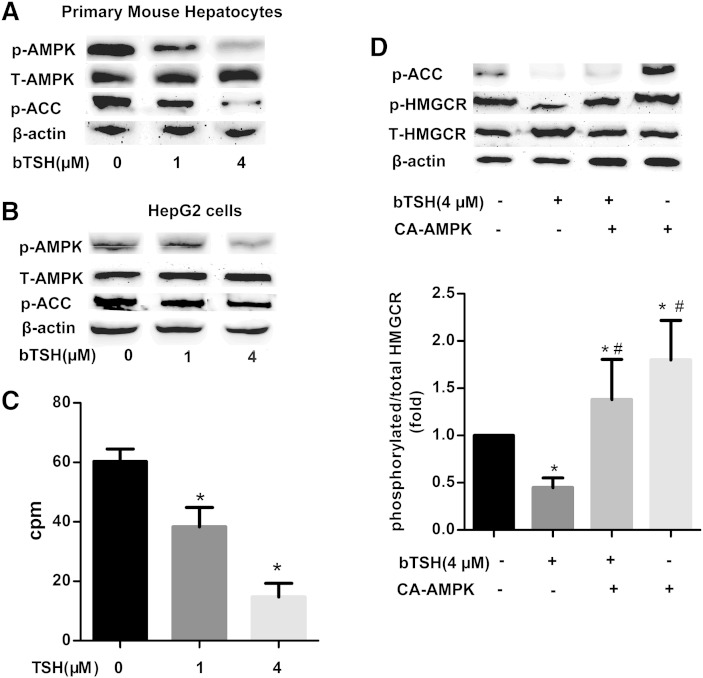
Hawley et al. (18) found that the AMPK seems to be the major kinase that targets the HMGCR in the liver. To assess whether the TSH could affect the activation of AMPK, we stimulated the hepatocytes with different TSH concentrations, then tested the expression of the p-AMPK (T172) and AMPK. As shown in Fig. 2A, the results showed that the treatment of the primary mouse hepatocytes with TSH decreased the expression of the phosphorylated AMPK (active form) in a concentration-dependent manner, compared with the control group. The amount of total AMPK was unaltered. Correspondingly, the phosphorylation of ACC, the best-characterized phosphorylation of AMPK, also decreased in a TSH concentration-dependent manner. Similar results were also found in the HepG2 cells (Fig. 2B, C).
Fig. 2.
TSH inhibits the phosphorylation of HMGCR via the decreased AMPK activity. The primary mouse hepatocytes (A) and HepG2 cells (B) were cultured in serum-free medium with or without different concentrations of bTSH (0, 1, and 4 μM) for 48 h. The protein levels of phosphorylated and total AMPK and phosphorylated ACC were determined by Western immunoblotting analysis, respectively. C: The phosphorylation of ACC was assayed using γ-32P in HepG2 cells with different concentrations of bTSH (0, 1, and 4 μM) stimulation for 48 h. D: HepG2 cells were nucleofected with CA-AMPK prior to 4 μM TSH treatment for an addictional 48 h. The phosphorylated protein levels of p-ACC and HMGCR, as well as total HMGCR, were determined by Western immunoblotting, respectively. Data were compiled from at least three independent experiments with triplicates in each experiment. *P < 0.05 versus untreated group. #P < 0.05 versus only the TSH stimulated group.
We examined whether AMPK was involved in a TSH-induced inhibition of the hepatic HMGCR phosphorylation. As shown in Fig. 2D, when the cells were nucleofected with recombinant pcDNA3 plasmids coding for CA-AMPK, the phosphorylated ACC, the best-characterized activation of AMPK, increased, and the phosphorylated HMGCR also elevated compared with the cells without any stimulation (P < 0.05). However, when TSH was supplemented, the TSH-induced change was dramatically blocked by AMPK activation. The ratio of p-HMGCR/HMGCR decreased by 20.1% in the cells nucleofected with CA-AMPK and then stimulated by TSH compared with the cells with CA-AMPK alone (P < 0.05).
The PKA inhibitor attenuates the inhibiting effect of TSH on AMPK activity
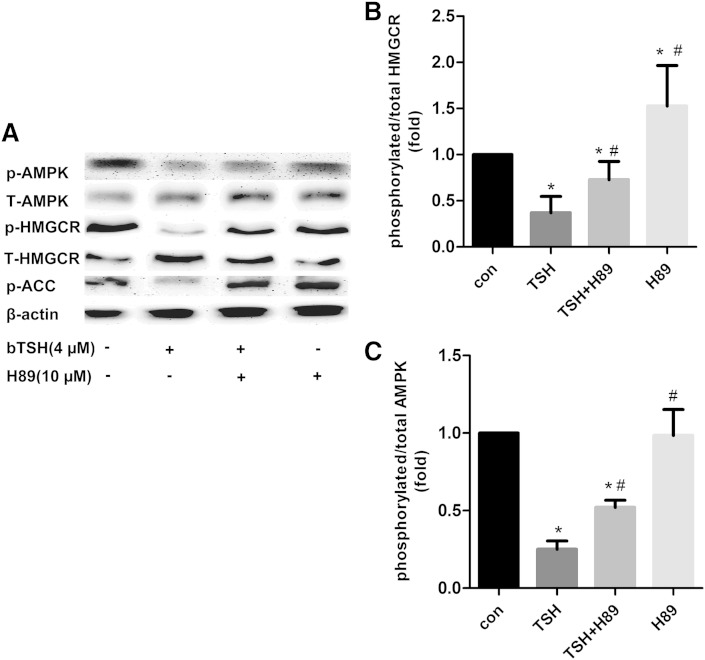
Our previous study had indicated that TSH could trigger the cAMP/PKA pathway (14). To investigate whether the TSH affects the AMPK activity associated the PKA effect, we treated the HepG2 cells with the PKA inhibitor, H89. The expression of p-AMPK, AMPK, p-HMGCR, and HMGCR were measured (Fig. 3A). The TSH alone decreased the ratio of p-AMPK/AMPK and p-HMGCR/HMGCR relative to the control (P < 0.05), but the pretreatment with H89 resisted this action by TSH, whereas the expression of the phosphorylated AMPK and the phosphorylated HMGCR recovered and increased compared with the cells stimulated by TSH alone (both P < 0.05) (Fig. 3B, C). These results indicated that the TSH has an inhibitory effect on the AMPK activity through the PKA/AMPK pathway in the hepatocytes.
Fig. 3.
The effects of the TSH on phosphorylation of HMGCR are inhibited by PKA inhibitor H89. HepG2 cells were pretreated with and without H89 (10 μM) for 1 h followed by incubation with bTSH for an additional 48 h. The protein levels of phosphorylated and total AMPK and HMGCR, as well as phosphorylated ACC, were determined by Western immunoblotting analysis, respectively. The results are given as the mean ± SD from at least three independent experiments. *P < 0.05 versus the untreated group. #P < 0.05 versus only the TSH stimulated group.
TSH decreases the liver HMGCR phosphorylation by AMPK via TSHRs
The characteristics of the Tshr-KO mice (aged 6–8 weeks) are shown in Table1. The serum T4, FT4, and TSH levels showed no differences between the wild-type and the Tshr-KO littermate mice (all P > 0.05). The body weights of Tshr-KO mice were significantly lower than wild-type mice (P < 0.05), however, the ratio of liver weight to body weight in Tshr-KO mice did not show significant differences compared with wild-type mice (P > 0.05). Compared with the littermate wild-type mice, the serum cholesterol levels in the Tshr-KO mice decreased by 35% (P < 0.05). Compared with the wild-type mice, the Tshr-KO mice had a significantly decreased level of HDL cholesterol. Although the level of the LDL cholesterol between the two groups had no significant difference, but the level in the Tshr-KO mice had a decreasing tendency.
TABLE 1.
General characteristics of the wild-type mice and Tshr-KO mice
| Wild-type Mice | Tshr-KO Mice | |
| N | 5 | 4 |
| BW (g) | 23.91 ± 0.94 | 20.77 ± 1.18a |
| LBI | 0.040 ± 0.004 | 0.038 ± 0.002 |
| FT4 (pmol/l) | 1.82 ± 0.11 | 1.59 ± 0.24 |
| T4 (μg/dl) | 4.43 ± 0.32 | 4.41 ± 0.45 |
| TSH (pg/ml) | 387.59 ± 56.42 | 382.28 ± 50.38 |
| Serum TC (mmol/l) | 2.93 ± 0.20 | 1.90 ± 0.15a |
| HDL-C (mmol/l) | 2.03 ± 0.07 | 1.38 ± 0.26a |
| LDL-C (mmol/l) | 0.65 ± 0.15 | 0.42 ± 0.14 |
| Liver TC (ug/mg protein) | 1.12 ± 0.08 | 0.74 ± 0.02a |
| TGs (mmol/l) | 0.78 ± 0.11 | 0.93 ± 0.15 |
| Glucose (mmol/l) | 6.04 ± 1.39 | 4.22 ± 1.29a |
| Insulin (mIU/l) | 8.24 ± 2.33 | 7.78 ± 2.54 |
| FFA (mmol/l) | 1.01 ± 0.40 | 1.47 ± 0.46a |
Values are expressed as mean ± SD. BW, body weight; LBI, liver weight/body weight index; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
P < 0.05 versus wild-type mice.
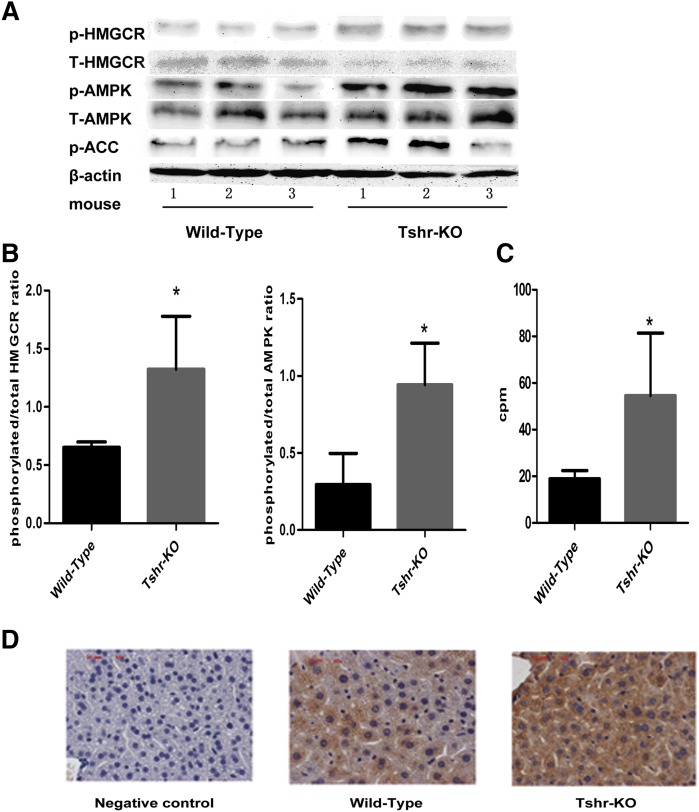
As shown in Fig. 4A, the HMGCR expression in the livers of the Tshr-KO mice, compared with the wild-type mice, decreased and the phosphorylated HMGCR increased (both groups, P < 0.05). The expression of p-AMPK was elevated by 3-fold (P < 0.05), but there was no significant difference of the total AMPK expression between the two groups. The expression of p-ACC proteins was increased by 100.5% (P < 0.05). Both the ratios of the phosphorylated HMGCR to total HMGCR expression and the phosphorylated AMPK to AMPK presented increase (P < 0.05, Fig. 4B) and the phosphorylation of the ACC also increased (P < 0.05, Fig. 4C) compared with the wild-type mice. In addition, immunochemistry staining showed the increased expression of the phosphorylated HMGCR located mainly in the cytolymph in the livers of Tshr-KO mice (Fig. 4D).
Fig. 4.
TSH decreases the liver HMGCR phosphorylation by AMPK via TSHRs. A: The expression of p-HMGCR, t-HMGCR, p-AMPK, t-AMPK, and p-ACC was analyzed using Western immunoblotting analysis in Tshr-KO and wild-type littermate mice, respectively. The same membranes were reprobed with anti-β-actin antibody to confirm the equal loading of proteins for each sample. The representation from three independent experiments is shown. B: The ratios of p-HMGCR/HMGCR and p-AMPK/AMPK in the liver of wild-type mice and Tshr-KO mice were assessed by semiquantitative analysis. C: ACC phosphorylation in the livers of wild-type mice and Tshr-KO mice was assayed by γ-32P. All data are expressed as mean ± SD. *P < 0.05 versus the wild-type mice. D: Liver tissues from Tshr-KO mice and wild-type mice were stained with primary antibodies specific for phosphorylated HMGCR (brown) or the primary antibodies were omitted (negative) and then were counterstained with hematoxylin (blue) to visualize nuclei using immune-histochemical analysis. The images are from a representative animal in each group. Magnification, ×400. Scale bar denotes 20 μM.
DISCUSSION
In the present study, we demonstrated a significant decrease in the ratios of p-HMGCR/HMGCR and p-AMPK/AMPK, which indicates the unactivated state of HMGCR and the activated state of AMPK, respectively, in the hepatocytes following bTSH stimulation. The changes above were inversed when the cells were treated with CA-AMPK plasmid or H89. In the Tshr-KO mice, the ratios of liver p-HMGCR/HMGCR and p-AMPK/AMPK were increased relative to the littermate wild-type mice. Our main finding in this study is that the TSH decreases the phosphorylation of HMGCR via AMPK in the liver.
As a complex pathway, cholesterol biosynthesis requires 20 enzymes to assemble 30 carbons from acetyl-CoA into a 27-carbon structure. HMGCR is the rate-limiting enzyme because it catalyzes an irreversible reaction at the beginning of the pathway. Thus, an increase or decrease in HMGCR activity can regulate the output of the overall pathway without accumulating unusable intermediates. The equilibrium of enzyme phosphorylation-dephosphorylation plays an important role in regulating the HMGCR activity. It has been shown that dephosphorylation of the HMGCR enhances its activity, whereas phosphorylation at serine872 leads to enzyme inactivation (29). Pallottini et al. (30) had shown that in aged rats the HMGCR is completely dephosphorylated in the liver, which is in agreement with the full activation of the enzyme; this report explained the increase in cholesterol production in the liver and a higher cholesterol content in the blood during aging. The decreased phosphorylation of the HMGCR led to an activation of the enzyme. To investigate whether the increased TSH was related to the elevated HMGCR activity, we stimulated the primary mouse hepatocytes and HepG2 cells with different doses of TSH. As expected, the expression of the phosphorylated HMGCR decreased in the TSH-stimulated hepatocytic cells, which suggests that the TSH has an association with HMGCR phosphorylation. We also found that, compared with the HepG2 cells stimulated without bTSH, a smaller increase in HMGCR activity was observed in HepG2 cells stimulated by bTSH isolated in the absence of NaF relative to those isolated with NaF, which indicated that TSH could decrease the HMGCR phosphorylation.
KO technology is one of the effective means of genetic function. Tshr-KO mice have been used commonly as an effective method in the research of TSH and TSHR (31, 32). The Tshr-KO mice were hypothyroid because of being unable to respond to pituitary TSH. If weaned at the usual time, the mice wasted and died, perhaps as a result of the small intestine defect seen in the thyroid hormone receptor (TR) KO mouse (33). However, Tshr-KO mice survived when weaned at day 21 onto a thyroid powder-supplemented diet. In contrast, thyroid-replaced animals had normal gross developmental patterns and were fertile, despite the absence of the TSHR. In our study, all Tshr-KO (homozygotic type) mice were fed a diet containing 100 ppm thyroid powder from the third week, testing the levels of thyroid hormones at the end of 6 and 8 weeks, respectively. Animal studies have shown that HMGCR exhibits a diurnal variation in activity (34–36). The HMGCR activity varies with the time of day and reaches a peak at the middle of the dark period (midnight) (37). So we harvested the blood and liver tissue at the midnight. We found the serum T4, FT4, and TSH levels showed no differences. The Tshr-KO mice, which had the ablation of TSHR, had decreased HMGCR expression, serum cholesterol, and liver cholesterol levels. However, the expression of the phosphorylated HMGCR increased compared with the wild-type mice. In addition, we failed to test the change of liver deiodinase expression (data not shown), thus, the results suggest the all changes we tested were independent of thyroid hormones.
Several protein kinases, such as AMPK, PKC, and Ca2+ calmodulin-dependent kinase, can phosphorylate the HMGCR in vitro (16, 17). However, AMPK seems to be the major kinase that regulates the phosphorylation of the HMGCR in the liver. AMPK is known to play a major role in energy homeostasis and is sensitive to the intracellular AMP/ATP ratio. AMPK is a heterotrimeric protein, consisting of three subunits α, β, and γ and possesses its full activity owing to the existing three subunits. The α subunit is the catalytic subunit, and its activation via the phosphorylation of the threonine residue 172 is crucial for AMPK activation under ATP-depleted conditions. AMPK is activated upon phosphorylation and, in turn, inactivates the HMGCR via phosphorylation of the enzyme (38, 39). In the present study, the AMPK activity, which was accompanied by an increased TSH level, decreased in the TSH-treated primary mouse hepatocytes and HepG2 cells. CA-AMPK increased the expression of phosphorylated HMGCR and phosphorylated ACC, which was decreased by the TSH treatment. In the Tshr-KO mice, the ratio of liver p-AMPK/AMPK was increased relative to the littermate wild-type mice. These results indicated that TSH-suppressed phosphorylated HMGCR expression was mediated via decreasing AMPK activation.
Benoit Viollet and colleagues have shown that in total and liver-specific AMPKα2−/− mice, plasma levels for total and HDL cholesterol are not statistically different compared with controls but have a tendency to be higher. This suggests that the remaining α1 subunit activity in AMPKα2−/− mice is sufficient to control hepatic cholesterol synthesis and that HMGCR is probably a target for both AMPK catalytic subunits (40). Further studies are needed to elucidate which subunit, AMPKα1, AMPKα2, or both, affect the activation of TSH on HMGCR phosphorylation.
It has been proposed that the ability of upstream kinases (LKB1, TAK1, and CAMKK) to activate AMPK is attenuated in response to agents that increase intracellular cAMP and induce PKA activation. The phosphorylation of the AMPK-Ser485/491 residues of AMPK decreases the accessibility of the AMPK-Thr172 site to its upstream kinase (41). Djouder et al. (19) found that PKA associates with and phosphorylates AMPKa1 at Ser173 to impede threonine (Thr172) phosphorylation and thus activation of AMPK. Interestingly, we found that stimulating TSH increased the level of p-AMPK (S173) expression in HepG2 cells (data not shown). These results indicated that PKA phosphorylates and inactivates AMPK.
The cAMP/PKA pathway plays an important role in TSH stimulation. In the current study, we noticed that the TSH inhibited AMPK phosphorylation in the HepG2 cells, but the effect was partly blocked (decreased by 56.5%) by the PKA inhibitor H89, providing evidence that the PKA pathway played the main role. However, the results also suggest that there were other molecules to take part in the process of TSH suppressing AMPK activation in a PKA-dependent manner. As we all know, TSH-induced dissociation of heterotrimeric G proteins leads to Gα and Gβγ activation. Zaballos, Garcia, and Santisteban (42) found that TSHR-mediated Gβγ activation could directly stimulate PI3K in rat thyrocytes leading to diminished sodium iodide symporter expression. Hahn-Windgassen et al. (43) showed in Akt1/Akt2 DKO cells that the AMP/ATP ratio was markedly elevated with a concomitant increase in AMPK activity, whereas in cells expressing activated Akt there was a dramatic decrease in AMP/ATP ratio and a decline in AMPK activity, demonstrating that Akt is a negative regulator of AMPK. At present, our group has found that TSH can increase the activated state of AKT in the liver (data not shown). Further studies are needed to elucidate whether the AKT participates in TSH downregulating AMPK activity.
In summary, our study demonstrates a novel mechanism in which TSH regulates hepatic HMGCR and manages elevated cholesterol levels. Hypercholesterolemia is a great problem in the world; our research showed that TSH could regulate the HMGCR phosphorylation via the AMPK in hepatocytes, establishing a potential mechanism for hypercholesterolemia involved in a direct action of the TSH in liver and indicated specific anti-TSHR in the liver as a potential therapeutic target of hypercholesteremia.
Note added in proof
The author Yingli Lu was inadvertently left out of the author list of the accepted version of this article. All other authors and the Journal’s accepting Associate Editor approved the addition after the article was in proof stage. Dr. Lu will appear as an author in all forms of the article except in the originally accepted Paper in Press.
Acknowledgments
The authors would like to thank Dave Carling for the gift of recombinant pcDNA3 plasmids coding for CA-AMPK and the teaching staff of the Science Center of Shandong Provincial Hospital for the excellent technical assistance they rendered to us during the conduct of this study.
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- AMPK
- AMP-activated protein kinase
- bTSH
- bovine thyroid-stimulating hormone
- CA-AMPK
- constitutively active AMP-activated protein kinase
- FT4
- free thyroxine
- HMGCR
- HMG-CoA reductase
- SCH
- subclinical hypothyroidism
- T4
- total thyroxine
- TC
- total cholesterol
- TSH
- thyroid-stimulating hormone
- TSHR
- thyroid-stimulating hormone receptor
This work was supported by grants from the National Basic Research Program (2012CB524900), the National Natural Science Foundation (81230018, 81170794, 81270869, 81270970, and 81300644), an international cooperation grant (2011) of Shandong Province, and the Jinan self-renovation plan for colleges, universities, and scientific research institutes (2012) of China. All of the authors gave their informed consent and report no conflicts of interest.
REFERENCES
- 1.Geelen M. J., Gibson D. M., Rodwell V. W. 1986. Hydroxymethylglutaryl-CoA reductase–the rate-limiting enzyme of cholesterol biosynthesis. A report of a meeting held at Nijenrode Castle, Breukelen, The Netherlands, August 24, 1985. FEBS Lett. 201: 183–186. [DOI] [PubMed] [Google Scholar]
- 2.Jurevics H., Hostettler J., Barrett C., Morell P., Toews A. D. 2000. Diurnal and dietary-induced changes in cholesterol synthesis correlate with levels of mRNA for HMG-CoA reductase. J. Lipid Res. 41: 1048–1054. [PubMed] [Google Scholar]
- 3.Ravid T., Doolman R., Avner R., Harats D., Roitelman J. 2000. The ubiquitin-proteasome pathway mediates the regulated degradation of mammalian 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 275: 35840–35847. [DOI] [PubMed] [Google Scholar]
- 4.Omkumar R. V., Darnay B. G., Rodwell V. W. 1994. Modulation of Syrian hamster 3-hydroxy-3-methylglutaryl-CoA reductase activity by phosphorylation. Role of serine 871. J. Biol. Chem. 269: 6810–6814. [PubMed] [Google Scholar]
- 5.Brown M. S., Goldstein J. L. 1980. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J. Lipid Res. 21: 505–517. [PubMed] [Google Scholar]
- 6.Feramisco J. D., Goldstein J. L., Brown M. S. 2004. Membrane topology of human insig-1, a protein regulator of lipid synthesis. J. Biol. Chem. 279: 8487–8496. [DOI] [PubMed] [Google Scholar]
- 7.Wu N., Sarna L. K., Siow Y. L., K. O. 2011. Regulation of hepatic cholesterol biosynthesis by berberine during hyperhomocysteinemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300: R635–R643. [DOI] [PubMed] [Google Scholar]
- 8.Sato R., Goldstein J. L., Brown M. S. 1993. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc. Natl. Acad. Sci. USA. 90: 9261–9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Istvan E. S., Palnitkar M., Buchanan S. K., Deisenhofer J. 2000. Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis. EMBO J. 19: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teixeira P. F., Reuters V. S., Ferreira M. M., Almeida C. P., Reis F. A., Buescu A., Costa A. J., Vaisman M. 2008. Lipid profile in different degrees of hypothyroidism and effects of levothyroxine replacement in mild thyroid failure. Transl. Res. 151: 224–231. [DOI] [PubMed] [Google Scholar]
- 11.Wanjia X., Chenggang W., Aihong W., Xiaomei Y., Jiajun Z., Chunxiao Y., Jin X., Yinglong H., Ling G. 2012. A high normal TSH level is associated with an atherogenic lipid profile in euthyroid non-smokers with newly diagnosed asymptomatic coronary heart disease. Lipids Health Dis. 11: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turhan S., Sezer S., Erden G., Guctekin A., Ucar F., Ginis Z., Ozturk O., Bingol S. 2008. Plasma homocysteine concentrations and serum lipid profile as atherosclerotic risk factors in subclinical hypothyroidism. Ann. Saudi Med. 28: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W., Tian L. M., Han Y., Ma H. Y., Wang L. C., Guo J., Gao L., Zhao J. J. 2009. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. J. Cell. Mol. Med. 13: 4636–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian L., Song Y., Xing M., Zhang W., Ning G., Li X., Yu C., Qin C., Liu J., Tian X., et al. 2010. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology. 52: 1401–1409. [DOI] [PubMed] [Google Scholar]
- 15.Henin N., Vincent M. F., Gruber H. E., Van den Berghe G. 1995. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 9: 541–546. [DOI] [PubMed] [Google Scholar]
- 16.Beg Z. H., Stonik J. A., Brewer H. B., Jr 1985. Phosphorylation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and modulation of its enzymic activity by calcium-activated and phospholipid-dependent protein kinase. J. Biol. Chem. 260: 1682–1687. [PubMed] [Google Scholar]
- 17.Beg Z. H., Stonik J. A., Brewer H. B., Jr 1987. Phosphorylation and modulation of the enzymic activity of native and protease-cleaved purified hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase by a calcium/calmodulin-dependent protein kinase. J. Biol. Chem. 262: 13228–13240. [PubMed] [Google Scholar]
- 18.Hawley S. A., Davison M., Woods A., Davies S. P., Beri R. K., Carling D., Hardie D. G. 1996. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 271: 27879–27887. [DOI] [PubMed] [Google Scholar]
- 19.Djouder N., Tuerk R. D., Suter M., Salvioni P., Thali R. F., Scholz R., Vaahtomeri K., Auchli Y., Rechsteiner H., Brunisholz R. A., et al. 2010. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 29: 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marians R. C., Ng L., Blair H. C., Unger P., Graves P. N., Davies T. F. 2002. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc. Natl. Acad. Sci. USA. 99: 15776–15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichurin P. N., Pichurina O., Marians R. C., Chen C. R., Davies T. F., Rapoport B., McLachlan S. M. 2004. Thyrotropin receptor knockout mice: studies on immunological tolerance to a major thyroid autoantigen. Endocrinology. 145: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 22.Casciano D. A. 2000. Development and utilization of primary hepatocyte culture systems to evaluate metabolism, DNA binding, and DNA repair of xenobiotics. Drug Metab. Rev. 32: 1–13. [DOI] [PubMed] [Google Scholar]
- 23.Honda A., Mizokami Y., Matsuzaki Y., Ikegami T., Doy M., Miyazaki H. 2007. Highly sensitive assay of HMG-CoA reductase activity by LC-ESI-MS/MS. J. Lipid Res. 48: 1212–1220. [DOI] [PubMed] [Google Scholar]
- 24.Brown M. S., Goldstein J. L., Dietschy J. M. 1979. Active and inactive forms of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the liver of the rat. Comparison with the rate of cholesterol synthesis in different physiological states. J. Biol. Chem. 254: 5144–5149. [PubMed] [Google Scholar]
- 25.Shapiro D. J., Imblum R. L., Rodwell V. W. 1969. Thin-layer chromatographic assay for HMG-CoA reductase and mevalonic acid. Anal. Biochem. 31: 383–390. [DOI] [PubMed] [Google Scholar]
- 26.Goldfarb S., Pitot H. C. 1972. Improved assay of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Lipid Res. 13: 797–801. [PubMed] [Google Scholar]
- 27.Jamil H., Madsen N. B. 1987. Phosphorylation state of acetyl-coenzyme A carboxylase. J. Biol. Chem. 262: 630–637. [PubMed] [Google Scholar]
- 28.Glass D. B., Masaracchia R. A., Feramisco J. R., Kemp B. E. 1978. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal. Biochem. 87: 566–575. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki S., Kawai K., Honjo Y., Nakamura H. 2006. Thyroid hormones and lipid metabolism [article in Japanese] Nihon Rinsho. 64: 2323–2329. [PubMed] [Google Scholar]
- 30.Pallottini V., Martini C., Cavallini G., Bergamini E., Mustard K. J., Hardie D. G., Trentalance A. 2007. Age-related HMG-CoA reductase deregulation depends on ROS-induced p38 activation. Mech. Ageing Dev. 128: 688–695. [DOI] [PubMed] [Google Scholar]
- 31.Elgadi A., Zemack H., Marcus C., Norgren S. 2010. Tissue-specific knockout of TSHr in white adipose tissue increases adipocyte size and decreases TSH-induced lipolysis. Biochem. Biophys. Res. Commun. 393: 526–530. [DOI] [PubMed] [Google Scholar]
- 32.Nakahara M., Mitsutake N., Sakamoto H., Chen C. R., Rapoport B., McLachlan S. M., Nagayama Y. 2010. Enhanced response to mouse thyroid-stimulating hormone (TSH) receptor immunization in TSH receptor-knockout mice. Endocrinology. 151: 4047–4054. [DOI] [PubMed] [Google Scholar]
- 33.Fraichard A., Chassande O., Plateroti M., Roux J. P., Trouillas J., Dehay C., Legrand C., Gauthier K., Kedinger M., Malaval L., et al. 1997. The T3R alpha gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 16: 4412–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke C. F., Fogelman A. M., Edwards P. A. 1984. Diurnal rhythm of rat liver mRNAs encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase. Correlation of functional and total mRNA levels with enzyme activity and protein. J. Biol. Chem. 259: 10439–10447. [PubMed] [Google Scholar]
- 35.Greene Y. J., Harwood H. J., Jr, Stacpoole P. W. 1985. Ascorbic acid regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol synthesis in guinea pig liver. Biochim. Biophys. Acta. 834: 134–138. [DOI] [PubMed] [Google Scholar]
- 36.Easom R. A., Zammit V. A. 1984. Diurnal changes in the fraction of 3-hydroxy-3-methylglutaryl-CoA reductase in the active form in rat liver microsomal fractions. Biochem. J. 220: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Máille E. R., Richards T. G., Short A. H. 1967. The influence of conjugation of cholic acid on its uptake and secretion: hepatic extraction of taurocholate and cholate in the dog. J. Physiol. 189: 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carling D., Clarke P. R., Zammit V. A., Hardie D. G. 1989. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur. J. Biochem. 186: 129–136. [DOI] [PubMed] [Google Scholar]
- 39.Clarke P. R., Hardie D. G. 1990. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 9: 2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephenne X., Foretz M., Taleux N., van der Zon G. C., Sokal E., Hue L., Viollet B., Guigas B. 2011. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 54: 3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurley R. L., Barre L. K., Wood S. D., Anderson K. A., Kemp B. E., Means A. R., Witters L. A. 2006. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J. Biol. Chem. 281: 36662–36672. [DOI] [PubMed] [Google Scholar]
- 42.Zaballos M. A., Garcia B., Santisteban P. 2008. Gbetagamma dimers released in response to thyrotropin activate phosphoinositide 3-kinase and regulate gene expression in thyroid cells. Mol. Endocrinol. 22: 1183–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn-Windgassen A., Nogueira V., Chen C. C., Skeen J. E., Sonenberg N., Hay N. 2005. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J. Biol. Chem. 280: 32081–32089. [DOI] [PubMed] [Google Scholar]