Abstract
Squalene synthase (SS) catalyzes the biosynthesis of squalene, the first specific intermediate in the cholesterol biosynthetic pathway. To test the feasibility of lowering plasma cholesterol by inhibiting hepatic SS, we generated mice in which SS is specifically knocked out in the liver (L-SSKO) using Cre-loxP technology. Hepatic SS activity of L-SSKO mice was reduced by >90%. In addition, cholesterol biosynthesis in the liver slices was almost eliminated. Although the hepatic squalene contents were markedly reduced in L-SSKO mice, the hepatic contents of cholesterol and its precursors distal to squalene were indistinguishable from those of control mice, indicating the presence of sufficient centripetal flow of cholesterol and/or its precursors from the extrahepatic tissues. L-SSKO mice showed a transient liver dysfunction with moderate hepatomegaly presumably secondary to increased farnesol production. In a fed state, the plasma total cholesterol and triglyceride were significantly reduced in L-SSKO mice, primarily owing to reduced hepatic VLDL secretion. In a fasted state, the hypolipidemic effect was lost. mRNA expression of liver X receptor α target genes was reduced, while that of sterol-regulatory element binding protein 2 target genes was increased. In conclusion, liver-specific ablation of SS inhibits hepatic cholesterol biosynthesis and induces hypolipidemia without increasing significant mortality.
Keywords: cholesterol/biosynthesis, enzymology/enzyme regulation, lipoproteins/metabolism, very low density lipoprotein, inborn errors of metabolism, Cre recombinase, gene targeting, knockout
Mammals have developed sophisticated and complex systems to maintain cellular content of cholesterol, an essential component of cellular membranes and a precursor of bile acids and steroid hormones (1). In addition to dietary intake, cholesterol is supplied by de novo synthesis from acetate. Squalene synthase (SS; farnesyl-diphosphate farnesyltransferase, EC2.5.1.21) catalyzes the reductive head-to-head condensation of two molecules of farnesyl diphosphate (FPP) to form squalene, the first committed intermediate in the cholesterol biosynthetic pathway (2, 3). SS contains ∼416 amino acids and is anchored to endoplasmic reticulum by a short C-terminal membrane-spanning domain, with its large N-terminal catalytic domain facing the cytosol, where water-soluble FPP and NADPH are present. Hepatic SS is highly regulated at the transcriptional level, not only by cellular cholesterol content (4) but also by proinflammatory cytokines: TNF-α and interleukin 1β (5). This enzyme has been an attractive target for cholesterol-lowering therapy because the inhibition of this step theoretically may not perturb the nonsterol pathway, which is a potential problem in the use of statins, inhibitors of HMG-CoA reductase (HMGCR). However, development of SS inhibitors such as zaragozic acid and lapaquistat acetate (TAK-475) has been halted because of safety concerns (6, 7).
To investigate the consequences of the systemic ablation of SS, we generated mice lacking SS in the whole body (8). The SS−/− mice exhibited developmental defects and did not survive beyond E12.5. Supplementation of the dams’ diet with squalene or cholesterol did not allow survival of SS−/− fetuses to term. In contrast, SS+/− mice were apparently normal and their plasma lipoprotein profiles were indistinguishable from those of wild-type mice, even though hepatic SS activity was reduced by 50%. These results suggest that fetal demands for cholesterol were not met by maternal supplies and/or that the accumulation of precursors of squalene such as FPP was toxic. In this context, it is noteworthy that naturally occurring inborn errors of cholesterol metabolism are frequently associated with severe developmental abnormalities particularly in the central nervous system (9).
Because deletion of SS theoretically does not block the nonsterol pathway, mice in which SS is specifically knocked out in the liver can be a viable model in which hepatic sterol pathway is selectively abrogated. To test this hypothesis and further examine the efficacy and safety of inhibition of SS, we used tissue-specific gene targeting with the Cre-loxP system to generate mice lacking SS in a liver-specific manner.
MATERIALS AND METHODS
The detailed procedures for the generation of liver-specific SS knockout (L-SSKO) mice and other assays are available in the supplementary Materials and Methods.
RESULTS
The heterozygous floxed SS (SS+/f; f denotes flanked by loxP) carrying one copy of the Cre recombinase gene under the control of the albumin gene promoter (Alb-Cre) (10) were interbred with SS+/f littermates lacking Alb-Cre to generate L-SSKO mice. L-SSKO mice and littermate controls [SS+/+, SS+/+Alb-Cre, and SSf/f (fSS)] were generated. Because there were no differences in growth curves or metabolic parameters such as plasma lipid and glucose levels between the SS+/+, SS+/+Alb-Cre, and SSf/f (fSS) mice, we used fSS mice as a control.
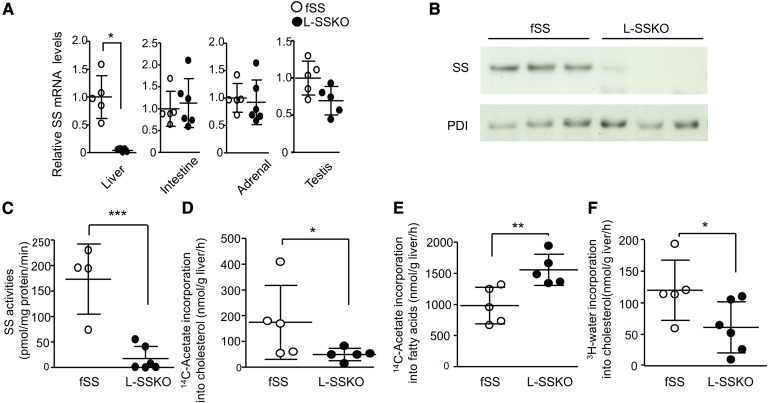
To determine whether SS expression was ablated in the liver, we performed a Southern blot analysis (supplementary Fig. 2A). At 4 weeks of age, the livers of L-SSKO mice contained both disrupted and floxed alleles with the former being more predominant (89% based on relative band density). Northern blot analysis (supplementary Fig. 2B) and real-time PCR (Fig. 1A) showed that the mRNA expression of SS in the livers of L-SSKO mice was reduced to 5% of that in the fSS mice. In contrast, the mRNA expressions of SS in small intestines, adrenal glands, and testes were not different between fSS and L-SSKO mice (Fig. 1A).
Fig. 1.
Conditional deletion of SS in the livers. A: Quantitative real-time PCR of SS mRNA levels from the livers, intestines, adrenal glands, and testes of control and L-SSKO male mice at the age of 12 weeks (n = 5∼6 in each group). The internal standards were GAPDH mRNA for livers and β actin mRNA for intestines, adrenal glands, and testes. B: Immunoblot analysis for SS protein of the liver of control and L-SSKO male mice at the age of 12 weeks (n = 3 in each group). Protein disulfide isomerase (PDI) was used as a loading control for SS protein levels. C: SS activity in the hepatic microsomal fractions of control (n = 4) and L-SSKO (n = 6) male mice at the age of 12 weeks. In vitro synthesis rate of cholesterol (D) and fatty acid (E) in whole liver slices from male mice at the age of 12 weeks (n = 5 in each group). Whole liver slices were incubated with [14C]acetate, and 14C-labeled cholesterol was determined. Two portions of liver from each mouse were used for the assay of liver slices. F: In vivo synthesis rate of cholesterol in liver from male mice at the age of 12 weeks (n = 5 in fSS and n = 6 in L-SSKO mice). Mice were injected with [3H]water intravenously. One hour later, the livers were removed for measurements of 3H-labeled cholesterol. Each value represents the mean ± SD. * P < 0.05, ** P< 0.01, and *** P < 0.001 by Student’s t-test.
In an immunoblot, SS protein was barely detectable in the livers of L-SSKO mice (Fig. 1B). Consistent with the profound reduction in SS at both the mRNA and protein levels, the SS activity in the livers of L-SSKO mice was <10% of that in fSS mice (Fig. 1C).
L-SSKO mice were born at a rate in accordance with the rule of Mendelian inheritance and survived at a rate that was not significantly different from that of the control mice up to 24 weeks of age.
Cholesterol synthesis was reduced by 71% in liver slices (Fig. 1D), while fatty acid synthesis was increased by 58% (Fig. 1E). In parallel, though less significantly, cholesterol synthesis from [3H]water injected intraperitoneally in vivo was decreased by 49% (Fig. 1F). Nevertheless, the hepatic levels of cholesterol were not decreased (Fig. 2H). A compensatory increase in the intestinal cholesterol absorption was not observed (supplementary Fig. 2C).
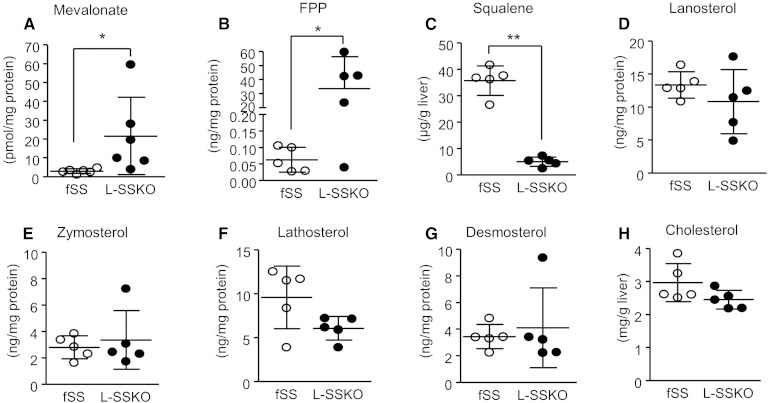
Fig. 2.
Hepatic contents of intermediates of the cholesterol synthetic pathway in control and L-SSKO mice. The liver tissue lysates from male mice at the age of 12 weeks (n = 5 in each group) were analyzed. Mevalonate content (A) was determined by the HMGCR enzymatic cycling assay. Squalene content (C) was determined by HPLC. Cholesterol content (H) was determined enzymatically. Other intermediates (B, D–G) were determined by LC/MS/MS method. Each value represents the mean ± SD. * P < 0.05 and ** P < 0.001 by Student’s t-test.
The hepatic contents of triglycerides, phospholipids, and fatty acids were not altered either (Table 1). The hepatic contents of intermediary metabolites of cholesterol biosynthesis proximal to SS such as mevalonate and FPP were increased, while those of squalene, the end product of SS, were reduced by 86% (Fig. 2A–C; supplementary Fig. 5). By contrast, the hepatic contents of intermediary metabolites of cholesterol biosynthesis distal to SS such as lanosterol, zymosterol, lathosterol, desmosterol, and cholesterol were not altered (Fig. 2D–H; supplementary Fig. 5).
TABLE 1.
Lipid contents in the plasma and livers
| Age 4 Weeks | Age 12 Weeks | |||
| fSS Genotype | L-SSKO Genotype | fSS Genotype | L-SSKO Genotype | |
| Plasma total cholesterol (mg/dl) | ||||
| Fed | 80.8 ± 9.0 (5) | 58.8 ± 15.1 (5)a | 85.8 ± 12.2 (6) | 55.6 ± 11.5 (7)b |
| 16 h fasted | 86.3 ± 1.44 (4) | 86.5 ± 15.0 (10) | 95.9 ± 13.2 (7) | 98.3 ± 26.2 (7) |
| Plasma triglyceride (mg/dl) | ||||
| Fed | 53.6 ± 22.3 (5) | 49.3 ± 17.0 (5) | 69.5 ± 33.2 (6) | 35.8 ± 13.2 (7)a |
| 16 h fasted | 103.8 ± 8.9 (4) | 81.1 ± 25.2 (10) | 106.2 ± 42.6 (7) | 102.5 ± 50.5 (7) |
| Plasma free fatty acid (mM) | ||||
| Fed | 0.44 ± 0.17 (5) | 0.56 ± 0.27 (5) | 0.41 ± 0.09 (6) | 0.43 ± 0.11 (7) |
| 16 h fasted | 2.13 ± 0.28 (4) | 1.63 ± 0.50 (10) | 1.50 ± 0.4 (7) | 1.58 ± 0.24 (7) |
| Hepatic cholesterol (mg/g) | 3.6 ± 0.1 (5) | 3.3 ± 0.5 (5) | 3.0 ± 0.5 (5) | 2.5 ± 0.3 (5) |
| Hepatic triglyceride (mg/g) | 4.2 ± 1.1 (5) | 6.0 ± 2.7 (5) | 5.7 ± 1.9 (5) | 6.2 ± 1.7 (5) |
| Hepatic phospholipid (mg/g) | 24.3 ± 2.1 (5) | 25.8 ± 2.1 (5) | 20.2 ± 1.8 (5) | 20.5 ± 1.8 (5) |
| Hepatic free fatty acid (μmol/g) | 4.7 ± 0.6 (5) | 4.4 ± 0.5 (5) | 5.4 ± 1.9 (5) | 4.0 ± 0.7 (5) |
Blood samples were taken from male mice fed a normal chow diet ad libitum or fasted for 16 h before the study. Each value represents the mean ± SD. Sample sizes are provided in parentheses.
P < 0.05 versus fSS mice with the same age by Student’s t-test.
P < 0.001 versus fSS mice with the same age by Student’s t-test.
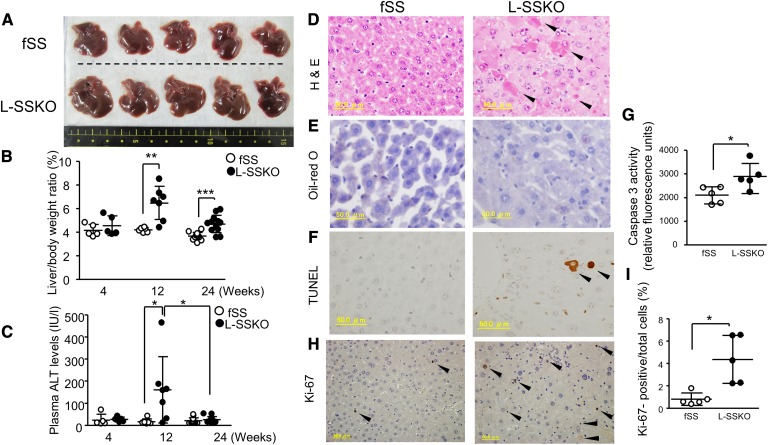
Next, we compared liver weight, liver functions, and plasma lipid levels between the control and L-SSKO mice (Fig. 3, Table 1, and supplementary Table 2). The livers of L-SSKO mice were larger than those of the control mice (Fig. 3A). The ratio of liver weight to body weight was 43% larger in L-SSKO mice at 12 weeks of age and 27% larger at 24 weeks of age (Fig. 3B). In proportion to enlargement of the liver, plasma levels of alanine aminotransferase (ALT) in the L-SSKO were significantly elevated compared with those in the control mice at 12 weeks of age (Fig. 3C). To determine the causes of the liver dysfunction associated with hepatomegaly, we performed a microscopic analysis of the liver. Hematoxylin and eosin (H and E) staining revealed cellular swelling and spotty necrosis of parenchymal cells (Fig. 3D). Oil Red O staining showed no evidence for accumulation of neutral lipids (Fig. 3E). The livers of the L-SSKO mice contained an increased number of deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)-positive parenchymal cells (lower panels), indicating apoptotic cell death (Fig. 3F). Consistently, liver caspase-3 activity in the L-SSKO mice was 50% higher than that in the fSS mice (Fig. 3G). In contrast, the number of Ki-67-positive hepatocytes was significantly increased in the L-SSKO mice (Fig. 3H, I), indicating that the hepatocytes regenerated at an increased rate.
Fig. 3.
Liver-specific deletion of SS causes transient hepatic toxicity with hepatomegaly. A: Representative gross appearance of the livers from male mice at the age of 12 weeks (n = 5 in each group). Liver/body weight ratio (B) and plasma ALT levels (C) in fSS and L-SSKO male mice at the ages of 4, 12, and 24 weeks (n = 5∼13 in each group). Liver sections from male mice at the age of 12 weeks were stained with H and E (D), Oil Red O (E), and TUNEL (F). Arrowhead indicates necrotic cells in H and E staining and apoptotic cells in TUNEL staining. G: Caspase-3 activity in the livers of fSS and L-SSKO male mice at the age of 12 weeks (n = 5 in each group). The liver tissue lysates were used for this assay. H: Ki-67-stained liver sections from control and L-SSKO male mice at the age of 12 weeks. Arrowhead indicates Ki-67-positive cells. I: Dot graph shows the percentages of Ki-67-positive cells relative to the total number of cells. Each value represents the mean ± SD. * P < 0.05, ** P < 0.01, and *** P < 0.001 by Student’s t-test.
The accumulation of FPP may be causally related to the increased apoptosis and proliferation of hepatocytes of L-SSKO mice. To verify this possibility, we evaluated the farnesol biosynthesis rate. The farnesol biosynthesis rate was significantly increased in L-SSKO mice by 12-fold and 4-fold at 12 and 24 weeks of age, respectively (supplementary Fig. 3A). The time course of the elevation of farnesol synthesis appears in parallel with that of the elevation of ALT, suggesting that the buildup of farnesol triggers apoptosis and/or proliferation. Indeed, increasing concentration of farnesol decreased the viability of the cultured hepatocytes in vitro (supplementary Fig. 3B). To examine whether extrinsic insults exaggerate the hepatocyte toxicity, we fed the mice a high-fat and high-sucrose diet for 12 weeks to make fatty liver. No difference was observed in the plasma ALT levels between fSS and L-SSKO mice (supplementary Fig. 3C).
In a fed state, plasma total cholesterol levels were decreased by ∼40% at 4 and 12 weeks of age (Table 1). Plasma triglyceride levels were decreased by 48% at 12 weeks of age. Plasma free fatty acid levels were not different between fSS and L-SSKO mice. After a 16 h fast, however, plasma levels of both total cholesterol and triglycerides were increased, and their differences between the control and L-SSKO mice disappeared.
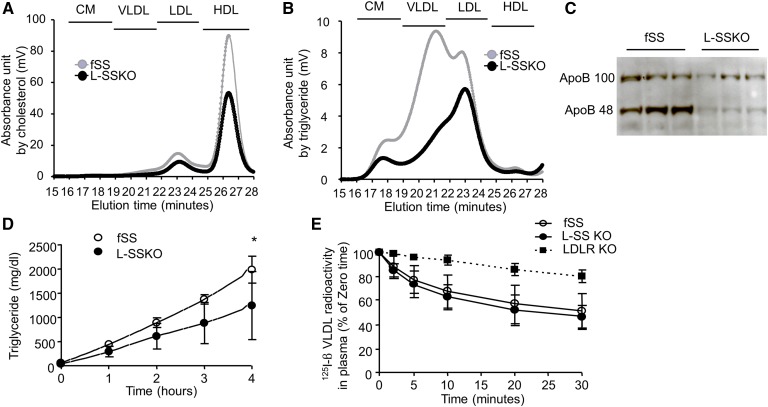
To determine which lipoproteins were affected by the abrogation of SS, we performed HPLC analyses of plasma lipoproteins. All of the lipoprotein fractions were affected by the elimination of SS synthesis: cholesterol contents were significantly decreased in the LDL and HDL fractions (Fig. 4A), and triglyceride contents were decreased in all the lipoprotein fractions (Fig. 4B). Consistent with the decreases in apoB-containing lipoproteins including chylomicron, VLDL, and LDL, plasma levels of apoB-100 and apoB-48 were decreased (Fig. 4C).
Fig. 4.
Plasma lipoprotein metabolism in control and L-SSKO mice. Total cholesterol (A) and triglyceride (B) contents in lipoproteins were determined by HPLC analysis. The chromatographic patterns of mean values from fSS (gray line, n = 5) and L-SSKO (black line, n = 5) male mice at 12 weeks of age. C: Immunoblot analysis of plasma apoB for fSS and L-SSKO male mice at the age of 12 weeks. Two microliters of plasma taken from mice in the regular chow-fed state was subjected to SDS-PAGE. Each lane represents an individual mouse (n = 3, in each group). D: Hepatic VLDL production rate after injection of Triton WR1339 in fSS (n = 4) and L-SSKO (n = 6) male mice at the age of 12 weeks. This rate is expressed as the increase in plasma triglyceride levels. E: Decay of 125I-labeled rabbit β-VLDL from the plasma in fSS, L-SSKO, and LDLR KO male mice at the age of 12 weeks (n = 5 in each groups). Each value represents the mean ± SD. * P < 0.01 by two-way repeated-measures ANOVA in E.
The mice were intravenously injected with Triton WR1339 to inhibit the plasma clearance of triglyceride-rich lipoprotein by interfering with lipoprotein lipase and the receptor-mediated lipoprotein uptake by the liver. The rate of increase in plasma triglyceride levels after the injection of Triton WR1339 was decreased by 38% in the L-SSKO mice, indicating that hepatic VLDL production was decreased (Fig. 4D).
β-VLDL clearance was severely delayed in LDL receptor (LDLR) KO mice compared with fSS mice, while it was not significantly different between the fSS and L-SSKO mice (Fig. 4E). Together, these results indicate that the hypolipidemia of L-SSKO mice primarily resulted from impaired secretion of VLDL by the liver.
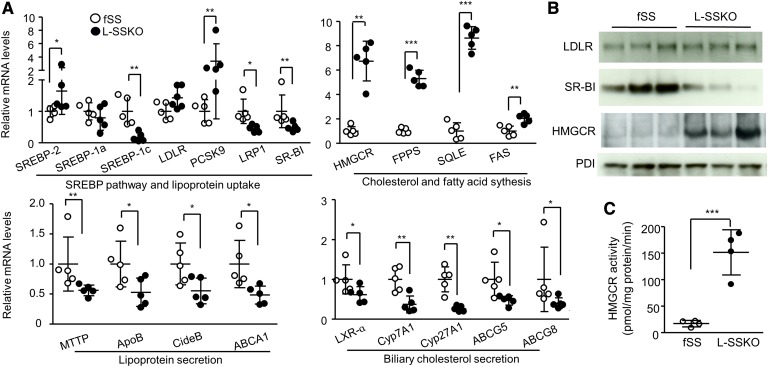
Eliminating SS synthesis in the liver affected the mRNA expressions of several genes involved in cholesterol and fatty acid metabolism (Fig. 5A and supplementary Fig. 5). Genes upregulated by a factor of 2 or more included HMGCR, FPP synthase (FPPS), squalene epoxidase (SQLE), proprotein convertase subtilisin/kexin type 9 (PCSK9), and FAS. Genes downregulated by a factor of 2 or more included sterol-regulatory element binding protein (SREBP) 1c, LDL receptor-related protein 1, scavenger receptor BI (SR-BI), apoB, cell death-inducing DNA fragmentation factor 45-like effector B (CideB), ABCA1, liver X receptor (LXR) α, cholesterol 7α-hydroxylase (Cyp7A1), ABCG5, and ABCG8. The mRNA expression of LDLR was not affected.
Fig. 5.
Hepatic expression of mRNA and proteins involved in lipid metabolism and hepatic HMGCR activity. A: Total RNA from the liver of male mice (n = 5 in each group) at the age of 12 weeks was subjected to quantitative real-time PCR. Relative mRNA levels were normalized to GAPDH. B: Immunoblot analysis of LDLR, SR-BI, and HMGCR of fSS and L-SSKO male mice at the age of 12 weeks. PDI was served as loading controls. Each lane represents an individual mouse. C: HMGCR activity (the rate of biosynthesis of mevalonate from HMG-CoA) in the liver microsomal fractions of fSS and L-SSKO male mice at 12 weeks of age (n = 4 in each group). Each value represents the mean ± SD. * P < 0.05, ** P < 0.01, and *** P < 0.001 by Student’s t-test. LRP1, LDL receptor-related protein 1; MTTP, microsomal triglyceride transfer protein.
The changes in protein expression of LDLR, SR-BI, and HMGCR were largely in parallel with the changes in mRNA expression: no change and drastic decrease and increase, respectively (Fig. 5B). Liver HMGCR activity, expressed as the rate of biosynthesis of mevalonate from HMG-CoA, was 8.9-fold higher in the L-SSKO mice than in the control mice (Fig. 5C). Consistent with the increased HMGCR activity, hepatic content of mevalonate was increased by 7.9-fold in the L-SSKO as compared with the control mice (Fig. 2A).
DISCUSSION
In contrast to the severe liver dysfunction and death resulting from the deficiency of HMGCR in the liver (11), the liver dysfunction of L-SSKO mice was mild and transient and did not compromise survival. More surprisingly, despite the almost complete absence of cholesterol synthesis ex vivo, hepatic cholesterol synthesis in vivo was suppressed only partially (49%), and hepatic contents of cholesterol and its precursors distal to squalene in L-SSKO mice were indistinguishable from those in fSS mice. Despite the apparent maintenance of cholesterol homeostasis in normally feeding L-SSKO mice, VLDL production was reduced, which thereby lowered the plasma concentration of apoB-containing lipoproteins.
Why was the hepatic cholesterol synthesis in vivo suppressed only partially? According to Turley et al. (12), 24% of cholesterol synthesis occurs in small intestine of rats, and a significant fraction of it is transported to liver (4.6 μmol/h) where nearly 50% of total cholesterol synthesis occurs (17.0 μmol/h). Probably a fraction of intraperitoneally injected [3H]water is used for cholesterol synthesis in the intestine and ultimately recovered in the liver, thereby diluting the effects of the elimination of cholesterol synthesis in the liver. Why was the reduction of cholesterol synthesis in vivo less prominent compared with that in the liver-specific SREBP-cleavage activating protein knockout (L-SCAPKO) mice where 82% of cholesterol synthesis was eliminated (13)? In contrast to L-SSKO mice, L-SCAPKO mice showed a significant decrease in the expression of LDLR. Conceivably, lower LDLR-mediated centripetal flux of cholesterol in L-SCAPKO mice might mitigate the contribution of cholesterol synthesis in the extrahepatic tissues, particularly in the intestine, to the overall cholesterol synthesis in vivo, thereby increasing the apparent contribution of the liver.
It is also surprising to note that hepatic contents of cholesterol and its precursors distal to squalene were not altered in L-SSKO mice (Fig. 2). As discussed above, they may be centripetally derived from the peripheral tissues such as the intestine, another potent cholesterogenic organ. According to Strandberg et al. (14), methyl sterols, most of which are cholesterol precursors, are preferentially released out of the intestinal cells compared with squalene. ABCA1 and HDL may not be involved in this process, because the centripetal movement of cholesterol was not altered in ABCA1 KO mice despite the virtual absence of HDL (15). The changes in the gene expression shown in Fig. 5A (decreases in the gene expressions of Cyp7A1, ABCG5, and ABCG8 and increases in the gene expressions of HMGCR, FPPS, and SQLE) also appear to favor the preservation of cholesterol in the liver.
Many of these changes may result from activation of the SREBP-2 pathway (Fig. 5A) probably owing to the reduced functional cholesterol or oxysterol pool in the livers of L-SSKO mice (16), as reported for HMGCR (17, 18) and PCSK9 (19) in hepatic cells or livers of rats treated with SS inhibitors. Attenuation of LXR-α signaling may also account for the changes in the expression of several genes: SREBP-1c, Cyp7A1, ABCA1, ABCG5, ABCG8 (20), SR-BI (21), and apoB (22). In addition to the reduced expression of LXR-α (Fig. 5A), deficiency of its endogenous ligands, oxysterols, may contribute to these changes. Certain oxysterols inhibit SREBP-2 activation while serving as ligands for LXR-α (23). Unlike common physiological oxysterols such as hydroxycholesterols, which are derived from cholesterol, 24(S), 25-epoxycholesterol (24,25EC), a potent LXR-α ligand with SREBP-2 suppressing activity (24, 25), is formed directly from squalene. Similarly, desmosterol, an immediate precursor of cholesterol, has been reported to affect both the LXR-α and SREBP-2 pathways (26). However, neither 24,25EC nor desmosterol contents were decreased in the livers of L-SSKO mice (supplementary Fig. 4 and Fig. 2G). Therefore, it is unlikely that the changes of gene and protein expressions in L-SSKO mice were caused by alterations of these oxysterols.
L-SSKO mice had decreased plasma levels of both apoB-containing lipoproteins and HDL in a fed state (Table 1; Fig. 4A, B). At the start of this study, we hypothesized that the low lipid levels in L-SSKO mice were caused by induction of expression of LDLR in the livers and subsequent accelerated catabolism of apoB-containing lipoproteins as occurs in the case of statins (27). However, LDLR expression was not significantly increased at either the mRNA level (Fig. 5A) or the protein level (Fig. 5B); consistently, plasma clearance of β-VLDL was not accelerated (Fig. 4D). Because mevalonate was found to downregulate LDLR mRNA in rat liver (28), the increased hepatic mavalonate contents (Fig. 2A) might have canceled the LDLR induction despite the activation of SREBP-2 pathway in L-SSKO mice.
The hypolipidemia was rather due to the decrease in hepatic VLDL production. Reduced VLDL production and subsequent plasma lipid lowering have also been reported in rats (29) or Watanabe heritable hyperlipidemic rabbits (30) treated with an SS inhibitor, TAK-475. Conversely, the mice overexpressing SS in the liver showed an opposite phenotype in terms of plasma lipoprotein metabolism: they were hyperlipidemic due to an increase in hepatic VLDL production (31).
How does SS activity determine hepatic VLDL production? The decreased expression of SREBP-1c, MTTP, apoB, and CideB appears to favor the decreased hepatic VLDL production (supplementary Fig. 5) because these genes regulate VLDL production (32–34). Although both expression of FAS mRNA (Fig. 5A) and fatty acid synthesis (Fig. 1E) were increased, overall hepatic triglyceride synthesis might be suppressed because of the decreased expression of SREBP-1c, the key transcriptional factor regulating lipogenesis (16), and accumulation of farnesol and its derivatives, which inhibit triglyceride biosynthesis (35). It is also noteworthy that expressions of many of LXR-α target genes were decreased as discussed before. Because LXR-α activity is a key determinant of VLDL production (22), it is reasonable to speculate that ablation of SS somehow suppresses LXR-α activity, thereby decreasing VLDL production. Because we failed to detect changes in hepatic contents of the oxysterols that can transactivate LXR-α, other pathways may underlie the suppression of LXR-α activity. In this context, it is interesting to note that the upstream nonsterol isoprenoid derivative of mevalonate, geranylgeranyl diphosphate (GGPP), and its alcohol derivative, geranylgeranyol, inhibit LXR-α signaling (36). This mechanism may play a dominant role because FPP, a precursor of GGPP, was robustly increased in the livers of L-SSKO mice (Fig. 2B).
The hypolipidemia observed in the L-SSKO mice in a fed state disappeared in a fasted state (Table 1). Fasting may mask the phenotypic manifestations caused by genetic inhibition of cholesterol biosynthesis, by suppressing the overall biosynthesis of cholesterol in the liver (37).
Finally, the transient liver dysfunction deserves discussion. Farnesol has been reported to cause cell-cycle arrest and apoptosis in some transformed cells (38). We confirmed a similar cytotoxicity on hepatocytes (supplementary Fig. 3B). Furthermore, liver dysfunction and increased synthesis of farnesol had similar time courses (Fig. 3C; supplementary Fig. 3A). Therefore, it is highly probable that the transient buildup of farnesol induced death of hepatocytes in L-SSKO mice. The increased cell proliferation might have occurred as a compensatory regeneration (Fig. 3H, I). The hepatotoxicity of lapaquistat acetate, or TAK-475, was the major reason for the suspension of its clinical development (7). If a buildup of farnesol is a causative factor, coadministration of statins can alleviate the liver toxicity by reducing the synthesis of FPP. A similar strategy has been proposed to avoid nonsterol isoprenoid depletion associated with treatment with statins (39). Judging from our results, the liver toxicity of SS inhibition is modest and self-limited even though the inhibition is complete. Therefore, it might be premature to give up the development of SS inhibitors as a novel strategy to treat hyperlipidemia and atherosclerosis.
In conclusion, L-SSKO mice are hypolipidemic due to a decrease in VLDL production. They develop mild and transient liver dysfunction. This animal model can be used to further define the role of hepatic cholesterol synthesis in the regulation of plasma lipoproteins and to understand the mechanism of toxicity of SS inhibition.
Supplementary Material
Acknowledgments
The authors thank Yukiko Hoshino, Mika Hayashi, and Nozomi Takatsudo for excellent technical assistance.
Footnotes
Abbreviations:
- Alb-Cre
- Cre recombinase gene under the control of the albumin gene promoter
- Cyp7A1
- cholesterol 7α-hydroxylase
- f
- flanked by loxP
- FPP
- farnesyl diphosphate
- H and E
- hematoxylin and eosin
- HMGCR
- HMG-CoA reductase
- LDLR
- LDL receptor
- L-SCAPKO
- liver-specific SREBP-cleavage activating protein knockout
- L-SSKO
- liver-specific SS knockout
- LXR
- liver X receptor
- SR-BI
- scavenger receptor BI
- SREBP
- sterol-regulatory element binding protein
- SS
- squalene synthase
- TUNEL
- deoxynucleotidyltransferase-mediated dUTP nick end labeling
This work was supported by a Grant-in-Aid for Scientific Research and the Program for the Strategic Research Foundation at Private Universities 2011–2015 “Cooperative Basic and Clinical Research on Circadian Medicine” and 2013–2017 “Non-communicable disease (NCD)” from the Ministry of Education, Culture, Sports, Science and Technology of Japan, JKA through its promotion funds from KEIRIN RACE, Jichi Medical University Young Investigator Award, Japan Heart Foundation & Astellas/Pfizer Grant for Research on Atherosclerosis Update, Banyu Life Science Foundation International, and unrestricted grants of from MSD, Pfizer, and Eli Lilly.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of Materials and Methods, five figures, and two tables.
REFERENCES
- 1.Goldstein J. L., DeBose-Boyd R. A., Brown M. S. 2006. Protein sensors for membrane sterols. Cell. 124: 35–46. [DOI] [PubMed] [Google Scholar]
- 2.Tansey T. R., Shechter I. 2000. Structure and regulation of mammalian squalene synthase. Biochim. Biophys. Acta. 1529: 49–62. [DOI] [PubMed] [Google Scholar]
- 3.Do R., Kiss R. S., Gaudet D., Engert J. C. 2009. Squalene synthase: a critical enzyme in the cholesterol biosynthesis pathway. Clin. Genet. 75: 19–29. [DOI] [PubMed] [Google Scholar]
- 4.Jiang G., McKenzie T. L., Conrad D. G., Shechter I. 1993. Transcriptional regulation by lovastatin and 25-hydroxycholesterol in HepG2 cells and molecular cloning and expression of the cDNA for the human hepatic squalene synthase. J. Biol. Chem. 268: 12818–12824. [PubMed] [Google Scholar]
- 5.Memon R. A., Shechter I., Moser A. H., Shigenaga J. K., Grunfeld C., Feingold K. R. 1997. Endotoxin, tumor necrosis factor, and interleukin-1 decrease hepatic squalene synthase activity, protein, and mRNA levels in Syrian hamsters. J. Lipid Res. 38: 1620–1629. [PubMed] [Google Scholar]
- 6.Vaidya S., Bostedor R., Kurtz M. M., Bergstrom J. D., Bansal V. S. 1998. Massive production of farnesol-derived dicarboxylic acids in mice treated with the squalene synthase inhibitor zaragozic acid A. Arch. Biochem. Biophys. 355: 84–92. [DOI] [PubMed] [Google Scholar]
- 7.Stein E. A., Bays H., O’Brien D., Pedicano J., Piper E., Spezzi A. 2011. Lapaquistat acetate: development of a squalene synthase inhibitor for the treatment of hypercholesterolemia. Circulation. 123: 1974–1985. [DOI] [PubMed] [Google Scholar]
- 8.Tozawa R., Ishibashi S., Osuga J., Yagyu H., Oka T., Chen Z., Ohashi K., Perrey S., Shionoiri F., Yahagi N., et al. 1999. Embryonic lethality and defective neural tube closure in mice lacking squalene synthase. J. Biol. Chem. 274: 30843–30848. [DOI] [PubMed] [Google Scholar]
- 9.Porter F. D., Herman G. E. 2011. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 52: 6–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yakar S., Liu J. L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. 1999. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA. 96: 7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagashima S., Yagyu H., Ohashi K., Tazoe F., Takahashi M., Ohshiro T., Bayasgalan T., Okada K., Sekiya M., Osuga J., et al. 2012. Liver-specific deletion of 3-hydroxy-3-methylglutaryl coenzyme a reductase causes hepatic steatosis and death. Arterioscler. Thromb. Vasc. Biol. 32: 1824–1831. [DOI] [PubMed] [Google Scholar]
- 12.Turley S. D., Andersen J. M., Dietschy J. M. 1981. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J. Lipid Res. 22: 551–569. [PubMed] [Google Scholar]
- 13.Kuriyama H., Liang G., Engelking L. J., Horton J. D., Goldstein J. L., Brown M. S. 2005. Compensatory increase in fatty acid synthesis in adipose tissue of mice with conditional deficiency of SCAP in liver. Cell Metab. 1: 41–51. [DOI] [PubMed] [Google Scholar]
- 14.Strandberg T. E., Tilvis R. S., Miettinen T. A. 1981. Squalene and sterol synthesis in isolated small-intestinal cells of the rat. Scand. J. Gastroenterol. 16: 801–810. [DOI] [PubMed] [Google Scholar]
- 15.Xie C., Turley S. D., Dietschy J. M. 2009. ABCA1 plays no role in the centripetal movement of cholesterol from peripheral tissues to the liver and intestine in the mouse. J. Lipid Res. 50: 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horton J. D., Goldstein J. L., Brown M. S. 2002. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez D., Chambers C. M., Keller R. K., Ness G. C. 1998. Compensatory responses to inhibition of hepatic squalene synthase. Arch. Biochem. Biophys. 351: 159–166. [DOI] [PubMed] [Google Scholar]
- 18.Kocarek T. A., Kraniak J. M., Reddy A. B. 1998. Regulation of rat hepatic cytochrome P450 expression by sterol biosynthesis inhibition: inhibitors of squalene synthase are potent inducers of CYP2B expression in primary cultured rat hepatocytes and rat liver. Mol. Pharmacol. 54: 474–484. [DOI] [PubMed] [Google Scholar]
- 19.Bedi M., Niesen M., Lopez D. 2008. Inhibition of squalene synthase upregulates PCSK9 expression in rat liver. Arch. Biochem. Biophys. 470: 116–119. [DOI] [PubMed] [Google Scholar]
- 20.Calkin A. C., Tontonoz P. 2012. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 13: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malerød L., Juvet L. K., Hanssen-Bauer A., Eskild W., Berg T. 2002. Oxysterol-activated LXRalpha/RXR induces hSR-BI-promoter activity in hepatoma cells and preadipocytes. Biochem. Biophys. Res. Commun. 299: 916–923. [DOI] [PubMed] [Google Scholar]
- 22.Basciano H., Miller A., Baker C., Naples M., Adeli K. 2009. LXRalpha activation perturbs hepatic insulin signaling and stimulates production of apolipoprotein B-containing lipoproteins. Am. J. Physiol. Gastrointest. Liver Physiol. 297: G323–G332. [DOI] [PubMed] [Google Scholar]
- 23.Peet D. J., Janowski B. A., Mangelsdorf D. J. 1998. The LXRs: a new class of oxysterol receptors. Curr. Opin. Genet. Dev. 8: 571–575. [DOI] [PubMed] [Google Scholar]
- 24.Peet D. J., Turley S. D., Ma W., Janowski B. A., Lobaccaro J. M., Hammer R. E., Mangelsdorf D. J. 1998. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 93: 693–704. [DOI] [PubMed] [Google Scholar]
- 25.Wong J., Quinn C. M., Gelissen I. C., Brown A. J. 2008. Endogenous 24(S),25-epoxycholesterol fine-tunes acute control of cellular cholesterol homeostasis. J. Biol. Chem. 283: 700–707. [DOI] [PubMed] [Google Scholar]
- 26.Yang C., McDonald J. G., Patel A., Zhang Y., Umetani M., Xu F., Westover E. J., Covey D. F., Mangelsdorf D. J., Cohen J. C., et al. 2006. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J. Biol. Chem. 281: 27816–27826. [DOI] [PubMed] [Google Scholar]
- 27.Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. 1981. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc. Natl. Acad. Sci. USA. 78: 1194–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ness G. C., Zhao Z., Keller R. K. 1994. Effect of squalene synthase inhibition on the expression of hepatic cholesterol biosynthetic enzymes, LDL receptor, and cholesterol 7 alpha hydroxylase. Arch. Biochem. Biophys. 311: 277–285. [DOI] [PubMed] [Google Scholar]
- 29.Nishimoto T., Amano Y., Tozawa R., Ishikawa E., Imura Y., Yukimasa H., Sugiyama Y. 2003. Lipid-lowering properties of TAK-475, a squalene synthase inhibitor, in vivo and in vitro. Br. J. Pharmacol. 139: 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amano Y., Nishimoto T., Tozawa R., Ishikawa E., Imura Y., Sugiyama Y. 2003. Lipid-lowering effects of TAK-475, a squalene synthase inhibitor, in animal models of familial hypercholesterolemia. Eur. J. Pharmacol. 466: 155–161. [DOI] [PubMed] [Google Scholar]
- 31.Okazaki H., Tazoe F., Okazaki S., Isoo N., Tsukamoto K., Sekiya M., Yahagi N., Iizuka Y., Ohashi K., Kitamine T., et al. 2006. Increased cholesterol biosynthesis and hypercholesterolemia in mice overexpressing squalene synthase in the liver. J. Lipid Res. 47: 1950–1958. [DOI] [PubMed] [Google Scholar]
- 32.Wiersma H., Nijstad N., Gautier T., Iqbal J., Kuipers F., Hussain M. M., Tietge U. J. 2010. Scavenger receptor BI facilitates hepatic very low density lipoprotein production in mice. J. Lipid Res. 51: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farese R. V., Jr, Ruland S. L., Flynn L. M., Stokowski R. P., Young S. G. 1995. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc. Natl. Acad. Sci. USA. 92: 1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye J., Li J. Z., Liu Y., Li X., Yang T., Ma X., Li Q., Yao Z., Li P. 2009. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 9: 177–190. [DOI] [PubMed] [Google Scholar]
- 35.Hiyoshi H., Yanagimachi M., Ito M., Yasuda N., Okada T., Ikuta H., Shinmyo D., Tanaka K., Kurusu N., Yoshida I., et al. 2003. Squalene synthase inhibitors suppress triglyceride biosynthesis through the farnesol pathway in rat hepatocytes. J. Lipid Res. 44: 128–135. [DOI] [PubMed] [Google Scholar]
- 36.Forman B. M., Ruan B., Chen J., Schroepfer G. J., Jr, Evans R. M. 1997. The orphan nuclear receptor LXRalpha is positively and negatively regulated by distinct products of mevalonate metabolism. Proc. Natl. Acad. Sci. USA. 94: 10588–10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomkins G. M., Chaikoff I. L. 1952. Cholesterol synthesis by liver. I. Influence of fasting and of diet. J. Biol. Chem. 196: 569–573. [PubMed] [Google Scholar]
- 38.Joo J. H., Jetten A. M. 2010. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett. 287: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wasko B. M., Smits J. P., Shull L. W., Wiemer D. F., Hohl R. J. 2011. A novel bisphosphonate inhibitor of squalene synthase combined with a statin or a nitrogenous bisphosphonate in vitro. J. Lipid Res. 52: 1957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.