Abstract
Pneumocystis jiroveci pneumonia (PCP) is frequently reported in lymphoma patients treated with rituximab-contained regimens. There is a trend toward a difference in PCP risk between bi- and tri-weekly regimens. The aims of this systemic review and meta-analysis were to estimate the risk for PCP in these patients, compare the impact of different regimens on the risk, and evaluate the efficacy of prophylaxis. The cohort studies with incept up to January 2014 were retrieved from the Cochrane Library, Medline, Embase, and Web of Science databases. Studies that compared the incidence of PCP in patients with and without rituximab treatment were conducted. Studies that reported the results of prophylaxis were concentrated to evaluate the efficacy of prophylaxis. Fixed effect Mantel-Haenszel model was chosen as the main analysis method. Funnel plots were examined to estimate the potential selection bias. Egger’s test and Begg’s test were used for the determination of possible small study bias. Eleven cohort studies that met the inclusion criteria were finally included. Results indicated that rituximab was associated with a significantly increased risk for PCP (28/942 vs 5/977; risk ratio: 3.65; 95% confidence interval 1.65 to 8.07; P=0.001), and no heterogeneity existed between different studies (I2=0%). Little significant difference in PCP risk was found between bi-weekly and tri-weekly regimens (risk ratio: 3.11; 95% confidence interval 0.92 to 10.52, P=0.068). PCP risk was inversely associated with prophylaxis in patients treated with rituximab (0/222 vs 26/986; risk ratio: 0.28; 95% confidence interval 0.09 to 0.94; P=0.039). In conclusion, PCP risk was increased significantly in lymphoma patients subjected to rituximab-contained chemotherapies. Difference in PCP risk between bi-weekly and tri-weekly regimens was not significant. Additionally, prophylaxis was dramatically effective in preventing PCP in rituximab-received lymphoma patients, suggesting that rituximab should be recommended for these patients.
Introduction
Pneumocystis jiroveci pneumonia (PCP) is an opportunistic infection which occurs in immunosuppressed patients such as those infected with the human immunodeficiency virus (HIV) [1]. In recent years, PCP has also been frequently reported in lymphoma patients treated with rituximab-contained regimens [2–4], and the increase of PCP in these patients was considered to be related to rituximab. Rituximab is a chimeric monoclonal antibody, which targets B cell-specific antigen CD20. It can reduce the number of B cells and remarkably enhance the efficacy of chemotherapy in non-Hodgkin lymphoma patients. Therefore, rituximab has been recommended as a first-line therapy for non-Hodgkin lymphoma since 2006 [5]. Along with the widespread application of rituximab, the incidence of PCP also increases rapidly [2–4]. Many studies show that the risk for PCP in patients with lymphoma increases with rituximab therapy [6–8]. The reported incidence rate in these patients could be as high as 10.04 to 13.04% [9,10]. Meanwhile, other studies claimed that rituximab was not a risk factor for PCP [11]. In contrast to recent reports, no PCP case was reported in a large-scale clinical trial of rituximab (n = 3,000) [12–16].
The clinical course of PCP in lymphoma patients subjected to rituximab can be quite fulminant with high mortality, which has been reported as high as 33.3% [17]. Sudden deaths have been reported in some patients given with anti-pneumocystis treatments [17,18]. In view of the increased incidence and potential fatality of PCP, the role of prophylaxis has been studied [19–22]. Prophylaxis was found to be highly efficient in preventing PCP [4,19,20] without the serious side effects of other anti-pneumocystis drugs [20]. Therefore, prophylaxis is strongly recommended for patients receiving therapies with rituximab [4,19,20]. Some researchers have argued against the use of universal prophylaxis since the incidence of PCP was not remarkably high and the use of the anti-pneumocystis drug, trimethoprim-suffamethoxazole (TMP/SMZ) might cause bone marrow suppression [22].
Therefore, it is still unclear whether prophylaxis should be recommended in lymphoma patients subjected to rituximab. There is also a need to study the risk of PCP associated with rituximab treatment and the exact incidence rate of PCP. Herein, we performed a systemic review and meta-analysis on clinical trial data to address these issues.
Methods
In Mar 2013, we reported two cases of patient with non-Hodgkin disease who developed PCP during rituximab-contained chemotherapy and reviewed related literature concentrating on the incidence of PCP in these patients [3]. We found that the incidence of PCP and the use of prophylaxis remained controversial. We also failed to find any randomized controlled trials for this query. Further discussion of the review design and protocol took place during the third quarter of 2013, based on the meta-analytical techniques to evaluate the correlation between rituximab and PCP in lymphoma patients. Final consensus on the protocol was reached at the end of December 2013, and the performance of this review began in January 2014. Meta-analyses of observational studies were performed following the standard criteria [23]. The study was approved by the independent ethics committee (IEC) of Anhui Provincial Hospital (No2013045).
Literature Search
We searched the Cochrane library, Medline, Web of Science, and Embase electronic databases from inception to January 2014 for relevant articles. Since PCP was an infrequent complication, which may not be the main purpose of the studies, a broad eligibility strategy was used to capture all potentially relevant data by using the following search string: “rituximab” and “pneumocystis pneumonia”. References of included studies were manually inspected for more trials. Standard guidelines for conducting and reporting meta-analyses of observational studies were followed. No language or publication restrictions were applied.
Study selection
Two reviewers independently screened the titles and abstracts of all possible relevant articles after removal of duplicates. Then, articles that were clearly not relevant-such as editorials, reviews, and single case study reports were removed. Potentially relevant articles were obtained in full text and read independently by two reviewers to determine the eligibility for inclusion. Trial inclusion criteria mandated the incidence of pneumocystis infection as an outcome. Only cohort studies that presented the numbers of PCP case in lymphoma patients treated with and without rituximab were included. For analyses of the effectiveness of prophylaxis, cohort studies that reported the results of prophylaxis were concentrated. As the incidence rate of PCP in lymphoma patients treated with rituximab was the focus of this review and there was immunological abnormality in patients with HIV infection, we excluded studies if, in which, the patient had concomitant HIV infection. We obtained copies of all articles identified as being potentially relevant, including contacting authors as necessary.
Data extraction
Two reviewers independently extracted the relevant characteristics and outcomes from eligible trials. Extracted data were directly imported to Microsoft excel sheets, which included predefined fields set up to capture aspects of study design and quality as well as all results (PCP case and total patient numbers, chemotherapy regimens, chemotherapy cycles). We also extracted additional data about age, sex, diagnostic methods, white blood cell count numbers, the onset time of PCP after rituximab treatment, pharmaceuticals used for prophylaxis, and outcome of treatment. When necessary, we contacted the corresponding authors for details on specific aspects of the data. For those that did not give the numbers of PCP cases and total patients directly, we calculated that from the original tables or article contents. Methodological quality of studies was evaluated using the Newcastle-Ottawa scale (NOS) for assessing non-randomized studies used in meta-analyses [24], as suggested by the Cochrane handbook of systematic reviews and meta-analyses [25]. Disagreements were resolved after rechecking the source articles and further discussion among the reviewers.
Statistical analysis
Software Stata 11.0 (Stata Corporation, College Station, TX) was used to conduct meta-analysis. Risk ratio (RR) with 95% confidence interval was calculated. Heterogeneity between studies was tested using the I 2 statistic [26,27]. Fixed effect Mantel-Haenszel model was chosen as the main analysis method when the heterogeneities were confirmed not statistically significant. Otherwise, random-effect model would be used and sensitivity analysis performed. Funnel plots were examined visually to estimate potential selection bias (publication or other factors). Egger’s test and Begg’s test were used for determine possible small study bias. Peto odds ratio (OR) analysis was also conducted and compared with Mantel-Haenszel model, because it can provide the best confidence interval coverage and be more powerful in dealing with low event rates in such circumstances compared with the validity of fixed effect assumption [28]. All the P values were two-sided, and statistical significance was defined at the 0.05 level.
Results
Literature search
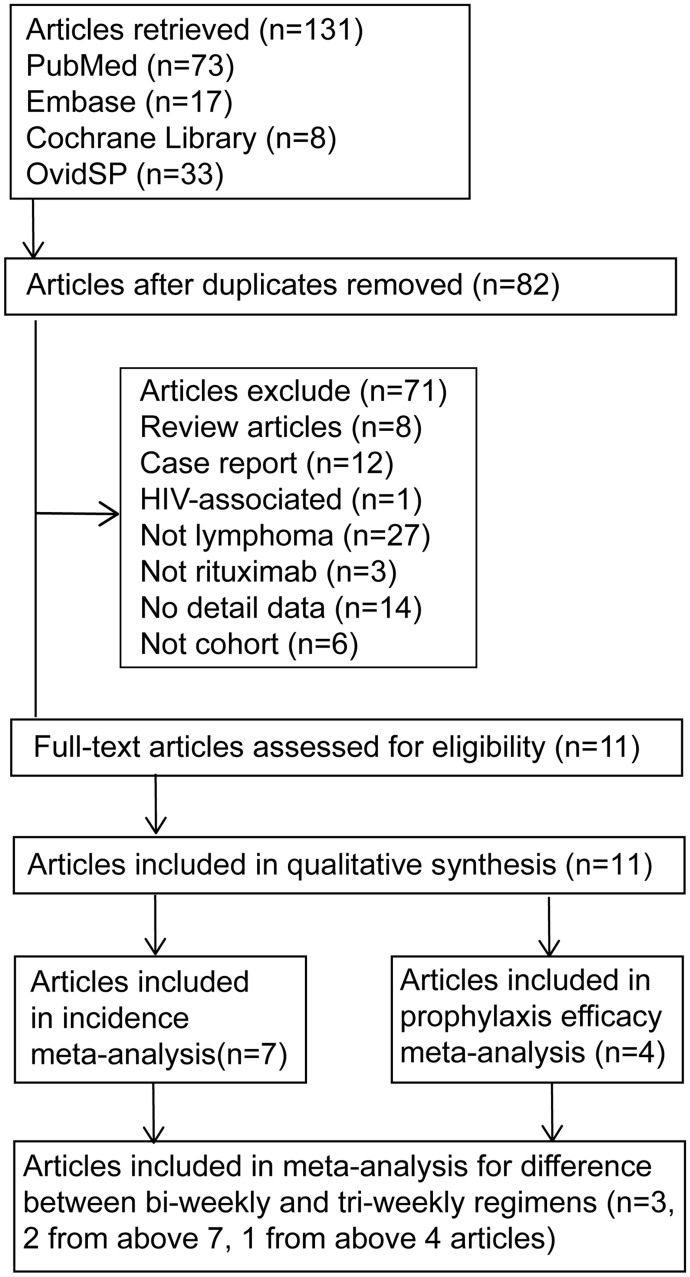
The flow diagram of article selection is shown in Fig 1. Initially, 131 articles were found. After removal of the duplicates, 82 articles were identified. Further, 71 studies were excluded based on our exclusion criteria. Of those excluded studies, there were eight reviews [29–36], six studies recruited patients with nephrotic syndrome [37,38], renal transplantation [37–42], three with other solid organ transplantation [43–45], six with systemic lupus erythematosus [46–51], seven with chronic lymphocytic leukemia [52–58], two with antibody autoimmune haemolytic anemia [59,60], two with macroglobulinemia [61,62], and one with Wegener’s granulomatosis [63]. Three studies in which rituximab was not used [64–66], 12 case reports [2–4,38,67–74], and six non cohort trials were also excluded [9,18,75–78]. Eleven studies were included for further investigation. Eventually, seven cohort studies that met the inclusion criteria were chosen for meta-analysis to compare PCP risk in patients with and without rituximab. One of the seven studies contained four groups of patients treated with different chemotherapy regimens and intervals. We treated this study as four trials. For one of the four trials presented no PCP case in neither of the two groups, it was excluded in meta-analysis [10]. Eventually, nine trials were included for meta-analysis. Three trials [10,17,20] that represented the occurrence of PCP in patients received R-CHOP tri-weekly (R-CHOP-21) and bi-weekly (R-CHOP-14) were included to assess the affect of different chemo-regimens and intervals on PCP risk. In addition, four articles [19–22] containing five trials that presented the results of prophylaxis were included for meta-analysis to evaluate the efficacy of prophylaxis.
Fig 1. Flow diagram of identification process for eligible studies.
Study characteristics
Seven cohort studies included 1919 participants, of whom 942 were treated with rituximab and 977 treated without rituximab. The characteristics of these trials are shown in Table 1. Six of the seven studies were from Asia. The other one was from Norway, in which, the authors reported four groups of patients treated with different regimens [10]. Six trials enrolled patients with non-Hodgkin lymphoma of miscellaneous types [6,7,10,11,17,79], while one study enrolled patients with diffuse large B cell lymphoma merely [80]. The cohort size of studies ranged from 100 to 529 cases. In diagnostic methods for PCP, PCR was used in three studies [6,7,10], microscopy examination in two studies [11,80], β-glucan detection in two studies [6,17], and direct fluorescentantibody assay used in one study [79]. Rituximab-added chemotherapy tri-weekly was administered in four studies and bi-weekly in two others. One study which included both bi-weekly and tri-weekly regimens was treated as two trials in meta-analysis [10]. In meta-analysis to assess the difference between bi-weekly and tri-weekly rituximab-added regimens in PCP incidence, three studies [10,17,20] were concluded. One of the studies that included CHOP and cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisolone (CHOEP) regimens was treated as two trials, Table 2. Four articles containing five trials were included in meta-analysis to determine the efficacy of prophylaxis [19–22]. The characteristics of these studies were shown in Table 3.
Table 1. Characteristics of cohort studies included in PCP risk meta-analysis for patients with and without rituximab.
| Author(year) | Country | Disease | Cohort size | Diagnostic method | Chemo-regimen | Chemo-cycle (day) | Onset time of PCP | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Cured | died | ||||||||
| Ennishi (2008) | Japan | NHL | 195 | PCR | (R-)CHOP | NG | 60–120d | 2 | 0 |
| Huang (2011) | China | DLBCL | 529 | microscopy | (R-)CHOP | 21 | 4(1–7)cycle | NG | |
| Kato (2011) | Japan | NHL | 103 | microscopy | (R-)CHOP | NG | 212d | 1 | 0 |
| Katsuya (2009) | Japan | NHL | 188 | PCR/β-glucan | (R-)CHOP | 21/14 | 4,6,7cycle | 2 | 1 |
| Kolstad a (2007) | Norway | NHL | 71 | PCR | (R-)CHOEP | 14 | NG | 6 | 0 |
| Kolstad b (2007) | Norway | NHL | 417 | PCR | (R-)CHOP | 21 | NG | 2 | 0 |
| Kolstad c (2007) | Norway | NHL | 81 | PCR | (R-)CHOP | 14 | NG | 2 | 0 |
| Kurokawa (2010) | Japan | NHL | 235 | PCR/β-glucan | (R-)CHOP | NG | 2,3,4,4,5cycle | 4 | 1 |
| Lim (2010) | Korea | NHL | 100 | DFA | (R-)CHOP | 21 | 27–117d | NG | |
DFA: direct fluorescent antibody assay; DLBCL: diffuse large B cell lymphoma; ND: not do; NG: not given; NHL: non-Hodgkin’s lymphoma; NOS: Newcastle-Ottawa quality assessment scale; PCR: polymerase chain reaction; R-CHOP: rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CHOEP: rituximab plus cyclophosphamide, doxorubicin, vincristine, prednisone and etoposide
Table 2. Characteristics of cohort studies reporting PCP occurrence in bi-weekly and tri-weekly rituximab-contained therapies.
| Author (year) | Country | Disease category | Cohort size | Diagnostic method | Regimens | No of pts in therapy | Onset time of PCP | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| bi-weekly | tri-weekly | Cured | det | died | |||||||
| Katsuya (2009) | Japan | NHL | 129 | PCR/β-glucan | R-CHOP | 2 | 127 | 4,6,7cycle | 2 | 0 | 1 |
| Kolstad a (2007) | Norway | NHL | 55 | PCR | R-CHOEP | 46 | 9 | NG | 6 | 0 | 0 |
| Kolstad b (2007) | Norway | NHL | 46 | PCR | R-CHOP | 32 | 14 | NG | 4 | 0 | 0 |
| Hardak (2012) | Israel | DLBCL | 132 | PCR | R-CHOP | 85 | 47 | 2,5,5,6,6cycle | 0 | 3 | 2 |
det: deteriorated; DLBCL: diffuse large B cell lymphoma; NHL: non-Hodgkin’s lymphoma; NG: not given; NOS: Newcastle-Ottawa quality assessment scale; pts: patients. PCR: polymerase chain reaction; R-CHO(E)P: rituximab plus cyclophosphamide, doxorubicin, vincristine, (etoposide), prednisone
Table 3. Characteristics of studies included for meta-analysis to determine the efficacy of prophylaxis.
| Author (year) | Country | Disease | Cohort size | Diagnostic method | Regimens | Chemo-cycle (day) | Onset time of PCP | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cured | det | died | ||||||||
| Haeusler (2013) | Australia | NHL | 66 | PCR | FCR | 28 | 3–6cycle | 1 | 0 | 0 |
| Hardak a (2012) | Israel | DLBCL | 85 | PCR | R-CHOP | 14 | 2,5,5,6,6cycle | NG | ||
| Hardak b (2012) | Israel | DLBCL | 47 | PCR | R-CHOP | 21 | 2,5,5,6,6cycle | NG | ||
| Hashimoto (2010) | Japan | NHL | 297 | PCR/CT/β-glucan | R-CHOP | 14 | 2,2,4,4,5,5cycle | NG | ||
| Kim (2012) | Korea | NHL | 713 | IF+imageology | R-CHOP | 21 | NG | 1 | 12 | 1 |
CT: computed tomography; det: deteriorated; DLBCL: diffuse large B cell lymphoma; FCR: fludarabine, cyclophosphamide and rituximab; IF: immunofluorescence, NG: not given; NHL: non-Hodgkin’s lymphoma; NOS: Newcastle-Ottawa quality assessment scale; PCR: polymerase chain reaction; R-CHOP: rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone
Study quality
The quality of trials included for meta-analysis was satisfactory and most trials were judged to be at low risk of bias. Out of seven studies, five studies had medium to high NOS scores, and the other two had medium to low scores: one article did not give the information of grouping method [80] and the other lacked sufficient information to determine an overall score (presented the results table only) [10]. Removal of studies with medium to low NOS scores did not greatly change the summary estimate. Two studies in meta-analysis for assessing the difference between bi-weekly and tri-weekly chemotherapies were scaled as high quality [17,20] while the other study could not be scaled [10]. Four studies [19–22] including five trials for meta-analysis to evaluate the efficacy of prophylaxis had high quality NOS scores too.
Incidence rate of PCP
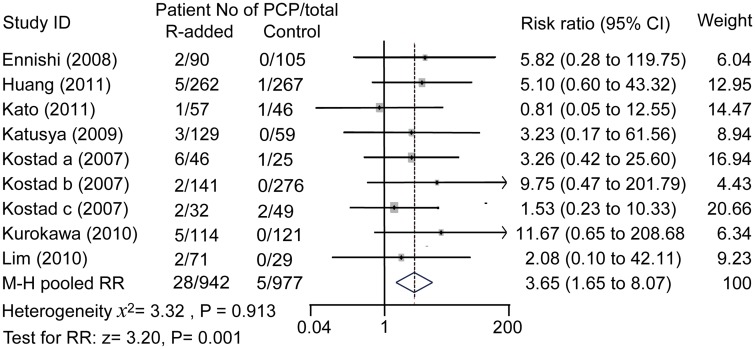
Results showed that the incidences of PCP in lymphoma patients with rituximab-contained regimens were higher than that in those without rituximab-contained regimens (28/942 vs 5/977). Forest figure results showed that patients subjected to rituximab had a higher risk of PCP (RR: 3.65, 95% confidence interval: 1.65 to 8.07; P = 0.001), and there was no heterogeneity between studies (I 2 = 0%, χ 2 = 3.32; P = 0.913, Fig 2).
Fig 2. Effect of rituximab treatment on PCP risk.
M-H pooled risk ratio = 3.65, fixed effect model method. R: rituximab. Rituximab increased the risk for PCP in lymphoma patients significantly.
Difference between bi-weekly and tri-weekly regimens
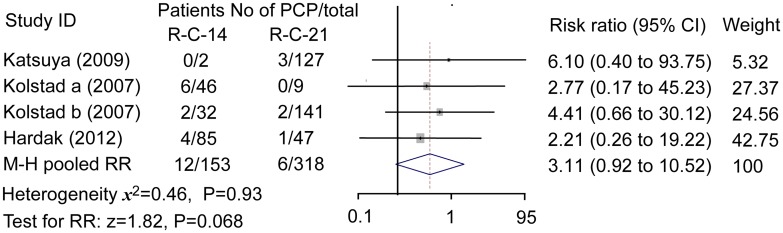
Data of three studies containing four trials were meta-analyzed. The incidence of PCP in patients treated with bi-weekly regimen seemed higher than that with tri-weekly regimen (12/165 vs 6/324). Fixed effect model showed M-H pooled RR was 3.11 (95% confidence interval 0.92 to 10.52, P = 0.068), which did not indicated a statistically significant difference between the two regimens although a tendency seemed existed, Fig 3.
Fig 3. PCP risk in bi-weekly and tri-weekly regimens.
M-H pooled risk ratio = 3.11; fixed effect model method. R-C-14: rituximab-added chemotherapy bi-weekly; R-C-21: rituximab-added chemotherapy tri-weekly. Patients treated with bi-weekly regimen seemed to have a higher risk for PCP but the difference between the two regimens was not statistically significant.
Effectiveness of prophylaxis
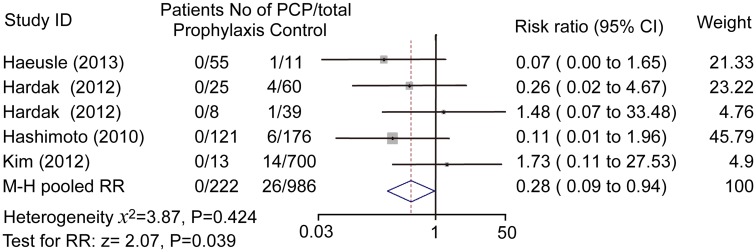
Prophylaxis was effective as there are remarkable differences between patients with and without prophylaxis (0/222 vs 26/986). Pooled estimate demonstrated a statistically difference in the two groups (RR: 0.28; 95% confidence interval 0.09 to 0.94; P = 0.039), Fig 4. Pooled odds ratio was 0.17 (95% confidence interval 0.05 to 0.55, P = 0.003), which was consistent to the result from Mantel-Haenszel fixed effect model.
Fig 4. Effect of prophylaxis on PCP risk in rituximab-received lymphoma patients.
M-H pooled risk ratio = 0.28, fixed effect model method. Prophylaxis dramatically reduced PCP risk in rituximab-received patients.
Heterogeneity and sensitivity
Evidence of statistical heterogeneity was lacking between the included cohort studies. Heterogeneity chi-square = 3.32, P = 0.913. I-squared (variation in RR attributable to heterogeneity) = 0%. Sensitivity analysis indicated that omission of any studies did not affect the pooled estimate significantly. No heterogeneity was found between bi-weekly and tri-weekly regimens (Heterogeneity chi-squared = 0.46, P = 0.927; I-squared = 0.0%), or received prophylaxis or not (Heterogeneity chi-squared = 3.87; P = 0.424; I-squared = 0.0%). We did not perform sensitivity analysis in the latter two meta-analyses due to their limited number of included trials.
Publication bias
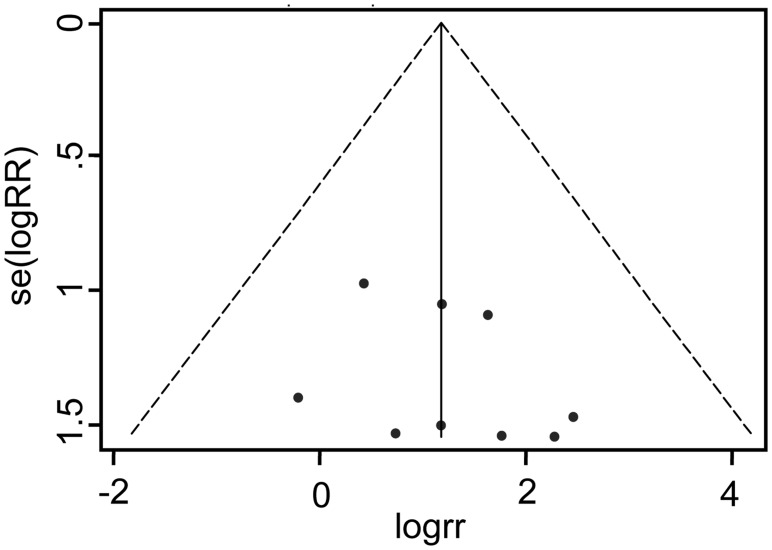
Funnel plot did not show a significant publication bias among cohort studies that presented PCP incidence in patients with or without rituximab treatment, Fig 5. Furthermore, no indication of publication bias was found in Egger's test (t = 0.95, P = 0.347) and Begg's test(z = 0.94, P = 0.348, continuity corrected)either. Similarly, no significant difference was found among the studies included for meta-analysis of bi-weekly or tri-weekly therapy and prophylaxis.
Fig 5. Funnel plot (with pseudo 95% confidence limits) for rituximab on PCP risk.
Discussion
This meta-analysis, based on data of cohort trials, demonstrated that rituximab was associated with a significant increase of PCP risk in lymphoma patients. Based on our analysis, no difference was found in PCP risk between different regimens; meanwhile, anti-pneumocystis prophylaxis was effective in these patients. There was no evidence of methodological heterogeneity or substantial evidence of publication bias.
The incidence of PCP in lymphoma patients subjected to rituximab treatment was a concern and warranted in daily practice. Rituximab can improve the efficacy of CHOP-like therapy by affecting B cells. However, as B cells play a vital role in generation of CD4+ T cells for defending Pneumocystis jiroveci infection in the lungs [81], reduction of B cells could lead to insufficient generation of CD4+ T cells and subsequently higher risk for Pneumocystis jiroveci infection. Rituximab has been reported to be associated with PCP in a chronic childhood autoimmune hemolytic anemia patient [60], and suspected to increase risk for PCP in lymphoma patients [2–4,8,68,69]. However, the correlation between PCP and rituximab and the exact incidence of PCP remain controversial. For example, one study claimed no correlation between rituximab and the increase of PCP [11], while others indicated a notable increase of PCP cases and presumed this increase was correlated with rituximab. Unsatisfactorily, many of these studies were case reports rather than randomized controlled trials or cohort trials. Therefore, the exact incidence of PCP remained under debate. In our meta-analysis of cohort trials, a significant increase of PCP risk was confirmed.
Many factors could affect the occurrence of PCP, such as administration of corticosteroids [59,79], shorter intervals of chemotherapy [10,17,20], and decrease of white blood cells [17,18] especially CD4+ lymphocytes [18,19,80]. Patients treated with bi-weekly regimen were reported to have a higher risk of PCP [20]. In our meta-analysis, a higher risk for PCP in patients treated with biweekly regimen was not established. However, bi-weekly and tri-weekly regimens are both routine therapies for lymphoma patients in daily practice. Due to the limited number of studies in our meta-analysis, further studies with large number of patients are needed to compare the difference between the two regimens.
There is also a need to investigate the role of prophylaxis since there are divergent views on whether anti-pneumocystis prophylaxis is necessary for patients subjected to rituximab-added chemotherapy. In view of the increased incidence and fatality of PCP, some researchers administered prophylaxis [9,10,20–22,76,78] and recommended it as a routine use [4,9,10,18,20]. It was demonstrated that with adequate prophylaxis, very few PCP occurred in those patients, and no adverse reactions from TMP-SMX were observed [6], suggesting the efficacy and safety of prophylaxis. Our meta-analysis also showed that pooled RR of PCP was reduced when prophylaxis was given. Similarly, a previous systematic review reported the efficacy of prophylaxis for PCP in immunocompromised non—HIV-infected patients [35]. Twelve randomized trials, which included 1245 patients who had undergone autologous bone marrow or solid organ transplant or had hematologic cancer, were included in the review. When TMP/SMZ was administered, the occurrence of PCP was reduced by 91%. These results were consistent with that of our meta-analysis although the patient populations were different.
Strengths of the study
To our knowledge, this is the first meta-study that covers the available evidence and performs a quantitative estimate of the impact of rituximab on the risk for PCP in lymphoma patients. The strengths of this meta-analysis lay in the broad search from multiple online databases, covering published literature from inception to newly updated, the high sensitivity terms that we used to obtain literature, and further hand searching combined with checking reference lists of included studies. We also contacted authors when necessary and carefully calculated data from articles when the data were not provided directly. Heterogeneity between studies was not obvious which indicated consistency in these studies. Besides, we did not find considerable evidence of publication bias, although this cannot be completely excluded. Our meta-analysis is robust based on the analysis from the funnel plot, Egger’s test and Begg’s test.
In meta-analysis for effectiveness of prophylaxis, RR and OR values were both calculated and compared. In contrast to the Mantel-Haenszel model, Peto method was more sensitive in estimating the true strength of the association for an event of low occurrence rate. As PCP is an opportunistic infection and the incidence might be lowered when prophylaxis was administered, we generated Peto method with OR rather than fixed effect model with RR in this meta-analysis, to improve the quality of our analysis.
Limitations of the review
Nevertheless, there were some limitations in our analysis due to the varied quality of retrieved articles. The included trials were all cohort studies, which are not as robust as randomized controlled trials. This might lead to some biases, particularly selection and attrition biases. In addition, there are differences in patient populations, sample size, number of patients in rituximab-added groups versus controls, chemotherapy cycles, intervals of therapy, length of follow up, and diagnostic methods for PCP among different studies. We could not analyze the importance of other possible factors in great depth either because of the lack of the details. The number of trials included to assess the difference between bi-weekly and tri-weekly regimens, and the effectiveness of prophylaxis was also limited. The estimates might not be precise owing to the fairly low event rate of PCP. To further assess PCP risk in different therapies such as bi-weekly or tri-weekly regimens and guide the duration of prophylaxis, further studies are needed.
Conclusions
Our meta-analysis demonstrated that the risk for PCP was associated with rituximab. Patients with lymphoma subjected to rituximab-added therapy had an increased risk of PCP. No difference was observed between bi-weekly and tri-weekly chemotherapies in PCP risk based on the limited number of studies included in our analysis. Prophylaxis was very effective in preventing this life-threatening complication. Therefore, clinicians should be more vigilant in view of the increased risk of PCP and prophylaxis should be recommended as a routine practice.
Supporting Information
(DOC)
Acknowledgments
We would like to thank Dr. Lim Keng Gat and Dr. Yufeng Chen from Cancer Therapeutics & Stratified Oncology, Genome Institute of Singapore, Agency for Science, Technology and Research (A-Star), Singapore, for their kind instructions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Taylor SM, Meshnick SR, Worodria W, Andama A, Cattamanchi A, Davis JL, et al. Low prevalence of Pneumocystis pneumonia (PCP) but high prevalence of pneumocystis dihydropteroate synthase (dhps) gene mutations in HIV-infected persons in Uganda. PLoS One. 2012; 7(11):e49991 10.1371/journal.pone.0049991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin-Garrido I, Carmona EM, Specks U, Limper AH. Pneumocystis pneumonia in patients treated with rituximab. Chest. 2013. July; 144(1):258–65. 10.1378/chest.12-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang XQ, Fang L, Mei XD, Wang XJ, Bao MH. Pneumocystis jiroveci pneumonia in patients with non-Hodgkin's lymphoma after Rituximab-containing regimen: two cases of report and literature review. J Thorac Dis. 2013. August; 5(4):E162–6. 10.3978/j.issn.2072-1439.2013.08.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venhuizen AC, Hustinx WN, van Houte AJ, Veth G, van der Griend R. Three cases of Pneumocystis jirovecii pneumonia (PCP) during first-line treatment with rituximab in combination with CHOP-14 for aggressive B-cell non-Hodgkin's lymphoma. Eur J Haematol. 2008. March; 80(3):275–6. . [DOI] [PubMed] [Google Scholar]
- 5. Zelenetz AD, Advani RH, Buadi F, Cabanillas F, Caligiuri MA, Czuczman MS, et al. Non-Hodgkin's lymphoma. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006. March; 4(3):258–310. . [DOI] [PubMed] [Google Scholar]
- 6. Kurokawa T, Kaya H, Yoshida T. Two cases of Pneumocystis jiroveci pneumonia with non-Hodgkin's lymphoma after CHOP-based chemotherapy containing rituximab. J Clin Exp Hematop. 2010; 50(2):159–62. . [DOI] [PubMed] [Google Scholar]
- 7. Ennishi D, Terui Y, Yokoyama M, Mishima Y, Takahashi S, Takeuchi K, et al. Increased incidence of interstitial pneumonia by CHOP combined with rituximab. Int J Hematol. 2008. May; 87(4):393–7. 10.1007/s12185-008-0066-7 [DOI] [PubMed] [Google Scholar]
- 8. Tam C, Seymour JF, Brown M, Campbell P, Scarlett J, Underhill C, et al. Early and late infectious consequences of adding rituximab to fludarabine and cyclophosphamide in patients with indolent lymphoid malignancies. Haematologica. 2005. May; 90(5):700–2. . [PubMed] [Google Scholar]
- 9. Kamel S, O'Connor S, Lee N, Filshie R, Nandurkar H, Tam CS. High incidence of Pneumocystis jirovecii pneumonia in patients receiving biweekly rituximab and cyclophosphamide, adriamycin, vincristine, and prednisone. Leuk Lymphoma. 2010. May; 51(5):797–801. 10.3109/10428191003699860 [DOI] [PubMed] [Google Scholar]
- 10. Kolstad A, Holte H, Fossa A, Lauritzsen GG, Gaustad P, Torfoss D. Pneumocystic jirovecii pneumonia in B-cell lymphoma patients treated with the rituximab-CHOEP-14 regimen. haematologica. 2007; 92:139–40. [DOI] [PubMed] [Google Scholar]
- 11. Kato H, Yamamoto K, Taji H, Oki Y, Chihara D, Seto M, et al. Interstitial pneumonia after autologous hematopoietic stem cell transplantation in B-cell non-hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2011. December; 11(6):483–9. 10.1016/j.clml.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 12. Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008. February; 9(2):105–16. 10.1016/S1470-2045(08)70002-0 [DOI] [PubMed] [Google Scholar]
- 13. Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006. May; 7(5):379–91. . [DOI] [PubMed] [Google Scholar]
- 14. Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006. July 1; 24(19):3121–7. . [DOI] [PubMed] [Google Scholar]
- 15. Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rube C, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004. August 1; 104(3):634–41. . [DOI] [PubMed] [Google Scholar]
- 16. Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993. April 8; 328(14):1002–6. . [DOI] [PubMed] [Google Scholar]
- 17. Katsuya H, Suzumiya J, Sasaki H, Ishitsuka K, Shibata T, Takamatsu Y, et al. Addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy has a high risk of developing interstitial pneumonia in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2009. November; 50(11):1818–23. 10.3109/10428190903258780 [DOI] [PubMed] [Google Scholar]
- 18. Kurokawa T, Hase M, Tokuman N, Yoshida T. Immune reconstitution of B-cell lymphoma patients receiving CHOP-based chemotherapy containing rituximab. Hematol Oncol. 2011. March; 29(1):5–9. 10.1002/hon.947 [DOI] [PubMed] [Google Scholar]
- 19. Haeusler GM, Slavin MA, Seymour JF, Lingaratnam S, Teh BW, Tam CS, et al. Late-onset Pneumocystis jirovecii pneumonia post-fludarabine, cyclophosphamide and rituximab: implications for prophylaxis. Eur J Haematol. 2013. August; 91(2):157–63. 10.1111/ejh.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hardak E, Oren I, Dann EJ, Yigla M, Faibish T, Rowe JM, et al. The increased risk for pneumocystis pneumonia in patients receiving rituximab-CHOP-14 can be prevented by the administration of trimethoprim/sulfamethoxazole: a single-center experience. Acta Haematol. 2012; 127(2):110–4. 10.1159/000334113 [DOI] [PubMed] [Google Scholar]
- 21. Hashimoto K, Kobayashi Y, Asakura Y, Mori M, Azuma T, Maruyama D, et al. Pneumocystis jiroveci pneumonia in relation to CD4+ lymphocyte count in patients with B-cell non-Hodgkin lymphoma treated with chemotherapy. Leuk Lymphoma. 2010. October; 51(10):1816–21. 10.3109/10428194.2010.506569 [DOI] [PubMed] [Google Scholar]
- 22. Kim T, Choi SH, Kim SH, Jeong JY, Woo JH, Kim YS, et al. Point prevalence of Pneumocystis pneumonia in patients with non-Hodgkin lymphoma according to the number of cycles of R-CHOP chemotherapy. Ann Hematol. 2012. January; 92(2):231–8. 10.1007/s00277-012-1592-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000. April 19; 283(15):2008–12. . [DOI] [PubMed] [Google Scholar]
- 24. Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ohri website. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2015 Feb 22. [Google Scholar]
- 25. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1.0. Cochrane Collaboration. 2011. [Google Scholar]
- 26. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003. September 6; 327(7414):557–60. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. British Medical Journal. 2007. November 3; 335(7626):914–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007. January 15; 26(1):53–77. . [DOI] [PubMed] [Google Scholar]
- 29. White DA. Drug-induced pulmonary infection. Clin Chest Med. 2004. March; 25(1):179–87. . [DOI] [PubMed] [Google Scholar]
- 30. Ram R, Ben-Bassat I, Shpilberg O, Polliack A, Raanani P. The late adverse events of rituximab therapy—rare but there! Leuk Lymphoma. 2009. July; 50(7):1083–95. 10.1080/10428190902934944 [DOI] [PubMed] [Google Scholar]
- 31. Tadmor T, McLaughlin P, Polliack A. A resurgence of Pneumocystis in aggressive lymphoma treated with R-CHOP-14: the price of a dose-dense regimen? Leuk Lymphoma. 2010. May; 51(5):737–8. 10.3109/10428191003715377 [DOI] [PubMed] [Google Scholar]
- 32. Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010. April; 47(2):187–98. 10.1053/j.seminhematol.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 33. Morrison VA. Infectious complications of chronic lymphocytic leukaemia: pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol. 2010. March; 23(1):145–53. 10.1016/j.beha.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 34. Kelesidis T, Daikos G, Boumpas D, Tsiodras S. Does rituximab increase the incidence of infectious complications? A narrative review. Int J Infect Dis. 2011. January; 15(1):e2–16. 10.1016/j.ijid.2010.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007. September; 82(9):1052–9. . [DOI] [PubMed] [Google Scholar]
- 36. Cornely OA, Ullmann AJ, Karthaus M. [Opportunistic infections after treatment with monoclonal antibodies]. Wien Med Wochenschr. 2004. May; 154(9–10):209–17. . [DOI] [PubMed] [Google Scholar]
- 37. Sato M, Ito S, Ogura M, Kamei K, Miyairi I, Miyata I, et al. Atypical Pneumocystis jiroveci pneumonia with multiple nodular granulomas after rituximab for refractory nephrotic syndrome. Pediatr Nephrol. 2013. January; 28(1):145–9. 10.1007/s00467-012-2286-6 [DOI] [PubMed] [Google Scholar]
- 38. Czarniak P, Zaluska-Lesniewska I, Zagozdzon I, Zurowska A. Difficulties in diagnosing severe Pneumocystis jiroveci pneumonia after rituximab therapy for steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2013. June; 28(6):987–8. 10.1007/s00467-013-2457-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boothpur R, Brennan DC. Didactic lessons from the serum lactate dehydrogenase posttransplant: a clinical vignette. Am J Transplant. 2008. April; 8(4):862–5. 10.1111/j.1600-6143.2008.02151.x [DOI] [PubMed] [Google Scholar]
- 40. Shelton E, Yong M, Cohney S. Late onset Pneumocystis pneumonia in patients receiving rituximab for humoral renal transplant rejection. Nephrology (Carlton). 2009. October; 14(7):696–9. 10.1111/j.1440-1797.2009.01168.x [DOI] [PubMed] [Google Scholar]
- 41. Kim MG, Kim YJ, Kwon HY, Park HC, Koo TY, Jeong JC, et al. Outcomes of Combination Therapy for Chronic Antibody-Mediated Rejection in Renal Transplantation. Nephrology (Carlton). 2013. September 5 . [DOI] [PubMed] [Google Scholar]
- 42. Kumar D, Gourishankar S, Mueller T, Cockfield S, Weinkauf J, Vethanayagam D, et al. Pneumocystis jirovecii pneumonia after rituximab therapy for antibody-mediated rejection in a renal transplant recipient. Transpl Infect Dis. 2009. April; 11(2):167–70. 10.1111/j.1399-3062.2008.00345.x [DOI] [PubMed] [Google Scholar]
- 43. Lamba M, Jabi M, Padmore R, Sengar DP, Veinot JP. Isolated pleural PTLD after cardiac transplantation. Cardiovasc Pathol. 2002. Nov-Dec; 11(6):346–50. . [DOI] [PubMed] [Google Scholar]
- 44. Perez-Ordono L, Hoyo I, Sanclemente G, Ricart MJ, Cofan F, Perez-Villa F, et al. Late-onset Pneumocystis jirovecii pneumonia in solid organ transplant recipients. Transpl Infect Dis. 2014. January 24 . [DOI] [PubMed] [Google Scholar]
- 45. Takeda K, Morioka D, Kumamoto T, Matsuo K, Tanaka K, Endo I, et al. A survival case of ABO-incompatible liver transplantation complicated with severe preoperative infection and subsequent overwhelming postsplenectomy infection. Transplant Proc. 2009. November; 41(9):3941–4. 10.1016/j.transproceed.2009.02.094 [DOI] [PubMed] [Google Scholar]
- 46. Tsai M-J, Tsai M-J, Chou C-W, Lin F-C, Chang S-C. Pneumocystis jiroveci pneumonia in patients with systemic lupus erythematosus after rituximab therapy. Lupus. 2013; 21:914–8. [DOI] [PubMed] [Google Scholar]
- 47. Teichmann LL, Woenckhaus M, Vogel C, Salzberger B, Scholmerich J, Fleck M. Fatal Pneumocystis pneumonia following rituximab administration for rheumatoid arthritis. Rheumatology (Oxford). 2008. August; 47(8):1256–7. 10.1093/rheumatology/ken234 [DOI] [PubMed] [Google Scholar]
- 48. Tsai MJ, Chou CW, Lin FC, Chang SC. Pneumocystis jiroveci pneumonia in patients with systemic lupus erythematosus after rituximab therapy. Lupus. 2012. July; 21(8):914–8. 10.1177/0961203312436855 [DOI] [PubMed] [Google Scholar]
- 49. Bonilla-Abadia F, Betancurt JF, Pineda JC, Velez JD, Tobon GJ, Canas CA. Pneumocystis jirovecii pneumonia in two patients with systemic lupus erythematosus after rituximab therapy. Clin Rheumatol. 2014. January 9 . [DOI] [PubMed] [Google Scholar]
- 50. Su GX, Wu FQ, Wang F, Zhou ZX, Huang XL, Lu J. [Rituximab therapy for severe pediatric systemic lupus erythematosus]. Zhonghua Er Ke Za Zhi. 2012. September; 50(9):697–704. . [PubMed] [Google Scholar]
- 51. Otremba MD, Adam SI, Price CC, Hohuan D, Kveton JF. Use of intravenous immunoglobulin to treat chronic bilateral otomastoiditis in the setting of rituximab induced hypogammaglobulinemia. Am J Otolaryngol. 2012. Sep-Oct; 33(5):619–22. 10.1016/j.amjoto.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 52. Badoux XC, Keating MJ, O'Brien S, Wierda WG, Faderl S, Estrov Z, et al. Final Analysis of a Phase 2 Study of Lenalidomide and Rituximab in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL). 21 ed pp. 448 2011. [Google Scholar]
- 53. Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S, et al. Cyclophosphamide, fludarabine, alemtuzumab, and rituximab as salvage therapy for heavily pretreated patients with chronic lymphocytic leukemia. Blood. 2011. August 25; 118(8):2085–93. 10.1182/blood-2011-03-341032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Conte GF, Aravena PC, Fardella PD, Alfaro JI, Araos DM, Flores CA, et al. Fludarabine as Therapy in Chronic Lymphocytic Leukemia (CLL) in Patients over 70 Years. 11 ed pp. Abstract-No, 5009. 2002. [Google Scholar]
- 55. Egle A, Steurer M, Melchardt T, Stoll M, Greil R The REVLIRIT CLL5 AGMT Study—a Phase I/II Trial Combining Fludarabine/Rituximab with Escalating Doses of Lenalidomide Followed by Rituximab/Lenalidomide in Untreated Chronic Lymphocytic Leukemia (CLL): Results of a Planned Interim Analysis. 22 ed pp. 1341–2. 2009. [Google Scholar]
- 56. Lin TS, Donohue KA, Lucas MS, Byrd JC, Bengtson EM, Peterson BL, et al. Consolidation therapy with subcutaneous (SC) alemtuzumab results in severe infectious toxicity in previously untreated CLL patients who achieve a complete response (CR) after fludarabine and rituximab (FR) induction therapy: Interim safety analysis of the CALGB study 10101. 11, Part 1 ed pp. 232A–3A. 2007. [Google Scholar]
- 57. Morrison VA, Peterson BL, Rai KR, Byrd JC, Larson RA Alemtuzumab increases serious infections in patients with previously untreated chronic lymphocytic leukemia (CLL) Receiving fludarabine-based therapy: A comparative analysis of 3 cancer and leukemia group B studies (CALGB 9011,9712,19901). 11, Part 1 ed pp. 233A 2007. [Google Scholar]
- 58. Zent CS, Call TG, Shanafelt TD, Tschumper RC, Jelinek DF, Bowen DA, et al. Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer. 2008. October 15; 113(8):2110–8. 10.1002/cncr.23824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bussone G, Ribeiro E, Dechartres A, Viallard JF, Bonnotte B, Fain O, et al. Efficacy and safety of rituximab in adults' warm antibody autoimmune haemolytic anemia: retrospective analysis of 27 cases. Am J Hematol. 2009. March; 84(3):153–7. 10.1002/ajh.21341 [DOI] [PubMed] [Google Scholar]
- 60. Motto DG, Williams JA, Boxer LA. Rituximab for refractory childhood autoimmune hemolytic anemia. Isr Med Assoc J. 2002. November; 4(11):1006–8. . [PubMed] [Google Scholar]
- 61. Owen RG, Rawstron AC, Osterborg A, Lundin J, Svensson G, Hillmen P Activity of alemtuzumab (Mabcampath) in relapsed/refractory Waldenstrom's macroglobulinemia. 11 ed pp. 644a–5a. 2003. [Google Scholar]
- 62. Treon SP, Branagan AR, Ioakimidis L, Soumerai JD, Patterson CJ, Turnbull B, et al. Long-term outcomes to fludarabine and rituximab in Waldenstrom macroglobulinemia. Blood. 2009. April 16; 113(16):3673–8. 10.1182/blood-2008-09-177329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hugle B, Solomon M, Harvey E, James A, Wadhwa A, Amin R, et al. Pneumocystis jiroveci pneumonia following rituximab treatment in Wegener's granulomatosis. Arthritis Care Res (Hoboken). 2010. November; 62(11):1661–4. 10.1002/acr.20279 [DOI] [PubMed] [Google Scholar]
- 64. Nosari A, Montillo M, Morra E. Infectious toxicity using alemtuzumab. Haematologica. 2004. December; 89(12):1415–9. . [PubMed] [Google Scholar]
- 65. Worth LJ, Dooley MJ, Seymour JF, Mileshkin L, Slavin MA, Thursky KA. An analysis of the utilisation of chemoprophylaxis against Pneumocystis jirovecii pneumonia in patients with malignancy receiving corticosteroid therapy at a cancer hospital. Br J Cancer. 2005. March 14; 92(5):867–72. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Harigai M, Tanaka Y, Maisawa S, Group JAS. Safety and efficacy of various dosages of ocrelizumab in Japanese patients with rheumatoid arthritis with an inadequate response to methotrexate therapy: a placebo-controlled double-blind parallel-group study. J Rheumatol. 2012. March; 39(3):486–95. 10.3899/jrheum.110994 [DOI] [PubMed] [Google Scholar]
- 67. Chang H, Shih LY, Wang CW, Chuang WY, Chen CC. Granulomatous Pneumocystis jiroveci pneumonia in a patient with diffuse large B-cell lymphoma: case report and review of the literature. Acta Haematol. 2010; 123(1):30–3. 10.1159/000261020 [DOI] [PubMed] [Google Scholar]
- 68. Yokoyama H, Watanabe T, Maruyama D, Kim SW, Kobayashi Y, Tobinai K. Progressive multifocal leukoencephalopathy in a patient with B-cell lymphoma during rituximab-containing chemotherapy: case report and review of the literature. Int J Hematol. 2008. November; 88(4):443–7. 10.1007/s12185-008-0168-2 [DOI] [PubMed] [Google Scholar]
- 69. Chang H, Yeh HC, Su YC, Lee MH. Pneumocystis jiroveci pneumonia in patients with non-Hodgkin's lymphoma receiving chemotherapy containing rituximab. J Chin Med Assoc. 2008. November; 71(11):579–82. 10.1016/S1726-4901(08)70173-4 [DOI] [PubMed] [Google Scholar]
- 70. Kumar N, Bazari F, Rhodes A, Chua F, Tinwell B. Chronic Pneumocystis jiroveci presenting as asymptomatic granulomatous pulmonary nodules in lymphoma. J Infect. 2011. June; 62(6):484–6. 10.1016/j.jinf.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 71. Beltz K, Kramm CM, Laws HJ, Schroten H, Wessalowski R, Gobel U. Combined trimethoprim and caspofungin treatment for severe Pneumocystis jiroveci pneumonia in a five year old boy with acute lymphoblastic leukemia. Klin Padiatr. 2006. May-Jun; 218(3):177–9. . [DOI] [PubMed] [Google Scholar]
- 72. Carter SJ, Bernstein SH, Friedberg JW, Barr PM. Pneumocystis jirovecii pneumonia as a complication of bendamustine in a patient receiving bendamustine plus rituximab for marginal zone lymphoma. Leuk Res. 2011. November; 35(11):e223–4. 10.1016/j.leukres.2011.07.014 [DOI] [PubMed] [Google Scholar]
- 73. Derungs A, Ratz Bravo AE, Kummer O. [Rash, fever, eosinophilia and elevated liver enzymes. DRESS syndrome (drug reaction or rash with eosinophilia and systemic symptoms)]. Praxis (Bern 1994). 2010. June 23; 99(13):767–77; quiz 76. 10.1024/1661-8157/a000199 [DOI] [PubMed] [Google Scholar]
- 74. Iguchi T, Yokoyama K, Mitsuishi M, Chen CK, Ikeda Y, Okamoto S. [Pulmonary tuberculosis and adenovirus-hemorrhagic cystitis after autologous peripheral blood stem cell transplantation for follicular lymphoma]. Rinsho Ketsueki. 2005. September; 46(9):1049–54. . [PubMed] [Google Scholar]
- 75. Kato H, Taji H, Ogura M, Kagami Y, Oki Y, Tsujimura A, et al. Favorable consolidative effect of high-dose melphalan and total-body irradiation followed by autologous peripheral blood stem cell transplantation after rituximab-containing induction chemotherapy with in vivo purging in relapsed or refractory follicular lymphoma. Clin Lymphoma Myeloma. 2009. December; 9(6):443–8. 10.3816/CLM.2009.n.087 [DOI] [PubMed] [Google Scholar]
- 76. Brusamolino E, Rusconi C, Montalbetti L, Gargantini L, Uziel L, Pinotti G, et al. Dose-dense R-CHOP-14 supported by pegfilgrastim in patients with diffuse large B-cell lymphoma: a phase II study of feasibility and toxicity. Haematologica. 2006. April; 91(4):496–502. . [PubMed] [Google Scholar]
- 77. Kavcic M, Fisher BT, Seif AE, Li Y, Huang YS, Walker D, et al. Leveraging administrative data to monitor rituximab use in 2875 patients at 42 freestanding children's hospitals across the United States. J Pediatr. 2013. June; 162(6):1252–8, 8 e1 10.1016/j.jpeds.2012.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Isidori A, Merli F, Angrilli F, Ferrara F, Alesiani F, Visani G. The incidence of Pneumocystis jirovecii pneumonia is not higher in patients receiving dose-dense therapy with rituximab, cyclophosphamide, non-pegylated liposomal doxorubicin, vincristine, and prednisolone and adequate Pneumocystis jirovecii pneumonia prophylaxis. Leuk Lymphoma. 2011. January; 52(1):148–9. 10.3109/10428194.2010.525726 [DOI] [PubMed] [Google Scholar]
- 79. Lim KH, Yoon HI, Kang YA, Lee KW, Kim JH, Bang SM, et al. Severe pulmonary adverse effects in lymphoma patients treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen plus rituximab. Korean J Intern Med. 2010. March; 25(1):86–92. 10.3904/kjim.2010.25.1.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huang YC, Liu CJ, Liu CY, Pai JT, Hong YC, Teng HW, et al. Low absolute lymphocyte count and addition of rituximab confer high risk for interstitial pneumonia in patients with diffuse large B-cell lymphoma. Ann Hematol. 2011. October; 90(10):1145–51. 10.1007/s00277-011-1268-2 [DOI] [PubMed] [Google Scholar]
- 81. Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006. May 15; 176(10):6147–54. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.