Abstract
IMPORTANCE
Discontinuation of bisphosphonate therapy after 3 to 5 years is increasingly considered, but methods to monitor fracture risk after discontinuation have not been established.
OBJECTIVE
To test methods of predicting fracture risk among women who have discontinued alendronate therapy after 4 to 5 years.
DESIGN, SETTING, AND PARTICIPANTS
The prospective Fracture Intervention Trial Long-term Extension (FLEX) study randomized postmenopausal women aged 61 to 86 years previously treated with 4 to 5 years of alendronate therapy to 5 more years of alendronate or placebo from 1998 through 2003; the present analysis includes only the placebo group. Hip and spine dual-energy x-ray absorptiometry (DXA) were measured when placebo was begun (FLEX baseline) and after 1 to 3 years of follow-up. Two biochemical markers of bone turnover, urinary type 1 collagen cross-linked N-telopeptide (NTX) and serum bone-specific alkaline phosphatase (BAP), were measured at FLEX baseline and after 1 and 3 years.
MAIN OUTCOMES AND MEASURES
Symptomatic spine and nonspine fractures occurring after the follow-up measurement of DXA or bone turnover.
RESULTS
During 5 years of placebo, 94 of 437 women (22%) experienced 1 or more symptomatic fractures; 82 had fractures after 1 year. One-year changes in hip DXA, NTX, and BAP were not related to subsequent fracture risk, but older age and lower hip DXA at time of discontinuation were significantly related to increased fracture risk (lowest tertile of baseline femoral neck DXA vs other 2 tertiles relative hazard ratio, 2.17 [95%CI, 1.38–3.41]; total hip DXA relative hazard ratio, 1.87 [95%CI, 1.20–2.92]).
CONCLUSIONS AND RELEVANCE
Among postmenopausal women who discontinue alendronate therapy after 4 to 5 years, age and hip BMD at discontinuation predict clinical fractures during the subsequent 5 years. Follow-up measurements of DXA 1 year after discontinuation and of BAP or NTX 1 to 2 years after discontinuation are not associated with fracture risk and cannot be recommended.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00398931
Bisphosphonate use reduces the risk of hip and spine fractures, particularly among individuals with osteoporosis defined by the presence of a vertebral fracture or bone mineral density (BMD) T score less than −2.5.1 Recent concerns about recognized adverse reactions (eg, esophagitis) and potential long-term safety issues, such as atypical femoral fractures, osteonecrosis of the jaw, and esophageal cancer—coupled with the possibility that fracture risk reduction may persist for months to years after long-term treatment is stopped—have resulted in heightened interest in interrupting or stopping bisphosphonate therapy after several years of treatment.2–4
The best evidence regarding the benefits and risks of discontinuation of bisphosphonate use comes from large trials in which participants who have received bisphosphonate therapy for a prolonged period are rerandomized to either continuation or discontinuation of the bisphosphonate therapy. The Fracture Intervention Trial Long-term Extension (FLEX) trial demonstrated that among older postmenopausal women who had used oral alendronate sodium for 5 years, those randomized to placebo for an additional 5 years had rates of nonspine and morphometric vertebral fractures similar to those randomized to receive an additional 5 years of alendronate therapy.5 During the 5 years of follow-up in FLEX, the rates of nonspine fractures were similar in women who continued or discontinued daily alendronate therapy (19.0% vs 18.9%), as were the rates of morphometric vertebral fractures (11.3% vs 9.8%), but the rate of clinical (symptomatic) vertebral fracture was significantly lower among those who continued alendronate therapy (5.3% vs 2.4%).5
The purpose of this post hoc analysis was to examine the utility of hip and spine dual-energy x-ray absorptiometry (DXA) and bone turnover marker (BTM) measurements at the time of discontinuation and after 1 to 3 years of follow-up for the 5-year prediction of fractures among women who have discontinued alendronate therapy after 4 to 5 years of treatment.
Methods
Participants
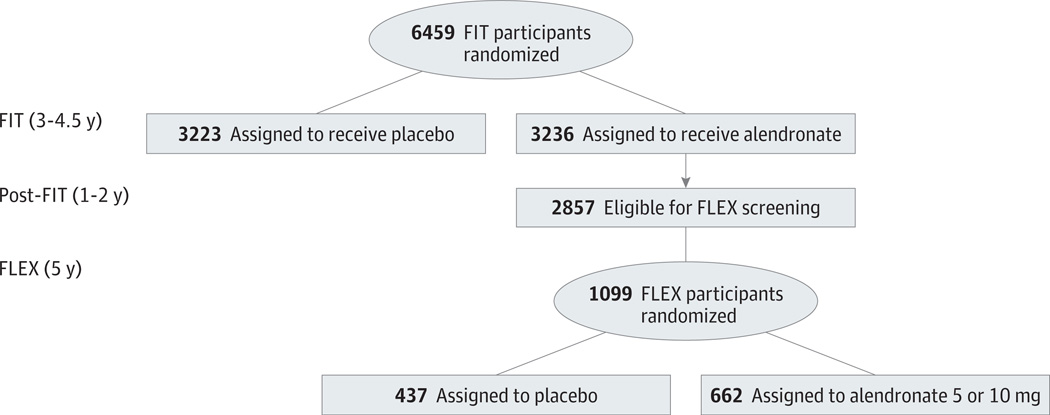
The design and results of the Fracture Intervention Trial (FIT) and the FLEX trial have been previously reported5–9 (Figure 1). All women provided written informed consent, and the protocol was approved by the appropriate institutional review boards. In FIT, postmenopausal women aged 55 to 81years with low femoral neck BMD (<0.68 g/cm2, equivalent to a T score of −1.6) were eligible to participate. Of 6459 enrolled participants, 2027 women with at least 1 prevalent vertebral deformity were enrolled in the vertebral fracture arm, and 4432 women with no existing vertebral deformity, in the clinical fracture arm. Within each arm, women were randomized to oral alendronate sodium (5 mg/day for 2 years, 10 mg/day thereafter; n = 3236) or placebo (n = 3223). Mean follow-up was 2.9 years in the vertebral fracture arm and 4.2 years in the clinical fracture arm. Up to 1 year of alendronate therapy (10 mg/day) was provided at no cost to all participants at the end of FIT, and although adherence was not assessed during this period, 78% of FLEX participants stated that they were taking alendronate at the time of randomization.
Figure 1. Design of Fracture Intervention Trial Long-term Extension (FLEX) Trial.
FIT indicates Fracture Intervention Trial.
Eligibility in FLEX was limited to women assigned to alendronate during FIT who completed a total of at least 3 years of treatment during the trial plus the subsequent open-label period. Women whose total hip BMD at FLEX baseline was less than 0.515 g/cm2 (T score, <−3.5)10 or whose total hip BMD was less than at FIT baseline were ineligible. Of the 3236 women enrolled in FIT and assigned to alendronate treatment, 2857 surviving women were contacted regarding participation in FLEX. Of these, a total of 1099 women (39%) were enrolled in FLEX. Compared with women who were eligible but did not participate in FLEX, randomized participants were slightly younger and less likely to have a fracture during FIT but had similar hip BMD.5,9
Treatment Allocation in FLEX
At FLEX baseline, participants were randomly assigned to receive alendronate sodium, 5 or 10 mg/d (n = 662 [60%]), or placebo (n = 437 [40%]) for 5 years. Each participant was also offered a daily supplement containing 500 mg calcium and 250 IU vitamin D3. The present analyses are limited to women who received placebo in FLEX.
DXA Measurements
Bone mineral density was measured at the total hip and its subregions and the posterior-anterior lumbar spine in all participants at FLEX baseline using DXA with the same Hologic QDR 2000 densitometers that were used for FIT (Hologic Inc). Hip DXA was repeated annually; spine DXA was repeated at the 36-month visit only. Quality control measures were the same as those used in FIT and have been outlined in detail elsewhere.8
The FLEX investigators were notified without revelation of treatment assignment if any participant experienced excessive bone loss (n = 54), defined as a decrease from the FLEX baseline visit of 8%ormore in total hip BMD over the first year with an additional2%decrease added for each subsequent year of follow-up, or 3 or more confirmed incident fractures (including hip or vertebral fractures). The clinical investigator then provided this information to the participant and counseled her on the risks and benefits of continued participation in the trial. In addition, if any follow-up total hip BMD in FLEX was at least 5% less than the participant’s total hip BMD at the FIT baseline examination (n = 16), discontinuation of study medication was mandatory.
Bone Turnover Measurements
Measurement of biochemical markers was performed in all FLEX participants at the baseline, 12-month, and 36-month visits in a central laboratory (Mayo Central Laboratories for Clinical Trials, Rochester, Minnesota). Fasting serum samples were analyzed for bone-specific alkaline phosphatase (BAP), an indicator of bone formation, using a commercially available assay (Ostase, Hybritech Inc).11
Fasting morning urine samples, preferably second void, were analyzed for urinary type 1 collagen cross-linked N-telopeptide (NTX), a biochemical marker of bone resorption, using a commercially available assay (Osteomark, Ostex International Inc).12 All NTX results are adjusted for urine creatinine concentration.
Fracture Outcomes
During FLEX follow-up, participants were queried about potential clinical fractures (nonspine and symptomatic vertebral fractures) every 3 months; self-reported fractures were confirmed by radiology or surgical reports. Fractures of the skull and those due to cancer or excessive trauma were excluded. If a vertebral fracture was diagnosed by a participant’s physician, usually following reported back pain, the spine radiograph used to diagnose the fracture was requested and compared with the radiograph obtained at FLEX baseline using semiquantitative grading.13
Statistical Analyses
These analyses are limited to women randomized to placebo in FLEX to mimic the clinical circumstance in which women discontinue alendronate therapy after 4 to 5 years. The primary outcome for this analysis was incident clinical fracture, defined as either a new nonspine fracture or a new clinical vertebral fracture during FLEX follow-up. Because acute fracture may affect both BMD and BTM measurements,14 we did not include clinical fractures occurring before the follow-up measurement of BMD or BTM in our primary analyses. The BTM levels were not normally distributed and are reported as geometric means (means after log transformation). The relationships between baseline BMD or BTM measurements and subsequent clinical fractures were analyzed with age-adjusted Cox models using fractures that occurred after the first year of follow-up. Analyses relating change in BMD or BTM to fracture outcomes were further adjusted for BMD or BTM levels at FLEX baseline, respectively, and for the presence or absence of an existing vertebral fracture. Cox model results are reported as relative hazard ratios (RHRs) and 95% confidence intervals. Secondary analyses examined 2-year changes in hip BMD and 3-year changes in BTM and hip and spine BMD using similar methods that excluded incident fractures occurring before the follow-up BTM or BMD measurement. Post hoc power calculations were not performed. All analyses were performed without knowledge of an individual’s fracture status using SAS, version 9.1.
Results
Baseline Characteristics and Fracture Outcomes in the FLEX Placebo Group
Of the 437 FIT participants (150 with existing vertebral fracture) randomized to receive placebo in FLEX, 94 (22%) experienced 1 or more clinical fractures during follow-up. Twelve women experienced clinical fracture before the first year when follow-up BMD and BTM measurements were repeated, and these fractures were excluded from the primary analysis. Women in the FLEX placebo group who experienced fracture after the first year of follow-up were older than those who did not (mean age, 76.2 vs 73.1 years; P < .001); however, BMI, duration of prior alendronate therapy, prevalence of vertebral fractures at FLEX baseline, and prevalence of confirmed nonspine fractures during FIT were similar (Table 1). Both femoral neck and total hip BMD were significantly lower at baseline among women who subsequently experienced fracture, but spine BMD and baseline BTM levels did not significantly differ among women who did and did not experience fracture (Table 1).
Table 1.
Characteristics of Women Who Discontinue Alendronate (Fracture Intervention Trial Long-term Extension [FLEX] Placebo Group) by Fracture Status After the First Year of FLEX Follow-up
| Variable | Fracture After FLEX Year 1 | P Value | |
|---|---|---|---|
| No (n = 355)a |
Yes (n = 82) |
||
| Age, mean (SD), y | 73.1 (5.7) | 76.2 (6.1) | <.001 |
| BMI, mean (SD) | 25.9 (4.2) | 25.4 (4.6) | .34 |
| Prior alendronate therapy, mean (SD), y | 5.0 (0.8) | 4.9 (0.6) | .27 |
| Clinical fracture during FIT, % | |||
| ≥1 | 9 | 12 | .43 |
| 1 | 8 | 10 | … |
| >1 | 0.6 | 2 | … |
| Prevalent vertebral fracture at FLEX baseline, % | 33 | 40 | .18 |
| Total hip BMD at FLEX baseline, mean (SD), g/cm2 | 0.73 (0.09) | 0.68 (0.08) | <.001 |
| Total hip T score <−2.5, % | 20 | 38 | .30 |
| Femoral neck BMD at FLEX baseline, mean (SD), g/cm2 | 0.62 (0.07) | 0.58 (0.07) | <.001 |
| Femoral neck T score <−2.5, % | 26 | 48 | .33 |
| Spine BMD at FLEX baseline, mean (SD), g/cm2 | 0.91(0.14) | 0.88 (0.14) | .08 |
| Spine T score <−2.5, % | 22 | 29 | .75 |
| NTX/Cr at FLEX baseline, geometric mean (SD), nmol/mmol | 19.7 (13.2) | 19.4 (11.8) | .86 |
| BAP at FLEX baseline, geometric mean (SD), ng/mL | 9.0 (3.4) | 9.5 (3.7) | .15 |
Abbreviations: BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ellipses, comparison not made; FIT, Fracture Intervention Trial; NTX/Cr, type 1 collagen cross-linked N-telopeptide to creatinine concentration ratio.
Includes 12 women with clinical fractures before first year of follow-up.
Changes in BMD and BTM Levels After Discontinuation of Alendronate Therapy
As previously reported,5 mean hip BMD decreased and BTMs increased in the FLEX placebo group (eFigure 1 and eFigure 2 in Supplement) to a greater extent than in those randomized to continue alendronate therapy, but there were no significant differences 1 year after discontinuation of alendronate therapy among those with and without clinical fracture during follow-up (Table 2).
Table 2.
Hip Bone Mineral Density (BMD) and Bone Turnover Marker (BTM) Levels Among Women Who Discontinue Alendronate Therapy by Fracture Status After the First Year of FLEX Follow-up
| Variable | Fracture After FLEX Year 1 | |
|---|---|---|
| No (n = 355) |
Yes (n = 82)a |
|
| BMD, mean (95% CI) | ||
| Femoral neck | ||
| Baseline, g/cm2 | 0.62 (0.61 to 0.63) | 0.58 (0.57 to 0.60) |
| After 1 year, g/cm2 | 0.61 (0.61 to 0.62) | 0.58 (0.56 to 0.59) |
| 1-year change, % | −0.71 (−1.12 to −0.29) | −1.05 (−1.91 to −0.18) |
| Total hip | ||
| Baseline, g/cm2 | 0.73 (0.72 to 0.74) | 0.69 (0.67 to 0.71) |
| After 1 year, g/cm2 | 0.72 (0.71 to 0.73) | 0.68 (0.66 to 0.70) |
| 1-year change, % | −1.32 (−1.59 to −1.05) | −1.49 (−2.06 to −0.92) |
| BTM, geometric mean (95% CI) | ||
| NTX/Cr | ||
| Baseline, nmol/mmol | 19.7 (18.7 to 20.9) | 19.1 (17.0 to 21.5) |
| After 1 year, nmol/mmol | 24.1 (22.9 to 25.3) | 24.7 (22.2 to 27.4) |
| 1-year change, % | 22.0 (15.5 to 28.9) | 29.1 (15.1 to 44.7) |
| BAP | ||
| Baseline, ng/mL | 9.0 (8.7 to 9.3) | 9.4 (8.7 to 10.2) |
| After 1 year, ng/mL | 10.5 (10.1 to 11.0) | 11.0 (10.2 to 12.0) |
| 1-year change, % | 17.1 (13.4 to 20.8) | 17.2 (9.7 to 25.1) |
Abbreviations: BAP, bone-specific alkaline phosphatase; FLEX, Fracture Intervention Trial Long-term Extension; NTX/Cr, type 1 collagen cross-linked N-telopeptide to creatinine concentration ratio.
Any nonspine or clinical vertebral fracture occurring after the 1-year visit.
BMD, BTMs, and Fracture Outcomes After Discontinuation of Alendronate Therapy
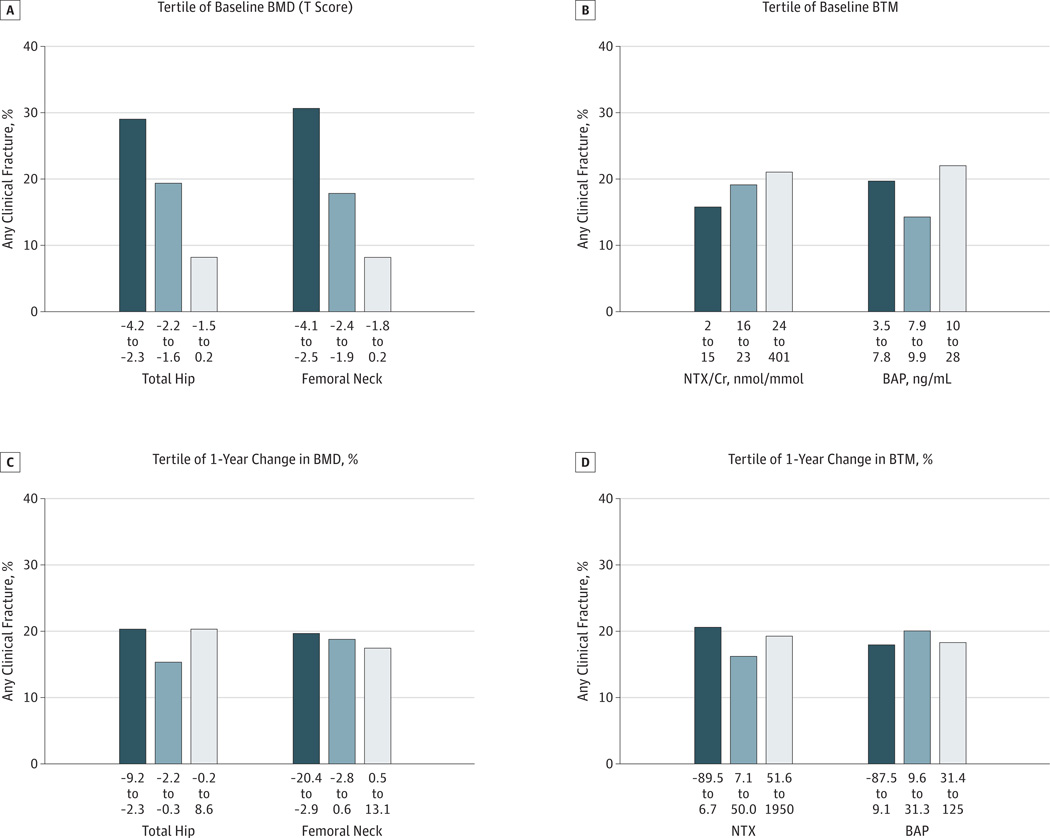
Examining fracture outcomes after the first year of FLEX follow-up by tertile of baseline BMD at the time of discontinuation revealed that those in the lowest tertile of femoral neck or total hip BMD were more likely to experience fracture than those in higher tertiles (Figure 2A). After adjustment for age (Table 3), the risk of fracture during FLEX in the lowest tertile of baseline total hip BMD was 87% higher compared with that in the other 2 tertiles (RHR, 1.87 [95% CI, 1.20–2.92]). Results were similar for femoral neck BMD. In separate analyses, baseline levels of NTX and BAP were not associated with fracture outcomes in the FLEX placebo group (Figure 2B and Table 3). Older age was independently associated with a greater risk of fracture (RHR, 1.54 [95% CI, 1.26–1.85] per 5-year increase) after discontinuation of alendronate therapy (Table 3).
Figure 2. Proportion of Women With Any Clinical Fracture After Discontinuation of Alendronate Therapy.
A, By tertile of hip bone mineral density (BMD) at Fracture Intervention Trial Long-term Extension (FLEX) baseline. P for trend < .001 for total hip BMD and for femoral neck BMD. B, By tertile of bone turnover marker at FLEX baseline. P for trend = .18 for urinary type 1 collagen cross-linked N-telopeptide to creatinine concentration ratio (NTX/Cr) and .40 for serum bone-specific alkaline phosphatase (BAP). C, By tertile of 1-year percent change in hip BMD. P for trend = .96 for total hip BMD and .81 for femoral neck BMD. D, By tertile of 1-year percent change in bone turnover marker. P for trend = .91 for NTX/Cr and .70 for BAP.
Table 3.
FLEX Baseline Bone Mineral Density (BMD), Bone Turnover Markers (BTMs), and Other Characteristics and Age- Adjusted Risk of Fracture Among Women Who Discontinued Alendronate Therapy
| Variables Measured at FLEX Baselinea |
Risk of Fracture, Relative Hazard Ratio (95% CI)b |
|---|---|
| Demographic and clinical characteristics | |
| Age, per 5-y increase, y | 1.54 (1.26–1.85)c |
| BMI, per SD increase | 1.10 (0.87–1.38) |
| Vertebral fracture | 1.11 (0.71–1.75) |
| Previous nonspine fracture | 1.24 (0.64–2.40) |
| BMD, lowest tertile vs other 2, T score | |
| Total hip | 1.87 (1.20–2.92)c |
| Femoral neck | 2.17 (1.38–3.41)c |
| BTMs, highest tertile vs other 2 | |
| NTX/Cr, nmol/mmol | 1.33 (0.84–2.10) |
| BAP, ng/mL | 1.39 (0.89–2.17) |
Abbreviations: BAP, bone-specific alkaline phosphatase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FLEX, Fracture Intervention Trial Long-term Extension; NTX/Cr, type 1 collagen cross-linked N-telopeptide to creatinine concentration ratio.
With the exception of age, each variable was examined in a separate age-adjusted model.
Any nonspine or clinical vertebral fracture occurring after the 1-year visit.
Statistically significant association.
One-year changes in BMD were not associated with the risk of clinical fracture after discontinuing alendronate therapy. The risk of fracture after the first year of follow-up did not differ across tertiles of 1-year change in femoral neck (P for trend = .81) or total hip BMD (P for trend = .96) (Figure 2C). The age- and baseline BMD–adjusted risk of fracture after the first year of follow-up among those in the tertile with the greatest total hip bone loss (1-year change in BMD, −2.3% to −9.2%) did not differ from those in the other 2 tertiles (RHR, 1.06 [95% CI, 0.67–1.68]) (Table 4). Results were similar for 1-year change in femoral neck BMD.
Table 4.
Change in Bone Mineral Density (BMD) or Bone Turnover Markers (BTMs) and Subsequent Age-Adjusted Risk of Clinical Fracture After Discontinuation of Alendronate Therapya
| Variable | Risk of Fracture, Relative Hazard Ratio (95% CI) | ||
|---|---|---|---|
| 1-Year Change | 2-Year Change | 3-Year Change | |
| BMD | |||
| Tertile with greatest decrease vs other 2 | |||
| Femoral neck | 0.98 (0.61–1.56) | 1.51 (0.93–2.44) | 1.32 (0.77–2.28) |
| Total hip | 1.07 (0.68–1.69) | 1.56 (0.97–2.52) | 1.68 (0.98–2.86) |
| Spine | NA | NA | 1.11 (0.63–1.97) |
| ≥3% decrease | |||
| Femoral neck | 1.12 (0.70–1.80) | 1.45 (0.90–2.35) | 1.39 (0.81–2.36) |
| Total hip | 1.36 (0.83–2.23) | 1.68 (1.05–2.72)b | 1.63 (0.95–2.27) |
| Spine | NA | NA | 0.77 (0.36–1.64) |
| BTMs | |||
| Tertile with greatest increase vs other 2 | |||
| NTX/Cr | 1.14 (0.70–1.86) | NA | 1.02 (0.55–1.91) |
| BAP | 1.03 (0.63–1.67) | NA | 0.79 (0.41–1.50) |
| ≥30% increase | |||
| NTX/Cr | 1.24 (0.77–1.99) | NA | 1.26 (0.69–2.32) |
| BAP | 0.94 (0.58–1.52) | NA | 0.83 (0.44–1.57) |
Abbreviations: BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; NA, not available; NTX/Cr, type 1 collagen cross-linked N-telopeptide to creatinine concentration ratio; RHR, relative hazard ratio.
Any nonspine or clinical vertebral fracture occurring after the 1-year (n = 82 fractures), 2-year (n = 70 fractures), or 3-year (n = 57 fractures) visits.
Statistically significant association.
One-year changes in BTMs were not associated with risk of fracture after discontinuation of alendronate therapy. The risk of fracture after 1 year of follow-up did not differ across tertiles of 1-year change in NTX (P for trend = .91) or BAP (P for trend = .70) (Figure 2D). The age- and baseline NTX–adjusted risk of fracture after the first year of follow-up among those in the tertile with the greatest increase in NTX (1-year increase in NTX, >51.6%) did not differ from those in the other 2 tertiles (RHR, 1.14 [95%CI, 0.70–1.86]) (Table 4). Results were similar for 1-year change in BAP.
Additional Analyses
We further classified 1-year changes in BMD and BTMs on the basis of published estimates of the “least significant change,” which represent the smallest difference in paired measurements that is unlikely to be due to chance (P < .05).15 Women with 3% or greater 1-year loss of femoral neck (29% of women) or total hip BMD (21% of women) did not have a statistically significant increase in fractures after the first year of follow-up (Table 4). Similarly, fracture risk after the first year of follow- up was similar among women with a 30%or greater 1-year increase in NTX (45% of women) or BAP (37% of women) compared with those with smaller increases in BTM (Table 4).
Similarly, most associations between 2- or 3-year changes in BMD and fracture risk were not statistically significant, although there were fewer fractures after the second (n = 70) and third (n = 57) annual visits included in the analysis (Table 4). The risk of fracture was elevated among those with greater total hip bone loss after 2 or 3 years of follow-up, but after adjustment for age and baseline BMD, only 2-year total hip bone loss greater than 3% was significantly associated with fracture risk (RHR, 1.68 [95% CI, 1.05–2.72]). Neither 2- or 3-year change in femoral neck BMD nor 3-year change in spine BMD was associated with fracture risk.
Three-year changes in BTMs after discontinuation of alendronate therapy were not associated with fracture risk (Table 4). For example, after adjustment for age and baseline NTX, the risk of facture among women in the highest tertile of 3-year change in NTX (>56%increase) did not differ significantly from those in the other 2 tertiles with smaller increases in NTX (RHR, 1.02 [95% CI, 0.55–1.91]).
Additional analyses were performed to assess the impact of duration of alendronate therapy, the presence of a vertebral fracture, and self-reported alendronate use at FLEX baseline, but the results were similar. For example, the total duration of alendronate therapy was not associated with bone loss or fracture risk, and fracture analyses that were further adjusted for duration of alendronate use or prevalent vertebral fractures gave similar results (data not shown). Similarly, results were qualitatively similar in analyses limited to the 78% of FLEX participants who reported alendronate use at FLEX baseline (data not shown).
Last, although we excluded fractures that occurred before the follow-up BMD or BTM measurement in our primary analyses, results were qualitatively similar when all nonspine and clinical vertebral fractures were analyzed. We did not plan separate analyses of the relationships between change in BMD or BTM and clinical vertebral fractures because of the small number (n = 23), but qualitatively the results were similar to those observed with nonspine fractures (data not shown).
Discussion
Bisphosphonates are highly effective antifracture treatments, particularly in patients with osteoporosis.1 Recently, on the basis of randomized trials that reported fracture end points after discontinuation of long-term bisphosphonate use5,16 and a US Food and Drug Administration Advisory Committee meeting about the benefits and risks of long-term bisphosphonate use for the treatment and prevention of osteoporosis,17 clinicians and patients are increasingly considering temporary or permanent discontinuation after 3 to 5 years of treatment.2,18 After discontinuation of bisphosphonate use, some experts recommend yearly measurements of BMD or BTMs, or both, to help identify those individuals in need of additional antifracture treatment.2,19–21 In this post hoc analysis of FLEX participants randomized to placebo after receiving 5 years of oral alendronate therapy, we found that older age and lower hip BMD at the time of discontinuation were associated with higher rates of clinical fracture during the next 5 years, but neither 1-year changes in hip DXA nor 1- and 3-year changes in BTM levels were associated with fracture risk. We did observe an increased risk of fracture among those with greater 2- or 3-year hip bone loss, but only 2-year change in total hip BMD was statistically significant. Thus, clinicians and patients contemplating a drug holiday after 5 years of alendronate therapy should be aware that short-term monitoring to detect individuals at higher risk who might resume bisphosphonate therapy, or initiate another therapy, may not add to risk prediction over and above age and BMD measured at the time of discontinuation of therapy.
These results build on and extend previous results from the FLEX study.5,22 The analyses detailed here are limited to the placebo-treated group in FLEX and were designed tomimic the clinical scenario in which the decision has been made to stop alendronate therapy after 4 to 5 years and begin a drug holiday. Because 22% of FLEX participants experienced fracture during the 5 years following discontinuation of alendronate use, it is important to document the utility of serial monitoring that attempts to detect those at highest risk of fracture after discontinuation. Previous FLEX analyses have found that bone loss after discontinuation of 4 to 5 years of alendronate therapy could not be easily predicted on the basis of clinical risk factors.23
Given the mechanism of action of bisphosphonates and well-documented treatment-related effects on bone turnover and BMD,24 it is somewhat surprising that short-term changes in these individual measurements are not associated with fracture risk after discontinuation of alendronate therapy, particularly BTM measurements, which increased 17% to 25% during the first year after discontinuation of alendronate use. Interestingly, greater individual treatment related reductions in bone turnover are associated with increased fracture risk among postmenopausal women treated with alendronate,25 risedronate sodium,26 and zoledronic acid.27 Conversely, some25,28 but not all29 studies have found that treatment-related changes in BMD are not associated with individual fracture risk.
Although our results were consistent and robust after accounting for numerous potential confounding factors, such as duration of treatment and previous vertebral fractures, several other factors may have contributed to the lack of significant associations between short-term changes in BMD or BTMs and subsequent fracture in this analysis. First, our results only apply to the eligible women who chose to participate in FLEX and may not apply to those who were not eligible or chose not to participate in FLEX. Second, the number of fractures during FLEX follow-up was relatively small, and for some analyses (particularly those examining 2- and 3-year changes in BMD or BTMs) confidence intervals were wide, indicating limited power to detect significant associations. Third, BTM measurements were only assessed once at each visit. Repeated measurements at each visit might have improved BTM precision and allowed identification of women at higher risk of fracture. Last, we examined tertile changes in BMD and BTM, as well as those that approximated the least significant change for those measurements, and the results for other cut points may have differed. Although an underlying assumption of the least significant change calculation is that no change in the true value of the measurement has occurred, it is frequently used in settings in which changes are likely (eg, changes in BMD or BTMs with antiresorptive therapy).30
Although short-term changes in BMD and BTM were not associated with fracture risk in FLEX, older age and lower BMD at the time of discontinuation were associated with the risk of fracture after discontinuation. Thus, although there are no accepted guidelines regarding continuation of bisphosphonate therapy or switching to another therapy, clinicians should be aware that older patients and those with low hip BMD after 5 years of alendronate therapy remain at high risk of fracture. Interestingly, we found that several other factors typically associated with higher fracture risk among untreated populations, such as lower BMI or previous fracture (including prevalent vertebral fracture),were not associated with fracture risk after discontinuation of alendronate therapy. Additional studies are needed to clarify the optimal management of patients who remain at risk of fracture after completing 3 to 5 years of bisphosphonate therapy.
Previous published studies have not examined the utility of serial BMD or BTM monitoring after discontinuation of bisphosphonate use, and these findings need confirmation with other bisphosphonates and in other settings. Additional strengths of this study include careful assessment of alendronate exposure and fracture outcomes, blinding to therapy assignment, and high-quality BMD and BTM measurements with rigorous quality control. Such efforts likely improve the accuracy and precision of BMD and BTM measurements compared with those obtained in clinical practice. Furthermore, to avoid acute and subacute fracture-related changes in BMD and BTM measurements, we excluded women with documented clinical fractures before the follow-up measurement of BMD or BTMs. Our study does have several additional weaknesses; as noted, the modest number of pooled fractures, particularly after the second and third annual visits, resulted in relatively wide confidence intervals and precluded analyses of specific fracture types, and fracture follow-up in FLEX was limited to 5 years. Finally, these results only apply to postmenopausal women treated with alendronate 5 to 10 mg/d for 5 years and not to other populations or to other bisphosphonates.
Conclusions
We found that after discontinuation of 4 to 5 years of alendronate therapy, 22% of women experience fracture during the subsequent 5 years. Older age and lower hip BMD at the time of discontinuation strongly predict fracture risk after discontinuation, but neither 1-year change in hip BMD nor 1- or 3-year change in NTX or BAP are associated with the risk of fracture after discontinuation. Women with greater total hip bone loss 2 or 3 years after discontinuation may be at increased risk of fracture, but these results need to be confirmed in other studies before routine measurement of BMD after discontinuation of alendronate therapy can be recommended. Future discontinuation studies should be designed to confirm these findings, test newer BTMs (such as serum procollagen type 1 N propeptide and C-terminal telopeptide of type 1 collagen30), and study long-term changes in hip BMD (eg, >5 years) and perhaps newer imaging modalities. These studies should focus on women whose hip BMD remains low after long-term treatment.31 In the meantime, short-term monitoring with BMD, BAP, or NTX after discontinuation of 4 to 5 years of alendronate therapy does not appear to improve fracture prediction.
Supplementary Material
Acknowledgments
Dr Schwartz has consulted for Merck. Ms Palermo has consulted for Nycomed. Dr Cauley has consulted for Merck. Dr Hochberg has consulted for Merck, Amgen, and Eli Lilly. Dr Santora is employed by Merck. Dr Cummings has consulted for Amgen, Eli Lilly, GlaxoSmithKline, and Merck. Dr Black has consulted for Amgen and Eli Lilly and has received honoraria from Amgen, Merck, and Novartis.
Funding/Support: The FLEX study was supported by contracts from Merck & Co; this analysis was designed and conducted by the non-Merck investigators without additional financial support. The study drug was manufactured and packaged by Merck.
Role of the Sponsor: Merck had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Bauer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bauer, Cummings, Black.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Bauer.
Critical revision of the manuscript for important intellectual content: Schwartz, Palermo, Cauley, Hochberg, Santora, Cummings, Black.
Statistical analysis: Palermo, Black.
Obtained funding: Cummings, Black.
Administrative, technical, or material support: Bauer, Cauley, Cummings.
Study supervision: Bauer, Cummings, Black.
Conflict of Interest Disclosures: No other disclosures are reported.
Contributor Information
Douglas C. Bauer, Department of Medicine, University of California, San Francisco; Department of Epidemiology and Biostatistics, University of California, San Francisco.
Ann Schwartz, Department of Epidemiology and Biostatistics, University of California, San Francisco.
Lisa Palermo, Department of Epidemiology and Biostatistics, University of California, San Francisco.
Jane Cauley, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, Pennsylvania.
Marc Hochberg, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore.
Art Santora, Merck and Co, Inc, Rahway, New Jersey.
Steven R. Cummings, California Pacific Research Foundation, San Francisco, California.
Dennis M. Black, Department of Epidemiology and Biostatistics, University of California, San Francisco.
REFERENCES
- 1.Favus MJ. Bisphosphonates for osteoporosis. N Engl J Med. 2010;363(21):2027–2035. doi: 10.1056/NEJMct1004903. [DOI] [PubMed] [Google Scholar]
- 2.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 3.Recker RR, Lewiecki EM, Miller PD, Reiffel J. Safety of bisphosphonates in the treatment of osteoporosis. Am J Med. 2009;122(2) suppl:S22–S32. doi: 10.1016/j.amjmed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E, Swern AS, Hustad CM, et al. Alendronate and atrial fibrillation: a meta-analysis of randomized placebo-controlled clinical trials. Osteoporos Int. 2012;23(1):233–245. doi: 10.1007/s00198-011-1546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black DM, Schwartz AV, Ensrud KE, et al. FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 7.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 8.Black DM, Reiss TF, Nevitt MC, Cauley J, Karpf D, Cummings SR. Design of the Fracture Intervention Trial. Osteoporos Int. 1993;3(suppl 3):S29–S39. doi: 10.1007/BF01623005. [DOI] [PubMed] [Google Scholar]
- 9.Ensrud KE, Barrett-Connor EL, Schwartz A, et al. Fracture Intervention Trial Long-term Extension Research Group. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res. 2004;19(8):1259–1269. doi: 10.1359/JBMR.040326. [DOI] [PubMed] [Google Scholar]
- 10.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 11.Garnero P, Delmas PD. Assessment of the serum levels of bone alkaline phosphatase with a new immunoradiometric assay in patients with metabolic bone disease. J Clin Endocrinol Metab. 1993;77(4):1046–1053. doi: 10.1210/jcem.77.4.8104954. [DOI] [PubMed] [Google Scholar]
- 12.Rosen HN, Dresner-Pollak R, Moses AC, et al. Specificity of urinary excretion of cross-linked N-telopeptides of type I collagen as a marker of bone turnover. Calcif Tissue Int. 1994;54(1):26–29. doi: 10.1007/BF00316285. [DOI] [PubMed] [Google Scholar]
- 13.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 14.Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res. 2007;22(8):1155–1164. doi: 10.1359/jbmr.070505. [DOI] [PubMed] [Google Scholar]
- 15.Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB. Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res. 2011;26(7):1662–1669. doi: 10.1002/jbmr.342. [DOI] [PubMed] [Google Scholar]
- 16.Black DM, Reid IR, Cauley JA, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2012;27(2):243–254. doi: 10.1002/jbmr.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Food and Drug Administration. Background Document for Meeting of Advisory Committee for Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee. [Accessed March 28, 2014]; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM270958.pdf. Published September 9, 2011.
- 18.Whitaker M, Guo J, Kehoe T, Benson G. Bisphosphonates for osteoporosis—where do we go from here? N Engl J Med. 2012;366(22):2048–2051. doi: 10.1056/NEJMp1202619. [DOI] [PubMed] [Google Scholar]
- 19.Lewiecki EM. Bisphosphonates for the treatment of osteoporosis: insights for clinicians. Ther Adv Chronic Dis. 2010;1(3):115–128. doi: 10.1177/2040622310374783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boonen S, Ferrari S, Miller PD, et al. Postmenopausal osteoporosis treatment with antiresorptives: effects of discontinuation or long-term continuation on bone turnover and fracture risk—a perspective. J Bone Miner Res. 2012;27(5):963–974. doi: 10.1002/jbmr.1570. [DOI] [PubMed] [Google Scholar]
- 21.McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126(1):13–20. doi: 10.1016/j.amjmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz AV, Bauer DC, Cummings SR, et al. FLEX Research Group. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res. 2010;25(5):976–982. doi: 10.1002/jbmr.11. [DOI] [PubMed] [Google Scholar]
- 23.McNabb BL, Vittinghoff E, Schwartz AV, et al. BMD changes and predictors of increased bone loss in postmenopausal women after a 5-year course of alendronate. J Bone Miner Res. 2013;28(6):1319–1327. doi: 10.1002/jbmr.1864. [DOI] [PubMed] [Google Scholar]
- 24.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK. Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149(6):404–415. [PubMed] [Google Scholar]
- 25.Bauer DC, Black DM, Garnero P, et al. Fracture Intervention Trial Study Group. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004;19(8):1250–1258. doi: 10.1359/JBMR.040512. [DOI] [PubMed] [Google Scholar]
- 26.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18(6):1051–1056. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 27.Delmas PD, Munoz F, Black DM, et al. HORIZON-PFT Research Group. Effects of yearly zoledronic acid 5mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. J Bone Miner Res. 2009;24(9):1544–1551. doi: 10.1359/jbmr.090310. [DOI] [PubMed] [Google Scholar]
- 28.Watts NB, Geusens P, Barton IP, Felsenberg D. Relationship between changes in BMD and nonvertebral fracture incidence associated with risedronate: reduction in risk of nonvertebral fracture is not related to change in BMD. J Bone Miner Res. 2005;20(12):2097–2104. doi: 10.1359/JBMR.050814. [DOI] [PubMed] [Google Scholar]
- 29.Hochberg MC, Ross PD, Black D, et al. Fracture Intervention Trial Research Group. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Arthritis Rheum. 1999;42(6):1246–1254. doi: 10.1002/1529-0131(199906)42:6<1246::AID-ANR22>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.Vasikaran S, Eastell R, Bruyère O, et al. IOF-IFCC Bone Marker Standards Working Group. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 31.Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051–2053. doi: 10.1056/NEJMp1202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.