Abstract
Objectives
To examine the agreement of multifrequency bioelectric impedance analysis (BIA) and anthropometry with reference methods for body composition assessment in children with intestinal failure (IF).
Methods
We conducted a prospective pilot study in children 14 years of age or younger with IF resulting from either short bowel syndrome (SBS) or motility disorders. Bland Altman analysis was used to examine the agreement between BIA and deuterium dilution in measuring total body water (TBW) and lean body mass (LBM); and between BIA and dual X-ray absorptiometry (DXA) techniques in measuring LBM and FM. Fat mass (FM) and percent body fat (%BF) measurements by BIA and anthropometry, were also compared in relation to those measured by deuterium dilution.
Results
Fifteen children with IF, median (IQR) age 7.2 (5.0, 10.0) years, 10 (67%) male, were studied. BIA and deuterium dilution were in good agreement with a mean bias (limits of agreement) of 0.9 (-3.2, 5.0) for TBW (L) and 0.1 (-5.4 to 5.6) for LBM (kg) measurements. The mean bias (limits) for FM (kg) and %BF measurements were 0.4 (-3.8, 4.6) kg and 1.7 (-16.9, 20.3)% respectively. The limits of agreement were within 1 SD of the mean bias in 12/14 (86%) subjects for TBW and LBM, and in 11/14 (79%) for FM and %BF measurements. Mean bias (limits) for LBM (kg) and FM (kg) between BIA and DXA were 1.6 (-3.0 to 6.3) kg and -0.1 (-3.2 to 3.1) kg, respectively. Mean bias (limits) for FM (kg) and %BF between anthropometry and deuterium dilution were 0.2 (-4.2, 4.6) and -0.2 (-19.5 to 19.1), respectively. The limits of agreement were within 1 SD of the mean bias in 10/14 (71%) subjects.
Conclusions
In children with intestinal failure, TBW and LBM measurements by multifrequency BIA method were in agreement with isotope dilution and DXA methods, with small mean bias. In comparison to deuterium dilution, BIA was comparable to anthropometry for FM and %BF assessments with small mean bias. However, the limits of agreement were wide and clinically unacceptable for some patients. BIA is a reliable method for TBW and LBM assessments in population studies. However, its reliability in individual patients, especially for FM assessments, cannot be guaranteed.
Keywords: Body composition, bioelectric impedance analysis, multifrequency, deuterium dilution, dual x-ray absorptiometry, short bowel syndrome, intestinal failure
Introduction
Children with intestinal failure (IF) may require prolonged periods of parenteral nutrition (PN) for sustenance and growth until intestinal adaptation allows adequate enteral intake 1. Lean body mass (LBM) or muscle mass preservation and accrual are important goals during this phase of nutritional rehabilitation. LBM status at admission has been inversely associated with length of stay in hospitalized patients 2. Preservation and accrual of LBM during illness has been shown to be an important predictor for clinical outcomes in a variety of settings, including patients with sepsis, cystic fibrosis, and malnutrition 3-5. The current practice of monitoring change in body weight as a nutritional index may be misleading. Despite stable weight, muscle mass depletion cannot be ruled out and may remain undetected 6. In a recent study of body composition in children with intestinal failure who were dependent on PN, limb muscle mass was lower than population reference values and total body as well as truncal fat mass index was excessive despite adequate weight gain 7. Hence, body composition measurements may provide important information when titrating nutritional intake in children with SBS. Routine anthropometry has been traditionally used to obtain body composition assessment, and requires specially trained personnel. Other validated methods of measuring body composition are not currently available outside the research environment. A noninvasive, simple and accurate in vivo technique that allows assessment of body composition in pediatric IF is desirable. Bioelectric impedance analysis (BIA) is a readily available and noninvasive technique for body composition monitoring 8,9.
We examined the agreement of multifrequency BIA for measuring total body water (TBW), LBM, fat mass (FM) and percent body fat (%BF) in children with IF, in comparison with deuterium dilution and dual energy x-ray absorptiometry (DXA) techniques. We hypothesized that BIA would provide estimates of TBW and body composition that are in agreement with reference methods in this cohort.
Materials and Methods
We performed a prospective pilot study in children 14 years of age or younger with intestinal failure (IF) followed at the Center for Advanced Intestinal Rehabilitation at Boston Children's Hospital (BCH). Patients with motility disorders or short bowel syndrome (SBS) with current or past PN dependence were eligible for enrollment. SBS was defined as a malabsorptive state resulting from congenital or acquired gastrointestinal disease leading to dependence on parenteral nutrition for more than 90 days. Children with SBS were enrolled during the adaptive phase of transition from PN to enteral nutrition.
Patients were excluded if they were older than 14 years, had an electrical device that might interfere with BIA (e.g., cardiac pacing device, implantable drug delivery pumps, vagal nerve stimulator or invasive cerebral perfusion monitor), central venous catheter-related infection or sepsis physiology, ongoing fluid imbalance, clinically evident shifts in fluid compartments (e.g., edema or ascites) or required ongoing fluid resuscitation (defined as daily fluid intake more than 150% of maintenance or fluid boluses more than 20 ml/kg/day) or evidence of renal insufficiency (defined by serum creatinine more than twice normal upper limit for age or need for renal replacement therapy). Due to the requirement for lying still during this test, DXA was only performed in children aged 5 years or older. Subjects underwent study procedures in the Clinical and Translational Study Unit. The BCH institutional review board approved the study and their parents or guardians gave written informed consent for the study.
Deuterium dilution
An enteral dose of 0.2 g/kg of deuterium-enriched water (2H2O) was administered via a feeding tube or orally (based on patient feeding status). The isotope was prepared in the Pharmacy Department and tested for sterility and pyrogenicity before administration. For patients receiving tube-feeds, enteral nutrition was stopped for 1 hour prior to and after the administration of isotope, and then resumed. Urine samples were obtained at baseline and then at 5 hours after the administration of deuterium. Urine specimens were centrifuged and stored immediately in a -80°C freezer until the time of analysis. Isotope enrichment was obtained by gas isotope ratio mass spectrometry (IRMS) using validated protocols in a commercial lab (Metabolic Solutions, Inc., Nashua, NH) 10. The delta deuterium values for the pre-dose (δpre) and post-dose samples (δpost) were determined. The deuterium dose was diluted with tap water. The amount of dose diluted and water used was recorded using standard scale to weigh the syringes. The deuterium content of the tap water (δtap) and diluted dose (δdose) were measured. Total body water (TBW) in moles was calculated from the dilution of the heavy isotope using the equation: TBW (moles) = WA/18.02a × (δdose−δtap)/(δpost−δpre); where W = Amount of water (grams) used to dilute the deuterium dose, A = Amount of deuterium dose (grams) administered to subject, a = amount of dose (grams) diluted for analysis. TBW (moles) was converted to TBW (kg) by multiplying with a factor of 18.02 and dividing by 1000 (g/kg). Deuterium oxide overestimates TBW by 4% 11. Therefore, to correct for the non- exchange of deuterium in the body, the TBW measurement was divided by 1.04. LBM (or fat free mass) was calculated from TBW using a hydration factor of 0.73. FM and %BF were then derived from LBM and total body weight. Hence, TBW was measured and LBM, FM and %BF are derived variables, using TBW and weight.
BIA measurements were obtained with subjects in the supine position, using a multifrequency impedance device (Bodystat Quadscan 4000®, Bodystat, Inc., Tampa, FL). Current-injector electrodes were placed just below the phalangeal-metacarpal joint in the middle of the dorsal side of the right hand and below the metatarsal arch on the superior side of the right foot. Detector electrodes were placed on the posterior side of the right wrist, midline to the pisiform bone of the medial (fifth phalangeal) side with the wrist semi flexed. Impedance was measured with a multi-frequency bioelectrical impedance analyzer using 5, 50, 100, and 200 kHz at oscillating current. An undisclosed proprietary equation developed by the manufacturer calculated TBW using the impedance at 5 kHz and 200 kHz, body weight, height, age and gender (information provided by manufacturer). Estimates of TBW from BIA were converted to kg by using a conversion factor equivalent to the density of water at 36°C (0.9937 g/cm3). Hence, TBW is the calculated variable from measured impedance values. LBM, FM and %BF values are calculated using TBW and body weight measurements.
Dual Energy X-Ray Absorptiometry (DXA) measurements were obtained in the anterior posterior supine position using a Hologic Discovery A® (Hologic, Inc.) fan beam scanner generating X-rays at 2 energy levels (100 and 70 kV). The device uses the differential attenuation of the X-ray beam at these two energies to calculate the bone mineral content and soft tissue composition in the scanned region. A whole body scan followed by a Hip/Spine scan was performed including measurements of bone density and body composition from the head to distal feet in the supine position. The scan included bone mass and body composition from the head to distal feet while in the supine position. Data were expressed as grams of fat (FM), grams of lean tissue mass (LBM), and percent body fat (%BF). Bone mineral density (g/cm2) and bone mineral content (g) were also recorded.
Anthropometric measurements, including skin folds, weight, and height, were recorded using standard devices and methods by 2 members of the nutrition staff dedicated to the study. An electronic digital scale® (ScaleTronics, White Plains, NY) accurate to 0.1 kg was used to measure body weight (kg). Standing height (cm) was measured by a Harpenden® stadiometer (Holtain Ltd., Crymych, UK) to the nearest 0.1 cm. Weight for age z-score (WAZ), weight for height z-score (WHZ) and height for age z-scores (HAZ) were calculated using the WHO standards 12. Triceps, biceps, iliac, and subscapular skinfold thickness (SF) were measured to the nearest 0.2 mm using Lange skin calipers. Mid-arm circumference (MUAC) was measured to the nearest 0.1 cm using a flexible non-stretchable plastic tape. Mid arm muscle area (MAMA) was calculated according to the equation. MAMA = (MUAC − 3.1416 * TSF)2 / 4*3.1416. Fat mass (kg) and %BF were calculated from published equations using 4 skinfold thickness measurements 13. All anthropometric measurements were performed in triplicate by a two independent observers, and the average value of these three measurements was recorded.
Statistical analysis
Categorical data were tabulated using frequency and percentage, and continuous data described using median and inter-quartile range (IQR). Bland Altman analysis was used to examine the agreement between BIA and deuterium dilution in measuring total body water (TBW) and lean body mass (LBM); and between BIA and dual X-ray absorptiometry (DXA) techniques in measuring LBM and FM 14,15. FM and %BF obtained by BIA and anthropometry, were also compared in relation to those by deuterium dilution. All analyses were conducted in SAS® Version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Fifteen children with intestinal failure, median (IQR) age 7.2 (5.0, 10.0) years, 10 (67%) male, were enrolled. Nine (60%) subjects were dependent on parenteral nutrition at the time of the first visit. The etiology of IF included SBS secondary to gastroschisis (20%), midgut volvulus (20%), necrotizing enterocolitis (13%) and motility disorders (17%). Patient characteristics, including the median (IQR) values for z-scores of anthropometric variables, are reported in Table 1. The cohort was mildly underweight (median WAZ of -0.9), stunted (median HAZ of -1.58) and had evidence of low muscle mass (median MAMA z score of -1.41). The body composition measurements by the different techniques are summarized in Table 2. Percent body fat by all four techniques (BIA, DXA, deuterium dilution and sum of four skinfolds method) averaged between 21 and 26%.
Table 1. Demographic and clinical characteristics of 15 children with intestinal failure at enrollment.
| Characteristic | Median (IQR) or n (%) |
|---|---|
| Age (y) | 7.2 (5.0, 10.0) |
| Male sex | 10 (67%) |
| Diagnosis | |
| Gastroschisis | 3 (20%) |
| Midgut volvulus | 3 (20%) |
| Necrotizing enterocolitis (NEC) | 2 (13%) |
| Pseudo-obstruction | 2 (13%) |
| Mesenteric vascular insufficiency | 2 (13%) |
| Jejunal atresia | 1 (7%) |
| Cloacal exstrophy | 1 (7%) |
| Hirschsprung's disease | 1 (7%) |
| Citrulline (umol/L) | 24 (14, 30) |
| PN dependent | 9 (60%) |
| Oral/enteral intake versus total energy intake (%) | |
| All subjects | 69 (22, 100) |
| Parenteral nutrition dependent | 29 (17, 52) |
| Weight (kg) | 19.9 (16.7, 25.7) |
| Weight for age z-score | -0.90 (-1.10, -0.30) |
| Height for age z-score | -1.58 (-2.24, -0.97) |
| Body mass index z-score | 0.49 (-0.72, 0.81) |
| Upper arm muscle area z-score | -1.41 (-1.97, -0.75) |
| Scapular skin-fold z-score | 0.16 (-0.26, 1.19) |
Table 2. Body composition in children with intestinal failure (n=15 subjects).
| Characteristic | N | Median (IQR) |
|---|---|---|
| Total weight (kg) | 15 | 19.9 (16.7, 25.7) |
| TBW (L) | ||
| BIA | 15 | 11.0 (8.9, 17.0) |
| Deuterium | 15 | 12.0 (8.4, 15.5) |
| LBM (kg) | ||
| BIA | 15 | 14.2 (11.6, 22.3) |
| Deuterium | 15 | 16.7 (11.7, 21.5) |
| DXA | 11 | 18.5 (12.0, 20.8) |
| Fat mass (kg) | ||
| Anthropometry | 15 | 3.9 (3.5, 5.4) |
| BIA | 15 | 5.3 (4.3, 6.1) |
| Deuterium | 15 | 4.3 (3.5, 6.3) |
| DXA | 11 | 5.3 (4.1, 6.5) |
| % Body fat | ||
| Anthropometry | 15 | 21.4 (15.8, 24.5) |
| BIA | 15 | 25.6 (19.2, 33.1) |
| Deuterium | 15 | 23.5 (17.0, 29.2) |
| DXA | 11 | 23.2 (19.7, 28.6) |
TBW – total body water, LBM = lean body mass, BIA = bioelectric impedance analysis, DXA = dual energy x-ray absorptiometry.
Bland Altman analyses (Figure 1) of body composition measurements by BIA and deuterium dilution technique showed comparable measures for total body water (L) and lean body mass (kg) measurements by the 2 methods, with mean bias (limits) of 0.9 (-3.2, 5.0) and 0.1 (-5.4 to 5.6) respectively. The limits of agreement were within 1 SD of the mean bias in 12/14 (86%) subjects. Of note, the limits of agreement were wider for LBM compared to TBW. The mean bias (limits) for FM (kg) and %BF values by the 2 methods was 0.4 (-3.8 to 4.6) kg and 1.7 (-16.9, 20.3) respectively. The limits of agreement were within 1 SD of the mean bias, in 11/14 (79%) subjects for both fat mass and percent body fat.
Figure 1. Total body water, lean body mass, fat mass and percent body fat measurements in children with intestinal failure – agreement between BIA and Deuterium dilution methods.
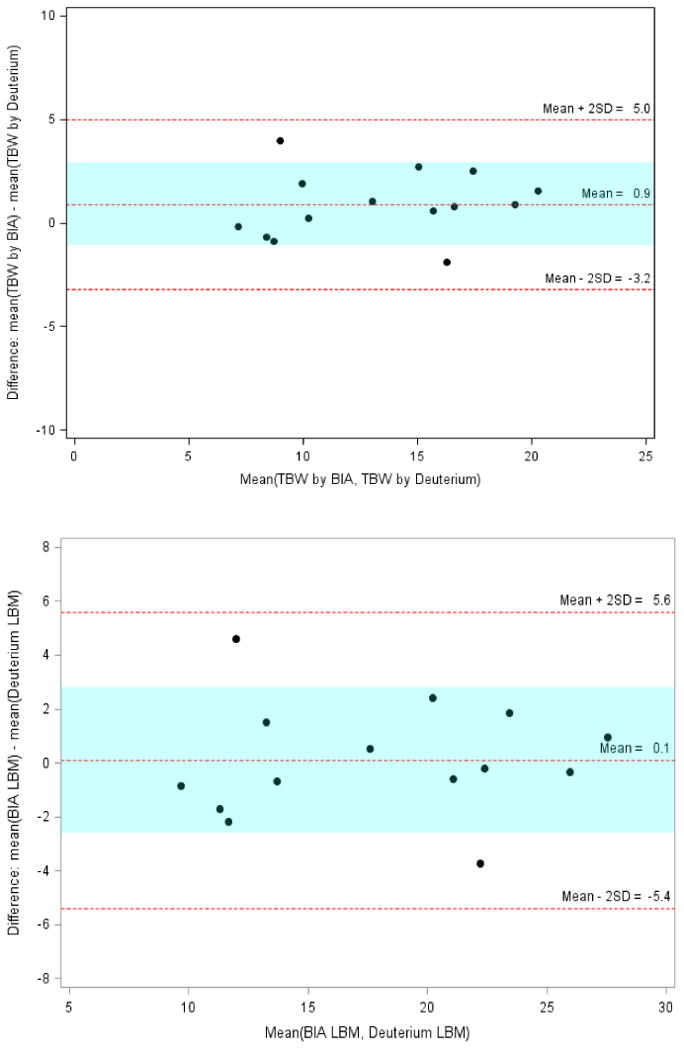
(Panel A: Total Body Water; Panel B: Lean body mass)
TBW = total body water, LBM = lean body mass,
BIA = bioelectric impedance analysis.
Colored band is at ±1SD.
The mean bias for FM (kg) and %BF values derived by anthropometry and deuterium dilution was 0.2 (limits -4.2, 4.6) and -0.2 (-19.5, 19.1). Figure 2 shows Bland Altman plots with mean bias (limits) of agreement for FM and %BF values between anthropometry and deuterium dilution techniques. The limits of agreement were within 1 SD of the mean bias, in 10/14 (71%) subjects for both fat mass and percent body fat. Hence, in our cohort the agreement for FM and %BF measurements with those by isotope dilution method was comparable between BIA and anthropometry.
Figure 2. Fat mass and percent body fat in children with intestinal failure – agreement between anthropometry and deuterium dilution.
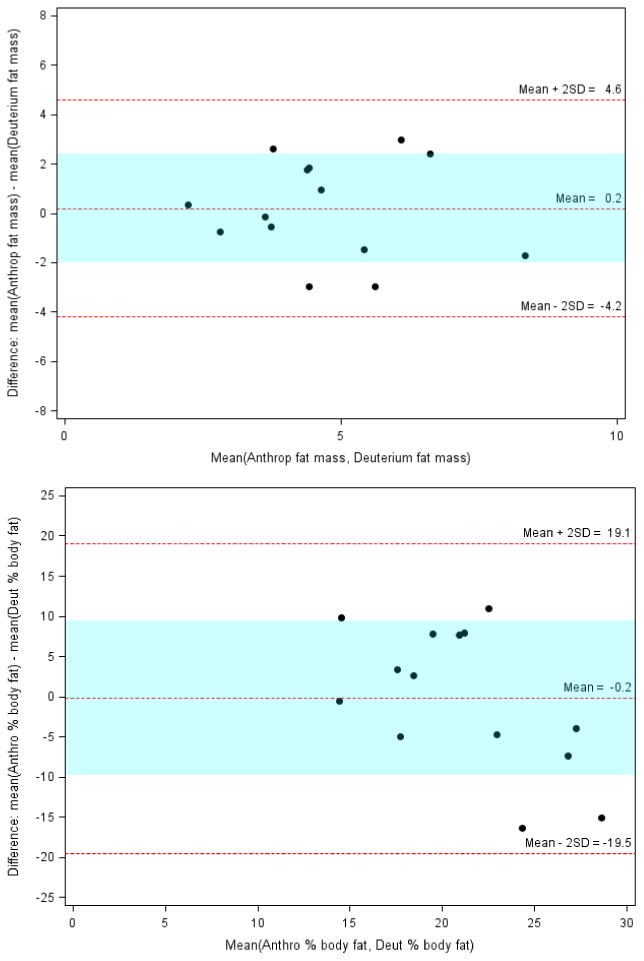
Panel A: Fat mass (FM)
Panel B: Percent body Fat (%BF)
FM: Anthropometry vs Deuterium: Mean bias (limits) = 0.2 (-4.2 to 4.6)
%BF: Anthropometry vs Deuterium: Mean bias (limits) = -0.2 (-19.5 to 19.1)
In subjects over 5 years of age (n=11), LBM (kg) and FM (kg) measurements by BIA were in agreement with these measurements by DXA scan, with mean bias (limits) of 1.6 (-3.0 to 6.3) kg and -0.1 (-3.2 to 3.1) kg, respectively. Fat mass (kg) and percent body fat derived by anthropometry were in agreement with DXA with mean bias (limits) of -0.4 (-3.8, 3.1) and -3.4 (-18.3, 11.4) respectively.
Discussion
Total body water (TBW) and lean body mass (LBM) assessments with BIA were in agreement with those measured by deuterium dilution and DXA scan techniques in our cohort of children with intestinal failure. The mean biases obtained by Bland Altman analysis of agreement between these techniques were small, suggesting that BIA may be suitable for population studies. However, the limits of agreement were wide, and individual values of TBW and LBM assessments by BIA must be interpreted with caution. The limits were slightly larger for LBM than for TBW for the entire cohort. When compared to deuterium dilution derived values of FM and %BF by BIA and anthropometric methods were comparable, with small mean biases. However, limits of agreement were wide for both methods, in some individual cases reaching clinically unacceptable levels.
A variety of techniques of body composition measurement, including body densitometry by underwater weighing, neutron activation analysis, total body potassium determination or dual-energy x-ray absorptiometry (DXA) have been described in the literature, but are not feasible for routine bedside use in pediatric patients 16-18. BIA provides an alternative that is safe, relatively easy, and can be applied to pediatric patients at the bedside to derive clinically relevant information 19-21. BIA has been previously reported to provide accurate measurement of TBW, when compared to deuterium dilution, in children 22,23. However, the limits of agreement in these studies are large or comparisons with reference standard method were made by correlation rather than analysis of agreement. Our current study shows similarly small mean bias of agreement between BIA and deuterium dilution techniques for TBW and LBM measurements, but with wide limits of agreement. For greater than 80% of subjects in our cohort, the bias for TBW and LBM by BIA was within 1 SD of the mean bias. For this subgroup, BIA would estimate TBW with a potential error of up to -8% or +20%. This could represent underestimation of up to 2kg and overestimation of up to 5 kg in a child with TBW of 25 kg. For LBM values by BIA, the potential error for this subgroup ranged from -14% to +14%. This would represent a potential BIA underestimation or overestimation of LBM by 3.5kg in a child with total LBM of 25 kg.
The validity of BIA in individual patients has been questioned in the past 24. In a study of adults with SBS, BIA estimates of TBW and fat free mass were examined for agreement with these estimates by DXA 25. Mean bias for FFM by the 2 techniques was -1.6. However, similar to our observation, the limits of agreement were wide (-10.7 to +7.4). Fluid shifts and intravenous fluid administration prior to the study procedures were thought to have influenced the hydration state and hence the accuracy of BIA estimates in this study. Similarly, disagreement between BIA and DXA estimates of fat free mass has been reported in patients with obesity, cirrhosis and ileostomies 26-28.
In the 2-component model of body composition, weight is considered composed of FM and FFM. Because water is associated predominantly with fat-free tissue, TBW can be used to provide an estimate of FFM. The water content of LBM is presumed to be constant and the body fat is anhydrous 29. Using these assumptions, TBW measures are used to estimate LBM by applying age-appropriate hydration factors 30,31. The underlying assumption that hydration of lean tissue is constant at 73% may be true for adults, but children may have a higher aqueous fraction of their fat free mass, and this factor may vary in different age groups 32,33. Hydration factor also varies dependent on nutritional status, and is thought to be higher in obese as well as undernourished individuals 34,35. Changes in body water content, the assumption of a fixed hydration factor and errors introduced by equations used to derive LBM from TBW, can result in unreliable BIA estimates of TBW and body composition measurement. TBW is measured directly by deuterium dilution, while it and LBM are calculated variables by BIA. The limits of agreement for LBM (by BIA versus deuterium dilution methods) were wider compared to those for TBW in our study. This may reflect the impact of the proprietary equation used by the BIA manufacturer to derive the LBM value. BIA provides measurements of electrical properties (impedance) that are calibrated against other reference methods (isotope dilution, DXA) to derive prediction equations for estimation of the components of body composition. A number of equations are used to estimate body composition from electrophysical measurements 36. Errors may be introduced when evaluating a population that is distinct from one that was used to derive the equations 8. Furthermore, the mathematical models or equations used to generate estimates of derives body composition values by commercial bioelectrical impedance devices such as the one used in our study, are not available 37. Future studies of BIA must examine the impact of using different hydration factors and proprietary equations for LBM derivation on its agreement with a reference standard.
In our current study, mean BIA measures were comparable to anthropometry as a measure of FM and %BF, in comparison with deuterium dilution. Anthropometric procedures require standardized techniques and an expert operator. Anthropometry may be difficult to obtain in children, involving manipulation of extremities and skinfolds, often in an uncooperative or distressed child 38. The results of our study suggest a role of BIA as an alternative to anthropometry to obtain assessments of body composition in children. Compared to skin folds measurements, BIA is noninvasive, relatively faster and may be better tolerated 36. However, the wide limits if agreement in our cohort suggests that FM and BF% assessments by both these bedside methods cannot be guaranteed in some individuals. The currently used equations used to derive these values from bioelectric measurements (BIA) and skin folds (anthropometry) may need to be revisited. An accurate bedside method for FM assessment remains elusive for this cohort.
Children with IF are characterized by diarrhea, malabsorption and risk of nutrient deficiencies and malnutrition 7,39,40. Children with SBS in our study had a median WAZ of -0.90 and HAZ of -1.58. Median values for BMIZ and upper arm muscle area z score were 0.49 and -1.41 respectively, suggesting a population with lower than average lean body mass. Pichler et al reported abnormal body composition in their cohort of 34 children with intestinal failure 41. Limb muscle mass was significantly lower than normal in this cohort and in patients completely dependent on PN, the total as well as truncal fat mass index was higher. These findings indicate a possibility of energy intake (prescription) in excess of actual requirement. We have recently reported significant variability in resting energy expenditure in a cohort of children with IF associated liver disease 42. Energy prescriptions based on standard equations may not be accurate and result in a potential for overfeeding in children with IF. Weight stability or improvement over time has been shown to mask underlying sarcopenia and may be misleading 43. Skeletal muscle mass loss may be offset by a corresponding increase in total body fat mass or fluid shifts, resulting in falsely reassuring weight stability or weight gain in these cases. In the future, bedside measure of body composition in addition to weight changes could help titrate and ensure optimal nutrient interventions in pediatric IF.
There are a number of limitations to our current study. We examined agreement between BIA and anthropometry methods of body composition assessment against DXA and deuterium dilution methods in pediatric SBS population. DXA and deuterium dilution techniques are accepted as accurate and validated methods against which the accuracy of BIA and anthropometry can be examined. Patients with SBS may have variable enteral absorption during periods of transition from parenteral to enteral nutrition. Hence, the absorption of enterally administered deuterium isotope in our study could be problematic. Our group has an extensive history of applying stable isotope techniques in surgical and critically ill patients 44-47. The amount of isotope was small and administered through the feeding tubes in most patients. Subjects with contraindications to EN were excluded and there were no cases of intolerance to the isotope dosage. Furthermore, altered total body fluid could lead to inaccurate determination of body composition 48. We enrolled subjects with no evidence of current illness, edema, fluid shifts, diuretic use or hospitalization. The error due to the fixed assumption of hydration factor on children may not become clinically significant in the absence of obesity 33. Hence, the hydration assumptions are unlikely to impact the estimation of TBW and lean body mass by BIA in our cohort. Finally, this was a convenience pilot study with a small sample size. The results of our study indicate a role for bedside body composition assessment in this group of patients that should be further elucidated in larger studies.
Conclusions
Children with intestinal failure may be at increased risk of nutritional deterioration during the rehabilitative phase of their illness, when parenteral nutrition is gradually replaced by enteral intake. A reliable bedside method for body composition assessment in this cohort is therefore desirable. BIA provided bedside TBW and LBM values that were in agreement with deuterium dilution and DXA techniques, with mean bias close to zero, in our cohort of pediatric intestinal failure. However, the limits of agreement between BIA values and the reference standard were wide, thus calling into question the applicability of this technique for individual patients. BIA and anthropometry were comparable for FM and %BF in relation to deuterium, with small bias. Hence, BIA may be a comparable alternative to anthropometry when skin fold measures are unavailable or not well tolerated.
Our results suggest a potential role for BIA when assessing TBW and LBM in population and cohort studies. Values in individual patients must be interpreted with caution and with awareness of the magnitude of potential error. Future studies should explore alternative methods of body composition in children with intestinal failure.
Acknowledgments
Funding: This project was funded in part by a) the Fred. Lovejoy Research Grant (NM), b) the MO1-RR02172 grant (NM) from the National Center for Research Resources, National Institutes of Health, to the Children's Hospital Boston General Clinical Research Center, which is now supported by Harvard Catalyst (UL1RR025758), and c) the NICHD K24HD058795 award (CD).
Footnotes
Conflicts: None
References
- 1.Andorsky DJ, Lund DP, Lillehei CW, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001 Jul;139(1):27–33. doi: 10.1067/mpd.2001.114481. [DOI] [PubMed] [Google Scholar]
- 2.Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr. 2004 Apr;79(4):613–618. doi: 10.1093/ajcn/79.4.613. [DOI] [PubMed] [Google Scholar]
- 3.Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987 Mar;27(3):262–266. doi: 10.1097/00005373-198703000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Sood M, Adams JE, Mughal MZ. Lean body mass in children with cystic fibrosis. Arch Dis Child. 2003 Sep;88(9):836. doi: 10.1136/adc.88.9.836-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brambilla P, Rolland-Cachera MF, Testolin C, et al. Lean mass of children in various nutritional states. Comparison between dual-energy X-ray absorptiometry and anthropometry. Ann N Y Acad Sci. 2000 May;904:433–436. doi: 10.1111/j.1749-6632.2000.tb06497.x. [DOI] [PubMed] [Google Scholar]
- 6.Schols AM, Mostert R, Soeters PB, Wouters EF. Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax. 1991 Oct;46(10):695–699. doi: 10.1136/thx.46.10.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pichler J, Chomtho S, Fewtrell M, Macdonald S, Hill S. Body composition in paediatric intestinal failure patients receiving long-term parenteral nutrition. Arch Dis Child. 2013 Oct 28; doi: 10.1136/archdischild-2012-303516. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004 Oct;19(5):433–446. doi: 10.1177/0115426504019005433. [DOI] [PubMed] [Google Scholar]
- 9.Wang XB, Ren JA, Li JS. Sequential changes of body composition in patients with enterocutaneous fistula during the 10 days after admission. World J Gastroenterol. 2002 Dec;8(6):1149–1152. doi: 10.3748/wjg.v8.i6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scrimgeour CM, Rollo MM, Mudambo SM, Handley LL, Prosser SJ. A simplified method for deuterium/hydrogen isotope ratio measurements on water samples of biological origin. Biol Mass Spectrom. 1993 Jul;22(7):383–387. doi: 10.1002/bms.1200220704. [DOI] [PubMed] [Google Scholar]
- 11.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982 May;35(5 Suppl):1169–1175. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 12.de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull. 2004 Mar;25(1 Suppl):S15–26. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- 13.Zemel BS, Riley EM, Stallings VA. Evaluation of methodology for nutritional assessment in children: anthropometry, body composition, and energy expenditure. Annu Rev Nutr. 1997;17:211–235. doi: 10.1146/annurev.nutr.17.1.211. [DOI] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–582. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–310. [PubMed] [Google Scholar]
- 16.Krzywicki HJ, Ward GM, Rahman DP, Nelson RA, Consolazio CF. A comparison of methods for estimating human body composition. Am J Clin Nutr. 1974 Dec;27(12):1380–1385. doi: 10.1093/ajcn/27.12.1380. [DOI] [PubMed] [Google Scholar]
- 17.Cohn SH, Ellis KJ, Wallach S. In vivo neutron activation analysis. Clinical potential in body composition studies. Am J Med. 1974 Nov;57(5):683–686. doi: 10.1016/0002-9343(74)90841-9. [DOI] [PubMed] [Google Scholar]
- 18.Johnson J, Dawson-Hughes B. Precision and stability of dual-energy X-ray absorptiometry measurements. Calcif Tissue Int. 1991 Sep;49(3):174–178. doi: 10.1007/BF02556113. [DOI] [PubMed] [Google Scholar]
- 19.Sherriff A, Wright CM, Reilly JJ, McColl J, Ness A, Emmett P. Age- and sex-standardised lean and fat indices derived from bioelectrical impedance analysis for ages 7-11 years: functional associations with cardio-respiratory fitness and grip strength. Br J Nutr. 2009 Jun;101(12):1753–1760. doi: 10.1017/S0007114508135814. [DOI] [PubMed] [Google Scholar]
- 20.Wright CM, Sherriff A, Ward SC, McColl JH, Reilly JJ, Ness AR. Development of bioelectrical impedance-derived indices of fat and fat-free mass for assessment of nutritional status in childhood. Eur J Clin Nutr. 2008 Feb;62(2):210–217. doi: 10.1038/sj.ejcn.1602714. [DOI] [PubMed] [Google Scholar]
- 21.Kyle UG, Piccoli A, Pichard C. Body composition measurements: interpretation finally made easy for clinical use. Curr Opin Clin Nutr Metab Care. 2003 Jul;6(4):387–393. doi: 10.1097/01.mco.0000078988.18774.3d. [DOI] [PubMed] [Google Scholar]
- 22.Nyboer J. Workable volume and flow concepts of bio-segments by electrical impedance plethysmography. TIT J Life Sci. 1972;2(1):1–13. [PubMed] [Google Scholar]
- 23.Littlewood RA, Trocki O, Cleghorn G. Measured and predicted total body water in children with myelomeningocele. J Paediatr Child Health. 2003 May-Jun;39(4):278–281. doi: 10.1046/j.1440-1754.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- 24.Ellis KJ, Shypailo RJ, Wong WW. Measurement of body water by multifrequency bioelectrical impedance spectroscopy in a multiethnic pediatric population. Am J Clin Nutr. 1999 Nov;70(5):847–853. doi: 10.1093/ajcn/70.5.847. [DOI] [PubMed] [Google Scholar]
- 25.Carlsson E, Bosaeus I, Nordgren S. Body composition in patients with short bowel syndrome: an assessment by bioelectric impedance spectroscopy (BIS) and dual-energy absorptiometry (DXA) Eur J Clin Nutr. 2004 Jun;58(6):853–859. doi: 10.1038/sj.ejcn.1601886. [DOI] [PubMed] [Google Scholar]
- 26.Carlsson E, Bosaeus I, Nordgren S. Body composition in patients with an ileostomy and inflammatory bowel disease: validation of bio-electric impedance spectroscopy (BIS) Eur J Clin Nutr. 2002 Jul;56(7):680–686. doi: 10.1038/sj.ejcn.1601378. [DOI] [PubMed] [Google Scholar]
- 27.Lehnert ME, Clarke DD, Gibbons JG, et al. Estimation of body water compartments in cirrhosis by multiple-frequency bioelectrical-impedance analysis. Nutrition. 2001 Jan;17(1):31–34. doi: 10.1016/s0899-9007(00)00473-1. [DOI] [PubMed] [Google Scholar]
- 28.Cox-Reijven PL, Soeters PB. Validation of bio-impedance spectroscopy: effects of degree of obesity and ways of calculating volumes from measured resistance values. Int J Obes Relat Metab Disord. 2000 Mar;24(3):271–280. doi: 10.1038/sj.ijo.0801123. [DOI] [PubMed] [Google Scholar]
- 29.Davies PS, Wells JC. Calculation of total body water in infancy. Eur J Clin Nutr. 1994 Jul;48(7):490–495. [PubMed] [Google Scholar]
- 30.Bunt JC, Lohman TG, Boileau RA. Impact of total body water fluctuations on estimation of body fat from body density. Med Sci Sports Exerc. 1989 Feb;21(1):96–100. doi: 10.1249/00005768-198902000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Friis-Hansen BJ, Holiday M, Stapleton T, Wallace WM. Total body water in children. Pediatrics. 1951 Mar;7(3):321–327. [PubMed] [Google Scholar]
- 32.Hewitt MJ, Going SB, Williams DP, Lohman TG. Hydration of the fat-free body mass in children and adults: implications for body composition assessment. Am J Physiol. 1993 Jul;265(1 Pt 1):E88–95. doi: 10.1152/ajpendo.1993.265.1.E88. [DOI] [PubMed] [Google Scholar]
- 33.Wells JC, Williams JE, Chomtho S, et al. Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y. Am J Clin Nutr. 2010 Mar;91(3):610–618. doi: 10.3945/ajcn.2009.28428. [DOI] [PubMed] [Google Scholar]
- 34.Bray GA, DeLany JP, Harsha DW, Volaufova J, Champagne CC. Evaluation of body fat in fatter and leaner 10-y-old African American and white children: the Baton Rouge Children's Study. Am J Clin Nutr. 2001 Apr;73(4):687–702. doi: 10.1093/ajcn/73.4.687. [DOI] [PubMed] [Google Scholar]
- 35.Beddoe AH, Streat SJ, Hill GL. Hydration of fat-free body in protein-depleted patients. Am J Physiol. 1985 Aug;249(2 Pt 1):E227–233. doi: 10.1152/ajpendo.1985.249.2.E227. [DOI] [PubMed] [Google Scholar]
- 36.Sproule DM, Montes J, Dunaway SL, et al. Bioelectrical impedance analysis can be a useful screen for excess adiposity in spinal muscular atrophy. J Child Neurol. 2010 Nov;25(11):1348–1354. doi: 10.1177/0883073810365185. [DOI] [PubMed] [Google Scholar]
- 37.Foster KR, Lukaski HC. Whole-body impedance--what does it measure? Am J Clin Nutr. 1996 Sep;64(3 Suppl):388S–396S. doi: 10.1093/ajcn/64.3.388S. [DOI] [PubMed] [Google Scholar]
- 38.Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl. 2006 Apr;450:38–46. doi: 10.1111/j.1651-2227.2006.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 39.Ubesie AC, Cole CR, Nathan JD, et al. Micronutrient deficiencies in pediatric and young adult intestinal transplant patients. Pediatr Transplant. 2013 Nov;17(7):638–645. doi: 10.1111/petr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ubesie AC, Heubi JE, Kocoshis SA, et al. Vitamin D deficiency and low bone mineral density in pediatric and young adult intestinal failure. J Pediatr Gastroenterol Nutr. 2013 Sep;57(3):372–376. doi: 10.1097/MPG.0b013e31829c10eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichler J, Chomtho S, Fewtrell M, Macdonald S, Hill S. Body composition in paediatric intestinal failure patients receiving long-term parenteral nutrition. Arch Dis Child. 2014 Feb;99(2):147–153. doi: 10.1136/archdischild-2012-303516. [DOI] [PubMed] [Google Scholar]
- 42.Duro D, Mitchell PD, Mehta NM, et al. Variability of Resting Energy Expenditure in Infants and Young Children With Intestinal Failure-Associated Liver Disease. J Pediatr Gastroenterol Nutr. 2013 Dec 19; doi: 10.1097/MPG.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallagher D, Ruts E, Visser M, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000 Aug;279(2):E366–375. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 44.Jaksic T, Shew SB, Keshen TH, Dzakovic A, Jahoor F. Do critically ill surgical neonates have increased energy expenditure? J Pediatr Surg. 2001 Jan;36(1):63–67. doi: 10.1053/jpsu.2001.20007. [DOI] [PubMed] [Google Scholar]
- 45.Jaksic T, Wagner DA, Burke JF, Young VR. Proline metabolism in adult male burned patients and healthy control subjects. Am J Clin Nutr. 1991 Aug;54(2):408–413. doi: 10.1093/ajcn/54.2.408. [DOI] [PubMed] [Google Scholar]
- 46.Shew SB, Beckett PR, Keshen TH, Jahoor F, Jaksic T. Validation of a [13C]bicarbonate tracer technique to measure neonatal energy expenditure. Pediatr Res. 2000 Jun;47(6):787–791. doi: 10.1203/00006450-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Shew SB, Keshen TH, Jahoor F, Jaksic T. Assessment of cysteine synthesis in very low-birth weight neonates using a [13C6]glucose tracer. J Pediatr Surg. 2005 Jan;40(1):52–56. doi: 10.1016/j.jpedsurg.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Kushner RF, Schoeller DA. Estimation of total body water by bioelectrical impedance analysis. Am J Clin Nutr. 1986 Sep;44(3):417–424. doi: 10.1093/ajcn/44.3.417. [DOI] [PubMed] [Google Scholar]


