Abstract
Birt-Hogg-Dubé syndrome (BHD) is a rare, autosomal dominant disorder characterized by the development of hair follicle tumors, renal tumors and pulmonary cysts. BHD is caused by heterozygous, predominantly truncating mutations in the folliculin (FLCN) gene located on chromosome 17, which encodes a highly conserved tumor suppressor protein. Although management of renal tumors of low malignant potential is the primary focus of longitudinal care, pulmonary manifestations including cyst formation and spontaneous pneumothorax are among the most common manifestations in BHD. Due to the lack of awareness, there is commonly a delay in the pulmonary diagnosis of BHD and patients are frequently mislabeled as having chronic obstructive lung disease, emphysema or common bullae/blebs. A family history of pneumothorax is present in 35 % of patients with BHD. Certain imaging characteristics of the cysts, including size, basilar and peripheral predominance, perivascular and periseptal localization, and elliptical or lentiform shape can suggest the diagnosis of BHD based on inspection of the chest CT scan alone. Recurrent pneumothoraces are common and early pleurodesis is recommended. A better understanding of role of FLCN in pulmonary cyst formation and long term studies to define the natural history of the pulmonary manifestations of BHD are needed.
Keywords: Birt-Hogg-Dubé, Pneumothorax, Pulmonary cysts, Folliculin
Introduction
Birt-Hogg-Dubé syndrome (BHD) is an autosomal dominant disorder characterized by the development of hair follicle tumors, renal tumors and pulmonary cysts (Online Mendelian Inheritance in Man # 135150). Hornstein and Knickenberg [1] were the first to report the syndrome in two siblings with ‘perifollicular fibromatosis cutis with polyps of the colon’ and skin lesions, bilateral renal cysts and unilateral pulmonary cysts in their father. Hornstein published two additional manuscripts on the subject in 1976 [2, 3] before Birt et al. [4] in 1977 reported a family with fibrofolliculomas, trichodiscomas and achrochordons, suggested an autosomal dominant pattern of inheritance for these findings, and named the disorder.
Pulmonary manifestations are a common finding in this group of patients, but the disorder is rare and unfamiliar to many physicians, so the diagnosis is frequently delayed. In this review we focus on the lung cysts and pneumothoraces that occur in BHD.
Genetics
BHD is caused by mutations in the folliculin (FLCN) gene which encodes the protein FLCN. In 2001, Khoo et al. [5] performed a genome-wide linkage analysis using polymorphic microsatellite markers on a large Swedish family with BHD and mapped the BHD locus on chromosome 17p12-q11.2. At about the same time, Schmidt et al. [6] reported a genome-wide scan in a large kindred with BHD and localized the BHD locus to pericentromeric region of chromosome 17p. In 2002, Nickerson et al. [7] did recombination mapping in families with BHD, localized the susceptibility locus to 700 kb on chromosome 17p11.2 and identified a novel gene encoding a protein called FLCN. Schmidt et al. [8] further analyzed 30 families with BHD and found germline FLCN mutations in 84 %. The majority of BHD mutations were predicted to truncate FLCN, suggesting a loss-of-function mechanism for phenotype development. Based on their results, they suggested that FLCN may act as a tumor suppressor gene. In 2008, Toro et al. [9] studied 50 new families with BHD and found an 88 % detection rate of germline mutations in the FLCN gene. Most of these patients had truncating mutations as well. Kunogi et al. [10] studied the FLCN gene for mutations in 36 Japanese patients presenting with pulmonary cysts and found germline mutations in the FLCN gene in 69.4 % patients. They also suggested a potential racial difference in clinical manifestations of BHD as their patient population had a very low incidence of skin and renal involvement. To date, 142 unique DNA mutations of the FLCN gene have been implicated in the pathogenesis of BHD [11] and its role as a tumor suppressor gene is well established.
Function of folliculin and tumorigenesis
The exact function of FLCN gene and the mechanism of its tumor suppressor actions, however, is still unclear. It is most likely involved in energy, metabolism, and nutrient sensing through the mammalian target of rapamycin (mTOR) pathway. Baba et al. [12] identified a FLCN-interacting protein (FNIP1) and demonstrated its interaction with 5′ AMP-activated protein kinase (AMPK), a key molecule for energy sensing that negatively regulates mTOR activity. Inactivating mutations in this pathway result in dysregulated cell growth and protein synthesis, thus providing potential insights into the molecular mechanisms for the BHD phenotype. Two years later another FNIP1, FNIP2, was identified and revealed to be a homologue of FNIP1 [13, 14]. Similarly, other studies have explored the mechanism by which activation of the mTOR pathway leads to development of renal tumors and cysts [15, 16]. The involvement of mTOR pathway in the pathogenesis of BHD is also suggested by its phenotypic similarities with tuberous sclerosis complex (TSC), which is known to be caused by dysregulation of the mTOR pathway. This association was explored in a number of different studies [17, 18].
However, regulation of mTOR activity is not the only signaling pathway implicated in the tumor suppressor action of FLCN gene. Hong et al. [19] demonstrated a role for FLCN in the regulation of key TGF-beta signaling, suggesting a potentially important role for the pathway in tumorigenesis in BHD syndrome. Hong et al. [20] have also demonstrated that inactivation of FLCN leads to activation of the transcription factor TFE3, which might also lead to development of renal tumors in patients with BHD. Other investigators have described a model in which FLCN acts as a mitochondrial regulator, and reduced FLCN function leads to overexpression of nuclear mitochondrial gene expression [21, 22]. Nookala et al. [23] showed that FLCN is distantly related to differentially expressed in normal cells and neoplasia (DENN) domain proteins, and may play a role in vesicle membrane trafficking.
The role of FLCN in development of pulmonary cysts is poorly understood. While flcn ± mouse models have proven to be useful for studying the renal tumors associated with BHD, they have been less informative with respect to development of pulmonary cysts [18]. The expression of FLCN gene has been consistently found in renal tumors as well as skin fibrofolliculomas, but has not been characterized in pulmonary cysts. Warren et al performed fluorescent in situ hybridization to measure the expression of BHD mRNA in normal and neoplastic human tissues. They were able to demonstrate strong expression of BHD mRNA in the stromal cells within the connective tissue of lung and a weak expression in type I pneumocytes [24]. Furuya et al. [25] studied the lungs of 11 patients from 9 families of BHD. They found that the cells constituting the cysts stained positively for phospho-S6 ribosomal protein expression, suggesting activation of the mTOR pathway. Based on these results, they proposed that pulmonary cysts associated with BHD are a distinct entity and can be considered as a hamartoma-like lesion associated with deranged mTOR signaling. Further studies are needed to understand the molecular mechanisms leading to the development of pulmonary cysts in BHD. This is especially important as there are phenotypic variants of BHD in which patients present with either isolated or predominantly pulmonary manifestations [10, 26–28].
Pulmonary manifestations of BHD
The pulmonary manifestations of BHD are mainly related to the formation of pulmonary cysts and development of pneumothoraces. Cystic lung lesions likely develop in early to mid adulthood (30–40 years) [10, 27], but have been described in patients ranging in age from 20 to 85 years [29]. There is no gender predilection and there is no information about the role of cigarette smoking in cyst development.
Radiologic findings in BHD
There is evidence that pulmonary cysts occur with nearly complete penetrance in BHD. Toro et al. [30] studied a cohort of 198 patients with BHD and found that on CT examination, 89 % of patients with BHD had lung cysts. In a study by Furuya et al. [25], all patients with BHD who underwent thoracic CT (n = 11) had pulmonary cysts. The cysts in patients with BHD are frequently mislabeled as common bullae or blebs. The location of cysts in cases of BHD is different from the typical apical location seen in cases of primary spontaneous pneumothorax or emphysema [30, 31]. Tobino et al. [31] reviewed thin section thoracic CT scans of 12 patients with BHD and conducted a comprehensive analysis of the radiologic characteristics of pulmonary cysts. They found that majority of the cysts are located in the basilar medial regions (58 %), followed next by the basilar lateral regions (27 %) of the lungs. The number of cysts in each patient was variable (range 29–407). The cyst size was also quite variable but small cysts (<1 cm) were seen most commonly (75.6 %). Most of the cysts (76.6 %) were irregularly shaped. Almost 40 % of the cysts abutted the pleural surfaces. Similar findings were also reported in another study by Tobino et al. [32]. Based on these reports, the characteristic chest CT findings of patients with BHD can be summarized as multiple, irregularly-shaped, thin-walled pulmonary cysts of various sizes, predominantly distributed to the lower medial and subpleural regions of the lung.
Histology of pleuropulmonary lesions in BHD
Although the pathology of renal and cutaneous manifestations of BHD has been well described, there are very few reports of the histological characteristics of pulmonary cysts in BHD. Butnor et al. [33] described the histopathological findings in 2 patients with BHD who had undergone bleb resection following a spontaneous pneumothorax and found that the histology was consistent with typical emphysematous changes. They suggested that the major distinguishing factor is the basilar localization of cysts rather than the typical apical localization seen in other causes of spontaneous pneumothorax. The validity of the histological findings has been questioned as they were based on examination of ruptured cystic lesions in patients who had suffered pneumothorax. Only fragmented pleural walls were resected in these cases and detailed microscopic examination of these specimens was not available [34]. Furuya and Koga et al. [25, 35] have described histopathological findings of unruptured BHD cysts. They found that the inner surface of cysts was lined by epithelial cells, sometimes with a predominance of type II pneumocyte-like cuboidal cells, without evidence for neoplastic proliferation, inflammation, fibrosis or atypical morphology. The cysts occasionally contained internal septa consisting of alveolar walls or showed an “alveoli within an alveolus” pattern. Thus the authors concluded that the histopathology of BHD pulmonary cysts differs from that of common blebs or bullae. Examination of ruptured cysts can be misleading, since post-rupture remodeling can result in mesothelial invagination and fibrosis, which can be virtually indistinguishable from bullous changes [25]. Based on these findings, they suggested that surgeons performing pulmonary wedge resections in patients suspected of having BHD should also sample unruptured cystic areas [34]. Further studies are needed to determine if the histological characteristics of BHD are sufficiently distinctive to allow an expert pathologist to distinguish them from those of emphysema.
Clinical features of pleuropulmonary involvement in BHD
The most common presentation of pulmonary involvement with BHD is the occurrence of a spontaneous pneumothorax. Zbar et al. [36] found a 50-fold increase in the rate of development of pneumothorax in patients with BHD after adjusting for age. They also found that age is inversely correlated with development of pneumothorax. In a study of 198 patients with BHD, Toro et al. found that while 89 % (177/198) of patients had cystic lesions, only 24 % patients with BHD had a history of pneumothorax. The median age of development of pneumothorax was 38 years (range 22-71 years). There were no gender differences with regards to development of pneumothorax. The majority of the patients (75 %) had recurrent pneumothoraces. Further risk factor analysis done by Toro et al. [30], revealed that the presence of lung cysts was significantly associated with pneumothorax. Other parameters that were positively associated with pneumothorax included cyst size and cyst volume. Interestingly, age and smoking status were not found to be associated with pneumothorax in this cohort.
Another interesting aspect of the syndrome which warrants further investigation is the possibility of increased risk of pulmonary malignancies. Hartman et al. [18] found incidental development of pulmonary adenoma and adenocarcinoma in FLCN heterozygous mice. Ayo et al. [37] found a small focus of low-grade adenomatous hyperplasia as an incidental finding in a biopsy from a patient with BHD. Recently, there was a report of a patient with BHD who was found to have a 1.2 cm pulmonary histiocytoma [29]. Furuya et al. [34] also published a case of bronchoalveolar cell carcinoma in a patient with BHD. Whether haploinsufficiency in FLCN predisposes to lung cancer is unclear but further studies are warranted.
Pulmonary function studies (PFTs) in patients with BHD
Little data is available about the results of pulmonary function tests (PFTs) in patients with BHD. In spite of the presence of multiple cysts, pulmonary mechanics and gas exchange are typically well preserved or only mildly abnormal in this patient population [38]. Among 14 patients with BHD a study by Tobino et al. [32], the mean spirometric values (FEV1, FVC and FEV1/FVC) were found to be within the normal range. However, diffusion capacity for carbon monoxide (adjusted for alveolar volume) was found to be mildly decreased in patients with BHD (73.6 ± 10.4 %). They also showed that absolute FEV1 was inversely correlated with the cyst area measured by CT scan. Further studies are needed to evaluate lung function in patients with BHD, especially longitudinal studies to better define the natural history of lung function decline in this disorder.
Diagnosis and screening in BHD
The diagnosis of BHD should be suspected in a young patient presenting with spontaneous pneumothorax, especially those with a personal or family history of pneumothorax, skin lesions or renal tumors. Menko et al. [38] have suggested a set of diagnostic criteria for diagnosing BHD (Table 1). In the study by Toro et al. [30], 93 % of patients with BHD in his cohort were ascertained through a kidney tumor protocol had characteristic skin fibrofolliculomas. Trichodiscomas and angiofibromas (rarely) are also found in patients with BHD. Achrochordons (also known as skin tags) are very common skin lesions and are not specific for BHD, although fibrofolliculomas can occasionally be found hidden in these lesions. It is important to perform punch biopsies rather than shave biopsies, because it can be very difficult to differentiate angiofibromas from the hair follicle tumors (fibrofolliculomas and trichodiscomas) with shallow samples. The presence of renal or abdominal tumors on CT or MRI is also a useful diagnostic clue. The presence of fat is consistent with an angiomyolipoma, which is common in TSC or LAM and rare in BHD. Serum VEGF-D is elevated in patients with LAM and not in patients with BHD [39]. Not all patients with BHD will have characteristic skin and/or renal findings. Kunogi et al. [10] studied 36 patients with lung cysts and found germline mutations in FLCN gene in 69.4 % patients, but their subgroup of patients had very low prevalence of skin and renal involvement. Similar findings have been reported in other pulmonary-based studies as well [26–28]. Painter et al. [26] studied a Finnish family with a dominantly inherited tendency to develop spontaneous pneumothoraces. They identified the causative mutation to be a 4 base pair deletion in the FLCN gene, and also found that it led to the development of bullous lesions with 100 % penetrance. No evidence of fibrofolliculomas or renal tumors was found in this family. Gunji et al. [27] studied 8 patients with lung cysts, without skin or renal involvement, and found germline mutations in the BHD gene in 5 out of those 8 patients. Graham et al. [28] studied 12 families with a family history of spontaneous pneumothorax and identified nonsense mutations in the FLCN gene in 2 out of the 12 families. None of the members from these 2 families had any known skin or renal manifestations of BHD. Thus, there are large differences in the prevalence of skin involvement based on mode of ascertainment. It is important that physicians maintain a high index of suspicion for BHD in young patients presenting with a spontaneous pneumothorax.
Table 1. Diagnostic criteria for BHD.
| Diagnostic Criteria for BHD | |
|---|---|
| Major criteria |
|
| Minor criteria |
|
Patients should fulfill one major or two minor criteria for diagnosis. Adapted from Menko et al. [38], Lancet Oncology 2009
High resolution CT scan of the chest is the modality of choice for the diagnosis of BHD. The differential diagnosis of cystic lung disease includes lymphangioleiomyomatosis (LAM), pulmonary langerhans cell histiocytosis (PLCH), lymphocytic interstitial pneumonia (LIP), follicular bronchiolitis, light chain deposition disease, Sjogren's syndrome and amyloidosis [40]. A proposed diagnostic algorithm for BHD is presented in Table 2. Perhaps, the most difficult to differentiate from BHD amongst the above mentioned diseases is LAM, especially when associated with tuberous sclerosis with renal and cutaneous involvement. Important clinical and radiological differences between these two diseases are summarized in Tables 3 and 4. [33]. The presence of a fat containing renal tumor on abdominal MRI or CT is suggestive of an angiomyolipoma, which is more typical for LAM than BHD. BHD, on the other hand, can cause a variety of renal tumors ranging from benign oncocytomas and chromophobe adenomas to renal cell carcinoma. Furthermore, the clinical features, especially, the cyst characteristics on high resolution CT scans can help differentiate between BHD and LAM. A strong family history of pneumothoraces can be a helpful clue to the diagnosis of BHD. Diseases such as Marfan syndrome, homocystinuria, Ehler-Danlos syndrome, and alpha-one antitrypsin deficiency can also present with a history of familial spontaneous pneumothorax (FSP) [41]. Most of these conditions have other diagnostically useful features and markers to assist clinicians who maintain a high index of suspicion. A list of causes of FSP along with their characteristic clinical findings is provided in Table 5.
Table 2. Proposed diagnostic criteria for BHD.
| Definite pulmonary BHD |
|
| Probable pulmonary BHD | |
| Possible pulmonary BHD | Compatible or characteristic HRCT |
Characteristic lung HRCT findings: Multiple thin-walled round, elliptical or lentiform well-defined air-filled cysts, without internal structure, in a basilar, medial and subpleural predominant distribution, with preserved or increased lung volume, and no other significant pulmonary involvement (specifically no interstitial lung disease)
Compatible HRCT findings: Thin walled cysts without the more typical elliptical shape or subpleural distribution
Failure to meet modified Gomez criteria for TSC. Other features that may exclude TSC and LAM include absence of positive serum VEGF-D, surgical lung biopsy negative for LAM, negative genetic testing for TSC, CT or MRI of the head negative for tubers, subependymal nodules or subependymal giant cell astrocytomas
Radiographic diagnosis based on fat content by MRI or CT acceptable
Table 3. Clinical characteristics of BHD versus LAM.
| BHD | LAM | |
|---|---|---|
| Inheritance pattern | Autosomal dominant | Autosomal dominant or sporadic |
| Percent of patients with cysts | About 90 % | Nearly 100 % |
| Incidence of pneumothorax | 24 % | 70 % |
| Average age at first pneumothorax | 38 | 35 |
| Rate of recurrent pneumothorax | 70 % | 70 % |
| Exacerbation by pregnancy | No | Yes |
| Gender | Women = men | Women > men |
Table 4. Cyst characteristics of BHD versus LAM.
| BHD | LAM | |
|---|---|---|
| Distribution | Basilar/peripheral/ subpleural | Diffuse |
| Size | 75 % <1 cm | 2 mm–2 cm |
| Shape | Elliptical, lentiform | Round |
| Epithelial lining | Continuous | Discontinuous |
| Smooth muscle infiltration | No | Yes |
| Relationship to vessels | Abut proximal arteries and veins | No relationship |
| Relationship to pleura | Abut pleural surfaces | No relationship |
| HMB-45 | Negative | Positive |
| Pathological examination diagnostic | No | Yes |
Table 5. Conditions associated with familial spontaneous pneumothorax.
| Disease | Gene | Clinical features |
|---|---|---|
| Marfan syndrome | Fibrillin 1 | Increased height, disproportionately long limbs and digits, subluxation of eye lens and dilatation of aortic root |
| Homocystinuria | Cystathionine b-synthase | Same skeletal and ocular features as Marfan along with mental retardation and vascular thrombosis |
| Ehlers-Danlos syndrome | Various | Hyperextensible skin, hypermobile joints, extensive bruising and intestinal/uterine rupture |
| Alpha-one antitrypsin deficiency | Alpha-one antitrypsin | Lower lobe predominant emphysema at a young age (3rd–5th decade), liver cirrhosis and necrotizing panniculitis |
| Birt-Hogg-Dubé syndrome | Folliculin | Skin fibrofolliculomas, renal tumors and pneumothorax |
Adapted from Chiu et al. [41], Current Opinion Pulmonary Medicine 2006
Patients suspected of having BHD may be offered genetic testing, which will not only confirm the diagnosis, but also facilitate presymptomatic testing of at-risk family members [38]. The potential insurance and privacy consequences of a genetic diagnosis should be considered, although the Genetic Information Nondiscrimination Act protects subjects with genetic disease from discrimination or harm. Referral to a qualified genetic counselor is recommended before BHD genetic studies are done.
Performance of screening chest CT scans on young patients who present to emergency departments with pneumothorax has the potential to identify patients with BHD (Fig. 1). Hagaman et al. [42] have shown that when employed in a targeted group of patients (nonsmoking women aged 25–54 years), high resolution CT scans to screen for LAM can be cost effective.
Fig. 1.
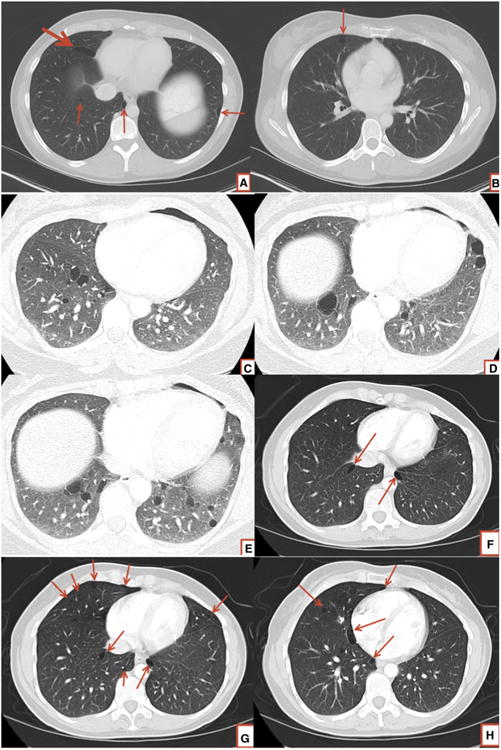
Chest CT scans in patients with BHD showing lower zone predominant cysts in various shapes and sizes. Note the elliptical/ lenticular shape of cysts seen commonly in BHD (a, f, g, h). BHD cysts have a predilection for abutting the pleural surfaces (a, b, d, e, f, g, h)
Another question with regards to screening is whether we should perform CT chest on patients with BHD diagnosed based on skin/renal findings. With studies showing 80–100 % penetrance of cysts [25, 30, 36], we feel it is prudent to perform a high resolution CT chest on all patients diagnosed with BHD to characterize the pulmonary involvement and facilitate education of patients about the risk of future pneumothoraces.
Management of pulmonary manifestations of BHD
The management of BHD from a pulmonary perspective is largely focused on the approach to treatment of pneumothoraces. As mentioned previously, 24 % of patients in the study by Toro et al. [30] had a history of pneumothorax. A similar percentage rate of pneumothorax (29 %) in patients with BHD has been recently reported by Houweling et al. [43]. The management of pneumothorax in patients with BHD differs from that recommended for primary spontaneous pneumothorax and is similar to that of LAM. In patients with primary spontaneous pneumothorax, the initial pneumothorax is usually managed with conservative measures (observation, aspiration, tube thoracostomy). Measures such as video assisted thoracoscopy (VATS) and mechanical or chemical pleurodesis are typically reserved for recurrent or non-healing cases [44]. However, like patients with LAM, patients with BHD are at very high risk for recurrent pneumothoraces. In the study by Toro et al. [30], 75 % patients had recurrent pneumothoraces. This is similar to the 73 % recurrence rate in patients with LAM [42, 45]. Almoosa et al. [45] have previously shown that chemical pleurodesis or surgery is equally effective and better than conservative therapy in preventing recurrence of pneumothorax in LAM. The estimated reduction in costs and morbidities of future hospitalizations and surgeries associated with future pneumothoraces was considerable in their analysis. These considerations led their group to conclude that pleurodesis should be considered for the initial pneumothorax in LAM patients, despite the potential for increased bleeding with future lung transplant in patients who suffer progressive respiratory failure. A unique method to prevent recurrent pneumothorax without adhesion to the chest wall, total pleural covering (TPC), is reported [46] but success is heavily dependent on local expertise.
Reassurance plays a key role in helping patients cope with the pulmonary aspects of BHD. They should be informed that BHD cystic lung disease typically does not result in respiratory failure. Patients with lung function impairment should be regularly followed by pulmonologists and periodic measurement of pulmonary function should be performed.
Other issues related to pulmonary management of patients with BHD relate to the question of air travel or diving as changes in ambient atmospheric pressure could predispose patients to develop pneumothoraces. While no disease specific data is available for BHD, The British Thoracic Society guidelines warn patients with lung cysts or bullae about the risk of barotrauma with diving and consider the presence of lung cysts a contraindication to diving [47]. With regards to air travel, we recommend that patients with lung function impairment, extensive cystic change or prior pneumothorax be evaluated by a pulmonologist prior to undertaking air travel and that patients should not board with unexplained chest pain or shortness of breath. In general, most patients with BHD should be able to undergo air travel safely. Exceptions may include patients with a limited pulmonary reserve or a history of multiple pneumothoraces.
Smoking is a known risk factor for development of primary spontaneous pneumothorax. Although no clear association has been found between smoking and the risk of pneumothorax in patients with BHD [30], it seems prudent to strongly discourage the use of tobacco in this patient population. These patients should get regularly immunized. Pneumococcal vaccination and annual influenza vaccination should be strongly encouraged in patients with BHD.
Once the diagnosis of BHD is established, it is important to screen patients for renal tumors. MRI and CT scanning are more accurate than renal ultrasound for this purpose. A proposed checklist of tasks to remember for patients with suspected BHD is presented in Table 6.
Table 6. Checklist of tasks for patients with suspected BHD.
| Diagnosis/baseline evaluation | Detailed personal and family history for skin lesions, pneumothorax, lung cysts and renal tumors |
| Abdominal CT/MRI to screen for renal tumors | |
| Genetic counseling followed by genetic testing for BHD, consider screening family members | |
| Skin exam and punch biopsy if lesions present | |
| Baseline pulmonary function testing | |
| HRCT scanning and review of cyst morphology and distribution by expert radiologist | |
| Studies and tests to rule out alternative diagnoses, such as modified Gomez criteria, CT/MRI head, serum VEGF-D, and possible lung biopsy for LAM, SSA and SSB for Sjogrens, a1 AT for emphysema, etc. | |
| Health care maintenance and follow up | Pneumococcal vaccination and annual influenza vaccination |
| Periodic pulmonary function testing if profusion of cysts sufficient to impair lung function | |
| Action plan for pneumothorax (Symptoms to recognize, TPC/pleurodesis after first event) | |
| Advise regarding air travel and scuba diving | |
| Annual screen for renal tumor growth | |
| Dermatologic consultation for disfiguring skin lesions |
Conclusion
There has been remarkable progress in our understanding of the genetic and cellular basis of BHD in the 35 years since the syndrome was first reported. As with many other disorders, advances in our ability to manage the clinical manifestations of the disease have lagged behind. Future studies should aim to better define the natural history of BHD pulmonary manifestations. Biomarkers that can be used for diagnosis, and prediction of risk for pneumothorax and lung function decline are needed. Understanding the molecular mechanisms by which FLCN deficiency leads to the development of pulmonary cysts may facilitate the development of therapeutic agents to prevent formation of the cystic pulmonary changes, and perhaps shed light on the pathophysiology of more common disorders such as pulmonary emphysema.
Footnotes
Conflict of interest: None.
Contributor Information
Nishant Gupta, Email: guptans@ucmail.uc.edu, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, University of Cincinnati, 231 Albert Sabin Way, MSB Room 6053, ML 0564, Cincinnati, OH 45267, USA.
Kuniaki Seyama, Division of Respiratory Medicine, Juntendo University, 2-1-1, Hongo, Bunkyo-Ku, Tokyo, Japan.
Francis X. McCormack, Division of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, University of Cincinnati, 231 Albert Sabin Way, MSB Room 6053, ML 0564, Cincinnati, OH 45267, USA
References
- 1.Hornstein OP, Knickenberg M. Perifollicular fibromatosis cutis with polyps of the colon—A cutaneo-intestinal syndrome sui generis. Arch Dermatol Res. 1975;253:161–175. doi: 10.1007/BF00582068. [DOI] [PubMed] [Google Scholar]
- 2.Hornstein OP. Generalized dermal perifollicular fibromas with polyps of the colon. Hum Genet. 1976;33:193–197. doi: 10.1007/BF00281897. [DOI] [PubMed] [Google Scholar]
- 3.Hornstein OP, Knickenberg M, Mörl M. Multiple dermal perifollicular fibromas with polyps of the colon—Report of a peculiar clinical syndrome. Acta Hepatogastroenterol (Stuttg) 1976;23:53–58. [PubMed] [Google Scholar]
- 4.Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibro-folliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–1677. [PubMed] [Google Scholar]
- 5.Khoo SK, Bradley M, Wong FK, Hedblad MA, Nordenskjöld M, Teh BT. Birt-Hogg-Dubé syndrome: mapping of a novel hereditary neoplasia gene to chromosome 17p12-q11.2. Oncogene. 2001;20:5239–5242. doi: 10.1038/sj.onc.1204703. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt LS, Warren MB, Nickerson ML, Weirich G, Matrosova V, Toro JR, Turner ML, Duray P, Merino M, Hewitt S, Pavlovich CP, Glenn G, Greenberg CR, Linehan WM, Zbar B. Birt-Hogg-Dubé syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. Am J Hum Genet. 2001;69:876–882. doi: 10.1086/323744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G, Turner ML, Duray P, Merino M, Choyke P, Pavlovich CP, Sharma N, Walther M, Munroe D, Hill R, Maher E, Greenberg C, Lerman MI, Linehan WM, Zbar B, Schmidt LS. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, Turner ML, Choyke PL, Sharma N, Peterson J, Morrison P, Maher ER, Walther MM, Zbar B, Linehan WM. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dubé syndrome. Am J Hum Genet. 2005;76:1023–1033. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toro JR, Wei MH, Glenn GM, Weinreich M, Toure O, Vocke C, Turner M, Choyke P, Merino MJ, Pinto PA, Steinberg SM, Schmidt LS, Linehan WM. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dubé syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321–331. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunogi M, Kurihara M, Ikegami TS, Kobayashi T, Shindo N, Kumasaka T, Gunji Y, Kikkawa M, Iwakami S, Hino O, Takahashi K, Seyama K. Clinical and genetic spectrum of Birt-Hogg-Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet Apr. 2010;47:281–287. doi: 10.1136/jmg.2009.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim DH, Rehal PK, Nahorski MS, Macdonald F, Claessens T, Van Geel M, Gijezen L, Gille JJ, Giraud S, Richard S, van Steensel M, Menko FH, Maher ER. A new locus-specific database (LSDB) for mutations in the folliculin (FLCN) gene. Hum Mutat. 2010;1:E1043–E1051. doi: 10.1002/humu.21130. [DOI] [PubMed] [Google Scholar]
- 12.Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, Esposito D, Gillette WK, Hopkins RF, 3rd, Hartley JL, Furihata M, Oishi S, Zhen W, Burke TR, Jr, Linehan WM, Schmidt LS, Zbar B. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci USA. 2006;103:15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasumi H, Baba M, Hong SB, Hasumi Y, Huang Y, Yao M, Valera VA, Linehan WM, Schmidt LS. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415:60–67. doi: 10.1016/j.gene.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takagi Y, Kobayashi T, Shiono M, Wang L, Piao X, Sun G, Zhang D, Abe M, Hagiwara Y, Takahashi K, Hino O. Interaction of folliculin (Birt-Hogg-Dubé gene product) with a novel Fnip1-like (FnipL/Fnip2) protein. Oncogene. 2008;27:5339–5347. doi: 10.1038/onc.2008.261. [DOI] [PubMed] [Google Scholar]
- 15.Baba M, Furihata M, Hong SB, Tessarollo L, Haines DC, Southon E, Patel V, Igarashi P, Alvord WG, Leighty R, Yao M, Bernardo M, Ileva L, Choyke P, Warren MB, Zbar B, Linehan WM, Schmidt LS. Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. J Natl Cancer Inst. 2008;100:140–154. doi: 10.1093/jnci/djm288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Futami K, Petillo D, Peng J, Wang P, Knol J, Li Y, Khoo SK, Huang D, Qian CN, Zhao P, Dykema K, Zhang R, Cao B, Yang XJ, Furge K, Williams BO, Teh BT. Deficiency of FLCN in mouse kidney led to development of polycystic kidneys and renal neoplasia. PLoS One. 2008;3:e3581. doi: 10.1371/journal.pone.0003581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Slegtenhorst M, Khabibullin D, Hartman TR, Nicolas E, Kruger WD, Henske EP. The Birt-Hogg-Dube and tuberous sclerosis complex homologs have opposing roles in amino acid homeostasis in Schizosaccharomyces pombe. J Biol Chem. 2007;282:24583–24590. doi: 10.1074/jbc.M700857200. [DOI] [PubMed] [Google Scholar]
- 18.Hartman TR, Nicolas E, Klein-Szanto A, Al-Saleem T, Cash TP, Simon MC, Henske EP. The role of the Birt-Hogg-Dubé protein in mTOR activation and renal tumorigenesis. Oncogene. 2009;28:1594–1604. doi: 10.1038/onc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong SB, Oh H, Valera VA, Stull J, Ngo DT, Baba M, Merino MJ, Linehan WM, Schmidt LS. Tumor suppressor FLCN inhibits tumorigenesis of a FLCN-null renal cancer cell line and regulates expression of key molecules in TGF-beta signaling. Mol Cancer. 2010;9:160. doi: 10.1186/1476-4598-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong SB, Oh H, Valera VA, Baba M, Schmidt LS, Linehan WM. Inactivation of the FLCN tumor suppressor gene induces TFE3 transcriptional activity by increasing its nuclear localization. PLoS One. 2010;5:e15793. doi: 10.1371/journal.pone.0015793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klomp JA, Petillo D, Niemi NM, Dykema KJ, Chen J, Yang XJ, Sääf A, Zickert P, Aly M, Bergerheim U, Nordenskjöld M, Gad S, Giraud S, Denoux Y, Yonneau L, Méjean A, Vasiliu V, Richard S, MacKeigan JP, Teh BT, Furge KA. Birt-Hogg-Dubé renal tumors are genetically distinct from other renal neo-plasias and are associated with up-regulation of mitochondrial gene expression. BMC Med Genomics. 2010;3:59. doi: 10.1186/1755-8794-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasumi H, Baba M, Hasumi Y, Huang Y, Oh H, Hughes RM, Klein ME, Takikita S, Nagashima K, Schmidt LS, Linehan WM. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN. J Natl Cancer Inst. 2012;104:1750–1764. doi: 10.1093/jnci/djs418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nookala RK, Langemeyer L, Pacitto A, Ochoa-Montaño B, Donaldson JC, Blaszczyk BK, Chirgadze DY, Barr FA, Bazan JF, Blundell TL. Crystal structure of folliculin reveals a hid-DENN function in genetically inherited renal cancer. Open Biol. 2012;2:120071. doi: 10.1098/rsob.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Steensel MA, Verstraeten VL, Frank J, Kelleners-Smeets NW, Poblete-Gutiérrez P, Marcus-Soekarman D, Bladergroen RS, Steijlen PM, van Geel M. Novel mutations in the BHD gene and absence of loss of heterozygosity in fibrofolliculomas of Birt-Hogg-Dubé patients. J Invest Dermatol. 2007;127:588–593. doi: 10.1038/sj.jid.5700592. [DOI] [PubMed] [Google Scholar]
- 25.Furuya M, Tanaka R, Koga S, Yatabe Y, Gotoda H, Takagi S, Hsu YH, Fujii T, Okada A, Kuroda N, Moritani S, Mizuno H, Nagashima Y, Nagahama K, Hiroshima K, Yoshino I, Nomura F, Aoki I, Nakatani Y. Pulmonary cysts of Birt-Hogg-Dubé syndrome: a clinicopathologic and immunohistochemical study of 9 families. Am J Surg Pathol. 2012;36:589–600. doi: 10.1097/PAS.0b013e3182475240. [DOI] [PubMed] [Google Scholar]
- 26.Painter JN, Tapanainen H, Somer M, Tukiainen P, Aittomäki K. A 4-bp deletion in the Birt-Hogg-Dubé gene (FLCN) causes dominantly inherited spontaneous pneumothorax. Am J Hum Genet. 2005;76:522–527. doi: 10.1086/428455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunji Y, Akiyoshi T, Sato T, Kurihara M, Tominaga S, Takahashi K, Seyama K. Mutations of the Birt Hogg Dube gene in patients with multiple lung cysts and recurrent pneumothorax. J Med Genet. 2007;44:588–593. doi: 10.1136/jmg.2007.049874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med. 2005;172:39–44. doi: 10.1164/rccm.200501-143OC. [DOI] [PubMed] [Google Scholar]
- 29.Tomassetti S, Carloni A, Chilosi M, Maffè A, Ungari S, Sverzellati N, Gurioli C, Casoni G, Romagnoli M, Gurioli C, Ravaglia C, Poletti V. Pulmonary features of Birt-Hogg-Dubé syndrome: cystic lesions and pulmonary histiocytoma. Respir Med. 2011;105:768–774. doi: 10.1016/j.rmed.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, Wei MH, Schmidt LS, Davis L, Zbar B, Choyke P, Steinberg SM, Nguyen DM, Linehan WM. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175:1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tobino K, Gunji Y, Kurihara M, Kunogi M, Koike K, Tomiyama N, Johkoh T, Kodama Y, Iwakami S, Kikkawa M, Takahashi K, Seyama K. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol Mar. 2011;77:403–409. doi: 10.1016/j.ejrad.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Tobino K, Hirai T, Johkoh T, Kurihara M, Fujimoto K, Tomiyama N, Mishima M, Takahashi K, Seyama K. Differentiation between Birt-Hogg-Dubé syndrome and lymphangioleiomyomatosis: quantitative analysis of pulmonary cysts on computed tomography of the chest in 66 females. Eur J Radiol. 2012;81:1340–1346. doi: 10.1016/j.ejrad.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 33.Butnor KJ, Guinee DG., Jr Pleuropulmonary pathology of Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2006;30:395–399. doi: 10.1097/01.pas.0000183571.17011.06. [DOI] [PubMed] [Google Scholar]
- 34.Furuya M, Nakatani Y. Birt-Hogg-Dube syndrome: clinicopathological features of the lung. J Clin Pathol. 2012 Dec 8; doi: 10.1136/jclinpath-2012-201200. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga S, Furuya M, Takahashi Y, Tanaka R, Yamaguchi A, Yasufuku K, Hiroshima K, Kurihara M, Yoshino I, Aoki I, Nakatani Y. Lung cysts in Birt-Hogg-Dubé syndrome: histopathological characteristics and aberrant sequence repeats. Pathol Int. 2009;59:720–728. doi: 10.1111/j.1440-1827.2009.02434.x. [DOI] [PubMed] [Google Scholar]
- 36.Zbar B, Alvord WG, Glenn G, Turner M, Pavlovich CP, Schmidt L, Walther M, Choyke P, Weirich G, Hewitt SM, Duray P, Gabril F, Greenberg C, Merino MJ, Toro J, Linehan WM. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. [PubMed] [Google Scholar]
- 37.Ayo DS, Aughenbaugh GL, Yi ES, Hand JL, Ryu JH. Cystic lung disease in Birt-Hogg-Dube syndrome. Chest. 2007;132:679–684. doi: 10.1378/chest.07-0042. [DOI] [PubMed] [Google Scholar]
- 38.Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, Nordenskjöld M, Hansen TV, Solly J, Maher ER European BHD Consortium. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–1206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 39.Young LR, Vandyke R, Gulleman PM, Inoue Y, Brown KK, Schmidt LS, Linehan WM, Hajjar F, Kinder BW, Trapnell BC, Bissler JJ, Franz DN, McCormack FX. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138:674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seaman DM, Meyer CA, Gilman MD, McCormack FX. Diffuse cystic lung disease at high-resolution CT. AJR Am J Roentgenol. 2011;196:1305–1311. doi: 10.2214/AJR.10.4420. [DOI] [PubMed] [Google Scholar]
- 41.Chiu HT, Garcia CK. Familial spontaneous pneumothorax. Curr Opin Pulm Med. 2006;12:268–272. doi: 10.1097/01.mcp.0000230630.73139.f0. [DOI] [PubMed] [Google Scholar]
- 42.Hagaman JT, Schauer DP, McCormack FX, Kinder BW. Screening for lymphangioleiomyomatosis by high-resolution computed tomography in young, nonsmoking women presenting with spontaneous pneumothorax is cost-effective. Am J Respir Crit Care Med. 2010;181:1376–1382. doi: 10.1164/rccm.200910-1553OC. [DOI] [PubMed] [Google Scholar]
- 43.Houweling AC, Gijezen LM, Jonker MA, van Doorn MB, Oldenburg RA, van Spaendonck-Zwarts KY, Leter EM, van Os TA, van Grieken NC, Jaspars EH, de Jong MM, Bongers EM, Johannesma PC, Postmus PE, van Moorselaar RJ, van Waesberghe JH, Starink TM, van Steensel MA, Gille JJ, Menko FH. Renal cancer and pneumothorax risk in Birt-Hogg-Dubé syndrome; an analysis of 115 FLCN mutation carriers from 35 BHD families. Br J Cancer. 2011;105:1912–1919. doi: 10.1038/bjc.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacDuff A, Arnold A, Harvey J BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii, 18–31. doi: 10.1136/thx.2010.136986. [DOI] [PubMed] [Google Scholar]
- 45.Almoosa KF, Ryu JH, Mendez J, Huggins JT, Young LR, Sullivan EJ, Maurer J, McCormack FX, Sahn SA. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006;129:1274–1281. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 46.Kurihara M, Kataoka H, Ishikawa A, Endo R. Latest treatments for spontaneous pneumothorax. Gen Thorac Cardiovasc Surg. 2010;58:113–119. doi: 10.1007/s11748-009-0539-5. [DOI] [PubMed] [Google Scholar]
- 47.British Thoracic Society Fitness to Dive Group, Subgroup of the British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines on respiratory aspects of fitness for diving. Thorax. 2003;58:3–13. doi: 10.1136/thorax.58.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]


