Abstract
Objective
Blood vessel hemodynamics have profound influences on function and structure of vascular cells. One of the main mechanical forces influencing vascular smooth muscle cells (VSMC) is cyclic stretch (CS). Increased CS stimulates reactive oxygen species (ROS) production in VSMC leading to their de-differentiation, yet the mechanisms involved are poorly understood. This study was designed to test the hypothesis that pathological CS stimulates Nox1-derived ROS via MEF2B, leading to VSMC dysfunction via a switch from a contractile to a synthetic phenotype.
Approach and Results
Using a newly developed isoform-specific Nox1 inhibitor and gene silencing technology, we demonstrate a novel pathway including MEF2B-Nox1-ROS is upregulated under pathological stretch conditions and this pathway promotes a VSMC phenotypic switch from a contractile to a synthetic phenotype. We observed that CS (10% at 1 Hz) mimicking systemic hypertension in humans increased Nox1 mRNA, protein levels, and enzymatic activity in a time-dependent manner and this upregulation was mediated by MEF2B. Furthermore, we show that stretch-induced Nox1-derived ROS upregulated a specific marker for synthetic phenotype (osteopontin), while downregulating classical markers for contractile phenotype (calponin1 and smoothelin B). In addition, our data demonstrated that stretch-induced Nox1 activation decreases actin fiber density and augments matrix metalloproteinase 9 activity, VSMC migration, and vectorial alignment.
Conclusions
These results suggest that CS initiates a signal through MEF2B that potentiates Nox1-mediated ROS production and causes VSMC to switch to a synthetic phenotype. The data also characterize a new Nox1 inhibitor as a potential therapy for treatment of vascular dysfunction in hypertension.
Keywords: Oxidative Stress, Vascular Remodeling, Vascular Dysfunction, Cyclic stretch, Nox1, MEF2B, Smooth Muscle Cell Differentiation
Introduction
Differentiated vascular smooth muscle cells (VSMC) are a major constituent of the blood vessel wall and play a vital role in the maintenance of vessel homeostasis. These highly-specialized cells regulate vessel tone, blood pressure, and blood flow distribution.1, 2 Mature VSMC that are fully differentiated exhibit a contractile phenotype characterized by low protein: DNA synthetic activity, reduced proliferation rate, and a unique set of contractile proteins and signaling molecules.3, 4 Unlike skeletal and cardiac muscle cells, mature VSMC retain a remarkable ability to modulate their phenotype and de-differentiate into a synthetic phenotype in response to changes in local environmental cues. 5, 6 The synthetic phenotype is characterized by increased VSMC migration, loss of contractility, and abnormal extracellular matrix production. These hallmarks have been observed clinically and in animal models of vascular injury and diseases, including systemic hypertension, angioplasty-induced restenosis, atherosclerosis, and aortic aneurysm formation.7, 8
VSMC phenotype is influenced by diverse hormonal and environmental cues, including cytokine stimulation, cell-cell contact, cellular adhesions, vascular injury, and increased mechanical force. In vivo, VSMC are constantly subjected to mechanical forces as a consequence of pulsatile blood flow and shear stress. Among multiple hemodynamic forces, VSMC are primarily subjected to pulsatile cyclic stretch (CS) in response to systolic-diastolic fluctuations in pressure. As such, in vitro CS serves as a model of pressure fluctuations in the vasculature with 10%, 1 Hz stretch mimicking hypertension.9 Indeed, CS is a well-established stimulus for VSMC de-differentiation and a switch to the synthetic phenotype10-12, yet the mechanism involved is incompletely understood.
Nox isozymes are expressed in vascular endothelial cells, smooth muscle cells, and adventitial fibroblasts in which they are major ROS producers and mediators of cardiovascular physiology and pathophysiology.13 Consistent with a role for Nox in stretch-induced de-differentiation, smooth muscle Nox isoform 1 (Nox1)-derived superoxide anion (O2.-) production is elevated in neointimal growth in response to balloon angioplasty.14,15
Myocyte Enhancer Factor 2 (MEF2) proteins are a family of transcription factors that play a pivotal role in the transduction of extracellular signals to the genome that control cell differentiation, proliferation, morphogenesis, survival, and apoptosis.16 MEF2s are evolutionarily conserved and serve as lynchpins in the transcriptional circuits that control cell differentiation and organogenesis of an ancient regulatory differentiation network. Importantly, MEF2s are involved in morphogenesis and myogenesis of skeletal, cardiac, and smooth muscle cells. 17, 18 Moreover, MEF2s are established contributors to growth, proliferation, and hypertrophy of multiple cell types.18 However, their role in VSMCs is less clear.
MEF2s are subject to multiple positive and negative control mechanisms, which serve to fine-tune the diverse transcriptional circuits in which these factors participate. In adult rat aortic VSMC (RASMC), three MEF2 isoforms (MEF2A, MEF2B and MEF2D) are expressed, whose levels are increased in vascular injury.19, 20 Interestingly, studies using RT-PCR (5’RACE) suggest that the Nox1 promoter region possesses a cis-regulatory element that is a consensus site for MEF2B.21 Despite the evidence for a role of MEF2s in developmental myogenesis and their upregulation in vessel injury, the role of MEF2s and their link to Nox/ROS, and adult smooth muscle cell differentiation in vascular disease are entirely unknown.
We postulated that CS via induction of MEF2B activity stimulates Nox1-derived O2.- production, leading to a switch from a contractile to synthetic smooth muscle cell phenotype. This includes marked changes in phenotypic markers including calponin1 (CNN1), smoothelin B and osteopontin (OPN), in concert with a decrease in F-actin fiber density, enhanced matrix metalloproteinase (MMP) activity, cell migration, and aberrant vectorial cell alignment.
Results
Uniaxial Cyclic Stretch (CS) Induces Nox-1 Expression and Activity
To determine the effect of uniaxial mechanical CS on Nox1 mRNA, protein levels, and activity in RASMC, cells were subjected to 10% CS (1 Hz) for different time points. Nox1 mRNA expression increased in a time-dependent response reaching a plateau of 3.8-fold versus static after 3 hr of stimulation (Figure 1A). Likewise, Nox1 protein levels increased in a time-dependent manner, yielding a maximum signal of 1.45-fold versus static after 24 hrs of stimulation (Figure 1B). As 24 hr yielded maximum Nox1 protein upregulation, we chose this time point for the remainder of experiments in this study. After 24 hr of mechanical stretch we observed an approximate 2-fold increase in O2.- production in stretched cells versus static control. Pre-incubation of RASMC with a recently developed isoform-specific Nox1 peptidic inhibitor, NoxA1ds 22, completely inhibited CS-induced O2.- generation to values observed in static conditions (Figure 1C). Additionally, a time course of optimal NoxA1ds effectiveness was tested (Supplemental Figure I), showing that administration of NoxA1ds 4 hr before the end of CS was maximally effective. The inhibitory effect of NoxA1ds in RASMC under control conditions and in response to classical Nox agonists, such as phorbol myristate acetate (PMA) and platelet-derived growth factor (PDGF), is shown in Supplemental Figure II. For detailed evidence of NoxA1ds isoform-specificity, efficacy, and mechanism of action please refer to Ranayhossaini et al.22
Figure 1.
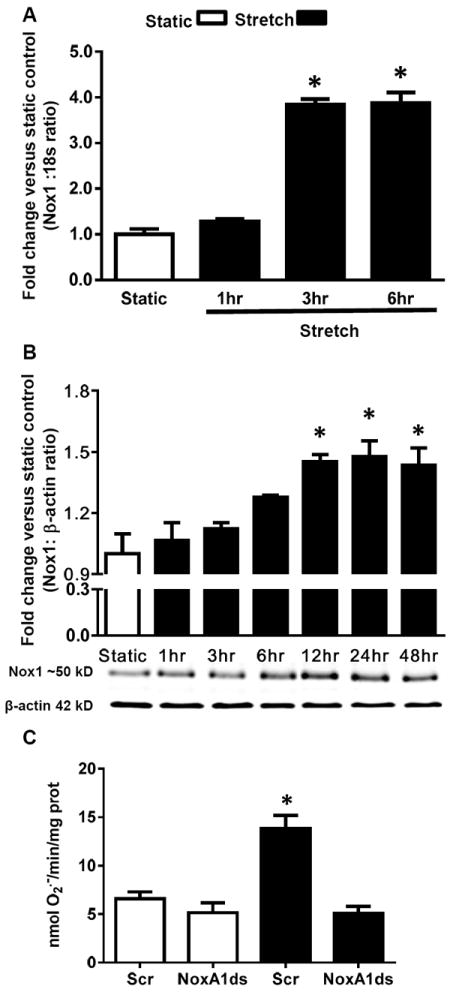
Uniaxial cyclic stretch induces Nox1 expression and activity. RASMCs were subjected to a time course of cyclic stretch. A) Nox1 mRNA was measured by qPCR (n = 5). B) Nox1 protein was measured by Western blot (n = 6). C) RASMC were incubated with NoxA1ds or scrambled (Scr) peptide (10 μM) and subjected to 24 hr cyclic stretch. SOD-inhibitable O2.- was measured by cytochrome c reduction (n = 9). Data are expressed as means + SEM. *P<0.05 versus static control.
Uniaxial Cyclic Stretch Induces MEF2B Promoter and Nox1 Expression
To evaluate whether MEF2B is activated under stretch conditions, cells were co-transfected with MEF2B firefly luciferase promoter (pMEF2B-pGL3) and activity was compared to control Renilla luciferase (pRL-CMV) promoter activity. pMEF2B-pGL3 promoter activity was increased ~2-fold following 1 hr CS (Figure 2A). In separate experiments, RASMCs were subjected to a time course of CS (1, 3, 6, 9, and 24 hr) and MEF2B protein expression was investigated by Western blot. CS gradually increased MEF2B protein expression over the course of 24 hr (Figure 2B). To investigate whether MEF2B activity regulates Nox1 protein expression, cells were transfected with siRNA against MEF2B or scrambled siRNA (Scr); after 24 hr of CS stimulation, Nox1 protein levels were evaluated by Western blot. We observed that under stretch conditions, cells that were treated with Scr siRNA displayed a 1.8-fold increase in Nox1. Gene silencing of MEF2B by 60% (data not shown) reverted Nox1 protein expression to static levels (Figure 2C). MEF2B siRNA showed no effect on basal Nox1 levels.
Figure 2.
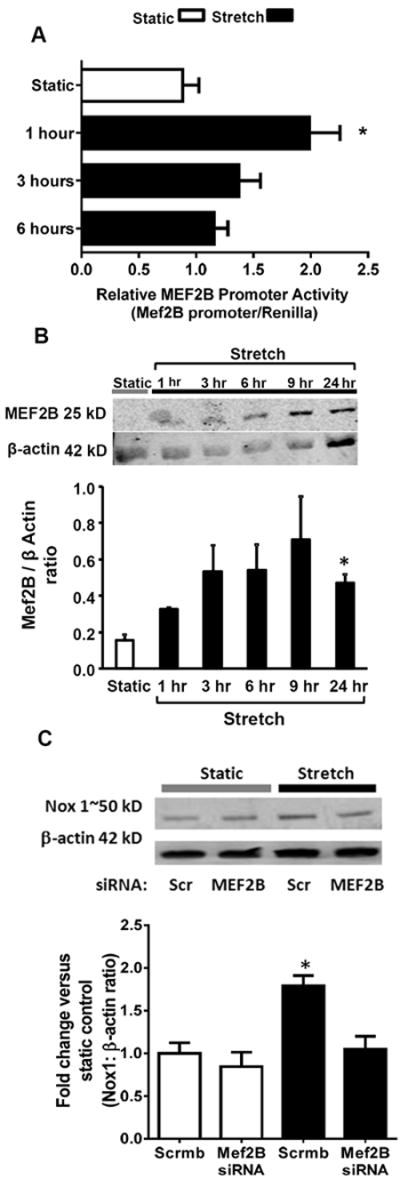
Uniaxial cyclic stretch induces MEF2B promoter activity, MEF2B protein expression, and MEF2B-dependent Nox1 expression. A) RASMCs were subjected to a time course of cyclic stretch and MEF2B promoter reported was measured by dual firefly luciferase reporter assay, using MEF2F firefly luciferase reporter and a control reporter expressing Renilla luciferase (n = 6). B) RASMCs were subjected to a time course of cyclic stretch and MEF2B protein expression was investigated by Western blot (n = 4). C) RASMCs were pretreated with MEF2B siRNA or scrambled (Scr) siRNA, subjected to 24 hr cyclic stretch. Nox1 protein expression was measured by Western blot (n = 6). Data are expressed as means + SEM. *P<0.05 versus vehicle or static condition.
Nox1 Mediates a Decrease in RASMC Contractile Marker Expression in Response to Uniaxial Cyclic Stretch
First, we tested whether the RASMC used for the experiments are homogeneous and express markers of fully differentiated SMCs using a confocal laser scanning microscope. The representative confocal images demonstrate that virtually all cells express SM α-actin, smoothelin, SM myosin heavy chain, and SM22α (Supplemental Figure III A-D).
In response to CS, expression of contractile phenotype markers CNN1 and smoothelin B were decreased [42 and 50% versus static, respectively (Figure 3A and 3C)]. When cells were pre-treated with NoxA1ds, CS-induced decrease in CNN1 and smoothelin B expression was normalized to values observed under non-stretched conditions. On the other hand, a Scr control for NoxA1ds had no effect on the expression of these phenotypic markers following CS. To verify these data, RASMC were pretreated with Nox1 siRNA or Scr siRNA and subjected to CS. Confirming the effectiveness of Nox1 siRNA, quantitative polymerase chain reaction showed levels of Nox1 mRNA were decreased by approximately 65% in Nox1 siRNA-treated vs. Scr control-treated cells. In contrast, we observed no change in Nox4 mRNA levels 23. Nox1 siRNA had no effect on CNN1 or smoothelin B levels under static conditions. CS induced a significant decrease in both markers in cells treated with Scr siRNA (Figures 3B and 3D). On the other hand, Nox1 siRNA abolished the decrease in both markers (Figure 3B and 3D). In contrast, CS (24 hr) had no effect on myosin heavy chain (MHC) expression in RASMC (MHC/β-actin ratio: 0.39±0.1 and 0.46±0.2 for static control and stretch, respectively, n = 3), perhaps suggesting that 24 hr CS is not sufficient to effect a change in MHC.
Figure 3.
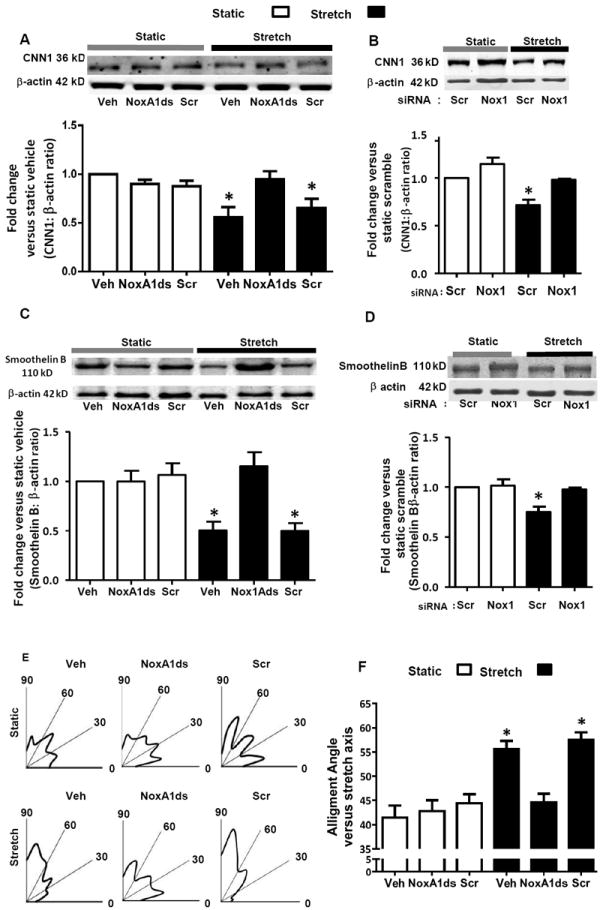
Nox1 mediates a decreased contractile phenotype in response to uniaxial cyclic stretch. RASMCs were subjected for 24 hr to stretch or static conditions. A and B) CNN1 protein expression measured by Western blot (n = 4). C and D) Smoothelin B protein expression measured by Western blot (n = 4). E) Cell nuclear perpendicular alignment versus the direction of the stretch vector represented by radial histograms (n = 5, over 130 cells). F) Summary of the average alignment angles (n = 5 over 130 cells). In figures A, C, E, and F, cells were pretreated with NoxA1ds or scrambled peptide (10 μM). In figures B and D, cells were pretreated with Nox1 siRNA or scrambled (Scr) siRNA. Data are expressed as means + SEM. *P<0.05 versus static control.
Separate experiments were performed to investigate whether Nox4 (Nox2 and Nox5 are not expressed in RASMC) is also involved in CS-induced changes in contractile proteins. Nox4 was gene silenced in RASMC using siRNA, cells exposed to CS for 24 hr, and CNN1 and OPN expression were analyzed by Western blot. Our data showed that gene silencing Nox4 in RASMC did not reverse CS-induced changes in CNN1 and OPN protein levels (Supplemental Figure IVA and B). In the case of CNN, Nox4 silencing had an effect to augment the reduction in CNN in response to stretch perhaps suggesting that Nox4 plays a role in attenuating this phenotypic change.
Nox1 Mediates a Shift in RASMC Cell Alignment in Response to Uniaxial Cyclic Stretch
In cell culture, CS causes a shift in the total population of cells toward perpendicular realignment of RASMC (higher cell percentage closer to 90°) relative to the stretch axis direction.12 As measured by nuclear orientation, radial histograms show a greater distribution of cells shifted toward 90° under CS. This effect was ablated by NoxA1ds but not scrambled control (Figure 3E and F). Neither NoxA1ds nor its Scr control had an effect on cell alignment under static conditions.
Nox1 Mediates an Increase in RASMC Synthetic Phenotype Markers in Response to Uniaxial Cyclic Stretch
To further assess the role of Nox1 in stretch-induced synthetic phenotype, osteopontin (OPN) protein expression was measured following 24 hr of CS. CS increased OPN protein levels by 1.5-fold versus static conditions, which was inhibited by NoxA1ds-pretreatment and Nox1 gene silencing but not scrambled controls (Figure 4A and B).
Figure 4.
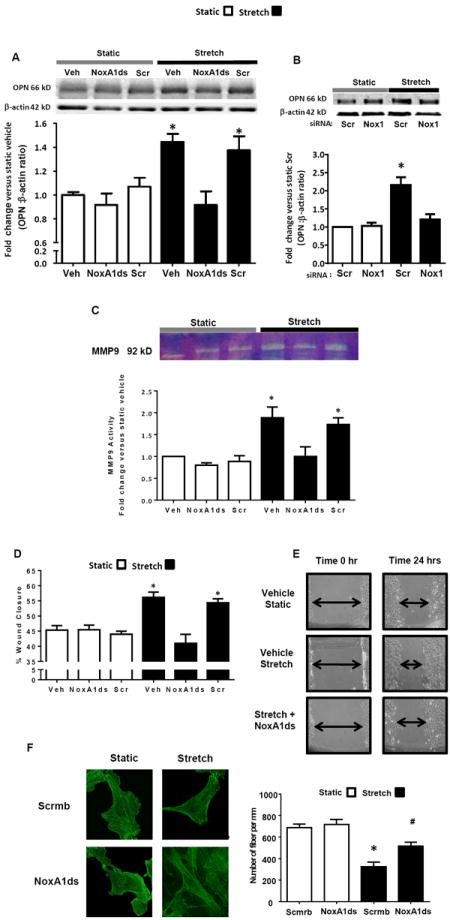
Nox1-mediated increase in synthetic phenotype in response to uniaxial cyclic stretch. RASMC were subjected for 24 hr to stretch or static conditions. A and B) OPN was measured by Western blot (n = 4). C, Extracellular MMP9 activity was measured by zymography (n = 5). D and E) Cell migration was measured by wound healing assay (n = 8). F) Uniaxial CS increases actin fiber density via Nox1. Fluorescence microscopy images of phalloidin-stained RASMC. Fiber density was assessed using ImageJ software and with the help of the Hessian matrix plug-in. The graph depicts mean values of 8 cells from 4 independent experiments. The green bars represent 20 μm. In figures A, C, D, E and F cells were treated with NoxA1ds or scrambled (Scr) peptide (10 μM). In B) cells were pretreated with Nox1 siRNA or scrambled siRNA (5 pmol/ml) for 48 hr. Data are expressed as means + SEM. *P<0.05 versus static control. #P<0.05 vs. stretch scrmb.
Nox1 Increases MMP9 Activity and Stimulates Migration in Response to CS
Increased matrix metalloproteinase (MMP) expression contributes to the de-differentiation process and plays a role in the migration of synthetic SMC 24,25. Thus, we determined whether Nox1 participates in CS-induced MMP activity and VSMC migration. CS stimulated MMP9 activity by ~ 2.5-fold versus static control (Figure 4C). NoxA1ds, but not Scr control, treatment reduced MMP9 activity to static levels (Figure 4C). Finally, CS induced a significant increase in RASMC migration compared to static, which was reduced following pharmacological inhibition of Nox1 (Figure 4D and 4E).
Finally, we tested whether CS induces changes in the architecture of F-actin network in SMC. CS significantly increased the density of actin filaments in SMC in a Nox1-dependent manner (Fig. 4F). In contrast, quantitative analysis of fiber thickness showed no significant actin thickening in response to CS.
Discussion
The present study illustrates for the first time that uniaxial stretch-induced phenotypic transitioning of vascular smooth muscle cells from a contractile to synthetic phenotype, as detected by changes in cytoskeletal proteins, F-actin density, MMP9 activity, cell migration, and cell orientation, is mediated by an early increase in MEF2B transcription and protein levels, upregulation of Nox1 expression, and increased Nox1-derived O2.- production. These novel findings indicate that MEF2B to Nox1 signaling causing alterations in cytoskeletal proteins and MMP9 activation are pivotal for the synthetic and hyperproliferative/pro-migratory VSMCs in response to cyclic stretch.
Blood vessels are continuously subjected to hemodynamic mechanical forces, including cyclic stretch and shear stress and these forces are highly dependent on the fluid dynamics of the blood, in particular, flow and viscosity.26 Increases in any of these conditions concomitantly lead to increased cyclic stretch and shear stress. Under physiological conditions, as in early vascular development, blood pressure and thus mechanical stress in the arterial wall regulate critical parameters of vascular function and maintain the balance between blood supply and tissue oxygen demand.27 In contrast to these physiological processes, sustained or chronic elevations in blood pressure and flow lead to phenotypic changes of the vascular wall and vascular remodeling.28
NADPH oxidases (Noxes) are well established as major sources of ROS in the vasculature as well as significant contributors to vascular pathologies, including neointima formation.13, 29-32 A previous study demonstrated that p22phox-expressing smooth muscle cells in the neointima, that have greater capability to produce ROS, are positive for SMemb but not for SM2, suggesting that ROS-producing cells could possess a synthetic rather than a contractile phenotype 33. The manner and means by which MEF-induced Nox-derived ROS induces a molecular shift away from contractile proteins and activity to a highly proliferative and synthetic phenotype are unknown.
We observed that uniaxial cyclic stretch (10%, 1Hz, conditions mimicking hypertension in humans) 9 increased Nox1 mRNA, protein expression, and activity in smooth muscle cells. Our findings illustrate an early upregulation of Nox1 mRNA at 3 - 4 hours of stretch followed by increased protein expression at 12-24 hours. A previous report suggested that Nox1 can be upregulated in smooth muscle cells under in vitro CS conditions34. Our data confirm this finding and go further in demonstrating a rise in Nox1 mRNA, protein expression, and specific activity in response to CS. Upon establishing optimal conditions for Nox1 upregulation, we measured O2.- production (via cyt c reduction assay) and observed a robust increase in SOD-inhibitable O2.- production after 24 hr of CS. Previous reports suggested that CS induces O2.- production 12, 35, or implicated a CS-induced O2.- effect on transduction pathways using broad-range flavoprotein inhibitors including DPI 36 or apocynin 37. Nevertheless, those data pointed to a role for O2.- in CS. Our findings are the first to our knowledge to identify the functional involvement of Nox1, or any Nox for that matter, in the stretch response of VSMC (utilizing two approaches; gene silencing and pharmacological inhibition using an isoform-specific Nox1 inhibitor, NoxA1ds).22 Previous work in our laboratory confirmed siRNA efficacy to inhibit Nox1 by 60-70% in this assay.38 Moreover, NoxA1ds was able to completely blunt Nox1 activity in RASMC. Taken together, these data strongly support that mimicking hypertensive conditions via CS causes an increase in Nox1 expression and Nox1-derived O2.- production. The time-frame of our results suggest a tightly regulated signaling role for Nox1.
Accumulating evidence suggests that the family of MEF2 transcription factors is involved in VSMC differentiation and disease 3, 17, 19. It has been proposed that Nox1 promoter region possesses a cis-regulatory element that is a consensus site for MEF2B binding 21. In order to corroborate the existence of this MEF2B regulatory element in the Nox1 gene, we evaluated the Nox1 promoter region using Ensembl Genome Browser and found a consensus sequence for MEF2B binding located at -438 bp upstream of the Nox1 transcription initiation codon (Suplemental Figure VI); demonstrating a putative association between MEF2B and Nox1. Furthermore, our results showed that there is a rapid increase in MEF2B promoter activity in stretch-stimulated RASMC and that inhibition of MEF2B expression using siRNA attenuates stretch-induced Nox1 expression, supporting the link between MEF2B and Nox1. These data suggest for the first time a tightly-regulated, time-dependent response to cyclic stretch that involves MEF2B, Nox1, and O2.- production.
VSMC phenotypic switching is a varied and complex process. While numerous reports have addressed diverse molecular mechanisms behind VSMC lineage determination and differentiation, there is a lack of information on how Nox-derived O2.- affects these processes. VSMC cytostructural proteins are commonly used to define contractile or synthetic phenotypes which each exhibit distinct proliferative and migratory manifestations.39-41 Generally, synthetic VSMC exhibit higher growth rates and higher migratory activity than contractile VSMC and are identified in part by detection of reduced contractile proteins.42 Our observations demonstrate that stretch-induced Nox1 activity leads to a major decrease in two of the classical contractile proteins: CNN1 (calponin 1) 43 and smoothelin B44 as well as changes in F-actin fiber density. The observed stretch-induced reduction in CNN1 and smoothelin B expression are reversed by an isoform-specific Nox1 inhibitor (NoxA1ds) and/or Nox1 siRNA. Osteopontin and fiber density, on the other hand, were increased in a Nox1-dependent manner. Taken together, these results are indicative of a less contractile and more synthetic VSMC phenotype. 45
Although these data indicate that Nox1 plays a major role in CS-induced phenotypic changes of smooth muscle cells, it is likely that other Nox isoforms and ROS-generating enzymes or even ROS-independent processes are involved in the transition of SMC into the proliferative, synthetic phenotype. A variety of signaling mediators have been associated with the phenotypic change of SMC some of which happen to be redox sensitive (ie: protein phosphatases and matrix metalloproteinases). In addition, the role of microRNAs (miRNAs) and intracellular Ca2+ signaling have recently been demonstrated in VSMC phenotype switching. 46,47,48 Other Nox-independent pathways initiated by cyclic stretch (ie. basic fibroblast growth factor, insulin-like growth factors, epidermal growth factor, etc.) are also expected to contribute to cyclic stretch-induced phenotypic changes. 49 Future studies are required to investigate whether one or more of these factors elicit phenotypic changes involving changes in the redox status of the cell. In the present study we investigated whether Nox4 (Nox2 and Nox5 are not expressed in RASMC) contributes to CS-induced changes in contractile proteins. Our data demonstrated that gene silencing Nox4 in RASMC does not rescue cyclic stretch-induced changes in CNN1 and OPN protein levels.
To further validate our observations of a decreased VSMC contractile phenotype, we proceeded to measure VSMC orientation with respect to the direction of the stretch stimulus. In vivo, arterial smooth muscle cells are aligned primarily in the circumferential direction in the media of artery, the circumferential orientation and structural network of the VSMC layers are key to maintaining mechanical strength and function of the arterial wall in response to increased wall stress and also provide the flexibility required for pulsatile blood flow. 50 This effect can be evaluated in cell culture probing an alignment response to persistent mechanical force. It has been reported that, in response to uniaxial CS, VSMC rapidly realign in an orientation perpendicular (90°) to the axis of the strain. 36,51 We postulated that under CS conditions there is a Nox1-dependent decrease in contractile proteins in concert with increased RASMC perpendicular realignment. Indeed, both effects were mediated by Nox1 and are indicative of an impaired contractile VSMC state.
One of several structural proteins shown to be increased in RASMC in a synthetic phenotype is OPN. OPN is a cytokine upregulated in diabetes which augments metalloproteinase (MMP) activation, promoting migration in vascular cells. 52 Overexpression of MMPs in the aortic wall is believed to play an important role in dilative aortic pathologies. MMP2 and MMP9 upregulation has been observed in aortic walls from patients with thoracic aortic dissection and thoracic aortic aneurysm.53 In addition, in vitro cultured VSMC derived from abdominal aortic aneurysmal wall exhibits an increased synthesis of MMP2 and MMP9.54 Our results show that OPN expression, MMP9 activation, and migration were increased during CS vs. static conditions. All of these cell changes were suppressed by Nox1 inhibition. Interestingly, CS did not increase MMP2 activation (Supplemental Figure V). Incidentally, these results are consistent with previous observations in vivo in which after two weeks of balloon angioplasty, there is an increased Nox1 expression within the neointima associated with MMP9 activation. 55
In summary, these findings highlight the involvement of a new signaling pathway originating at mechanical stretch, stimulating MEF2B activity, and upregulating a Nox1-mediated shift to a synthetic and migratory VSMC phenotype. This includes marked Nox1-mediated shifts in CNN1, smoothelin B and OPN, and MMP9 activity along with Nox1-enhanced F-actin density, cell migration and aberrant cell orientation. VSMC migration and proliferation are key processes in neointima formation in multiple vascular diseases including atherosclerosis, restenosis, and vein graft failure56, suggesting that our findings have broad implications for hyperproliferative vascular diseases. Moreover, the data provide new insight into mechanisms controlling vascular dysfunction initiated by hemodynamic alterations and are expected to elucidate the mechanisms regulating vascular responses to elevations in mean arterial blood pressure and pulse pressure.57,58 The findings also further support MEF2B and Nox1 as therapeutic targets in ameliorating vascular dysfunction in hypertension and multiple other cardiovascular disorders.
Supplementary Material
Significance.
This is the first study to establish a link between MEF2B and Nox1 and identify both factors as mediators of vascular smooth muscle cell phenotypic changes during mechanical stretch. Our findings illustrate that mechanical stretch stimulates MEF2B transcription, leading to Nox1 upregulation, and increased reactive species production. The data also demonstrate that under pathological cyclic stretch, Nox1 mediates vascular smooth muscle cell contractile to synthetic phenotypic switch manifested by alterations in key cytoskeletal proteins, matrix metalloproteinase 9 activation, disorientation of cell alignment, and augmented cell migration. Furthermore, the data demonstrate the feasibility of a novel isoform-specific inhibitor to ameliorate these phenotypic changes and provide additional proof of its viability as a therapeutic agent in vascular disease (see appendix Ranayhossaini et al. 22).
Acknowledgments
We would like to thank Dr. Joseph Miano (University of Rochester Medical Center) for supplying us with the MEF2 luciferase reporter plasmid (pMEF2-pGL3) and for his critical feedback in the writing of the manuscript. The authors wish to thank Jessyca Pavanelli (University of Sao Paulo) for her help with the Ensembl Genome Browser. We also thank Laura Pliske (University of Pittsburgh) for her assistance with the editing of this manuscript.
Sources of Funding
This work was supported by the following: P.J.P. receives support from National Institutes of Health (R01HL079207, P01HL103455–01) and is an Established Investigator of the American Heart Association. G.C. receives support from National Institutes of Health (K99HL114648). All Vascular Medicine Institute investigators receive support from the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania, PA.
Non-standard Abbreviations
- CNN1
Calponin 1
- CS
Cyclic Stretch
- MMP9
Matrix Metalloproteinase 9
- MEF2
Myocyte Enhanced Factor 2
- Nox1
NADPH oxidase isoform 1
- OPN
Osteopontin
- ROS
Reactive Oxygen Species
- O2.-
Superoxide anion
- NoxA1ds
NADPH oxidase 1 inhibitor
- PMA
Phorbol Myristate Acetate
- SM
Smooth Muscle
- MHC
Myosin Heavy Chain
- p22phox
p22 phagocyte oxidase
Footnotes
Disclosures
None
References
- 1.Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiological genomics. 2010;42A:169–187. doi: 10.1152/physiolgenomics.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogut O, Brozovich FV. Regulation of force in vascular smooth muscle. Journal of molecular and cellular cardiology. 2003;35:347–355. doi: 10.1016/s0022-2828(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 3.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 4.Miano JM. Mammalian smooth muscle differentiation: Origins, markers and transcriptional control. Results and problems in cell differentiation. 2002;38:39–59. doi: 10.1007/978-3-540-45686-5_2. [DOI] [PubMed] [Google Scholar]
- 5.Spin JM, Maegdefessel L, Tsao PS. Vascular smooth muscle cell phenotypic plasticity: Focus on chromatin remodelling. Cardiovasc Res. 2012;95:147–155. doi: 10.1093/cvr/cvs098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annual review of physiology. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 7.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis-Dusenbery BN, Wu C, Hata A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler Thromb Vasc Biol. 2011;31:2370–2377. doi: 10.1161/ATVBAHA.111.226670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maul TM, Chew DW, Nieponice A, Vorp DA. Mechanical stimuli differentially control stem cell behavior: Morphology, proliferation, and differentiation. Biomechanics and modeling in mechanobiology. 2011;10:939–953. doi: 10.1007/s10237-010-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shyu KG. Cellular and molecular effects of mechanical stretch on vascular cells and cardiac myocytes. Clin Sci (Lond) 2009;116:377–389. doi: 10.1042/CS20080163. [DOI] [PubMed] [Google Scholar]
- 11.Shaw A, Xu Q. Biomechanical stress-induced signaling in smooth muscle cells: An update. Curr Vasc Pharmacol. 2003;1:41–58. doi: 10.2174/1570161033386745. [DOI] [PubMed] [Google Scholar]
- 12.Zhu JH, Chen CL, Flavahan S, Harr J, Su B, Flavahan NA. Cyclic stretch stimulates vascular smooth muscle cell alignment by redox-dependent activation of notch3. Am J Physiol Heart Circ Physiol. 2011;300:H1770–1780. doi: 10.1152/ajpheart.00535.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, et al. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, et al. Upregulation of nox-based nad(p)h oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 15.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of nadph oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katoh Y, Molkentin JD, Dave V, Olson EN, Periasamy M. Mef2b is a component of a smooth muscle-specific complex that binds an a/t-rich element important for smooth muscle myosin heavy chain gene expression. The Journal of biological chemistry. 1998;273:1511–1518. doi: 10.1074/jbc.273.3.1511. [DOI] [PubMed] [Google Scholar]
- 17.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (mef2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 18.Potthoff MJ, Olson EN. Mef2: A central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 19.Firulli AB, Miano JM, Bi W, Johnson AD, Casscells W, Olson EN, et al. Myocyte enhancer binding factor-2 expression and activity in vascular smooth muscle cells. Association with the activated phenotype. Circ Res. 1996;78:196–204. doi: 10.1161/01.res.78.2.196. [DOI] [PubMed] [Google Scholar]
- 20.Martin JF, Miano JM, Hustad CM, Copeland NG, Jenkins NA, Olson EN. A mef2 gene that generates a muscle-specific isoform via alternative mrna splicing. Molecular and cellular biology. 1994;14:1647–1656. doi: 10.1128/mcb.14.3.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsuyama M, Ozgur Cevik M, Arakawa N, Kakehi T, Nishinaka T, Iwata K, et al. Myocyte enhancer factor 2b is involved in the inducible expression of nox1/nadph oxidase, a vascular superoxide-producing enzyme. FEBS J. 2007;274:5128–5136. doi: 10.1111/j.1742-4658.2007.06034.x. [DOI] [PubMed] [Google Scholar]
- 22.Ranayhossaini DJ, R AI, Chen BB, Mallampalli RK, Kelley EE, Csanyi G, Sahoo S, Gladwin MT, Romero G, Pagano PJ. Selective recapitulation of conserved and non-conserved contiguous regions in putative noxa1 activation domain confers isoform-specificity of nox1 oxidase inhibition. J Biol Chem. 2013;288:36437–36450. doi: 10.1074/jbc.M113.521344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Ghouleh I, Frazziano G, Rodriguez AI, Csanyi G, Maniar S, St Croix CM, et al. Aquaporin 1, nox1, and ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc Res. 2013;97:134–142. doi: 10.1093/cvr/cvs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, et al. Mt1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. The Journal of experimental medicine. 2005;202:663–671. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehti K, Rose NF, Valavaara S, Weiss SJ, Keski-Oja J. Mt1-mmp promotes vascular smooth muscle dedifferentiation through lrp1 processing. Journal of cell science. 2009;122:126–135. doi: 10.1242/jcs.035279. [DOI] [PubMed] [Google Scholar]
- 26.Kirby BJ. Micro- and nanoscale fluid mechanics: Transport in microfluidic devices. Cambridge: University Press; 2010. [Google Scholar]
- 27.Mongardon N, Dyson A, Singer M. Pharmacological optimization of tissue perfusion. British journal of anaesthesia. 2009;103:82–88. doi: 10.1093/bja/aep135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, et al. Impaired tissue perfusion: A pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968–976. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 29.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: Regulation and signaling leading to dysfunction. Experimental biology and medicine (Maywood, N J) 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 30.Cascino T, Csanyi G, Al Ghouleh I, Montezano AC, Touyz RM, Haurani MJ, et al. Adventitia-derived hydrogen peroxide impairs relaxation of the rat carotid artery via smooth muscle cell p38 mitogen-activated protein kinase. Antioxidants & redox signaling. 2011;15:1507–1515. doi: 10.1089/ars.2010.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lassegue B, Clempus RE. Vascular nad(p)h oxidases: Specific features, expression, and regulation. American journal of physiology Regulatory, integrative and comparative physiology. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 32.Sedeek M, Hebert RL, Kennedy CR, Burns KD, Touyz RM. Molecular mechanisms of hypertension: Role of nox family nadph oxidases. Current opinion in nephrology and hypertension. 2009;18:122–127. doi: 10.1097/MNH.0b013e32832923c3. [DOI] [PubMed] [Google Scholar]
- 33.Azumi H, Inoue N, Takeshita S, Rikitake Y, Kawashima S, Hayashi Y, et al. Expression of nadh/nadph oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 34.Ohmine T, Miwa Y, Takahashi-Yanaga F, Morimoto S, Maehara Y, Sasaguri T. The involvement of aldosterone in cyclic stretch-mediated activation of nadph oxidase in vascular smooth muscle cells. Hypertens Res. 2009;32:690–699. doi: 10.1038/hr.2009.76. [DOI] [PubMed] [Google Scholar]
- 35.Wang BW, Chang H, Shyu KG. Regulation of resistin by cyclic mechanical stretch in cultured rat vascular smooth muscle cells. Clin Sci (Lond) 2010;118:221–230. doi: 10.1042/cs20090155. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Li W, Quan Z, Sumpio BE. Modulation of vascular smooth muscle cell alignment by cyclic strain is dependent on reactive oxygen species and p38 mitogen-activated protein kinase. Journal of vascular surgery. 2003;37:660–668. doi: 10.1067/mva.2003.95. [DOI] [PubMed] [Google Scholar]
- 37.Paravicini TM, Montezano AC, Yusuf H, Touyz RM. Activation of vascular p38mapk by mechanical stretch is independent of c-src and nadph oxidase: Influence of hypertension and angiotensin ii. Journal of the American Society of Hypertension : JASH. 2012;6:169–178. doi: 10.1016/j.jash.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, et al. Thrombospondin-1 regulates blood flow via cd47 receptor-mediated activation of nadph oxidase 1. Arterioscler Thromb Vasc Biol. 2012;32:2966–2973. doi: 10.1161/ATVBAHA.112.300031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Zhang J, Fu W, Guo D, Jiang J, Wang Y. Association of smooth muscle cell phenotypes with extracellular matrix disorders in thoracic aortic dissection. Journal of vascular surgery. 2012;56:1698–1709. doi: 10.1016/j.jvs.2012.05.084. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Zheng J, Du Y, Huang Y, Li J, Liu B, et al. Cartilage oligomeric matrix protein maintains the contractile phenotype of vascular smooth muscle cells by interacting with alpha(7)beta(1) integrin. Circ Res. 2010;106:514–525. doi: 10.1161/CIRCRESAHA.109.202762. [DOI] [PubMed] [Google Scholar]
- 41.Tsai MC, Chen L, Zhou J, Tang Z, Hsu TF, Wang Y, et al. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor alpha/delta activations by prostacyclin released by sheared endothelial cells. Circ Res. 2009;105:471–480. doi: 10.1161/CIRCRESAHA.109.193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nobrega MA, Shiozawa M, Koike G, Jacob HJ, Miano JM. Gene structure and chromosomal mapping of the rat smooth muscle calponin gene. Mammalian genome : official journal of the International Mammalian Genome Society. 2000;11:115–119. doi: 10.1007/s003350010023. [DOI] [PubMed] [Google Scholar]
- 44.Rensen SS, Niessen PM, Long X, Doevendans PA, Miano JM, van Eys GJ. Contribution of serum response factor and myocardin to transcriptional regulation of smoothelins. Cardiovasc Res. 2006;70:136–145. doi: 10.1016/j.cardiores.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Christen T, Bochaton-Piallat ML, Neuville P, Rensen S, Redard M, van Eys G, et al. Cultured porcine coronary artery smooth muscle cells. A new model with advanced differentiation. Circ Res. 1999;85:99–107. doi: 10.1161/01.res.85.1.99. [DOI] [PubMed] [Google Scholar]
- 46.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. Mir-143 and mir-145: Molecular keys to switch the phenotype of vascular smooth muscle cells. Circulation Cardiovascular genetics. 2011;4:197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 47.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the mir143/145 gene cluster. The Journal of clinical investigation. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matchkov VV, Kudryavtseva O, Aalkjaer C. Intracellular ca(2)(+) signalling and phenotype of vascular smooth muscle cells. Basic & clinical pharmacology & toxicology. 2012;110:42–48. doi: 10.1111/j.1742-7843.2011.00818.x. [DOI] [PubMed] [Google Scholar]
- 49.Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular regulation of contractile smooth muscle cell phenotype: Implications for vascular tissue engineering. Tissue engineering Part B, Reviews. 2010;16:467–491. doi: 10.1089/ten.teb.2009.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobsen JC, Holstein-Rathlou NH. A life under pressure: Circumferential stress in the microvascular wall. Basic & clinical pharmacology & toxicology. 2012;110:26–34. doi: 10.1111/j.1742-7843.2011.00796.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu B, Qu MJ, Qin KR, Li H, Li ZK, Shen BR, et al. Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro. Biophysical journal. 2008;94:1497–1507. doi: 10.1529/biophysj.106.098574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lai CF, Seshadri V, Huang K, Shao JS, Cai J, Vattikuti R, et al. An osteopontin-nadph oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res. 2006;98:1479–1489. doi: 10.1161/01.RES.0000227550.00426.60. [DOI] [PubMed] [Google Scholar]
- 53.Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. The Annals of thoracic surgery. 2004;78:2106–2110. doi: 10.1016/j.athoracsur.2004.05.088. discussion 2110-2101. [DOI] [PubMed] [Google Scholar]
- 54.Patel MI, Melrose J, Ghosh P, Appleberg M. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. Journal of vascular surgery. 1996;24:82–92. doi: 10.1016/s0741-5214(96)70148-9. [DOI] [PubMed] [Google Scholar]
- 55.Xu S, Shriver AS, Jagadeesha DK, Chamseddine AH, Szocs K, Weintraub NL, et al. Increased expression of nox1 in neointimal smooth muscle cells promotes activation of matrix metalloproteinase-9. Journal of vascular research. 2012;49:242–248. doi: 10.1159/000332958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh NK, Kundumani-Sridharan V, Kumar S, Verma SK, Kotla S, Mukai H, et al. Protein kinase n1 is a novel substrate of nfatc1-mediated cyclin d1-cdk6 activity and modulates vascular smooth muscle cell division and migration leading to inward blood vessel wall remodeling. The Journal of biological chemistry. 2012;287:36291–36304. doi: 10.1074/jbc.M112.361220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 58.Liu S, Li Y, Zhang Z, Xie F, Xu Q, Huang X, et al. Alpha(1)-adrenergic receptors mediate combined signals initiated by mechanical stretch stress and norepinephrine leading to accelerated mouse vein graft atherosclerosis. Journal of vascular surgery. 2013;57:1645–1656. doi: 10.1016/j.jvs.2012.09.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


