Abstract
Background
Alzheimer disease is characterized by cognitive decline, senile plaques of β-amyloid (Aβ) peptides, neurofibrillary tangles composed of hyperphosphorylated τ proteins and neuronal loss. Aβ and τ are useful markers in the cerebrospinal fluid (CSF). C-Jun N-terminal kinases (JNKs) are serine-threonine protein kinases activated by phosphorylation and involved in neuronal death.
Methods
In this study, Western blots, enzyme-linked immunosorbent assay and histological approaches were used to assess the concentrations of Aβ, τ and JNK isoforms in postmortem brain tissue samples (10 Alzheimer disease and 10 control) and in CSF samples from 30 living patients with Alzheimer disease and 27 controls with neurologic disease excluding Alzheimer disease. Patients with Alzheimer disease were followed for 1–3 years and assessed using Mini–Mental State Examination scores.
Results
The biochemical and morphological results showed a significant increase of JNK3 and phosphorylated JNK levels in patients with Alzheimer disease, and JNK3 levels correlated with Aβ42 levels. Confocal microscopy revealed that JNK3 was associated with Aβ in senile plaques. The JNK3 levels in the CSF were significantly elevated in patients with Alzheimer disease and correlated statistically with the rate of cognitive decline in a mixed linear model.
Limitations
The study involved different samples grouped into 3 small cohorts. Evaluation of JNK3 in CSF was possible only with immunoblot analysis.
Conclusion
We found that JNK3 levels are increased in brain tissue and CSF from patients with Alzheimer disease. The finding that increased JNK3 levels in CSF could reflect the rate of cognitive decline is new and merits further investigation.
Introduction
Alzheimer disease is a neurodegenerative disorder that leads to progressive cognitive decline with memory loss and dementia. Neuropathological lesions are characterized by extracellular accumulations of senile plaques, formed by β-amyloid (Aβ) peptide, and intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated τ proteins.1 According to the amyloid cascade hypothesis, neurodegeneration in Alzheimer disease could be linked to an abnormal amyloid precursor protein (APP) processing through the activity of the β-site APP cleaving enzyme 1 (BACE1) and presenilin 1. All these processes lead to the production of toxic Aβ oligomers that accumulate in fibrillar Aβ peptides before forming Aβ plaques. Aβ accumulation can lead to synaptic dysfunction, altered kinase activities resulting in NFT formation, neuronal loss and dementia.2,3 Over the past 20 years, several biomarkers of Alzheimer disease obtained from cerebrospinal fluid (CSF) have been extensively studied: CSF concentrations of Aβ42 have been reported to be decreased, whereas CSF total τ (T-τ) and phosphorylated-τ on threonine 181 (p181τ) are augmented.4 A previous study has reported significant correlations between CSF biomarker levels and neuropathological load for Aβ42 and τ.5 In addition, we have previously shown that the levels of the pro-apoptotic double-stranded RNA-dependent protein kinase (PKR) are increased in the brains and the CSF of patients with Alzheimer disease6 and correlate with cognitive decline.7
C-Jun N-terminal kinases (JNKs) are a family of serine-threonine protein kinases encoded by 3 genes (JNK1, JNK2, and JNK3) and expressed as 10 different isoforms due to alternative splicing of messenger RNA (mRNA). Each isoform is detected as a short (46 kDa) and a long form (54 kDa).8 Whereas JNK1 and JNK2 are ubiquitous, JNK3 is mainly expressed in the brain.9 JNKs are activated by phosphorylation (pJNK) through mitogen-activated protein (MAP) kinase kinase activation by extracellular stimuli, such as ultraviolet light, cytokines and Aβ peptides. They have multiple functions, including regulation of gene expression, inflammation, cell proliferation and apoptosis.10,11 In general, in the brain, JNK1 and JNK2 isoforms seem to have a role in development, whereas JNK3 seems principally implicated in neurodegeneration.12 Previous studies implicating JNKs in the pathogenesis of Alzheimer disease have shown that JNKs, particularly JNK3, can phosphorylate APP, enhancing Aβ production.13,14 JNK activity can also raise BACE1 expression15 and is detected in granulovacuolar degeneration.16 Activated JNKs are increased in Alzheimer disease brains17 as well as some upstream JNK activators, such as JKK1.18 JNK3 is implicated in neurodegeneration, and its deletion has a neuroprotective effect against ischemia19 and excitotoxicity.20,21 Aβ-induced cell death is reduced in cultures of cortical neurons from JNK3 knockout (KO) mice,22 and JNKs have been implicated in experimental models of Alzheimer and Parkinson diseases.14,23,24
JNK3 may be detrimental to neurons, but no study has evaluated the levels of JNK3 in Alzheimer disease brains and in the CSF of living patients with Alzheimer disease. To our knowledge, our study is the first to show that JNK3 levels are increased in the CSF of patients with Alzheimer disease and that they are linked to cognitive decline in affected patients.
Methods
Patients
We obtained written informed consent from all patients assessed at Lariboisiere Hospital Paris France (Paris North Memory Center) who provided CSF samples. The project was approved by the Ethical Committee of Paris Diderot University Hospitals (CEERB Bichat University Hospital, Paris, France). Probable Alzheimer disease diagnosis was made during life according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s disease and Related Disorders (NINCDS-ADRDA) criteria.25 We obtained frozen and fixed brain samples (Alzheimer disease and controls) from the Brain Banks of Geneva University Hospital, Geneva, Switzerland, or Lariboisière Hospital, Paris, France.
For the CSF study, we retrospectively included, from our out-patient memory clinic, 30 consecutive patients with clinically probable Alzheimer disease and 27 consecutive controls with neurologic disease. Our patients were consecutive and were not age-matched. The control group was composed of patients with psychiatric disorders (n = 12), alcohol-related dementia (n = 6), vascular dementia (n = 3), normal pressure hydrocephaly (n = 4), sleep apnea syndrome (n = 1) and Lewy Body disease (n = 1). We confirmed the absence of Alzheimer disease from the control group based on imaging and CSF results. Clinical diagnosis was made by a multidisciplinary team specialized in cognitive disorders using all available clinical data, including extensive neuropsychological assessment and MRI. The medical team was unaware of the CSF results, and their interpretation was not part of the initial diagnostic process. All patients with Alzheimer disease were treated with cholinesterase inhibitors and were followed up clinically for 1–3 years.
Human brain samples
For immunoblots and Aβ42 enzyme-linked immunosorbent assay (ELISA) analysis, one of us (C.B.) provided 10 Alzheimer disease and 10 control frozen frontal cortices. For immunoblots and Aβ42 enzyme-linked immunosorbent assay (ELISA) analysis, samples from 10 Alzheimer disease and 10 control fixed frontal cortices were collected. For immunohistochemistry, samples of 8 Alzheimer disease and 9 control fixed frontal cortices were collected. The neuropathological diagnosis of Alzheimer disease was made according to standard procedures,1 and this diagnosis was excluded in control brains after a careful neuropathological examination. Clinical characteristics of Alzheimer disease and control samples are given in the Appendix, Table S1, available at jpn.ca. Postmortem intervals (PMI) never exceeded 24 hours.
CSF samples
For all included patients, CSF samples were provided by the Research Memory Centre Paris Nord Ile de France (Lariboisière Hospital, France). It was collected by lumbar punctures performed on fasting patients in the month following their clinical diagnosis, as previously reported.26 Each CSF sample was first centrifuged at 1000g for 10 minutes at 4°C. A small amount of CSF was used to perform routine analyses, including total cell count, bacteriological exam, total protein and glucose levels to exclude other anomalies.
Antibodies
For immunoblotting, we used mouse anti-panJNK (Santa Cruz Biotechnology), rabbit anti-pJNKThr183/Tyr185;Thr221/Tyr223 (Millipore), mouse anti-JNK1 (BD Biosciences), rabbit anti-JNK2 (Cell Signaling), rabbit anti-JNK3 (Millipore), mouse anti-serum albumin (Santa Cruz Biotechnology) and mouse anti-α-actin (Cell Signaling). We used IR Dye 800CW conjugated anti-rabbit IgG and IR Dye 800CW conjugated anti-mouse (Rockland Immunochemical Inc.) as secondary antibodies.
For immunohistochemistry, we used rabbit anti-pJNKThr183/Tyr185 (Cell Signaling), rabbit anti-JNK3 (Millipore) and mouse anti-Aβ42 (Waco Chemicals) as primary antibodies and biotinylated anti-rabbit and anti-mouse (Vector Laboratories) as secondary antibodies. For immunofluorescence, we used donkey anti-mouse Alexa Fluor488 (Life Technology) or goat anti-rabbit Cy3 (Jackson Laboratory) as secondary antibodies.
The specificity of the JNK3 antibody was assessed by comparison of JNK3 immunostaining of the same Alzheimer disease frontal cortex section incubated with either normal JNK3 antibody or with saturated JNK3 antibody. We achieved antibody saturation of JNK3 binding epitopes by previous incubation with 1.25 μg/mL of recombinant JNK3 protein (Millipore) at 4°C overnight. The same comparison was done for immunoblot on Alzheimer disease CSF samples (Appendix, Fig. S1). The specificity of the JNK3 antibody toward JNK1 and JNK2 isoforms was assessed by immunoblot using human embryonic kidney (HEK) cell lysates expressing only JNK1 (provided by Dr. Hervé Enslen, Cochin Institute, Paris) or JNK2 (Santa Cruz Biotechnology; Appendix, Fig. S1).
Neuropathological study
For all assessments, the researchers were blind to results from the clinical data. Neuropathological evaluations were in agreement with postmortem neuropathological consensus criteria for Alzheimer disease diagnosis.1 Braak stages of neurofibrillary degeneration were assessed according to previous reports.27 Selected sections of frontal cortex were immunostained for Aβ and phosphorylated τ. The Alzheimer disease and control cases were matched as closely as possible for age and sex.
Human brain immunoblotting
Frozen frontal cortex samples were homogenized in Laemmli sample buffer, 5% sodium dodecyl sulfate, 1X protease and phosphatase inhibitors cocktail (Roche). Lysates were then sonicated and centrifuged at 15 000g for 15 minutea at 4°C. The protein concentration in the supernatant was determined using a Micro BCA protein assay kit (Thermo Scientific). We used 30 μg of the supernatant for immunoblot analysis, as previously described.28 Bound proteins were visualized with the Odyssey Imaging System (Li-COR Biosciences) and quantified with MultiGauge software (Fuji). Assays were performed in duplicate.
Human brain immunoprecipitation
Protein immunoprecipitation was performed to detect the level of each phosphorylated form of the 3 JNK isoforms in 3 Alzheimer disease brains and 3 control brains. We suspended 200 μg of SDS-frontal cortex homogenates in IP buffer (PBS, 5 mM EDTA, 0.5% NP40 vol/vol, 0.5% SDS weight/vol, 1X protease inhibitor) and incubated with 2 μg primary antibody or 2 μg control IgG rabbit or IgG mouse (Santa Cruz Biotechnology) for negative controls overnight at 4°C. Homogenates were then incubated in the presence of A/G-agarose beads (Santa Cruz Biotechnology) for 60 minutes at 4°C. The beads were washed 3 times, and the precipitated proteins were extracted at 95°C using sodium dodecyl sulfate poly-acrylamide gel (SDS–PAGE) sample buffer (Invitrogen). Proteins were revealed by immunoblot analysis using the primary and secondary antibodies described earlier.
Brain Aβ42 quantification
We measured Aβ42 concentration in duplicate in all SDS-frontal cortex homogenates using a Human Aβ42 ELISA kit (Invitrogen) and following the manufacturer’s instructions.
Immunohistochemistry and confocal microscopy
Frontal brain sections (4 μm) from paraffined human brains (8 Alzheimer disease and 9 control) were used, as previously described.29,30 Quantification of immunohistochemistry staining (% area stained of total area examined) was performed using Image J version 1.6 software (National Institutes of Health). At least 15 images taken in a zigzag sequence at a magnification of ×20, were captured from each slide of tissue for quantification. We used an Olympus AX70 Provis microscope. For confocal microscopy, paraffined Alzheimer disease brains sections were incubated in formic acid for 30 minutes in addition to the citrate buffer unmasking method. Sections were permeabilized with tris-buffered saline (TBS) Triton 0.2% (vol/vol) before the blocking procedure and incubated with the 2 primary antibodies anti-Aβ and anti-JNK3 at 4°C. Sections were incubated in Autofluorescence Eliminator Reagent (Millipore) for 5 minutes at the end of the protocol. Confocal imaging was performed at a magnification of ×60 on an Olympus Fluoview FV10i confocal microscope using MetaMorph software (Roper Scientific).
CSF Procedures
Biological analyses of Aβ42, T-τ and p181τ CSF levels were performed using the ELISA method according to manufacturer’s procedures (Innogenetics) in the Department of Biochemistry, Lariboisière Hospital, France. The CSF Alzheimer disease profile was defined as an Innotest Amyloid Tau Index less than 0.8 and 3 abnormal biomarkers according to our optimal cut off: Aβ42 less than 500 pg/mL; T-τ greater than 290 pg/mL and p181τ greater than 66 pg/mL. These cut-offs are based on a previous publication from the Memory Center.26
To assess CSF JNK levels, we removed albumin and immunoglobulin from CSF samples before performing the immunoblot analysis using the ProteoExtract Albumin/IgG Removal Kit (Calbiochem), as previously described.6 The serum albumin removal efficiency results are discussed in the Appendix, Figure S2. Protein concentrations in the depleted CSF samples were determined with the Micro BCA Protein Assay Reagent Kit (Thermo Scientific). Evaluations of the different JNK levels were obtained using immunoblot analysis. All CSF analyses were carried out in duplicate.
Statistical analysis
Brain study
For immunohistochemical analysis, quantification of immunostaining obtained for the Alzheimer disease and control groups were compared using the Student t test. For immunoblot analysis, quantifications were normalized to α-actin levels before performing the test. Spearman correlation coefficients were used to analyze brain Aβ42 and JNKs levels.
CSF study
Clinical and biomarkers characteristics were compared using the χ2 statistic for categorical variables and analysis of variance for continuous variables. To determine the efficacy of JNK3 as a discriminatory biomarker between Alzheimer disease and control patients, we analyzed the receiver operating characteristic (ROC) curve. JNK3 Alzheimer disease–control discriminatory power was symbolized by the value of the area under the ROC curve. We identified optimum cut-offs for all biomarkers by calculating the Youden Index and determined the sensitivity and specificity. Correlations between the different biomarkers were calculated using Pearson correlation coefficients. In the Alzheimer disease group, linear mixed models adjusted for age and sex or MRI abnormalities (the Fazekas score, measuring the presence of lesions of microvascular origin,31 and the Scheltens scale, measuring hippocampal atrophy32) were used to study the cross-sectional and longitudinal associations between baseline levels of CSF biomarkers and CSF JNK3 with repeated measurements of the Mini–Mental State Examination (MMSE) scores, the neuropsychological test used in our memory centre. Mixed models allow all available data to be used in the analysis to estimate the intercept (cross-sectional effect) and the slope (longitudinal effect). They are estimated together, with the correlation between them taken into account in the model. The intercept and slope were treated as random effects, allowing them to vary among individuals. Time in years since baseline (lumbar puncture) was included as a continuous linear term after verification that a quadratic term did not improve model fit. Results are shown using tertiles of patients (10 patients per group) according to CSF JNK3 at baseline.
All statistical analyses for brain and CSF studies were performed using SAS version 9.2 software (SAS Institute). For all comparisons, we considered results to be significant at p < 0.05.
Results
Increased JNK3 level in the Alzheimer disease frontal cortex
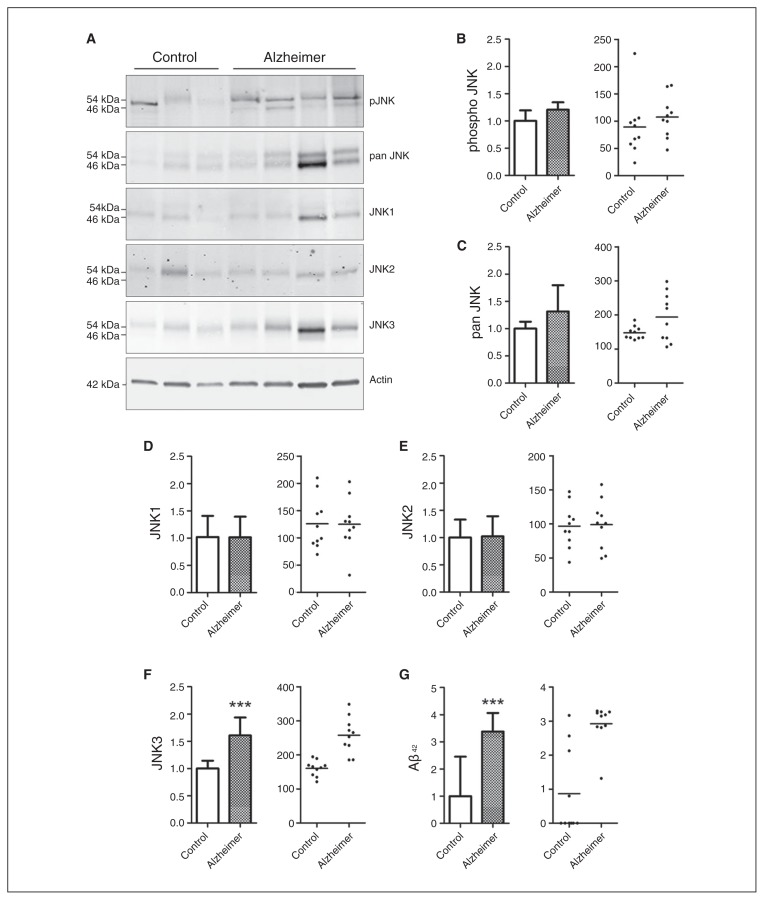
The characteristics of Alzheimer disease and control brains used for immunoblot study are detailed in the Appendix, Table S1. There was no statistical difference in age or sex between the groups. Using immunoblot analysis of the frontal cortex (Fig. 1A), we detected a significant increase of 61% for JNK3 levels (p < 0.001) in the frontal cortex of patients with Alzheimer disease compared with controls (Fig. 1F). We did not observe any difference in the level of pJNK or pan-JNK (Fig. 1B and C). Similarly, the analysis of JNK1 and JNK2 did not show any variation between the groups (Fig. 1D and E), and no correlation was found between the levels of JNK3 and sex, age and PMI of patients with Alzheimer disease.
Fig. 1.
Levels of total pan JNK, pJNK, the different full form of JNK isoforms, and amyloid-β (Aβ42) in the postmortem frontal cortex of patients with Alzheimer disease (n = 10) and controls (n = 10). (A) Immunoblot analysis of pJNK, total JNK, JNK1, JNK2 and JNK3 in frontal cortex samples from patients with Alzheimer disease and controls. Corresponding histograms and scatter plots, in optical density units (ODU), of (B) pJNK, (C) total JNK, (D) JNK1, (E) JNK2 and (F) JNK3 protein levels, showing that the JNK3 isoform is significantly increased in the frontal cortex of patients with Alzheimer disease. (G) Aβ42 measured in the brain, showing an increase in the frontal cortex of patients with Alzheimer disease. ***p < 0.001. Error bars indicate standard errors of the mean.
Brain Aβ42 levels
Results of the ELISA showed that Aβ42 concentrations were increased in Alzheimer disease brains compared with control brains, although several control brains had high Aβ42 levels (p < 0.001; Fig. 1G). A significant correlation between brain Aβ42 and JNK3 levels was observed in all samples (p = 0.023; z = 0.5929).
JNK3 and pJNK immunostainings
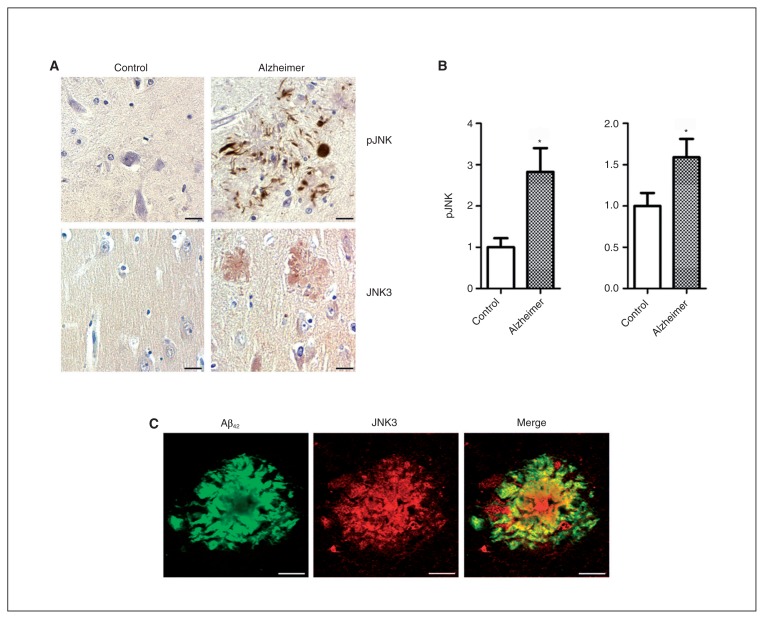
The characteristics of Alzheimer disease and control brains used for immunohistochemistry are detailed in the Appendix, Table S1. There was no statistical difference in age or sex between the groups. Immunohistochemical results revealed that pJNK in Alzheimer disease brains was detected in the peripheral rim of senile plaques, the neurofibrillary tangles (Fig. 2A) and the granulovacuolar degenerations (data not shown), as previously reported.17,33 We rarely observed pJNK immunolabelling in control brains. Neurons were modestly marked in the cytoplasm and in the nucleus in Alzheimer disease brains.
Fig. 2.
Expression and localization of pJNK and JNK3 full in Alzheimer disease and control brains. (A) Immunohistochemical studies in the frontal cortex of patients with Alzheimer disease (n = 8) and controls (n = 9) showed pJNK staining localization around senile plaques and neurofibrillary tangles (top) and JNK3 immunostaining at senile plaques (bottom). (B) The pJNK and JNK3 expression was increased in the frontal cortex of patients with Alzheimer disease compared with the same regions in controls. (C) Confocal analysis showed an association of JNK3 and amyloid-β (Aβ) labelling in senile plaques in the frontal cortex of patients with Alzheimer disease. *p < 0.05. Scale bars = 10 μm. Error bars indicate standard errors of the mean.
In Alzheimer disease brains, JNK3 immunostaining was detected in the centre and around senile plaques as well as in the cytoplasm of neurons (Fig. 2A). Confocal imaging of senile plaques revealed an association between Aβ42 and JNK3 staining (Fig. 2C), suggesting that some JNK3 proteins may accumulate during the formation of amyloid aggregates.
JNK3 and pJNK colocalization has been indirectly validated by immunostaining of the same senile plaques on 2 consecutive Alzheimer disease frontal cortex sections (data not shown).
Quantification of histological results in Alzheimer disease (n = 8) and control (n = 9) brains confirmed the increase of JNK3 staining (+59%, p = 0.042; Fig. 2B) and pJNK staining (+182%, p = 0.039; Fig. 2B) in the frontal cortex of Alzheimer disease brains compared with control brains.
Increased JNK3 level in Alzheimer disease CSF
The clinical characteristics and biomarker levels of patients with Alzheimer disease and controls are shown in Table 1. Controls were slightly younger than patients with Alzheimer disease. Fazekas scores did not differ between the groups. Scheltens scores correlated with Alzheimer disease diagnosis (p = 0.005). Aβ42, T-τ and p181τ CSF levels differed significantly between the groups (p < 0.001).
Table 1.
Baseline characteristics of controls with neurologic disease and patients with Alzheimer disease
| Group; no. (%) or mean ± SD | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Overall, n = 57 | Control, n = 27 | Alzheimer disease, n = 30 | p value |
| Age, yr | 68.2 ± 9.7 | 65.2 ± 8.8 | 70.9 ± 9.9 | 0.024 |
| Sex, female | 32 (56.1) | 15 (55.6) | 17 (56.7) | 0.83 |
| MMSE | 20.9 ± 7.8 | 25.6 ± 2.6 | 20.2 ± 6.0 | < 0.001 |
| Educational level* | 0.66 | |||
| Low | 15 (27.8) | 8 (33.3) | 7 (23.3) | |
| Medium | 18 (33.3) | 8 (33.3) | 10 (33.3) | |
| High | 21 (38.9) | 8 (33.3) | 13 (43.3) | |
| MRI characteristics | ||||
| Fazekas score | 1.04 (0.56) | 0.97 (0.63) | 1.14 (0.47) | 0.29 |
| Scheltens scale | 1.75 (1.06) | 1.27 (0.95) | 2.10 (1.02) | 0.005 |
| ApoE4 carriers‡ | 15 (34.9) | 1 (6.3) | 14 (51.9) | 0.002 |
| CSF biomarkers, pg/mL | ||||
| Aβ42 | 632.2 ± 281.8 | 828.2 ± 196.7 | 455.9 ± 231.2 | < 0.001 |
| T-τ | 409.8 ± 310.5 | 200.2 ± 75.9 | 598.5 ± 326.6 | < 0.001 |
| p181τ | 75.8 ± 44.7 | 43.3 ± 17.3 | 102.2 ± 46.1 | < 0.001 |
| CSF JNK3, ODU | 76.2 ± 21.0 | 67.9 ± 19.4 | 83.6 ± 20.2 | 0.004 |
Aβ = amyloid-β; CSF = cerebrospinal fluid; MMSE = Mini–Mental State Examination; ODU = optical density units; p181τ = τ phosphorylated at threonine 181; SD = standard deviation; T-τ = total τ.
Low = unschooled; medium = middle school or high school; high = postsecondary or more.
Average between Scheltens scale from right and left sides.
ApoE score was missing for 15 patients.
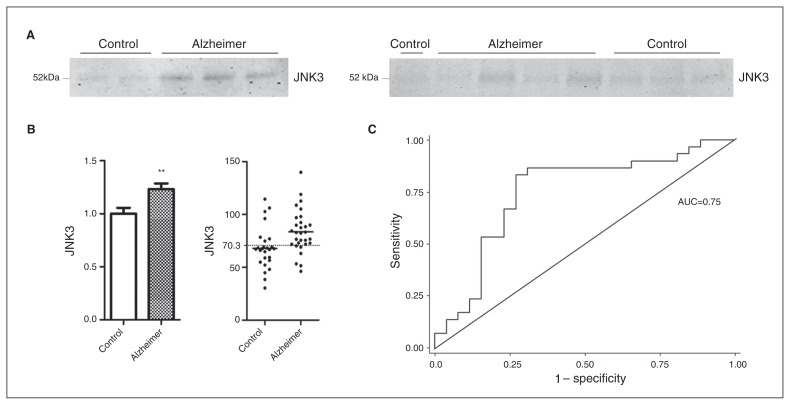
JNK1, JNK2 and pJNK proteins were not detectable in the CSF. The results revealed increased CSF JNK3 levels in the Alzheimer disease compared with the control group (+23%, p = 0.004; Fig. 3A, B). Optimal cut-offs, Younden index, sensitivity, specificity and areas under the curve (AUC) are shown in Table 2. The JNK3 value of 70.3 optical density units (ODU) had a sensitivity of 0.80 and a specificity of 0.73, and the AUC was 0.75 (Fig. 3C). No correlations were found between Alzheimer disease CSF JNK3 levels and age, sex, MMSE scores or the CSF levels of Aβ42, T-τ, and p181τ (Appendix, Table S2).
Fig. 3.
Level of total JNK3 in the cerebrospinal fluid (CSF) of patients with Alzheimer disease (n = 30) and controls with neurologic disease (n = 27). (A) Immunoblot analyses of total CSF JNK3 in patients with Alzheimer disease and controls. (B) Histogram showing that JNK3 is increased in the CSF of patients with Alzheimer disease, and scatter plot showing the CSF JNK3 levels of patients with Alzheimer disease. The dotted line at 70.3 optimal density units (ODU) represents the optimal threshold value, which allows us to distinguish patients in 1 of the 2 observed categories. (C) Representation of the receiver operating characteristic (ROC) curve for JNK3 level in the CSF. The ROC curve distinguishes between patients with Alzheimer disease and controls with neurologic disease. The area under curve (AUC) for JNK3 is 0.75. **p < 0.01. Error bars indicate standard errors of the mean.
Table 2.
Receiver operating characteristics curve evaluation for the different cerebrospinal fluid biomarkers in controls with neurologic disease and patients with Alzheimer disease
| CSF biomarkers | AUC (SE) | Threshold value | Sensitivity, % | Specificity, % | Youden Index |
|---|---|---|---|---|---|
| Aβ42, pg/mL | 0.89 (0.05) | 585 | 0.83 | 0.92 | 1.76 |
| T-τ, pg/mL | 0.91 (0.04) | 345 | 0.83 | 0.96 | 1.79 |
| p181τ, pg/mL | 0.91 (0.04) | 73 | 0.80 | 0.92 | 1.72 |
| JNK3, ODU | 0.75 (0.07) | 70.3 | 0.80 | 0.73 | 1.56 |
Aβ = amyloid-β; AUC = area under the curve; CSF = cerebrospinal fluid; ODU = optical density units; p181τ = τ phosphorylated at threonine 181; SE = standard error; T-τ = total τ.
No correlation was detected between MRI abnormalities and CSF JNK3 levels, using Fazekas scores or Scheltens scales (Appendix, Tables S2 and S3). No association was found between CSF JNK3 and educational level (Appendix, Table S3).
CSF JNK3 levels are linked to cognitive decline
Patients with Alzheimer disease were followed for a mean period of 1.8 (± 1.3) years. During the follow-up, clinicians performed 4.7 (± 2.25) MMSE tests per patient. Using linear mixed models, we assessed the association between baseline CSF Aβ42, T-τ, p181τ and JNK3 levels and the MMSE score at baseline as well as the evolution of the MMSE scores. Table 3 summarizes the results of this linear mixed model adjusted for age and sex. The mean decline of MMSE score was 1.80 ± 0.83 points per year (p = 0.049). We found that low CSF Aβ42 levels and pτ:τ ratio were associated with a lower MMSE score at baseline (data not shown), whereas there was no association with CSF JNK3 levels. For the longitudinal analysis, tertiles of JNK3 levels were defined as displayed in Table 3. We found that patients in the third tertile (> 89 ODU) experienced a significant decline in MMSE score over time (p = 0.041). The association between JNK3 and Alzheimer disease was maintained after adjusting for age, sex, educational level and MRI abnormalities (Appendix, Table S4).
Table 3.
Estimates of mixed linear models adjusted for age, sex and JNK3 CSF level and their associations with baseline or follow-up MMSE scores of patients with Alzheimer disease
| CSF JNK3 | Estimate (SE) | p value |
|---|---|---|
| MMSE score at baseline | ||
| Intercept | 21.8 (2.0) | < 0.001 |
| Age, yr | 0.09 (0.11) | 0.41 |
| Sex, men v. women | −0.83 (2.32) | 0.72 |
| JNK3 tertiles | ||
| 73.1 ODU | Ref. | |
| 73.2–89 ODU | −1.41 (2.65) | 0.60 |
| > 89 ODU | −0.66 (0.81) | 0.81 |
| Change in MMSE over the follow-up | ||
| Time, yr | −1.80 (0.83) | 0.028 |
| Age × time | 0.05 (0.05) | 0.31 |
| Sex × time | −0.59 (1.09) | 0.59 |
| JNK3 tertiles × time | ||
| 73.1 ODU | Ref. | |
| 73.2–89 ODU | −0.11 (1.04) | 0.92 |
| > 89 ODU | 2.44 (1.18) | 0.041 |
Aβ = amyloid-β; CSF = cerebrospinal fluid; MMSE = Mini–Mental State Examination; ODU = optical density units; SE = standard error.
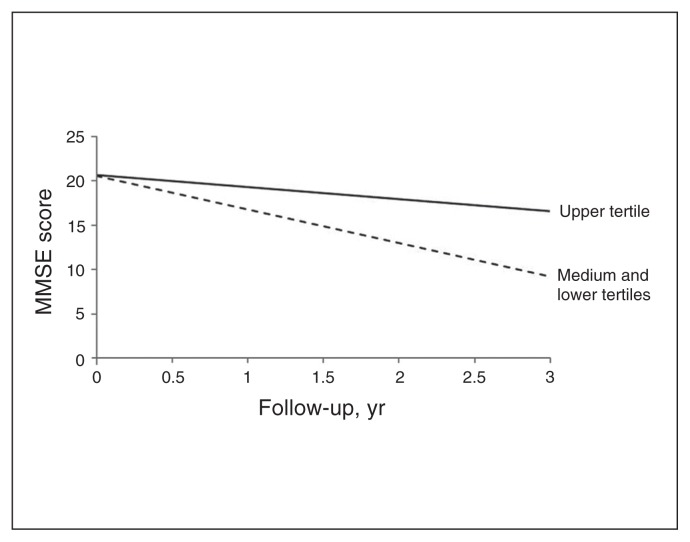
Comparison among the tertiles revealed that patients in the 2 lowest tertiles (< 89 ODU) experienced a more rapid decline in MMSE scores over time (p = 0.018) than those in the upper tertile. The predicted progression of MMSE scores according to JNK3 CSF tertiles during follow-up is presented in Figure 4. The clinical characteristics of patients with Alzheimer disease grouped by JNK3 tertiles are presented in the Appendix, Table S5.
Fig. 4.
Predicted trajectories of Mini–Mental State Examination (MMSE) scores of patients with Alzheimer disease according to tertiles of CSF JNK3 levels. Upper tertile of JNK3: > 89 optical density units (ODU); lower tertiles of JNK3 combined: < 89 ODU. Cognitive decline was most pronounced in patients with Alzheimer disease who had low levels of CSF JNK3 (dashed line; p = 0.018).
Discussion
This study has 4 major findings. First, JNK3 levels are increased in Alzheimer disease brains. Second, brain JNK3 levels correlate with Aβ levels. Third, CSF JNK3 levels are enhanced in patients with Alzheimer disease compared with controls. Finally, patients with Alzheimer disease who have medium and low levels of CSF JNK3 experience more severe cognitive decline.
Immunohistochemical results revealed a significant increase of pJNK in the frontal cortices of postmortem Alzheimer disease brains compared with control brains. Morphologically, JNK3 immunostaining colocalized with Aβ in the core of senile plaques, whereas pJNK labelling was mostly associated with NFT and dystrophic neurites around senile plaques.
These results raise several questions. First, what is the mechanism of JNK3 protein increase in Alzheimer disease brains? Little is known about the regulation of JNK, and in particular JNK3 gene expression in human cells. Experimentally, increased JNK3 expression has been detected in mice after brain ischemia, but this result was shown in a transgenic JNK1 KO mouse model and could have been due to a compensatory mechanism linked to the lack of JNK1.20 Enhanced JNK3 levels could be linked to Aβ-induced endoplasmic reticulum (ER) stress, which has been reported to be increased in Alzheimer disease brains.14,34 In addition, ER stress is implicated in JNK3 regulation in cellular models.14 A previous systematic search for global gene expression in Alzheimer disease also showed an increase of JNK cascade cluster in patients in late Braak stages.35 In addition, it has been shown that nuclear factor κ B (NFκB) can control BACE1 levels under the influence of Aβ and this process could also be involved for JNK3.36 Increased JNK3 could also be explained by a posttranscriptional mechanism involving the phosphorylation of the translation initiation factor eIF2α. This possibility has been demonstrated in Alzheimer disease mouse models and Alzheimer disease brains.28 The phosphorylation of eIF2α by kinases, including PKR, leads to an inhibition of protein synthesis, as seen in an Alzheimer disease mouse model,37,38 except for proteins with a peculiar reading frame that are putatively implicated in stress-response signalling pathways, such as BACE1.39 Our result is in agreement with that of a previous work showing an increase of JNK3 activity in Alzheimer disease brains.14 These 2 results revealed that JNK3 protein and JNK3 activity are both enhanced in Alzheimer disease brains compared with control brains and may explain the detrimental consequences on neuronal survival. Interestingly, our study shows that the levels of both JNK3 and Aβ are correlated in the brains of controls and patients with Alzheimer disease, suggesting that both molecules might belong to the same pathological pathway.
Second, a correlation between brain JNK3 and Aβ levels was detected in our entire sample. We have shown that brain Aβ levels were increased not only in patients with Alzheimer disease, but also in some controls devoid of τ lesions. This correlation suggests that JNK3 protein may accumulate in association with Aβ even when the complete set of Alzheimer disease neuropathological lesions is not present in control brains.
Third, immunohistochemical results revealed a significant increase of pJNK in the frontal cortices of Alzheimer disease brains without any significant difference of pJNK levels in the same structure on immunoblotting. This finding could be explained by the rather small number of evaluated patients, taking into consideration that 1 control patient had a very high level of pJNK — higher than the highest level in the Alzheimer disease group — probably because of an unknown brain inflammatory disease. Previous studies have shown an increase of pJNK in Alzheimer disease brains,17,33 but, unlike us, those authors used the temporal cortex,17,33 entorhinal cortex and hippocampus of Alzheimer disease brains.33
A fourth question raised by the results is related to the differences between JNK3 and pJNK immunostaining. Phosphorylated JNK immunoreactivity distribution is roughly the same as phosphorylated τ staining, whereas JNK3 labelling is localized in neurons and more in the core of senile plaques. The JNK3 antibody saturation assay revealed the specificity of the JNK3 antibody. Moreover our immunoprecipitation study showed that JNK3 is the principal activated JNK isoform (Appendix, Fig. S3). There are several possible explanations for these differences. Depending on the maturity of the senile plaque, the staining could be different. Diffuse plaques could be labelled with either pJNK or JNK3, whereas mature plaques could be labelled better with JNK3. The phosphorylated JNK3 epitope might be partially hidden in the core of senile plaques, unable to be recognized by the pJNK antibody, although formic acid was used. Or the form of JNK3 found inside senile plaques could be in a total form and not phosphorylated. Tangles and dystrophic neurites labelled by pτ and pJNK could also be mainly composed of JNK1 and JNK2 isoforms. A specific antibody directed to phosphorylated JNK3 could be useful, but seems very difficult to obtain in relation to the comparable sequences of amino acid residues of the concerned regions of JNK isoforms.40
Fifth, the CSF results reveal a significant increase of the JNK3 protein in patients with Alzheimer disease compared with controls, with a threshold value of 70.3 ODU that differentiates 2 populations of patients, with a good sensitivity (0.80) and a valid specificity (0.73). No correlations were detected between CSF Aβ and JNK3 levels, possibly because JNK3 protein could be released into the CSF but could also be trapped in senile plaques.
How can JNK3 protein be present in the CSF? As proposed for other proteins, such as τ or PKR,6 neuronal death detected in Alzheimer disease brains could release JNK3 in extracellular spaces. It is known that activated JNK can accumulate in degenerating neurons as Alzheimer disease progresses.17 We cannot rule out that JNK3 could also be released from nondying cells in Alzheimer disease brains, as seen for τ protein in experimental Alzheimer disease models or for PKR in patients with Alzheimer disease.6,41–43
In addition, we found an association between CSF JNK3 levels and the prospective cognitive decline in patients with Alzheimer disease. Patients in the 2 lowest tertiles of CSF JNK3 level (< 89 ODU) experienced a faster decline than patients in the upper tertile. We did not find this association with other CSF biomarkers, such as Aβ42, T-τ and pτ. Although CSF JNK3 levels were increased in patients with Alzheimer disease, why did patients with low levels of CSF JNK3 experience a more rapid decline in cognitive function? It is possible that JNK3 protein, accumulating in the core of a large number of senile plaques could be partially prevented from release into the CSF. Furthermore, this effect was observed only for patients with Alzheimer disease in the upper tertile of CSF JNK3 levels, which may suggest a threshold effect. Our hypothesis is summarized in the Appendix, Figure S4.
Limitations
Our study has some limitations. The survey was performed on 3 small cohorts of different samples for biochemical, histological and CSF assessments because we could not follow the living patients for a long period of time. Furthermore, CSF assessments were performed in patients with mild Alzheimer disease, whereas immunohistochemistry and immunoblot analyses were performed in deceased patients at the terminal stages of the disease. It will be necessary to perform our CSF assessments in a confirmatory cohort of patients with Alzheimer disease, followed for many years. The CSF analysis using immunoblots is well validated, but it requires a large quantity of CSF samples. The development of an ELISA test specifically for JNK3, which is not currently proposed by biotechnology companies, will help to validate our findings.
Finally, experimental research on JNK3-specific inhibition is becoming a new major goal in the panel of Alzheimer disease pharmacology,44,45 and the CSF assessment of JNK3 and phosphorylated JNK3 could become a useful surrogate marker for future clinical trials using JNK inhibition. In addition, the use of multitarget therapy able to inhibit several stress kinases, including JNK3 and PKR, could be proposed as a disease-modifying approach and as a way to enhance memory, as shown previously in experimental paradigms.46,47
Conclusion
By measuring the JNK3 levels in CSF from patients with Alzheimer disease and controls with various neuropsychiatric diseases, we found a higher level of JNK3 in the Alzheimer disease group, which corresponds with the increase of JNK3 we observed in the Alzheimer disease cortex. This result could be due to the increase of neuronal death during the course of the disease, in the same manner as for τ proteins. Surprisingly, in our exploratory cohort, patients with Alzheimer disease who had the highest levels of CSF JNK3 appeared to experience a less rapid decline than those with lower levels. We advanced the hypothesis that JNK3 could be trapped in senile plaques, which is supported by our JNK3–Aβ colocalization analysis in frontal cortex samples. In the more severe case of Alzheimer disease, patients would present more senile plaques, trapping JNK3 and preventing it from being released into the CSF. If our hypothesis is confirmed, JNK3 could become an additional biomarker for the diagnosis of Alzheimer disease, with a prognostic interest.
Acknowledgements
The authors thank members of the INSERM Units 839 and 942 and members of the Research Memory Center Paris North for their contributions to the study.
Footnotes
Competing interests: C. Paquet declares a grant from Fondation Phillippe Chatrier and has been paid for travel expenses by Novartis. J. Dumurgier declares grants from Institut Servier and Phillips Foundataion and has been paid for travel expenses by Novartis. J. Hugon is a consultant for Xigen, Roche and Sanofi Laboratories. No other competing interests were declared.
Contributors: C. Bouras, F. Gray, J.-L. Laplanche, E. Meurs, F. Mouton-Liger and J. Hugon designed the study. S. Gourmaud, C. Paquet and J. Dumurgier acquired and analyzed the data. C. Pace also acquired the data, and F. Mouton-Liger and J. Hugon also analyzed the data. S. Gourmaud and J. Hugon wrote the article, which all authors reviewed and approved for publication.
References
- 1.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 3.Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet. 2010;19:R12–20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blennow K, Hampel H, Weiner M, et al. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 5.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–9. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 6.Mouton-Liger F, Paquet C, Dumurgier J, et al. Increased cerebrospinal fluid levels of double-stranded RNA-dependant protein kinase in Alzheimer’s disease. Biol Psychiatry. 2012;71:829–35. doi: 10.1016/j.biopsych.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Dumurgier J, Mouton-Liger F, Lapalus P, et al. Cerebrospinal fluid PKR level predicts cognitive decline in Alzheimer’s disease. PLoS ONE. 2013;8:e53587. doi: 10.1371/journal.pone.0053587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 9.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 10.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei W, Norton DD, Wang X, et al. Abeta 17–42 in Alzheimer’s disease activates JNK and caspase-8 leading to neuronal apoptosis. Brain. 2002;125:2036–43. doi: 10.1093/brain/awf205. [DOI] [PubMed] [Google Scholar]
- 12.Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal kinases (JNKs): differences revealed by gene targeting. BioEssays. 2006;28:923–34. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- 13.Colombo A, Bastone A, Ploia C, et al. JNK regulates APP cleavage and degradation in a model of Alzheimer’s disease. Neurobiol Dis. 2009;33:518–25. doi: 10.1016/j.nbd.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Yoon SO, Park DJ, Ryu JC, et al. JNK3 perpetuates metabolic stress induced by Abeta peptides. Neuron. 2012;75:824–37. doi: 10.1016/j.neuron.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamagno E, Guglielmotto M, Aragno M, et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104:683–95. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagalwar S, Berry RW, Binder LI. Relation of hippocampal phospho-SAPK/JNK granules in Alzheimer’s disease and tauopathies to granulovacuolar degeneration bodies. Acta Neuropathol. 2007;113:63–73. doi: 10.1007/s00401-006-0159-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhu X, Raina AK, Rottkamp CA, et al. Activation and redistribution of C-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem. 2001;76:435–41. doi: 10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Ogawa O, Wang Y, et al. JKK1, an upstream activator of JNK/SAPK, is activated in Alzheimer’s disease. J Neurochem. 2003;85:87–93. doi: 10.1046/j.1471-4159.2003.01645.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuan CY, Whitmarsh AJ, Yang DD, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–9. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brecht S, Kirchhof R, Chromik A, et al. Specific pathophysiological functions of JNK isoforms in the brain. Eur J Neurosci. 2005;21:363–77. doi: 10.1111/j.1460-9568.2005.03857.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang DD, Kuan CY, Whitmarsh AJ, et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–70. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 22.Morishima Y, Gotoh Y, Zieg J, et al. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the C-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001;21:7551–60. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunot S, Vila M, Teismann P, et al. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:665–70. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu X, Lee HG, Raina AK, et al. The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals. 2002;11:270–81. doi: 10.1159/000067426. [DOI] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Dumurgier J, Vercruysse O, Paquet C, et al. Intersite variability of CSF Alzheimer’s disease biomarkers in clinical setting. Alzheimers Dement. 2013;9:406–13. doi: 10.1016/j.jalz.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 28.Mouton-Liger F, Paquet C, Dumurgier J, et al. Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2alpha pathway. Biochim Biophys Acta. 2012;1822:885–96. doi: 10.1016/j.bbadis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Bose A, Mouton-Liger F, Paquet C, et al. Modulation of tau phosphorylation by the kinase PKR: implications in Alzheimer’s disease. Brain Pathol. 2011;21:189–200. doi: 10.1111/j.1750-3639.2010.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paquet C, Mouton-Liger F, Meurs EF, et al. The PKR activator PACT is induced by Abeta: involvement in Alzheimer’s disease. Brain Pathol. 2012;22:219–29. doi: 10.1111/j.1750-3639.2011.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–6. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 32.Scheltens P, Barkhof F, Valk J, et al. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease. Evidence for heterogeneity. Brain. 1992;115:735–48. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- 33.Pei JJ, Braak E, Braak H, et al. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer’s disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis. 2001;3:41–8. doi: 10.3233/jad-2001-3107. [DOI] [PubMed] [Google Scholar]
- 34.Hoozemans JJ, van Haastert ES, Nijholt DA, et al. The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am J Pathol. 2009;174:1241–51. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bossers K, Wirz KT, Meerhoff GF, et al. Concerted changes in transcripts in the prefrontal cortex precede neuropathology in Alzheimer’s disease. Brain. 2010;133:3699–723. doi: 10.1093/brain/awq258. [DOI] [PubMed] [Google Scholar]
- 36.Chami L, Buggia-Prevot V, Duplan E, et al. Nuclear factor-kappaB regulates betaAPP and beta- and gamma-secretases differently at physiological and supraphysiological Abeta concentrations. J Biol Chem. 2012;287:24573–84. doi: 10.1074/jbc.M111.333054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–56. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 38.Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16:3–12. doi: 10.1016/j.semcdb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 39.O’Connor T, Sadleir KR, Maus E, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S, Barrett T, Whitmarsh AJ, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–70. [PMC free article] [PubMed] [Google Scholar]
- 41.Kim W, Lee S, Hall GF. Secretion of human tau fragments resembling CSF-tau in Alzheimer’s disease is modulated by the presence of the exon 2 insert. FEBS Lett. 2010;584:3085–8. doi: 10.1016/j.febslet.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 42.Kim W, Lee S, Jung C, et al. Interneuronal transfer of human tau between Lamprey central neurons in situ. J Alzheimers Dis. 2010;19:647–64. doi: 10.3233/JAD-2010-1273. [DOI] [PubMed] [Google Scholar]
- 43.Simon D, Garcia-Garcia E, Royo F, et al. Proteostasis of tau. Tau overexpression results in its secretion via membrane vesicles. FEBS Lett. 2012;586:47–54. doi: 10.1016/j.febslet.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Resnick L, Fennell M. Targeting JNK3 for the treatment of neurodegenerative disorders. Drug Discov Today. 2004;9:932–9. doi: 10.1016/S1359-6446(04)03251-9. [DOI] [PubMed] [Google Scholar]
- 45.Waetzig V, Herdegen T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharmacol Sci. 2005;26:455–61. doi: 10.1016/j.tips.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Sclip A, Antoniou X, Colombo A, et al. C-Jun N-terminal kinase regulates soluble Abeta oligomers and cognitive impairment in AD mouse model. J Biol Chem. 2011;286:43871–80. doi: 10.1074/jbc.M111.297515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu PJ, Huang W, Kalikulov D, et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-gamma-mediated disinhibition. Cell. 2011;147:1384–96. doi: 10.1016/j.cell.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]