Abstract
Background
Vortioxetine was approved by the U.S. Food and Drug Administration (FDA) in September 2013 for treating major depressive disorder (MDD). Thus far, a number of randomized, double-blind, placebo-controlled clinical trials (RCTs) of vortioxetine have been conducted in patients with MDD. We performed a meta-analysis to increase the statistical power of these studies and enhance our current understanding of the role of vortioxetine in the treatment of MDD.
Methods
We performed an extensive search of databases and the clinical trial registry. The mean change in total scores on the 24-item Hamilton Rating Scale for Depression (HAM-D) and the Montgomery–Åsberg Depression Rating Scale (MADRS) from the baseline were the primary outcome measures. The secondary efficacy measures were the response and remission rates, as defined by a 50% or greater reduction in HAM-D/MADRS total scores and as a score of 10 or less in the MADRS and 7 or less in the HAM-D total scores at the end of treatment.
Results
We included 7 published and 5 unpublished short-term (6–12 wk) RCTs in our meta-analysis. Vortioxetine was significantly more effective than placebo, with an effect size (standardized mean difference [SMD]) of −0.217 (95% confidence interval [CI] −0.313 to −0.122) and with odds ratios (ORs) for response and remission of 1.652 (95% CI 1.321 to 2.067) and 1.399 (95% CI 1.104 to 1.773), respectively. Those treated with vortioxetine did not differ significantly from those treated with selective norepinephrine reuptake inhibitors/agomelatine with regard to the SMD of the primary outcome measure (0.081, −0.062 to 0.223) or for response (OR 0.815, 95% CI 0.585 to 1.135) and remission (OR 0.843, 95% CI 0.575 to 1.238) rates. Discontinuation owing to lack of efficacy (OR 0.541, 95% CI 0.308 to 0.950) was significantly less common among those treated with vortioxetine than among those who received placebo, whereas discontinuation owing to adverse events (AEs; OR 1.530, 95% CI 1.144 to 2.047) was significantly more common among those treated with vortioxetine than among those receiving placebo. There was no significant difference in discontinuation rates between vortioxetine and comparators owing to inefficacy (OR 0.983, 95% CI 0.585 to 1.650), whereas discontinuation owing to AEs was significantly less common in the vortioxetine than in the comparator group (OR 0.728, 95% CI 0.554 to 0.957).
Limitations
Studies examining the role of vortioxetine in the treatment of MDD are limited.
Conclusion
Although our results suggest that vortioxetine may be an effective treatment option for MDD, they should be interpreted and translated into clinical practice with caution, as the meta-analysis was based on a limited number of heterogeneous RCTs.
Introduction
Major depressive disorder (MDD), a common debilitating illness, is one of the leading causes of disability and disease worldwide.1 Despite the availability of diverse antidepressants, many patients with depression do not achieve proper treatment outcomes.2,3 Although many drugs relying on mechanisms of action that are not related to monoamine have been tested, the targets of approved antidepressants are still based on the monoamine hypothesis.4 As a result, increasing remission and response rates have been associated with greater reliance on polypharmacy strategies that involve combining antidepressants and augmenting them with other agents.5–9 However, this approach has increased concerns about adverse events (AEs) and health care costs.10 Therefore, new pharmacological agents with novel mechanisms of action are needed for patients who do not respond to conventional antidepressant treatments.6,11–14
The development of vortioxetine, an antidepressant with a novel mechanism of action, which was approved by the U.S. Food and Drug Administration (FDA) in September 2013 for the treatment of MDD, is timely.15 Vortioxetine is a selective serotonin reuptake inhibitor (SSRI) that binds to the presynaptic serotonin reuptake site, increasing the level of serotonin (5-HT) in the neuronal synapse and selectively binding to a variety of other serotonin receptors. It selectively binds to and acts as an antagonist of 5-HT3, 5-HT1D, and 5-HT7 receptors; as a partial agonist to 5-HT1B receptors; and as an agonist of 5-HT1A receptors.16 The efficacy, safety and tolerability of vortioxetine have been investigated in a number of short-term (6–12 wk), randomized, double-blind, placebo-controlled clinical trials (RCTs), including a trial involving elderly patients, and other longer RCTs, including an international relapse-prevention RCT of up to 64 weeks in duration and a 52-week open-label extension study.
Systematic reviews and meta-analyses, especially of newly approved drugs, are important as they can overcome the limitations of small sample sizes, increase the generalizability of results by including many trials conducted in various populations, increase the statistical power for group comparisons, investigate potential publication biases, and quantify and analyze inconsistencies in results across clinical studies.17–19
To synthesize the available trial evidence, we performed a meta-analysis of short-term RCTs of vortioxetine in patients with MDD. We aimed to identify the properties of vortioxetine by assessing its efficacy, discontinuation rate and side effects with respect to the treatment of MDD.
Methods
Sources of data
We repeatedly searched PubMed, Embase, Medline, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science and the Cochrane Central Register of Controlled Trials from January to April 2014 using only 1 key term: “vortioxetine (Lu AA21004).” We also searched ClinicalTrials.gov because it includes the results of both publicly and privately supported clinical studies of human participants conducted worldwide. Reference lists from identified articles and reviews were manually searched to find additional studies. Two of us (S.M.W. and S.J.L.) independently reviewed the abstracts identified from the literature search; potentially eligible papers were then re-evaluated by 2 other authors (C.H. and C.U.P.) to determine whether they clearly met the selection criteria. If a disagreement occurred, the article in question was discussed and a consensus was reached by the second set of review authors.
Inclusion criteria
Clinical trials testing the efficacy of vortioxetine for the short-term treatment (6–12 wk) of MDD were eligible for inclusion. Included studies had to be RCTs comparing vortioxetine with placebo and/or another antidepressant. Patients needed to meet the criteria for MDD used in the individual trials. We considered trials that recruited patients for evaluation of other outcomes if they also met the aforementioned criteria for MDD and included data for outcomes of MDD. Studies were excluded if the main outcome was prevention of relapse or if treatment outcomes based on rating scales of MDD were not available. There were no requirements or restrictions regarding the severity of MDD, sex, age, number of participants, study location or inpatient versus outpatient treatment. No restrictions regarding the pharmaceutical form or dose regimen (fixed v. flexible) were applied.
Data extraction
We collected data on participant characteristics, treatment details, study procedures, efficacy measures, dropout rates and AEs. These data included, for example, age, sex, severity/treatment outcomes of depression (based on primary and secondary end points), type of comparator (active v. placebo), dose, study location (United States, multiple locations, or outside the United States), publication status and outpatient versus inpatient treatment.
Outcome data related to the characteristics of the individual trial and the reported results were extracted for each trial. For instance, the mean changes or reported numbers for the primary and secondary end points were extracted from the individual study when appropriate. In addition, the quality of the RCT was also assessed as recommended by the Cochrane Review. Data extraction was initially performed by C.U.P. and then reassessed independently by C.H.
Efficacy measures
The primary efficacy measures were the mean change from baseline in total scores on the 24-item Hamilton Rating Scale for Depression (HAM-D) and the Montgomery–Åsberg Depression Rating Scale (MADRS), as defined by the individual study. The secondary efficacy measures were the response and remission rates, as defined by a 50% or greater reduction from baseline in HAM-D/MADRS total scores and a score of 10 or less in the MADRS total score or 7 or less in the HAM-D total score at the end of treatment, as indicated by the individual study.20,21
Safety and tolerability measures
Data on the number of dropouts (for any reason), lack of efficacy and incidence of AEs were included in the analysis.
Data synthesis and statistical analysis
In terms of continuous measures, data on the mean change from baseline to end point, the standard deviation (SD) or standard error, and the number of patients were extracted for the primary and secondary efficacy measures. In terms of dichotomous measures, data on the number of patients treated, the number of patients rated as having responded and remitted, and the number of patients leaving the study early were collected for the secondary efficacy measures and safety/tolerability evaluation, respectively.
The effect sizes for continuous data related to the primary and secondary efficacy measures used in each study are presented as the standardized mean difference (SMD) using Hedges g with 95% confidence intervals (CIs), because this statistical tool allowed us to combine the scores from different rating scales. Cohen classification can be used to evaluate the magnitude of the overall effect size, where an SMD of 0.2 is a small effect size, an SMD of 0.5 is a medium effect size, and an SMD greater than 0.8 is a large effect size. The SMD was calculated using the following equation: (end point mean efficacy score − baseline efficacy score) ÷ pooled SD of each treatment group. We used odds ratios (ORs) to assess binary outcomes, such as response and remission rates, including dropout rates.
We performed separate analyses for each comparison of placebo and/or antidepressants with vortioxetine. Studies that evaluated more than 1 dose of vortioxetine were combined to form a composite measure to reduce multiple comparisons with a common placebo control arm, as recommended by Cochrane review.22
Intent-to-treat (ITT) with a last-observation-carried-forward (LOCF) analysis was performed to evaluate efficacy among all randomized patients who received at least 1 study medication and 1 postbaseline assessment. With regard to missing data, we tried to contact the author of each study to acquire additional data. Safety analysis was based on the all-patients-treated set.
Fixed- and random-effects models were applied to the analyses of primary and secondary measures when appropriate. When the I2 index reflected significant heterogeneity between the study results (I2 > 50% and p < 0.05), we used a random-effects model to evaluate the primary and secondary end points. The random-effects model grants more balance than does the fixed-effects model, because it allows for sampling variability with and between studies, and smaller studies are weighted more, whereas larger studies are weighted less. In general, a random-effects model is used to combine subgroups and yield the overall effect. All data extracted from the individual studies included in the present meta-analysis were entered into Comprehensive Meta-analysis version 2.0 software for the final analysis.
Heterogeneity analysis, sensitivity analysis and meta-regression
Heterogeneity among studies was assessed using the I2 statistic. This measure evaluates how much of the variance among studies can be attributed to the actual differences among the studies rather than to chance. A magnitude of considerable heterogeneity is usually I2 = 75%–100%. We considered an I2 value higher than 50% and a p < 0.05 to indicate heterogeneity.
We conducted sensitivity analyses to test the robustness of the impact of a single study on the overall results. If we found statistical heterogeneity, then sensitivity analyses (by eliminating 1 study at a time) were performed to explore the possible reasons for this heterogeneity. These included judgments regarding whether a single study had a significant impact on the overall estimate.
A meta-regression was also performed to assess the influence of the following moderators on the overall estimate: duration of treatment (< 6 wk v. ≥ 6–12 wk), type of treatment (outpatient v. inpatient/unclear), publication status (published v. unpublished), study location (United States only v. outside the United States/mixed location), primary end point (HAM-D v. MADRS), MADRAS cut-off point at baseline (≥ 30 v. 22–26), type of comparator (serotonin–norepinephrine reuptake inhibitors [SNRIs] v. agomelatine), and the doses of vortioxetine under investigation (high doses [e.g., 15 and 20 mg/d] v. other doses); these were included as independent parameters influencing the primary and secondary end points.
Risk of bias
Two authors (C.U.P. and C.H.) independently assessed the risk of bias in individual studies, and any disagreements were resolved by consensus. According to recommendations from the Cochrane Review, the risk of bias associated with sequence generation, allocation concealment, the blinding of participants and investigators, the blinding of outcome assessments, incomplete outcome data, selective outcome reporting and other sources were evaluated using specific and detailed criteria (see the Appendix, Table S1, available at jpn.ca). In addition, we assessed the quality of the RCTs using the Jadad score (Table 1),23 which assesses RCTs based on randomization, blindness and attrition. A score of 3 or higher indicates high quality, whereas a score lower than 3 indicates low quality.
Table 1.
Summary of currently available short-term randomized, double-blind, placebo-controlled clinical trials of vortioxetine for the treatment of patients with major depressive disorder*
| Study; drug, mg/d | Jadad score | Mean age, yr | Sex, % female | Duration, wk | No. treated | Baseline HAM-D/MADRS score | Baseline CGI-S score | Study location | Primary end point | Entry Score† |
|---|---|---|---|---|---|---|---|---|---|---|
| Alvarez et al.24 | 4 | 6 | Europe/Asia | MADRS | ≥30 | |||||
| PBO | 42.0 | 65.7 | 105 | 33.9 ± 2.7 | 5.1 ± 0.7 | |||||
| VTX, 5 | 43.8 | 64.8 | 108 | 34.1 ± 2.6 | 5.2 ± 0.7 | |||||
| VTX, 10 | 42.3 | 66.0 | 100 | 34.0 ± 2.8 | 5.1 ± 0.7 | |||||
| VFX, 225 | 45.0 | 54.9 | 113 | 34.2 ± 3.1 | 5.2 ± 0.7 | |||||
| Henigsberg et al.27 | 4 | 8 | Europe/Asia/Africa | HAM-D | ≥ 26 | |||||
| PBO | 46.4 | 61.4 | 140 | 32.7 ± 4.4 | 4.8 ± 0.8 | |||||
| VTX, 1 | 45.4 | 66.4 | 140 | 32.5 ± 5.1 | 4.8 ± 0.7 | |||||
| VTX, 5 | 47.3 | 62.1 | 140 | 32.1 ± 5.0 | 4.7 ± 0.7 | |||||
| VTX, 10 | 46.4 | 60.7 | 139 | 33.1 ± 4.8 | 4.9 ± 0.8 | |||||
| Baldwin et al.25 | 5 | 8 | Europe/Asia | MADRS | ≥ 26 | |||||
| PBO | 43.4 | 69.6 | 148 | 29.8 ± 5.1 | 4.8 ± 0.7 | |||||
| VTX, 2.5 | 46.0 | 71.0 | 155 | 29.6 ± 5.8 | 4.8 ± 0.7 | |||||
| VTX, 5 | 44.7 | 66.2 | 157 | 31.3 ± 5.8 | 4.8 ± 0.7 | |||||
| VTX, 10 | 45.2 | 68.1 | 151 | 30.4 ± 5.4 | 4.8 ± 0.7 | |||||
| DLX, 60 | 45.3 | 67.7 | 155 | 29.9 ± 5.8 | 4.7 ± 0.7 | |||||
| Katona et al.30‡ | 4 | 8 | USA/Europe/Asia | HAM-D | ≥ 26 | |||||
| PBO | 70.3 | 62.1 | 145 | 29.4 ± 5.1 | 4.7 ± 0.7 | |||||
| VTX, 5 | 70.5 | 68.6 | 156 | 29.2 ± 5.0 | 4.8 ± 0.7 | |||||
| DLX, 60 | 70.9 | 66.2 | 151 | 28.5 ± 4.9 | 4.7 ± 0.8 | |||||
| Mahableshwarkar et al.29 | 5 | 8 | USA | HAM-D§ | ≥ 22 | |||||
| PBO | 42.6 | 60.8 | 153 | 29.5 ± 6.1 | 4.5 ± 0.6 | |||||
| VTX, 2.5 | 42.6 | 64.1 | 153 | 29.8 ± 5.4 | 4.6 ± 0.6 | |||||
| VTX, 5 | 43.1 | 69.3 | 153 | 29.8 ± 4.5 | 4.6 ± 0.7 | |||||
| DLX, 60 | 42.7 | 59.9 | 152 | 29.4 ± 4.4 | 4.5 ± 0.7 | |||||
| Jain et al.28 | 5 | 6 | USA | HAM-D§ | ≥ 30 | |||||
| PBO | 42.4 | 54.7 | 298 | 32.2 ± 5.5 | 4.8 ± 0.7 | |||||
| VTX, 5 | 42.5 | 62.0 | 299 | 32.7 ± 5.4 | 4.8 ± 0.7 | |||||
| Boulenger et al.26 | 4 | 8 | Europe | MADRS | ≥ 26 | |||||
| PBO | 48.1 | 69.6 | 158 | 31.5 ± 3.6 | 4.9 ± 0.7 | |||||
| VTX, 15 | 47.0 | 64.2 | 151 | 31.8 ± 3.4 | 4.9 ± 0.6 | |||||
| VTX, 20 | 46.2 | 60.3 | 151 | 31.2 ± 3.4 | 4.8 ± 0.7 | |||||
| DLX, 60 | 45.6 | 69.4 | 147 | 31.2 ± 3.5 | 4.8 ± 0.7 | |||||
| Mahableshwarkar et al.32 | 4 | 8 | USA | MADRS | ≥ 26 | |||||
| PBO | 42.4 | 72.0 | 161 | 31.6 ± 4.18 | 4.6 ± 0.6 | |||||
| VTX, 15 | 43.1 | 70.7 | 147 | 31.9 ± 4.08 | 4.5 ± 0.6 | |||||
| VTX, 20 | 42.8 | 74.0 | 154 | 32.0 ± 4.36 | 4.5 ± 0.6 | |||||
| DLX, 60 | 43.4 | 78.3 | 152 | 32.9 ± 4.39 | 4.5 ± 0.6 | |||||
| Jacobsen et al.31 | 4 | 8 | USA | MADRS | ≥ 26 | |||||
| PBO | 42.3 | 70.1 | 157 | 32.0 ± 4.0 | 4.5 ± 0.6 | |||||
| VTX10 | 43.1 | 76.1 | 155 | 32.3 ± 4.5 | 4.5 ± 0.6 | |||||
| VTX20 | 43.1 | 71.3 | 150 | 32.4 ± 4.3 | 4.5 ± 0.5 | |||||
| Mahableshwarkar et al.33 | 4 | 8 | USA | MADRS | ≥ 26 | |||||
| PBO | 46.2 | 67.5 | 160 | 33.4 ± 4.5 | 4.7 ± 0.6 | |||||
| VTX10 | 45.2 | 72.0 | 154 | 34.1 ± 4.1 | 4.7 ± 0.6 | |||||
| VTX15 | 43.8 | 71.1 | 151 | 33.7 ± 4.5 | 4.6 ± 0.6 | |||||
| NCT0125578734 | 4 | 8 | Europe/Asia | MADRS | ≥ 26 | |||||
| PBO | 43.6 | 59.9 | 152 | 31.6 ± 3.6 | 4.7 ± 0.7 | |||||
| VTX, 5 | 44.2 | 68.1 | 144 | 31.6 ± 3.7 | 4.7 ± 0.7 | |||||
| VTX, 10 | 45.7 | 62.0 | 148 | 31.8 ± 4.0 | 4.7 ± 0.7 | |||||
| VTX, 20 | 44.0 | 62.0 | 150 | 31.7 ± 3.7 | 4.7 ± 0.7 | |||||
| Dragheim et al.35 | 4 | 12 | Europe | MADRS | ≥ 22 | |||||
| VTX, 10–20 | 47.0 | 77.1 | 253 | 29.1 ± 4.4 | 4.4 ± 0.6 | |||||
| AGO, 25–50 | 45.6 | 72.3 | 242 | 28.7 ± 4.0 | 4.4 ± 0.6 |
AGO = agomelatine; CGI-S = Clinical Global Impression — Severity scale; DLX = duloxetine; HAM-D = Hamilton Rating Scale for Depression; MADRS = Montgomery–Åsberg Depression Rating Scale; PBO = placebo; VTX =vortioxetine.
Based on randomized set or all-patients-treated set.
By MADRS total score.
Elderly population.
Remission criteria by 50% reduction in MADRS total score at the end point.
Publication bias
Visual inspection of funnel plots and the Egger test were used to evaluate publication bias. These methods were adopted because the Egger linear regression method quantifies the bias captured by a funnel plot using the actual values and precision of the effect sizes, whereas the Begg and Mazumdar test uses ranks.
Results
Description of included studies
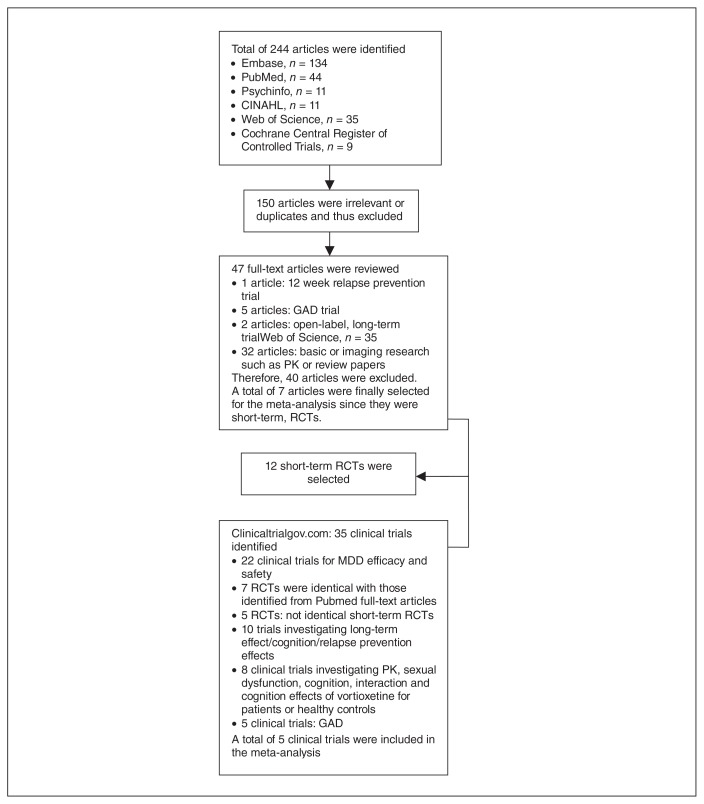
Of the 244 records identified by the search of the databases, 150 were excluded as they were irrelevant to our meta-analysis. The remaining 47 studies were retrieved for more detailed evaluation (Fig. 1).
Fig. 1.
Studies selected for inclusion in the present meta-analysis. CINAHL = Cumulative Index to Nursing and Allied Health Literature; GAD = generalized anxiety disorder; MDD = major depressive disorder; PK = pharmacokinetic; RCT = randomized, double-blind, placebo-controlled clinical trial.
Seven studies,24–29 including 1 RCT with an elderly population,30 met the inclusion criteria. In addition, of the 35 records obtained from ClinicalTrials.gov, 5 short-term RCTs31–35 on MDD were not duplicates of those identified by the aforementioned search and met our inclusion criteria (Fig. 1). Therefore, a total of 12 short-term RCTs were included in the present meta-analysis. Of these, 7 were published,24–30 4 were presented in abstract form at scientific meetings,31–33,35 and 1 was a clinical study report.34
The major characteristics of these 12 studies are presented in Table 1. Studies were multicentred and conducted throughout the world. All study comparisons included 100 or more patients per treatment arm, and the duration of follow-up ranged from 6 to 12 weeks. The diagnosis of primary MDD was made according to DSM-IV-TR criteria. Studies had slightly different inclusion criteria with regard to the severity of MDD based on MADRS total scores (i.e., total scores of 22, 26, and 30 were used; Table 1). Patients with treatment-resistant depression or who had diagnoses of other potentially confounding comorbid psychiatric conditions or clinically important comorbid physical conditions were generally excluded.
Vortioxetine was tested at doses of 1, 2.5, 5, 10, 15 and 20 mg/d, administered once daily. Of these 12 RCTs, 6 included an active control for assay sensitivity as well as a placebo arm, either venlafaxine extended-release 225 mg/d24 or duloxetine 60 mg/d.25,26,29,30,32 Another RCT35 directly compared vortioxetine (10–20 mg/d) with agomelatine (25–50 mg/d), without a placebo arm. Five RCTs compared vortioxetine with a placebo.27,28,31,33,34 Five RCTs included low doses of vortioxetine (1, 2.5 and 5 mg/d) in the treatment arm,25,27–30,34 whereas 6 included higher doses of vortioxetine (15 and 20 mg/d) in the treatment arm.26,31–35
All studies included a preponderance of female participants, with proportions ranging from 54.7% to 77.1%. All participants included in each treatment arm were considered moderately/severely ill at baseline, with mean HAM-D or MADRS total scores ranging from 28.5 to 34.1. All studies were financially supported by the manufacturer.
Eleven pairwise comparisons with a placebo and 7 comparisons with other antidepressants were performed in the 12 RCTs included in our meta-analysis.
Risk of bias
Table 2 compares the risk of bias of individual studies (see the Appendix, Fig. S1, for the overall risk of bias of the included studies). The risk of bias was considered low or unclear in all studies based on evaluations of all domains, and no study presented a high risk of bias in all domains. Overall, all included studies were of good methodological quality.
Table 2.
Risk of bias in individual studies included in the meta-analysis
| Study | Sequence generation | Allocation concealment | Blinding of participants, personnel and outcome assessors | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|---|---|---|---|---|---|
| Alvarez et al.24 | + | + | + | ? | + | + |
| Henigsberg et al.27 | + | + | ? | ? | ? | ? |
| Baldwin et al.25 | + | + | + | + | + | ? |
| Katona et al.30 | + | + | + | ? | ? | ? |
| Mahableshwarkar et al.29 | + | + | + | ? | + | ? |
| Jain et al.28 | + | + | ? | + | + | + |
| Boulenger et al.26 | + | + | + | ? | + | + |
| Mahableshwarkar et al.32 | + | + | + | ? | + | ? |
| Jacobsen et al.31 | + | + | + | ? | + | ? |
| Mahableshwarkar et al.33 | + | + | + | ? | + | ? |
| NCT0125578734 | + | + | + | ? | + | ? |
| Dragheim et al.35 | + | + | + | ? | + | ? |
+ = clear; ? = unclear, based on Cochrane systematic review values.
Efficacy
Vortioxetine versus placebo
Primary end point overall efficacy
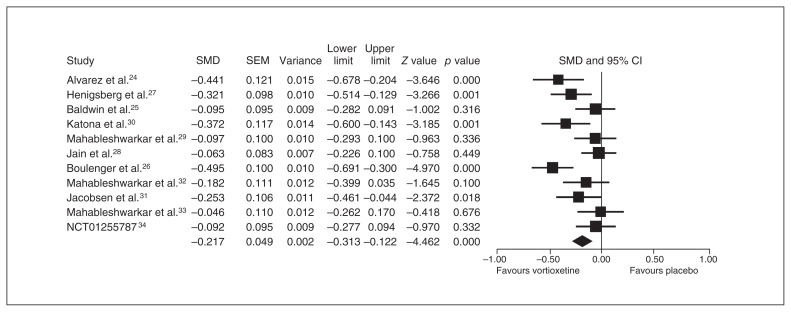
Eleven studies provided an ITT sample of 4947 patients for the primary analysis (vortioxetine, n = 3276 v. placebo, n = 1671). The results of the meta-analysis regarding the primary end point are presented as a forest plot (Fig. 2). Vortioxetine was significantly more effective than placebo, with an SMD of −0.217 (95% CI −0.313 to −0.122).
Fig. 2.
Meta-analysis of the mean changes from baseline in the primary end point between vortioxetine and placebo. CI = confidence interval; SEM = standard error of the mean; SMD = standardized mean difference.
Sensitivity analysis, heterogeneity and publication bias
The heterogeneity among studies was significant according to the SMD (I2 = 59.8%). The pooled SMDs were repeatedly calculated and analyzed with the omission of 1 study at a time to perform a sensitivity analysis; the results of this analysis were consistent, indicating that no single study strongly affected them. The Egger test on the SMDs indicated a statistically marginal difference (p = 0.06).
Meta-regression
We found significant differences among the pooled SMDs according to 1 moderator, as the study location significantly influenced the results (Z = 2.665, p = 0.007) (outside the United States/mixed location > United States only). However, when we performed a subanalysis of the studies conducted in the United States, the SMD between vortioxetine and placebo treatment reflected a significant difference in favour of vortioxetine (−0.120, 95% CI −0.208 to −0.032).
Secondary end point overall efficacy
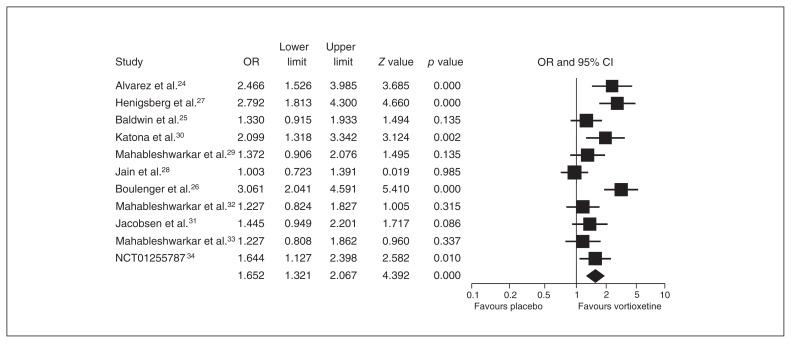
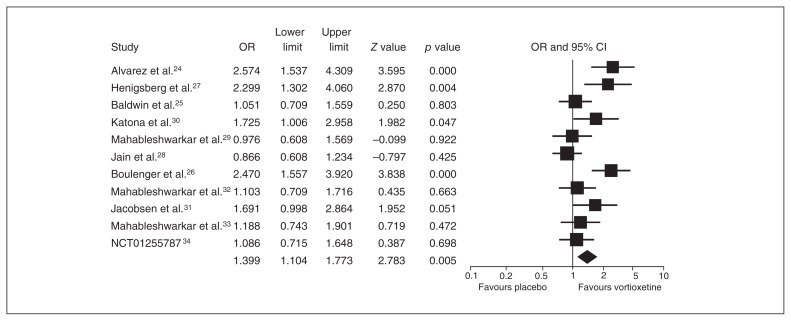
The ORs for votioxetine for response and remission were 1.652 (95% CI 1.321–2.067) and 1.399 (95% CI 1.104–1.773), respectively (Figs. 3 and 4).
Fig. 3.
Meta-analysis of the response rate between vortioxetine and placebo. CI = confidence interval; OR = odds ratio.
Fig. 4.
Meta-analysis of the remission rate between vortioxetine and placebo. CI = confidence interval; OR = odds ratio.
Sensitivity analysis, heterogeneity and publication bias
Heterogeneity was found in the secondary end point analysis for response (I2 = 70.1%) and remission (I2 = 65.4%) rates. The pooled ORs for response and remission rates were repeatedly calculated and analyzed with the omission of 1 study at a time to perform a sensitivity analysis; these results did not change, indicating that no single study strongly impacted them. The Egger test showed significant differences for response and remission rates (p = 0.016 and p = 0.003, respectively).
Meta-regression
In terms of the ORs for the response (Z = −4.291, p < 0.001) and remission (Z = −2.887, p = 0.004) rates, we found a significant effect for study location that favoured studies outside the United States/mixed location over those performed only in the United States. When we performed a subanalysis of the studies conducted only in the United States, the OR for response still favoured vortioxetine over placebo treatment (1.215, 95% CI 1.021–1.447), whereas the ORs for remission with vortioxetine versus placebo no longer differed significantly (1.078, 95% CI 0.885–1.313).
Vortioxetine versus other antidepressants (SNRIs and agomelatine)
Primary end point overall efficacy
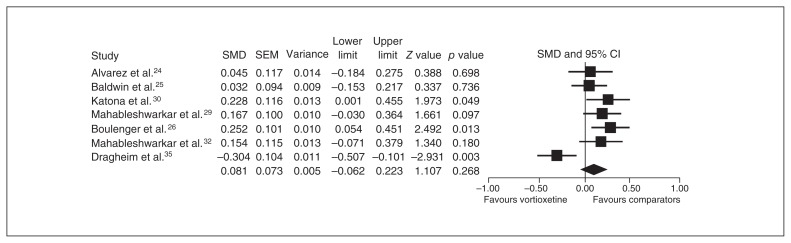
Seven studies provided an ITT sample of 2843 patients for the primary analysis (vortioxetine, n = 1847, v. other antidepressants, n = 996), and the SMD (0.081, 95% CI −0.062 to 0.223) for the comparison indicated no significant difference between the treatment groups (Fig. 5).
Fig. 5.
Meta-analysis of the mean changes from baseline in the primary end point between vortioxetine and comparators. CI = confidence interval; SEM = standard error of the mean; SMD = standardized mean difference.
Sensitivity analysis, heterogeneity and publication bias
In terms of heterogeneity, the SMDs differed significantly among studies (I2 = 69.6%). The pooled SMDs were repeatedly calculated and analyzed with the omission of 1 study at a time to perform a sensitivity analysis, and 1 study (NCT01488071) by Dragheim and Nielsen,35 that compared vortioxetine with agomelatine was found to significantly change the results (0.144, 95% CI 0.059–0.229, p = 0.001). The Egger test showed no significant SMD differences among studies (p = 0.37).
Meta-regression
We found significant differences among the SMDs according to publication status and type of comparators favouring comparators over vortioxetine (Z = −2.673, p = 0.008, and Z = −3.987, p < 0.001, respectively). When we reanalyzed the studies excluding the one by Dragheim and Nielsen (NCT01488071), the moderator effects on the SMD disappeared, and comparators (SNRIs) were superior to vortioxetine (See the Appendix, Fig. S2).
Secondary end point overall efficacy
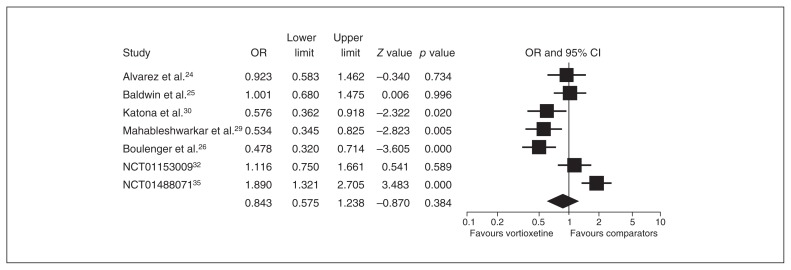
Seven studies provided an ITT sample of 2843 patients for the primary analysis (vortioxetine, n = 1847, v. other antidepressants, n = 996). Compared with SNRIs/agomelatine, the ORs for response (0.815, 95% CI 0.585–1.135) and remission (0.843, 95% CI 0.575–1.238) rates did not differ significantly between the 2 treatment groups (Figs. 6 and 7).
Fig. 6.
Meta-analysis of the response rate in the secondary end point between vortioxetine and comparators. CI = confidence interval; OR = odds ratio.
Fig. 7.
Meta-analysis of the remission rate in the secondary end point between vortioxetine and comparators. CI = confidence interval; OR = odds ratio.
Sensitivity analysis, heterogeneity and publication bias
The heterogeneity among studies was significant in terms of the ORs for response (I2 = 77.8%) and remission (I2 = 83.5%) rates. The ORs for response and remission rates were repeatedly calculated and analyzed with the omission of 1 study at a time to perform a sensitivity analysis, and the study by Dragheim and Nielsen (NCT01488071) was found to significantly change the results (OR 0.706, 95% CI 0.595–0.838, p < 0.001, and OR 0.734, 95% CI 0.543–0.991, p = 0.043, respectively). The Egger test showed no significant differences among studies in the ORs of the response and remission rates (p = 0.19 and p = 0.05, respectively).
Meta-regression
The moderators of study location (Z = −2.22, p = 0.026), publication status (Z = 2.693, p = 0.007) and type of comparator (Z = 4.544, p < 0.001) had a significant impact on the ORs for response rate, whereas publication status (Z = 4.750, p < 0.001) and type of comparator (Z = 4.615, p < 0.001) had a significant effect on the OR for remission rate. When we reanalyzed the studies excluding the one by Dragheim and Nielsen (NCT01488071), the significance of the effects of all moderators on the ORs for response and remission rates disappeared; comparators (SNRIs) were superior to vortioxetine regarding response and remission rates (see the Appendix, Figs. S3 and S4).
When we performed a subanalysis of the studies by publication status, comparators (SNRIs) were superior to vortioxetine with respect to the response (OR 0.719, 95% CI 0.595–0.869) and remission (OR 0.672, 95% CI 0.495–0.912) rates reported by published studies, whereas no significant differences in this regard were found in unpublished studies (SNRI/agomelatine). When we performed a subanalysis based on study location, comparators (SNRIs) were superior to vortioxetine regarding response rates (OR 0.776, 95% CI 0.377–1.599) in studies conducted only in the United States, whereas this difference was not found in studies conducted outside the United States/mixed location. When we performed a subanalysis of the studies by comparators (SNRIs or agomelatine), the comparators (SNRIs) were superior to vortioxetine (see the Appendix, Figs. S3 and S4), whereas vortioxetine was superior to agomelatine with regard to response and remission rates (OR 1.818, 95% CI 1.256–2.633, and OR 1.890, 95% CI 1.321–2.705, respectively).
Safety and tolerability
Data on overall discontinuation were available for 11 comparisons involving placebo (vortioxetine, n = 3519, placebo, n = 1738) and 7 comparisons involving comparators (vortioxetine, n = 1996, comparators, n = 1115).
No significant difference was observed between the vortioxetine and placebo groups regarding the likelihood of discontinuation for any reason (OR 1.057, 95% CI 0.840–1.331), whereas the discontinuation rate due to AEs was significantly higher in the vortioxetine group than in the placebo group (OR 1.530, 95% CI 1.144–2.047; see the Appendix, Fig. S5), and the discontinuation rate owing to lack of efficacy was significantly lower in the vortioxetine group than in the placebo group (OR 0.541, 95% CI 0.308–0.950, see the Appendix, Fig. S6). The main effects of discontinuation owing to AEs derived from 2 studies by Mahableshwarkar and colleagues32 (NCT01153009) and Jacobsen and colleagues31 (NCT01163266); when these studies were excluded, the difference between the vortioxetine and placebo groups disappeared.
We found no significant differences between vortioxetine and comparators in the rates of discontinuation for any reason or for lack of efficacy (OR 0.915, 95% CI 0.753–1.113, and OR 0.983, 95% CI 0.585–1.650, respectively), whereas the discontinuation rate owing to AEs was significantly lower in the vortioxetine group than in the comparator group (OR 0.728, 95% CI 0.554–0.957; see the Appendix, Fig. S7).
Discussion
The present meta-analysis demonstrated the superior efficacy of vortioxetine compared with placebo for the treatment of MDD in terms of mean changes in HAM-D or MADRS total scores compared with baseline (−SMD = 0.217) and in response (OR 1.652) and remission (OR 1.399) rates. Regarding acceptability, discontinuation owing to lack of efficacy was significantly more common in the group receiving placebo than in the group receiving vortioxetine, whereas the rate of discontinuation owing to AEs was significantly higher in the vortioxetine group than in the placebo group. The efficacy of vortioxetine was also similar to that of comparators, such as SNRIs/agomelatine, whereas the acceptability of vortioxetine was superior to that of comparators in terms of rates of discontinuation owing to AEs. In terms of overall efficacy, vortioxetine performed better than placebo, and its performance was equal to that of other antidepressants, with little likelihood of important differences in efficacy measures.
It is questionable whether the overall SMD of −0.22 between the vortioxetine and placebo groups is sufficiently large to translate into clinical significance. These data meet the small-to-medium effect size criteria proposed by Cohen (0.2 = small, 0.5 = medium, 0.8 = large). The SMD of −0.34 between SSRIs and placebo on the HAM-D was considered statistically significant by the National Institute for Health and Clinical Evidence (NICE); however, it is doubtful that this effect size is clinically important.36 Among patients with severe depression, the SMD of 0.61 clearly separates SSRIs from placebo, and this can be considered a clinically important reduction in depressive symptoms, as measured by the HAM-D; however, this result is difficult to achieve with antidepressant treatment for MDD.36,37 According to the previous meta-analyses of agomelatine, which was also recently approved for the treatment of MDD, the SMDs were 0.18–0.2638,39; these results are similar to those of the present meta-analysis.
The SMD of −0.217 achieved in the present meta-analysis corresponds approximately to a −2.0 point mean difference in total MADRS scores between drug and placebo groups. In fact, debate persists about the definition of a minimal clinically important difference (MCID) in drug–placebo comparisons in RCTs evaluating antidepressants for the treatment of MDD. According to Duru and Fantino,40 a drug–placebo MCID between antidepressants may yield an MADRS difference of 2 points or a response-rate difference of 10% (corresponding with a number-needed-to-treat [NNT] of 10). This finding has been supported by previous research.41 Based on this proposal regarding the MCID, the efficacy of vortioxetine may meet the marginal standard criterion for an antidepressant to be considered effective for treating MDD. The ORs for response and remission rates in comparison with placebo were approximately 1.7 and 1.4, respectively, in the present meta-analysis; these results are consistent with those of other RCTs of antidepressants.42 The NNTs for the response and remission rates for vortioxetine versus placebo were 8.8 (95% CI 7.1–11.7) and 18.3 (95% CI 12.6–34.1), respectively. In fact, the drug–placebo differences in the RCTs of antidepressants for the treatment of MDD have been decreasing dramatically for several decades; these results have been accompanied by the selectively increasing clinical improvement associated with placebo treatment.43
With regard to comparative efficacy, vortioxetine was equal to SNRIs/agomelatine in general. The NNTs for response and remission rates for comparators versus vortioxetine were 16 (95% CI 10.3–41.3) and 22 (95% CI 12.2–108.5), respectively. However, when we excluded the comparative study involving agomelatine, vortioxetine was not as efficacious as SNRIs in terms of all efficacy measures, indicating a possible differential efficacy between vortioxetine and SNRIs. However, only 1 study involving venlafaxine and 5 involving duloxetine were compared in the present meta-analysis. In addition, the SMD between vortioxetine and duloxetine was only 0.160 (95% CI 0.068–0.251), which is unlikely to reflect a clinically meaningful difference; similarly, this difference was not significant in comparison with venlafaxine alone. Furthermore, when we excluded the study involving duloxetine by Boulenger and colleagues,26 the SMD decreased further, to 0.135 (95% CI 0.032–0.238). Although this criterion has not yet been firmly established, the MADRS scores for a particular antidepressant would need to be 2 points higher than that of a comparator to be considered superior.44 However, the SMDs of 0.135 and 0.16 reflect a difference in MADRS scores that is considerably lower than 2 points. Interestingly, vortioxetine clearly showed its superiority over agomelatine in all efficacy measures when 6 other RCTs were excluded (SMD −0.304, 95% CI −0.507 to −0.101); vortioxetine was directly compared with agomelatine, whereas SNRIs were not directly compared but served as active controls. One ground-breaking meta-analysis including 117 RCTs of antidepressants (n = 25 928) conducted between 1991 and 2007 by Cipriani and colleagues45 found that potential differences in both efficacy and acceptability may exist between commonly prescribed antidepressants; in particular, these differences favoured escitalopram and sertraline. However, these results have not been replicated consistently. Some meta-analyses have reported the superiority of a particular antidepressant, whereas other studies have reported opposite or different results. For instance, the meta-analysis performed by Kennedy and colleagues46 involving 16 RCTs (n = 4549) found that escitalopram was significantly superior to SSRIs and SNRIs, such as duloxetine and venlafaxine; however, these results have not been supported by other meta-analyses.45,47,48 Likewise, a recent meta-analysis comparing agomelatine with SSRIs49 reported different results than previous meta-analyses.38,39 It is important to remember that the findings of meta-analyses depend on many factors, such as the availability of RCTs of individual antidepressant at the time of the meta-analysis, the criteria for study inclusion and the power of the meta-analyses to detect differences.47 Differences in study inclusion criteria, such as the duration of trials or the noninclusion of trials with treatment-resistant patients, may account for differences in findings. Inclusion of unpublished data ensures a larger, more comprehensive sample, but there is no certain way to ensure that all unpublished data are included,47 and this substantially affects the results of meta-analyses, as clearly seen in studies of agomelatine.11,38,39 Hence, it is premature to conclude that vortioxetine may not be equal to SNRIs but may be more efficacious than agomelatine based on data from currently available studies. A sufficient number of trials that include adequately powered, direct comparisons of vortioxetine with other antidepressants are required to ultimately address its comparative efficacy.
One intriguing finding of the present meta-analysis is that study location significantly influenced treatment effects, favouring studies conducted outside the United States over those conducted in only the United States. Likewise, among 5 RCTs conducted in the United States exclusively, 2 failed to show the superiority of vortioxetine over placebo. According to efficacy data from 81 RCTs (n = 21 611) submitted to the U.S. FDA,50 the American trials had higher success rates than those conducted outside the United States (58% v. 33%), which is the opposite of the results of the present meta-analysis. According to that study, baseline disease severity was a more important contributor to study outcome than study duration, dosing regimen, sample size or time and location of the study. Indeed, there were no substantial differences between studies conducted outside the United States and those conducted in the United States exclusively regarding the baseline parameters. Hence, the reasons for the differences between our findings and those reported by previous studies remain elusive. Possible explanations may involve differences in the location of participants (onsite recruitment v. recruitment via advertisement), disease characteristics (neurotic v. melancholic, more psychological v. more physical symptoms), diagnostic criteria (inflated baseline symptoms v. rigorous entry criteria), rater qualifications (training) or clinical care patterns. Therefore, international multicentre trials should devote more attention to the design and conduct of RCTs, including the patient population, diagnostic criteria, patient assessments and clinical practices used.29
The likelihood of early dropouts owing to AEs was significantly higher in the vortioxetine than in the placebo group, but significantly higher in the comparator than in the vortioxetine group. However, there were no robust differences between each dose of vortioxetine and placebo at the level of individual studies. Nausea, vomiting, diarrhea and dry mouth were the most common AEs reported, with an incidence that was significantly higher in the vortioxetine than in the placebo group. Nausea was the single most common AE reported as a reason for discontinuation of vortioxetine, and its frequency showed a trend toward a dose–response relationship. The majority of such AEs were mild to moderate in intensity and were not dose-dependent. The number needed to harm (NNH) for the rates of discontinuing vortioxetine versus placebo for all causes owing to AEs and to lack of efficacy were 28 056 (95% CI −48 to 46), 47 (95% CI 30.0–113), and −54 (95% CI 33.8–117.3), respectively. The NNHs for discontinuing vortioxetine versus comparators due to all causes, AEs, and lack of efficacy of were 107 (95% CI −53 to 26), −51 (95% CI −2410 to 24), and 749 (95% CI −82 to 91), respectively.
Limitations
Despite the major strength of this analysis, its inclusion of all published and unpublished short-term RCT data regarding the use of vortioxetine to treat MDD available from major contemporary databases, the present study also has numerous limitations, as is the case for many other previous meta-analyses. First, we included only short-term RCTs; the duration of most of the trials was less than 12 weeks, which is an important limitation because patients with MDD typically require long-term pharmacological treatment. According to a relapse-prevention study,51 vortioxetine was effective in preventing the relapse of MDD and was well tolerated; 13% of patients receiving vortioxetine relapsed, whereas 26% of those receiving placebo relapsed. In addition, vortioxetine was also effective and tolerable as maintenance treatment according to 2 long-term studies.52,53 Hence, future meta-analyses should include long-term RCTs when available.
Second, we combined all doses of vortioxetine, which may have resulted in heterogeneity in the evaluation of effect sizes. However, our analysis at the level of individual studies did not yield consistent results showing clear and robust dose–response relationships between outcome and dose, as the 2 doses used were invariably nearly identical, and the data revealed no clear pattern of the superior effectiveness of 1 dose. Nonetheless, it is interesting to note that 2 RCTs31,32 found significant differences in the primary end point with a dose of 20 mg/d but not with 10 mg/d or 15 mg/d of vortioxetine compared with placebo. When we excluded all failed RCTs25,28,29,33 and all studies24,30 that failed to show greater symptom reduction according to dose, the SMD changed from −0.217 to −0.322 (95% CI −0.423 to −0.220), increasing by 48.4%, indicating a potential dose–response relationship in vortioxetine treatment.26,27,31,32 Hence, conclusions about the dose-related efficacy of vortioxetine must wait until more definite and consistent results are available.
Third, a relatively high level of heterogeneity was found. There was considerable difference among the individual studies with regard to the observed SMDs in the mean change in the total scores on the primary end points, indicating that hidden clinical heterogeneity may have existed among the studies owing to unidentified variations in study and population characteristics. Finally, although the use of effect sizes to compare treatments is generally considered to be superior to the use of qualitative comparisons among studies, this method also has inherent pitfalls.
Finally, vortioxetine was found to be more effective than placebo for reducing depressive symptoms, although the magnitude of this difference was relatively small. This weak anti-depressant effect has been consistently reported in the relevant research.13 Despite the existence of a number of possible reasons for a weak antidepressant effect, such as a high placebo response rate, we should also consider the potential effect of clinical and biological heterogeneity of MDD, which was observed in the present meta-analysis. Currently available evidence suggests that MDD involves abnormalities in different neurotransmitter systems, such as serotonin, norepinephrine, dopamine, γ-aminobutyric acid (GABA), and glutamate, as well as in neurotrophic factors (e.g., brain-derived neurotrophic factor [BDNF]).13,54 In addition, clinical heterogeneity is probably linked to different biological aberrations, such as the stress response systems modulated by the hypothalamic–pituitary–adrenal (HPA) axis (e.g., hyperactivation and hypoactivation of the HPA axis in melancholic and atypical depression, respectively)55 and altered immune system functioning (e.g., proinflammatory cytokines).56,57 Furthermore, clinical heterogeneity has also been reflected in a corresponding variety in the relative resting regional brain activity in MDD.
Conclusion
We found that vortioxetine may be another treatment option for MDD. However, our results should be interpreted and translated into clinical practice with caution owing to the limited number, small effect sizes of and substantial heterogeneity of the clinical trials included in present the meta-analysis. Adequately powered, well-designed, direct-comparison clinical trials should also more clearly address the comparative efficacy of vortioxetine and different antidepressants.
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C0003).
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study. C. Pae, S.-M. Wang, C. Han and S.-J. Lee acquired and analyzed the data, which P. Masand and A. Serretti also analyzed. C. Pae, S.-M. Wang, C. Han, S.-J. Lee and A. Patkar wrote the article, which all authors reviewed and approved for publication.
References
- 1.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papakostas GI. The efficacy, tolerability, and safety of contemporary antidepressants. J Clin Psychiatry. 2010;71(Suppl E1):e03. doi: 10.4088/JCP.9058se1c.03gry. [DOI] [PubMed] [Google Scholar]
- 3.Warden D, Rush AJ, Trivedi MH, et al. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449–59. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- 4.Massart R, Mongeau R, Lanfumey L. Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philos Trans R Soc Lond B Biol Sci. 2012;367:2485–94. doi: 10.1098/rstb.2012.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhi GS, Hitching R, Berk M, et al. Pharmacological management of unipolar depression. Acta Psychiatr Scand Suppl. 2013;(443):6–23. doi: 10.1111/acps.12122. [DOI] [PubMed] [Google Scholar]
- 6.Wang SM, Han C, Lee SJ, et al. A review of current evidence for acetyl-l-carnitine in the treatment of depression. J Psychiatr Res. 2014;53:30–7. doi: 10.1016/j.jpsychires.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Han C, Wang SM, Seo HJ, et al. Aripiprazole augmentation, antidepressant combination or switching therapy in patients with major depressive disorder who are partial- or non-responsive to current antidepressants: a multi-center, naturalistic study. J Psychiatr Res. 2014;49:75–82. doi: 10.1016/j.jpsychires.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Han C, Wang SM, Kato M, et al. Second-generation antipsychotics in the treatment of major depressive disorder: current evidence. Expert Rev Neurother. 2013;13:851–70. doi: 10.1586/14737175.2013.811901. [DOI] [PubMed] [Google Scholar]
- 9.Pae CU, Patkar AA, Jun TY, et al. Aripiprazole augmentation for treatment of patients with inadequate antidepressants response. Depress Anxiety. 2007;24:522–6. doi: 10.1002/da.20244. [DOI] [PubMed] [Google Scholar]
- 10.Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–88. doi: 10.2147/PPA.S29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pae CU. Agomelatine: A new option for treatment of depression? Expert Opin Pharmacother. 2014;15:443–7. doi: 10.1517/14656566.2014.877889. [DOI] [PubMed] [Google Scholar]
- 12.Marks DM, JT, Pae CU. Innovations in clinical research design and conduct in psychiatry: shifting to pragmatic approaches. Psychiatry Investig. 2009;6:1–6. doi: 10.4306/pi.2009.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks DM, Pae CU, Patkar AA. Triple reuptake inhibitors: a premise and promise. Psychiatry Investig. 2008;5:142–7. doi: 10.4306/pi.2008.5.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pae CU, Marks DM, Han C, et al. Does minocycline have antidepressant effect? Biomed Pharmacother. 2008;62:308–11. doi: 10.1016/j.biopha.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Gibb A, Deeks ED. Vortioxetine: first global approval. Drugs. 2014;74:135–45. doi: 10.1007/s40265-013-0161-9. [DOI] [PubMed] [Google Scholar]
- 16.Dubovsky SL. Pharmacokinetic evaluation of vortioxetine for the treatment of major depressive disorder. Expert Opin Drug Metab Toxicol. 2014;10:759–66. doi: 10.1517/17425255.2014.904286. [DOI] [PubMed] [Google Scholar]
- 17.Cohn LD, Becker BJ. How meta-analysis increases statistical power. Psychol Methods. 2003;8:243–53. doi: 10.1037/1082-989X.8.3.243. [DOI] [PubMed] [Google Scholar]
- 18.Finckh A, Tramer MR. Primer: strengths and weaknesses of meta-analysis. Nat Clin Pract Rheumatol. 2008;4:146–52. doi: 10.1038/ncprheum0732. [DOI] [PubMed] [Google Scholar]
- 19.Pae CU, Lim HK, Peindl K, et al. The atypical antipsychotics olanzapine and risperidone in the treatment of posttraumatic stress disorder: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Int Clin Psychopharmacol. 2008;23:1–8. doi: 10.1097/YIC.0b013e32825ea324. [DOI] [PubMed] [Google Scholar]
- 20.Keller MB. Remission versus response: the new gold standard of antidepressant care. J Clin Psychiatry. 2004;65(Suppl 4):53–9. [PubMed] [Google Scholar]
- 21.Riedel M, Moller HJ, Obermeier M, et al. Response and remission criteria in major depression–a validation of current practice. J Psychiatr Res. 2010;44:1063–8. doi: 10.1016/j.jpsychires.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. Hoboken (NJ): Wiley-Blackwell; 2009. Multiple comparisons within a study. [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez E, Perez V, Dragheim M, et al. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15:589–600. doi: 10.1017/S1461145711001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldwin DS, Loft H, Dragheim M. A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD) Eur Neuropsychopharmacol. 2012;22:482–91. doi: 10.1016/j.euroneuro.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29:138–49. doi: 10.1097/YIC.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henigsberg N, Mahableshwarkar AR, Jacobsen P, et al. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. 2012;73:953–9. doi: 10.4088/JCP.11m07470. [DOI] [PubMed] [Google Scholar]
- 28.Jain R, Mahableshwarkar AR, Jacobsen PL, et al. A randomized, double-blind, placebo-controlled 6-wk trial of the efficacy and tolerability of 5 mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol. 2013;16:313–21. doi: 10.1017/S1461145712000727. [DOI] [PubMed] [Google Scholar]
- 29.Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin. 2013;29:217–26. doi: 10.1185/03007995.2012.761600. [DOI] [PubMed] [Google Scholar]
- 30.Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27:215–23. doi: 10.1097/YIC.0b013e3283542457. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen PL, Mahableshwarkar AR, Serenko M, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of vortioxetine 10 mg and 20 mg in adults with major depressive disorder ( NCT01163266) [poster]. American Psychiatric Association 166th Annual Meeting; 2013 May 18–22; San Francisco. [Google Scholar]
- 32.Mahableshwarkar AR, Jacobsen PL, Serenko M, et al. A duloxetine-referenced, fixed-dose study comparing efficacy and safety of 2 vortioxetine doses in the acute treatment of adult MDD patients ( NCT01153009) [poster]. American Psychiatric Association 166th Annual Meeting; 2013 May 18–22; San Francisco. [Google Scholar]
- 33.Mahableshwarkar AR, Jacobsen PL, Serenko M, et al. A randomized, double-blind, parallel-group, placebo-controlled, fixed-dose study comparing the efficacy and safety of 2 doses of vortioxetine (Lu AA21004) in acute treatment of adults with major depressive disorder ( NCT01179516) [poster]. American Psychiatric Association 166th Annual Meeting; 2013 May 18–22; San Francisco. [Google Scholar]
- 34.Efficacy and safety study of vortioxetine (Lu AA21004) for treatment of major depressive disorder. NCT01255787. [accessed 2014 Apr. 1]. Available: http://clinicaltrials.gov/show/NCT01255787.
- 35.Dragheim M, Nielsen RZ. A randomized, double-blind, study of vortioxetine versus agomelatine in adults with major depressive disorder (MDD) switched after inadequate response to SSRI or SNRI treatment ( NCT01488071) [poster]. NCDEU 53rd Annual Meeting; 2013 May 28–31; Hollywood, FL. [Google Scholar]
- 36.National Institute for Health and Care Excellence. Depression: the treatment and management of depression in adults. London (UK): NICE; 2009. [accessed 2014 Feb. 8]. Available: www.nice.org.uk/CG90fullguideline.pdf. [Google Scholar]
- 37.Thase ME, Larsen KG, Kennedy SH. Assessing the ‘true’ effect of active antidepressant therapy v. placebo in major depressive disorder: use of a mixture model. Br J Psychiatry. 2011;199:501–7. doi: 10.1192/bjp.bp.111.093336. [DOI] [PubMed] [Google Scholar]
- 38.Taylor D, Sparshatt A, Varma S, et al. Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ. 2014;348:g1888. doi: 10.1136/bmj.g1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15:417–28. doi: 10.1017/S1461145711001301. [DOI] [PubMed] [Google Scholar]
- 40.Duru G, Fantino B. The clinical relevance of changes in the Montgomery-Asberg Depression Rating Scale using the minimum clinically important difference approach. Curr Med Res Opin. 2008;24:1329–35. doi: 10.1185/030079908x291958. [DOI] [PubMed] [Google Scholar]
- 41.Ali MK, Lam RW. Comparative efficacy of escitalopram in the treatment of major depressive disorder. Neuropsychiatr Dis Treat. 2011;7:39–49. doi: 10.2147/NDT.S12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thase ME, Pritchett YL, Ossanna MJ, et al. Efficacy of duloxetine and selective serotonin reuptake inhibitors: comparisons as assessed by remission rates in patients with major depressive disorder. J Clin Psychopharmacol. 2007;27:672–6. doi: 10.1097/jcp.0b013e31815a4412. [DOI] [PubMed] [Google Scholar]
- 43.Schalkwijk S, Undurraga J, Tondo L, et al. Declining efficacy in controlled trials of antidepressants: effects of placebo dropout. Int J Neuropsychopharmacol. 2014;17:1343–52. doi: 10.1017/S1461145714000224. [DOI] [PubMed] [Google Scholar]
- 44.Montgomery SA, Moller HJ. Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol. 2009;24:111–8. doi: 10.1097/YIC.0b013e32832a8eb2. [DOI] [PubMed] [Google Scholar]
- 45.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–58. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy SH, Andersen HF, Thase ME. Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin. 2009;25:161–75. doi: 10.1185/03007990802622726. [DOI] [PubMed] [Google Scholar]
- 47.de Silva VA, Hanwella R. Efficacy and tolerability of venlafaxine versus specific serotonin reuptake inhibitors in treatment of major depressive disorder: a meta-analysis of published studies. Int Clin Psychopharmacol. 2012;27:8–16. doi: 10.1097/YIC.0b013e32834ce13f. [DOI] [PubMed] [Google Scholar]
- 48.Gartlehner G, Thaler K, Hansen RA, et al. The general and comparative efficacy and safety of duloxetine in major depressive disorder: a systematic review and meta-analysis. Drug Saf. 2009;32:1159–73. doi: 10.2165/11318930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Huang KL, Lu WC, Wang YY, et al. Comparison of agomelatine and selective serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors in major depressive disorder: a meta-analysis of head-to-head randomized clinical trials. Aust N Z J Psychiatry. 2014;48:663–71. doi: 10.1177/0004867414525837. [DOI] [PubMed] [Google Scholar]
- 50.Khin NA, Chen YF, Yang Y, et al. Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applications. J Clin Psychiatry. 2011;72:464–72. doi: 10.4088/JCP.10m06191. [DOI] [PubMed] [Google Scholar]
- 51.Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26:1408–16. doi: 10.1177/0269881112441866. [DOI] [PubMed] [Google Scholar]
- 52.Baldwin DS, Hansen T, Florea I. Vortioxetine (Lu AA21004) in the long-term open-label treatment of major depressive disorder. Curr Med Res Opin. 2012;28:1717–24. doi: 10.1185/03007995.2012.725035. [DOI] [PubMed] [Google Scholar]
- 53.Alam MY, Jacobsen PL, Chen Y, et al. Safety, tolerability, and efficacy of vortioxetine (Lu AA21004) in major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2014;29:36–44. doi: 10.1097/YIC.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann JJ. Clinical pleomorphism of major depression as a challenge to the study of its pathophysiology. World Psychiatry. 2010;9:167–8. doi: 10.1002/j.2051-5545.2010.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pae CU, Tharwani H, Marks DM, et al. Atypical depression: a comprehensive review. CNS Drugs. 2009;23:1023–37. doi: 10.2165/11310990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 56.Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664–75. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 57.Lee SY, Lee SJ, Han C, et al. Oxidative/nitrosative stress and anti-depressants: targets for novel antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:224–35. doi: 10.1016/j.pnpbp.2012.09.008. [DOI] [PubMed] [Google Scholar]