Abstract
Background
Schizophrenia is highly comorbid with cannabis use disorders (CUDs), and this comorbidity is associated with an unfavourable course. Early onset or frequent cannabis use may influence brain structure. A key question is whether comorbid CUDs modulate brain morphology alterations associated with schizophrenia.
Methods
We used surface-based analysis to measure the brain volume, cortical thickness and cortical surface area of a priori–defined brain regions (hippocampus, amygdala, thalamus, caudate, putamen, orbitofrontal cortex, anterior cingulate cortex, insula, parahippocampus and fusiform gyrus) in male patients with schizophrenia or related disorders with and without comorbid CUDs and matched healthy controls. Associations between age at onset and frequency of cannabis use with regional grey matter volume were explored.
Results
We included 113 patients with (CUD, n = 80) and without (NCUD, n = 33) CUDs and 84 controls in our study. As expected, patients with schizophrenia (with or without a CUD) had smaller volumes of most brain regions (amygdala, putamen, insula, parahippocampus and fusiform gyrus) than healthy controls, and differences in cortical volume were mainly driven by cortical thinning. Compared with the NCUD group, the CUD group had a larger volume of the putamen, possibly driven by polysubstance use. No associations between age at onset and frequency of use with regional grey matter volumes were found.
Limitations
We were unable to correct for possible confounding effects of smoking or antipsychotic medication.
Conclusion
Patients with psychotic disorders and comorbid CUDs have larger putamen volumes than those without CUDs. Future studies should elaborate whether a large putamen represents a risk factor for the development of CUDs or whether (poly)substance use causes changes in putamen volume.
Introduction
In patients with recent onset schizophrenia, cannabis use disorders (CUDs) are highly comorbid and associated with an unfavourable outcome.1–3 In several studies, patients who used cannabis were found to have an earlier age at onset of their first psychotic episode,4–7 suggesting that cannabis may trigger the onset of schizophrenia.
Patients with schizophrenia have morphological differences compared with healthy individuals, including several smaller brain regions8 and decreased cerebral lateralization.9 However, an important question is whether these volume reductions are modulated by a comorbid CUD. Two systematic reviews10,11 evaluated studies on differences in brain morphology between patients with and without CUDs. However, no definite conclusions could be drawn owing to differences between studies in duration of cannabis use, presence of other comorbid (substance use) disorders, medication use, sex and differences in disease characteristics, which have all been found to be related to grey matter volume decreases in schizophrenia.10 The use of a more homogeneous sample (i.e., including only patients with recent onset schizophrenia) would diminish these confounding effects, but so far few studies in patients with recent onset schizophrenia have been published. Some studies show smaller volumes in the CUD group,12–15 whereas others found no volumetric differences between patients with and without (NCUD) CUDs16–20 or an increase in grey matter density.21 Importantly, all studies included in these reviews had small samples (6–30) of patients with CUDs.
Because an earlier age at onset of cannabis use and a higher frequency of cannabis use may increase the risk for schizophrenia, 22–25 it is possible that these factors influence brain morphology. In nonschizophrenic participants with a CUD, grey matter volume was positively correlated with age at onset but not with duration of cannabis use, 26 suggesting that the association between cannabis use and brain volume resulted from abnormal brain development rather than neurodegeneration caused by cannabis. However, Cousijn and colleagues27 found that volume reductions between cannabis users and controls did not differ significantly, but still correlated with the amount of cannabis used and the severity of cannabis dependence. This finding was corroborated by Battistella and colleagues,28 who reported an effect of dosage as well, since regular cannabis users had decreased grey matter volumes in the temporal pole and parahippocampal gyrus compared with occasional users. Lorenzetti and colleagues29,30 described in their reviews a trend for decreases in brain volumes in samples with high- but not low-frequency cannabis use, all of which suggests an adverse effect of cannabis.
Most studies on brain abnormalities in (recent onset) schizophrenia used volumetric techniques to assess grey matter differences between patients and controls. However, these techniques cannot differentiate between cortical thickness and cortical surface area. As cortical volume is a product of both measures, cortical volume changes can result from changes in cortical thickness, surface area or both. This distinction is important because changes in cortical thickness and cortical surface area occur at different stages of development.31–33 While cortical surface area seems to develop according to a fixed maturation course,34 cortical thickness is also influenced by external factors, such as substance use.32,35 In addition, there is a reduced cortical cerebral asymmetry in patients with schizophrenia,9 which can also be influenced by substance use, as there is decreased cortical thickness and altered thickness asymmetry in individuals with cocaine dependence.35
The aim of the present study was 2-fold. First, we sought to identify differences in brain volume, cortical thickness, cortical surface area and cortical symmetry in a large and relatively homogeneous group of male patients with recent onset schizophrenia with and without comorbid CUDs and a group of matched healthy controls. Second, we investigated the association between brain volume and age of onset and frequency of cannabis use in patients with schizophrenia. We hypothesized that both patient groups would show decreased regional brain volumes compared with healthy controls in regions previously found to be related to schizophrenia.8,36 Also, we hypothesized that patients in the CUD group would show decreased brain volumes compared with those in the NCUD group in regions previously found to be related to cannabis use and CUDs.10,12,19,37 Regions of interest (ROIs) were the hippocampus, amygdala, anterior cingulate gyrus, orbitofrontal gyrus, fusiform gyrus, insula, parahippocampus, thalamus, caudate and putamen. Furthermore, we hypothesized that these differences in regional brain volumes would be driven by differences in cortical thinning rather than by differences in cortical surface area38 and that patients would show decreased cerebral lateralization, reflected by decreased cortical symmetry, compared with controls and that this lateralization would be even more pronounced in patients with a CUD.9 Finally, we hypothesized that an earlier age of onset and a higher frequency of regular cannabis use would both be related to larger decreases in these ROIs.
Methods
Participants and clinical assessments
Data were extracted from the charts of patients admitted to the Early Psychosis Department of the Academic Medical Center, Amsterdam, the Netherlands, between June 2004 and December 2011. The ethics committee of the Academic Medical Center gave permission to use the fully anonymized, routinely collected patient data. Before MRI assessment, we obtained verbal informed consent from patients; whenever participants with psychosis were deemed not capable of giving informed consent, we obtained consent from their caretakers. From the patient charts, we obtained information from diagnostic interviews based on the Comprehensive Assessment of Symptoms and History39 conducted by experienced clinicians. The DSM-IV diagnoses of schizophrenia, schizophreniform, schizoaffective and other psychotic disorders, which we hereafter refer to as schizophrenia for reasons of brevity) were based on this interview and on additional interviews with parents and available patient history. In addition, we collected information on cannabis use and other substance use disorders. Nicotine use was dichotomously assessed as current use or no current use. Because our sample included only 2 female patients with CUDs, we decided to include only male patients. This diminished heterogeneity, as sex has been found to influence brain morphology in patients with schizophrenia40 and in healthy cannabis users.41 Patients were included in the CUD group if they had a DSM-IV diagnosis of cannabis abuse or dependence; they were included in the NCUD group if they had a maximum lifetime use of cannabis of 5 times.
We selected age-matched healthy controls from a series of different studies performed at the Academic Medical Center between June 2004 and December 2011 for which the ethics committee provided approval. Written informed consent was obtained from all controls. Controls were excluded if they met criteria for any lifetime DSM-IV Axis-1 disorder, including CUDs, or if they used any psychotropic drug at the time of scanning.
MRI acquisition and processing
All structural MRI scans were acquired on the same 3 T MRI scanner (Intera, Philips Healthcare) with a phased array SENSE 6/8-channel receiver head coil. For each participant, a T1-weighted structural MRI image was acquired. We performed cortical reconstruction and volumetric segmentation using the FreeSurfer image analysis suite version 5.0.0 (http://surfer.nmr.mgh.harvard.edu/) on the e-Bioinfra Gateway,42 a Web application that provides facilitated access to the Dutch Grid infrastructure to analyze large data sets. In some scans, manual editing of pial surfaces was necessary; this was carefully conducted according to established guidelines.
Statistical analysis
Demographic characteristics were compared between patient groups and healthy controls using the χ2 or Mann–Whitney U tests for discrete variables and 2-sample t tests for normally distributed continuous variables. The FreeSurfer processing steps generate different output variables for each participant, which we extracted from the FreeSurfer output files using Matlab version 7.8.0.347 (R2009a) and transferred into SPSS version 18 (SPSS Inc.). We selected the ROIs before analysis according to the literature.10,19,37 We calculated the mean volume and surface measures as well as the mean thickness measures (weighted for cortical surface area) of the left and right hemispheres.
For each brain region, the effect of group (CUD, NCUD, control) was assessed using a linear regression model with brain volume as the dependent variable and group, age, slice thickness and appropriate global measure as independent variables. The global measure included in the model for volume was intracranial volume, for surface area it was the sum of the total left and right surface area, and for cortical thickness it was the mean cortical thickness, weighted by surface area. Slice thickness and pixel spacing both differed between groups; however, they were strongly correlated, so only slice thickness was included as an additional predictor in the model. All possible interaction terms were first added to the model and subsequently removed in a backward elimination procedure when they were not significant. We performed similar statistical analyses to assess differences in cortical thickness and cortical surface area for each region. To control for the number of statistical tests we used a Bonferroni correction based on the number of ROIs, adjusted for the correlation between the ROIs.43,44 For the volumes (11 ROIs with an average r of 0.359 between the regions) this resulted in a critical α of 0.01. For the cortical thickness (5 ROIs with an average r of 0.45) and surface area (5 ROIs with an average r of 0.57) calculations the critical α were 0.02 and 0.03, respectively.
We report the overall effect of group status on regional brain volume and, if significant, the separate estimate and standard error values per pairwise comparison. To compare the magnitudes of the significant effects, we calculated Cohen d scores. These analyses were repeated after exclusion of patients who used illicit substances other than cannabis. In addition, we repeated the analyses with nicotine use (yes v. no) as an additional covariate. Owing to missing data, these analyses were performed in a smaller sample (n = 76 in the CUD group, 5% missing values; n = 33 in the NCUD group; no missing values; n = 49 in the control group, 42% missing values).
Within the CUD group, a similar statistical procedure was used to assess the association between grey matter volumes and the frequency and age of onset of cannabis use. For frequency of use, patients were divided in 2 groups: high frequency (daily) and low frequency (weekly or less than weekly use of cannabis).
To assess cortical symmetry, we separated the raw cortical thickness data of the left and right hemispheres from the total cortical thickness and 5 cortical ROIs (orbitofrontal cortex [OFC], anterior cingulate cortex (ACC), insula, parahippocampus and fusiform gyrus). We performed a paired t test for all of these regions for each group (CUD, NCUD, control) separately.45
Results
Demographic and clinical variables
We included 113 patients with schizophrenia (80 in the CUD group and 33 in the NCUD group) and 84 healthy controls in our study. There were no significant differences in age among the groups (CUD: 22.18 ± 2.74 yr; NCUD: 22.15 ± 3.04 yr; control: 23.19 ± 3.48 yr, χ22 = 3.96, p = 0.14). Also, no differences were found between the CUD and NCUD groups for any demographic or clinical characteristics (Table 1). Remarkably, the age at onset of first psychosis was very similar in the CUD and NCUD groups. With regard to nicotine use, patients in the CUD group were more likely to smoke cigarettes (71 smokers v. 5 nonsmokers) than those in the NCUD group (7 smokers v. 26 nonsmokers) and healthy controls (10 smokers v. 39 nonsmokers, χ22 = 84.46, p < 0.001).
Table 1.
Demographic and clinical characteristics in patients with schizophrenia with and without comorbid cannabis use disorders
| Group, no.(%)* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | CUD, n = 80 | NCUD, n = 33 | Statistic | p value |
| Age, mean ± SD, yr | 22.18 ± 2.7 | 22.15 ± 3.0 | t111 = 0.040 | 0.97 |
| Age onset first psychosis, mean ± SD, yr | 20.40 ± 3.3 | 20.24 ± 3.4 | t111 = 0.228 | 0.82 |
| Duration of positive symptoms, median, mo | 11 | 14 | U = 1156 | 0.30 |
| Duration of psychotic disorder, median, yr | 1 | 1 | U = 1258 | 0.69 |
| GAF score, mean ± SD | 34.29 ± 10.6 | 34.55 ± 11.0 | t111 = −0.117 | 0.91 |
| Nicotine use | 71 (89.0) | 7 (21.2) | χ21 = 58.95 | < 0.001 |
| Education | χ24 = 2.207 | 0.82 | ||
| Primary education | 1 (1.3) | — | ||
| Lower secondary professional education | 17 (21.3) | 5 (15.2) | ||
| Higher secondary education | 42 (52.5) | 15 (45.5) | ||
| Higher professional education | 11 (13.8) | 7 (21.2) | ||
| University | 9 (11.3) | 5 (15.2) | ||
| Missing | — | 1 (3.0) | ||
| Specific psychotic disorders | χ24 = 2.411 | 0.66 | ||
| Schizophrenia | 57 (71.3) | 24 (72.7) | ||
| Schizophreniform disorder | 7 (8.8) | 5 (15.2) | ||
| Schizoaffective disorder | 13 (16.3) | 4 (12.1) | ||
| Substance induced psychotic disorder | 2 (2.5) | — | ||
| Psychotic disorder NOS | 1 (1.3) | — | ||
| Number of psychotic episodes | χ22 = 1.08 | 0.58 | ||
| 1 | 67 (83.8) | 29 (87.9) | ||
| 2 | 12 (15.0) | 3 (9.1) | ||
| 3 | 1 (1.3) | 1 (3.0) | ||
| Medication use | χ22 = 0.247 | 0.88 | ||
| Atypical antipsychotics | 65 (81.3) | 28 (84.9) | ||
| Typical antipsychotics | 7 (8.8) | 3 (9.1) | ||
| None | 7 (8.8) | 2 (6.1) | ||
| Duration of medication use, median, mo | 4 | 5 | U = 903 | 0.47 |
| Comorbid DSM-IV diagnoses | χ26 = 5.026 | 0.54 | ||
| Affective disorder | 2 (2.5) | 1 (3.0) | ||
| Anxiety disorder | — | 1 (3.0) | ||
| Obsessive–compulsive disorder | 1 (1.3) | 2 (6.1) | ||
| Posttraumatic stress disorder | 1 (1.3) | 1 (3.0) | ||
| Pervasive developmental disorder | 1 (1.3) | 1 (3.0) | ||
| ADHD | 3 (3.8) | — | ||
| Other | 3 (3.8) | 1 (3.0) | ||
| Comorbid DSM-IV drug diagnoses | χ25 = 5.577 | 0.35 | ||
| Alcohol abuse | 10 (12.5) | 4 (12.1) | ||
| Alcohol dependency | 5 (6.3) | — | ||
| Cocain abuse | 2 (2.5) | — | ||
| Cocain dependency | 1 (1.3) | — | ||
| Amphetamine abuse | — | — | ||
| Amphetamine dependency | 2 (2.5) | — | ||
| Multiple | 7 (8.8) | — | ||
ADHD = attention-deficit/hyperactivity disorder; CUD = cannabis use disorder; GAF = Global Assessment of Functioning; NCUD = no cannabis use disorder; NOS = not otherwise specified; SD = standard deviation.
Unless otherwise indicated.
Regarding the frequency of cannabis use, 57 patients in the CUD group were daily users (71.3%), 17 (21.3%) were weekly users and 5 (6.3%) used less than weekly. The mean age at onset of regular cannabis use was 15.81 ± 3.10 years.
MRI data
Results of the scanning secquences are summarized in Table 2. We found no significant differences in total cortical grey matter volume among the groups (Table 3). Also, there were no associations between total grey matter volume and age at onset or frequency of cannabis use in the CUD group (Appendix, Tables S1 and S2, available at jpn.ca).
Table 2.
Scanning sequences of all participants (n = 197), by group
| Group, no. (%)* | |||||
|---|---|---|---|---|---|
|
|
|||||
| Parameter | CUD, n = 80 | NCUD, n = 33 | Control, n = 84 | Statistic | p value |
| Pixel spacing, mean ± SD, mm† | 0.84 ± 0.2 | 0.79 ± 0.2 | 0.96 ± 0.1 | Welch t2,65 = 28.51 | < 0.001 |
| TR, mean ± SD (range), ms | 9.6 ± 0.3 (9.0–9.8) | 9.6 ± 0.3 (9.0–9.8) | 9.6 ± 0.2 (9.0–9.7) | Welch t2,73 = 0.001 | 0.99 |
| TE, mean ± SD (range), ms | 4.4 ± 0.4 (3.5–4.6) | 4.4 ± 0.4 (3.7–4.6) | 4.5 ± 0.3 (3.5–4.6) | Welch t2,81 = 3.13 | 0.049 |
| Slice thickness, mm | χ22 = 7.99 | 0.018 | |||
| 1.0 | 16 (20.0) | 8 (24.2) | 6 (7.1) | ||
| 1.2 | 64 (80.0) | 24 (72.7) | 78 (92.9) | ||
| Flip angle of 8° | 80 (100) | 33 (100) | 84 (100) | ||
CUD = cannabis use disorder; NCUD = no cannabis use disorder; SD = standard deviation; TE = echo time; TR = repetition time.
Unless otherwise indicated.
Pixel spacing is the same in both dimensions (i.e., x and y).
Table 3.
Mixed model analyses of the effect of group on brain volume, cortical thickness and surface area*
| Group, mean ± SD† | |||||
|---|---|---|---|---|---|
|
|
|||||
| Brain region | CUD, n = 80 | NCUD, n = 33 | Control, n = 84 | F | p value‡ |
| Total grey matter volume§ | 480399 ± 48604 | 507985 ± 47218 | 496952 ± 44090 | 4.12 | 0.018 |
| Hippocampus volume§ | 4272 ± 465 | 4340 ± 469 | 4337 ± 417 | 0.09 | 0.92 |
| Amygdala volume§ | 1754 ± 238 | 1670 ± 190 | 2042 ± 224 | 45.34 | < 0.001 |
| Thalamus volume§ | 7333 ± 778 | 7525 ± 793 | 7157 ± 639 | 3.65 | 0.028 |
| Caudate volume§ | 4084 ± 484 | 4031 ± 518 | 4197 ± 420 | 2.62 | 0.08 |
| Putamen volume§ | 6363 ± 808 | 5958 ± 727 | 6885 ± 687 | 20.28 | < 0.001 |
| OFC | |||||
| Volume | 13272 ± 1518 | 14282 ± 1558 | 13511 ± 1361 | 3.29 | 0.039 |
| Cortical thickness¶ | 2.57 ± 0.21 | 2.61 ± 0.23 | 2.54 ± 0.16 | 2.07 | 0.13 |
| Surface area¶ | 4671 ± 524 | 4980 ± 692 | 4789 ± 570 | 6.52 | 0.002 |
| ACC | |||||
| Volume¶ | 4746 ± 718 | 4796 ± 703 | 4908 ± 880 | 1.73 | 0.018 |
| Cortical thickness¶ | 2.76 ± 0.25 | 2.81 ± 0.22 | 2.66 ± 0.23 | 5.58 | 0.004 |
| Surface area¶ | 1525 ± 213 | 1525 ±242 | 1656 ± 279 | 3.59 | 0.029 |
| Insula | |||||
| Volume¶ | 7090 ± 720 | 7507 ± 765 | 7551 ± 879 | 7.84 | 0.001 |
| Cortical thickness¶ | 3.02 ± 0.20 | 3.01 ± 0.20 | 3.19 ± 0.18 | 38.54 | < 0.001 |
| Surface area¶ | 2302 ± 280 | 2469 ± 277 | 2305 ± 320 | 8.24 | < 0.001 |
| Parahippocampal gyrus | |||||
| Volume¶ | 2325 ± 316 | 2186 ± 277 | 2566 ± 321 | 24.53 | < 0.001 |
| Cortical thickness¶ | 2.78 ± 0.28 | 2.64 ± 0.25 | 3.01 ± 0.33 | 22.82 | < 0.001 |
| Surface area¶** | 727 ± 94 | 727 ± 98 | 736 ± 101 | 0.86 | 0.42 |
| Fusiform gyrus | |||||
| Volume¶ | 10359 ± 1454 | 10385 ± 1171 | 11415 ± 1191 | 18.27 | < 0.001 |
| Cortical thickness¶ | 2.65 ± 0.20 | 2.64 ± 0.18 | 2.84 ± 0.19 | 39.40 | < 0.001 |
| Surface area¶ | 3434 ± 451 | 3480 ± 429 | 3510 ± 407 | 1.64 | 0.20 |
ACC = anterior cingulate cortex; CUD = cannabis use disorder; NCUD = no cannabis use disorder; OFC = orbitofrontal cortex; SD = standard deviation.
Measures are adjusted for respectively intracranial volume, mean cortical thickness weighted by surface area and total surface area. In addition, all results are adjusted for age and slice thickness.
Volumes are measured in cubic millimetres, surface area is measured in square millimetres, and cortical thickness is measured in millimetres.
Volumes significant from p < 0.01, cortical thickness significant from p < 0.02 and surface area significant from p < 0.03.
Based on the FreeSurfer segmentation output.
Based on the FreeSurfer Desikan-Killiany Atlas.
Significant interaction between group status (i.e., CUD, NCUD or HC) and age.
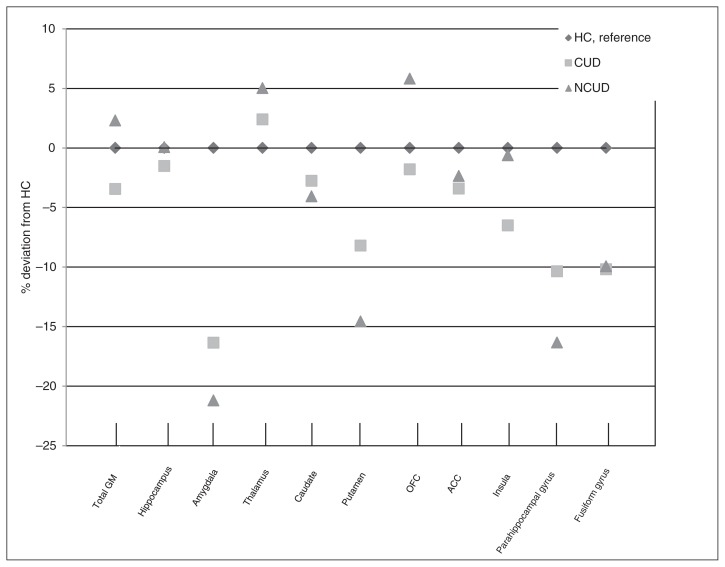
Figure 1 summarizes the results of the volume analyses. It depicts the percentage deviation of the mean volume of the control group per ROI. For the subcortical volumes, we found an effect of group on the volume of the amygdala and putamen (Table 3). The pairwise comparisons (Table 4) showed that both patient groups had smaller amygdala volumes (CUD v. control: d = 1.24; NCUD v. control: d = 1.73) and putamen volumes (CUD v.control: d = 0.70; NCUD v. control: d = 1.33) than healthy controls. Patients with CUDs showed significantly larger putamen volumes than NCUD patients (d = 0.51). All analyses were run a second time using voxel-based morphometry in SPM8 (default settings, including modulation). Results were similar except that the CUD group showed higher grey matter density in the left (t = 3.79, cluster size = 224, pFWEcor = 0.016) and right thalamus (t = 3.81, cluster size = 225, pFWEcor = 0.016). Effect sizes were comparable after exclusion of multiple substance users (n = 50 in the CUD and n = 33 in the NCUD groups) and in the analyses including nicotine use as a covariate. However, in both these analyses the difference between the CUD and NCUD groups in the putamen no longer reached significance (Appendix, Table S3 and S4).
Fig. 1.
Mean volumes of the regions of interest per group, shown as percentage deviation of the healthy control (HC) group. ACC = anterior cingulate cortex; CUD = cannabis use disorder; GM = grey matter; NCUD = no cannabis use disorder; OFC = orbitofrontal cortex.
Table 4.
Pairwise comparisons of the regions with a significant effect of group on volume*
| CUD v. control | NCUD v. control | CUD v. NCUD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Brain region | β† | SEβ | p value | d‡ | β† | SEβ | p value | d‡ | β† | SEβ | p value | d‡ |
| Amygdala§ | −276.00 | 34.50 | < 0.001 | 1.24 | −355.55 | 45.50 | < 0.001 | 1.73 | −79.60 | 45.46 | 0.08 | |
| Putamen§ | −505.79 | 117.45 | < 0.001 | 0.70 | −926.14 | 154.97 | < 0.001 | 1.33 | −420.36 | 154.83 | 0.007 | 0.52 |
| Insula¶ | −427.10 | 107.9 | < 0.001 | 0.57 | −197.00 | 142.40 | 0.17 | 230.13 | 142.27 | 0.11 | ||
| Parahippocampal gyrus | −241.71 | 48.25 | < 0.001 | 0.76 | −409.40 | 63.66 | < 0.001 | 1.23 | −167.69 | 63.60 | 0.009 | |
| Fusiform gyrus¶ | −1024.10 | 195.80 | < 0.001 | 0.80 | −1238.32 | 258.38 | < 0.001 | 0.87 | −214.19 | 258.14 | 0.41 | |
CUD = cannabis use disorder; NCUD = no cannabis use disorder; SEβ = standard error of β.
All results are adjusted for age, slice thickness and intracranial volume. Significant from p < 0.01.
Adjusted mean difference between the 2 groups.
Calculated only for significant regions of interest.
Based on the FreeSurfer segmentation output.
Based on the FreeSurfer Desikan-Killiany Atlas.
In the CUD group, we observed a positive trend for the correlation between the frequency of cannabis use and the volume of the caudate (F = 5.70, p = 0.019; Appendix, Table S2), which did not survive the multiple comparison correction. We found no significant correlation between regional volume and age at onset of regular cannabis use (Appendix, Table S1).
For the cortical volumes, we found an effect of group on brain volume in the insula, parahippocampus and fusiform gyrus (Fig. 1, Table 3). Pairwise comparisons showed significant differences between patients and healthy controls in the insula (CUD v. control: d = 0.57), parahippocampus (CUD v. control: d = 0.76; NCUD v. control: d = 1.23) and fusiform gyrus (CUD v. control: d = 0.80; NCUD v. control: d = 0.87), with smaller volumes in the patient groups (Table 4). Effect sizes of these differences were comparable after exclusion of multiple substance users and in the analyses including nicotine use as a covariate (Appendix, Table S3 and S4). There were no significant correlations with age at onset or frequency of cannabis use in any of the assessed regions (Appendix, Tables S1 and S2).
When assessing the contribution of cortical thickness and cortical surface area to the volume differences between patients and healthy controls, we found an effect of group in the anterior cingulate gyrus, insula, parahippocampus and fusiform gyrus (Table 3). Pairwise comparisons showed significantly thinner cortices in most ROIs (insula, parahippocampus and fusiform gyrus) for both patient groups compared with controls and in the anterior cingulate gyrus for the CUD group compared with controls (Appendix, Table S5). With respect to the cortical surface areas, we found an effect of group for the OFC and the insula (Table 3). Pairwise comparisons of the OFC showed smaller cortical surface areas for the NCUD compared with the control group (d = 0.31) and for the CUD compared with the NCUD group (d = 0.54). Significantly smaller cortical surface areas were found in the insula for both patient groups compared with controls (CUD v. control: d = 0.01; NCUD v. control: d = 0.53; Appendix, Table S6).
We assessed cortical symmetry in all 3 groups separately (Appendix, Table S7), showing cortical asymmetry in 3 of 5 regions in the CUD group (ACC: t79 = 2.58, p = 0.012; insula: t79 = −2.40, p = 0.019; parahippocampus: t79 = 5.11, p < 0.001) and in the control group (OFC: t83 = 7.26, p < 0.001; ACC: t83 = 2.02, p = 0.047; insula: t83 = −8.09, p < 0.001). In the NCUD group, all 5 ROIs showed left/right symmetry, as none of the regions significantly differed between the left and right hemisphere.
Discussion
In the present study we evaluated differences in grey matter volume, cortical thickness and cortical surface area in a large sample of patients with recent onset schizophrenia with and without comorbid cannabis use disorders and healthy controls using an ROI approach. As expected, patients with schizophrenia had significantly smaller volumes of most a priori–defined brain regions than healthy controls. Contrary to our expectations, patients in the CUD group had a larger volume of the putamen than those in the NCUD group, while differences in the other regions did not reach significance. Inconsistent results have been reported in the literature regarding volume differences between patients with and without CUDs, with most studies showing no differences or smaller volumes in patients with CUDs.10,16,46 Similar to our results, however, 2 studies reported larger volumes in the CUD group than in the NCUD group.21,47 A possible explanation is that patients with CUDs represent a subgroup of patients that is intrinsically less vulnerable for schizophrenia than patients who never used cannabis (i.e., NCUD patients are a group in whom schizophrenia developed without the extra risk factor of cannabis use and therefore could be intrinsically more vulnerable). This idea is supported by our finding of reduced asymmetry in the NCUD group compared with the CUD and the control groups. Decreased cerebral lateralization in patients with schizophrenia could be caused by a deviation of the genetic mechanism underlying cerebral dominance (the hypothesized “right-shift factor”) which may cause a vulnerability to schizophrenia.9 In line with this explanation, it has consistently been reported that patients who use cannabis perform better in several cognitive domains than patients who do not use cannabis.21,48,49 Based on these results, a neuroprotective role of cannabis has been suggested. However, in a longitudinal study by Rais and colleagues,50 volume decreases in patients who use cannabis appeared at 5-year follow-up, whereas no volume differences were apparent at baseline, suggesting that long-term cannabis use is more likely neurodegenerative. This adverse effect of cannabis use is supported by the findings of a longitudinal study by González-Pinto and colleagues.51 Although at baseline no differences in clinical characteristics were found between the CUD and NCUD groups, patients who stopped using cannabis had a significantly better functional outcome than patients who never used cannabis and those who continued to use cannabis.51 In summary, these studies show that patients with CUDs could represent a subgroup of patients with schizophrenia who have a lower intrinsic vulnerability, but that continued cannabis use in these patients may have neurotoxic effects and is associated with a worse functional outcome. However, for the present cohort no long-term follow-up data are available, so this theory cannot be corroborated by our findings.
Other possible explanations for our finding of enlarged putamen volumes in the CUD group cannot be ruled out; for example, cannabis may have a direct neuroprotective effect on brain volumes. This could especially be the case when using cannabis with a high concentration of cannabidiol.52 In addition, Malchow and colleagues53 found an increased N-acetyl aspartate:choline ratio using magnetic resonance spectroscopy in the left putamen in patients with comorbid cannabis use, indicating a neuro- and membrane-protective effect of tetrahydrocannabinol.
However, given our finding that polysubstance use may contribute to the difference in putamen volume between the CUD and NCUD groups, another possible explanation is the role of this structure in addiction. The putamen — as part of the dorsal striatum — is a crucial structure in habit formation in individuals with chronic addictive behaviours. According to Everitt and Robbins,54 early stages of addiction are characterized by voluntary drug use related to its rewarding and motivational effects, represented by hyperactivity of the ventral striatum. Later stages of addiction are characterized by involuntary habitual or compulsive drug use, represented by hyperactivation of the dorsal striatum.54 The dorsal striatum (putamen), therefore, plays an important role in the progression from the initial reinforcing effects of drug use to habitual, compulsive substance use,54,55 which might explain the larger volume of this area in the cannabis-dependent patient group.
With regard to the age at onset of regular cannabis use, we found no association with cortical brain volume, which is in accordance with findings of most studies published so far.30 In line with findings in nonschizophrenic patients with CUDs,29 we found a trend association in our sample between caudate volume and frequency of use, with smaller volumes associated with more frequenct use. Other studies, however, did not include the caudate as an ROI. The lack of associations in other brain regions may be explained by the low variance in frequency of use (71% of the cannabis users were daily cannabis users). Studies included in the review by Lorenzetti and colleagues29 included more diverse samples with varying frequencies of cannabis use.
As hypothesized, cortical thinning mainly drove volumetric differences between patients and controls. However, we found no differences between the CUD and NCUD groups in cortical thickness. For cortical surface area, differences between the CUD and NCUD groups were found only in the OFC. Volumetric differences between the CUD and NCUD groups were expected to depend on cortical thinning, because environmental factors may influence cortical thickness more heavily than cortical surface area. 31 In our sample, volumetric differences might have been too subtle to distinguish effects of thickness and surface area.
Limitations
Our findings should be viewed in light of several limitations. First, although patients were in the early phase of a psychotic disorder, most of them used antipsychotic medication, which may have confounded our results, especially with regard to subcortical volumes. However, because the CUD and NCUD groups did not differ with respect to their use of antipsychotic medication, it is not likely that medication effects would explain the volume differences between these groups. The same goes for other potential confounders, such as age, presence of comorbid disorders (including DSM-IV drug diagnoses), duration of schizophrenia and other clinical variables.
A second limitation is that it was difficult to correct for nicotine use, because data were missing for 39 of 197 participants. This may be problematic, because nicotine use has been shown to affect regional brain volumes in healthy controls and patients with schizophrenia. In healthy individuals, smoking seems mainly related to grey matter volume loss in the prefrontal regions and possibly in the insula and the olfactory gyrus.56–58 However, findings in patients with schizophrenia are inconsistent. One ROI study reported smaller grey matter volumes in the hippocampus and the dorsolateral prefrontal cortex,58 whereas another study reported larger grey matter volumes in the lateral prefrontal cortex and the superior temporal gyri,57 and a recent study found no grey matter volume differences between smoking and non-smoking patients with schizophrenia.59 To control for the possible confounding effect of nicotine use, we decided to add nicotine use to the model as a covariate. The effect sizes remained very similar, but the difference in putamen volume between the CUD and NCUD groups was no longer significant. A possible explanation is that polysubstance and nicotine use contribute to the difference in putamen volume between patients with and without CUDs. However, a more likely explanation for this combination of findings (similar effect sizes and loss of significance) is the reduced statistical power due to missing entries. In summary, the presence of a CUD seems independently associated with putamen volume, but we cannot completely rule out that the group differences in putamen volume were caused by nicotine rather than cannabis use; therefore, future studies should include healthy, nicotine-dependent controls.
A third limitation is the use of different scan protocols. Although all participants were scanned on the same MRI scanner, there were slight differences in scanning protocols. However, surface-based models like FreeSurfer have a strong robustness for differences in field strength, scanner upgrades and scanner manufacturer.60 In addition we included slice thickness in our statistical model to reduce this confounding effect. However, it was not possible to create a subset of better-matched participants. Future studies should include participants undergoing exactly the same scanning protocol.
The strengths of our study include the large sample of patients with recent onset schizophrenia with and without CUDs. The few studies published so far on the influence of cannabis use on brain volumes in recent onset schizophrenia all have small sample sizes of fewer than 30 patients. In addition, our sample had a relatively short history of antipsychotic medication intake, minimizing the influence of antipsychotic medication on brain volumes, especially compared with studies examining chronic patient cohorts.
Conclusion
Patients with schizophrenia (with and without a CUD) had smaller volumes of most brain regions than healthy controls. However the CUD and NCUD groups were mostly indistinguishable in the early phase of the disease, except for the larger putamen volumes in the CUD group. A possible explanation for these results is that cannabis use promotes the onset of schizophrenia in less vulnerable individuals and that patients with schizophrenia and a comorbid CUD represent a subgroup of patients who could have a relatively favourable outcome if they stopped using cannabis.49 This explanation is supported by our finding of reduced asymmetry in the NCUD group compared with the CUD and control groups, which could be caused by a genetic vulnerability to schizophrenia.9 Another possible explanation is the role of the putamen in habitual, compulsive substance use,54,55 which is supported by our finding that polysubstance use may drive the difference in putamen volume between groups. Therefore, future studies should elaborate on whether this finding represents differences in brain development between groups or whether addiction affects putamen volume.
Acknowledgments
The authors thank the patients and healthy controls who participated in this study. This study was funded with grants from ZonMW (grant numbers: 3160007, 91676084, 31160003, 31180002, 31000056, 2812412, 100001002, 100002034), NWO (grant numbers: 90461193, 40007080, 48004004, 40003330), and additional grants from the Amsterdam Brain Imaging Platform, Neuroscience Campus Amsterdam and the Dutch Brain foundation. The processing with FreeSurfer was performed on the Dutch e-Science Grid through the BiG Grid project and COMMIT project “e-Biobanking with imaging for healthcare,” which are funded by the Netherlands Organization for Scientific Research (NWO). The funding agency played no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Competing interests: None declared.
Contributors: L. Koenders and M. Machielsen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. L. Koenders, M. Machielsen, C. Meyer and L. de Haan designed the study. L. Koenders, M. Machielsen, A. van Gasselt, M. Caan, J. Cousijn, A. den Braber, D. van ‘t Ent, M. Rive, A. Schene, E. van de Giessen, C. Huyser, B. de Kwaasteniet and D. Veltman acquired the data, which L. Koenders, M. Machielsen, F. van der Meer, C. Meyer, W. van den Brink, M. Koeter, M. Caan, J. Cousijn, D. Veltman and L. de Haan analyzed. L. Koenders, M. Machielsen and F. van der Meer wrote the article, which all authors reviewed and approved for publication.
References
- 1.Caspari D. Cannabis and schizophrenia: results of a follow-up study. Eur Arch Psychiatry Clin Neurosci. 1999;249:45–9. doi: 10.1007/s004060050064. [DOI] [PubMed] [Google Scholar]
- 2.Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry. 1994;51:273–9. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- 3.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–28. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 4.Bersani G, Orlandi V, Kotzalidis GD, et al. Cannabis and schizophrenia: impact on onset, course, psychopathology and outcomes. Eur Arch Psychiatry Clin Neurosci. 2002;252:86–92. doi: 10.1007/s00406-002-0366-5. [DOI] [PubMed] [Google Scholar]
- 5.Bühler B, Hambrecht M, Loffler W, et al. Precipitation and determination of the onset and course of schizophrenia by substance abuse–a retrospective and prospective study of 232 population-based first illness episodes. Schizophr Res. 2002;54:243–51. doi: 10.1016/s0920-9964(01)00249-3. [DOI] [PubMed] [Google Scholar]
- 6.Dekker N, Meijer J, Koeter M, et al. GROUP Investigators. Age at onset of non-affective psychosis in relation to cannabis use, other drug use and gender. Psychol Med. 2012;42:1903–11. doi: 10.1017/S0033291712000062. [DOI] [PubMed] [Google Scholar]
- 7.Mueser KT, Yarnold PR, Levinson DF, et al. Prevalence of substance abuse in schizophrenia: demographic and clinical correlates. Schizophr Bull. 1990;16:31–56. doi: 10.1093/schbul/16.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd AM, Laurens KR, Matheson SL, et al. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–56. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Sommer I, Ramsey N, Kahn R, et al. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry. 2001;178:344–51. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- 10.Malchow B, Hasan A, Fusar-Poli P, et al. Cannabis abuse and brain morphology in schizophrenia: a review of the available evidence. Eur Arch Psychiatry Clin Neurosci. 2013;263:3–13. doi: 10.1007/s00406-012-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James A, James C, Thwaites T. The brain effects of cannabis in healthy adolescents and in adolescents with schizophrenia: a systematic review. Psychiatry Res. 2013;214:181–9. doi: 10.1016/j.pscychresns.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 12.James A, Hough M, James S, et al. Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS) Schizophr Res. 2011;128:91–7. doi: 10.1016/j.schres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Szeszko PR, Robinson DG, Sevy S, et al. Anterior cingulate grey-matter deficits and cannabis use in first-episode schizophrenia. Br J Psychiatry. 2007;190:230–6. doi: 10.1192/bjp.bp.106.024521. [DOI] [PubMed] [Google Scholar]
- 14.Bangalore SS, Prasad KMR, Montrose DM, et al. Cannabis use and brain structural alterations in first episode schizophrenia — a region of interest, voxel based morphometric study. Schizophr Res. 2008;99:1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Rapp C, Walter A, Studerus E, et al. Cannabis use and brain structural alterations of the cingulate cortex in early psychosis. Psychiatry Res. 2013;214:102–8. doi: 10.1016/j.pscychresns.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Cahn W, Hulshoff Pol HE, Caspers E, et al. Cannabis and brain morphology in recent-onset schizophrenia. Schizophr Res. 2004;67:305–7. doi: 10.1016/S0920-9964(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 17.Wobrock T, Czesnik D, Malchow B. Schizophrenia and comorbid substance abuse. In: Ritsner MS, editor. Pathophysiological and therapeutic approaches. Springer; Netherlands: 2011. [accessed 2014 Dec. 15]. pp. 321–63. Available: http://dx.doi.org/10.1007/978-94-007-0834-115. [Google Scholar]
- 18.Cohen M, Rasser PE, Peck G, et al. Cerebellar grey-matter deficits, cannabis use and first-episode schizophrenia in adolescents and young adults. Int J Neuropsychopharmacol. 2012;15:297–307. doi: 10.1017/S146114571100068X. [DOI] [PubMed] [Google Scholar]
- 19.Kumra S, Robinson P, Tambyraja R, et al. Parietal lobe volume deficits in adolescents with schizophrenia and adolescents with cannabis use disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:171–80. doi: 10.1016/j.jaac.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Haller S, Curtis L, Badan M, et al. Combined grey matter VBM and white matter TBSS analysis in young first episode psychosis patients with and without cannabis consumption. Brain Topogr. 2013;26:641–7. doi: 10.1007/s10548-013-0288-8. [DOI] [PubMed] [Google Scholar]
- 21.Schnell T, Kleiman A, Gouzoulis-Mayfrank E, et al. Increased gray matter density in patients with schizophrenia and cannabis use: a voxel-based morphometric study using DARTEL. Schizophr Res. 2012;138:183–7. doi: 10.1016/j.schres.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Arseneault L, Cannon M, Poulton R, et al. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–3. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen M, Solowij N, Carr V. Cannabis, cannabinoids and schizophrenia: integration of the evidence. Aust NZ J Psychiatry. 2008;42:357–68. doi: 10.1080/00048670801961156. [DOI] [PubMed] [Google Scholar]
- 24.Hall WD. Cannabis use and the mental health of young people. Aust NZ J Psychiatry. 2006;40:105–13. doi: 10.1080/j.1440-1614.2006.01756.x. [DOI] [PubMed] [Google Scholar]
- 25.Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol. 2010;160:511–22. doi: 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson W, Mathew R, Turkington T, et al. Brain morphological changes and early marijuana use. J Addict Dis. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- 27.Cousijn J, Wiers RW, Ridderinkhof KR, et al. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59:3845–51. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 28.Battistella G, Fornari E, Annoni JM, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014;39:2041–48. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzetti V, Lubman DI, Whittle S, et al. Structural MRI findings in long-term cannabis users: what do we know? Subst Use Misuse. 2010;45:1787–808. doi: 10.3109/10826084.2010.482443. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzetti V, Solowij N, Fornito A, et al. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr Pharm Des. 2014;20:2138–67. doi: 10.2174/13816128113199990435. [DOI] [PubMed] [Google Scholar]
- 31.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–6. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 32.Rimol LM, Nesvag R, Hagler DJ, Jr, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71:552–60. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Wierenga LM, Langen M, Oranje B, et al. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–6. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rentería ME. Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Research and Human Genetics. 2012;15:401–13. doi: 10.1017/thg.2012.13. [DOI] [PubMed] [Google Scholar]
- 36.Levitt JJ, Bobrow L, Lucia D, et al. A Selective Review of Volumetric and Morphometric Imaging in Schizophrenia. In: Swerdlow NR, editor. Behavioral Neurobiology of Schizophrenia and Its Treatment. Springer; Berlin Heidelberg: 2010. pp. 243–81. [DOI] [PubMed] [Google Scholar]
- 37.Solowij N, Walterfang M, Lubman DI, et al. Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophr Res. 2013;143:179–84. doi: 10.1016/j.schres.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 38.Habets P, Marcelis M, Gronenschild E, et al. Genetic risk and outcome of psychosis (G.R.O.U.P). Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiatry. 2011;69:487–94. doi: 10.1016/j.biopsych.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Andreasen NC, Flaum M, Arndt S. The comprehensive assessment of symptoms and history (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–23. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 40.Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–28. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- 41.Medina KL, McQueeny T, Nagel BJ, et al. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. 2009;14:457–68. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahand S, Santcroos M, van Kampen AHC, et al. A grid-enabled gateway for biomedical data analysis. J Grid Computing. 2012;10:725–42. [Google Scholar]
- 43.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–42. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 44.Li W, van Tol MJ, Li M, et al. Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Hum Brain Mapp. 2014;35:238–47. doi: 10.1002/hbm.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haller S, Borgwardt SJ, Schindler C, et al. Can cortical thickness asymmetry analysis contribute to detection of at-risk mental state and first-episode psychosis? A pilot study. Radiology. 2009;250:212–21. doi: 10.1148/radiol.2501072153. [DOI] [PubMed] [Google Scholar]
- 46.Wobrock T, Sittinger H, Behrendt B, et al. Comorbid substance abuse and brain morphology in recent-onset psychosis. Eur Arch Psychiatry Clin Neurosci. 2009;259:28–36. doi: 10.1007/s00406-008-0831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potvin S, Mancini-Marïe A, Fahim C, et al. Increased striatal gray matter densities in patients with schizophrenia and substance use disorder: a voxel-based morphometry study. Psych Research. 2007;154:275–9. doi: 10.1016/j.pscychresns.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Meijer JH, Dekker N, Koeter MW, et al. Cannabis and cognitive performance in psychosis: a cross-sectional study in patients with non-affective psychotic illness and their unaffected siblings. Psychol Med. 2012;42:705–16. doi: 10.1017/S0033291711001656. [DOI] [PubMed] [Google Scholar]
- 49.Yücel M, Bora E, Lubman DI, et al. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull. 2012;38:316–30. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rais M, van Haren NEM, Cahn W, et al. Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2010;20:855–65. doi: 10.1016/j.euroneuro.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 51.González-Pinto A, Alberich S, Barbeito S, et al. Cannabis and first-episode psychosis: different long-term outcomes depending on continued or discontinued use. Schizophr Bull. 2011;37:631–9. doi: 10.1093/schbul/sbp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demirakca T, Sartorius A, Ende G, et al. Diminished gray matter in the hippocampus of cannabis users: possible protective effects of cannabidiol. Drug Alcohol Depend. 2011;141:242–5. doi: 10.1016/j.drugalcdep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 53.Malchow B, Hasan A, Schneider-Axmann T, et al. Effects of cannabis and familial loading on subcortical brain volumes in first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013;263:155–68. doi: 10.1007/s00406-013-0451-y. [DOI] [PubMed] [Google Scholar]
- 54.Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–54. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 56.Fritz H, Wittfeld K, Schmidt CO, et al. Current smoking and reduced gray matter volume: a voxel-based morphometry study. Neuropsychopharmacology. 2014;39:2594–600. doi: 10.1038/npp.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider CE, White T, Hass J, et al. Smoking status as a potential confounder in the study of brain structure in schizophrenia. J Psychiatr Res. 2014;3:84–91. doi: 10.1016/j.jpsychires.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tregellas JR, Shatti S, Tanabe JL, et al. Gray matter volume differences and the effects of smoking on gray matter in schizophrenia. Schizophr Res. 2007;97:242–9. doi: 10.1016/j.schres.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Van Haren NEM, Koolschijn PCMP, Cahn W, et al. Cigarette smoking and progressive brain volume loss in schizophrenia. Eur Neuropsychopharmacol. 2010;20:454–8. doi: 10.1016/j.euroneuro.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]