Abstract
Purpose
Establish differences in intracortical facilitation (ICF) and inhibition (ICI) between survivors of stroke and healthy individuals.
Methods
Fourteen chronic stroke survivors and 19 healthy subjects were investigated using single and paired-pulse transcranial magnetic stimulation (TMS). TMS was applied over the motor cortex in thelesioned (stroke survivors) or left (healthy subjects) hemisphere. Motor evoked potentials (MEPs) were collected from the contra lateral first dorsal interosseus. Subjects received 40 pseudo-randomized trials consisting of 10 trials for each: conditioning stimulus, test stimulus (TS), ICF, and ICI. Between the groups, we compared MEP amplitudes for TS, ICF, and ICI, motor threshold (MT), and ICF/ICI ratio.
Results
Compared to healthy individuals, the stroke group exhibited higher MT and lower ICI; the difference ICF neared significance. The ICF/ICI ratio was significantly lower in the stroke group and close to 1, indicating little difference between ICF and ICI responses. These differences demonstrate that motor cortex excitatory and inhibitory mechanisms are impaired for individuals in the chronic post-stroke recovery phase.
Conclusions
Compared to healthy individuals, both global and intracortical TMS measures reveal reduced motor cortex excitability in survivors of stroke. Interventions that normalize motor cortex excitability may promote better neurophysiological conditions for motor recovery to occur.
Keywords: transcranial magnetic stimulation, stroke, cerebral vascular accident, brain, intracortical facilitation, intracortical inhibition
Introduction
Motor dysfunction is a common residual impairment that leads to inability to independently perform activities of daily living (Edwards and Fregni 2008). Only 12% of survivors of stroke regain complete motor recovery (Dafotakis, Grefkes, Eickhoff et al. 2008), and 30%-60% of hemiplegic stroke patients never regain function of the paretic upper extremity (UE) (Kwakkel, Kollen and Krebs 2008). Although many parts of the central nervous system contribute to voluntary movement, paretic UE motor control is typically severely impacted by motor cortex dysfunction in the lesioned hemisphere (LH) (Hodics, Cohen and Cramer 2006).
Following stroke, the LH motor cortex commonly has a depressed level of excitation, which is often associated with severe impairments of motor function. This phenomenon is especially true in the acute and sub-acute recovery stages. Over the long-term and particularly if the survivor of stroke experiences some recovery of motor function, LH motor cortex excitability has been shown to increase. This association between enhanced motor cortex activation and recovery has resulted in recent investigations of brain stimulation methods to promote greater motor cortex excitability that result in better motor function. Indeed, this work has demonstrated that brain stimulation combined with focused movement rehabilitation can significantly enhance motor outcomes compared to rehabilitation alone. The neurophysiological basis of this approach, however, has primarily focused on raising excitability with less consideration of the status of inhibitory mechanisms or the balance of excitation to inhibition. To this end, research is needed to establish differences in excitability, inhibition, and the balance of excitation to inhibition when comparing stroke-affected versus healthy motor cortex function. Furthermore, while neurophysiological function has been investigated in the acute and sub-acute recovery stages, less is known about the persistence of post-stroke effects on motor cortex function during the chronic phase of recovery.
Transcranial magnetic stimulation (TMS) has been used to measure both excitatory (also referred to as facilitative) and inhibitive neuronal systems. Intracortical facilitation (ICF) is a process in which the activity of one neuron will facilitate the activity of another neuron, whereas intracortical inhibition (ICI) involves a presynaptic neuron inhibiting the firing of another neuron (Saladin, 2010). ICF is believed to reflect activity in the glutamatergic system (Chen et al., 1998); and ICI, the GABAA system (Kujirai, Caramia, Rothwell et al. 1993, Chen, Tam, Bütefisch et al. 1998, Ziemann, Tergau, Wassermann et al. 1998). In ICF and ICI paired-pulse TMS protocols, a suprathreshold test stimulus (TS) is delivered over the primary motor cortex, preceded by a subthreshold conditioning stimulus (Chen and Garg 2000). The length of the inter-stimulus interval determines whether intracortical facilitative or inhibitory circuits are activated and measured (Kujirai, Caramia, Rothwell et al. 1993). When the TS is applied at a short interstimulus interval (e.g., 2ms) after the CS, the MEP is partially inhibited, i.e. reflecting ICI (Kujirai, Caramia, Rothwell et al. 1993, Bütefisch, Wessling, Netz et al. 2008). Alternatively, the MEP is facilitated (ICF) when the TS is delivered at longer ISIs (e.g, 15ms) after the CS (Kujirai, Caramia, Rothwell et al. 1993, Chen, Tam, Bütefisch et al. 1998).
Using TMS, researchers have measured mechanisms, such as ICI and ICF, within the primary motor cortex to understand differences between people who have recently (acute or sub-acute stage) had a stroke as compared to those who have not. These studies have generally found the lesioned hemisphere to be less excitable than the non-lesioned hemisphere (Bütefisch 2004, Liepert, Hamzei and Weiller 2004, Di Lazzaro, Pilato, Dileone et al. 2008, Cramer, Sur, Dobkin et al. 2011). Other common TMS measures (e.g., motor threshold and MEP amplitude) often reveal reduced motor cortex excitation in survivors of stroke. Others have examined either inhibition or facilitation (but not both) in stroke and healthy populations. For example, using paired-pulse TMS, Liepert and colleagues (Liepert, Hamzei and Weiller 2000) found stroke survivors to have significantly reduced ICI when compared to healthy subjects, concluding that motor cortex disinhibition post-stroke is a naturally occurring compensatory mechanism in the recovery process. Similarly, Butefisch and colleagues (Bütefisch, Wessling, Netz et al. 2008) found that survivors of stroke demonstrated increased short interval cortical excitability compared to subjects without stroke as a result of greater disinhibition not seen in healthy individuals. This previous work has primarily focused on patients in the acute and (to a lesser extent) early sub-acute phase of recovery. Comparison of motor cortex neurophysiological function between survivors of stroke in the chronic phase and healthy controls are needed.
The purpose of this study was to establish differences in motor threshold, MEP amplitude, ICI, ICF, and the ICF to ICI ratio when comparing chronic stroke survivors to individuals unaffected by stroke. The following questions were investigated during this study:
Are there significant differences in motor threshold (MT) and amplitude of MEPs during supra-threshold, single-pulse TMS between people who have had a stroke as compared people who have not had a stroke?
Are there significant differences ICF and ICI between people who have had a stroke as compared to subjects without stroke?
Does the ratio of ICF to ICI differ significantly between individuals with and those without stroke?
Methods
Subjects
The Colorado State University Institutional Review Board approved this investigation prior to the initiation of any study procedures and all subjects provided written informed consent prior to their study involvement. Two groups were studied in this investigation: 14 individuals in the chronic post-stroke phase and 19 healthy controls. The stroke group was a convenience sample consisting of volunteers recruited through stroke support groups, and clinician offices. The inclusion criteria for the group with stroke included being 40 years or older and UE hemiparesis resulting from a stroke that occurred ≥9 months prior to study participation. Furthermore, these subjects were required to meet the following motor criteria for the hemiparetic hand: active extension of at least 20° at the wrist, and 10° at the metacarpophalangeal and interphalangeal joints of at least two fingers and thumb. Subjects were excluded if they had a history of seizures, epilepsy, head trauma leading to loss of consciousness, mental retardation, poorly-controlled psychiatric or mental illness, bipolar disorder, increased intracranial pressure, alcohol or drug abuse within the past year, implanted pacemaker or medication pump, metal plate or metal objects in the eye or the skull, aneurism clips, cochlear implant, intracardiac lines, significant history of heart disease, or were pregnant. Fourteen stroke survivors participated (demographic details provided in Table 1).
Table 1. Demographic data for stroke group participants.
| Subject | Gender | Age (yrs.) | Post-stroke time (mos.) | Lesion side | Lesion details | Fugl-Meyer upper limb motor score |
|---|---|---|---|---|---|---|
| S01 | F | 56 | 65 | R | midbrain + pons | 51 |
| S02 | F | 51 | 12 | R | MCA territory | 43 |
| S03 | F | 66 | 80 | R | internal capsule | 32 |
| S04 | M | 70 | 12 | R | lacunar infarct | 24 |
| S05 | M | 82 | 22 | R | posterior frontal periventricular infarct | 21 |
| S06 | F | 71 | 38 | R | lacunar infarct | 30 |
| S07 | F | 40 | 12 | L | anterior MCA territory | 41 |
| S08 | M | 57 | 20 | L | posterior parietal | 54 |
| S09 | M | 72 | 24 | L | medulla | 31 |
| S10 | F | 61 | 12 | L | corona radiata into posterior limb of internal capsule | 42 |
| S11 | F | 47 | 16 | L | insula and temporal/parietal | 56 |
| S12 | F | 61 | 9 | R | basal ganglia/midbrain junction | 20 |
| S13 | F | 77 | 59 | R | pons | 17 |
| S14 | M | 60 | 9 | L | pons + cerebellum | 55 |
The non-stroke group was a convenience sample comprised of 19 participants between the ages of 21-35 years old (mean: 25.7 ± 3.4 years; ten female, nine male). In addition to the exclusion criteria included for the aforementioned stroke group, subjects were excluded if they had evidence of mass brain lesions, hemorrhagic stroke, arteriovenous malformation, intracortical hemorrhage, subarachnoid hemorrhage, or bilateral cerebrovascular disease. Subjects were also excluded if pregnant or were left-handed.
Subject set-up
Each subject was seated in a semi-reclined dental chair with a pillow behind the neck and a pillow beneath the forearm and hand contralateral to the stimulated hemisphere. A cloth cap was placed on the participant's head so that TMS coil reference points could be marked. Surface electromyography (EMG) electrodes were applied to the first dorsal interosseus (FDI) contralateral to stimulation: over the FDI muscle of the stroke affected hand for the subjects with stroke and over the FDI of the right hand for subjects without stroke. EMG electrodes were connected to and activity was recorded by a Nicolet Viking Select (Nicolet Biomedical, USA) electromyograph. EMG silence was monitored and trials contaminated by voluntary muscle activity were rejected.
Transcranial magnetic stimulation
Excitability and inhibition of the primary motor cortex were investigated using single and paired-pulse transcranial magnetic stimulation (TMS) delivered by Magstim 2002 magnetic stimulators (Magstim Ltd, UK) through a 7cm figure-of-eight shaped coil centered over the area of the primary motor cortex controlling the hand. The coil handle was oriented 45 degrees from the mid-sagittal line to produce an induced current flow in the anteromedial direction, which is approximately perpendicular to the central sulcus (Laakso, Hirata and Ugawa 2014) and has been demonstrated as optimal for generating MEPs in intrinsic hand muscles (Mills, Boniface and Schubert 1992, Laakso, Hirata and Ugawa 2014). TMS was administered to the left motor cortex in non-stroke subjects, and the lesioned hemisphere motor cortex in survivors of stroke. Motor threshold (MT) was established first, and was determined as the minimum output of the Magstim 2002 necessary to elicit an MEP in the relaxed FDI in 5 out of 10 trials (Kujirai, Caramia, Rothwell et al. 1993, Chen, Tam, Bütefisch et al. 1998). To assess ICI and ICF, we used 2ms and 15ms interstimulus intervals, respectively. For both ICI and ICF, CS and TS intensities were 90% and 116% of motor threshold, respectively (Chen, Tam, Bütefisch et al. 1998). Ten (10) trials each of ICF, ICI, CS-only, and TS-only were carried out in pseudorandom order with a 6s inter-trial interval (Massie, Tracy and Malcolm 2012). MEP wave forms were analyzed for peak-to-peak amplitude for each trial using Lab Chart 7 Pro (ADInstruments Ltd., USA). The onset and offset for each MEP were determined; then peak amplitude was determined as the greatest mV between the MEP positive and negative peaks.
Dependent measures
Dependent measures included MT, MEP amplitude single-pulse TMS, ICI, ICF, and the ICF to ICI. Because CS trials did not result in MEPs, no values were reported in this study. The ratio of ICF to ICI had not been used previously to report excitability in the primary motor cortex. It was utilized in this study as an index of excitability, considering both excitation and inhibition, in order to characterize both excitability and inhibition in the cortices of adults who have had a stroke as compared to adults without stroke (Massie, Tracy and Malcolm 2012). A higher ratio indicates more facilitation, while a ratio of one indicates no difference in MEP amplitudes obtained during ICI versus ICF conditions.
Statistical analysis
This was a non-randomized, group-comparison study based on the attribute variable stroke versus non-stroke. For each subject, MEP amplitudes for the 10 trials of TS were averaged. The ICF MEPs for each trial were divided by the average TS MEPs in order to normalize the data (Massie, Tracy and Malcolm 2012). Normalization of these data allowed for comparison of variables as well as comparison of groups. ICI trials were averaged and normalized using the same method. The ICF-to-ICI ratio is the ratio of normalized ICF to normalized ICI. Values in Figures 1 and 2 are normalized to the MEP obtained with single-pulse TMS at TS intensity. IBM SPSS Statistics version 20 was utilized to run statistics. Descriptive statistics were run for each group individually and Box and Whisker plots were created to determine outliers for each group. Outliers were removed for each variable. Based on comparing the two groups on multiple dependent measures, a multivariate analysis of variance (MANOVA) was used to determine significant differences between groups. In order to test the assumption of homogeneity of variance in the two groups for each dependent measure, the Levene's test was used. Considering that in three of the four dependent measures homogeneity was not found, the t-test was used for univariate between-groups comparisons, owing to its transparent presentation of adjustment and significance based on equality of variance assumed versus not assumed. Given that multiple comparisons were performed, we adjusted our significance level to α=.01 using the Bonferroni correction (i.e., .05/5 = .01).
Figure 1. Supra-Threshold Single-Pulse MEP Amplitude Group Comparison.
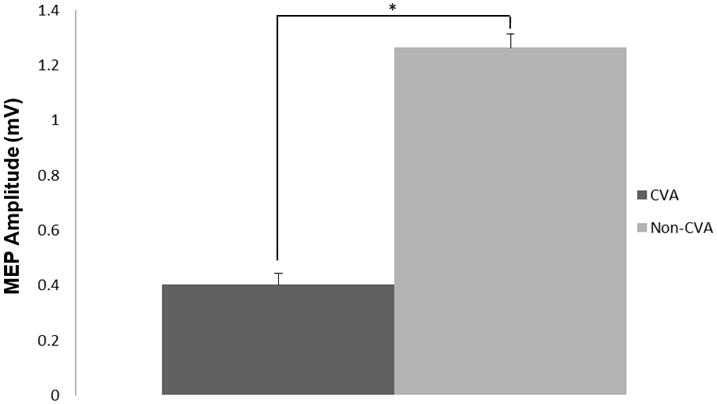
Group comparisons of motor evoked potentials (MEP) obtained duringsupra-threshold, single-pulse TMS. * indicates significant difference between groups (p<.01).
Figure 2. ICF and ICI Group Comparisons.
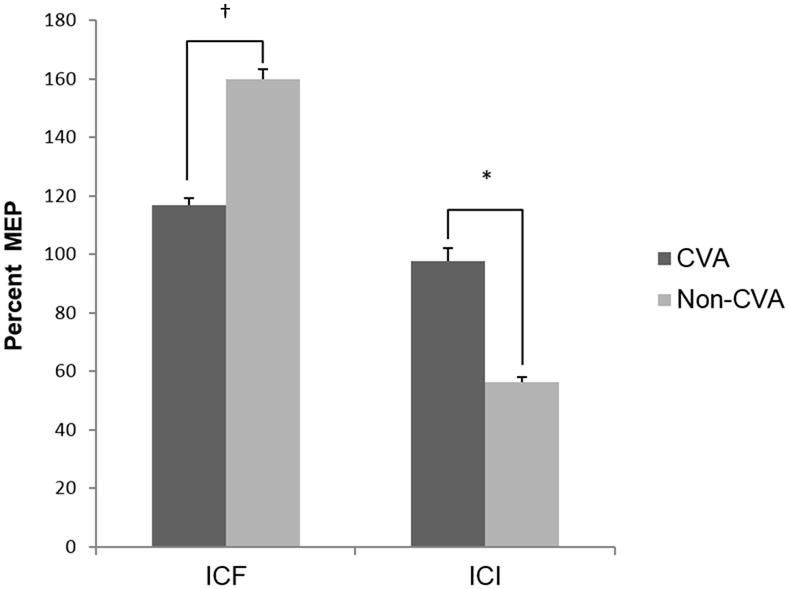
Chart depicting group comparisons of normalized motor evoked potential (MEP) amplitudes for intracortical facilitation (ICF) and intracortical inhibition (ICI) between a group with CVA and a group without CVA. Note that a higher MEP for ICI indicates decreased intracortical inhibition. * indicates significant difference between groups (p<.01), †p<.05.
Results
There were no adverse events or effects of the TMS, nor did any subject report discomfort with the procedures.
The multivariate analysis indicated significant differences between groups (F5, 17 = 8.57, p<0.001) considering all variable together. Between-groups comparisons based on subsequent univariate analyses are summarized in Figures 1, 2, and 3; and specific mean values per variable are listed in Table 2. In comparison to the non-stroke group, survivors of stroke had a significantly higher motor threshold (t1,17.1 = 4.126, p=0.001) and lower mean MEP amplitude during single-pulse (TS) trials (t26.2= 3.45, p=0.002). Compared to the non-stroke group, survivors of stroke exhibited less ICF (smaller MEP amplitudes during ICF trials) although this difference, after adjusting alpha level was not significant (t26.1= 2.4, p=0.02) and significantly less ICI (larger MEP amplitudes during ICI trials)(t26 = 2.89, p=0.008). Survivors of stroke had a significantly lower normalized ICF to ICI ratio (t19.7 = 3.93, p = 0.001) than the non-stroke group. For the stroke group, the ICF:ICI ratio was close to 1, indicating that varying paired-pulse intervals had a similar effect on MEP amplitudes whether testing ICF or ICI. Additionally, for the stroke group, both 2ms (ICI) and 15ms (ICF) paired-pulse intervals appear to have facilitated MEPs in comparison to single-pulse (TS) MEPs. Figure 4 displays MEPs obtained during single-pulse (TS), ICF, and ICI trials for a representative non-stroke subject and stroke subject.
Figure 3. Ratio of Normalized ICF to Normalized ICI Group Comparison.
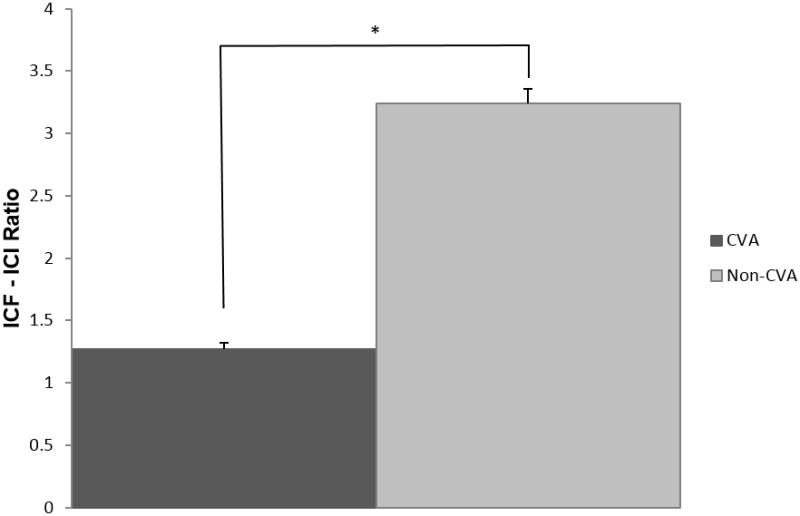
Chart depicting group comparison of normalized intracortical facilitation (ICF) to normalized intracortical inhibition (ICI) ratio. * indicates significant difference between groups.
Table 2. Between-groups comparisons with variable means and standard deviations.
| Variable | Stroke group (n = 14) Mean ± SD | Non-stroke (n = 19) Mean ± SD | Test Statistic (degrees of. freedom) | Significance |
|---|---|---|---|---|
| MT‡ | 66.29 ± 17.02 (n=14) | 46.11 ± 7.84 (n=19) | F=20.893 (df=1,31) | p=0.001 |
| TS† | 0.4018 ± 0.4878 (n=12) | 1.2624 ± 0.8489 (n=17) | F=9.941 (df=1, 27) | p=0.001 |
| ICF* | 116.80 ± 28.93 (n=12) | 159.87 ± 69.15 (n=19) | F=4.152 (df=1, 29) | p=0.040 |
| ICI* | 97.77 ± 42.78 (n=10) | 56.31 ± 32.52 (n=18) | F=8.340 (df=1, 26) | p=0.008 |
| ICF/ICI | 1.2677 ± 0.5096 (n=9) | 3.2444 ± 1.9504 (n=17) | F=8.768 (df=1,24) | p=0.003 |
MT Means ± SD expressed as % of stimulator output with 0.50Hz at 01% to 49% and 0.33Hz at 50% to 75% Power Output;
TS Means ± SD expressed as MEP amplitude (mV);
ICI and ICF Means ± SD expressed as %TS MEP
Figure 4. Representative MEPs of a non-stroke subject and stroke subject.
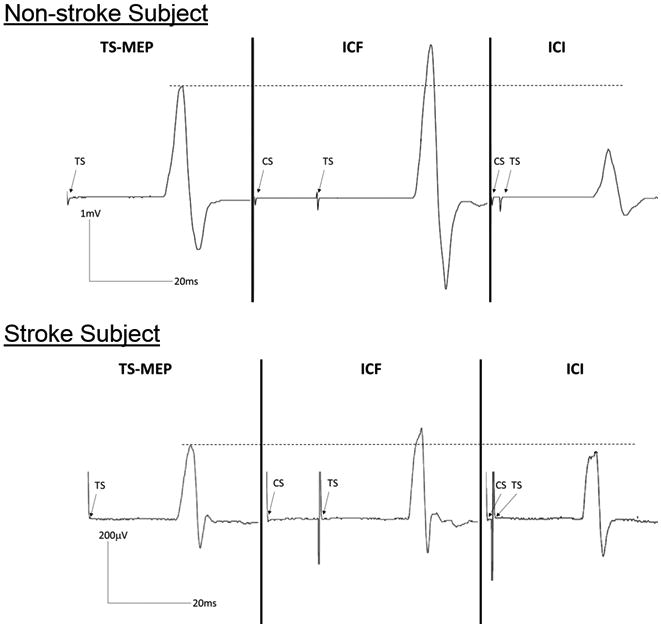
This representative non-stroke subject (top panel) demonstrated the characteristic intracortical facilitation (ICF) and intracortical inhibition (ICI) compared to a motor evoked potential (MEP) obtained with single-pulse TMS at the test stimulus (TS) intensity. In comparison, the stroke subject (bottom panel) demonstrated minimal ICF and ICI compared to an MEP obtained with single-pulse TMS at the TS intensity.
Discussion
The results of this study reveal that the motor cortex in the stroke-lesioned hemisphere is generally less excitable than the neurologically healthy motor cortex. This finding is not surprising, as other previous research has similarly shown motor cortex hypo-excitability, which is commonly linked to movement dysfunction in survivors of stroke. As with other work, we specifically found that survivors of stroke presented with elevated motor threshold and reduced-amplitude MEPs during single-pulse TMS when compared to healthy controls. However, beyond these oft-reported global excitability measures, the extent of our understanding of motor cortex dysfunction in these stroke survivors was deepened by considering more discrete neurophysiological measures, namely intracortical facilitation and inhibition and the ratio of ICF to ICI. Investigating these facilitative and inhibitory intracortical mechanisms in stroke survivors in the chronic phase of recovery is needed, as previous work has focused on patients in the acute and early sub-acute post-stroke stage.
We consider motor threshold and MEP amplitude to be broad measures of motor cortex excitability, based upon their presumed neurophysiological bases. Motor threshold represents the minimum stimulation intensity needed to elicit an MEP approximately 50% of trials. It is believed to reflect the global excitability of cortical synapses involving excitatory inputs and corticospinal neurons, as well as spinal cord level synapses between the corticospinal and alpha motor neurons (Talelli, Greenwood and Rothwell 2006). Accordingly, motor threshold is influenced by the excitatory state of several elements in the motor nervous system. Similarly, MEP amplitude is believed to reflect the regional corticospinal system excitability (Pell, Roth and Zangen 2011). In the case of both of these global measures of excitability, one must recognize that they represent very indirect measures of excitability at a neuronal level.
To investigate more discrete neural mechanisms of motor cortex function, we employed a paired-pulse TMS paradigm to assess ICF and ICI, as well as the ratio between these measures. In contrast to the aforementioned global measures, ICF and ICI are presumed to be tied to more specific neurotransmitter systems. More specifically, ICF is believed to reflect the activity of excitatory glutamergic synapses, (Chen 2004) while pharmacological interventions indicate that ICI is mediated by GABAA-ergic activity (Talelli, Greenwood and Rothwell 2006). Considerable evidence exists to indicate that these mechanisms occur at a cortical rather than subcortical level (for review, see Chen, 2004). By employing discrete intracortical measures alongside common global measures of excitability, a goal of this investigation was to offer a relatively thorough picture of differences in motor cortex neurophysiology observed when comparing survivors of stroke and healthy individuals.
We found that survivors of stroke presented with significantly less ICF compared to healthy individuals, which was somewhat surprising as the results of previous studies have shown a similar degree of ICF when comparing survivors of stroke to healthy controls (Liepert, Hamzei and Weiller 2000, Manganotti, Patuzzo, Cortese et al. 2002). In the present study, however, we investigated survivors of stroke in the chronic phase of recovery (e.g., ≥ 9 months post-stroke), while the previous work has studied those in primarily in the acute (and to a lesser extent) sub-acute phase of recovery. Three potential factors may underlie the reduced ICF observed in the survivors of stroke in the chronic phase: (1) damage to or loss of glutamergic intracortical neurons in the motor cortex, (2) damage to or loss of excitatory inputs to glutamergic intracortical neurons, and/or (3) reduced excitability of surviving inputs to glutamergic intracortical neurons. Further, the reduced ICF in survivors of stroke in the chronic phase may contribute to the “cortical portion” of reduced global excitability, i.e., elevated motor threshold and reduced TS-evoked MEP amplitude. One explanation for the stroke versus non-stroke difference in ICF based upon time-since-stroke may be a gradual reduction in motor system facilitation due to persistent disuse of the paretic muscles.
Similar to investigations on acute stroke patients, our results revealed that the survivors of stroke presented with reduced ICI as compared to the healthy controls. In fact, when normalized to MEPs obtained at TS intensity, MEPs obtained during ICI trials of survivors of stroke were, on average, 42% larger than the ICI MEPs of the healthy controls. This finding suggests that the stimulated motor cortex region in the lesioned hemisphere of these stroke survivors possessed a substantial reduction in intracortical inhibition to the extent that this paired-pulse paradigm actually yielded paired-pulse MEPs with nearly identical amplitude as unconditioned (single-pulse) MEPs. In the acute post-stroke stage, reduced ICI has been suggested to be an important disinhibitory, compensatory mechanism important to facilitating cortical plasticity in support of movement recovery (Cicinelli, Pasqualetti, Zaccagnini et al. 2003). In the present study, we found that this reduced ICI persisted into the chronic phase of recovery—suggesting that this is not a short-term phenomenon in survivors of stroke.
The presence of significantly reduced, if not entirely absent, ICI in the survivors of stroke raises an important question: Why is global motor cortex excitability so much greater in healthy individuals? To help answer this question, we also considered the ratio of ICF to ICI MEP amplitudes as a measure of the balance between excitatory and inhibitory inputs to the corticospinal system. In the healthy controls, we found that this ratio of 3.24 was very much in the direction of excitation, as reflects the typical paired-pulse TMS responses, i.e., that longer interstimulus intervals (e.g., 15ms) facilitate MEPs while shorter intervals (e.g., 2ms) inhibit MEPs, producing a > 1 ICF:ICI ratio. In the stroke survivors, however, we found this ratio to be approximately equal to 1. This finding is likely the result of very minimal MEP facilitation during ICF trials and no inhibition of MEPs during ICI trials, such that the amplitudes of MEPs were minimally affected by a conditioning stimulus in the survivors of stroke. Therefore, reduced global motor nervous system excitability in this sample of survivors of stroke appears to be influenced by reduced intracortical facilitation. At the same time, our findings demonstrate that these survivors of stroke also presented with an impaired inhibitory mechanism that would have otherwise not been evident in only assessing measures of excitation.
Limitations
Some limitations in the present study should be considered when examining its results. First, although we used a sizeable control group, we did not age-match these healthy individuals with our stroke survivors. Previously, Peinemann and colleagues (Peinemann, Lehner, Conrad et al. 2001) showed a reduction in intracortical excitability that was mediated by age. However, these findings were not supported by subsequent studies (Smith, Ridding, Higgins et al. 2009), to the extent that several excitability measures do not significantly differ when comparing young and older subjects (Oliviero, Profice, Tonali et al. 2006). We acknowledge that age-related cortical changes may have influenced our comparisons between stroke survivors and healthy controls (despite conflicting reports in the literature), and that future investigations should age-match controls. A second limitation of the study is our lack of control over lesion location. While all stroke survivors had an ischemic stroke, our entrance criteria did not specify lesion location (e.g., cortical or sub-cortical). This may be an important factor to consider given that increased peri-lesional excitability has been demonstrated in cortical strokes (albeit this has mostly been demonstrated in the acute recovery stage; (Bütefisch 2004).
Conclusions
Recovery of paretic arm movement in survivors of stroke is intimately tied to the neurophysiological function of the motor cortex in the lesioned hemisphere. In the present study, we observed that abnormalities in global motor system excitability and intracortical inhibition and excitation persist into the chronic post-stroke stage. Accordingly, the reduced output of the motor system results from a combination of decreased facilitation and inhibition. In the case of our stroke subjects, even significant disinhibition (i.e., elevated ICI MEP amplitude) was insufficient in elevating global and intracortical excitatory measures to more normal levels. To this end, both excitatory and inhibitory mechanisms may be important targets for intervention strategies aimed at normalizing motor cortex functions in survivors of stroke in the chronic stage of recovery.
Acknowledgments
Funding: This research was supported by NIH R21HD053718 and AHA 10GRNT4580008.
References
- Bütefisch CM. Plasticity in the human cerebral cortex: lessons from the normal brain and from stroke. Neuroscientist. 2004;10(2):163–173. doi: 10.1177/1073858403262152. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, et al. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22(1):4–21. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Experimental Brain Research. 2004;154(1):1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. Journal of Neurophysiology. 2000;83(3):1426–1434. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Chen R, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. Journal of Neurophysiology. 1998;80(6):2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, et al. Interhemispheric Asymmetries of Motor Cortex Excitability in the Postacute Stroke Stage A Paired-Pulse Transcranial Magnetic Stimulation Study. Stroke. 2003;34(11):2653–2658. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- Cramer SC, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(6):1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafotakis M, et al. Effects of rTMS on grip force control following subcortical stroke. Experimental neurology. 2008;211(2):407–412. doi: 10.1016/j.expneurol.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, et al. Modulating cortical excitability in acute stroke: a repetitive TMS study. Clinical Neurophysiology. 2008;119(3):715–723. doi: 10.1016/j.clinph.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Edwards D, Fregni F. Modulating the healthy and affected motor cortex with repetitive transcranial magnetic stimulation in stroke: development of new strategies for neurorehabilitation. NeuroRehabilitation. 2008;23(1):3–14. [PubMed] [Google Scholar]
- Hodics T, et al. Functional imaging of intervention effects in stroke motor rehabilitation. Archives of physical medicine and rehabilitation. 2006;87(12):36–42. doi: 10.1016/j.apmr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Kujirai T, et al. Corticocortical inhibition in human motor cortex. The Journal of physiology. 1993;471(1):501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkel G, et al. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabilitation and neural repair. 2008;22(2):111–121. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso I, et al. Effects of coil orientation on the electric field induced by TMS over the hand motor area. Physics in Medicine and Biology. 2014;59(1):203–218. doi: 10.1088/0031-9155/59/1/203. [DOI] [PubMed] [Google Scholar]
- Liepert J, et al. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle & nerve. 2000;23(11):1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Liepert J, et al. Lesion-induced and training-induced brain reorganization. Restorative neurology and neuroscience. 2004;22(3):269–277. [PubMed] [Google Scholar]
- Manganotti P, et al. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clinical Neurophysiology. 2002;113(6):936–943. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- Massie CL, et al. Functional repetitive transcranial magnetic stimulation increases motor cortex excitability in survivors of stroke. Clinical Neurophysiology. 2012 doi: 10.1016/j.clinph.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Mills KR, et al. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85(1):17–21. doi: 10.1016/0168-5597(92)90096-t. [DOI] [PubMed] [Google Scholar]
- Oliviero A, et al. Effects of aging on motor cortex excitability. Neuroscience Research. 2006;55(1):74–77. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Peinemann A, et al. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neuroscience Letters. 2001;313(1-2):33–36. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- Pell GS, et al. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Progress in neurobiology. 2011;93(1):59–98. doi: 10.1016/j.pneurobio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Smith AE, et al. Age-related changes in short-latency motor cortex inhibition. Experimental Brain Research. 2009;198(4):489–500. doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- Talelli P, et al. Arm function after stroke: Neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clinical Neurophysiology. 2006;117(8):1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Ziemann U, et al. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. The Journal of physiology. 1998;511(1):181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


