Abstract
Background
Vein bypass is an essential therapy for patients with advanced peripheral and coronary artery disease despite development of neointimal hyperplasia. We have shown that stimulation of the receptor tyrosine kinase Eph-B4 with its ligand Ephrin-B2 prevents neointimal hyperplasia in murine vein grafts. This study determines whether Eph-B4 in human veins is capable of phosphorylation, activation of downstream signaling pathways, and functional to release nitric oxide and prevent neointimal hyperplasia in vitro.
Methods
Discarded human saphenous veins were taken from the operating room and placed in organ culture without or with Ephrin-B2/Fc (2 μg/ml; 14 days) and the neointima:media ratio was measured in matched veins. Primary human umbilical vein endothelial cells (HUVEC) were treated with Ephrin-B2/Fc (2 μg/ml) and examined with qPCR, Western blot, immunoassays and for release of nitric oxide. Ephrin-B2/Fc (2 μg/ml) was placed in pluronic gel on the adventitia of saphenous veins treated with arterial shear stress for 24 hours in a bioreactor and activated Eph-B4 examined with immunofluorescence.
Results
The baseline intima:media ratio in saphenous vein rings was 0.456 ± 0.097 which increased to 0.726 ± 0.142 in untreated veins after 14 days in organ culture, but only to 0.630 ± 0.132 in veins treated with Ephrin-B2/Fc (p=.017; n=19). Ephrin-B2/Fc stimulated Akt, eNOS and caveolin-1 phosphorylation and NO release (p=0.007) from HUVEC (n=6). Ephrin-B2/Fc delivered to the adventitia stimulated endothelial Eph-B4 phosphorylation after 24 hours of arterial stress in a bioreactor (n=3).
Discussion
Eph-B4 is present and functional in adult human saphenous veins, with intact downstream signaling pathways capable of nitric oxide release and prevention of neointimal hyperplasia in vitro. Adventitial delivery of Ephrin-B2/Fc activates endothelial Eph-B4 in saphenous veins treated with arterial shear stress in vitro. These results suggest that stimulation of Eph-B4 function may be a candidate strategy for translation to human clinical trials designed to inhibit venous neointimal hyperplasia.
Keywords: Eph-B4, Ephrin-B2, neointimal hyperplasia, vein graft adaptation
Despite the recent rise in percutaneous interventions, vein bypass surgery remains an important therapy for patients with advanced peripheral and coronary artery disease, with approximately 400,000 coronary bypasses and 80,000 lower extremity bypasses performed yearly.1,2 Autologous saphenous vein remains the most commonly used and durable conduit for peripheral arterial reconstruction despite a significant portion of saphenous vein grafts eventually failing due to excessive remodeling, particularly neointimal hyperplasia.3,4 Although much research has suggested that smooth muscle cell proliferation and migration are critical steps in the development of neointimal hyperplasia, inhibition of these processes with the E2F transcription factor decoy edifoligide did not improve vein graft patency.4,5 These disappointing failures of large scale, multicenter randomized trials to improve vein graft patency suggest that our knowledge of venous biology, and particularly how veins adapt to and remodel in the arterial environment, remains incomplete.
In recent years a critical role has emerged for the erythropoietin producing hepatocellular carcinoma (Eph) family of tyrosine kinases and their Ephrin ligands in the embryonic determination of venous and arterial identity.6-9 Activation of the Eph-B4 receptor by its specific ligand Ephrin-B2 results in phosphorylation of Eph-B4 as well as downstream targets including caveolin-1, Akt, and eNOS, stimulating nitric oxide (NO) production with effects on cell proliferation and migration in mouse and human cell lines.10-14 Eph-B4 and Ephrin-B2 expression persists in the adult vasculature, although the functional significance of these markers of venous and arterial identity, respectively, is not known.11,15 In particular, roles for Eph B receptors in adult physiological angiogenesis are thought to be limited, with most research showing a role for Eph B receptors in adult pathological angiogenesis including diabetic retinopathy and tumor angiogenesis.16
We have previously demonstrated that Eph-B4 expression is lost, but Ephrin-B2 expression is not gained, during human and adult rat vein graft adaptation, e.g. venous identity is lost but arterial identity is not gained in vein grafts.17 We subsequently showed that Eph-B4 signaling can be stimulated via its ligand Ephrin-B2 to reduce intimal thickening in a murine vein graft model.13 Since these findings suggest that Eph-B4 is functional in adult murine veins, we hypothesized that Eph-B4 receptors are also functional in human veins. If this hypothesis proves to be true, then stimulation of Eph-B4 receptors might be an alternative new strategy to improve the outcome of venous bypass grafts.
Methods
Saphenous Vein Organ Culture
Saphenous veins that were harvested but not needed for clinical use were obtained as previously described; no patient-specific information was collected and no informed consent was obtained, in accordance with the Yale Human Investigation Committee approval.17 Veins were immediately cut into 5-10 mm cross sectional rings and placed into culture as previously described.18,19 Baseline samples were fixed in formalin. Additional samples from adjacent sections of the vein were placed in 12 well tissue culture dishes with Roswell Park Memorial Institute medium (RPMI 1640; Gibco) supplemented with 30% fetal bovine serum, 1% L-glutamine, 1% penicillin/streptomycin for 2 weeks at 37°C. Vein rings were supplemented with recombinant human ephrin-B2/Fc chimera (0 or 2 μg/ml; R&D Systems, Minneapolis, MN); all control and treated veins were matched from the same specimen, and coded to treat in blinded fashion. The media was changed every 2-3 days.
After 14 days samples were fixed in formalin or frozen in optimal cutting temperature compound (Tissue-Tek Sakura Finetek, Torrance, CA) for further analysis. Samples were embedded, sectioned, and stained with the Verhoeff-Van Gieson (VVG) elastin stain, and digital pictures were taken. The intima and media areas were measured by computer morphometry in blinded fashion, in sections stained for elastin, using ImageJ software (NIH, Bethesda, MD); the vessel was divided into 4 quadrants, and the areas of each quadrant was measured, and a mean of the 4 measurements was calculated for each vein segment. Neointima was defined as the area on the luminal side of the internal elastic lamina, when present, and included the area of disordered smooth muscle cell proliferation luminal to the ordered, linear smooth muscle cell layer. The neointima area was divided by the area of the media to calculate the neointima:media ratio.
Proliferation within the intima and media of saphenous vein rings was measured by counting the proportion of cells staining positively for Ki-67, in vein rings after 3 days in organ culture. Apoptosis within whole saphenous vein rings was assessed by Western blot analysis of cleaved caspase-3 expression in vein ring lysates after 3 days in organ culture; densitometry was normalized to GAPDH expression. All samples were examined in paired fashion, e.g. both untreated and treated vein rings were derived from the same specimen.
Human umbilical vein endothelial cell (HUVEC) culture
Primary HUVEC cells were obtained from the VBT core facility at Yale University (New Haven, CT) and were cultured in Endothelial Basal Medium supplemented with Bulletkit growth factors (Lonza, Basel, Switzerland), 15% fetal bovine serum, and 1% penicillin/streptomycin. Cells were grown on either glass coverslips or 6 well plates coated with 0.1% gelatin. Cells were assayed between passages 2-5. Prior to stimulation with Ephrin-B2/Fc, growth medium was removed and cells were placed in serum free starvation media for at least 3 hours. To measure proliferation, equal numbers of cells were placed in 6-well plates, allowed to adhere for 24 hours, and placed in media with 0.05% fetal bovine serum without or with Ephrin-B2/Fc (2 ug/ml) for 48 hours; media with 4% fetal bovine serum was used as a positive control. After 48 hours, cells were trypsinized and counted using a hemocytometer.
Western blot and immunoprecipitation
Ephrin-B2/Fc was pre-clustered with human IgG/Fc (Sigma Aldrich, St. Louis, MO) at a 5:1 molar ratio for 20 minutes at 37°C prior to use. Following stimulation, cells were washed with ice cold PBS and then lysed using a RIPA buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO) and phosphatase inhibitors (Millipore, Billerica, MA). Protein concentrations were assessed using a colorimetric assay (Bio-rad, Hercules, CA) and equal amounts of protein were run on a SDS-PAGE gel and then transferred to a PVDF membrane. Following blocking with 2% bovine serum albumin, membranes were probed overnight with primary antibodies, listed below. Membranes were developed using Supersignal West Pico kit (Thermo Scientific, Waltham, MA). Immunoprecipitation was performed by incubating at least 200 μg of protein with a phospho-tyrosine antibody-bound sepharose bead overnight and then Western blot conducted as above.
Immunofluorescence microscopy
HUVEC cells were plated on glass coverslips, serum starved, and treated. They were fixed in 10% neutral buffered formalin for 15 minutes, washed, and then treated with Triton-X100 and blocked with goat serum prior to exposure to primary antibody overnight, and afterwards treated with alexa-flouro 488, 568, and 647 secondary antibodies and mounted with a SlowFade Gold anti-fade reagent with DAPI mounting media (Life Technologies, Carlsbad, CA). Samples were photographed using an AxioImager A1 (Carl Zeiss Inc, Oberkochen, Germany). Vein samples undergoing immunofluorescence were fixed with formalin and then embedded in paraffin and cut into 5 μm sections and placed on slides. Antigen retrieval was conducted by boiling with 10mM citric acid with 0.05 Tween (pH 6) for 20 minutes. Blocking was performed with goat serum and primary antibody was applied overnight. Secondary antibodies were applied as above. Autofluoresence Eliminator Reagent (Millipore, Billerica, MA) was used and then sections were mounted in DAPI containing mounting media and imaged as above.
Antibodies
Primary antibodies to the following antigens, and their working dilutions, were obtained as follows: Eph-B4 #64820 (1:500 WB; 1:50 IF), caveolin-1 #17052 (1:500 WB; 1:50 IF), Ki-67 #15580 were obtained from Abcam (Cambridge, England). Antibodies to phosphorylated AKT ser 473 #9271 (1:1500), phosphorylated eNOS ser 1177 #9571 (1:500), GAPDH #2118 (1:1000), phosphorylated-tyrosine conjugated to sepharose beads #9419 (1:100) and cleaved caspase-3 #9661 (1:500) were obtained from Cell Signaling Technologies (Danvers, Ma). Antibody to phospho-tyrosine #05-1050 (1:100) was obtained from Millipore (Billerica, MA). Antibody to total eNOS #BD610296 (1:1000) and phosphorylated caveolin-1 (tyr14) #BD611338 (1:500) were obtained from BD Biosciences (Franklin Lakes, NJ).
Nitric oxide release
HUVEC cells were serum starved and cells incubated with starvation media supplemented with sepiapterin (10 uM). After 30 minutes, 100 ul of media was withdrawn to determine basal secretion rates and 100ul of starvation media were added containing pre-clustered ephrin-B2/Fc. After 30 minutes 100 ul of media was again withdrawn. NO2− in the media was converted to NO with sodium iodide/glacial acetic acid and then concentrations were measured via chemiluminescence after reaction with ozone as previously described.13,20
Pluronic gel application and bioreactor
Ephrin-B2/Fc was dissolved in a 30% pluronic gel (Sigma-Aldrich, St Louis, MO). Saphenous vein was placed in a custom bioreactor fabricated at the Yale Scientific Glass Shop (New Haven, CT) with the vein section attached to a glass connector as previously described.21 Pluronic gel was allowed to harden on the adventitia of the vein for 20 minutes prior to initiation of flow and submersion in extraluminal fluid. A digital programmable roller-pump (Cole Parmer, Vernon Hills, IL) was used to push media through the system (20 dyne/cm2) which was placed in a 37°C incubator. Intraluminal fluid consisted of Endothelial Growth Medium (EGM-2 Lonza, Basel, Switzerland) thickened with Xanthum Gum (Sigma-Aldrich, St Louis, MO) to a viscosity of 3.8 cP; extraluminal fluid consisted of DMEM supplemented with 30% fetal bovine serum. Specimens were harvested avoiding the approximately 1 cm from each attachment site to avoid areas of turbulent flow at this transition point.
Quantitative reverse transcriptase polymerase chain reaction
RNA was isolated from vein rings by taking OCT embedded samples and collecting approximately 50 10 μm thick sections cut on a microtome. These sections were washed twice with cold, RNAse, DNAse free water to dissolve OCT. The veins were then homogenized by repeatedly aspirating the lysate through a 22-gauge needle in beta-mercaptoethanol containing lysis buffer (RNeasy Mini Kit; Qiagen, Hilden, Germany). The remainder of the isolation and purification steps were carried as specified in the manufacturer’s instructions. RNA isolation from cell culture was also carried out according to manufacturer’s instructions using the RNeasy Mini Kit. Reverse transcription was performed using the SuperScript III First-Strand Synthesis Supermix (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was performed using SYBR Green Supermix and the iQ5 Real-Time PCR Detection system (Bio-Rad Laboratories, Hercules, CA). Primers for specific genes are presented in Table 1. Primers were designed using Primer3 software.22 Oligonucleotides were synthesized by the Keck Oligo Synthesis Facility (New Haven, CT). Correct target size amplification and exclusion of nonspecific amplification was confirmed by performing electrophoresis on amplification products on 2% agarose gel as well as performing melt curve analysis. Reactions were normalized to GAPDH as a housekeeping gene.
Table 1.
Primers used in qPCR analysis.
| Gene | Sequence | |
|---|---|---|
| GAPDH | 5′->3′ | GAGAAGGCTGGGGCTCATTT |
| 3′->5′ | AGTGATGGCATGGACTGTGG | |
| Eph-B4 | 5′->3′ | ATGCCCGTCATGATTCTCAC |
| 3′->5′ | GGAAAGGCCAAAGTCAGACA | |
| Ephrin-B2 | 5′->3′ | AACTGTGCCAAACCAGACCA |
| 3′->5′ | GCAGAACTTGCATCTTGTCCA | |
| Osteopontin (OPN) | 5′->3′ | CTGCCAGCAACCGAAGTTTT |
| 3′->5′ | TCCTCGCTTTCCATGTGTGA | |
| Collagen 1 (Col1A1) | 5′->3′ | ACCTGGTCCACAAGGTTTC |
| 3′->5′ | ACCATCCAAACCACTGAAGC | |
| Matrix metalloproteinase 2 (MMP2) | 5′->3′ | CAGGGCACCTCCTACAACAG |
| 3′->5′ | ACTTGTTGCCCAGGAAAGTG |
Statistics
Statistical analyses were performed using GraphPad version 6.0a for MAC OS X, GraphPad Software (La Jolla, CA). Means are reported as arithmetic means with standard errors of the means following. Student’s t-test and paired t-tests were used to compare mean values between groups. A p-value of less than 0.05 was considered significant.
Results
Ephrin-B2/Fc-stimulated human saphenous veins have reduced neointimal thickness
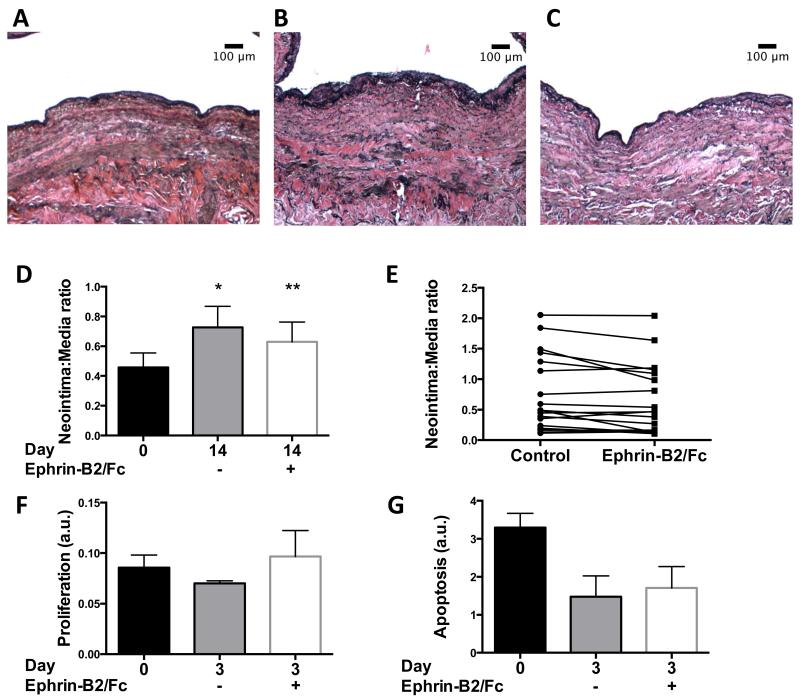
Ephrin-B2/Fc treatment of mouse vein grafts prevents neointimal thickening in vivo.13 To determine if Ephrin-B2/Fc treatment may be functional in human vein grafts, human saphenous veins were obtained freshly from the operating room and segmental rings were immediately examined or placed in organ culture with Ephrin-B2/Fc (2 μg/ml) for 14 days; each treatment group was compared to control vein segments from the same donor. Histological examination showed reduced neointimal thickness in human veins treated with Ephrin-B2/Fc for 14 days compared to control veins after 14 days (Figures 1A-C). At baseline, the mean intima:media ratio of saphenous veins was 0.456 ± 0.097 (n=19); during the 14 days in organ culture, the neointima:media ratio increased in control veins to 0.726 ± 0.142 (78% increase; n=19) but only to 0.630 ± 0.132 (53% increase; n=19) in veins treated with Ephrin-B2/Fc (p=.017; Figure 1D). As expected, examination of individual veins showed variability in the response to treatment, with 13 of 19 (68%) veins responding to Ephrin-B2/Fc treatment (e.g. >0% response) and 6 veins not responding compared to control samples (Figure 1E); the mean response in the 13 veins was 47% reduction, with the 6 non-responders having a 29% increase (p=0.002, t-test). Interestingly, veins that were thickened at baseline with an I:M ratio >0.4 all responded to Ephrin-B2/Fc (6/6, 100% response; mean I:M ratio = 0.19 ± 0.05), whereas veins with an I:M ratio <0.4 at baseline had marginal response (7/13, 54% response; mean I:M ratio = 0.24 ± 0.04; p=0.47, t-test). The reduction in neointimal thickness in saphenous veins treated with Ephrin-B2/Fc was neither due to diminished proliferation (Figure 1F) nor to increased apoptosis (Figure 1G) in treated veins.
Figure 1.
Ephrin-B2/Fc reduces neointimal thickness in human saphenous vein organ culture. A-C) Representative photomicrographs of (A) saphenous vein at day 0, or the same vein after 14 days in organ culture and either (B) control or (C) treated with Ephrin-B2/Fc (2 μg/ml); elastin von Gieson stain. Scale bar, 100 μm. D) Bar graph shows mean neointima:media ratio for vein rings, day 0 or 14, matched samples treated without or with Ephrin-B2/Fc. n=19. *, p=0.001; **, p=0.048 vs. day 0; **, p=0.017 vs. day 14 untreated; paired t-test. E) Graph shows the neointima:media ratio in matched vein segments after 14 days. F) Bar graph shows proportion of Ki-67 positive nuclei in vein rings after 3 days in organ culture. p=0.396, day 3 untreated vs. day 0; p=0.798, day 3 treated vs. day 0; p=0.384, day 3 untreated vs. treated; paired t-test. n=3. G) Bar graph shows densitometry of Western blot analysis of cleaved caspase-3 expression, normalized to GAPDH, in vein rings, day 0 or 3. p=0.079, day 3 untreated vs. day 0; p=0.055, day 3 treated vs. day 0; p=0.827, day 3 untreated vs. treated; paired t-test. n=3. AU, arbitrary units.
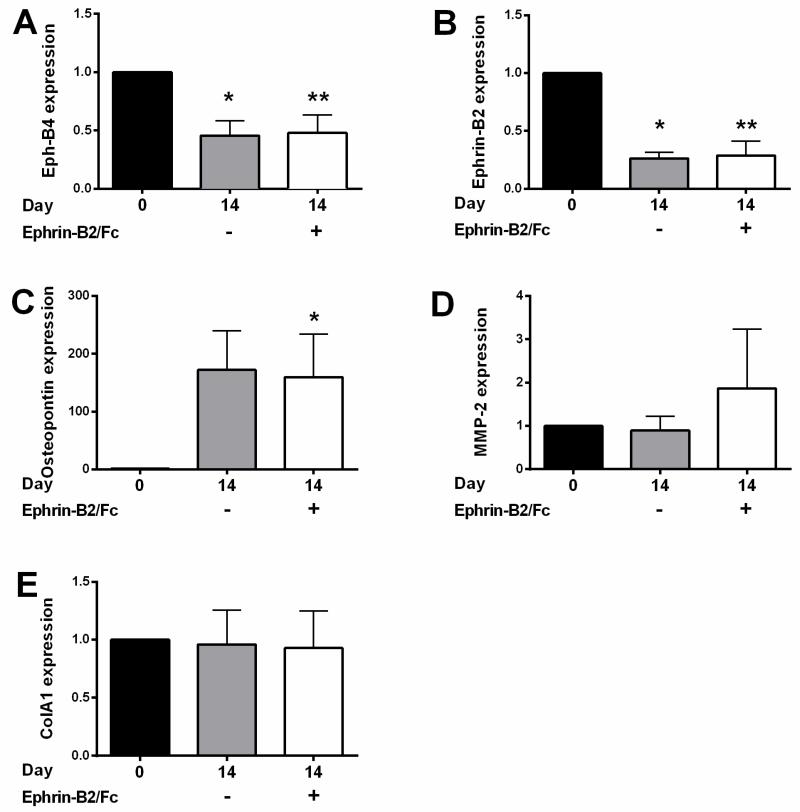
Since vein graft adaptation is associated with reduced Eph-B4 and Ephrin-B2 expression in mice, as well as increased osteopontin expression,13 we determined whether similar expression patterns were present in human saphenous vein rings in organ culture. Both Eph-B4 and Ephrin-B2 mRNA expression was reduced (Figures 2A, 2B), and osteopontin expression was increased (Figure 2C) after 14 days in vitro. However, there was no change in expression in vein rings treated with Ephrin-B2/Fc (Figures 2A-C). We have previously shown that expression of both collagen, a key structural component of the extracellular matrix, as well as MMP2, a key regulator of matrix metabolism, are increased during venous adaptation to the arterial environment.23 There was no difference between Col1A1 and MMP2 expression in vein rings without or with Ephrin-B2/Fc (Figures 2D, 2E).
Figure 2.
mRNA expression in human saphenous vein organ culture. A) Bar graph shows relative number of Eph-B4 mRNA transcripts at day 0 or 14, without or with Ephrin-B2/Fc, in matched samples. n=7. *, p=0.005; **, p=0.015 vs. day 0; **, p=0.599 vs. day 14 untreated. B) Bar graph shows relative number of Ephrin-B2 mRNA transcripts at day 0 or 14, without or with Ephrin-B2/Fc in matched samples. n=7. *, p<0.001; **, p=0.001 vs. day 0. p=0.740 vs. day 14 untreated. C) Bar graph shows relative number of osteopontin mRNA transcripts at day 0 or 14, without or with Ephrin-B2/Fc in matched samples. n=7. *, p=0.044. p=0.078 vs. day 0; p=0.746 vs. day 14 untreated. D) Bar graph shows relative number of MMP-2 mRNA transcripts at day 0 or 14, without or with Ephrin-B2/Fc in matched samples. n=7. p=0.763, day 0 vs. day 14 untreated; p=0.547, day 0 vs. day 14 treated; p=0.391, day 14 untreated vs. treated. E) Bar graph shows relative number of Col1A1 mRNA transcripts at day 0 or 14, without or with Ephrin-B2/Fc in matched samples. n=7. p=0.895, day 0 vs. day 14 untreated; p=0.833, day 0 vs. day 14 treated; p=0.906, day 14 untreated vs. treated. Paired t-tests were used for all comparisons. All expression is in arbitrary units relative to the control.
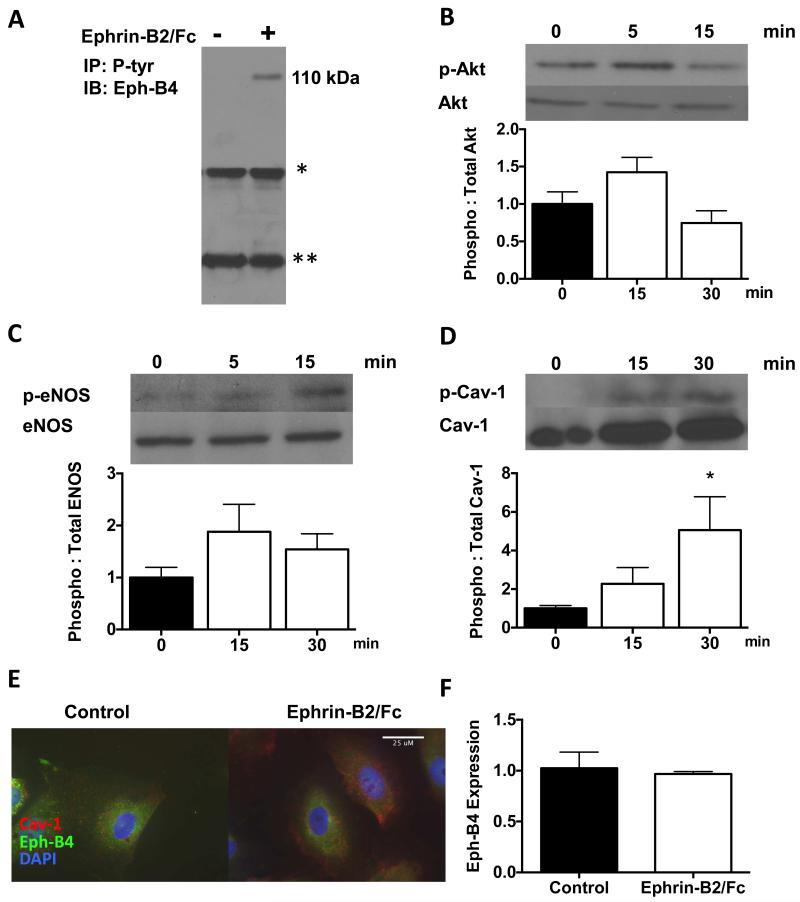
Ephrin-B2/Fc stimulates Eph-B4 phosphorylation and downstream signaling in human endothelial cells
Since Ephrin-B2/Fc stimulates Eph-B4 phosphorylation in mouse endothelial cells,13 we determined whether Ephrin-B2/Fc stimulates Eph-B4 phosphorylation in human endothelial cells. Ephrin-B2/Fc stimulated Eph-B4 phosphorylation rapidly in HUVEC (Figure 3A). Ephrin-B2/Fc also stimulated phosphorylation of proteins downstream of Eph-B4, Akt (Figure 3B), eNOS (Figure 3C), and caveolin-1 (Figure 3D). Ephrin-B2/Fc also induced co-localization of Eph-B4 and caveolin-1 in HUVEC (Figure 3E), similar to its function in mouse endothelial cells.13 As expected, there was no increase in Eph-B4 expression in response to Ephrin-B2/Fc treatment (Figure 3F).
Figure 3.
Ephrin-B2/Fc stimulates Eph-B4 phosphorylation and downstream signaling in HUVEC. A) Immunoprecipitation analysis of tyrosine-phosphorylated Eph-B4 (110 kDa) in HUVEC treated without or with Ephrin-B2/Fc. *, IgG heavy chain; **, IgG light chain. IP, immunoprecipitation; IB, immunoblot. B) Phosphorylated (ser473) and total Akt expression in HUVEC treated with Ephrin-B2/Fc (0, 5, 15 minutes), representative Western blot analysis and bar graph showing relative densitometry (n=6; p=0.16, 0 vs. 15 min; unpaired t-test). C) Phosphorylated (ser1177) and total eNOS expression in HUVEC treated with Ephrin-B2/Fc (0, 5, 15 minutes), representative Western blot analysis and bar graph showing relative densitometry (n=6; p=0.15, 0 vs. 15 min; unpaired t-test). D) Phosphorylated (tyr14) and total caveolin-1 expression in HUVEC treated with Ephrin-B2/Fc (0, 15, 30 minutes), representative Western blot analysis and bar graph showing relative densitometry (n=6; *, p=0.04, 0 vs. 30 min; unpaired t-test). E) Representative immunofluorescence images of HUVEC without (left panel) and with (right panel) Ephrin-B2/Fc (2 μg/ml), 5 min, n=3. Red, caveolin-1; Green, Eph-B4; Blue, DAPI. White arrow shows co-localization of Eph-B4 and Cav-1. Scale bar, 25 μm F) Bar graph shows relative Eph-B4 mRNA transcript numbers, normalized to GAPDH, in HUVEC treated without or with Ephrin-B2/Fc (2 μg/ml) for 12hrs (n=3). P=0.745, unpaired t-test.
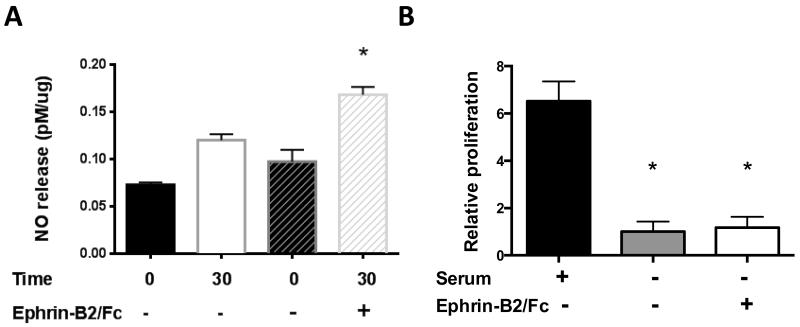
Since Ephrin-B2/Fc stimulates eNOS phosphorylation, we determined whether this phosphorylation induced a functional change, e.g. release of NO. During 30 minutes of culture, control HUVEC released 0.047 ± 0.005 pM of NO per ug of protein (n=3), whereas Ephrin-B2/Fc treated HUVEC released 0.071 ± 0.014 pM of NO per ug of protein (n=6; p=0.007; Figure 4A). Despite stimulation of Eph-B4, Akt, eNOS, and Cav-1 phosphorylation, Ephrin-B2/Fc did not stimulate HUVEC proliferation (Figure 4B).
Figure 4.
Ephrin-B2/Fc stimulates NO release but not proliferation in HUVEC. A) Bar graph showing NO release in HUVEC treated without or with Ephrin-B2/Fc (2 μg/ml, 30 min), normalized to total protein (n=6; *, p=0.007 vs. 30 min untreated, unpaired t-test). Two bars for time 0 are shown, matched to each of the two time 30 runs. B) Bar graph shows relative cell numbers in HUVEC stimulated with serum or Ephrin-B2/Fc (72 hr). n=3. * p<.05, unpaired t-test.
Adventitial delivery of Ephrin-B2/Fc stimulates endothelial Eph-B4 phosphorylation
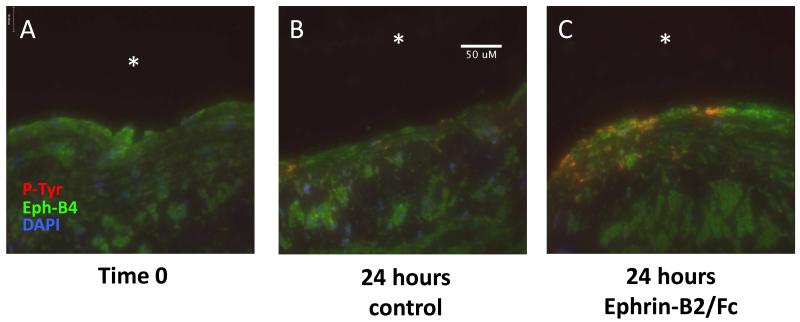
Since our data shows that Ephrin-B2/Fc stimulates Eph-B4 phosphorylation in human endothelial cells, in order to determine the translational relevance of using Ephrin-B2/Fc to stimulate Eph-B4 in human veins, we examined whether adventitial delivery of Ephrin-B2/Fc would induce phosphorylation of Eph-B4 in endothelial cells of saphenous veins. We have previously characterized a bioreactor that controls delivery of shear stress and pressure to veins, including arterial magnitudes of shear stress.21 Segments of human saphenous vein were placed in the bioreactor and exposed to arterial shear stress. Prior to initiation of flow, pluronic gel without or with Ephrin-B2/Fc, was placed on the adventitial surface of the vein. After 24 hours of exposure to arterial stress, veins treated with adventitial Ephrin-B2/Fc showed co-localization of Eph-B4 and phospho-tyrosine in the endothelium that was not present either at time 0 or in the untreated vein (Figure 5). These results suggest that treatment of human saphenous veins with Ephrin-B2/Fc stimulates Eph-B4 phosphorylation, even if delivered via the venous adventitia and not directly to the endothelium, and thus of translational interest for human patients.
Figure 5.
Adventitial delivery of Ephrin-B2/Fc stimulates endothelial Eph-B4 phosphorylation. Immunofluorescence of human saphenous vein treated on its adventitial surface with pluronic gel without or with Ephrin-B2/Fc (2 μg/ml) and placed in a bioreactor; analysis of matched samples. (A) Saphenous vein prior to initiation of arterial flow. (B) Saphenous vein treated with pluronic gel alone (24 hr). (C) Saphenous vein treated with Ephrin-B2/Fc (24 hr). Red, phosphotyrosine; Green, Eph-B4; Blue, DAPI. White arrows show co-localization of Eph-B4 and phosphotyrosine in the endothelium. White asterisks, vessel lumen. N=3. Scale bar, 50 μm.
Discussion
We show that stimulation of Eph-B4 receptors with the ligand Ephrin-B2/Fc is associated with less neointima in human saphenous vein rings (Figure 1). Human saphenous vein rings show reduced Eph-B4 expression and increased osteopontin expression (Figure 2), similar to the changes that occur during vein graft adaptation in vivo, at least in animal models.13,24
Stimulation of Eph-B4 in human endothelial cells induces phosphorylation of Eph-B4 and caveolin-1 as well as induces Eph-B4-caveolin-1 colocalization (Figure 3), similar to previous results in animal and human cells.12,13,25 Eph-B4 activation also induces release of NO (Figure 4), another functional effect. Finally we show that adventitial delivery of a single dose of Ephrin-B2/Fc followed by 24 hours of arterial shear stress shows increased endothelial Eph-B4 phosphorylation (Figure 5). These results show that Eph-B4-mediated reduction of vein graft thickness previously shown in mice may be translatable to human veins.
We use the saphenous vein ring assay to show that stimulation of Eph-B4 with Ephrin-B2/Fc prevents wall thickening (Figures 1A-D). A strength of our study is the use of saphenous veins from patients having cardiac bypass procedures, and thus these veins would be similar to those being used in patients having cardiac or peripheral vein bypasses. Animal models show the utility of Ephrin-B2/Fc treatment in preventing vein graft thickening,13 and our study shows that the animal data translates to relevant human tissue. Although the effect of Ephrin-B2/Fc treatment in the saphenous vein ring in vitro assay is modest, with approximately 32% reduction of neointima, this is not much less than the effects of other inhibitors using this model;19 nevertheless, stimulation of Eph-B4 in the vein graft in vivo model shows approximately 60% reduced wall thickness, an effect size of importance.13 Although the saphenous vein organ culture model does not include the effects of arterial shear stress, host invading cells, and the geometry of an anastomosis,26-29 this model is in common use as it is one of the very few options to assay intact human vessels.30,31 The reduction in neointima with Ephrin-B2/Fc treatment was not universal, with some variability of effect (Figure 1E), as is often typical with human samples. Some of this variability may be explained by the genetic differences between humans as well as differences in degree of trauma from surgical harvest, ischemic time, and vein quality that may alter venous relaxation, reactive oxygen species generation, and histologic appearance.18,32-34 Nonetheless, the majority of samples showed some effect of Ephrin-B2/Fc on neointimal thickness (Figure 1D). Interestingly, all saphenous veins that were thick at baseline, with an I:M ratio > 0.4 responded to Ephrin-B2/Fc treatment, suggesting that patients in need of vein graft placement but who have diseased veins may still be candidates for therapy based on stimulation of the Eph-B4 receptor, e.g. this pathway is still active in diseased human saphenous veins.
Another advantage of using the human saphenous vein is the ability to test it in a bioreactor that delivers arterial shear stress, e.g. mimics the vein graft after implantation into the arterial environment.21 Using the bioreactor we show that a single dose of Ephrin-B2/Fc applied to the adventitia stimulates endothelial Eph-B4 phosphorylation that persists 24 hours (Figure 5). Adventitial drug delivery is a common strategy, and may be useful in the operating room.35-37 A strategy of vein immersion in drug-containing solution, such as used in the PREVENT trials, could increase the delivery above that delivered by a purely adventitial-based strategy, and thus may be even more effective. This data also confirms our previous finding in mouse vein grafts that stimulation of the Eph-B4 pathway prevents down-regulation of Eph-B4 expression, suggesting a novel feed-forward mechanism of Eph-B4 expression in human veins grafts as well.13
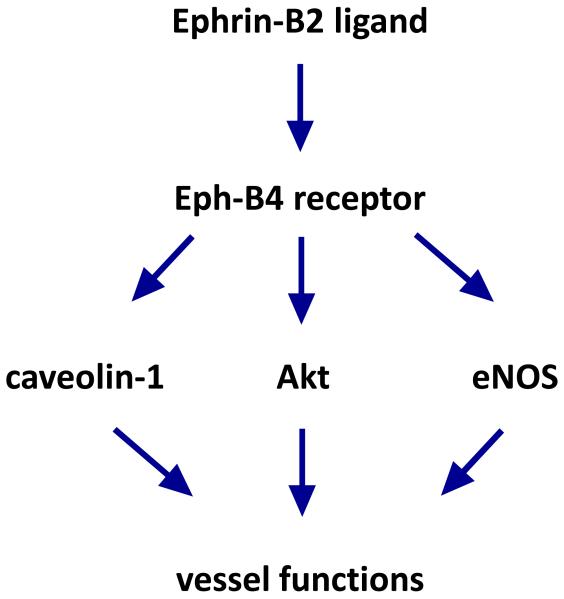
The mechanisms by which Eph-B4 prevents neointimal hyperplasia are still not well understood; since Eph-B4 is ordinarily down-regulated during vein graft adaptation, therapy directed towards downstream effectors may be more effective clinically. Eph-B4 activation leads to Akt phosphorylation and NO release that may be one pathway regulating wall thickness (Figure 6).12,38 Eph-B4 activation also stimulates caveolin-1 phosphorylation and co-localization of with Eph-B4 (Figure 6).12,13 Caveolae are small vesicles near the cell membrane that play important roles in signal transduction.39 Loss of caveolin-1 eliminates the Ephrin-B2/Fc-induced reduction in wall thickness in mouse vein grafts, showing the critical importance of caveolin-1 in Eph-B4 downstream signal transduction.13 Interestingly endothelial cells derived from caveolin-1-knockout mice have increased nitric oxide production,40 suggesting that Eph-B4 may reduce intimal hyperplasia via both Akt and caveolin-1 regulation of nitric oxide. As additional information regarding the signaling pathways downstream from Eph-B4 becomes available, it is likely that more specific targets for therapy will be identified.
Figure 6.
Schematic of Eph-B4 signal transduction. Ephrin-B2 ligand (Ephrin-B2/Fc in the experiments) binds to the Eph-B4 receptor, activating it by dimerization and phosphorylation. Activated Eph-B4 stimulates additional downstream signaling pathways, including caveolin-1, Akt, and eNOS signaling among others, leading to additional functions in the blood vessel.
Conclusion
We show that Eph-B4 receptors are functional in the human saphenous vein, and that they can be stimulated with Ephrin-B2/Fc applied to the adventitial surface. It is possible that this strategy will be translatable to reduce vein graft failure, and may be generally applicable to venous adaptation. We believe that stimulation of Eph-B4 signaling will be a viable strategy to alter human venous remodeling.
Clinical Relevance.
Neointimal hyperplasia remains the Achilles’ heel of vein grafts, with the disappointing failure of the PREVENT trials showing our incomplete knowledge of endothelial cell biology. Promising results in mice suggest that stimulation of Eph-B4, the embryonic determinant of veins in undifferentiated cells, may prevent neointimal hyperplasia in adult vein grafts. This study shows that human veins also have functional Eph-B4 receptors and that stimulation of these Eph-B4 receptors maintains activation even under arterial flow conditions. These results suggest that stimulation of Eph-B4 function may be a worthwhile treatment to test in human vein grafts.
Acknowledgements
This work was supported by the National Institutes of Health R01-HL095498 (AD), the Doris Duke Foundation Clinical Fellowship (DW), the Yale Department of Surgery Ohse award (KY, CP), as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT (KY, AD). The authors appreciate the help and support of Kota Yamamoto, Michael Hall, Roland Assi, and Kirstyn Brownson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Survey CNNHD . National Hospital Discharge Survey: 2010 Table, Procedures by Selected Patient Characteristics - Number by Procedure Category and Age. 2010. [Google Scholar]
- 2.Bhasin M, Huang Z, Pradhan-Nabzdyk L, Malek JY, LoGerfo PJ, Contreras M, Guthrie P, Csizmadia E, Andersen N, Kocher O, Ferran C, LoGerfo FW. Temporal Network Based Analysis of Cell Specific Vein Graft Transcriptome Defines Key Pathways and Hub Genes in Implantation Injury. PloS one. 2012;7:e39123. doi: 10.1371/journal.pone.0039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary Bypass Graft Fate and Patient Outcome: Angiographic Follow-up of 5,065 Grafts Related to Survival and Reoperation in 1,388 Patients During 25 Years. Journal of the American College of Cardiology. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 4.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS. Results of Prevent Iii: A Multicenter, Randomized Trial of Edifoligide for the Prevention of Vein Graft Failure in Lower Extremity Bypass Surgery. Journal of vascular surgery. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr., Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT. Efficacy and Safety of Edifoligide, an E2f Transcription Factor Decoy, for Prevention of Vein Graft Failure Following Coronary Artery Bypass Graft Surgery: Prevent Iv: A Randomized Controlled Trial. JAMA: the journal of the American Medical Association. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 6.Torres-Vazquez J KM, Weinstein BM. Molecular Distinction between Arteries and Veins. Cell Tissue Res. 2003;314:43–59. doi: 10.1007/s00441-003-0771-8. [DOI] [PubMed] [Google Scholar]
- 7.Adams R, Wilknison G, Weiss C, Diella F, Gale N, Deustsch U, Risau W, Klein R. Roles of Eprhinb Ligands and Ephb Receptors in Cardiovascular Development. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerety SS WH, Chen ZF, Anderson DJ. Symmetrical Mutant Phenotypes of the Receptor Ephb4 and Its Specific Transmembrane Ligand Ephrin-B2 in Cardiovascular Development. Mol Cell. 1999 Sep;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang Hu CZ, Anderson DJ. Molecular Distinction and Angiogenic Interaction between Embryonic Arteries and Vein Revealed by Ephrin-B2 and Its Receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 10.Jadlowiec CC, Feigel A, Yang C, Feinstein AJ, Kim ST, Collins MJ, Kondo Y, Muto A, Dardik A. Reduced Adult Endothelial Cell Ephb4 Function Promotes Venous Remodeling. American journal of physiology. Cell physiology. 2013;304:C627–635. doi: 10.1152/ajpcell.00333.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C, Guo Y, Jadlowiec CC, Li X, Lv W, Model LS, Collins MJ, Kondo Y, Muto A, Shu C, Dardik A. Vascular Endothelial Growth Factor-a Inhibits Ephb4 and Stimulates Delta-Like Ligand 4 Expression in Adult Endothelial Cells. The Journal of surgical research. 2013;183:478–486. doi: 10.1016/j.jss.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinle JJ MC, Forough R, Wu G, Wu MH, Granger HJ. Eph B4 Receptor Signaling Mediates Endothelial Cell Migration and Proliferation Via the Phosphatidylinositol 3-Kinase Pathway. The Journal of biological chemistry. 2002;277:43830–43835. doi: 10.1074/jbc.M207221200. [DOI] [PubMed] [Google Scholar]
- 13.Muto A, Yi Y, Harrison KD, Dávalos A, Fancher TT, Ziegler KR, Feigel A, Kondo Y, Nishibe T, Sessa WC, Dardik A. Ephb4 Prevents Venous Adaptive Remodeling in the Adult Arterial Environment. The Journal of experimental medicine. 2011;208:561–567. doi: 10.1084/jem.20101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao Z, Carrasco R, Kinneer K, Sabol D, Jallal B, Coats S, Tice D. Ephb4 Promotes or Suppresses Ras/Mek/Erk Pathway in a Context-Dependent Manner: Implications for Ephb4 as a Cancer Target. Cancer Biol Ther. 2012;13:630–637. doi: 10.4161/cbt.20080. [DOI] [PubMed] [Google Scholar]
- 15.Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, GD Y. Ephrin-B2 Selectively Marks Arterial Vessels and Neovascularization Sites in the Adult, with Expression in Both Endothelial and Smooth-Muscle Cells. Dev Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 16.Salvucci O, Tosato G. Essential Roles of Ephb Receptors and Ephrinb Ligands in Endothelial Cell Function and Angiogenesis. Advances in cancer research. 2012;114:21–57. doi: 10.1016/B978-0-12-386503-8.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Frald TN, Westvik TS, Frattini JC, Breuer CK, Cha CH, Nishibe T, Tellides G, Sessa WC, Dardik A. Venous Identity Is Lost but Arterial Identity Is Not Gained During Vein Graft Adaptation. Arterioscler. Thromb. Vasc. Biol. 2007;27:1562–1571. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- 18.Li F, Sexton K, Hocking K, Osgood M, Eagle S, Cheung-Flynn J, Brophy C, Komalavilas P. Intimal Thickness Associated with Endothelial Dysfunction in Human Vein Grafts. The Journal of surgical research. 2013;180:55–62. doi: 10.1016/j.jss.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muto A, Panitch A, Kim N, Park K, Komalavilas P, Brophy CM, Dardik A. Inhibition of Mitogen Activated Protein Kinase Activated Protein Kinase Ii with Mmi-0100 Reduces Intimal Hyperplasia Ex Vivo and in Vivo. Vascular pharmacology. 2012;56:47–55. doi: 10.1016/j.vph.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, Iwakiri Y, Sessa WC. Targeting of Endothelial Nitric-Oxide Synthase to the Cytoplasmic Face of the Golgi Complex or Plasma Membrane Regulates Akt- Versus Calcium-Dependent Mechanisms for Nitric Oxide Release. The Journal of biological chemistry. 2004;279:30349–30357. doi: 10.1074/jbc.M402155200. [DOI] [PubMed] [Google Scholar]
- 21.Model L, Hall M, Wong F, Muto A, Kondo Y, Ziegler K, Feigel A, Quint C, Nklason L, Dardik A. Arterial Shear Stress Reduces Eph-B4 Expression in Adult Human Veins. Yale J Biol Med. In Press. [PMC free article] [PubMed] [Google Scholar]
- 22.Steve Rozen HJS. Primer3. 1998 Code Available at Http://Www-Genome.Wi.Mit.Edu/Genome_Software/Other/Primer3.Html.
- 23.Hall MR, Yamamoto K, Protack CD, Tsuneki M, Kuwahara G, Assi R, Brownson KE, Bai H, Madri J, Dardik A. Temporal Regulation of Venous Extracellular Matrix Components During Arteriovenous Fistula Maturation. J Vasc Access. 2014 doi: 10.5301/jva.5000290. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abeles D, Kwei S, Stavrakis G, Zhang Y, Wang ET, Garcia-Cardena G. Gene Expression Changes Evoked in a Venous Segment Exposed to Arterial Flow. Journal of vascular surgery. 2006;44:863–870. doi: 10.1016/j.jvs.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 25.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of Nitric Oxide Synthase in Endothelial Cells by Akt-Dependent Phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 26.Osterberg K, Mattsson E. Intimal Hyperplasia in Mouse Vein Grafts Is Regulated by Flow. J Vasc Res. 2005;42:13–20. doi: 10.1159/000082802. [DOI] [PubMed] [Google Scholar]
- 27.Owens CD, Gasper WJ, Rahman AS, Conte MS. Vein Graft Failure. Journal of vascular surgery. 2013 doi: 10.1016/j.jvs.2013.08.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassiouny HS, White S, Glagov S, Choi E, Giddens DP, Zarins CK. Anastomotic Intimal Hyperplasia: Mechanical Injury or Flow Induced. Journal of vascular surgery. 1992;15:708–716. doi: 10.1067/mva.1992.33849. discussion 716-707. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Freedman NJ, Brian L, Peppel K. Graft-Extrinsic Cells Predominate in Vein Graft Arterialization. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:470–476. doi: 10.1161/01.ATV.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]
- 30.Soyombo AA, Angelini GD, Bryan AJ, Jasani B, Newby AC. Intimal Proliferation in an Organ Culture of Human Saphenous Vein. The American journal of pathology. 1990;137:1401–1410. [PMC free article] [PubMed] [Google Scholar]
- 31.Porter KE, Varty K, Jones L, Bell PR, London NJ. Human Saphenous Vein Organ Culture: A Useful Model of Intimal Hyperplasia? European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 1996;11:48–58. doi: 10.1016/s1078-5884(96)80134-1. [DOI] [PubMed] [Google Scholar]
- 32.Conte M. Technical Factors in Lower-Extremity Vein Bypass Surgery: How Can We Improve Outcomes? Semin Vasc Surg. 2009;22:227–233. doi: 10.1053/j.semvascsurg.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Lovren F, Pan Y, Yanagawa B, Deb S, Karkhanis R, Quan A, Teoh H, Feder-Elituv R, Moussa F, Souza DS, Fremes SE. Pedicled No-Touch Saphenous Vein Graft Harvest Limits Vascular Smooth Muscle Cell Activation: The Patent Saphenous Vein Graft Study. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2014;45:717–725. doi: 10.1093/ejcts/ezt560. [DOI] [PubMed] [Google Scholar]
- 34.Wilbring M, Tugtekin SM, Zatschler B, Ebner A, Reichenspurner H, Matschke K, Deussen A. Even Short-Time Storage in Physiological Saline Solution Impairs Endothelial Vascular Function of Saphenous Vein Grafts. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2011;40:811–815. doi: 10.1016/j.ejcts.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Shi X, Chen G, Guo LW, Si Y, Zhu M, Pilla S, Liu B, Gong S, Kent KC. Periadventitial Application of Rapamycin-Loaded Nanoparticles Produces Sustained Inhibition of Vascular Restenosis. PloS one. 2014;9:e89227. doi: 10.1371/journal.pone.0089227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J, Zhang Y, Zhang X, Rudic RD, Bauer PM, Altieri DC, Sessa WC. Endothelium Derived Nitric Oxide Synthase Negatively Regulates the Pdgf-Survivin Pathway During Flow-Dependent Vascular Remodeling. PloS one. 2012;7:e31495. doi: 10.1371/journal.pone.0031495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dourron HM, Jacobson GM, Park JL, Liu J, Reddy DJ, Scheel ML, Pagano PJ. Perivascular Gene Transfer of Nadph Oxidase Inhibitor Suppresses Angioplasty-Induced Neointimal Proliferation of Rat Carotid Artery. American journal of physiology. Heart and circulatory physiology. 2005;288:H946–953. doi: 10.1152/ajpheart.00413.2004. [DOI] [PubMed] [Google Scholar]
- 38.Kibbe MR, Tzeng E, Gleixner SL, Watkins SC, Kovesdi I, Lizonova A, Makaroun MS, Billiar TR, Rhee RY. Adenovirus-Mediated Gene Transfer of Human Inducible Nitric Oxide Synthase in Porcine Vein Grafts Inhibits Intimal Hyperplasia. Journal of vascular surgery. 2001;34:156–165. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 39.Gratton JP BP, Sessa WC. Caveolae and Caveolins in the Cardiovascular System. Circ Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 40.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of Caveolae, Vascular Dysfunction, and Pulmonary Defects in Caveolin-1 Gene-Disrupted Mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]