Abstract
The widespread use of organophosphate (OP) pesticides has resulted in ubiquitous exposure in humans, primarily through their diet. Exposure to OP pesticides may have adverse health effects, including neurobehavioral deficits in children. The optimal design of new studies requires data on the reliability of urinary measures of exposure.
In the present study, urinary concentrations of six dialkyl phosphate (DAP) metabolites, the main urinary metabolites of OP pesticides, were determined in 120 pregnant women participating in the Generation R Study in Rotterdam. Intra-class correlation coefficients (ICCs) across serial urine specimens taken at <18, 18–25, and >25 weeks of pregnancy were determined to assess reliability.
Geometric mean total DAP metabolite concentrations were 229 (GSD 2.2), 240 (GSD 2.1), and 224 (GSD 2.2) nmol/g creatinine across the three periods of gestation. Metabolite concentrations from the serial urine specimens in general correlated moderately. The ICCs for the six DAP metabolites ranged from 0.14 to 0.38 (0.30 for total DAPs), indicating weak to moderate reliability.
Although the DAP metabolite levels observed in this study are slightly higher and slightly more correlated than in previous studies, the low to moderate reliability indicates a high degree of within-person variability, which presents challenges for designing well-powered epidemiologic studies.
Keywords: organophosphate pesticide metabolites, environmental exposure, variability, reliability, cohort study, epidemiologic studies
INTRODUCTION
After intake, most organophosphate (OP) pesticides are rapidly metabolized to one or more of six dialkyl phosphate (DAP) metabolites (Duggan et al., 2003). Due to the short half-life these are mostly excreted in urine within 24 hours (Huen et al., 2012). Therefore, levels of DAP metabolites in urine reflect recent exposure to one or more OP pesticides (Barr et al., 2004; Bradman et al., 2005; Duggan et al., 2003). Furthermore, OP pesticides can degrade in the environment, be metabolized by plants or broken down during food processing, leading to the presence of those metabolites in food and the environment (Morgan et al., 2005). Thus, urinary OP metabolite levels represent exposure to parent OP pesticides as well as their metabolites, and are considered to be non-specific markers of OP pesticide exposure.
The potential health effects of low-level exposure to OP pesticides is an active area of investigation. The widespread use of OP pesticides results in human exposure through a variety of pathways. In urban settings, exposure to OP pesticides or their metabolites mainly occurs through consumption of conventionally grown fruits and vegetables (Lu et al., 2008). Other sources of exposure may be the spraying of insecticides in houses, common spaces, and around building exteriors (Simcox et al., 1995; Narayan et al., 2013). In rural settings, residents may be also exposed to pesticides when applied to agriculture in the vicinity of their homes, or when passing by (Koch et al., 2002). Given the many possible exposure pathways, recent epidemiologic studies have often relied on urinary biomarkers of pesticides to obtain an integrated exposure estimate. Human exposure to OP pesticides has been associated with adverse neurobehavioral outcomes in rural, urban, and occupational settings (Bouchard et al., 2011; Engel et al., 2007; Eskenazi et al., 2007, Eskenazi et al., 2010; Handal et al., 2008; Young et al., 2005). Most data specifically address prenatal exposure (Bouchard et al., 2011; Engel et al., 2007; Eskenazi et al., 2007; Eskenazi et al., 2010; Marks et al., 2010; Young et al. 2005). The timing of exposure to OP pesticides during pregnancy may be of significance. For instance, in one study urinary DAP concentrations during the second half of pregnancy were more strongly related to a lower IQ at age 7 years than during the first half, and only prenatal (and not postnatal) urinary DAP concentrations were associated with lower IQ at age 7 (Bouchard et al., 2011).
Use of DAPs as biomarkers of exposure to OP pesticides (Sudakin et al., 2011) presents a design challenge for epidemiologic studies because of the high degree of within-person variability in measured concentrations due to the intermittent nature of exposure and rapid renal elimination (Eskenazi et al., 2007; Rauch et al., 2012; Whyatt et al., 2009). In the present study, the concentrations of six OP pesticide metabolites in two or three serial urine specimens from 120 pregnant women were determined to investigate their reliability. In this study the term reliability is used to refer to how well the DAP concentrations in one urine specimen correlate with those in urine specimens collected at other times in pregnancy (White et al., 2008). As a secondary aim, determinants of DAP concentrations were studied.
METHODS
Study population
The Generation R Study is a population-based birth cohort in the city of Rotterdam, The Netherlands. All mothers who resided in the study area and had a delivery date between April 2002 and January 2006 were eligible. Mothers were enrolled during pregnancy or in the first months after the birth of their child when newborns visited the routine child health centers. Among the 9778 mothers who participated in the study, 91% (n=8880) were enrolled during pregnancy. Their offspring are currently being followed to young adulthood. The study addresses four primary areas, namely growth and physical development, behavioral and cognitive development, diseases in childhood, and health and health care for pregnant women and children (Jaddoe et al., 2012). This study protocol underwent human subjects review at Erasmus Medical Center, Rotterdam, The Netherlands (IRB Registration #: IRB00001482).
From February 2004 to January 2006, women enrolled during pregnancy were asked to provide a spot urine sample during early (<18 weeks of gestational age), middle (gestational age 18–25 weeks) and late (gestational age >25 weeks) pregnancy, when they presented for ultrasound examinations and were asked to complete questionnaires. In total, 2083 women provided a complete set of three urine specimens, 1161 women provided two specimens, and one urine specimen was available for 754 women. From the 2083 women with three urine specimens and the 1161 women with 2 urine specimens, women with missing data on maternal age, ethnicity, education level, pre-pregnancy body mass index (BMI), perinatal smoking habits, gravidity, parity, and gestational age at child’s birth were excluded. Women for whom child’s birth weight and Child Behavior Checklist scores at age 3 years were missing were also excluded, resulting in a total of 1041 women with three urine specimens and 510 women with 2 urine specimens and complete data. These exclusions were applied to select a reliability sample that would be most generalizable to future studies in the Generation R cohort. For the present reliability study, 80 out of the 1041 women with three urine specimens and 40 out of the 510 women with two urine specimens were selected at random.
Urine collection and analysis of DAP metabolites
Details of biological specimen collection have been described elsewhere (Jaddoe et al., 2007). All samples were collected between 8 a.m. and 8 p.m. in 100 mL polypropylene urine collection containers that were kept for a maximum of 20 hours in a cold room (4°C) before being frozen at −20°C in 20 mL portions in 25 mL polypropylene vials.
Measurements of six nonspecific DAP metabolites of OP pesticides were conducted at the Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of Ruhr-University Bochum, Germany, using gas chromatography coupled with tandem mass spectrometry (GC–MS/MS) (Bravo et al., 2004; Barr et al. 2010). Three dimethyl (DM) metabolites (dimethylphosphate [DMP], dimethylthiophosphate [DMTP], and dimethyldithiophosphate [DMDTP]) were determined, as well as three diethyl (DE) metabolites (diethylphosphate [DEP], diethylthiophosphate [DETP], and diethyldithiophosphate [DEDTP]). In short, acidified urine samples (2 mL) were spiked and mixed with isotope labeled internal standards (DMP-d6, DMTP-d6, DMDTP-d6, DEP-d6, DETP-d10 and DEDTP-13C4) and freeze-dried overnight. The lyophilized urine was dissolved and extracted with diethylether and acetonitrile, and metabolites were derivatized using pentafluorobenzylbromide (PFBBr) at 40°C for 15 hours. After further liquid–liquid extraction with hexane, analytes were detected and quantified by GC–MS/MS. The limit of quantification (LOQ) was 0.01 µg/L for DEDTP and 0.1 µg/L for the other five DAP metabolites. The recovery of the method was determined by analyzing six different native urine samples both unspiked and spiked with 10 and 40 µg of the respective DAPs per liter. Relative recoveries ranged from 91% to 115%. Quality control samples (prepared from pooled urine samples DAPs spiked at 10 and 40 µg/L) were analyzed blindly among the study samples. The between-day coefficients of variation (CV) were between 3.5 to 9.6 % for all 6 DAPs at both concentration levels.
Molar concentrations were used to facilitate comparison of our results with those from other studies, based on the following molecular weights: DMP 126.0 g/mol, DMTP 142.1-g/mol, DMDTP 158.2 g/mol, DEP 154.1 g/mol, DETP 170.2 g/mol, and DEDTP 186.2 g/mol. Urinary creatinine concentrations were determined according to Larsen (1972), with a limit of detection (LOD) of 100 mg/L. Day-to-day precision varied between 1.31 and 2.48 CV%, within-series precision varied between 0.62 and 0.96 CV%.
Three dimethyl (DM) metabolites (DMP, DMTP, and DMDTP) were summed as total DM (nmol/L) and three diethyl (DE) metabolites (DEP, DETP, and DEDTP) were summed as total DE (nmol/L). Total DAP concentrations (nmol/L) were calculated by summing all six metabolites. Urinary values were calculated with and without adjustment for creatinine.
Dietary information
One of the questionnaires administered to the participants comprised a semi-quantitative food frequency questionnaire (SFFQ). The SFFQ included 290 food items, and was based on the validated 170-item SFFQ used in the Dutch ERGO study, a community based prospective cohort study in Rotterdam, The Netherlands (Klipstein-Grobusch et al., 1998). The questionnaire referred to diet during the preceding 3 months, and was filled in during week 30 of pregnancy. To estimate the mean amounts in grams per day from completed surveys, the programs ‘Measures and weights book’ (Donders-Engelen et al., 2003) and ‘Nevo’, the Dutch food composition database (Netherlands Nutrition Center, 2006), were used. The SFFQ was completed by 7229 of the 9778 women enrolled in Generation R (81%).
Statistical analysis
Data were analyzed with SAS statistical software (version 9.3; SAS Institute, Cary, NC, USA). Values below the LOQ for DAP metabolites and the LOD for creatinine were imputed using the Richardson-Ciampi method, in which one imputes E[x|x≤LOQ] (defined as the expectation of x given x below or equal LOQ) (Richardson et al., 2003). Because the distributions of the DAP metabolite concentrations were right-skewed, values were natural log transformed to obtain a more normal distribution.
Crude descriptive statistics were calculated as geometric mean (GM), geometric standard deviation (GSD), median, percentiles, and range for the various DAP metabolites and combinations of metabolites. Possible differences between the mean metabolite levels for the different periods were tested with an analysis of variance (PROC ANOVA). Between-person and day-to-day (within-person) variance in DAP metabolite concentrations were determined by fitting mixed effects models (PROC MIXED), in which the identity of the mother was introduced as a random factor in order to account for possible correlation between repeated measurements in the same person. To estimate reliability across all three points in time during pregnancy, the intraclass correlation coefficient (ICC) was estimated as [between-person variance / (between-person + within-person variance)]. Values can range from 0 to 1, where 1 indicates perfect reliability (Fleiss et al., 2004). Furthermore, Spearman correlation coefficients between pairs of urinary DAP metabolite concentrations across pregnancy were estimated.
The associations between maternal characteristics (as listed in Table 1) and metabolite concentrations were also examined by introducing these characteristics as fixed effects in bivariate mixed effects models. The analyses were performed for the total DM, total DE and total DAP metabolite concentrations, and were based on lognormal transformed DAP values. After that, multiple regression analyses were performed (241 measurements from 91 mothers), by including all the covariates that were found to be predictive of total DM, total DE and/or total DAP metabolite levels in the bivariate models (p-value for one of the categories <0.1).
Table 1.
Characteristics of the 120 randomly selected women compared with the Generation R cohort
| Variable | DAP subset (n=120) |
Generation R cohort (n=9778) |
|---|---|---|
| Age mother at enrollment (years, mean ± SD) | 31.8 ± 4.3 | 29.9 ± 5.4* |
| Ethnicity mother (n (%)) | ||
| Dutch | 78 (65.0%) | 4546 (46.5%) |
| European | 12 (10.0%) | 729 (7.5%) |
| Moroccan | 3 (2.5%) | 605 (6.2%) |
| Turkish | 6 (5.0%) | 797 (8.2%) |
| Dutch Antilles | 2 (1.7%) | 307 (3.1%) |
| Surinamese | 11 (9.2%) | 804 (8.2%) |
| Indonesian | 4 (3.3%) | 269 (2.8%) |
| Other | 1029 (10.5%) | |
| Not stated (missing) | 692 (7.1%) | |
| Mother, highest education level finished (n (%)) | ||
| No education finished | 25 (0.3%) | |
| Primary | 7 (5.8%) | 937 (9.6%) |
| Secondary | 35 (29.2%) | 3935 (40.2%) |
| Higher | 78 (65.0%) | 3661 (37.4%) |
| Not stated (missing) | 1220 (12.5%) | |
| Marital status (n (%)) | ||
| Married / living together | 108 (90.0%) | 7327 (74.9%) |
| No partner | 11 (9.2%) | 1239 (12.7%) |
| Not stated (missing) | 1 (0.8%) | 1212 (12.4%) |
| Net income household (n (%)) | ||
| ≤2200 euro per month | 34 (28.3%) | 3064 (31.3%) |
| >2200 euro per month | 75 (62.5%) | 3649 (37.3%) |
| Not stated (missing) | 11 (9.2%) | 3065 (31.3%) |
| Description of work status (n (%)) | ||
| Paid work | 84 (70.0%) | 4591 (47.0%) |
| Self employed | 7 (5.8%) | 334 (3.4%) |
| Other | 14 (11.7%) | 1863 (19.1%) |
| Not stated (missing) | 15 (12.5%) | 2990 (30.6%) |
| Working with pesticides / agricultural chemicals during pregnancy (n (%)) | ||
| Yes | 2 (1.7%) | 45 (0.5%) |
| No | 100 (83.3%) | 6313 (64.6%) |
| Don’t know | 1 (0.8%) | 125 (1.3%) |
| Not stated (missing) | 17 (14.2%) | 3295 (33.7%) |
| Previous pregnancies (parity) (n (%)) | ||
| 0 | 64 (53.3%) | 5178 (53.0%) |
| 1 | 45 (37.5%) | 2836 (29.0%) |
| ≥2 | 11 (9.2%) | 1387 (14.2%) |
| Not stated (missing) | 377 (3.9%) | |
| Alcohol consumption during pregnancy (n (%)) | ||
| Never alcohol in pregnancy | 42 (35.0%) | 3808 (38.9%) |
| Alcohol until pregnancy was known | 23 (19.2%) | 1045 (10.7%) |
| Alcohol continued in pregnancy | 55 (45.8%) | 2786 (28.5%) |
| Not stated (missing) | 2139 (21.9%) | |
| Maternal smoking during pregnancy (n (%)) | ||
| Never smoked in pregnancy | 101 (84.2%) | 6055 (61.9%) |
| Smoked until pregnancy was known | 8 (6.7%) | 706 (7.2%) |
| Continued smoking during pregnancy | 11 (9.2%) | 1483 (15.2%) |
| Not stated (missing) | 1534 (15.7%) | |
| Food intake (g/day) (mean ± SD) | ||
| Potatoes and other tubers | 48 ± 33 | 59 ± 46 |
| Vegetables | 143 ± 55 | 160 ±72 |
| Legumes | 3.9 ± 5.0 | 5.2 ± 8.0 |
| Fruits | 206 ± 113 | 182 ± 119 |
| Not stated (missing) | (n=18) | (n=3376) |
| Season of collection (n (%)) ** | Unknown for whole cohort | |
| Spring (<18 / 18–25 / > 25 weeks) | 86 (23.9%) (31 /39 / 16) | |
| Summer (<18 / 18–25 / > 25 weeks) | 92 (25.6%) (30 / 25 / 37) | |
| Autumn (<18 / 18–25 / > 25 weeks) | 71 (19.7%) (19 / 29 / 23) | |
| Winter (<18 / 18–25 / > 25 weeks) | 68 (18.9%) (26 /13 / 29) | |
| Missing (<18 / 18–25 / > 25 weeks) | 43 (11.9%) (14 / 14 / 15) | |
Information missing for one person
Based on 360 collected urine samples
SD = standard deviation
RESULTS
Sample characteristics
The 120 women selected for the present study represent a random sample of the 1041 women with 3 urine samples and 510 women with 2 urine samples and complete data. Women in this subset tended to be older, were more likely to be Dutch or of European nationality, and were generally more educated and more likely to have paid work compared with the women enrolled in the Generation R Cohort study (n=9778) (Table 1).
The age of the 120 mothers ranged from 20 to 42 years, with more than half of them (65%) of Dutch nationality, and 70% had paid work. Only two of the mothers indicated that they had worked with pesticides during their pregnancy. The mean intake of potatoes and other tubers was 48 gram/day, of vegetables was 143 gram/day, of legumes was 3.9 grams/day and of fruit was 206 grams/day (Table 1).
DAP metabolite levels in urine
Of the 120 women, 78 had OP metabolite levels measured in 3 urines and 42 women had measures in 2 urines. Unfortunately, for 2 women selected to have 3 urine samples available, no analytical results were available for one of the 3 samples. On average (± SD), maternal urine specimens were collected at 13.2±1.7, 20.4±0.8 and 30.2±0.8 weeks of gestation. Urinary creatinine concentrations averaged 623 (range 77–1921) mg/L for <18 weeks, 689 (range 77–2719) mg/L for 18–25 weeks, and 651 (77–2094) mg/L for >25 weeks of pregnancy, and were not significantly different between the three time periods (p>0.05).
The urinary OP metabolite levels are presented on a creatinine basis in Table 2. At least one DAP metabolite was detectable in all urine specimens, with the lowest detection frequency for DEDTP (below the LOD in approximately 25–40% of samples taken at the three pregnancy time points). Geometric mean total DAP metabolite concentrations were 229 (geometric standard deviation (GSD) 2.2), 240 (GSD 2.1) and 224 (GSD 2.2) nmol/g creatinine for the three sampling periods. For all metabolites, variability in levels was high and no statistically significant differences in the creatinine-adjusted levels between the time periods were observed. Total DM metabolites were present at higher concentrations than total DE, and accounted for 75–80% of the total DAP metabolites. DMP and DMTP were the DAP metabolites present at the highest levels. DMDTP was the DM metabolite with the lowest concentration, comprising 1.7–2.4% of the total DM metabolites. DEP was the major DE metabolite, comprising 71–75% of the total DE metabolites.
Table 2.
DAP metabolite levels for subset of 120 pregnant women from the Generation R cohort (nmol/g creatinine)*
| Meatbolite/ Period |
N | # <LOQ |
AM | GM | GSD | min | p5 | p25 | p50 | p75 | p95 | max |  |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP | 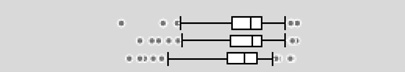 |
||||||||||||
| <18 weeks | 106 | 17 | 122.59 | 76.34 | 3.21 | 0.61 | 6.06 | 47.14 | 98.51 | 154.52 | 406.07 | 636.00 | |
| 18–25 weeks | 106 | 6 | 125.73 | 77.15 | 3.30 | 1.27 | 6.85 | 45.60 | 104.68 | 152.48 | 394.75 | 613.71 | |
| >25 weeks | 105 | 9 | 97.46 | 58.86 | 3.60 | 0.83 | 3.85 | 40.99 | 80.74 | 129.50 | 237.44 | 513.75 | |
| DMTP | 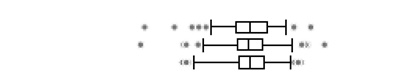 |
||||||||||||
| <18 weeks | 106 | 2 | 122.94 | 80.37 | 2.73 | 1.30 | 17.89 | 47.30 | 80.82 | 159.55 | 347.93 | 924.31 | |
| 18–25 weeks | 106 | 2 | 140.10 | 80.17 | 2.95 | 1.11 | 13.35 | 48.96 | 82.04 | 140.01 | 465.71 | 1592.7 | |
| >25 weeks | 105 | 1 | 126.68 | 80.34 | 2.81 | 5.83 | 8.89 | 53.20 | 81.02 | 142.05 | 401.71 | 630.4 | |
| DMDTP | 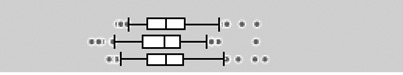 |
||||||||||||
| <18 weeks | 106 | 20 | 8.72 | 4.21 | 2.99 | 0.57 | 0.86 | 1.83 | 3.77 | 8.18 | 33.02 | 139.99 | |
| 18–25 weeks | 106 | 23 | 6.22 | 3.13 | 3.05 | 0.20 | 0.50 | 1.53 | 3.45 | 6.54 | 19.83 | 134.62 | |
| >25 weeks | 105 | 20 | 10.00 | 4.06 | 3.28 | 0.41 | 0.62 | 1.80 | 3.82 | 7.41 | 38.80 | 191.94 | |
| Total DM | 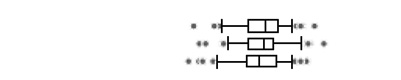 |
||||||||||||
| <18 weeks | 106 | - | 254.25 | 182.97 | 2.36 | 12.43 | 37.09 | 108.82 | 204.56 | 343.24 | 611.47 | 1470.37 | |
| 18–25 weeks | 106 | - | 272.04 | 187.03 | 2.36 | 15.52 | 47.05 | 109.10 | 197.92 | 297.93 | 869.27 | 2126.93 | |
| >25 weeks | 105 | - | 234.14 | 167.49 | 2.42 | 10.13 | 30.51 | 100.97 | 169.13 | 317.14 | 596.04 | 1098.79 | |
| DEP | 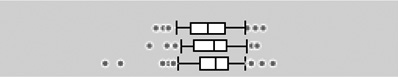 |
||||||||||||
| <18 weeks | 106 | 0 | 36.10 | 24.91 | 2.42 | 3.13 | 6.71 | 12.24 | 25.19 | 49.11 | 108.27 | 217.52 | |
| 18–25 weeks | 106 | 0 | 39.08 | 28.32 | 2.27 | 2.43 | 8.47 | 13.69 | 30.84 | 49.17 | 116.43 | 171.26 | |
| >25 weeks | 105 | 1 | 43.68 | 29.67 | 2.67 | 0.42 | 7.07 | 18.19 | 33.05 | 54.15 | 111.00 | 319.32 | |
| DETP | 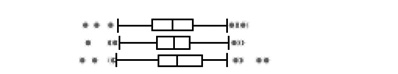 |
||||||||||||
| <18 weeks | 106 | 18 | 13.62 | 6.26 | 3.54 | 0.20 | 0.71 | 2.90 | 6.20 | 14.16 | 54.92 | 113.66 | |
| 18–25 weeks | 106 | 9 | 12.33 | 6.40 | 3.21 | 0.22 | 0.76 | 3.30 | 6.59 | 12.19 | 56.68 | 96.28 | |
| >25 weeks | 105 | 14 | 18.02 | 7.85 | 3.77 | 0.18 | 0.66 | 3.63 | 7.68 | 20.67 | 55.06 | 257.50 | |
| DEDTP |  |
||||||||||||
| <18 weeks | 106 | 31 | 0.69 | 0.26 | 3.94 | 0.01 | 0.02 | 0.10 | 0.22 | 0.67 | 2.40 | 13.73 | |
| 18–25 weeks | 106 | 26 | 0.60 | 0.26 | 3.96 | 0.01 | 0.03 | 0.10 | 0.27 | 0.80 | 2.45 | 4.06 | |
| >25 weeks | 105 | 0 | 1.02 | 0.23 | 4.78 | 0.01 | 0.02 | 0.08 | 0.17 | 0.84 | 2.64 | 43.16 | |
| Total DE | 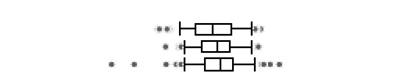 |
||||||||||||
| <18 weeks | 106 | - | 50.41 | 34.15 | 2.50 | 3.78 | 8.90 | 16.79 | 33.38 | 69.35 | 158.35 | 222.30 | |
| 18–25 weeks | 106 | - | 52.01 | 37.94 | 2.23 | 5.18 | 10.34 | 21.19 | 38.50 | 65.34 | 158.09 | 203.31 | |
| >25 weeks | 105 | - | 62.71 | 41.87 | 2.66 | 0.61 | 10.32 | 24.23 | 44.53 | 73.59 | 177.91 | 465.45 | |
| Total DAP |  |
||||||||||||
| <18 weeks | 106 | - | 304.66 | 228.50 | 2.23 | 29.23 | 50.30 | 133.62 | 261.70 | 405.26 | 698.17 | 1502.50 | |
| 18–25 weeks | 106 | - | 324.05 | 240.23 | 2.10 | 43.91 | 78.19 | 144.23 | 249.43 | 357.34 | 1072.21 | 2315.43 | |
| >25 weeks | 105 | - | 296.85 | 223.95 | 2.23 | 15.85 | 59.64 | 144.38 | 233.22 | 379.71 | 693.49 | 1204.26 | |
The boxplots represent the 5th, 25th, 50th, 75th and 95th percentiles (vertical lines) as well as the outliers (dots) of the underlying distribution
Reliability between measurements of DAP metabolites
Pairs of specific DAP metabolite concentrations across pregnancy were weakly to moderately correlated, with the correlation coefficients varying between 0.07 and 0.44 (Table 3). Spearman correlations of total DAP creatinine-adjusted metabolite levels were 0.31 between period 1 (<18 weeks) and period 2 (18–25 weeks), 0.38 between period 2 and period 3 (>25 weeks) and 0.31 between period 1 and 3. Correlations were similar for total DM and total DE metabolites. In general, the correlation between periods 2 and 3 was somewhat higher than for the other paired time points, though not across all metabolites. Limiting the correlation analyses to only those women with a relatively short duration between the 3 periods (≤ median duration), did not result in an overall increase in correlation coefficients (Table S3 in the supplementary information) Overall, no clear pattern with regard to stronger correlations between measurements closer in time could be observed.
Table 3.
Pairwise spearman correlation coefficients of metabolite concentrations per mother measured during the 3 periods, variance components and ICCs for DAP metabolite levels for subset of 120 pregnant women from the Generation R cohort (nmol/g creatinine)
| Metabolite | Correlation coefficients | Variance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Period 1–2 (n=92) |
Period 2–3 (n=91) |
Period 1–3 (n=92) |
Between- person variance |
Within- person variance |
Total variance |
ICC | ||||
| Corr. | (p) | Corr. | (p) | Corr. | (p) | |||||
| DMP | 0.33 | (0.001) | 0.42 | (0.000) | 0.23 | (0.028) | 0.48 | 0.99 | 1.47 | 0.33 |
| DMTP | 0.27 | (0.009) | 0.29 | (0.005) | 0.34 | (0.001) | 0.22 | 0.85 | 1.07 | 0.21 |
| DMDTP | 0.21 | (0.044) | 0.07 | (0.498) | 0.28 | (0.006) | 0.18 | 1.12 | 1.29 | 0.14 |
| Total DM | 0.27 | (0.009) | 0.36 | (0.001) | 0.27 | (0.010) | 0.21 | 0.54 | 0.75 | 0.28 |
| DEP | 0.25 | (0.016) | 0.35 | (0.001) | 0.28 | (0.007) | 0.20 | 0.60 | 0.80 | 0.25 |
| DETP | 0.26 | (0.012) | 0.41 | (0.001) | 0.18 | (0.086) | 0.39 | 1.19 | 1.58 | 0.25 |
| DEDTP | 0.44 | (0.000) | 0.43 | (0.000) | 0.30 | (0.004) | 0.79 | 1.29 | 2.08 | 0.38 |
| Total DE | 0.28 | (0.007) | 0.30 | (0.004) | 0.23 | (0.030) | 0.20 | 0.62 | 0.81 | 0.24 |
| Total DAP | 0.31 | (0.002) | 0.38 | (0.000) | 0.31 | (0.003) | 0.18 | 0.43 | 0.61 | 0.30 |
| Range* | 0.21–0.44 | 0.07–0.43 | 0.18–0.34 | 0.18–0.79 | 0.43–1.19 | 0.61–2.08 | 0.14–0.38 | |||
Range as presented is for the 6 individual DAPS as well as the 3 summed groupings.
For all specific DAP metabolites the within-person variability (range 0.18–0.79) exceeded the between-person variability (range 0.43–1.19), resulting in ICCs for creatinine-basis metabolite levels of 0.14 to 0.38, indicating poor to moderate reliability. For total DM, total DE and total DAP ICCs of 0.28, 0.24 and 0.30 were estimated. The wet-weight metabolite levels (in nmol/L), which showed comparable results, are presented in the supplementary information (Tables S1 and S2 and Figure S1).
To investigate whether the relatively low creatinine levels affected the reliability, we conducted a sensitivity analysis in which 17 out of 328 urine samples were excluded (from 14 women) with creatinine levels below 100 mg/L (the LOD for creatinine, values below that were imputed; 10 in the period <18 weeks, 5 in the period 18–25 weeks and 2 in the period >25 weeks). Results were comparable regardless of exclusion based on low creatinine concentration (Table S4 in the supplementary information).
Determinants of urinary OP pesticide metabolite levels
Table 4 shows a multiple regression model in which all parameters that showed associations in bivariate analyses (ethnicity, education, smoking during pregnancy, work status, working with pesticides, age mother, food intake; bivariate results are briefly discussed in the Supplementary information) were included, and explained 8–12% of the between-person variance. Higher total DM and total DAP levels were observed in those of Dutch origin. Higher total DE and total DAP levels were observed in women with a high fruit intake. Lower total DE, total DM and total DAP levels were seen in those women who continued smoking, and lower total DM and total DAP levels were observed in self-employed women. In the multiple regression model in which the food intake parameters were included on a categorical scale (Table S5 in the supplementary information), in general the direction of the effects were similar, though intake of potatoes was associated with lower DAP concentrations.
Table 4.
Results of multiple regression analysis of determinants of exposure (based on lognormal transformed metabolite levels in nmol/g creatinine)
| Total DM (eβ)3 | Total DE (eβ)3 | Total DAP (eβ)3 | ||
|---|---|---|---|---|
| Combination of determinants1 | ||||
| Between-person variance | 0.1507 (7.5%) | 0.1269 (9.4%) | 0.1171 (11.5%) | |
| Within-person variance | 0.4976 (−0.6%) | 0.5928 (0.1%) | 0.4002 (−0.3%) | |
| Intercept | 151.4**** | 27.7**** | 193.8**** | |
| Ethnicity2 | Dutch | 1.49*** | 1.11 | 1.34* |
| European | 1.55* | 1.11 | 1.36 | |
| Other | Ref | Ref | Ref | |
| Education2 | Primary | 0.99 | 0.86 | 0.99 |
| Secondary | 0.84 | 0.86 | 0.83* | |
| Higher | Ref | Ref | Ref | |
| Smoking during pregnancy | Until known | 0.69 | 0.76 | 0.72 |
| Continued | 0.70* | 0.55*** | 0.67** | |
| Never | Ref | Ref | Ref | |
| Work status2 | Paid work | 0.91 | 1.09 | 0.92 |
| Self-employed | 0.59 * | 0.97 | 0.65* | |
| Other | Ref | Ref | Ref | |
| Working with pesticides | Don’t know | 1.91 | 2.98* | 1.94 |
| Yes | 0.84 | 1.28 | 0.92 | |
| No | Ref | Ref | Ref | |
| Age mother2 <30 years | 1.05 | 1.09 | 1.07 | |
| 30–35 years | 1.12 | 1.09 | 1.12 | |
| >35 years | Ref | Ref | Ref | |
| Potatoes(g/day) | 0.998 | 0.998 | 0.998 | |
| Vegetables(g/day) | 0.999 | 0.999 | 0.999 | |
| Legumes(g/day) | 1.013 | 1.001 | 1.011 | |
| Fruits(g/day) | 1.001 | 1.002*** | 1.001* | |
Based on part of the dataset with complete data, 241 (out of 318) measurements for 91 (out of 120) women
Based on combination of categories, to limit the number of categories within each parameter in the determinant analysis and have a relevant number of individuals in each category
Between brackets the percentage of explained between- and within-person variability. Please note that the exponentiated coefficients (eβ) are presented here, as the analysis was performed on lognormal transformed data.
Ref: reference category
p<0.0001,
0.0001<p<0.05,
0.05<p<0.10,
0.10<p<0.20
DISCUSSION AND CONCLUSIONS
Six DAP metabolites were measured in two or three urine samples in 120 women from the Generation R Study. The geometric mean total DAP metabolite concentrations were 229 nmol/g creatinine at <18 weeks, 240 nmol/g creatinine at 18–25 weeks and 224 nmol/g creatinine at >25 weeks, and DM metabolites accounted for 75–80% of the total DAP metabolites. For all DAP metabolites the within-person variability exceeded the between-person variability, resulting in ICCs of 0.14–0.38, indicating poor to moderate reliability. Furthermore, pairs of DAP metabolite concentrations were moderately correlated, with the strongest correlation between period 2 and 3, although no clear pattern with regard to stronger correlations between measurements closer in time could be observed.
Our results on reliability are similar to those in previous studies of pregnant women. In the CHAMACOS cohort, two pregnancy total DAP concentrations were weakly correlated (r=0.14, p=0.005) and did not differ from each other (Eskenazi et al. 2007). Within the HOME cohort, the two creatinine-standardized measures were also weakly correlated (r=0.22) (Rauch et al., 2012). In the present study, the correlations between the total DAP concentrations were somewhat higher, between 0.31 and 0.38. It should be noted that previous studies of OP pesticide metabolites have been limited to one or two maternal specimens as a measure of prenatal “in utero” exposure. The weak correlation between the samples from the same mother emphasizes the need to use multiple urine samples in order to reduce misclassification of exposure and increase power, if the exposure of interest is an average of gestational exposure.
Studies of the reliability of urinary metabolites of organophosphate pesticides in children and adults have also reported high within-person variance relative to between-person variance (Attfield et al., 2014; Meeker et al., 2005). This high ratio of within to between person variance is found even among specimens collected within a day or two (Meeker et al., 2005), and appears, in the present case, to be due primarily to variation in diet as well as variation in the concentration of pesticide residues on a given type of food (see below).
Total DAP concentrations in the present study were slightly lower than those reported in a previous study of another subset of the Generation R cohort, who had only one urine specimen collected after 20 weeks of pregnancy (Ye et al., 2008; Ye et al., 2009) (see Table 5). It is possible that women who have had three urine specimens rather than one differed in ways not captured by the variables in the multiple regression analysis. Comparing the DAP metabolite concentrations in the Generation R Study to other cohorts in which urine OP concentrations during pregnancy were reported (Table 5), total DM, DE, and DAP concentrations (in nmol/L) indicated that levels were more or less similar to those observed in the Californian CHAMACOS study (USA) (Eskenazi et al., 2007), and appeared to be slightly higher compared to participants in the HOME study in Ohio (USA) (Rauch et al., 2012) and the Mount Sinai study in New York (USA) (Engel et al., 2007). Furthermore, the levels reported here were higher than those reported in the Norwegian MoBa study and the American NHANES study (in nmol/g creatinine) (Ye et al., 2009). A possible reason for the higher OP metabolite levels in the Generation R cohort compared to the NHANES cohort may be a higher fruit and vegetable intake (median of ± 323 gram/day compared to median of 176 gram/day for NHANES adult women), although a direct comparison is imprecise due to the different methods used to ascertain diet (Agudo et al., 2005; Kimmons et al., 2009; U.S. EPA, 2011). This may also partly explain the comparable metabolite levels as observed in the CHAMACOS study, in which 42% of mothers worked in agriculture during pregnancy (as source of exposure) (Eskenazi et al., 2004), compared to 2 out of the 120 mothers reporting to have worked with pesticides during pregnancy in this selection of the Generation R cohort.
Table 5.
Comparison dialkyl phosphate metabolites in maternal urine specimens of various birth cohorts
| Cohort | Generation R1 | Generation R2 | MoBa3 | NHANES4 | Mt. Sinai5 | Home6 | CHAMACOS7 |
|---|---|---|---|---|---|---|---|
| Location | Rotterdam, Netherlands | Rotterdam, Netherlands | Norway | USA | New York City, NY, USA | Cincinnati, OH, USA | Salinas Valley, CA, USA |
| # women | 120 | 100 | 11 | 126 | 285–297 | 344 | 445 |
| Statistic | GM | GM | GM | Median | Median | Median | Median |
| Metabolite Concentrations in nmol/g creatinine | |||||||
| Total DAP | 223–240 | 282 | 145 | 72 | |||
| Total DM | 167–187 | 240 | 112 | 45 | |||
| Total DE | 50–63 | 31 | 12 | 22 | |||
| Metabolite concentrations in nmol/L | |||||||
| Total DAP | 97–124 | 183 | 87 | 52 | 82 | 81 | 115 |
| Total DM | 78–97 | 157 | 79 | 29 | 48 | 57 | 82 |
| Total DE | 15–21 | 20 | 8 | 16 | 25 | 18 | 18 |
Current study, 120 persons with 2 or 3 urine samples
Previous study, 100 mothers with one urine spot sample after 20–30 weeks of gestation, mean age 30 (18–41) (Ye et al., 2008; Ye et al., 2009)
Ten pools of one 1 ml urine samples from 11 women at ~17 weeks of gestation, mean age 30 (15–53) years (Ye et al., 2009). In the table approximate values are given, based on GM values in µg/g creatinine
Urine samples of 126 pregnant women in 2001–2002, mean age 27 (16–40) years from the NHANES study, which is a slightly different dataset as presented in the paper of Ye et al. (2009) (n=119) but otherwise the same data.
Multiethnic pregnancy prospective cohort study in Mount Sinai Children’s Environmental health Center, New York City, urine spot sample at mean gestational age of 31.2 weeks (Engel et al., 2007)
Prospective birth cohort in Cincinnati metropolitan area, urine spot samples at 16 and 26 weeks of gestation Rauch et al., 2012)
Prospective cohort study in Salinas Valley, California, with primarily Latino children, metabolite levels as average of baseline and 26-week maternal pregnancy measures
In general, urinary OP metabolite levels represent exposure to parent OP pesticides and their metabolites from dietary intake, and use of pesticides at work, at home (indoors or in the garden), or in the surrounding environment (Koch et al., 2002; Lu et al., 2008; Morgan et al., 2005). To our knowledge, data on residential organophosphate pesticide use in the Netherlands are not available. However, the marketing of biocides (non-agricultural pesticides) has been regulated in the Netherlands since 1998 by Directive 98/8/EC of the European Parliament. Under this Directive, organophosphate pesticides were never approved for use as biocides. Furthermore, because the cohort participants all live in an urban area, bystander exposure from agricultural application OP pesticides was not very likely. Therefore, it is assumed that most of the exposure to OP pesticides in this study was through diet, via consumption of fruits and vegetables with OP pesticide residues.
The European pesticide residue monitoring program has shown a decrease in the overall percentage of food samples with detectible residues since 1999. Furthermore, the percentage of samples with residues detected at or below the maximum risk limits (MRLs) is increasing, and these MRLs are more frequently exceeded in foods imported from developing countries (6.4%) than in foods produced in the EU (2.2%). Furthermore, the percentage of fruit, vegetable and cereal samples with detectable residues of multiple pesticides has increased as well (European Commission, 2008). In the present study, higher potato consumption was associated with lower metabolite levels, and higher fruit consumption correlated with higher metabolite levels. An explanation for the lower metabolite concentrations among those with high potato consumption is not obvious. Characteristics such as ethnicity, education, and work status are thought to be proxies for differences in food consumption patterns between nationalities and/or socioeconomic groups. Nicotine is known to influence the metabolism and toxicity of OP pesticides, and our results suggest future studies of OP pesticide toxicity may need to consider effect modification by smoking status (Lee et al., 2010).
Although the total variance observed was mainly within-person variability, the multiple regression models explained none of the day-to-day variability due to the fact that no specific information on variation of the determinants within the mothers during their pregnancy was available. For instance, the limited associations found with regard to diet can be partially explained by the fact that we had information that addressed the longer-term diet of the mothers during pregnancy, rather than the diet consumed shortly before urine sampling took place. However, limiting the bivariate determinant analysis to only the metabolite concentrations from the third period (>25 weeks), which is most in line with the period over which information on food intake was gathered (week 17–30 of pregnancy), showed in general comparable results (results not shown). Due to the short half-life of the OP metabolites the consumption of fruit with a relatively large amount of pesticide residues shortly before sampling will have a large effect on the measured OP metabolites in the urine for a day or two, which is one of the explanations of the large within-person variability in the measured OP metabolite levels. Since detailed information on short-term food intake was not available, the information on food intake can only be used as a general indication of dietary sources of OP pesticide exposure.
Diet, especially fruit and vegetable intake, has been associated with OP pesticide exposure in many previous studies. For example, in a study investigating exposure to OP pesticides in the general adult population in Israel, DAP metabolite levels were positively associated with age, income, female sex, and fruit consumption (Berman et al., 2013). Among children in the CHAMACOS birth cohort, daily servings of fruit and vegetables and temporal and spatial proximity to agricultural use were strong predictors of DAP metabolite levels (Bradman et al., 2011). In a study of U.S. men, intake of grapes and cheese was related to the concentration in urine of a metabolite of chlorpyrifos, an organophosphate pesticide. In a study of U.S. children from Ohio, diet was shown to be a more important source of chlorpyrifos metabolite than of chlorpyrifos itself (Morgan et al., 2011).
A number of neurodevelopmental and behavioral outcomes have been associated with indices of OP pesticide exposure. For instance, associations have been observed between higher maternal pregnancy urine OP concentrations and an increased number of abnormal reflexes (Engel et al., 2007; Young et al., 2005), poorer scores on the Bayley Scale of Infant Development, and with screening positive for pervasive developmental disorder (Eskenazi et al., 2007). Within the CHAMACOS cohort, higher maternal DAP concentrations were associated with ADHD-like behavioral scores in some analyses (Burns et al., 2013), and within the NHANES study, higher OP pesticide metabolites in urine were associated with attention-deficit hyperactivity disorder (Bouchard et al. 2010). Another cross-sectional study among children in Canada, however, did not support and adverse effect on behavior (Oulhote et al., 2013). Nonetheless, compared to postnatal exposure to OP pesticides, prenatal exposure may be more strongly associated with adverse health outcomes. For instance, as noted earlier, in the CHAMACOS cohort prenatal (maternal) but not postnatal urinary DAP concentrations were associated with poorer intellectual development in 7-year-old children (Bouchard et al., 2011). While the associations of OP pesticide exposure with cognitive development are more consistent than those for behavioral outcomes, additional research is needed on the influence of low-level exposure to OP pesticides and child cognitive and behavioral development (Gray et al., 2011).
While characterizing the reliability of the DAP metabolites concentrations in urine during pregnancy will be useful for power calculations for future Generation R Studies, a number of limitations can be identified. Having all samples from the critical window of exposure would be advantageous, but the timing of this window is unknown. Thus, more samples per subject would have been better. As noted above, this investigation of determinants of DAP metabolite concentration in urine would have been more informative if data on diet in the day or two before the urine collection would have been obtained. Data on time of day the urine was collected and time since last void would have added precision to the estimates of exposure. Changes in diet as the pregnancy progressed could have affected the estimates of reliability. In calculating the ICC it is assumed that the 3 urine specimens were collected at random and that the exposure patterns were similar across the three time periods (i.e., that the 3 time points were exchangeable). While the concentrations of DAPs were not statistically different across the 3 time points, if there were undetected systematic differences in exposure, e.g., due to dietary changes during pregnancy, the estimates of the ICC could be inaccurate, though the direction of bias would be hard to predict. Environmental and dietary specimens could have allowed verification of the assumption that most exposure was via diet, and could have allowed determination of how much exposure was to parent compounds rather than metabolites, since DAP metabolites are not specific regarding the pesticide from which they were derived. During pregnancy, urine volume increases, with a 25% increase by the third trimester (Maikranz et al., 1989). However, urinary creatinine and creatinine clearance are relatively stable during the period of pregnancy studied here (van Buul et al., 1995). Thus the changes in excretion related to pregnancy would have had little, if any, effect on our estimates of reliability, and expressing results on a creatinine basis facilitated comparison of these results to other studies on organophosphate metabolites in urine from pregnancy (Ye et al., 2008). Strengths of the study were that it had a fairly large number of subjects for a study of this type, and it provided reliability data for a European population, for whom few data of this type are available.
In this study a relatively large variation in DAP metabolite concentrations during pregnancy was observed, and for all DAP metabolites the within-person variability exceeded the between-person variability, resulting in only poor to moderate reliability. Furthermore, no clear pattern with regard to stronger correlations between measurements closer in time could be observed, and the available information on determinants of OP pesticide exposure explained only a small proportion of the observed exposure variance. The relatively large within-person variability presents a challenge for designing epidemiologic studies with sufficient statistical power. Analysis of more than one urine specimen per subject will reduce the sample size needed to detect a given association. For example, with the ICC measured in this population, with exposure based on the average in repeated measures, for a desired power, increasing the number of specimens per subject from 2 to 3 meant about 20% fewer subjects were needed (Dupont et al., 1998; Lagakos, 1998).
Supplementary Material
ACKNOWLEDGEMENTS
The Generation R study is conducted by the Erasmus MC, University Medical Center, Rotterdam, the Netherlands in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of general practitioners, hospitals, midwives, and pharmacies in Rotterdam. The first phase of the Generation R study is made possible by financial support from the Erasmus MC, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development (ZonMw). We also wish to thank Edwin van Wijngaarden for reviewing previous versions of the manuscript.
This research received support from the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences, and grants K12 ES019852 and P30 001247 from the National Institutes of Health (NIH, USA). We are grateful to R. Hauser of Harvard University for the suggestion to collect multiple urine specimens from subjects.
REFERENCES
- Agudo A. ISBN 9241592826. World Health Organization (WHO); 2005. Measuring intake of fruit and vegetables. [Google Scholar]
- Attfield KR, Hughes MD, Spengler JD, Lu C. Within- and between-child variation in repeated urinary pesticide metabolite measurements over a 1-year period. Environ. Health Perspect. 2104;122(2):201–206. doi: 10.1289/ehp.1306737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Olsson AO, Caudill SP, Schober SE, Pirkle JL, Sampson EJ, Jackson RJ, Needham LL. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the US population. Environ. Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr D, Wittassek M, Schettgen T, Hoppe H-W, Angerer J. Dialkyl phosphates. The MAK Collection for Occupational Health and Safety. Part IV: Biomonitoring Methods. 2010;12:185–209. [Google Scholar]
- Berman T, Goldsmith R, Göen T, Spungen J, Novack L, Levine H, Amitai Y, Shohat T, Grotto I. Urinary concentrations of organophosphate pesticide metabolites in adults in Israel: Demographic and dietary predictors. Environ. Int. 2013;60:183–189. doi: 10.1016/j.envint.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Bradman A, Eskenaz Bi, Barr DB, Bravo R, Castorina R, Chevrier J, Kogut K, Harnly ME, McKone TE. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ. Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Castorina R, Barr DB, Chevrier J, Harnly ME, Eisen EA, McKone TE, Harley K, Holland N, Eskenazi B. Determinants of organophosphorus pesticide urinary metabolite levels in Young Children Living in an Agricultural Community. Int. J. Environ. Res. Public Health. 2011;8:1061–1083. doi: 10.3390/ijerph8041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Caltabiano LM, Weerasekera G, Whitehead RD, Fernandez C, Needham LL, Bradman A, Barr DB. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. J. Expo. Anal. Environ. Epidemiol. 2004;14:249–259. doi: 10.1038/sj.jea.7500322. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125(6):e1270–e1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B. Prenatal Exposure to Organophosphate Pesticides and IQ in 7-Year-Old Children. Environ. Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders-Engelen M, Heijden van der L, Hulshof KF. Maten, gewichten en codenummers. Wageningen: Human Nutrition of TNO & Wageningen University; 2003. [Google Scholar]
- Duggan A, Charnley G, Chen W, Chukwudebe A, Hawk R, Krieger RI, Ross J, Yarborough C. Di-alkyl phosphate biomonitoring data: assessing cumulative exposure to organophosphate pesticides. Regul. Toxicol. Pharmacol. 2003;37:382–395. doi: 10.1016/s0273-2300(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Dupont WD, Plummer WD. Power and sample size calculations for studies involving linear regression. Controlled Clinical Trials. 1998;19:589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JB, Wolff MS. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am. J. Epidemiol. 2007;165(12):1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, 1 Barr DB, Furlong CE, Holland NT. Association of in Utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ. Health Perspect. 2004;112:1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Huen K, Marks A, Harley KG, Bradman A, Barr DB, Holland N. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environ. Health Perspect. 2010;118:1775–1781. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. Monitoring of Pesticide Residues in products of plant origin in the European Union, Norway, Iceland and Liechtenstein 2006. Commission of the European communities, commission staff working document, SEC(2008) 2902 final, Brussels. 2008 Nov 20; [Google Scholar]
- Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. Third Edition. Hoboken, NJ, USA: John Wiley & Sons, Inc.; Chapter 18. The Measurement of Interrater Agreement. [Google Scholar]
- Freire C, Koifman S. Pesticides, depression and suicide: A systematic review of the epidemiological evidence. Int. J. Hyg. Environ. Health. 2013;216:445–460. doi: 10.1016/j.ijheh.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Gray K, Lawler CK. Strength in Numbers: Three separate studies link in utero organophosphate pesticide exposure and cognitive development. Environ. Health Perspect. 2011;119(8):a328–a329. doi: 10.1289/ehp.1104137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handal AJ, Harlow SD, Breilh J, Lozoff B. Occupational exposure to pesticides during pregnancy and neurobehavioral development of infants and toddlers. Epidemiology. 2008;19:851–859. doi: 10.1097/EDE.0b013e318187cc5d. [DOI] [PubMed] [Google Scholar]
- Huen K, Bradman A, Harley K, Yousefi P, Barr DB, Eskenazi B, Holland N. Organophosphate pesticide levels in blood and urine of women and newborns living in an agricultural community. Environ. Res. 2012;117:8–16. doi: 10.1016/j.envres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaddoe VW, Bakker R, van Duijn CM, van der Heijden AJ, Lindemans J, Mackenbach JP, Moll HA, Steegers EAP, Tiemeier H, Uitterlinden AG, Verhulst FC, Hofman A. The Generation R Study Biobank: a resource for epidemiological studies in children and their parents. Eur. J. Epidemiol. 2007;22:917–923. doi: 10.1007/s10654-007-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaddoe VW, van Duijn CM, Franco OH, van der Heijden AJ, van IJzendoorn MH, de Jongste JC, van der Lugt A, Mackenbach JP, Moll HA, Raat H, Rivadenaira F, Steegers EAP, Tiemeier H, Uitterlinder AG, Verhulst FC, Hofman A. The Generation R Study: design and cohort update 2012. Eur. J. Epidemiol. 2012;27:739–756. doi: 10.1007/s10654-012-9735-1. [DOI] [PubMed] [Google Scholar]
- Kimmons J, Gillespie C, Seymour J, Serdula M, Michels Blanck H. Fruit and vegetable intake among adolescents and adults in the United States: Percentage meeting individualized recommendations. Medscape J. Med. 2009;11(1):26. [PMC free article] [PubMed] [Google Scholar]
- Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, Witteman JC. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur. J. Clin. Nutr. 1998;52:588–596. doi: 10.1038/sj.ejcn.1600611. [DOI] [PubMed] [Google Scholar]
- Koch D, Lu C, Fisker-Andersen J, Jolley L, Fenske RA. Temporal association of children's pesticide exposure and agricultural spraying: report of a longitudinal biological monitoring study. Environ, Health Perspect. 2002;110(8):829–833. doi: 10.1289/ehp.02110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagakos SW. Effects of mismodeling and mismeasuring explanatory variables on tests of their association with a response variable. Statistics in Medicine. 1988;7:257–274. doi: 10.1002/sim.4780070126. [DOI] [PubMed] [Google Scholar]
- Larsen K. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clinica Chimica Acta. Int. J. Clin. Chem. 1972;38(2):475–476. doi: 10.1016/0009-8981(72)90146-5. [DOI] [PubMed] [Google Scholar]
- Lee S, Poet TS, Smith JN, Busby-Hjerpe AL, Timchalk C. Effect of in vivo nicotine exposure on chlorpyrifos pharmacokinetics and pharmacodynamics in rats. Chem. Biol. Interact. 2010;184(3):449–457. doi: 10.1016/j.cbi.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ. Health Perspect. 2008;116:537–542. doi: 10.1289/ehp.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maikranz P, Holley JL, Parks JH, Lindheimer MD, Nakagawa Y, Coe FL. Gestational hypercalciuria causes pathological urine calcium oxalate supersaturations. Kidney Int. 1989;36(1):108–113. doi: 10.1038/ki.1989.168. [DOI] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children. Environ. Health Perspect. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Ryan L, Herrick RF, Bennett DH, Bravo R, Hauser R. Temporal variability of urinary levels of nonpersistent insecticides in adult men. J. Expo. Anal. Environ. Epidemiol. 2005;15(3):271–281. doi: 10.1038/sj.jea.7500402. [DOI] [PubMed] [Google Scholar]
- Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, Wilson NL, Christopher WL. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J. Expo. Anal. Environ. Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- Morgan MK, Sheldon LS, Jones PA, Croghan CW, Chuang JC, Wilson NK. The reliability of using urinary biomarkers to estimate children's exposures to chlorpyrifos and diazinon. J Expo Sci Environ Epidemiol. 2011;21(3):280–290. doi: 10.1038/jes.2010.11. [DOI] [PubMed] [Google Scholar]
- Narayan S, Liew Z, Paul K, Lee P-C, Sinsheimer JS, Bronstein JM, Ritz B. Household organophosphorus pesticide use and Parkinson’s disease. Int. J. Epi. 2013;42:1476–1485. doi: 10.1093/ije/dyt170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherlands Nutrition Center. Nevo: Dutch food composition database 2006. The Hague, The Netherlands: Netherlands Nutrition Centre; 2006. [Google Scholar]
- Oulhote Y, Bouchard MF. Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in canadian children. Environ. Health Perspect. 2013;121(11–12):1378–1384. doi: 10.1289/ehp.1306667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss R, Koch HM, Angerer J. Biological monitoring of the five major metabolites of di-(2-ethylhexyl)phthalate (DEHP) in human urine using column-switching liquid chromatography–tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;816:269–280. doi: 10.1016/j.jchromb.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano MA, Yolton K, Lanphear BP. Associations of prenatal exposure to erganophosphate pesticide metabolites with gestational age and birth weight. Environ. Health Perspect. 2012;120:1055–1060. doi: 10.1289/ehp.1104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DB, Ciampi A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am. J. Epidemiol. 2003;157(4):355–363. doi: 10.1093/aje/kwf217. [DOI] [PubMed] [Google Scholar]
- Simcox NJ, Fenske RA, Woltz SA, Lee I-C, Kalman DA. Pesticides in household dust and soil: Exposure pathways for children of agricultural families. Environ. Health Perspect. 1995;103(12):1226–1234. doi: 10.1289/ehp.951031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin DL, Stone DL. Dialkyl phosphates as biomarkers of organophosphates: The current divide between epidemiology and clinical toxicology. Clin. Toxicol. 2011;49:771–781. doi: 10.3109/15563650.2011.624101. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (EPA) Exposure Factors Handbook: 2011 Edition. Washington, DC: National Center for Environmental Assessment; 2011. EPA/600/R-09/052F, Available from the National Technical Information Service, Springfield, VA (online at http://www.epa.gov/ncea/efh). [Google Scholar]
- van Buul EJ, Steegers EA, Jongsma HW, Eskes TK, Thomas CM, Hein PR. Haematological and biochemical profile of uncomplicated pregnancy in nulliparous women; a longitudinal study. Neth. J. Med. 1995;46(2):73–85. doi: 10.1016/0300-2977(94)00104-h. [DOI] [PubMed] [Google Scholar]
- White E, Armstrong BK, Saracci R. Principles of exposure measurement in epidemiology: Collecting, evaluating, and improving measures of disease risk factors. 2nd edition. Oxford: Oxford University Press; 2008. p. 98. [Google Scholar]
- Whyatt RM, Garfinkel R, Hoepner LA, Andrews H, Holmes D, Williams MK, Reyes A, Diaz D, Perera FP, Camannand DE, Barr DB. A Biomarker Validation Study of Prenatal Chlorpyrifos Exposure within an Inner-City Cohort during Pregnancy. Environ. Health Perspect. 2009;117(4):559–567. doi: 10.1289/ehp.0800041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VWV, Mackenbach JP, Steegers EAP, Tiemeier H, Longnecker MP. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R study. Environ. Res. 2008;108:260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Angerer J, Meltzer HM, Jaddoe VWV, Tiemeier H, Hoppin JA, Longnecker MP. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa) Int. J. Hyg. Environ. Health. 2009;212:481–491. doi: 10.1016/j.ijheh.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, Barr DB, Furlong CE, Holland NT. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26(2):199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


