Abstract
Background
Clinical stage T2c (cT2c) is an indeterminate factor in prostate cancer (PC) risk stratification. In D’Amico grouping and AUA guidelines, cT2c is high-risk, whereas NCCN and EAU classify cT2c as intermediate-risk. We assessed whether cT2c tumors, without other high-risk factors (cT2c not otherwise specified (cT2c-nos)), behave as intermediate or high-risk by analyzing biochemical recurrence (BCR) after radical prostatectomy.
Methods
We analyzed 2,759 men from SEARCH and 12,900 men from Johns Hopkins Hospital (JHH) from 1988–2011 and 1982–2012, respectively. Patients were grouped into low (PSA<10ng/mL, Gleason sum≤6, and cT1-T2a), intermediate (PSA 10–20ng/mL, Gleason sum 7, or cT2b) and high-risk PC (PSA>20ng/mL, Gleason sum 8–10, or cT3). Men with cT2c who were not otherwise high-risk (i.e. PSA<20 ng/mL and Gleason sum<8) were placed into a separate category termed cT2c -nos. Associations between cT2c-nos and intermediate-risk, and high-risk patients and BCR were tested using log-rank test and Cox proportional analyses models.
Results
99 men (4%) from SEARCH and 202 (2%) from JHH were cT2c-nos. cT2c-nos patients had similar BCR risk as intermediate-risk (SEARCH p=0.27; JHH p=0.23), but significantly lower BCR vs. high-risk (SEARCH p<0.001; JHH p<0.001). When specifically compared to intermediate and high-risk patients, and after adjusting for year and center, cT2c-nos patients had outcomes comparable to intermediate-risk (SEARCH p=0.53; JHH p=0.54), but significantly better than high-risk patients (SEARCH p=0.003; JHH p<0.001).
Conclusions
Patients with cT2c without other high-risk features had similar outcomes as intermediate-risk and significantly better than high-risk PC. These findings suggest men with cT2c should be considered intermediate-risk.
Keywords: biochemical recurrence, clinical staging, D’Amico risk stratification, Gleason score, prostate cancer, prostate specific antigen, radical prostatectomy
INTRODUCTION
In 2008 there were an estimated 899,000 new cases of prostate cancer (PC) and 258,000 deaths worldwide; 72% of the cases and 53% of the deaths occurred in developed countries.1 D’Amico et al. previously developed a risk stratification grouping that defined low (prostate-specific antigen (PSA) <10 ng/ml and Gleason sum 6 and clinical stage T1 or T2a) intermediate (PSA 10–20 ng/ml or Gleason sum 7 or clinical stage T2b) and high-risk (PSA >20 ng/ml or biopsy Gleason sum 8–10 or clinical stage ≥T2c disease) PC, which was shown to be predictive of biochemical recurrence (BCR) and cancer specific mortality rates following radical prostatectomy (RP), external beam radiation therapy (EBRT) or brachytherapy.2 Oncologic and urologic governing bodies, including the National Comprehensive Cancer Network (NCCN), American Urological Association (AUA) and European Urological Association (EAU), have also incorporated PC risk stratification schema into their specific guidelines.3–5
Although PC guidelines are similar among the NCCN, AUA and EAU, inconsistent classification of clinical stage T2c (cT2c) exists. According to the original D’Amico risk stratification schema and the AUA guidelines, cT2c is defined as high-risk, whereas the NCCN and EAU guidelines classify cT2c as intermediate-risk.2–5 Though many clinicians in clinical practice have moved away from a three-tiered risk stratification category towards multivariable models, most guidelines continue to use three-tiered risk groupings. As such, what are the clinical implications of classifying cT2c patients as either intermediate- or high-risk for recurrence after primary treatment? Differences in classification may determine the extent of lymph node dissection at the time of RP, the duration of androgen deprivation therapy (ADT) given concomitantly with EBRT, or eligibility for enrolment in clinical trials. Furthermore, as diagnostic work up with the use of magnetic resonance imaging continues to escalate, clinical staging may become more significant.6–8
Since determining whether cT2c is intermediate- or high-risk has important implications for treatment decisions, it is essential to define what exact risks cT2c portends. The objective of this study was to assess whether cT2c tumors (without associated other high-risk factors for PSA or biopsy Gleason sum) behave as intermediate- or high-risk by analyzing BCR after RP. Using the United States’ Veterans Affairs Medical Center based Shared Equal Access Regional Cancer Hospital (SEARCH) database and a tertiary-care referral center (Johns Hopkins Hospital (JHH)) we tested the hypothesis that men with cT2c tumors behave similarly to intermediate-risk PC patients.
MATERIALS AND METHODS
SEARCH Database Study Population
After obtaining Institutional Review Board approval, data on patients treated with RP from 1988 to 2011 at Veterans Affairs Medical Centers in West Los Angeles, San Diego and Palo Alto, California; Durham and Asheville, North Carolina; and Augusta, Georgia, were combined into the SEARCH database.9 This database includes information on patient age at the time of surgery, race, height, weight, clinical stage, grade of cancer on diagnostic biopsies, preoperative serum PSA value, surgical specimen pathology (specimen weight, tumor grade, tumor volume, stage and surgical margin status), and follow-up serum PSA data. Patients that were treated with either preoperative ADT or radiation therapy were excluded from the database. Clinical T stage was determined by preoperative digital rectal examination. Specifically, cT2c categorization was based on bilateral palpable disease as per the digital rectal examination and not based on biopsy findings in a patient without bilateral palpable disease. Biochemical recurrence was defined as a single PSA above 0.2 ng/mL, 2 concentrations at 0.2 ng/mL or secondary treatment for an elevated PSA.
Of the 3,928 patients in the SEARCH Database, we excluded 202 patients with missing data for preoperative serum PSA values, 428 patients with missing data for biopsy Gleason score, 538 patients with missing data for clinical stage, and one patient for missing data for ethnicity, resulting in a study population of 2,759 patients. There were 22 patients without follow-up data that were not included in BCR analysis. Patients were grouped into low (PSA <10 ng/mL, Gleason sum ≤6, and cT1-T2a), intermediate (PSA 10–20 ng/mL, Gleason sum 7, or cT2b) and high-risk PC (PSA > 20 ng/mL, Gleason sum 8–10, or cT3). Men with clinical stage T2c but who were not otherwise high-risk (i.e. PSA <20 ng/mL and Gleason sum <8) were placed into their own separate category termed cT2c, not otherwise specified (cT2c-nos).
Johns Hopkins Hospital Study Population
After obtaining IRB approval and informed consent when appropriate, consecutive patients treated with RP from 1982 to 2012 at JHH were identified (n=20,795). Men that were treated with either preoperative hormonal therapy or radiation therapy were excluded (n=274). We also excluded patients with missing data for preoperative serum PSA, biopsy Gleason score, clinical stage, and patients with missing data for determining BCR (PSA ≥ 0.2 ng/mL) (n=7621), resulting in a study population of 12,900 patients. Patients were grouped into four risk groups as described above. Both databases undergo routine quality checks to ensure data accuracy.
Statistical Analysis
The distribution of clinicopathologic characteristics for cT2c-nos patients and PC disease risk groups was compared using chi-square analysis for categorical variables, and ANOVA or Kruskal-Wallis test for continuous variables. BCR was examined using the cumulative incidence method, and comparisons between cT2c-nos patients and intermediate and high-risk patients were performed using log-rank test. The association between PC disease risk groups and time to BCR was examined using competing risk Cox proportional hazards analyses. Furthermore, since 1998 (early PSA era), stage and grade migration has occurred and thus PC disease risk groups were stratified by year (≤1998 and >1998) and time to BCR was examined using Cox proportional hazards analyses. Given changes in PC over the years we mutually adjusted for year of surgery (continuous). To adjust for case mix among the centers in SEARCH we included a categorical term for each center (SEARCH only). All statistical analyses were performed using STATA 13.0 (Stata Corp., College Station, Texas) with p<0.05 defined as statistically significant.
RESULTS
Baseline Patient Characteristics
Table 1 lists the clinicopathological characteristics of the patient population as stratified by disease risk group and database (SEARCH vs. JHH). In the SEARCH database, there were 1,124 (41%) low-risk, 1,082 (39%) intermediate-risk and 454 (16%) high-risk patients, and 99 (4%) patients with cT2c-nos tumors. In the JHH cohort, there were 7,541 (58%) low-risk, 4,210 (33%) intermediate-risk and 947 (7%) high-risk patients, and 202 (2%) patients with cT2c-nos tumors. In both the SEARCH and JHH cohort, patients with increasingly higher risk disease were older (p<0.001) and had higher serum PSA levels (p<0.001). Furthermore, black patients were more likely to have high-risk disease and white patients were more likely to be cT2c-nos (SEARCH p=0.048; JHH p<0.001). Patients with cT2c-nos had median preoperative serum PSA values more comparable to intermediate than high-risk patients (Table 1).
Table 1.
Clinical and pathologic features of men undergoing radical prostatectomy.
| SEARCH Database | Johns Hopkins Hospital | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Low Risk | Intermediate Risk |
High Risk | cT2c-nos | p value | Low Risk | Intermediate Risk |
High Risk | cT2c-nos | p value |
| Patients, n | 1124 (41) | 1082 (39) | 454 (16) | 99 (4) | 7541 (58) | 4210 (33) | 947 (7) | 202 (2) | ||
| Age at surgery, mean ± SD | 61.2 ± 6.2 | 62.0 ± 6.2 | 62.4 ± 6.0 | 61.9 ± 7.5 | <0.001* | 57.4 ± 6.5 | 59.2 ± 6.5 | 59.3 ± 6.8 | 59.6 ± 6.1 | <0.001* |
| Median yr surgery | 2003 | 2004 | 2003 | 2002 | 0.09# | 2001 | 2001 | 2000 | 1995 | <0.001# |
| Race, n | 0.048$ | <0.001$ | ||||||||
| White | 678 (60) | 592 (55) | 260 (57) | 68 (69) | 6731 (89) | 3664 (87) | 820 (86) | 190 (94) | ||
| Black | 386 (35) | 420 (39) | 167 (37) | 25 (25) | 518 (7) | 361 (9) | 95 (10) | 9 (4) | ||
| Other | 60 (5) | 70 (6) | 27 (6) | 6 (6) | 292 (4) | 184 (4) | 32 (4) | 3 (2) | ||
| PSA (ng/mL), median (Q1–Q3) | 5.3 (4.2–6.9) | 8.2 (5.3–11.8) | 11.9 (6.0–24.7) | 6.3 (4.6–9.5) | <0.001# | 4.9 (3.6–6.4) | 6.7 (4.6–10.9) | 11.6 (5.7–24.3) | 6.6 (4.6–9.7) | <0.001# |
| Clinical T Stage, n | <0.001$ | <0.001$ | ||||||||
| T1 | 821 (73) | 621 (57) | 247 (54) | 0 | 5954 (79) | 2332 (55) | 458 (48) | 0 | ||
| T2 | 303 (27) | 461 (43) | 189 (42) | 99 (100) | 1587 (21) | 1878 (45) | 416 (44) | 202 (100) | ||
| T3 | 0 | 0 | 18 (4) | 0 | 0 | 0 | 73 (8) | 0 | ||
| Biopsy Gleason sum, n | <0.001$ | <0.001$ | ||||||||
| 2–6 | 1124 (100) | 330 (31) | 82 (18) | 59 (60) | 7541 (100) | 1497 (36) | 231 (25) | 132 (65) | ||
| 7 | 0 | 752 (69) | 83 (18) | 40 (40) | 0 | 2709 (64) | 165 (17) | 70 (35) | ||
| 8–10 | 0 | 0 | 289 (64) | 0 | 0 | 0 | 551 (58) | 0 | ||
SEARCH = Shared Equal Access Regional Cancer Hospital; SD = standard deviation; PSA = prostate specific antigen
Values are number (percentage) unless otherwise staged
p value from analysis of variance
p value from Kruskal-Wallis equality-of-populations rank test
p value from chi-square
Prostate Cancer Risk Groups and BCR
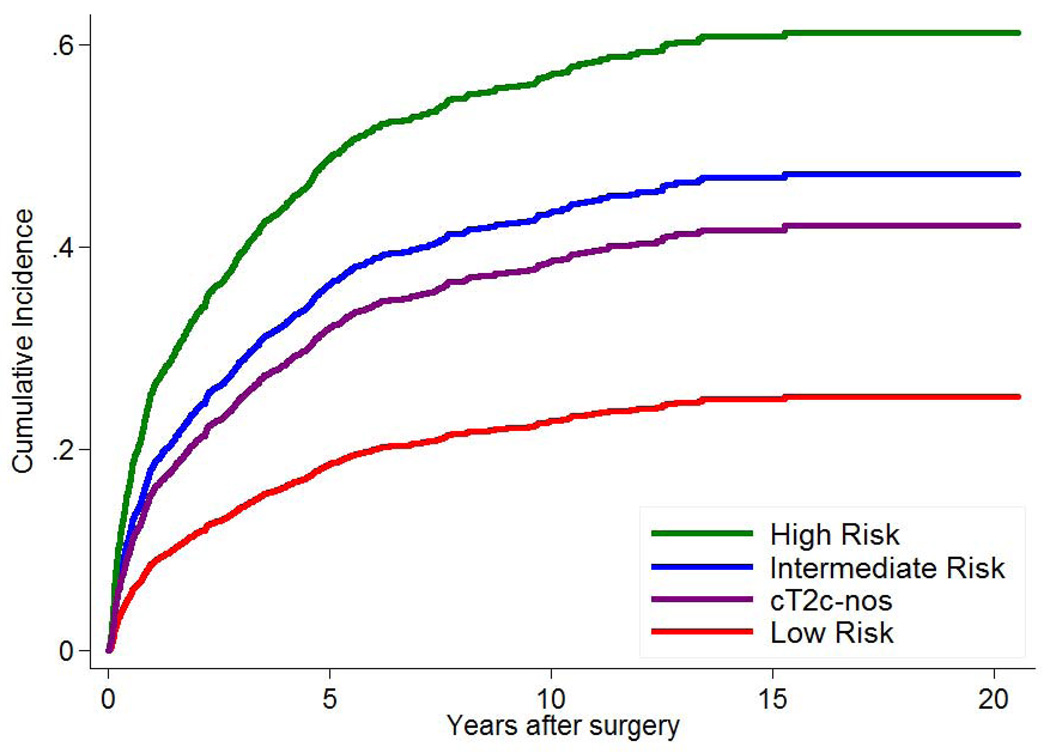
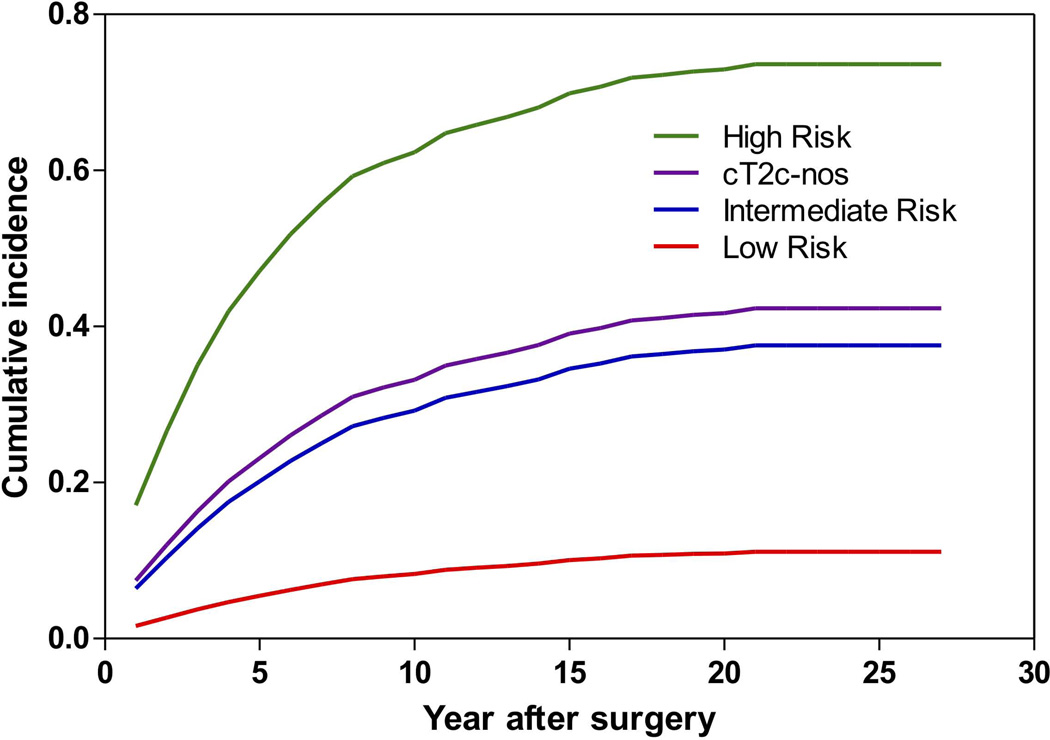
During a median follow-up of 66 months (IQR: 34–101 months) for the SEARCH cohort there were 247 (22%) low-risk, 432 (40%) intermediate-risk and 231 (51%) high-risk patients, and 37 (37%) cT2c-nos patients that experienced BCR. During a median follow-up of 48 months (IQR: 24–108 months) for the JHH cohort there were 424 (6%) low-risk, 870 (21%) intermediate-risk and 463 (49%) high-risk patients, and 60 (30%) cT2c-nos patients that experienced BCR. As expected, patients with high-risk disease had the worst outcomes, those with low-risk the best and those with intermediate risk were in the middle. When examining cT2c-nos patients, they had similar BCR risk as intermediate-risk patients (log-rank; SEARCH p=0.27; JHH p=0.23), but significantly lower BCR risk compared to high-risk patients (log-rank; SEARCH p<0.001; JHH p<0.001) (Figure 1A–B).
Figure 1.
Cumulative incidence curve of biochemical recurrence by prostate cancer risk for (A) the SEARCH database and (B) Johns Hopkins Hospital.
Overall patterns were unchanged after adjusting for year and surgical center (Table 2). Specifically, high-risk men did the worst with intermediate doing worse than low-risk. Of note, relative to low-risk patients, the HRs for BCR for the cT2c-nos patients were nearly identical to those for intermediate-risk disease (SEARCH HR=1.89 vs. 2.21; Hopkins HR=4.26 vs. 3.93). When specifically compared to intermediate- and high-risk patients, and after adjusting for year and center, again, cT2c-nos patients had outcomes comparable to intermediate-risk (SEARCH p=0.53; Hopkins p=0.54), but significantly better than high-risk patients (SEARCH p=0.003; Hopkins p<0.001; Table 3).
Table 2.
Risk of biochemical recurrence among patients undergoing radical prostatectomy stratified by prostate cancer disease risk with competing risk of death.
| SEARCH Database | Johns Hopkins Hospital | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk | Univariable HR (95% CI) |
p value | Multivariable HR (95% CI)* |
p value | Univariable HR (95% CI) |
p value | Multivariable HR (95% CI)** |
p value |
| Low | Reference | - | Reference | - | Reference | - | Reference | - |
| Intermediate | 2.13 (1.83, 2.49) | <0.001 | 2.21 (1.89, 2.58) | <0.001 | 4.00 (3.56, 4.48) | <0.001 | 3.93 (3.51, 4.41) | <0.001 |
| High | 3.18 (2.65, 3.82) | <0.001 | 3.28 (2.72, 3.95) | <0.001 | 11.30 (9.92, 12.87) | <0.001 | 10.89 (9.55, 12.42) | <0.001 |
| cT2c-nos | 1.84 (1.31, 2.58) | <0.001 | 1.89 (1.33, 2.68) | <0.001 | 4.67 (3.58, 6.09) | <0.001 | 4.26 (3.26, 5.58) | <0.001 |
SEARCH = Shared Equal Access Regional Cancer Hospital; HR = hazard ratio; CI = confidence interval
Cox proportional hazards analysis adjusted for year of surgery and center
Cox proportional hazards analysis adjusted for year of surgery
Table 3.
Comparison of biochemical recurrence among patients undergoing radical prostatectomy with intermediate-risk or high-risk disease or cT2c disease, not otherwise specified with competing risk of death.
| SEARCH Database | Johns Hopkins Hospital | |||
|---|---|---|---|---|
| Risk | Multivariable HR (95% CI)* | p value | Multivariable HR (95% CI)** | p value |
| Intermediate | Reference | - | Reference | - |
| cT2c-nos | 0.95 (0.80, 1.12) | 0.53 | 1.08 (0.84, 1.40) | 0.54 |
| High | Reference | - | Reference | - |
| cT2c-nos | 0.59 (0.42, 0.84) | 0.003 | 0.39 (0.30, 0.51) | <0.001 |
SEARCH = Shared Equal Access Regional Cancer Hospital; HR = hazard ratio; CI = confidence interval
Cox proportional hazards analysis adjusted for year of surgery and center
Cox proportional hazards analysis adjusted for year of surgery
When assessing risk of BCR among PC risk groups stratified by year of surgery, cT2c-nos patients were similar in BCR risk to intermediate-risk patients in both cohorts and both time periods (all HR 0.91–1.23, all p≥0.39) (Supplementary Table 1 and 2). In contrast, cT2c-nos patients had better outcomes than high-risk patients in both cohorts and both eras (all HR ≤0.62), though due to small numbers of some of these subsets, the results did not always reach statistical significance (all p≤0.14).
DISCUSSION
Given the discordance between various guidelines of whether cT2c PC should be classified as intermediate- or high-risk disease, we sought to analyze two large patient cohorts to further delineate the true behavior of cT2c tumors. We unequivocally found that cT2c-nos tumors were more closely congruent with intermediate-risk patients when assessing for BCR after RP. The two patient populations analyzed (SEARCH – veteran population; JHH – tertiary referral center) were demographically distinct and provide a degree of generalizability for these findings. Our findings also corroborate the findings from the CaPSURE group of community-based men that those with cT2c disease do not warrant high-risk classification.10
Pre-treatment risk stratification of PC patients allows clinicians to tailor treatment algorithms appropriately and counsel patients regarding treatment expectations, risk of recurrence and probability of disease progression. However, three of the most common groups that issue PC guidelines have incongruent definitions of what characteristics portend high-risk disease. The AUA, based upon the original D’Amico risk grouping, classifies high-risk patients as cT2c or Gleason 8–10 or PSA > 20 ng/mL.2,4 Conversely, the EAU and NCCN classify high-risk patients as cT3a or Gleason score 8–10 or PSA >20 ng/mL.3,5 These differences in classification can make comparison of studies and clinical trial outcomes difficult due to the heterogeneity of the patient populations.
Previous studies have suggested that clinical staging of PC is often inaccurate and that clinical stage may add minimal information when risk stratifying patients.11–13 To overcome these limitations, other risk stratification schemas have been proposed and externally validated to provide more accurate risk assessment.14–20 For example, Cancer of the Prostate Risk Assessment Score (CAPRA) provides a predictor of disease recurrence after RP and incorporates PSA, biopsy Gleason score, clinical T stage, percent positive biopsy and age into the point-calculated algorithm.15 In the initial analysis of 1,439 men with PC, the authors reported a concordance index of 0.66; in this cohort clinical stage T1 and T2 patients were given equal point weighting. Subsequently, the CAPRA score was externally validated using the SEARCH database population with a reported concordance index of 0.68.16 Many other models and nomograms to predict recurrence have been published and validated, which all use multivariable models not just segregating patients into low, intermediate, and high-risk.21–22 More recent studies have incorporated molecular markers to improve contemporary risk stratification.18–20
Despite these advances in PC risk stratification, particularly at the molecular level, clinical stage remains an integral entity for risk stratification and has important clinical implications. Firstly, the EAU and NCCN have specific guidelines for determining the indication for pelvic lymph node dissection (PLND) at the time of RP, both of which utilize clinical stage in their algorithm. The EAU recommends a PLND for patients with a >7% risk of lymph node invasion (LNI), calculated using the Briganti et al. nomogram; this nomogram takes into account clinical stage, PSA and Gleason biopsy score.23 NCCN utilizes the Cagiannos et al. nomogram to assess whether patients would benefit from a PLND.24 Patients with a >2% risk of LNI are recommended to have a PLND, which is calculated based on the patient’s pre-treatment PSA, age, biopsy primary and secondary Gleason grade, number of positive biopsy cores, and clinical tumor stage. Secondly, clinical tumor stage is also important for patients who choose EBRT as their primary treatment. Patients who have intermediate-risk PC typically receive 4–6 months of ADT, while those with high-risk disease receive ≥ 2 years of ADT. Multiple randomized controlled trials, including those from the Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC) have demonstrated a survival and progression benefit for patients with more extensive localized disease to receive longer duration ADT.25–28 However, inaccurately upstaging a patient’s risk based on clinical stage subjects the patient to longer-term ADT and the potential long-term consequences of prolonged ADT.29–30 Thirdly, whether a patient is classified as intermediate- or high-risk may determine clinical trial eligibility and recruitment. For example, our finding that cT2c behaves more like intermediate-risk means these men should not be included in trials for high-risk men. Doing so would not only expose these men to potentially more toxic treatments than their disease aggressiveness would warrant but also means enrolling a lower risk cohort than desired which leads to fewer end-points reached, long time for study follow-up, and potentially underpowering the clinical trial. As such, our findings regarding the prognostic value of cT2c have multiple important clinical implications.
The strengths of the current study include the patient diversity of the two datasets with mature, well-established, long-term detailed outcome data. The main limitation of the current study is its retrospective design. In addition, cT2c is uncommon and there is recognizable interobserver variability associated with a digital rectal examination to determine clinical stage. Finally, we acknowledge that these are surgical data and may not directly apply to patients managed with radiation. A recent study highlights the heterogeneous behavior of intermediate-risk PC in men undergoing dose-escalated EBRT31, suggesting that patients with favorable intermediate-risk PC may only need monotherapy comparable to low-risk PC, whereas men with unfavorable intermediate-risk PC may need combination ADT similar to high-risk PC.
CONCLUSIONS
Among patients undergoing RP, risk of BCR for patients with clinical stage T2c disease was comparable to men with intermediate-risk disease and significantly better than men with high-risk PC. These findings suggest men with cT2c PC should be counseled appropriately and offered treatment options for intermediate-risk disease. Classifying patients with cT2c as intermediate-risk and standardizing risk stratification between the AUA, EAU and NCCN will allow meaningful and accurate comparison of clinical outcomes across studies. With recent advances in genomics and pre-treatment staging imaging, the long-term durability of clinical staging remains to be determined, however at the present time, PC risk stratification governing bodies may consider reclassification of cT2c to intermediate-risk disease.
Supplementary Material
Acknowledgments
Funding Support - Research support was received from the Department of Veterans Affairs, the National Institutes of Health (NIH) (grant R01CA100938 to Dr. Aronson), NIH Specialized Programs of Research Excellence Grant P50 CA92131-01A1 (to Dr. Aronson), the Georgia Cancer Coalition (to Dr. Terris), and NIH grant K24CA160653 (to Dr. Freedland).
Footnotes
Financial Disclosures – no disclosures for any authors.
REFERENCES
- 1.Center MM, Jemal A, Lortet-Tieulent J, et al. International Variation in Prostate Cancer Incidence and Mortality Rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL, Armstrong AJ, Bahson RR, et al. Oncology – Prostate Cancer, Version 4. National Comprehensive Cancer Network; 2013. [Accessed Aug 6, 2013]. NCCN Clinical Practice Guidelines. Available from URL: www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. [Google Scholar]
- 4.Thompson I, Thrasher JB, Aus G, et al. Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bastian PJ, Bellmunt J, et al. Guidelines on Prostate Cancer – 2013. European Urological Association; [Accessed July 30, 2013]. Available from URL: www.uroweb.org/gls/pdf/09_Prostate_Cancer_LR.pdf. [Google Scholar]
- 6.Shukla-Dave A, Hricak H, Akin O, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int. 2012;109:1315–1322. doi: 10.1111/j.1464-410X.2011.10612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van As NJ, de Souza NM, Riches SF, et al. A study of diffusion-weighted magnetic resonance imaging in men with untreated localized prostate cancer on active surveillance. Eur Urol. 2009;56:981–987. doi: 10.1016/j.eururo.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 8.D’Amico AV, Whittington R, Malkowicz SB, et al. Role of percent positive biopsies and endorectal coil MRI in predicting prognosis in intermediate-risk prostate cancer patients. Cancer J Sci Am. 1996;2:343–350. [PubMed] [Google Scholar]
- 9.Allott EH, Abern MR, Gerber L, et al. Metformin does not affect risk of biochemical recurrence following radical prostatectomy: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2013;16:391–397. doi: 10.1038/pcan.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooperberg MR, Cowan J, Broering JM, et al. High-risk prostate cancer in the United States, 1990–2007. World J Urol. 2008;26:211–218. doi: 10.1007/s00345-008-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reese AC, Sadetsky N, Carroll PR, et al. Inaccuracies in Assignment of Clinical Stage for Localized Prostate Cancer. Cancer. 2011;117:283–289. doi: 10.1002/cncr.25596. [DOI] [PubMed] [Google Scholar]
- 12.Reese AC, Cooperberg MR, Carroll PR. Minimal Impact of Clinical Stage on Prostate Cancer Prognosis Among Contemporary Patients with Clinically Localized Disease. J Urol. 2010;184:114–119. doi: 10.1016/j.juro.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Gosselaar C, Kranse R, Roobol MJ, et al. The Interobserver Variability of Digital Rectal Examination in a Large Randomized Trial for the Screening of Prostate Cancer. The Prostate. 2008;68:985–993. doi: 10.1002/pros.20759. [DOI] [PubMed] [Google Scholar]
- 14.Eifler JB, Feng Z, Lin BM, et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2012;111:22–29. doi: 10.1111/j.1464-410X.2012.11324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment Score: A Straightforward and Reliable Preoperative Predictor of Disease Recurrence After Radical Prostatectomy. J Urol. 2005;173:1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Freedland SJ, Pasta DJ, et al. Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006;107:2384–2391. doi: 10.1002/cncr.22262. [DOI] [PubMed] [Google Scholar]
- 17.Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 18.Cuzick J, Swanson GP, Fisher G, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: A retrospective study. Lancet Oncol. 2011;12:245–255. doi: 10.1016/S1470-2045(10)70295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuzick J, Berney DM, Fisher G, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer. 2012;106:1095–1099. doi: 10.1038/bjc.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooperberg MR, Simko JP, Cowan JE, et al. Validation of a Cell-Cycle Progression Gene Panel to Improve Risk Stratification in a Contemporary Prostatectomy Cohort. J Clin Oncol. 2013;31:1428–1434. doi: 10.1200/JCO.2012.46.4396. [DOI] [PubMed] [Google Scholar]
- 21.Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715–717. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briganti A, Chun FK, Salonia A, et al. Validation of a nomogram predicting the probability of lymph node invasion among patients undergoing radical prostatectomy and an extended lymphadenectomy. Eur Urol. 2006;49:1019–1026. doi: 10.1016/j.eururo.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Cagiannos I, Karakiewicz P, Eastham JA, et al. A preoperative nomogram identifying decreased risk of positive lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–1803. doi: 10.1097/01.ju.0000091805.98960.13. [DOI] [PubMed] [Google Scholar]
- 25.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): A phase III randomized trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 26.Hanks GE, Pajak TF, Porter A, et al. A phase III trial of long term adjuvant androgen suppression following neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The RTOG protocol 92-02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow up of a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer: RTOG 92-02. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 28.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 29.Alibhai SM, Gogov S, Allibhai Z. Long-term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: a systematic literature review. Crit Rev Oncol Hematol. 2006;60:201–215. doi: 10.1016/j.critrevonc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Tsai HK, D’Amico AV, Sadetsky N, et al. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 31.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.