Abstract
Alzheimer dementia (AD) is an important clinical problem that appears to be closely tied to comorbid cardiovascular disease, making it a relevant topic for the clinical cardiologist. Determinants of cardiovascular health, especially midlife dyslipidemia, are associated with an increased risk of dementia based on molecular and epidemiologic data. Given the potential role of dyslipidemia in the development of dementia, statins have been investigated as potential therapeutic options to slow or prevent disease. This review discusses the role of dyslipidemia and other cardiovascular risk factors in the pathogenesis of AD, with a focus on the existing evidence for the use of statin medications in the treatment and prevention of AD from observational studies and randomized clinical trials. Clinical questions for the practicing cardiologist are addressed.
Introduction
Alzheimer dementia (AD) is an important clinical problem that cardiologists in particular will commonly see, as it is associated with cardiovascular disease (CVD) risk factors as well as atherosclerotic vascular disease. Alzheimer dementia is now estimated to affect 5.2 million adults in the United States, equating to roughly 1 in 9 individuals age >65 years.1 The prevalence of AD is expected to increase 40% by 2025, reflecting the growth of an aging group of baby boomers and the expanding proportion of the population age >65 years.
Observational data have noted an increased burden of CVD in patients with AD,2 and cardiovascular risk factors, especially dyslipidemia, are associated with an increased risk of AD development.3 Statins, or 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase inhibitors, are the most widely prescribed cardiovascular therapy4 and are first‐line agents for lipid‐lowering pharmaceutical therapy.5 Cohort data suggest that statins are associated with a lower risk of dementia and have generated consideration of their therapeutic potential to reduce AD.6
The sheer prevalence of AD, combined with the increased burden of CVD among those with the condition and observations that modification of lipids may play a role in attenuating disease development, make this an important topic for clinical cardiologists and cardiovascular researchers. The aim of this review is to present an overview of the existing evidence for the role of lipids and other cardiovascular risk factors in AD pathogenesis and current data for the potential of statins in the prevention and treatment of AD.
Alzheimer Dementia and the Cholesterol Hypothesis
The neuropathologic hallmark of AD in the brain consists of 2 microscopic observations: the development of extracellular neuritic plaques consisting of amyloid β (Aβ) protein, and neurofibrillary tangles within cells consisting of phosphorylated tau protein. These abnormalities are thought to disrupt synaptic transmission through structural interference and to invoke inflammatory cytotoxic pathways that ultimately lead to neuronal cell death.7
Observational studies have shown elevated cholesterol at midlife to be an independent risk factor for the development of AD.8 In one large retrospective cohort study, high total cholesterol (TC) >240 mg/dL 30 years prior to diagnosis carried a 57% higher risk for AD (hazard ratio [HR]: 1.57, 95% confidence interval [CI]: 1.23‐2.01).9 Elevated low‐density lipoprotein cholesterol (LDL‐C) and TC levels earlier in life have also been found to be associated with the discovery of Aβ plaques within the brain at autopsy,10 even in individuals as young as age 40 to 55 years.11 On the basis of such epidemiologic and observational data, some have suggested that cholesterol‐dependent mechanisms are at play in the pathogenesis of AD.12
Cellular and molecular data also implicate cholesterol pathways in AD development. Apolipoprotein E (ApoE) is the major cholesterol transporter in the brain, and one of the major advances in the understanding of AD pathogenesis was the discovery of the ApoE4 allele. Increased expression of this form of the gene is associated with increased risk of disease development and a younger age at AD presentation, especially in ApoE4/E4 homozygotes.13
The Aβ protein that comprises senile plaques is produced from cleavage of the amyloid precursor protein, but this protein is capable of undergoing differential processing via competing enzymatic pathways. Cleavage of the amyloid precursor protein by the β‐secretase pathway results in the Aβ seen in AD, whereas cleavage via α‐secretase produces a benign, soluble product.14 Decreased cholesterol levels have been shown in vitro to increase α‐secretase activity and tip the balance away from the nonsoluble Aβ product.15
The relationship between serum cholesterol concentration and the development of AD is likely more complex, especially given that cholesterol does not readily cross the blood‐brain barrier and most of the brain's cholesterol is synthesized de novo.16 Additionally, not all observational studies have noted increased serum cholesterol levels among those who develop AD pathology,17 and some investigators remain skeptical of a causative relationship.12 Nevertheless, cholesterol‐dependent pathways remain a major focus in the investigation of AD and have provided the impetus for the exploration of potential therapeutic targets.
The Role of Other Cardiovascular Risk Factors in the Development of Alzheimer Dementia
Epidemiologic studies have also implicated other cardiovascular risk factors such as obesity, hypertension, smoking, diabetes mellitus, and peripheral arterial disease in the development of cognitive decline and AD. Kivipelto et al analyzed health parameters of nondemented subjects who were followed for a mean of 21 years as part of the Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) study. They found that midlife obesity (body mass index >30 kg/m2) was associated with an increased risk of incident AD (odds ratio: 2.4, 95% CI: 1.2‐5.1).18 This effect appeared to be additive with midlife hypertension (systolic blood pressure >140 mm Hg) and elevated TC (>251 mg/dL). Men enrolled in the Honolulu‐Asia Aging Study with untreated diastolic hypertension (>95 mm Hg) at study onset were more likely to develop AD 2 decades later (odds ratio: 4.47, 95% CI: 1.53‐13.09), though this association was not observed in those who had received treatment for hypertension.19 A recent analysis of 13 476 patients in the Atherosclerotic Risk In Communities (ARIC) cohort found hypertension diagnosed between the age of 48 and 67 years to be associated with significantly worse performance on a variety of cognitive batteries 20 years later.20 This association was attenuated by antihypertensive treatment, and there was no association between late‐life hypertension and cognitive change.
Participants of the Cardiovascular Health Study (CHS) cohort with a prior diagnosis of peripheral arterial disease were likewise more likely to develop incident AD after 5 years of follow‐up (HR: 2.2, 95% CI: 1.1‐4.5).2 Furthermore, midlife tobacco use of >2 packs per day carried a 2‐fold risk of later AD development (HR: 2.57, 95% CI: 1.63‐4.03), even after controlling for obesity, hypertension, diabetes mellitus, and cholesterol in another prospective study.21 Diabetes has also been identified as a significant independent risk factor in multiple studies.22, 23 In those with already‐established AD, vascular comorbidities including hypertension and atrial fibrillation have been associated with more precipitous decline in cognitive performance compared with those without such conditions.24
Given shared risk factors, it is not surprising that there is considerable overlap between CVD and cognitive impairment. Recent data from survivors of myocardial infarction have shed light on this. Gharacholou et al examined 772 patients age >65 years in the Translational Research Investigating Underlying Disparities in Recovery From Acute Myocardial Infarction: Patients' Health Status (TRIUMPH) Registry of myocardial infarction survivors using telephone cognitive assessments 1 month postinfarction.25 They found at least some degree of cognitive impairment in 56% of the 772 patients studied, suggesting an intimate relationship between cognitive status and CVD.
Many of the studies that found an increased risk of AD in the presence of cardiovascular risk factors also found an increased risk for vascular dementia, where cognitive decline is the result of sequential cerebral infarctions.19, 21, 22, 23 Indeed, one of the major challenges in exploring the relationship between AD and cardiovascular risk factors is distinguishing AD from vascular dementia, where the role of factors affecting vessel health is perhaps more obvious. The distinction is not straightforward, and in one review of postmortem dementia pathology, 30% of those with a diagnosis of AD had cerebrovascular disease, whereas a similar proportion of those diagnosed with vascular dementia also demonstrated hallmarks of AD pathology.26 The 2 entities are often difficult to differentiate clinically27 and can even coexist in some patients.1 It may ultimately be that the observed diagnostic and pathologic overlap between AD and vascular dementia occurs because the disruption of vascular integrity is a common etiologic pathway in both diseases.
Statins as Targets for Therapeutic Intervention
On the basis of intriguing basic science data and observational studies supporting the cholesterol hypothesis, investigation of the potential therapeutic benefit of statin medications has been pursued with vigor. A series of case‐control studies suggested a protective effect for statins,6, 28, 29, 30, 31 but the most supportive data have come from several prospective observational cohort studies analyzing statin use and later AD development.
In their 2009 analysis of the Rotterdam Study, Haag et al studied 6992 Dutch patients age >55 years for a mean follow‐up time of 9 years.32 Among statin users, they found a 43% lower incidence of AD (HR: 0.57, 95% CI: 0.37‐0.90), which persisted even after controlling for TC. Li et al followed 3099 subjects for a mean of 6.1 years and found a HR of 0.62 (95% CI: 0.40‐0.97) for the use of statins and incident AD.33 In this study, the strength of association decreased with advancing age, and the association was nonsignificant for individuals age >80 years.
Betterman et al conducted a secondary analysis of 3069 patients in the Ginkgo Evaluation of Memory Study (GEMS) and found a similar protective association (HR: 0.57, 95% CI: 0.39‐0.85) after a mean follow‐up period of 6 years.34 Additional prospective observational studies have agreed with these findings,35 though some36, 37 did not exclude other forms of dementia in their analyses. Some cohorts have failed to observe an association.38, 39, 40, 41 A 2013 meta‐analysis of 8 prospective cohort studies found an overall relative risk reduction of 0.62 (95% CI: 0.43‐0.81).42 This analysis included studies that were not specific to AD, a reminder that the distinction between AD, vascular dementia, and other diagnoses is not always straightforward. Another meta‐analysis weighing 8 of the highest‐quality prospective studies found an overall HR of 0.71 (95% CI: 0.61‐0.82; Figure 1).43 In the most recent systematic review, Richardson et al found a relative risk reduction specific to AD of 0.79 (95% CI: 0.63‐0.99) in a pooled analysis of 10 cohort studies.44
Figure 1.
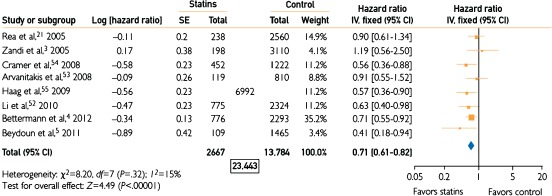
Forest plot of quantitative synthesis for long‐term cognition showing incidence of dementia in the statin vs placebo groups. The total N (6992) for the study of Haag et al is presented; it is not differentiated between statins and control because the analysis was performed at the drug‐exposure level rather than patient level. Combined with the Ns in the statins and control columns, the total N for analysis was 23 443. Reproduced with permission from Swiger et al.43 Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
Although most investigators controlled for comorbid conditions, a risk of indication bias remains in such observational studies. Statin use may, for example, simply be a marker for increased contact with medical care or other demographic and lifestyle factors. A case‐control study by Rockwood et al sought to address this specific question. Although statin use was protective for the development of AD, it was not associated with differences in exercise, educational level, or self‐reported health.30 Therefore, although indication bias was not supported by this analysis, it remains theoretically possible.
Potential Mechanisms of Statin Protection
Support for statin effect has come from laboratory data as well, and numerous mechanisms have been proposed to explain their potential benefit in AD pathology (Figure 2). The most basic is that modifications in serum and brain cholesterol promote a nonamyloidogenic processing pathway at the level of the cell surface.15, 45 Animal data have shown a reduction in the formation of Aβ product with statin treatment,46 whereas investigators have found a decrease in postmortem AD pathology in humans treated with statins.47
Figure 2.
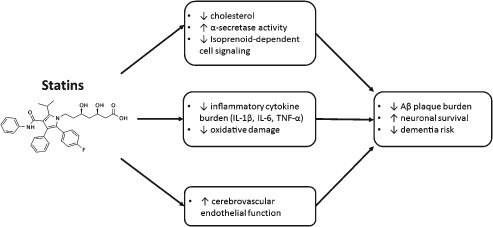
Proposed mechanisms for statin effects in the CNS. Abbreviations: Aβ, amyloid β; CNS, central nervous system; IL‐1β, interleukin‐1β; IL‐6, interleukin‐6; TNF‐α, tumor necrosis factor‐α.
However, central nervous system effects are unlikely to be explained by lipid reduction alone. Statins have been shown to reduce inflammatory cytokines in the brains of mice, including interleukin‐1β, interleukin‐6, and tumor necrosis factor‐α.48 Other potential targets include reduced oxidative and nitrosative stress.49 Just as statins promote nitric oxide synthase in the coronary endothelium, they may also work to improve and protect cerebral blood flow,50 and further data are emerging strengthening the association between vessel health and Aβ deposition, providing biologic plausibility to the overlap between vascular disease and AD.51
Finally, there is evidence that statin effects in the central nervous system may be mediated by their ability to modify upstream products in the cholesterol synthesis pathway. As statins inhibit the conversion of HMG‐CoA to mevalonate, they block not only the production of the cholesterol end‐product, but also other mevalonate‐derived products called isoprenoids, including farnesyl pyrophosphate and geranyl pyrophosphate. These compounds are attached through post‐translational modification to alter the activity of cell‐signaling proteins like Ras and Rho, which may in turn affect neuronal metabolic activity and amyloid processing.52
Concerns Regarding Statin Effect on Short‐term Cognition
In 2012, the US Food and Drug Administration released a safety label change for statin medications describing the potential for reversible cognitive side effects including “memory loss” and “forgetfulness,”53 citing observational analyses of patients who experienced such symptoms. For example, Evans et al surveyed a group of 171 patients who reported varying cognitive side effects with statin use.54 These effects were generally reversible with discontinuation and not related to the development of dementia or other long‐term cognitive pathology. However, because the brain is a cholesterol‐rich environment relying upon lipids for cellular activities and neurotransmission,52 the concern that intense lipid reduction may actually lead to adverse cognitive effects is worth investigating.
Two recent analyses have addressed this issue. In addition to examining the relationship between statin use and AD, Richardson et al also analyzed the effect on cognitive performance in cognitively intact patients treated with statins.44 After examining 1 large controlled trial and numerous cohort studies that provided initial and follow‐up cognitive assessments in those without a previous deficit, no negative effect was observed. Swiger et al examined 8 high‐quality studies in their 2013 meta‐analysis and found no adverse signal in short‐term cognitive outcomes among statin treatment groups.43
In the Evans et al report, the mean total cholesterol in those reporting cognitive side effects was not particularly low (191 mg/dL), suggesting that any such effects are unlikely to be related to extremely low cholesterol levels.54 Proprotein convertase subtilisin‐kexin type 9 inhibitors are a new generation of lipid‐lowering medications capable of dramatic reductions in serum cholesterol. Early trials with these agents have driven LDL‐C levels <60 mg/dL without any reported cognitive side effects at 1 year.55 When combined with high‐intensity statins (atorvastatin 80 mg/d), LDL‐C levels may even drop below 40 mg/dL, with early data showing no significant increase in reported neurocognitive adverse events.56 Further follow‐up of studies utilizing these novel medications may shed more light on the potential of lipid‐lowering medications to cause reversible short‐term cognitive side effects in a select group of patients.
Randomized Trials for Statins in the Treatment of Alzheimer Dementia
Observational and laboratory data have provided the impetus for several clinical trials of statins in the treatment of already‐established AD. Most trials tracked therapeutic benefit through follow up neuropsychiatric evaluations using the Alzheimer Disease Assessment Scale–Cognitive (ADAS‐Cog). This is a validated measurement tool to assess disease severity ranging from 0 to 70 points, with higher scores indicating increased cognitive impairment.57 The results of these relatively small trials have been generally disappointing (Table 1).
Table 1.
Randomized Clinical Trials of Statins in Established AD
| Trial | Subjects | Intervention Group | Interval | Outcome |
|---|---|---|---|---|
| ADCLT58 | 67 patients with mild‐moderate AD | Atorvastatin, 80 mg/d | 1 year | Slight improvement in ADAS‐Cog at 6 months (3.5 points; P = 0.003). No significant difference at 1 year. |
| LEADe60, 61 | 640 patients with mild‐moderate AD | Atorvastatin, 80 mg/d | 18 months | No significant change in ADAS‐Cog or other inventories |
| CLASP62 | 406 patients with mild‐moderate AD | Simvastatin, 40 mg/d | 18 months | No significant change in ADAS‐Cog or other measures of cognition |
Abbreviations: AD, Alzheimer dementia; ADAS‐Cog, Alzheimer Disease Assessment Scale–Cognitive; ADCLT, Alzheimer Disease Cholesterol‐Lowering Treatment trial; CLASP, Cholesterol‐Lowering Agent to Slow the Progression of AD; LEADe, Lipitor's Effect in Alzheimer Dementia study.
The 2006 Alzheimer's Disease Cholesterol‐Lowering Treatment (ADCLT) trial randomized 67 patients with mild‐to‐moderate AD to receive atorvastatin 80 mg daily or placebo and measured ADAS‐Cog scores 6 months and 1 year after treatment.58 At 6 months, the investigators found a statistically significant improvement in ADAS‐Cog scores in the treatment group (3.5 points; P = 0.003), though the change was below the 4‐point threshold generally considered to be clinically meaningful.59 Moreover, this effect lost statistical significance at 1 year of follow‐up.
The Lipitor's Effect in Alzheimer's Dementia (LEADe) study randomized 640 patients with mild‐to‐moderate AD to atorvastatin 80 mg daily vs placebo.60, 61 Participants in the study were already taking acetylcholinesterase inhibitors (donepezil) as treatment for their dementia. After 18 months of follow‐up, there was no observed difference in cognitive performance between the 2 groups as measured by ADAS‐Cog scores or other neuropsychiatric inventories.
Finally, the most recent Cholesterol‐Lowering Agent to Slow the Progression of AD (CLASP) trial randomized 406 individuals with mild‐to‐moderate AD to receive simvastatin 20 mg daily for 6 weeks followed by 40 mg daily vs placebo.62 Again, after 18 months of treatment, there were no significant differences in ADAS‐Cog scores or other measures of disease progression.
It should not be surprising that such attempts to attenuate disease development in mild‐to‐moderate AD have been unsuccessful. Laboratory data demonstrate that statins may prevent the formation of new senile neuritic plaques46; however, data have not indicated that statin therapy can regenerate neurons that have already been damaged beyond repair or adequately dissolve existing plaques. As Rosenblum suggests, trying to prevent further Aβ production in the setting of an already heavy burden of disease is likely a futile endeavor.63 Randomized trials of statins in established AD may have missed the window of opportunity in the natural history of the disease, intervening too late, when damage is already irreversible or there is little left to preserve. Because already‐demented patients may not be the most appropriate group to target, new research is being directed toward earlier intervention.
The Trial of Simvastatin in Amnestic Mild Cognitive Impairment (MCI) Patients (SIMaMCI; http://www.clinicaltrials.gov NCT00842920) is an ongoing multicenter trial in Germany seeking to address the utility of earlier intervention with statin therapy in those at highest risk of developing AD before they progress to full‐blown disease.64 Eligible subjects include individuals age 55 to 90 years who have mild impairments in memory but no other evidence of dementia. Patients will be randomized to simvastatin 60 mg daily vs placebo and followed for a minimum of 48 months to assess for conversion to dementia using the Clinical Dementia Rating (CDR) scale and other secondary measures including the ADAS‐Cog. The hope is that earlier intervention may yield more encouraging results.
Statins as Primary Prevention for Cognitive Decline: Data From Randomized Trials
Although the therapeutic benefit of statins in the treatment of established AD has been disappointing, the role of statins in the primary prevention of AD has not been adequately addressed in a randomized trial designed for this purpose. The only existing data come from trials designed primarily to assess cardiac endpoints. The Medical Research Council/British Heart Foundation (MRC/BHF) Heart Protection Study (HPS) examined 20 536 patients in the United Kingdom age 40 to 80 years randomized to simvastatin 40 mg/d or placebo.65 Dementia and cognitive impairment were exploratory outcomes based on reported diagnosis and end‐of‐trial telephone cognitive questionnaire performance. The trial found no differences between the 2 groups in the incidence of cognitive impairment after 5 years (23.7% of the simvastatin group vs 24.2% of the placebo group). In addition, equal numbers of patients developed incident dementia in each treatment arm, although the numbers were very low (31 patients, or 0.3%, respectively).
The Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER) trial randomized 5804 individuals age 70 to 82 years to pravastatin 40 mg/d or placebo.66, 67 Participants underwent repeat cognitive testing at regular intervals to assess memory and executive function. Though new diagnoses of dementia were not reported, there was no statistically significant difference in cognitive function between the treatment and control groups after an average of 42 months of follow‐up. Other randomized trials of statins in nondemented subjects have focused on indirect outcomes, such as AD biomarkers, and were not continued long enough to assess for a difference in AD development between treatment groups.68, 69
How can the data from these existing statin trials be reconciled with multiple observational studies that support a solid risk reduction in the development of AD? Dementia was an exploratory outcome in the HPS trial, and after 5 years of follow‐up, only 31 subjects developed dementia in the control and treatment arms, respectively. As a result, it was vastly underpowered to detect a significant difference in incident dementia. The PROSPER trial followed participants for an even shorter time interval (42 months), and all subjects in the trial were already of advanced age (mean age, 75.4 years). Like trials of statins in existing AD, the timing of intervention may again be too late to alter the disease process for these elderly patients. Aβ plaque formation associated with hypercholesterolemia seems to be a relatively early process,11 and as Li et al note in their prospective cohort study, statin use seems to lose its protective effect with increasing age.33
Future Directions in Alzheimer Dementia and Lipid Research
Given that midlife cardiovascular risk factors are important predictors of AD and the benefit of statins occurs through lipid modification and other pleiotropic effects earlier in life, the primary prevention of AD in a younger population may be a better target for research. The question is how this might be investigated with a randomized controlled trial, and whether or not such a trial would be feasible. Patients would ideally be recruited in their fifth and sixth decades of life. To achieve the statistical power to replicate the 30% relative risk reduction noted in observational studies, the trial would need ≥2700 participants followed for over 2 decades. The practical challenges of ensuring follow‐up over such a long interval are obvious. Additionally, the amount of crossover between the statin and control groups would likely be substantial as those within the control group develop comorbid conditions that are primary indications for statin use.
Another important question is whether the broader prescribing of statin medications in accordance with new guidelines5 will be reflected in a change in new AD cases over the coming years. Indeed, emerging data have suggested that the incidence of dementia may actually be decreasing in some populations.70 Whether this is the result of increased statin use or an increased emphasis on controlling cardiovascular risk factors broadly is unclear. The number of soon‐to‐be elderly individuals who came of age during the era of statin development and increased prescribing may provide some insight into this issue. Until then, the cardiologist has limited high‐quality data with which to guide recommendations for patients inquiring about the long‐term cognitive benefits of these drugs (Box).
Box 1. Clinical Scenarios.
Question 1
A 55‐year‐old man with a family history of Alzheimer dementia (AD) in both parents presents to clinic. His total cholesterol is 170 mg/dL with a 10‐year atherosclerotic CVD (ASCVD) risk of 4.5%,71 and he inquires whether statin therapy may be indicated in his case for protection from dementia.
Answer: Inform the patient that the ability of statins to prevent dementia is currently an active area of research, with many observational studies showing that their use is associated with a reduced risk of AD. However, the cardiologist should note that there is stronger evidence for prevention of ASCVD. According to recent American College of Cardiology/American Heart Association guidelines, a 10‐year ASVCD risk of <5% may still warrant consideration of statin initiation, especially in the presence of clinical risk factors.5 A patient‐clinician risk discussion is advised, including review of risk factors, the role of further testing for risk stratification, opportunities for lifestyle improvement, and the possible role of pharmacotherapy.
Question 2
An 83‐year‐old woman with moderate aortic stenosis but no significant coronary artery disease or other vascular risk factors presents to clinic with her family. She was recently diagnosed with moderate AD and her daughter wonders whether aggressive treatment with statin therapy may protect against cognitive decline.
Answer: Inform the patient that although lipid reduction through statin therapy earlier in life may be associated with a decreased risk of AD development, there is currently no role for statins in treatment of established AD. Three short‐term randomized trials of statin therapy in such patients failed to demonstrate a lasting and clinically significant benefit in measures of disease progression.
Question 3
A well‐informed 62‐year‐old patient with a history of diabetes presents to clinic for further evaluation and management of coronary artery disease. He has been prescribed a high‐intensity statin but is concerned about US Food and Drug Administration labeling suggesting the potential for reversible cognitive side effects including “memory loss” and “forgetfulness” with statin therapy.53
Answer: Encourage the patient to report any perceived side effects, but provide reassurance and inform the patient that such side effects are rare, if they occur at all. Two recent meta‐analyses43, 44 failed to show any significant adverse effects from statins in a variety of cognitive domains, including memory and executive function, despite labeling changes.
Conclusion
Midlife dyslipidemia appears to play an important role in the development of AD among a host of other risk factors that affect vascular health. Results from observational cohorts have been mixed, though many of the highest‐quality studies have found a protective effect for statins. Laboratory data have supported numerous potential mechanisms for statin benefit including lipid reduction, vascular protection, and changes in cell signaling and amyloid processing. However, short‐term clinical trials of statins in AD patient populations have failed to show a sustained benefit in cognitive outcomes, perhaps due to an already advanced and irreversible disease process. Trials have not specifically addressed statins' role in the primary prevention of AD, and such trials would be very challenging, if not impossible, to conduct. Future studies should assess statin use during a critical period of risk in midlife to explore effects on the later development of AD.
S.S.M. is supported by the Pollin Cardiovascular Prevention Fellowship, Marie‐Josée and Henry R. Kravis endowed fellowship, and a National Institutes of Health training grant (T32HL07024). R.S.B. is supported by the Kenneth Jay Pollin Professorship in Cardiology. S.S.M. is listed as a co‐inventor on a pending patent filed by the Johns Hopkins University for a method of low‐density lipoprotein cholesterol estimation.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Thies W, Bleiler L; Alzheimer's Association. 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:208–245. [DOI] [PubMed] [Google Scholar]
- 2. Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer's disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc. 2005;53:1101–1107. [DOI] [PubMed] [Google Scholar]
- 3. Fonseca AC, Resende R, Oliveira CR, et al. Cholesterol and statins in Alzheimer's disease: current controversies. Exp Neurol. 2010;223:282–293. [DOI] [PubMed] [Google Scholar]
- 4. Top US pharmaceutical products by spending, 2012. IMS Health Inc. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/Press%20Room/Top_line_data/Top_Products_by_RX.pdf. Last updated February 23, 2012. Accessed January 26, 2014.
- 5. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25 suppl 2):S46–S48]. Circulation. 2014;129(25 suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 6. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia [published correction appears in Lancet. 2001;357:562]. Lancet. 2000;356:1627–1631. [DOI] [PubMed] [Google Scholar]
- 7. McGeer PL, McGeer EG. The amyloid cascade–inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126:479–497. [DOI] [PubMed] [Google Scholar]
- 8. Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late‐life Alzheimer disease. Ann Intern Med. 2002;137:149–155. [DOI] [PubMed] [Google Scholar]
- 9. Solomon A, Kivipelto M, Wolozin B, et al. Midlife serum cholesterol and increased risk of Alzheimer's and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsuzaki T, Sasaki K, Hata J, et al. Association of Alzheimer disease pathology with abnormal lipid metabolism: the Hisayama Study. Neurology. 2011;77:1068–1075. [DOI] [PubMed] [Google Scholar]
- 11. Pappolla MA, Bryant‐Thomas TK, Herbert D, et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61:199–205. [DOI] [PubMed] [Google Scholar]
- 12. Wood WG, Li L, Müller WE, et al. Cholesterol as a causative factor in Alzheimer's disease: a debatable hypothesis. J Neurochem. 2014;129:559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 14. Silva T, Teixeira J, Remião F, et al. Alzheimer's disease, cholesterol, and statins: the junctions of important metabolic pathways. Angew Chem Int Ed Engl. 2013;52:1110–1121. [DOI] [PubMed] [Google Scholar]
- 15. Kojro E, Gimpl G, Lammich S, et al. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α‐secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch‐Reinshagen V, Burgess BL, Wellington CL. Why lipids are important for Alzheimer disease? Mol Cell Biochem. 2009;326:121–129. [DOI] [PubMed] [Google Scholar]
- 17. Stewart R, White LR, Xue QL, et al. Twenty‐six‐year change in total cholesterol levels and incident dementia: the Honolulu‐Asia Aging Study. Arch Neurol. 2007;64:103–107. [DOI] [PubMed] [Google Scholar]
- 18. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. [DOI] [PubMed] [Google Scholar]
- 19. Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu‐Asia Aging Study. Neurobiol Aging. 2000;21:49–55. [DOI] [PubMed] [Google Scholar]
- 20. Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20‐year cognitive change: the Atherosclerosis Risk In Communities neurocognitive study. JAMA Neurol. 2014;71:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rusanen M, Kivipelto M, Quesenberry CP Jr, et al. Heavy smoking in midlife and long‐term risk of Alzheimer disease and vascular dementia. Arch Intern Med. 2011;171:333–339. [DOI] [PubMed] [Google Scholar]
- 22. Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population‐based neuropathologic study. Neurology. 2010;75:1195–1202. [DOI] [PubMed] [Google Scholar]
- 23. Ohara T, Doi Y, Ninomiya T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011;77:1126–1134. [DOI] [PubMed] [Google Scholar]
- 24. Mielke MM, Rosenberg PB, Tschanz J, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69:1850–1858. [DOI] [PubMed] [Google Scholar]
- 25. Gharacholou SM, Reid KJ, Arnold SV, et al. Cognitive impairment and outcomes in older adult survivors of acute myocardial infarction: findings from the Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status Registry. Am Heart J. 2011;162:860.e1–869.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(suppl 3):S115–S123. [DOI] [PubMed] [Google Scholar]
- 27. Mathias JL, Burke J. Cognitive functioning in Alzheimer's and vascular dementia: a meta‐analysis. Neuropsychology. 2009;23:411–423. [DOI] [PubMed] [Google Scholar]
- 28. Zamrini E, McGwin G, Roseman JM. Association between statin use and Alzheimer's disease. Neuroepidemiology. 2004;23:94–98. [DOI] [PubMed] [Google Scholar]
- 29. Hajjar I, Schumpert J, Hirth V, et al. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol A Biol Sci Med Sci. 2002;57:M414–M418. [DOI] [PubMed] [Google Scholar]
- 30. Rockwood K, Kirkland S, Hogan DB, et al. Use of lipid‐lowering agents, indication bias, and the risk of dementia in community‐dwelling elderly people. Arch Neurol. 2002;59:223–227. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez EG, Dodge HH, Birzescu MA, et al. Use of lipid‐lowering drugs in older adults with and without dementia: a community‐based epidemiological study. J Am Geriatr Soc. 2002;50:1852–1856. [DOI] [PubMed] [Google Scholar]
- 32. Haag MD, Hofman A, Koudstaal PJ, et al. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity: the Rotterdam study. J Neurol Neurosurg Psychiatry. 2009;80:13–17. [DOI] [PubMed] [Google Scholar]
- 33. Li G, Shofer JB, Rhew IC, et al. Age‐varying association between statin use and incident Alzheimer's disease. J Am Geriatr Soc. 2010;58:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bettermann K, Arnold AM, Williamson J, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the Ginkgo Evaluation of Memory study. J Stroke Cerebrovasc Dis. 2012;21:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sparks DL, Kryscio RJ, Sabbagh MN, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5:416–421. [DOI] [PubMed] [Google Scholar]
- 36. Beydoun MA, Beason‐Held LL, Kitner‐Triolo MH, et al. Statins and serum cholesterol's associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health. 2011;65:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cramer C, Haan MN, Galea S, et al. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ancelin ML, Carrière I, Barberger‐Gateau P, et al. Lipid lowering agents, cognitive decline, and dementia: the three‐city study. J Alzheimers Dis. 2012;30:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62:1047–1051. [DOI] [PubMed] [Google Scholar]
- 40. Arvanitakis Z, Schneider JA, Wilson RS, et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008;70(19 part 2):1795–1802. [DOI] [PubMed] [Google Scholar]
- 41. Smeeth L, Douglas I, Hall AJ, et al. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song Y, Nie H, Xu Y, et al. Association of statin use with risk of dementia: a meta‐analysis of prospective cohort studies. Geriatr Gerontol Int. 2013;13:817–824. [DOI] [PubMed] [Google Scholar]
- 43. Swiger KJ, Manalac RJ, Blumenthal RS, et al. Statins and cognition: a systematic review and meta‐analysis of short‐ and long‐term cognitive effects. Mayo Clin Proc. 2013;88:1213–1221. [DOI] [PubMed] [Google Scholar]
- 44. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688–697. [DOI] [PubMed] [Google Scholar]
- 45. Urano Y, Hayashi I, Isoo N, et al. Association of active γ‐secretase complex with lipid rafts. J Lipid Res. 2005;46:904–912. [DOI] [PubMed] [Google Scholar]
- 46. Shinohara M, Sato N, Kurinami H, et al. Reduction of brain beta‐amyloid (Abeta) by fluvastatin, a hydroxymethylglutaryl‐CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C‐terminal fragments (APP‐CTFs) and Abeta clearance. J Biol Chem. 2010;285:22091–22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–885. [DOI] [PubMed] [Google Scholar]
- 48. Zhang YY, Fan YC, Wang M, et al. Atorvastatin attenuates the production of IL‐1β, IL‐6, and TNF‐α in the hippocampus of an amyloid β1‐42–induced rat model of Alzheimer's disease. Clin Interv Aging. 2013;8:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barone E, Cenini G, Di Domenico F, et al. Long‐term high‐dose atorvastatin decreases brain oxidative and nitrosative stress in a preclinical model of Alzheimer disease: a novel mechanism of action. Pharmacol Res. 2011;63:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laufs U, La Fata V, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. [DOI] [PubMed] [Google Scholar]
- 51. Hughes TM, Kuller LH, Barinas‐Mitchell EJ, et al. Arterial stiffness and β‐amyloid progression in nondemented elderly adults. JAMA Neurol. 2014;71:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Martins IJ, Berger T, Sharman MJ, et al. Cholesterol metabolism and transport in the pathogenesis of Alzheimer's disease. J Neurochem. 2009;111:1275–1308. [DOI] [PubMed] [Google Scholar]
- 53. US Food and Drug Administration . FDA drug safety communication: important safety label changes to cholesterol‐lowering statin drugs. http://www.fda.gov/drugs/drugsafety/ucm293101.htm. Updated July 2012. Accessed January 26, 2014.
- 54. Evans MA, Golomb BA. Statin‐associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29:800–811. [DOI] [PubMed] [Google Scholar]
- 55. Blom DJ, Wasserman SM, Stein EA. Evolocumab in hyperlipidemia. N Engl J Med. 2014;371:877–878. [DOI] [PubMed] [Google Scholar]
- 56. Robinson JG, Nedergaard BS, Rogers WJ, et al; LAPLACE‐2 Investigators. Effect of evolocumab or ezetimibe added to moderate‐ or high‐intensity statin therapy on LDL‐C lowering in patients with hypercholesterolemia: the LAPLACE‐2 randomized clinical trial. JAMA. 2014;311:1870–1882. [DOI] [PubMed] [Google Scholar]
- 57. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 58. Sparks DL, Connor DJ, Sabbagh MN, et al. Circulating cholesterol levels, apolipoprotein E genotype and dementia severity influence the benefit of atorvastatin treatment in Alzheimer's disease: results of the Alzheimer's Disease Cholesterol‐Lowering Treatment (ADCLT) trial. Acta Neurol Scand Suppl. 2006;185:3–7. [DOI] [PubMed] [Google Scholar]
- 59. Oremus M. Does the evidence say a 4‐point change in ADAS‐Cog score is clinically significant? Alzheimers Dement. 2014;10:416–417. [DOI] [PubMed] [Google Scholar]
- 60. Feldman HH, Doody RS, Kivipelto M, et al; LEADe Investigators. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74:956–964. [DOI] [PubMed] [Google Scholar]
- 61. Jones RW, Kivipelto M, Feldman H, et al; LEADe Investigators. The Atorvastatin/Donepezil in Alzheimer's Disease Study (LEADe): design and baseline characteristics. Alzheimers Dement. 2008;4:145–153. [DOI] [PubMed] [Google Scholar]
- 62. Sano M, Bell KL, Galasko D, et al. A randomized, double‐blind, placebo‐controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rosenblum WI. Why Alzheimer trials fail: removing soluble oligomeric beta amyloid is essential, inconsistent, and difficult. Neurobiol Aging. 2014;35:969–974. [DOI] [PubMed] [Google Scholar]
- 64. Charité University, German Federal Ministry of Education and Research . Trial of simvastatin in amnestic mild cognitive impairment (MCI) patients (SIMaMCI). http://clinicaltrials.gov/show/NCT00842920. Accessed August 15, 2014.
- 65. Heart Protection Study Collaborative Group . MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20 536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002;360:7–22.12114036 [Google Scholar]
- 66. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 67. Trompet S, van Vliet P, de Craen AJ, et al. Pravastatin and cognitive function in the elderly: results of the PROSPER study. J Neurol. 2010;257:85–90. [DOI] [PubMed] [Google Scholar]
- 68. Riekse RG, Li G, Petrie EC, et al. Effect of statins on Alzheimer's disease biomarkers in cerebrospinal fluid. J Alzheimers Dis. 2006;10:399–406. [DOI] [PubMed] [Google Scholar]
- 69. Carlsson CM, Gleason CE, Hess TM, et al. Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle‐aged adults at risk for Alzheimer's disease. J Alzheimers Dis. 2008;13:187–197. [DOI] [PubMed] [Google Scholar]
- 70. Qiu C, von Strauss E, Bäckman L, et al. Twenty‐year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80:1888–1894. [DOI] [PubMed] [Google Scholar]
- 71. Goff DC Jr, Lloyd‐Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;63(25 part B):3026]. J Am Coll Cardiol. 2014;63(25 part B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]


