Abstract
Purpose
There is a negative prognostic impact of young age at diagnosis on outcome in breast cancer (BC). We sought to determine if there is a differential effect of race and examined mortality trends according to race and age.
Methods
SEER was used to identify women <50 with invasive BC diagnosed between 1990 and 2009. Multivariate regression analyses were performed to determine the risk-adjusted likelihood of survival for whites and blacks. Annual hazards of BC death according to race and calendar period, and adjusted relative hazards of death for whites and blacks stratified by age were computed.
Results
162,976 women were identified; 126,573 whites, 20,405 blacks, and 15,998 other races. At a median follow-up of 85 months, five-year disease specific survival rates were 90.1% for whites, 79.3% for blacks. Annual hazards for death in whites decreased by 26% at 5 years after diagnosis, in contrast to the hazards in blacks decreasing by only 19%. With 1990 as referent, the adjusted relative hazards for death in women <40 in year 2005 were 0.55 (95% CI 0.46-0.66) and 0.68 (95% CI 0.49-0.93) for whites and blacks, respectively. In women 40-49, adjusted hazards for death were 0.53 (95% CI 0.47-0.60) and 0.78 (95% CI 0.61-0.99) for whites and blacks.
Conclusion
Among young women diagnosed with BC, blacks have a worse outcome than whites. Mortality declines have been observed over time in both groups, although more rapid gains have occurred in whites. Emphasis should be placed on improving outcomes for young BC patients.
Keywords: breast cancer, young age, survival, trends
Introduction
Among adolescent and young women, breast cancer (BC) ranks as the most frequently diagnosed invasive cancer, and represents one quarter of BC seen in all women diagnosed in the United States.1, 2 The biology of BC in young women remains poorly understood and appears to be different from BC seen in older women. It is associated with more aggressive histopathologic characteristics, such as, higher grade tumors, more advanced stage, and lower hormone receptor positivity.1, 3 Additionally BC in young women has a worse clinical course than in older women.4-7 The incidence of BC in young women also varies by race with young black women having a much higher incidence compared to white women in the same age group.8-11
BC mortality trends suggest that rates have decreased by approximately 2% per year over the last two decades.12 For women under the age of 50 years, death rates have decreased by 3% per year as opposed to a yearly decrease of 1.9% in women ≥ 50 years, presumably as a result of more aggressive systemic treatment in younger women and less comorbidity. Since a larger proportion of black women present with earlier onset and more aggressive BC than whites, it is unclear whether the mortality gains seen in young women overall are due to improvements in whites alone. It is also unknown whether the disadvantages in clinical outcome in young BC patients and the negative effect black race exerts on survival are cumulative for young black patients. Consequently, we sought to examine the impact of race on BC outcomes as well as time trends in survival according to race, in a United States population-based cohort of young women diagnosed with BC.
Patients and methods
Study population
Data for this study were obtained from the Surveillance, Epidemiology and End Results (SEER) database which represents the largest population-based cancer registry in the United States. It covers 28% of the population and captures 97% of incident cancer cases in regions participating in SEER.12 The SEER registries collect information on demographics, primary tumor site and characteristics, first course of treatment, and survival from medical records, but do not have central pathologic review. The program started in 1973 and thus provides the greatest longevity for cancer statistics. The analyses included records for females aged 18-49 years who had been diagnosed with invasive BC between 1990 and 2009. Individuals who were diagnosed at death or autopsy only, had other first primary cancers, or did not have a record of race were excluded from this analysis (Figure 1). After exclusions were applied, data on 162,976 women were available for analysis.
Figure 1.
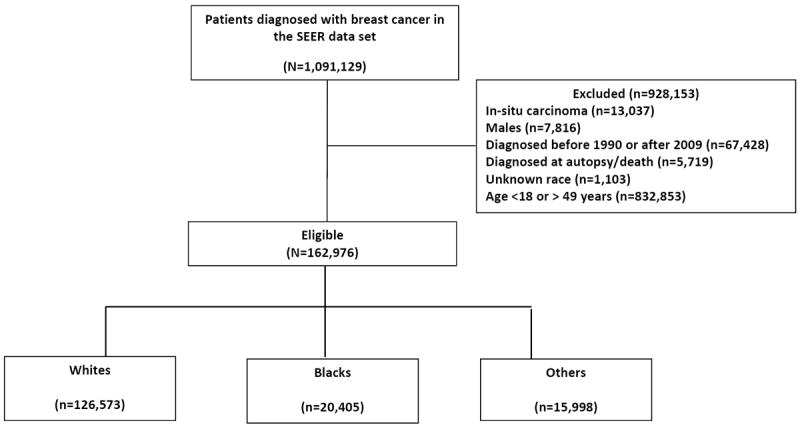
Patients with breast cancer included in the analytical cohort.
Consort diagram showing number of patients with breast cancer diagnosed between 1990 and 2009 that were included or excluded from the analytical cohort
Statistical analysis
The distribution of patient and clinical characteristics between race groups was compared using the Chi-square test or two-sample t-test, as appropriate. Overall survival (OS) was defined as the time from the date of diagnosis to death due to any cause. Survivors were censored at the date of last contact. Disease-specific survival (DSS) was defined as the time from diagnosis to death due to BC. Survival curves by race groups were estimated using the Kaplan-Meier product-limit method and compared by log-rank test. Univariate Cox proportional hazard models were fit to identify other demographic and clinical characteristics significantly related to OS. Multivariate Cox models were constructed to assess whether race was an independent predictor of survival, while adjusting other patient/clinical characteristics that were significant in the univariate analyses. Two-way interaction terms between race and other factors in the multivariate Cox model were assessed. To better understand the change of mortality risk over time, smoothed hazard functions were also estimated in each race group at different periods of diagnosis using non-parametric kernel-based methods.13 All analyses were two-sided and significance was set at a P-value of 0.05. The estimation of smoothed hazard was implemented using “muhaz” library in the statistical package R, while all the other analyses were performed using SAS 9.2 (SAS Institutes, Cary, NC).
Results
Patient characteristics
Among the study patients, 126,573 (77.7%) were white, 20,405 (12.5%) were black, and 15,998 (9.8%) were categorized as other races. Characteristics for the entire cohort are summarized in Table 1. Differences between patient characteristics by race were observed with respect to age at diagnosis, American Joint Committee on Cancer (AJCC) stage, grade, estrogen receptor (ER), and nodal status. A progressively higher number of patients overall were diagnosed from 1990-1994 to 2005-2009 (12.9%, 16.3%, 34.9% and 35.7%, respectively). This was evident in all racial categories. Patients who were categorized as other races, had clinicopathologic features similar to white patients. For example, approximately 76%, 16%, and 3% of both whites and others had AJCC stage I/II, III, and IV disease respectively.
Table 1.
Patients’ characteristics by race
| Variable | White | Black | Others | P value | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
|
|
||||||||
| 126573 | 77.7 | 20405 | 12.5 | 15998 | 9.8 | |||
|
| ||||||||
| Age | <30 | 2422 | 1.9 | 676 | 3.3 | 352 | 2.2 | <.0001 |
| 30-39 | 26184 | 20.7 | 5095 | 25.0 | 3728 | 23.3 | ||
| 40-49 | 97967 | 77.4 | 14634 | 71.7 | 11918 | 74.5 | ||
|
| ||||||||
| ER status | Positive | 80992 | 64.0 | 9755 | 47.8 | 10738 | 67.1 | <.0001 |
| Negative | 30851 | 24.4 | 7626 | 37.4 | 3526 | 22.1 | ||
| Unknown | 14730 | 11.6 | 3024 | 14.8 | 1734 | 10.8 | ||
|
| ||||||||
| PR status | Positive | 73575 | 58.1 | 8535 | 41.8 | 9894 | 61.9 | <.0001 |
| Negative | 36604 | 29.0 | 8609 | 42.2 | 4134 | 25.8 | ||
| Unknown | 16394 | 12.9 | 3261 | 16.0 | 1970 | 12.3 | ||
|
| ||||||||
| AJCC1 stage | I | 48334 | 38.2 | 5500 | 26.9 | 6098 | 38.1 | <.0001 |
| II | 46869 | 37.0 | 8254 | 40.4 | 6126 | 38.3 | ||
| III | 20555 | 16.2 | 4193 | 20.6 | 2480 | 15.5 | ||
| IV | 3762 | 3.0 | 1093 | 5.4 | 460 | 2.9 | ||
| Unknown | 7053 | 5.6 | 1365 | 6.7 | 834 | 5.2 | ||
|
| ||||||||
| Grade | 1 | 16258 | 12.8 | 1422 | 6.9 | 1984 | 12.4 | <.0001 |
| 2 | 43866 | 34.7 | 5215 | 25.6 | 5834 | 36.5 | ||
| 3 | 50846 | 40.2 | 10861 | 53.2 | 6370 | 39.8 | ||
| 4 | 2825 | 2.2 | 503 | 2.5 | 337 | 2.1 | ||
| Unknown | 12778 | 10.1 | 2404 | 11.8 | 1473 | 9.2 | ||
|
| ||||||||
| Tumor size (cm) | <2 | 59912 | 47.3 | 7039 | 34.5 | 7247 | 45.3 | <.0001 |
| 2-5 | 48887 | 38.6 | 9096 | 44.6 | 6502 | 40.6 | ||
| >5 | 8770 | 6.9 | 2321 | 11.4 | 1223 | 7.6 | ||
| Unknown | 9004 | 7.1 | 1949 | 9.5 | 1026 | 6.4 | ||
|
| ||||||||
| Nodes involved | Negative | 67228 | 53.1 | 9045 | 44.3 | 8797 | 55.0 | <.0001 |
| Positive | 48194 | 38.1 | 8414 | 41.2 | 5871 | 36.7 | ||
| Unknown | 11151 | 8.8 | 2946 | 14.4 | 1330 | 8.3 | ||
|
| ||||||||
| Histology | Ductal | 98011 | 77.4 | 16402 | 80.4 | 12880 | 80.5 | <.0001 |
| Lobular | 7693 | 6.1 | 839 | 4.1 | 601 | 3.8 | ||
| Mixed | 12478 | 9.9 | 1434 | 7.0 | 1336 | 8.4 | ||
| Inflammatory | 1461 | 1.1 | 347 | 1.7 | 102 | 0.6 | ||
| others | 6930 | 5.5 | 1383 | 6.8 | 1079 | 6.7 | ||
|
| ||||||||
| Diagnosis year | 1990-94 | 16690 | 13.2 | 2478 | 12.1 | 1908 | 11.9 | <.0001 |
| 1995-99 | 20682 | 16.3 | 3078 | 15.1 | 2875 | 18.0 | ||
| 2000-04 | 44621 | 35.3 | 7211 | 35.3 | 5188 | 32.4 | ||
| 2005-09 | 44580 | 35.2 | 7638 | 37.4 | 6027 | 37.7 | ||
American Joint Committee on Cancer
Impact of race on outcomes
At a median follow up of 85 months, a total of 32,506 (20%) women had died. The 5 year OS rates were 87.9% for whites, 74.9% for women blacks, and 88.5% for others. The 10 year OS rates were 79.5% for whites, 64.3% for blacks, and 80.7% for others. Median OS was not reached in any race category. Unadjusted Kaplan-Meier estimates indicated differences in OS by race (Figure 2). Cox regression analysis for OS demonstrated the association of black race, ER and progesterone receptor (PR) negativity, inflammatory BC, higher grade disease, more advanced stage, and nodal involvement as independent prognostic factors for survival (Table 2). Similar results were seen with DSS.
Figure 2.
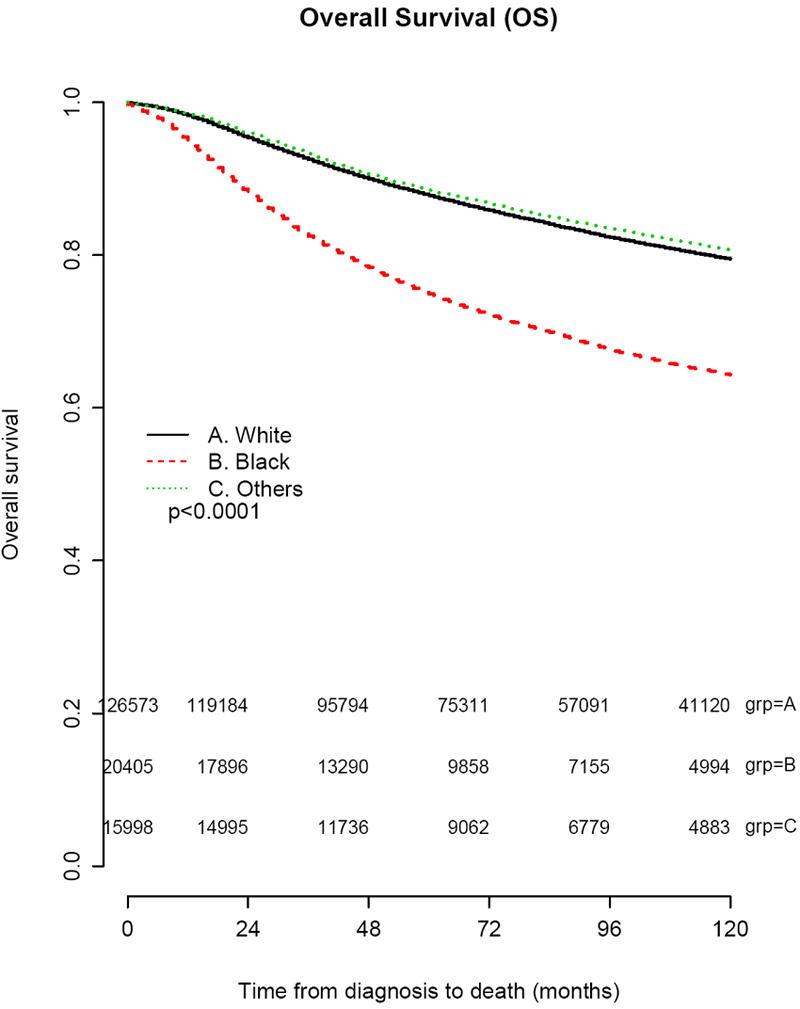
Kaplan-Meier survival curves showing estimates of overall survival by race for all patients included in the analytical cohort
Table 2.
Multivariate model results for overall (OS) and disease specific survival (DSS)
| Variable | OS | DSS |
|---|---|---|
|
| ||
| Hazard ratio (95% CI) P | Hazard ratio (95% CI) P | |
|
| ||
| Race (1ref: white) | ||
| • Black | 1.59 (1.55-1.64) <.0001 | 1.56 (1.52-1.62) <.0001 |
| • Others | 0.98 (0.94-1.02) .2966 | 0.99 (0.94-1.03) 0.5468 |
|
| ||
| Age group (ref:<30) | ||
| • 30-39 | 0.86 (0.81-0.92) <.0001 | 0.84 (0.78-0.90) <.0001 |
| • 40-49 | 0.79 (0.74-0.84) <.0001 | 0.71 (0.66-0.76) <.0001 |
|
| ||
| Histology (ref:ductal) | ||
| • Lobular | 0.96 (0.91-1.01) 0.1467 | 0.90 (0.85-0.96) <.0001 |
| • Mixed | 0.98 (0.94-1.02) 0.4066 | 0.97 (0.92-1.02) 0.1940 |
| • Inflammatory | 1.56 (1.47-1.67) <.0001 | 1.57 (1.46-1.68) <.0001 |
| • Others | 0.66 (0.63-0.70) <.0001 | 0.62 (0.58-0.66) <.0001 |
|
| ||
| ER (ref:negative) | ||
| • Positive | 0.74 (0.72-0.77) <.0001 | 0.73 (0.71-0.76) <.0001 |
|
| ||
| PR (ref:negative) | ||
| • Positive | 0.81 (0.78-0.83) <.0001 | 0.80 (0.77-0.83) <.0001 |
|
| ||
| Grade (ref:1) | ||
| • 2 | 1.60 (1.51-1.71) <.0001 | 2.06 (1.89-2.24) <.0001 |
| • 3 | 2.12 (2.03-2.29) <.0001 | 2.87 (2.64-3.11) <.0001 |
| • 4 | 2.19 (2.02-2.39) <.0001 | 2.94 (2.64-3.26) <.0001 |
|
| ||
| Stage (ref:I) | ||
| • II | 1.15 (1.09- 1.21) <.0001 | 1.37 (1.29-1.45) <.0001 |
| • III | 2.39 (2.26-2.52) <.0001 | 2.99 (2.81-3.20) <.0001 |
| • IV | 7.83 (7.38-8.31) <.0001 | 10.23 (9.53-10.98) <.0001 |
|
| ||
| Node (ref:negative) | ||
| • Positive | 1.74 (1.68-1.80) <.0001 | 1.87 (1.79-1.95) <.0001 |
|
| ||
| Tumor size (ref:<2cm) | ||
| • 2-5 | 1.46 (1.42-1.51) <0.001 | 1.58 (1.52-1.64) <.0001 |
| • >5 | 1.73 (1.65-1.81) <.0001 | 1.89 (1.81-1.99) <.0001 |
|
| ||
| Diagnosis year (ref:1990-94) | ||
| • 1995-1999 | 0.89 (0.86-0.92) <.0001 | 0.86 (0.82-0.89) <.0001 |
| • 2000-2004 | 0.80 (0.78-0.83) <.0001 | 0.77 (0.74-0.79) <.0001 |
| • 2005-2009 | 0.66 (0.64-0.69) <.0001 | 0.62 (0.59-0.65) <.0001 |
Reference
Time trends in mortality
Smoothed annual hazards for mortality according to race and period of diagnosis are shown in Figure 3. Both whites and blacks showed improvements over calendar period of diagnosis. During all periods of diagnosis, there was a sharp rise in the annual hazard of death for the first 2-3 years after diagnosis for whites and blacks; however, for each time period from initial diagnosis, blacks had a correspondingly higher hazard of death than whites. For instance, at 5 years after initial diagnosis, the annual risk in whites decreased from 2.7% per year for patients diagnosed in 1990-1994 to 1.9% per year for 2005-2009, representing a decline of 26%. Conversely, in blacks there was a more modest decline of only 19% (4.7% per year for 1990-1994 versus 3.8% per year in 2005-2009). Specific annual risk for years 1, 3, and 5 post diagnoses are shown in Table 3.
Figure 3.
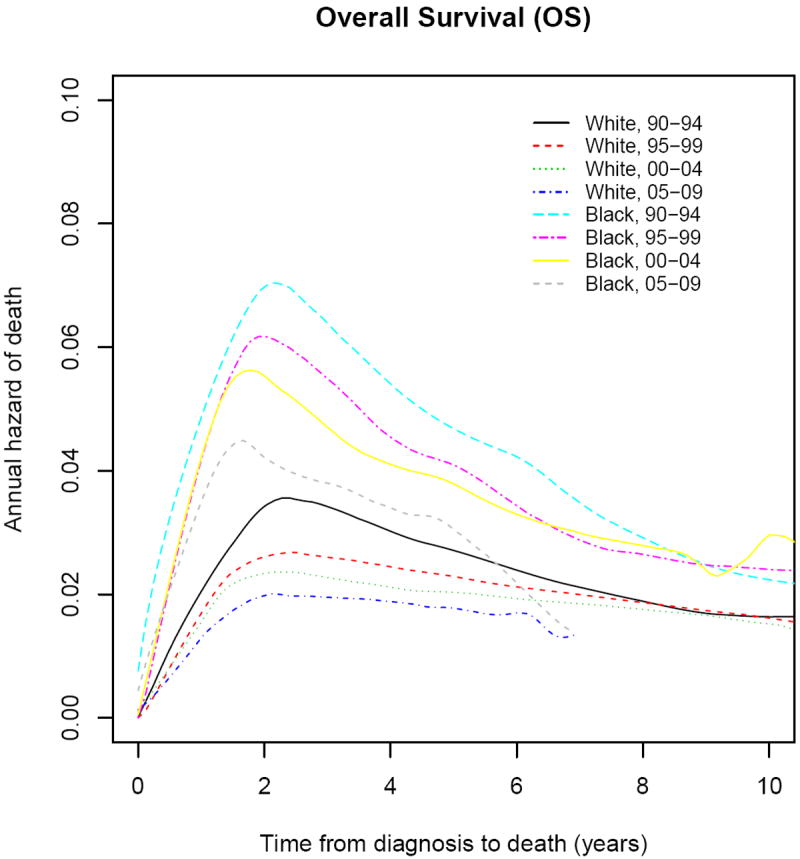
Smoothed Annual Hazard Rates of Breast Cancer Death according to Race and Calendar Period. Curves show absolute death rates at a stated time period after diagnosis.
Table 3.
Annual Hazard Rates for Mortality at 1, 3, and 5 Years after Diagnosis
| Period of Diagnosis | Year 1 | Year 3 | Year 5 | |||
|---|---|---|---|---|---|---|
| White | Black | White | Black | White | Black | |
| 1990-1994 | 2.21 | 5.1 | 3.4 | 6.4 | 2.7 | 4.7 |
| 1995-1999 | 1.8 | 4.4 | 2.6 | 5.5 | 2.3 | 4.1 |
| 2000-2004 | 1.7 | 4.4 | 2.3 | 4.7 | 2.0 | 3.8 |
| 2005-2009 | 0.8 | 2.4 | 1.9 | 4.5 | 1.9 | 3.8 |
Annual hazard rates are % per year hazard of death
Figure 4 shows the hazard ratio (HR) of death over time, stratified by race and age at diagnosis (<40 and ≥40 years), and adjusting for stage, grade, histology, node status, tumor size, ER and PR (Table 2). Adjusted HR declined over time for both races, although there was much more variability in blacks than whites. Compared with 1990, the HR for mortality in whites < 40 years diagnosed in 2009 was 0.53 (0.41-0.69), and 0.80 (0.54-1.19) in blacks, representing a 47% and 20% decrease from 1990 respectively (Table 4). Similar findings were seen in the ≥ 40 age group as well. Although mortality improvements were observed in blacks over time, those improvements were largely not statistically significant unlike those seen in whites.
Figure 4.
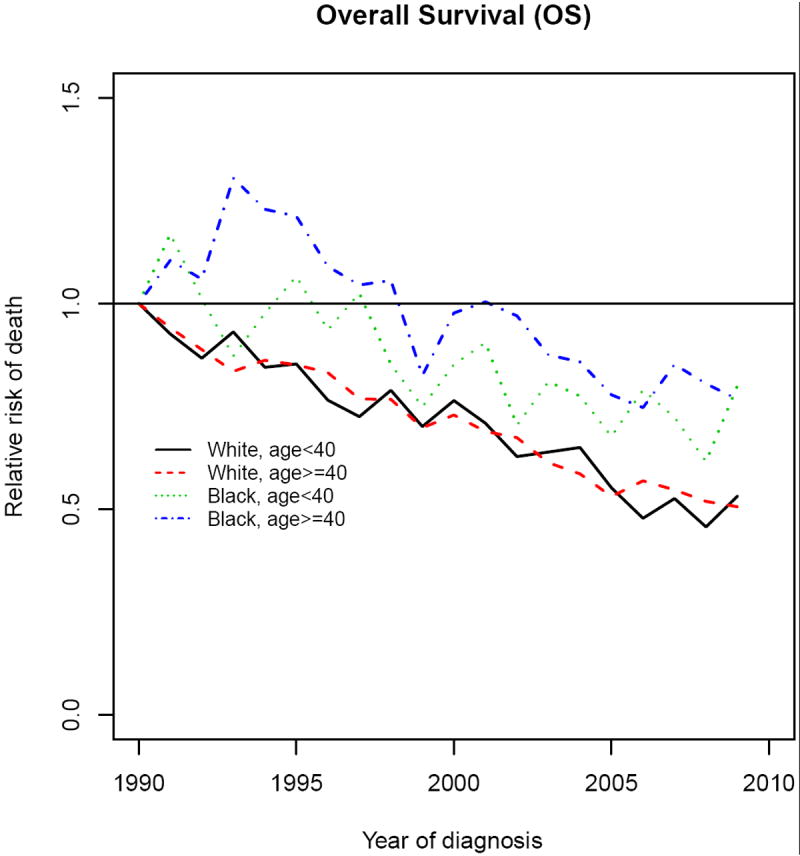
Relative Risk of Breast Cancer Death according to Race and Age at Diagnosis. RR are adjusted for stage, ER, PR, histology, age, diagnosis year, tumor size, nodal status, and grade.
Table 4.
Adjusted relative risks of death over time according to race and age group
| Variable | Whites RR1 (95% CI) | Blacks RR (95% CI) | |
|---|---|---|---|
| < 40 years | 19952 | 0.85 (0.72-1.02) | 1.07 (0.77-1.47) |
| 2000 | 0.76 (0.65-0.89) | 0.85 (0.65-1.12) | |
| 2005 | 0.55 (0.46-0.66) | 0.68 (0.49-0.93) | |
| 2009 | 0.53 (0.41-0.69) | 0.80 (0.54-1.19) | |
| 40-49 years | 1995 | 0.85 (0.76-0.96) | 1.21 (0.93-1.57) |
| 2000 | 0.73 (0.66-0.81) | 0.98 (0.78-1.22) | |
| 2005 | 0.53 (0.47-0.60) | 0.78 (0.61-0.99) | |
| 2009 | 0.51 (0.43-0.60) | 0.77 (0.56-1.05) | |
Relative risk
All years have 1990 as referent
Analysis adjusted for tumor size, grade, histology, lymph node status, ER, PR, and stage.
Discussion
In this study, we aimed to determine the relationship between race and survival in a population-based cohort of patients with early-onset BC. To achieve this aim, we analyzed 162,976 patients under age 50 years from the SEER database. Several findings emerged. First, it is evident that young black women with BC continue to have a worse outcome than young white women. Although this dilemma has been observed in patients with BC in general, only one other study has looked at this relationship specifically in young women.14 That study by Newman et al. also found race-related differences in survival favoring whites in a cohort of 500 blacks and 1400 whites with BC under age 40. The survival disadvantage conferred by race in young women is likely multifactorial. Black women are more likely to be diagnosed with basal-like BC, which has a poorer outcome, partly due to lack of therapeutic targeted drugs. However, even when biology is held constant, outcome differences are still seen as observed in this present study. Numerous studies have also shown racial discrepancies in treatment adherence,15-17 receipt of appropriate local therapies, and barriers in accessing care due to socioeconomic factors,18-20 which impact survival outcomes in BC. In clinical trials, it appears that black women derive similar benefits from adjuvant chemotherapy,21 although efficacy does not always translate to effectiveness in the general non-trial population.22 Another possible mechanism for poorer outcomes is the differences in immune-related genes between blacks and whites with BC. Although this has been shown to influence breast carcinogenesis,23, 24 it also appears that there may be a link between BC progression and racial differences in immune-related genes.25 This concept becomes more important to consider in view of the fact that trends in racial differences in mortality have been consistent over time, perhaps suggesting a genetic rather than environmental etiology.26 In addition, similarities in biology and clinical outcome in indigenous blacks in Africa and blacks from the United States or other developed countries suggest a common molecular factor contributing to poorer outcomes.27, 28
Consistent with other studies,22 we also observed that mortality trends have improved over time for all patients, although racial gaps previously seen in survival have not closed. In addition for all young women the highest risk of recurrence and mortality appears to be in the first 2-3 years followed by a progressive gradual decline. This is similar to patterns of recurrences seen with ER negative cancers. ER status undoubtedly influences recurrences and survival in young patients over time,29 however, the degree to which the traditional prognostic factors such as ER influence outcome in young BC patients is unclear, considering that over 60% of patients in our study had ER positive disease (whites 64%, blacks 48%, others 67%). Earlier relapses and mortality in young women suggest that there may be other unknown biologic differences that may account for the more aggressive behavior.
Thirdly, consistent with observations we and other have made, the incidence of invasive BC in younger women of all races appear to be increasing.30-32 This might be explained by stage migration, the change in reproductive risks profile over time such as women having first pregnancies at later ages and breast feeding for shorter durations, or increased awareness and consequently higher uptake of screening in those 40 years and older.
Our data must be interpreted in the light of its limitations. It is possible that the inability to include data on HER2 status may confound the interpretation of these results. However, there is no scientific evidence to suggest that trends in HER2 positivity have changed over time in young women. Similarly, this analytic cohort has missing data on ER in 11-15% of patients. Consistent with other studies, black patients appeared to have a slightly higher proportion of missing ER than whites.33 It is unclear what directional changes to the results these biases may have introduced. Although others have used statistical approaches to impute missing data, we have not done so in this particular analysis due to our hesitation in employing such techniques to reassign unknown ER status, a crucial classifier of BC. Information on systemic treatment is also not collected in SEER; however, most young women are generally treated more aggressively with chemotherapy. The cutoff age of 49 selected for the purposes of these analyses is somewhat arbitrary as there is no unanimously established definition of ‘early-onset’ BC. Finally, it is now quite clear that the molecular classification of BC34 is probably more pertinent in clinical outcome than ER, PR, and HER2, however, this information is not yet used in routine clinical practice.
In conclusion, young black women have higher mortality rates than other young women with BC irrespective of stage or hormone receptors. Further studies investigating the unique tumor biology that leads to black women having the greatest risk of death are warranted.
Acknowledgments
This publication was made possible by Grant Number 1K12CA167540 through the National Cancer Institute (NCI) at the National Institutes for Health (NIH) and Grant Number UL1 TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Cancer for Advancing Translational Sciences (NCATS) at the NIH. The authors also wish to acknowledge the support of the Siteman Cancer Center Biostatistics Core and the NCI Cancer Center Support Grant P30 CA091842. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCI, NCATS, or NIH.
Footnotes
Conflicts of Interest: None
References
- 1.Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8:288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 2.Breast Cancer Facts & Figures 2013-2014. 2013 [Google Scholar]
- 3.Gabriel CA, Domchek SM. Breast cancer in young women. Breast Cancer Res. 2010;12:212. doi: 10.1186/bcr2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 5.de la Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 6.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–347. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Althuis MD, Brogan DD, Coates RJ, et al. Breast cancers among very young premenopausal women (United States) Cancer Causes Control. 2003;14:151–160. doi: 10.1023/a:1023006000760. [DOI] [PubMed] [Google Scholar]
- 9.Hall IJ, Moorman PG, Millikan RC, Newman B. Comparative analysis of breast cancer risk factors among African-American women and White women. Am J Epidemiol. 2005;161:40–51. doi: 10.1093/aje/kwh331. [DOI] [PubMed] [Google Scholar]
- 10.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97:134–147. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 11.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 12.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009 2012. [April 19, 2012]; Available from URL: http://seer.cancer.gov/csr/1975_2009_pops09/index.html.
- 13.Muller HG, Wang JL. Hazard rate estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. [PubMed] [Google Scholar]
- 14.Newman LA, Bunner S, Carolin K, et al. Ethnicity related differences in the survival of young breast carcinoma patients. Cancer. 2002;95:21–27. doi: 10.1002/cncr.10639. [DOI] [PubMed] [Google Scholar]
- 15.Freedman RA, Hughes ME, Ottesen RA, et al. Use of adjuvant trastuzumab in women with human epidermal growth factor receptor 2 (HER2)-positive breast cancer by race/ethnicity and education within the National Comprehensive Cancer Network. Cancer. 2013;119:839–846. doi: 10.1002/cncr.27831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeder-Hayes KE, Meyer AM, S BD, Liu H, Wheeler SB. Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast Cancer Res Treat. 2014;145:743–751. doi: 10.1007/s10549-014-2957-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipscomb J, Gillespie TW, Goodman M, et al. Black-white differences in receipt and completion of adjuvant chemotherapy among breast cancer patients in a rural region of the US. Breast Cancer Res Treat. 2012;133:285–296. doi: 10.1007/s10549-011-1916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272:947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 19.Heimann R, Ferguson D, Powers C, Suri D, Weichselbaum RR, Hellman S. Race and clinical outcome in breast cancer in a series with long-term follow-up evaluation. J Clin Oncol. 1997;15:2329–2337. doi: 10.1200/JCO.1997.15.6.2329. [DOI] [PubMed] [Google Scholar]
- 20.Shi R, Mills G, McLarty J, Burton G, Shi Z, Glass J. Commercial insurance triples chances of breast cancer survival in a public hospital. Breast J. 2013;19:664–667. doi: 10.1111/tbj.12185. [DOI] [PubMed] [Google Scholar]
- 21.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001:36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458. [DOI] [PubMed] [Google Scholar]
- 22.Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst. 2009;101:993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quan L, Gong Z, Yao S, et al. Cytokine and cytokine receptor genes of the adaptive immune response are differentially associated with breast cancer risk in American women of African and European ancestry. Int J Cancer. 2014;134:1408–1421. doi: 10.1002/ijc.28458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Z, Quan L, Yao S, et al. Innate immunity pathways and breast cancer Risk in African American and European-American women in the Women’s Circle of Health Study (WCHS) PLoS One. 2013;8:e72619. doi: 10.1371/journal.pone.0072619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray JL, Thompson P, Yoo SY, et al. Prognostic value of single nucleotide polymorphisms of candidate genes associated with inflammation in early stage breast cancer. Breast Cancer Res Treat. 2013;138:917–924. doi: 10.1007/s10549-013-2445-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ademuyiwa FO, Olopade OI. Racial differences in genetic factors associated with breast cancer. Cancer Metastasis Rev. 2003;22:47–53. doi: 10.1023/a:1022259901319. [DOI] [PubMed] [Google Scholar]
- 27.Bowen RL, Duffy SW, Ryan DA, Hart IR, Jones JL. Early onset of breast cancer in a group of British black women. Br J Cancer. 2008;98:277–281. doi: 10.1038/sj.bjc.6604174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huo D, Ikpatt F, Khramtsov A, et al. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27:4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copson E, Eccles B, Maishman T, et al. Prospective observational study of breast cancer treatment outcomes for UK women aged 18-40 years at diagnosis: the POSH study. J Natl Cancer Inst. 2013;105:978–988. doi: 10.1093/jnci/djt134. [DOI] [PubMed] [Google Scholar]
- 30.Ademuyiwa FO, Groman A, Hong CC, et al. Time-trends in survival in young women with breast cancer in a SEER population-based study. Breast Cancer Res Treat. 2013;138:241–248. doi: 10.1007/s10549-013-2425-1. [DOI] [PubMed] [Google Scholar]
- 31.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309:800–805. doi: 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
- 32.Bouchardy C, Fioretta G, Verkooijen HM, et al. Recent increase of breast cancer incidence among women under the age of forty. Br J Cancer. 2007;96:1743–1746. doi: 10.1038/sj.bjc.6603783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krieger N, Chen JT, Ware JH, Kaddour A. Race/ethnicity and breast cancer estrogen receptor status: impact of class, missing data, and modeling assumptions. Cancer Causes Control. 2008;19:1305–1318. doi: 10.1007/s10552-008-9202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]


