Abstract
Diet has an important role in the development of colorectal cancer. In the past few decades, findings from extensive epidemiologic and experimental investigation have linked consumption of several foods and nutrients to the risk of colorectal neoplasia. Calcium, fiber, milk, and whole grain have been associated with a lower risk of colorectal cancer, and red meat and processed meat with an increased risk. There is substantial evidence for the potential chemopreventive effects of vitamin D, folate, fruits and vegetables. Nutrients and foods may also interact, as a dietary pattern, to influence colorectal cancer risk. Diet likely influences colorectal carcinogenesis through several interacting mechanisms. These include the direct effects on immune responsiveness and inflammation, and the indirect effects of over-nutrition and obesity—risk factors for colorectal cancer. Emerging evidence also implicates the gut microbiota as an important effector in the relationship between diet and cancer. Dietary modification therefore has the promise of reducing colorectal cancer incidence.
Keywords: diet, colorectal cancer, microbiota, prevention
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the fourth leading cause of cancer death in the world1. Although screening reduces the incidence and mortality from CRC2-4, the routine use of screening has been stymied, particularly in resource-limited settings5. Preventative measures, including dietary and lifestyle modifications, are therefore an attractive strategy to reduce the global burden of CRC.
In 1981, Doll and Peto “guestimated” that as many as 35% of cancer-related deaths and 90% of stomach and large bowel cancer-related deaths could be attributed to dietary factors6. Over the past several decades, numerous observational studies and randomized clinical trials (RCTs) have been conducted to identify potential dietary contributors to CRC risk7, 8. However, data from these studies have shown either inconsistent or only modest associations. This is in contrast to the robust evidence linking diet with cardiovascular disease and diabetes and despite experimental data uncovering specific mechanisms relating diet to CRC. This perhaps should not be surprising given the complexity intrinsic to the etiopathogenesis of cancer. The intricate interactions of genetic, epigenetic and environmental determinants of risk9-11, the prolonged latency period required for carcinogenesis12, 13, as well as the etiologic heterogeneity of CRC as reflected by its anatomic, histologic, and molecular variability14, 15 pose inherent challenges to nutritional cancer epidemiology. Nonetheless, a critical appraisal of human data gathered thus far within the context of our mechanistic understanding of diet and CRC may serve as an initial step in realizing the promise of dietary chemoprevention.
Although there have been numerous reviews of diet and CRC, most have been specific to one or a few exposures16-21. Herein, we review all major dietary factors that may have a role in CRC, summarizing the most recent epidemiologic and clinical trial evidence, with a focus on potential mechanisms (Table 1). In addition, based on our synthesis of the evidence, we offer some recommendations, where possible, for clinicians to consider in their counseling of patients who may be interested in modifying their diet for the purpose of cancer prevention. Given the methodological limitations of retrospective case-control studies13, 22-24, we focus on prospective studies and RCTs, prioritizing those with large size and robust design and analysis. Although RCTs are usually regarded as the gold standard for establishing causality, trials of dietary interventions have been challenging. Beyond issues of adequate compliance and enrollment of generalizable populations, dietary intervention RCTs have suffered from lack of knowledge regarding appropriate dose, form, and route of administration of dietary factors, the minimum duration of treatment, and optimal timing of exposure and endpoint ascertainment. Thus, we discuss data from RCTs within the context of observational studies as well as experimental evidence13, with a focus on those nutrients with the most compelling mechanisms. We emphasize mechanisms associated with inflammation and immune dysregulation given their established role in colorectal carcinogenesis25, 26 and the increasing appreciation of a role for the microbiota in CRC27, 28 (Figure 1).
Table 1. Summary of evidence and mechanisms relating nutrients and foods to colorectal cancer.
| Nutrient or Food | Level of Evidencea | Estimate of Relative Risk (RR)b | Proposed Mechanisms |
|---|---|---|---|
| Nutrient | |||
| Calcium | Probable | RR=0.92 (0.89–0.95) per 300 mg/day increase of total calcium intake [ref 74] | Binding to fatty acids and free bile acids; suppression of cell proliferation; promotion of cell differentiation and apoptosis; inhibition of oxidative DNA damage; modulation of colorectal cancer-related cell signaling pathways |
| Vitamin D | Limited-suggestive | RR=0.95 (0.93–0.98) per 100 IU/day increase of dietary vitamin D intake; 0.96 (0.94–0.97) per 2.5 ng/mL (6.25 nmol/L) increase of circulating 25(OH)D [ref 7] | Anti-proliferation, pro-differentiation and apoptosis, anti-inflammation, inhibition of invasion and metastasis, and suppression of angiogenesis |
| Fiber | Convincing | RR=0.90 (0.86–0.94) per 10 g/day increase of dietary fiber intake [ref131] | Increased stool weight, decreased transit time, dilution of colonic carcinogenic content, decreased adiposity, and anticancer properties of short-chain fatty acids produced by bacterial fermentation of resistant starch |
| Folate | Limited-suggestive | RR=0.99 (0.93–1.05) per 100 μg/d increase of dietary folate intake; 0.98 (0.94–1.03) per 100μg/day increase of total folate intake [ref 7] | Essential nutrient for DNA methylation and DNA synthesis, critical processes in carcinogenesis |
| Vitamin B6 | N/A | RR=0.90 (0.75–1.07) comparing the highest to the lowest categories of vitamin B6 intake; 0.51 (0.38–0.69) per 100 pmol/mL increase of blood level of pyridoxal 5′-phosphate [ref 218] | One-carbon metabolism, critical for DNA synthesis and DNA methylation |
| Methionine | N/A | RR=0.89 (0.79–1.00) comparing the highest to the lowest categories [ref 228] | One-carbon metabolism, critical for DNA synthesis and DNA methylation; inhibition of cell growth and reduced inflammation |
| Vitamin A, C, E | Limited-no conclusion | RR=0.93 (0.79–1.10) for total vitamin A, 0.86 (0.74–1.00) for total vitamin C and 0.83 (0.70–0.99) for total vitamin E, comparing the highest to the lowest categories [ref 255] | Antioxidative, inhibition of cell proliferation, pro-apoptosis, and reduced inflammation |
| Selenium | Limited-no conclusion | RR=0.81 (0.71–0.92) comparing the highest to the lowest categories of selenium concentrations in serum, plasma or toenails [ref 253] | Antioxidative, inhibition of cell proliferation, pro-apoptosis, and reduced inflammation |
| Total fat | Limited-no conclusion | RR=0.99 (0.89–1.09) comparing the highest to the lowest categories [ref 307] | Increased intestinal level of bile acids, which can be metabolized by bacteria to deoxycholic acid to promote CRC development |
| Omega-3 polyunsaturated fatty acids | Limited-no conclusion | RR=0.97 (0.86–1.10) comparing the highest to the lowest categories [ref 314] | Reduced inflammation through inhibition of arachidonic acid-derived eicosanoid biosynthesis, and modulation of transcription factor activity, gene expression and signal transduction; improved insulin sensitivity; altered cell membrane fluidity |
| Food | |||
| Red and processed meat | Convincing | RR=1.16 (1.04–1.30) per 100 g/day increase of red and processed meat intake [ref 7] | Carcinogenic effect of heme iron, N-nitro compounds, and heterocyclic amines generated during cooking at high temperature. Pro-neoplastic effect of increased adiposity and insulin |
| Milk | Probable | RR=0.91 (0.85–0.94) per 200 g/day increase of total milk intake [ref 419] | Antineoplastic effect of calcium, vitamin D (for fortified milk), conjugated linoleic acid, butyric acid, and lactose |
| Fruits | Limited-suggestive | RR=0.97 (0.94–0.99) per 100 g/day increase of fruit intake [ref 7] | Anticarcinogenic compounds, such as folate, vitamins, fiber, minerals, and flavonoids; decreased adiposity |
| Non-starchy vegetables | Limited-suggestive | RR=0.98 (0.96–0.99) per 100 g/day increase of vegetable intake [ref 7] | Anticarcinogenic compounds, such as folate, vitamins, fiber, minerals, flavonoids, and glucosinolates contained in cruciferous vegetables; decreased adiposity |
| Whole grains | Convincing | RR=0.83 (0.78–0.89) per 3 servings/day increase of whole grains [ref 131] | Anticancer properties of fiber, antioxidants and phytochemicals; decreased adiposity; improved insulin sensitivity and decreased insulin level |
Source: Continuous Update Project Report on Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer, World Cancer Research Fund/American Institute for Cancer Research, 20117.
The estimates are derived from the 2011 review by World Cancer Research Fund/American Institute for Cancer Research7, except for the nutrients that were not covered in that review (i.e., vitamin B6, methionine, total fat, and omega-3 polyunsaturated fatty acids), nutrients for which observational studies were not summarized in that review (i.e., vitamins A, C and E, and selenium), and exposures for which more recent meta-analyses were available (i.e., calcium, fiber, milk, and whole grain).
Figure 1. Proposed canonical and non-canonical inflammatory mechanisms relating nutrients to colorectal cancer.

Consumption of saturated or trans-fats increases free fatty acids and bile acid levels. Bile acids are metabolized by gut microbes to generate secondary bile acids, which can promote colorectal carcinogenesis through increased pro-inflammatory effectors and oxidative stress mediated via engagement of nuclear factor-κB (NFκB) and cyclooxygenase-2 (COX2/prostaglandin synthase-2) pathways. Sulfur-containing amino acids and inorganic sulfur are also metabolized by gut microbes to produce hydrogen sulfide (H2S), which can be toxic to the colorectal mucosa and induce oxidative stress. As reactive oxygen species scavengers, antioxidant nutrients can protect colonocytes from oxidative stress. B vitamins also counteract oxidative stress through lowering homocysteine. Calcium may inhibit colorectal carcinogenesis through direct effects on cell proliferation, differentiation and apoptosis, and binding to free fatty acids and bile acids. Vitamin D exerts anti-inflammatory effects through bile acid catabolism, suppression of NFκB and COX2 signaling, and enhanced production of interleukin-10 (IL10). Omega-3 fatty acids can inhibit inflammation and promote resolution through lipid mediators (i.e., lipoxin, resolvin and protectin) and directly via G protein-coupled receptor 120 (GPR120) signaling.
Nutrients
Calcium
Calcium is an essential nutrient for bone and dental health. The ability of ionized calcium to form insoluble soaps with tumor-promoting free fatty acids and bile acids in the colonic lumen led to the hypothesis that calcium was anti-neoplastic29. A prospective cohort study with nearly 20 years of follow-up observed an approximately 70% lower risk of CRC comparing the highest to the lowest quartiles of calcium intake30. Fairly consistent but more modest inverse associations have subsequently been reported in most31-46, although not all47-52, cohort studies for CRC and colorectal adenomas. These findings prompted RCTs of calcium supplementation in which a significant reduction in adenoma recurrence53, 54, particularly histologically advanced lesions55, was observed. Additional mechanisms through which calcium might affect CRC risk include: inhibition of cell proliferation, promotion of cell differentiation and apoptosis56-59, suppression of oxidative DNA damage60, and modulation of CRC-related cell signaling pathways61 (see reviews62, 63). These effects are likely mediated by extracellular calcium-sensing receptor (CaR) signaling which contributes to the integrity of intestinal barrier function and homeostasis between gut microbes and the immune response64 (Figure 2).
Figure 2. Metabolism and antineoplastic pathways of calcium and vitamin D.

Vitamin D signaling via vitamin D receptor (VDR) and retinoid X receptor (RXR) regulates the transcription of numerous genes involved in carcinogenesis through effects on inflammation, cell proliferation, differentiation, apoptosis and angiogenesis. Elevated intracellular calcium, together with diacyl glycerol (DAG), results in the activation of protein kinase C (PKC) that can inhibit cell proliferation and promote differentiation through multiple downstream signals. CaR signaling may also inhibit cell proliferation through inactivation of the β-catenin pathway. Vitamin D is an essential regulator of calcium homeostasis through the negative feedback system involving parathyroid hormone (PTH). The secretion of PTH is enhanced as circulating calcium concentration decreases, resulting in increased synthesis of 1,25(OH)2D3. Elevated 1,25(OH)2D3 in turn promotes calcium absorption in the intestine and calcium release from bone, which suppresses PTH production.
In contrast with adenoma recurrence RCTs, the Women's Health Initiative (WHI), the largest RCT with CRC incidence as a secondary outcome, did not find any reduction in CRC after a mean of 7 years of supplementation with calcium and vitamin D65. However, several limitations may have contributed to the null findings, including: high calcium intake at baseline, poor compliance, complex factorial design, and insufficient duration of treatment and/or follow-up66-68. In a reanalysis, a 17% reduction of CRC incidence with calcium supplementation was observed among WHI participants not already taking calcium or vitamin D at randomization69. Findings from other smaller trials have been similarly inconclusive70, 71. Furthermore, contrary to prior adenoma RCTs, the initial results of another RCT of calcium and vitamin D supplementation failed to detect a reduction in risk of recurrent adenoma among 2,259 individuals, adding complexity to the calcium-CRC relationship72.
Several reasons may explain these discrepancies. A threshold effect of calcium on neoplasia risk may mask a benefit in an already calcium-replete population. In cohort studies, calcium intake beyond approximately 700–1000 mg/day was found to have minimal incremental effect on lowering CRC risk32, 36, 38, 46, 73, 74 and supplemental calcium did not further benefit participants with high dietary calcium intake31, 35, 36. Also, the association between calcium intake and colorectal neoplasms may differ by anatomic location, with stronger associations observed for cancers in the distal colon or rectum36, 39, 41, 45, 52, 73, 75. Anatomic site heterogeneity may be driven by microbial composition or metabolic variation in the colonic lumen76, 77. Calcium may have a differential effect based on other dietary factors or genetic background. The inverse association between calcium and colorectal neoplasms may be confined to individuals with a high vitamin D level35, 36, 78, reflective of the regulation of calcium level by vitamin D62, 79, or a low calcium/magnesium intake ratio80, 81, due to competition between these metals for luminal absorption82, 83. There is also evidence for differential associations based on vitamin D receptor (VDR) polymorphisms84-86 or nonsteroidal anti-inflammatory drug use85.
In summary, predominant evidence indicates an increased CRC risk among individuals with calcium intake lower than 700-1000 mg/day. It would therefore be reasonable to encourage individuals to increase their calcium intake to a level above this range, recognizing that data are inconsistent, including limited findings that calcium supplementation may be weakly associated with cardiovascular hazards87. The potential modification of other factors on the calcium-CRC relationship warrants further investigation.
Vitamin D
In 1980, Garland and colleges proposed that vitamin D status accounted for the high mortality rate of CRC in populations with low solar UV-B radiation exposure88. Subsequent cohort studies tested this hypothesis using a range of surrogates for vitamin D status, including solar radiation89, 90, circulating 25-hydroxyvitamin D [25(OH)D] level45, 91-98, dietary and supplement intake34, 35, 39, 47, 99-102, and predicted 25(OH)D based on major determinants of vitamin D status103. The predominant evidence from these studies supports an inverse association of vitamin D with adenoma incidence104, CRC incidence, and mortality105, 106. Based on a systematic review, one study suggested that the benefits associated with serum 25(OH)D may require at least 75 nmol/L (30 ng/mL), with optimal levels between 90-100 nmol/L (36–40 ng/mL) for multiple health outcomes including CRC107. Because this level of sufficiency cannot typically be achieved with currently recommended vitamin D intake of 600 and 800 IU/d for younger and older adults108, respectively, some authorities have called for an increase in recommended vitamin D intake to ≥1000 IU/d109, 110.
Accumulating preclinical and clinical studies suggests an anticancer activity of vitamin D111, 112. Vitamin D functions by binding to and activating the nuclear VDR. A polymorphism in VDR, BsmI, has been consistently associated with CRC113. Vitamin D may directly or indirectly regulate 3–5% of the human genome111 and has been implicated in a wide spectrum of anticancer activities: anti-proliferation, induction of differentiation and apoptosis, anti-inflammation, inhibition of invasion and metastasis, and suppression of angiogenesis112 (Figure 2).
Among these mechanisms, the anti-inflammatory and immune regulatory effects of vitamin D are particularly compelling and may mediate its role in vascular, neurologic, autoimmune, and infectious diseases114. In mice with colitis, a CRC risk factor, consumption of a high vitamin D diet attenuated inflammation, indicating that vitamin D may have an important role in inflammation-associated carcinogenesis115. High plasma 25(OH)D was strongly associated with lower risk of colorectal tumors with high lymphocyte counts, but not tumors with low counts116. Given the well-established role of vitamin D in immunity117, 118 and the ability of immune cells to generate biologically active vitamin D119-121, these data provide evidence for the importance of host immunity in vitamin D-mediated CRC prevention.
Despite this compelling epidemiologic evidence and biologic plausibility, the results of vitamin D supplementation have been disappointing in all 65, 71, 122 but one70 RCT that assessed cancer incidence as a secondary outcome. However, similar to calcium RCTs, limitations in study design still leave open the possibility that vitamin D may indeed have a chemopreventive role. A recent systematic review hypothesized that confounding by inflammatory processes involved in both CRC development and 25(OH)D deficiency might account for the inverse association between vitamin D and CRC in observational studies123. However, we recently found that rigorous adjustment for inflammatory markers had little effect on the robust inverse association observed between 25(OH)D and CRC risk, indicating that confounding by inflammation was not a sufficient explanation124.
In summary, compelling data from epidemiologic and experimental studies support the potential chemopreventive effects of vitamin D against CRC development, although the evidence from RCTs is inconclusive. A large ongoing RCT of vitamin D supplementation that includes cancer as a primary outcome, the VITamin D and OmegA-3 TriaL125, will hopefully provide more direct data on this topic. Until then, it would be reasonable to ensure that patients obtain a sufficient level of vitamin D to maintain a plasma level of 25 (OH)D greater than at least 36 ng/mL.
Fiber
In the 1970s, Burkitt hypothesized that high fiber intake protected against CRC based on observations of the low CRC incidence among Africans who consumed a high fiber diet126. Proposed mechanisms for this hypothesis included: reduced concentrations of intestinal carcinogens due to increased stool mass, decreased transit time, and bacterial fermentation of resistant starch to short-chain fatty acids (SCFAs)126. Butyrate, the major SCFA produced by colonic fermentation, is the preferred energy source for colonocytes127 and may enhance apoptosis and inhibit proliferation of cancer cells128. In addition, SCFAs have immune modulatory and anti-inflammatory effects129, acting to influence gastrointestinal and perhaps systemic health130.
Despite substantial experimental evidence, human studies of fiber and CRC are equivocal131. Fiber intake was not associated with CRC risk in 14 cohort studies32, 46, 50, 132-140, whereas in 9 other cohorts an inverse association was detected at least among some subgroups or for certain types of fiber141-150. There are several explanations for this discrepancy. Given the close link of fiber intake with other behaviors, the fiber-CRC association may be confounded to a varied degree across studies. Indeed, adjustment for other dietary and lifestyle factors, such as intake of folate, red and processed meat and physical activity, substantially attenuated the inverse association between fiber and CRC in several137, 146, 151 but not all152 cohorts. Also, dietary sources of fiber vary enormously, with cereals as the major sources in European cohorts, and fruits and vegetables in American cohorts151. There is evidence that fiber from cereals may be more strongly associated with CRC146, 149-151, although the mechanisms remain unclear. Large variations in fiber consumption across populations may also lead to inconsistent findings. Although a linear dose-response relationship was reported in a recent meta-analysis131, some studies support a threshold effect, with an increased risk for CRC among very low consumers of fiber and no apparent benefit of fiber intake among those with high consumption138, 144, 147, 151. Measurement error in assessing fiber intake may also influence results; dietary fiber intake assessed by food diaries, but not food frequency questionnaires, was inversely associated with CRC risk in a case-control study nested within 7 cohorts148. Although food diaries may be more accurate compared to food frequency questionnaire, the 2 tools differ in assessment time frame (short term vs long term), range of covered food items, and sources of errors (recording/coding vs recall)153. Other reasons for disparate results include: differences in study populations, definition of fiber, length of follow-up, and tumor site heterogeneity. Colon cancer, especially distal tumors, may be more strongly associated with fiber than rectal cancer142, 147-150, although cereal fiber has been related to rectal cancer in some studies146, 150, 151.
Six RCTs testing fiber supplementation among patients with a history of colorectal polyps54, 154-158 failed to detect any benefit except for a marginal reduction in adenoma recurrence among women in one small study155. However, several limitations, including poor compliance with the intervention159-161 and the relatively short follow-up period162, complicate interpretation. A resistant starch RCT in individuals with Lynch syndrome found no reduction in CRC incidence163. However, the relevance of this finding to CRC prevention in the general population is unclear.
Given the biological plausibility and data from observational studies, fiber has been judged by the expert panel to protect against CRC with convincing evidence7. Recently, a meta-analysis summarized the prospective evidence and reported a 10% decreased risk of CRC per additional 10 g/day total dietary fiber intake131. In the absence of data to indicate any adverse consequences to a high fiber intake, it is reasonable to recommend individuals consume a high-fiber diet, particularly as it has been associated with other improved health outcomes164, 165.
B vitamins and methionine
Genetic studies further support a role for folate in CRC. Individuals with high folate intake are less likely to develop tumors with hypomethylation either on a genome-wide level or at specific loci190, 191. Consistent with the role of folate deficiency in promoting p53 mutation, the inverse association between folate intake and CRC appears restricted to p53-mutated tumors192. In addition, a common mutation in the methylenetetrahydrofolate reductase (MTHFR) gene shows a folate-dependent association with CRC193-201. MTHFR converts 5,10-methylenetetrahydrofolate, the required cofactor for DNA synthesis, to 5-methyltetrahydrofolate, the predominant form of folate in the circulation and the primary methyl donor for DNA methylation. The 677C→T(Ala→Val) MTHFR mutation impairs enzymatic activity, leading to 5,10-methylenetetrahydrofolate accumulation and lower 5-methyltetrahydrofolate levels202. When dietary methyl supply is high (i.e., no or low alcohol consumption, high folate and methionine intake), MTHFR mutation-positive individuals have a reduced CRC risk because higher levels of 5,10-methyltetrahydrofolate prevent imbalances of nucleotide pools during DNA synthesis. In contrast, when the methyl supply is low, there may be less compensation for the dysfunctional MTHFR enzyme and increased susceptibility to impaired DNA methylation that contributes to increased CRC risk193-201.
B vitamins, including folate (vitamin B9), riboflavin (vitamin B2), pyridoxine (vitamin B6) and cobalamin (vitamin B12), and methionine are essential for DNA methylation, synthesis, stability and repair. Folate has received the most investigation as a cancer preventive agent. In addition to being consumed through diet or supplement use, large quantities of folate can also be produced by gut bacteria166, 167. Folate deficiency results in genomic hypomethylation and defects in DNA synthesis, both of which can contribute to colonic carcinogenesis168-172 (Figure 3). Dietary intake and circulating levels of folate have been inversely associated with CRC173-182 and adenoma183-185 risk in observational studies. This association appears more marked among alcohol drinkers173, 175, 177, 183, 186-188 and low-methionine consumers182, 189, consistent with alcohol's impairment of one-carbon metabolism and methionine's role in folate-mediated DNA methylation.
Figure 3. Folate, methionine and other B vitamins in DNA methylation and synthesis.
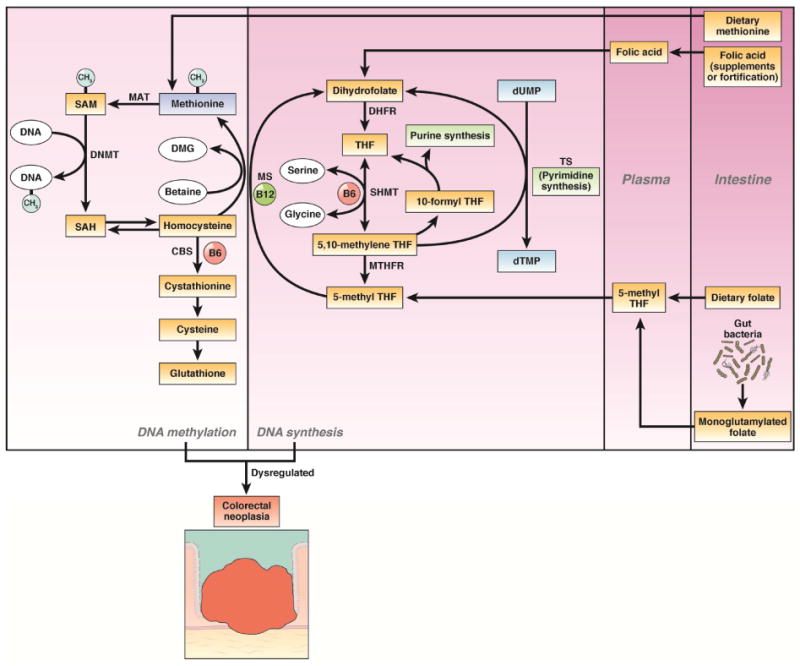
Folate from dietary intake, supplement use and synthesis by gut bacteria is converted to its predominant circulating form, 5-methyl tetrahydrofolate (THF), which can be metabolized by methionine synthase (MS) to form methionine from homocysteine. Vitamin B6 and B12 are also critical cofactors in DNA methylation and synthesis. Dysregulated DNA methylation and DNA synthesis contribute to colorectal carcinogenesis. Abbreviations: DNMT, DNA methyltransferase; MAT, S-adenosylmethonine synthase; SAH, S-adenosylhomocysteine; TS, thymidylate synthase; VDRE, vitamin D response element.
A potential dual-modulator role of folate adds more complexity to its relationship with CRC203. Contrary to the antineoplastic role in healthy tissues, folate may induce growth and progression of preexisting neoplasms through enhanced DNA synthesis in rapidly replicating neoplastic cells204, 205 and stimulation of inflammatory immune response pathways206, 207. This idea has fueled debate about whether mandatory folic acid fortification introduced in the US and Canada in 1996–1998 is responsible for the transient increase in CRC incidence from 1996–1998208. However, the short temporal proximity between fortification and the uptick in CRC incidence, as well as unchanged mortality, argues against this hypothesis209. Moreover, in 2 large cohort studies, high folate intake during the post-fortification period was still associated with lower CRC risk175, 176. In a recent meta-analysis of 13 RCTs, neither overall nor CRC-specific incidence was increased by daily 2.0 mg folic acid supplementation, an order of magnitude greater than the dose typically delivered by fortification210. Given the potential dual role of folate in normal tissues and neoplasms, the timing of folate introduction may be a critical determinant of anti-cancer benefit211, 212. Indeed, folate intake 12–16 years before diagnosis rather than recent intake was associated with lower CRC risk and a strong inverse association with adenoma was observed for folate intake 4–8 years before diagnosis in 2 large cohorts213. This may also explain the largely null effects214-216, or an even increased risk of advanced lesions216 in RCTs of folic acid supplementation among patients with a history of adenoma. Another important determinant of folate's effects might be the baseline intake. Some RCTs have found that folic acid supplementation reduces adenoma recurrence only among individuals with low baseline folate levels214, 217.
In summary, the investigation of the folate-CRC relationship provides an excellent example of the complex role of nutrients in human health and disease. Despite the inconsistent evidence, recommending adequate folate intake may abrogate the increased risk of CRC consistently observed with folate deficiency. In contrast to folate, data regarding other B vitamins and CRC are scant. Vitamin B6 as assessed by dietary intake and blood pyridoxal 5′-phosphate, the major circulating form, has been associated with lower CRC risk in a meta-analysis218. Vitamin B2 and B12 remain inconclusive32, 187, 189, 219-227. For methionine, high intake has been associated with lower risk of CRC in a recent meta-analysis of 8 cohort studies228. Similar findings have also been reported for circulating methionine220, 229. In addition to a role in one-carbon metabolism, metabolites of methionine may block mitogenic signaling in colon cancer cells230 and reduce inflammation-associated colon tumorigenesis through multiple signaling pathways231.
Antioxidant nutrients
Antioxidants, reactive oxygen species scavengers, protect cells from oxidative stress that can initiate and promote carcinogenesis by inducing gene mutations, DNA damage, genome instability, cell proliferation, and inflammation232. Carotenoids (e.g., beta carotene, vitamin A precursor, and lycopene), vitamin C, and vitamin E have potent anti-oxidative and anti-inflammatory properties. Selenium has no such action itself but is required for the anti-oxidative activity of selenoenzymes. Therefore, these vitamins and minerals are referred to as antioxidant nutrients.
Early ecologic233-235, case-control226, 236, 237 and small prospective studies141, 238-241 have shown that antioxidant nutrient intake is inversely associated with CRC risk. These data prompted small RCTs in which supplementation with antioxidant vitamins reduced adenoma recurrence154, 242-244. However, these data were not corroborated in subsequent cohort studies that assessed dietary intake32, 219, 245-248, supplemental use249, 250 or biomarker levels35, 245, 248, 251-253 of antioxidants. In pooled analyses of more than 10 cohort studies, dietary intake of carotenoids254, and vitamins A, C and E were not significantly associated with CRC risk, and any inverse association for total intake of vitamins A, C and E were attenuated after accounting for folate intake255. Larger RCTs that tested tumor chemopreventive efficacy of antioxidant supplementation also yielded null results156, 256-266, although a potential benefit of beta-carotene was detected in one trial among a subgroup who neither smoked cigarettes nor drank alcohol267. Selenium supplementation reduced CRC incidence by 61% in the secondary analysis of a RCT among patients with a history of non-melanoma skin cancer (the National Prevention of Cancer (NPC) study)268. However, no such benefit was observed in the large Selenium and Vitamin E Cancer Prevention Trial (SELECT)264. In addition to the different formulations of selenium used in the 2 trials, the lower baseline selenium level among participants of the NPC study may have contributed to the observed benefit. There is evidence for a U-shaped relationship between selenium status and protection from cancer, with an optimal circulating level of selenium within the range of 130–150 μg/L269-271. Selenium requirements may vary among individuals due to genetic variation in selenoenzymes272, 273. Although some genetic mutations in selenoproteins have been associated with colorectal neoplasm risk274-276, the findings remain inconclusive253, 277, 278 and require replication in larger studies.
Given differences in selenium metabolism279-283 and the modifying impact of estrogen284, a sex difference hypothesis emerged for the selenium effect on neoplasia. Some data including a CRC meta-analysis253, a prospective adenoma study285, a pooled analysis of 3 RCTs286, and the NPC study287, indicate that any selenium benefit in colorectal tumors may be restricted to men. However, these findings seem at odds with the null results of the SELECT trial, which only included men264, and a recent analysis of the large cohort in which the inverse associations with CRC for serum selenium and selenoprotein P levels were more apparent in women than in men288.
In summary, although adequate antioxidant intake may be essential for overall health, recommending routine use of antioxidant supplements is unlikely to prevent CRC, particularly in populations without significant nutrient deficiencies.
Fats
The hypothesis that high-fat diets cause CRC derives from the striking correlation between per capita consumption of meat or animal fat and national rates of the disease289, 290. High-fat diets increase the intestinal excretion of bile acids, which can be metabolized by the gut bacteria to cancer-promoting agents291-293. A high-fat diet can also induce marked changes in microbial community composition and function with concomitant or subsequent changes in gut immune and inflammatory effectors294-297 implicated in the intestinal tumorigenesis298. In 1990, findings from the Nurses' Health Study (NHS) indicated that high intake of total fat, specifically animal saturated and monounsaturated fat, but not vegetable fat, linoleic acid or cholesterol, increased colon cancer risk299. However, these results were not consistently replicated in subsequent studies46, 50, 300-306. A meta-analysis of 13 prospective studies found that neither total fat nor specific types of fat was associated with CRC risk307. In accordance with the observational findings, the WHI RCT did not detect any reduction in CRC incidence after over 8 years of low-fat (20% of energy intake) dietary modification304.
Among specific types of fat, omega-3 polyunsaturated fatty acids (PUFAs) have garnered particular attention due to their potent anti-inflammatory effects in experimental models308-311. A placebo-controlled RCT showed that omega-3 PUFA administration of 2 g daily for 6 months decreased polyp number, size and overall burden in patients with familial adenomatous polyposis312. A larger RCT, the seAFOod Polyp Prevention Trial, is currently underway to investigate the effect of omega-3 PUFA supplementation alone or in combination with aspirin on adenoma recurrence313. Despite these promising data, findings from epidemiologic studies are inconclusive314. Some studies suggest that the inverse association between omega-3 PUFAs and CRC is restricted to aspirin nonusers315, 316, although the results are mixed317-319. Recent studies also suggest that the omega-3-PUFA-CRC association may vary by tumor site, with an inverse association for proximal colon or rectal cancer, but a positive association for distal colon cancer318, 320. Differential associations are plausible given the considerable anatomic variation in biochemical milieu (e.g., pH), molecular features, and gut microbiota observed throughout the colon321-323. Several lines of evidence also support that omega-3 PUFAs may exert their effects only in the early stages of carcinogenesis. Dietary intake of omega-3 PUFAs more than 10 years before cancer diagnosis, but not recent intake, has been associated with a lower risk of CRC318, 324. Similarly, high levels of omega-3 PUFAs in the circulation or erythrocyte membranes have been associated with lower adenoma risk325-327.
Laboratory data indicate that omega-6 PUFAs have pro-inflammatory effects; these are attributed to antagonism of omega-3 PUFAs. However, human studies have not provided convincing data for the detrimental effects of omega-6 PUFAs on cancer outcomes, including CRC305, 306, 315-317, 328, and heart disease329, raising questions about the plausibility of this hypothesis330, 331. Another fatty acid, trans fat, has been associated with an increased risk for cardiometabolic diseases332. Some of the postulated mechanisms of trans fat also involve cancer risk, such as systemic inflammation, insulin resistance, and adiposity333. However, findings from epidemiologic studies have been inconsistent on the association between trans-fat intake and increased CRC risk334-337.
In summary, there is limited evidence that total fat or specific types of fat are associated with CRC risk. Thus, recommendations to alter one's fat intake for the specific purpose of CRC prevention do not appear warranted without additional data—particularly the results of the RCT of omega-3 PUFA supplementation125.
Sulfur
Sulfur in the diet can arise from inorganic sulfate used in the preservation of processed foods and beverages, and the sulfur-containing amino acids from protein, such as methionine, cysteine, and taurine338. These sulfur compounds are metabolized to hydrogen sulfide (H2S) by gut bacteria through reduction and fermentation reactions339-341 (Figure 4). H2S has been implicated in inflammatory disorders associated with risk of CRC, such as ulcerative colitis,342-344 and directly with CRC345-351. In a prospective study, high intake of sulfur and sulfate is associated with increased likelihood of relapse of ulcerative colitis352. Dietary sources of sulfur, such as red meat, animal protein and wine, have also been associated with ulcerative colitis onset and relapse353-356. In the colon, excess chronic H2S exposure is associated with factors that promote carcinogenesis, such as impaired colonocyte nutrition, DNA damage, epithelial hyperproliferation, inflammation, and alterations in immune cell populations and function341, 347, 357-359.
Figure 4. Proposed mechanisms relating sulfur-containing foods to colorectal cancer.
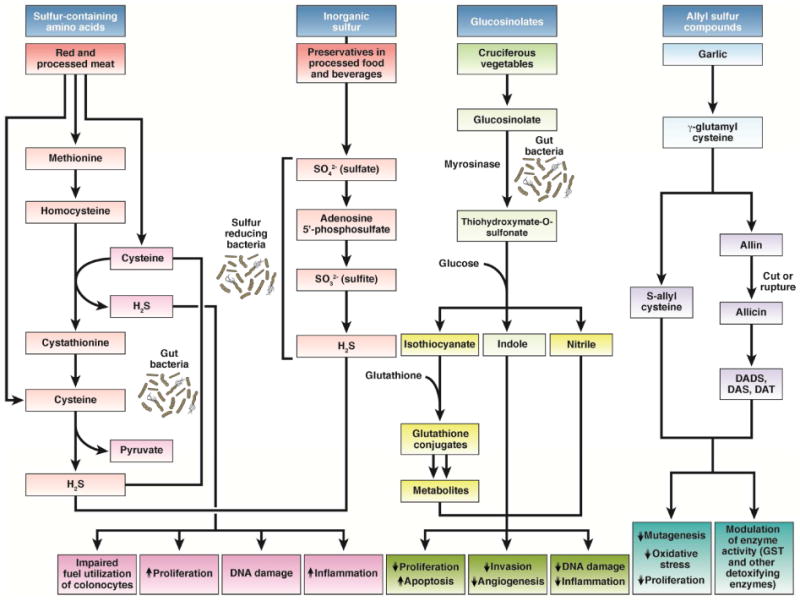
Sulfur-containing amino acids and inorganic sulfur may have procarcinogenic properties, whereas glucosinolates and allyl sulfur compounds possess antineoplastic activities. Gut bacteria can ferment sulfur-containing amino acids to produce hydrogen sulfide (H2S). Gut sulfate-reducing bacteria can reduce inorganic sulfur in processed food and beverages to produce H2S. Several mechanisms that may mediate the pro-carcinogenic effects of H2S include: impaired fuel utilization of butyrate by colonocytes, increased cell proliferation, DNA damage, and inflammation. Cruciferous vegetables are rich in glucosinolates that can be metabolized by myrosinase-expressing gut bacteria to thiohydroxymate-O-sulfonate, which can be further converted to isothiocyanates, indole and nitrile. These metabolites and their downstream products have a wide diversity of anticarcinogenic effects. Allium vegetables have high levels of allyl sulfur compounds, mainly γ-glutamylcysteines, which can be converted to S-allylcysteine, or hydrolyzed and oxidized to form alliin. After processing, alliin decomposes to diallyl disulfide (DADS), diallyl sulfide (DAS), and diallyl trisulfide (DAT), which have been related to garlic's cancer-preventive effects.
Other sources of sulfur include the allyl sulfur components from garlic360 and sulfur-containing glycosides (mainly glucosinolates) found in cruciferous vegetables, such as cabbage, brussel sprouts, and broccoli361. In contrast to the inorganic sulfur and sulfur-containing amino acids, allyl sulfur compounds and glucosinolates possess antineoplastic effects via multiple mechanisms including: inhibition of carcinogen-activating enzymes, detoxification of carcinogens, induction of apoptosis, arrest of cell cycle progression, modulation of inflammation, and suppression of angiogenesis362-366. High garlic intake has been associated with a modest reduction of CRC incidence in cohort studies367, although such findings were not replicated in a recent meta-analysis368. Animal studies also indicate a protective effect of garlic on CRC369. Cruciferous vegetables and their interaction with genetic variations may affect CRC risk as well370 (see fruits and vegetables section).
In summary, sulfur-containing foods may have divergent effects on CRC development depending on the specific sulfur-associated compounds. However, at present there is insufficient human evidence to recommend modification of sulfur intake for the prevention of CRC.
Foods
Because various nutrients are consumed in foods, investigation of foods accounting for the interaction among multiple nutrients may not only facilitate the formulation of public health recommendations, but also provide additional insight into the role of dietary factors in colorectal carcinogenesis. Here, we focus on foods with the greatest epidemiologic evidence in CRC (Figure 5).
Figure 5. Biologic mechanisms relating foods to colorectal cancer.
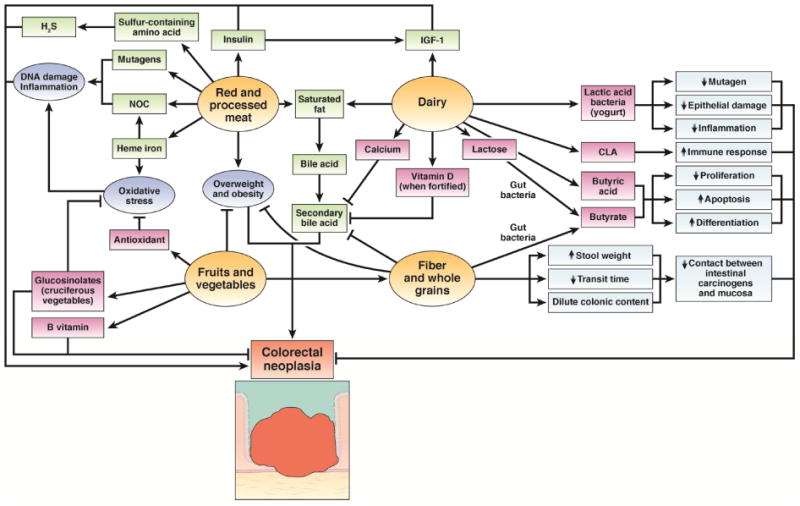
Red and processed meat may contribute to CRC through generation of carcinogens, including N-nitroso compounds (NOC), heme iron, sulfur-containing amino acids, and saturated fat. Overweight or obesity as well as chronically increased levels of insulin may also have a critical role. Despite high saturated fat content and its role in increasing insulin-like growth factor 1 (IGF1), dairy products may inhibit colorectal carcinogenesis via a variety of bioactive constituents: calcium, vitamin D (when fortified), conjugated linoleic acid (CLA), butyric acid, lactose, and lactic acid bacteria in fermented milk products. Fruits and vegetables contain various components with antineoplastic potential: fiber, antioxidants, B vitamins, and glucosinolates (in cruciferous vegetables). Potential anti-cancer mechanisms of fiber include: binding or diluting secondary bile acids, increased stool weight, decreased transit time, and bacterial fermentation leading to reduced colonic luminal pH and production of butyrate which has antineoplastic properties. Fiber, fruits and vegetables may also influence carcinogenesis through decreased adiposity.
Meat
In its systematic review of diet and cancer, the World Cancer Research Fund concluded that there is convincing evidence that red meat and processed meat increase CRC risk7. A recent meta-analysis indicated an approximately 20% higher risk of CRC per 100 g/day increase in red meat and 50 g/day increase in processed meat. The risk increases linearly with increasing intake of red and processed meats up to approximately 140 g/day; beyond this level, the risk increase is less pronounced371. Similar findings have been reported for colorectal adenomas372. Remote rather than recent intake of red meat may be significantly associated with CRC, suggesting an initiating role in colorectal carcinogenesis373. In addition, associations appear to be more pronounced for distal colon and rectosigmoid adenocarcinomas than other sites373-375.
Several mechanisms may underlie the relationship between red meat and CRC. Effects may be mediated through some of the nutrients already described. Meat is an abundant source of sulfur-containing amino acids, saturated fats and, in the case of processed meat, inorganic sulfur used as a preservative. Heme iron in red meat can induce oxidative stress376, colonocyte proliferation377 and the endogenous formation of N-nitroso compounds (NOCs), which are potent carcinogens in the gastrointestinal tract378. NOCs are also introduced exogenously in processed meats from nitrates and nitrites added in the preserving process379. Meat cooked at high temperature is also a source of other mutagens, including heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs)380-383. High consumption of heme iron (but not other forms of iron)384-386, NOCs385, 387, 388, HCAs385, 389-391, and PAHs385, 392 have all been associated with increased risk of colorectal tumors with a few exceptions393, 394. Consistent with their mutagenic effects, processed meat and heme iron have been more strongly associated with the risk of colorectal neoplasia harboring somatic mutations in KRAS and APC395-397. Genetic variations in NOC and HCA metabolism may modify the relationship between red meat intake and CRC risk as well391, 398-401. Individuals with genotypes that lead to rapid activation of N-acetyltransferases 1 and 2, important enzymes for carcinogen activation, have greater risk associated with high red meat intake, compared to those with slow activation genotypes391, 398-401, although the evidence is inconclusive402, 403.
Poultry404 and fish405 has been associated with a modest reduction of CRC incidence in contrast to red meat. This is concordant with the notion that components other than fat and protein in red and processed meat contribute to the carcinogenic effects304, 406. Thus, based on current evidence it would be reasonable to recommend substitution of poultry or fish for red and processed meat as a strategy for CRC prevention299, 407.
Dairy products
Dairy products may protect against colorectal neoplasia because of their high content of calcium, other micronutrients and bioactive constituents56, 408, 409. However, epidemiologic evidence is inconsistent33, 34, 44, 48, 51, 52, 73, 101, 102, 406, 410-418. In a pooled analysis of 10 cohort studies, milk consumption was associated with lower risk for CRC—particularly for distal colon and rectal cancers. Despite the moderate-to-high correlation between milk and calcium intake (r ranges 0.38-0.78), accounting for calcium only partially attenuated this association, indicating calcium-independent and -dependent mechanisms of cancer prevention73. A recent meta-analysis of 19 cohort studies indicate a nonlinear, inverse association between milk intake and CRC risk, with no substantial change in CRC incidence below ∼200 g/day and the greatest reduction over consumption of 500-800 g/day419. Constituents in milk other than calcium may also contribute to the antineoplastic activity, including conjugated linoleic acid (CLA) that has antioxidant, anti-inflammatory and immune modulatory properties420-424. CLA supplementation has been shown to inhibit colorectal carcinogenesis in animal models425-427. The first human evidence in support of beneficial CLA effects was reported in a Swedish cohort in which high CLA intake was associated with lower CRC risk and partly accounted for the inverse relationship between high-fat dairy food consumption and CRC incidence414. Butyric acid, a SCFA, present in or generated by consumption of dairy products may also have a protective effect against CRC possibly through its diverse effects on cellular function428, 429.
Contrary to adult dairy consumption, high childhood dairy intake was associated with 3-fold increase of CRC risk in a longitudinal study430. This association may be mediated by attained adult height which is positively associated with both childhood dairy intake and CRC risk431, or may be a marker of a programing effect of early-life nutrition on host growth signaling pathways432 and the gut microbiome433. Given the paucity of data, further investigation is needed to confirm these observations and elucidate the mechanisms whereby early- and later-life nutritional exposures may influence CRC risk434.
Fermented dairy products, such as yogurt, have also been associated with CRC. The lactic acid bacteria of fermented milk products may alter the composition or function of a host's endogenous gut microbiota435, reduce absorption of mutagens from cooked foods436, inactivate intestinal carcinogens437, and dampen intestinal inflammation438. However, epidemiologic evidence relating yogurt consumption to CRC risk is conflicting33, 48, 418, 439, 440. Possible reasons include the challenge of adequately accounting for confounding by lifestyle factors associated with yogurt consumption, limited variation in the amount of intake, and varied bacterial and nutritional yogurt composition in different populations. For example, yogurt was weakly associated with lower CRC risk in a pooled analysis of studies mostly from the U.S.73, where yogurt may be heat treated after fermentation to destroy most microbes and extend shelf-life. In contrast, a strong inverse association was reported in European countries where yogurt typically contains high counts of live lactic acid bacteria418, 440. Similarly, an inverse association with CRC was reported for high lactose intake51, a sugar present in milk that can be fermented by gut bacteria to produce butyrate441. Genetic variation associated with lactase persistence has also been shown to influence CRC risk442. There are limited data on other dairy products, including cheese33, 38, 48, 51, 52, 136, 414, 418, high-fat dairy products34, 305, 414, low-fat dairy products101, 305, cottage cheese33, 38, 51, 413, non-yogurt fermented dairy products (e.g., kefir)46, 48, 52, 101, 102 and butter51, 414. No association with CRC risk was reported for any of these dairy products in the meta-analysis419.
In summary, there is probable evidence that milk consumption protects against CRC. The potential anti-CRC effect of yogurt also deserves further investigation. Thus, it may be reasonable to encourage intake of milk, and possibly yogurt, for CRC prevention.
Fruits and vegetables
Fruits and vegetables may protect against CRC because of high levels of several potential anticarcinogenic compounds that we have already discussed, including: fiber, folate, other B vitamins, minerals, and antioxidants443. However, epidemiologic studies have yielded inconsistent findings, possibly because of the large variations across studies in consumption amount, production methods, storage conditions, nutrient content, and cooking and preparation as well as variation in accounting for confounding factors444, 445. In 11 of 21 cohort studies134, 136, 142, 413, 446-452, a weak inverse association was reported between fruit or vegetable intake and CRC; in other studies no association was detected46, 133, 417, 453-459. The relationship seems more evident for distal colon cancer than for other anatomic sites460. In some studies, a stronger association was observed among certain subgroups, such as obese or physically inactive individuals452, never and former smokers451, nondrinkers of alcohol460, and low consumers of red meat460. As suggested by some studies134, 144, 449, a recent meta-analysis found a significant nonlinear relationship between fruit and vegetable intake and CRC incidence, with the greatest risk reduction associated with increasing fruit intake up to about 100 g/day and vegetable intake to about 100-200 g/day, with little evidence for further reduction with higher intake444. These findings agree with the results of the Polyp Prevention Trial in which extremely high consumption of fruits and vegetables (as well as high fiber and low fat) did not result in appreciable reductions in adenoma recurrence158. This nonlinear relationship may explain the discrepant findings, as in some studies the lowest level of intake was already above the beneficial effect range448, 452, 456. In addition, there is some evidence that high fruit and vegetable intake during adolescence and mid-life, independent of adult diet461, 462, may confer additional benefit, suggesting that exposures over the life cycle plays a role in CRC development.
Of all vegetables, cruciferous vegetables are of particular interest given their high glucosinolate content that can be metabolized to isothiocyanates (ITCs) and indole-3-carbinol (I3C) by myrosinase-expressing colonic bacteria366. The multifaceted antineoplastic activities of ITCs and I3Cs have been suggested in animal studies365 (see Figure 4 and the sulfur section). A potential benefit of high cruciferous vegetable intake or high ITC levels on colorectal neoplasms has also been noted463. A recent meta-analysis reported 16% reduction of CRC risk comparing the highest to the lowest categories of cruciferous vegetable intake370. Genetic studies offer supporting evidence, in which genetic variations in glutathione S-transferase, a critical enzyme in ITC metabolism, modify the association of cruciferous vegetables or ITCs with colorectal tumors464-470. Given the importance of gut microbiota in glucosinolate metabolism, further investigation is needed to uncover the potential interplay between genetic susceptibility, gut bacterial composition, and dietary intake in determining CRC risk.
In summary, there is limited evidence that high intake of fruits and vegetables protects against CRC. However, considering the well-established cardiometabolic benefits of adequate fruit and vegetable intake471, 472, it would be reasonable to recommend increasing intake among populations with very low consumption.
Whole grain
In contrast with refined grains that retain only the endosperm, whole grains contain germ and bran, which are rich sources of various substances with anticancer properties, including fiber, antioxidants, and phytochemicals473. High intake of whole grain has been related to lower CRC risk in all146, 474-480 but 2 cohort studies46, 144. Of note, a particular challenge of studying whole-grain intake is the difficulty of accurate measurement owing to the wide variation in whole grain content among products and absent universal standards for whole grains or whole-grain products481. To address this issue, a circulating biomarker of whole grain intake, alkylresorcinol, has been developed. Alkylresorcinols, phenolic lipids found exclusively in the bran part of wheat and rye482, are not affected by food processing483 and can be measured in blood plasma with moderate validity and reproducibility484-489, 490. In a large nested case-control study, the highest quartile of plasma alkylresorcinol was associated with decreased risk of distal colon cancer, but not proximal colon or rectal cancer491. In another study, plasma alkylresorcinol, but not whole grain intake assessed by food frequency questionnaires, was associated with CRC, particularly of the distal colon480.
In summary, based on convincing evidence, it would be reasonable to recommend increasing intake of whole grains to help reduce risk of CRC. In addition to the high content of bioactive compounds, whole grain also represents a source of high-quality carbohydrate, as assessed by a low glycemic index, due its slow digestion and absorption492. Although the epidemiologic evidence on glycemic index and CRC is inconclusive493, consumption of whole grains has been associated with decreased fasting insulin level and improved insulin sensitivity494. Given the well-established role of insulin in promoting colonic growth495, whole grain may exert its beneficial effect on colorectal carcinogenesis by lowering insulin.
Dietary Pattern
Emerging data suggest that consideration of a combination of nutrients and foods may demonstrate stronger associations with CRC risk compared with specific nutrients or food types. This may reflect the interplay of various components of diet, and that not only the addition of beneficial dietary factors but the substitution of detrimental dietary constituents with such factors is critical. For example, in accordance with vitamin D's regulatory role in calcium metabolism, there is evidence that high intake of calcium and vitamin D interact to influence CRC risk35, 36, 78. In addition, increasing folate consumption may mitigate alcohol-induced folate deficiency496 and exert stronger protection among alcohol drinkers173, 177, 187, 188. Given the importance of inflammation in CRC development, various nutrients with either pro- or anti-inflammatory activities may work together to influence CRC risk through intertwined pathways, including gut microbiota co-metabolism294.
Human studies have also focused on dietary patterns, which represent the combination of highly correlated foods consumed in a certain population that can be derived from either an a posteriori (data-driven) approach by factor or cluster analysis, or a priori approach based on dietary recommendations497, 498. The data-driven approach has identified at least two general patterns: the western pattern, featuring high consumption of processed and red meats, refined grains, soda and sweets, and the prudent pattern featuring high intakes of fruits, vegetables, fish, poultry and whole-grain products. The western pattern has been associated with increased CRC risk474, 499-503, whereas the prudent pattern, has been less consistently associated with lower risk140, 474, 499, 503-508. In addition, a prudent pattern during adolescence, independent of adult dietary pattern, was associated with lower risk of rectal adenomas, whereas a western pattern was associated with higher risk461. Among CRC patients, higher intake of a western dietary pattern after diagnosis may increase the risk of cancer recurrence and mortality509.
Several a priori dietary patterns have also been assessed. A Mediterranean diet has been associated with lower cancer mortality510 and lower CRC risk511-513. The Dietary Approaches to Stop Hypertension (DASH) diet, originally designed for blood pressure control514, has been associated with a lower CRC risk478, 515. Other indexes, such as Healthy Eating Index-2005, Alternate Healthy Eating Index, and Recommended Food Score, have also been related to CRC risk512.
Energy Balance
Given the critical role of diet in energy balance, nutritional factors may influence the development of CRC through their effects on adiposity and its associated metabolic derangements. Long-term energy imbalance, represented by an excess of energy intake over expenditure, leads to overweight and obesity516. An extensive body of literature has implicated overweight and obesity, especially abdominal adiposity, as one of the critical factors, if not the most important factor, for CRC. The risk increases by 2-3% per unit increase of body mass index and per inch increase of waist circumference7.
Although the exact mechanisms that underlie this association remain unclear, several lines of evidence indicate that insulin resistance and increased signaling of insulin and insulin-like growth factor (IGF) may mediate the effect495, 517. Adipose tissue itself is a metabolically active organ, producing molecules that potentially modulate carcinogenesis, including: inflammatory cytokines, adipokines and sex hormones518, 519. Interested readers are encouraged to refer to other excellent reviews on the mechanisms underlying the relationship between obesity and cancer518, 519, in particular CRC520. Several components of a western diet have been associated with obesity and weight gain, including high intake of red meat and processed meat, and low consumption of fruits, vegetables, fiber, and whole grains521. Greater intake of red and processed meat has been related to increased levels of inflammatory and dysregulated metabolic biomarkers522. Fruits and vegetables, or biomarkers of their intake, have been inversely associated with metabolic syndrome523 and concentrations of fasting insulin and glucose524-526. Similarly, higher fiber and whole grain intake has been associated with improved insulin sensitivity527, 528.
In contrast to the equivocal findings on individual nutrients or foods, the strong, consistent association between obesity and CRC (at least in men) further underscores the predominant importance of combined, integrated effects of nutrients/foods over their individual effects. These effects probably reflect both the excess quantity of energy intake over expenditure, and the suboptimal quality of the diet associated with obesity.
Interaction between Diet and the Gut Microbiota
The explosion of research in host-microbe interaction supports a potential role of gut microbiota and their interplay with diet in human health and disease, through regulation of host metabolism and immune function294, 529-535. An altered microbial community as well as enrichments of select bacteria, such as Fusobacterium nucleatum, have been implicated in the development of colorectal adenomas and adenocarcinomas536-545. Studies have also associated changes in relative proportions of gut bacterial clades with obesity530, 546, 547, metabolic syndrome548 and type 2 diabetes549, 550, all of which are conditions that increase CRC risk. Accumulating evidence suggests a profound effect of diet on gut microbiota296, 531, 551-553. Dietary fiber can favor the growth of butyrate-producing bacteria over other bacteria, beneficially shaping the structure of the human gut microbiota554. Children from rural Africa, where diet is high in fiber, have a strikingly different composition of gut microbiota than those from urban Europe characterized by a western diet552. Switching from a low-fat, plant-rich diet to a western diet changed microbial composition, and metabolic pathways and gene expression in the gut microbiome553.
The progressive maturation and balanced composition of intestinal microbes may be important for establishment and regulation of immune and inflammatory responses through bacteria-derived products, such as SCFAs, bile acids and vitamins. Decreased levels of such products because of either dysbiosis or an imbalanced diet may contribute to inflammation and subsequent disease development in the host130, 294, 555. Synbiotic intervention with prebiotic resistant starch and beneficial bacteria has been shown to lower the incidence of colonic neoplasms in rats556.
However, more research in animal models as well as robust epidemiologic and well-controlled interventional studies in human are required.
Conclusion and Future Directions
Although there are few conclusive data from RCTs to support the efficacy of dietary modification or nutrient supplementation for CRC prevention, several foods and nutrients, including red meat and processed meat, fiber, milk, calcium, and whole grains, have been related to CRC with reasonable consistency in prospective cohort studies. Taken together with biological mechanisms derived from experimental studies linking these factors with CRC, there is a strong rationale to continue investigation into dietary strategies for CRC prevention.
Several questions remain to be addressed. Given evidence supporting clear anatomic and molecular heterogeneity of colorectal tumors, how do nutritional factors differentially influence the risk of cancers that arise from distinctive etiologic pathways? What are the optimal dose, formulation, and time window for exposure to specific nutrients, foods, or dietary patterns to interrupt or reverse carcinogenesis? How do dietary components interact with the gut microbiota as well as other host factors to influence CRC risk? Although we remain at an early stage of addressing these questions, technologic advances in the form of novel experimental models and methodologic tools (e.g, metabolomics, microbial meta'omics) will undoubtedly yield mechanistic and causal insights that can be used to inform public health recommendations557 and curtail CRC incidence and mortality558.
Acknowledgments
We thank Dr. Edward Giovannucci for his helpful insights on this manuscript.
Funding: This work was supported by the National Institutes of Health (R01 CA137178, K24 DK098311, and R01 CA154426).
Footnotes
No conflicts of interest to disclose for the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0. Lyon, France: International Agency for Research on Cancer; 2013. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] [Google Scholar]
- 2.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, et al. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:659–69. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holme O, Loberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. Jama. 2014;312:606–15. doi: 10.1001/jama.2014.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014;106:dju032. doi: 10.1093/jnci/dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–308. [PubMed] [Google Scholar]
- 7.World Cancer Research Fund / American Institute for Cancer Research. Continuous Update Project Report Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. 2011 [Google Scholar]
- 8.World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 9.Thomas D. Gene--environment-wide association studies: emerging approaches. Nat Rev Genet. 2010;11:259–72. doi: 10.1038/nrg2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004;4:769–80. doi: 10.1038/nrc1453. [DOI] [PubMed] [Google Scholar]
- 11.Herceg Z. Epigenetics and cancer: towards an evaluation of the impact of environmental and dietary factors. Mutagenesis. 2007;22:91–103. doi: 10.1093/mutage/gel068. [DOI] [PubMed] [Google Scholar]
- 12.Willett W. Nutrition and cancer: the search continues. Nutr Cancer. 2008;60:557–9. doi: 10.1080/01635580802380370. [DOI] [PubMed] [Google Scholar]
- 13.Martinez ME, Marshall JR, Giovannucci E. Diet and cancer prevention: the roles of observation and experimentation. Nat Rev Cancer. 2008;8:694–703. doi: 10.1038/nrc2441. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–54. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60:131–44. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perdigon G, de Moreno de LeBlanc A, Valdez J, et al. Role of yoghurt in the prevention of colon cancer. Eur J Clin Nutr. 2002;56(Suppl 3):S65–8. doi: 10.1038/sj.ejcn.1601490. [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132:2350S–2355S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 19.Nelson RL. Iron and colorectal cancer risk: human studies. Nutr Rev. 2001;59:140–8. doi: 10.1111/j.1753-4887.2001.tb07002.x. [DOI] [PubMed] [Google Scholar]
- 20.Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20:2209–18. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- 21.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E, Stampfer MJ, Colditz GA, et al. A comparison of prospective and retrospective assessments of diet in the study of breast cancer. Am J Epidemiol. 1993;137:502–11. doi: 10.1093/oxfordjournals.aje.a116703. [DOI] [PubMed] [Google Scholar]
- 23.Giovannucci E, Stampfer MJ, Colditz GA, et al. Recall and selection bias in reporting past alcohol consumption among breast cancer cases. Cancer Causes Control. 1993;4:441–8. doi: 10.1007/BF00050863. [DOI] [PubMed] [Google Scholar]
- 24.Malila N, Virtanen M, Pietinen P, et al. A comparison of prospective and retrospective assessments of diet in a study of colorectal cancer. Nutr Cancer. 1998;32:146–53. doi: 10.1080/01635589809514733. [DOI] [PubMed] [Google Scholar]
- 25.Terzic J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 26.Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–71. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 27.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–28. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–72. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 29.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984;72:1323–5. [PubMed] [Google Scholar]
- 30.Garland C, Shekelle RB, Barrett-Connor E, et al. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1:307–9. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Murphy SP, Wilkens LR, et al. Calcium and vitamin D intake and risk of colorectal cancer: the Multiethnic Cohort Study. Am J Epidemiol. 2007;165:784–93. doi: 10.1093/aje/kwk069. [DOI] [PubMed] [Google Scholar]
- 32.Shin A, Li H, Shu XO, et al. Dietary intake of calcium, fiber and other micronutrients in relation to colorectal cancer risk: Results from the Shanghai Women's Health Study. Int J Cancer. 2006;119:2938–42. doi: 10.1002/ijc.22196. [DOI] [PubMed] [Google Scholar]
- 33.Kesse E, Boutron-Ruault MC, Norat T, et al. Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int J Cancer. 2005;117:137–44. doi: 10.1002/ijc.21148. [DOI] [PubMed] [Google Scholar]
- 34.Bostick RM, Potter JD, Sellers TA, et al. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women's Health Study. Am J Epidemiol. 1993;137:1302–17. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]
- 35.Zheng W, Anderson KE, Kushi LH, et al. A prospective cohort study of intake of calcium, vitamin D, and other micronutrients in relation to incidence of rectal cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7:221–5. [PubMed] [Google Scholar]
- 36.Wu K, Willett WC, Fuchs CS, et al. Calcium intake and risk of colon cancer in women and men. J Natl Cancer Inst. 2002;94:437–46. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- 37.Flood A, Peters U, Chatterjee N, et al. Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiol Biomarkers Prev. 2005;14:126–32. [PubMed] [Google Scholar]
- 38.Larsson SC, Bergkvist L, Rutegard J, et al. Calcium and dairy food intakes are inversely associated with colorectal cancer risk in the Cohort of Swedish Men. Am J Clin Nutr. 2006;83:667–73. doi: 10.1093/ajcn.83.3.667. [DOI] [PubMed] [Google Scholar]
- 39.Ishihara J, Inoue M, Iwasaki M, et al. Dietary calcium, vitamin D, and the risk of colorectal cancer. Am J Clin Nutr. 2008;88:1576–83. doi: 10.3945/ajcn.2008.26195. [DOI] [PubMed] [Google Scholar]
- 40.Martinez ME, Marshall JR, Sampliner R, et al. Calcium, vitamin D, and risk of adenoma recurrence (United States) Cancer Causes Control. 2002;13:213–20. doi: 10.1023/a:1015032215779. [DOI] [PubMed] [Google Scholar]
- 41.Peters U, Chatterjee N, McGlynn KA, et al. Calcium intake and colorectal adenoma in a US colorectal cancer early detection program. Am J Clin Nutr. 2004;80:1358–65. doi: 10.1093/ajcn/80.5.1358. [DOI] [PubMed] [Google Scholar]
- 42.Oh K, Willett WC, Wu K, et al. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol. 2007;165:1178–86. doi: 10.1093/aje/kwm026. [DOI] [PubMed] [Google Scholar]
- 43.Massa J, Cho E, Orav EJ, et al. Total calcium intake and colorectal adenoma in young women. Cancer Causes Control. 2014;25:451–60. doi: 10.1007/s10552-014-0347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCullough ML, Robertson AS, Rodriguez C, et al. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States) Cancer Causes Control. 2003;14:1–12. doi: 10.1023/a:1022591007673. [DOI] [PubMed] [Google Scholar]
- 45.Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. Bmj. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietinen P, Malila N, Virtanen M, et al. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control. 1999;10:387–96. doi: 10.1023/a:1008962219408. [DOI] [PubMed] [Google Scholar]
- 47.Martinez ME, Giovannucci EL, Colditz GA, et al. Calcium, vitamin D, and the occurrence of colorectal cancer among women. J Natl Cancer Inst. 1996;88:1375–82. doi: 10.1093/jnci/88.19.1375. [DOI] [PubMed] [Google Scholar]
- 48.Lin J, Zhang SM, Cook NR, et al. Intakes of calcium and vitamin D and risk of colorectal cancer in women. Am J Epidemiol. 2005;161:755–64. doi: 10.1093/aje/kwi101. [DOI] [PubMed] [Google Scholar]
- 49.Heilbrun LK, Nomura A, Hankin JH, et al. Dietary vitamin D and calcium and risk of colorectal cancer. Lancet. 1985;1:925. doi: 10.1016/s0140-6736(85)91694-0. [DOI] [PubMed] [Google Scholar]
- 50.Gaard M, Tretli S, Loken EB. Dietary factors and risk of colon cancer: a prospective study of 50,535 young Norwegian men and women. Eur J Cancer Prev. 1996;5:445–54. [PubMed] [Google Scholar]


