Abstract
Background
The largest growth in differentiated thyroid cancer (DTC) diagnosis is in low-risk cancers. Trends in imaging after DTC diagnosis are understudied. Hypothesizing a reduction in imaging utilization due to rising low-risk disease, we evaluated post-diagnosis imaging patterns over time and patient characteristics that are associated with likelihood of imaging.
Methods
Using the Surveillance Epidemiology and End Results-Medicare database, we identified patients diagnosed with localized, regional or distant DTC between 1991 and 2009. We reviewed Medicare claims for neck ultrasound, I-131 scan, or PET scan within 3 years post-diagnosis. Using regression analyses we evaluated trends of imaging utilization. Multivariable logistic regression was used to estimate the likelihood of imaging based on patient characteristics.
Results
23,669 patients were included. Patients diagnosed during 2001-2009, compared to 1991-2000, were more likely to have localized disease (p<0.001) and tumors less than 1cm (p<0.001). Use of neck ultrasound and I-131 scan increased in patients with localized disease (p=<0.001 and p=0.003, respectively), regional disease (p<0.001 and p<0.001), and distant metastasis (p=0.001 and p=0.015). Patients diagnosed after 2000 were more likely to undergo neck ultrasound (OR 2.15, 95% CI 2.02-2.28) and I-131 scan (OR 1.44, 95% CI 1.35-1.54). PET scan use from 2005-2009, compared to 1996-2004, increased 32.4-fold (p=<0.001) in localized patients, 13.1-fold (p<0.001) in regional disease patients, and 33.4-fold (p<0.001) in patients with distant DTC.
Conclusion
Despite a rise in low-risk disease, the use of post-diagnosis imaging increased in all stages of disease. The largest growth was in use of PET scan after 2004.
Keywords: Differentiated thyroid cancer, surveillance imaging, neck ultrasound, radioiodine scan, PET scan
Introduction
Across a variety of payer systems, utilization of advanced imaging techniques has exploded in recent years.1, 2 This contributes to both radiation exposure and increased health care costs.3 One growing area of imaging utilization is post-treatment surveillance for malignancy. In most cancers, increased imaging has not translated into improved survival.4, 5 Additionally, studies have demonstrated poor adherence to clinical guidelines and a tendency to obtain non-indicated surveillance imaging.6, 7
Thyroid cancer is one of the fastest growing malignancies in the United States, with the majority of cases comprising differentiated thyroid cancer (DTC).8, 9 Small, low-risk tumors account for most of the increase in diagnosis, and mortality has remained relatively stable. 8 Six to 12 months after treatment for DTC, a neck ultrasound and thyroglobulin level is obtained to evaluate for persistent disease. If thyroglobulin is elevated but there is no abnormality on neck ultrasound, diagnostic radioiodine (I-131) scan is the preferred test; Positron Emission Tomography (PET) scan can be used if the I-131 scan is negative and non-iodine avid disease is suspected.10-12 While imaging has increased in the surveillance of other malignancies, imaging trends after diagnosis of thyroid cancer are understudied.
There remains uncertainty regarding the utility of imaging in thyroid cancer surveillance, especially in patients with low-risk disease.13, 14 Given the rapid growth in the incidence of DTC, it is important to understand post-treatment imaging trends, the associated costs and potential radiation exposure. We hypothesized that, in contrast to other malignancies, utilization of post-treatment imaging in DTC would decrease over time, as a consequence of the rise in low-risk disease. In this study, we examined the trends in imaging utilization after the diagnosis of DTC and determined the patient characteristics that increased the likelihood of undergoing an imaging study.
Methods
Data Source and Study Population
The Surveillance Epidemiology and End Results (SEER) database, a project of the National Cancer Institute since 1971, collects cancer incidence, survival and demographic data for every cancer reported in 20 geographic areas throughout the United States. It is the largest population based cancer database available in the United States and covers approximately 28% of the population15. Starting in 1991, data from SEER was linked with Medicare reimbursement claims to create a dataset that included information on services performed before and after a cancer diagnosis.
Data from 25,649 patients with thyroid cancer diagnosed from January 1, 1991 until December 31, 2009 were extracted from the SEER-Medicare database. Only those with DTC, including tumor histology of papillary, follicular, or Hurthle cell, were retained for the final analysis. In total, 23,669 patients were included in the final analysis.
Institutional Review Board approval was not required since this study used publically available data and could not be tracked to human subjects.
Measures
Patient age was stratified into three Medicare-appropriate groups: <65, 65-74 and ≥75 years old. Patient race/ethnicity was categorized by the SEER database as White, Black, Hispanic, Asian, North American Native or Other. Ethnicities of Hispanic, Asian, North American Native and Other were grouped together as “Other.” As measures of socioeconomic status, the median household income in the geographic region (<$35,000, $35,000-$59,999 and ≥$60,000) and the percent of the population over age 25 with only a high school diploma (<20%, 20-29.9%, ≥30%) were collected. Geographic region was based on Census 2000 tracts, where available; otherwise, zip codes were used to prevent missing data. Tumor size was categorized as <1cm, 1cm-1.9cm, 2.0-3.9cm and ≥4.0cm, according to definitions used by the American Joint Committee on Cancer16. Tumor histology was limited to International Classification of Disease for Oncology classification codes for papillary, follicular and Hurthle cell histology.17 Patients were stratified by their SEER stage at diagnosis: localized disease, confined to the thyroid; regional disease, spread to regional lymph nodes; and distant disease is metastatic.18 The incidence of missing data was: 0.2% for race, education and income data, and 11% for tumor size. There was no missing data for age, sex, stage or histology.
Using Current Procedure Terminology (CPT) codes, we identified the percent of patients that underwent a neck ultrasound, I-131 scan or PET scan. The CPT code used to identify neck ultrasound was 76536. The CPT codes used to identify I-131 scans were 78015, 78016, 78017, 78018, 78020, 78800, 78801, 78802 and 78804. The CPT codes used to identify PET scans were 78810, 78811, 78812, 78813, 78814, 78815 and 78816.
To avoid over-representing patients that were diagnosed at an earlier time, and therefore had longer follow up, only imaging studies done during the first three years after diagnosis were included. Additionally, because we did not have claims data for patients diagnosed before 1991, we started our analysis with the year 1993, the first year in which there was data for patients diagnosed that year and the two prior years. Sixty percent of neck ultrasounds, 74% of I-131 scans and 46% of PET scans were done in the 3 years post-diagnosis.
Statistical Analysis
To identify changes in patient characteristics over time, demographics were analyzed in two cohorts: patients diagnosed from 1991-2000 and from 2001-2009. Tumor size and patient age were categorized using a-priori established cutoffs, and all characteristics were compared across the two cohorts using the χ2 test.
For each year from 1993-2009, we calculated the percent of patients who underwent a neck ultrasound, I-131 scan or PET scan. To evaluate the trends in use of neck ultrasound and I-131 scan over time, we regressed percentages of use on the year of medical claim. For PET scan, due to large increase in usage in 2005, we calculated the fold-increase in percent usage, comparing mean usage in 1996-2004 with 2005-2009. A permutation test was used to calculate a P-value for the fold-increase.
To identify which patient characteristics influence the likelihood of undergoing an imaging test, we performed a multivariable logistic regression for each of the three imaging tests. For neck ultrasound and I-131 scan, we included all years from 1991-2009, but for PET scan we included only 2005-2009 due to few PET scans being performed before 2005. Covariates in the model included patient age, gender, race, geographic educational attainment, geographic median family income, SEER stage, and tumor histology. Year of diagnosis (1991-2000 or 2001-2009) was included for neck ultrasound and I-131 scan, but not for PET scan. Tumor size was not included as a covariate due to its relatively high degree of missing values. We evaluated the goodness-of-fit for our models with the Hosmer and Lemeshow test at the 0.05 significance level; all three models fit the data well.
All statistical analyses were performed using SAS 9.3 and R 3.1.1.
Results
The demographics of the study population are shown in Table 1. Compared to those diagnosed from 1991-2000, patients diagnosed with DTC after 2000 were more likely to be older (p<0.001), have localized disease (p<0.001) and smaller tumor size (p<0.001). Additionally, those diagnosed after 2000 tended to come from areas with lower median household income (p<0.001) and lower educational achievement (p<0.001).
Table 1.
Differentiated Thyroid Cancer Patient and Tumor Characteristics by Decade of Diagnosis
| Characteristic | Year of Diagnosis
|
P-value | ||
|---|---|---|---|---|
| 1991-2000 (N=7,138) N (%) |
2001-2009 (N=16,531) N (%) |
|||
| Age | <65 | 3,915 (54.8) | 6,418 (38.8) | <.0001 |
| 65-74 | 2,000 (28.0) | 6,419 (38.8) | ||
| ≥75 | 1,223 (17.1) | 3,694 (22.3) | ||
|
| ||||
| Sex | Male | 2,155 (30.2) | 5,045 (30.5) | 0.6148 |
| Female | 4,983 (69.8) | 11,486 (69.5) | ||
|
| ||||
| Race | White | 5,583 (78.4) | 13,232 (80.3) | <.0001 |
| Black | 475 (6.7) | 1,341 (8.1) | ||
| Other | 1,067 (15.0) | 1,914 (11.6) | ||
|
| ||||
| HS only %* | <20% | 2,306 (33.6) | 4,507 (27.3) | <.0001 |
| 20-29.9% | 2,329 (33.9) | 5,572 (33.7) | ||
| ≥30% | 2,227 (32.5) | 6,438 (39.0) | ||
|
| ||||
| Median Household Income** | <$35,000 | 1,363 (19.9) | 4,086 (24.7) | <.0001 |
| $35,000-$59,999 | 3,392 (49.4) | 7,655 (46.4) | ||
| ≥$60,000 | 2,105 (30.7) | 4,774 (28.9) | ||
|
| ||||
| Stage | Localized | 4,063 (56.9) | 10,486 (63.4) | <.0001 |
| Regional | 2,614 (36.6) | 5,137 (31.1) | ||
| Distant | 461 (6.5) | 908 (5.5) | ||
|
| ||||
| Histology | Papillary | 5,920 (82.9) | 14,196 (85.9) | <.0001 |
| Hurthle cell | 405 (5.7) | 893 (5.4) | ||
| Follicular | 813 (11.4) | 1,442 (8.7) | ||
|
| ||||
| Tumor size | <1cm | 1,769 (29.2) | 5,217 (34.7) | <.0001 |
| 1-1.9cm | 1,337 (22.1) | 3,660 (24.3) | ||
| 2-3.9cm | 1,890 (31.2) | 3,764 (25.0) | ||
| ≥4cm | 1,059 (17.5) | 2,390 (15.9) | ||
Percent of people ≥25 years in geographic area with only high school education.
Median household income by geographic region.
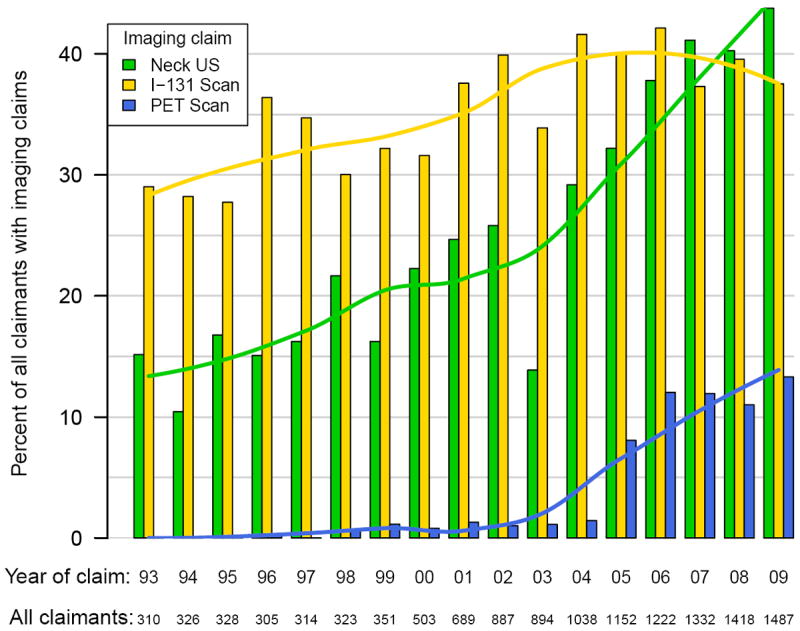
The use of imaging in the surveillance of DTC increased overall during the study period, as summarized in Figures 1-3. In linear regression analysis between 1993 and 2009, there was a significant increase in the use of ultrasound for those with localized (p<0.001), regional (p<0.001), and metastatic cancer (p=0.001). The increase in I-131 scans was smaller than neck ultrasound, but also increased by linear regression analysis in localized (p=0.003), regional (p<0.001) and metastatic disease (p=0.013).
Figure 1. Imaging Claims in Patients with Localized Differentiated Thyroid Cancer by Year.
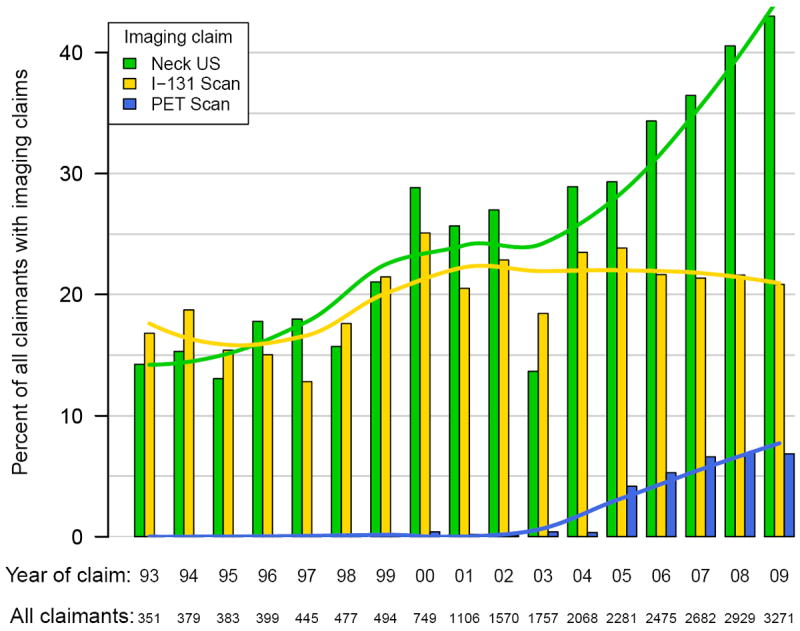
Figure 3. Imaging Claims in Patients with Distant Differentiated Thyroid Cancer by Year.
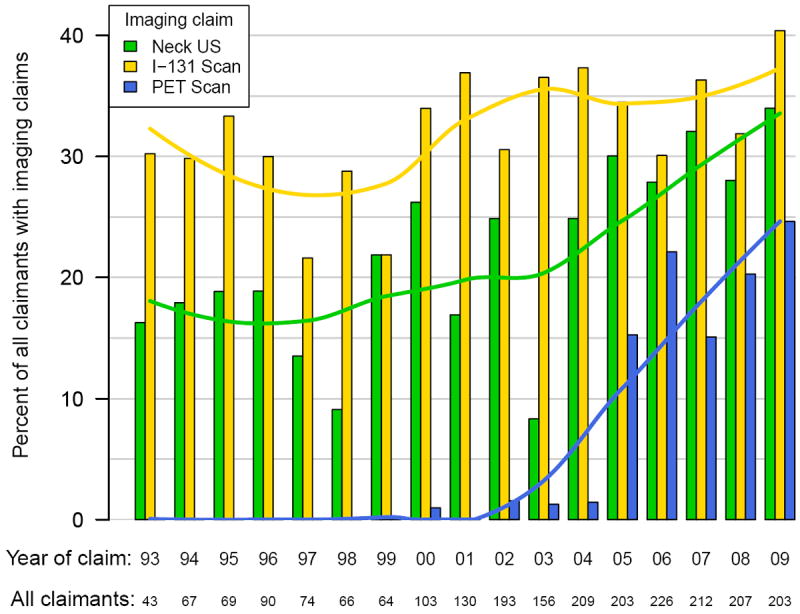
There was a significant increase in the number of claims for PET scan after the year 2004. From 2005-2009, compared to 1996-2004, claims for PET scan increased 32.4-fold (p<0.001) in those with localized DTC. Those with regional disease had a 13.1-fold (p<0.001) growth in the number of PET scans performed. Finally, claims for patients with distant disease increased 33.4-fold (p<0.001).
In multivariable analysis, shown in Table 2, patients who were above the age of 65 were more likely to undergo imaging studies than those <65. Patients age 65-74 years were more likely to undergo neck ultrasound (OR 2.50, 95% CI 2.35-2.65), I-131 scan (OR 2.81, 95% CI 2.64-3.00), and PET scan (OR 1.54, 95% CI 1.34-1.77). A similar effect was seen for those age 75 and above, with a higher likelihood of undergoing neck ultrasound (OR 2.10, 95% CI 19.6-2.26), I-131 scan (OR 1.80, 95% CI 1.67-1.95), and PET scan (OR 1.80, 95% CI 1.54-2.09). Women were more likely than men to undergo neck ultrasound (OR 1.35, 95% CI 1.27-1.43), but were less likely to have a PET scan (OR 0.73, 95% CI 0.65-0.82). Compared to Caucasians, African Americans were less likely to have a neck ultrasound (OR 0.81, 95% CI 0.73-0.90), I-131 scan (OR 0.88, 95% CI 0.79-0.99), or PET scan (OR 0.63, 95% CI 0.49-0.80). Other non-Caucasians were also less likely to undergo neck ultrasound (OR 0.86, 95% CI 0.79-0.93) and I-131 scan (OR 0.88, 09% CI 0.81-0.96).
Table 2.
Association of Patient and Tumor Characteristics with Likelihood of an Imaging Claim
| Characteristic | Neck Ultrasound | I-131 Scan | PET Scan*** | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Age | <65 | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||
| 65-74 | 2.50 (2.35-2.65) | <.0001 | 2.81 (2.64-3.00) | <.0001 | 1.54 (1.34-1.77) | <.0001 | |
| ≥75 | 2.10 (1.96-2.26) | <.0001 | 1.80 (1.67-1.95) | <.0001 | 1.80 (1.54-2.09) | <.0001 | |
|
| |||||||
| Sex | Male | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||
| Female | 1.35 (1.27-1.43) | <.0001 | 1.10 (1.03-1.17) | 0.0028 | 0.73 (0.65-0.82) | <.0001 | |
|
| |||||||
| Race | White | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||
| Black | 0.81 (0.73-0.90) | <.0001 | 0.88 (0.79-0.99) | 0.0268 | 0.63 (0.49-0.80) | 0.0002 | |
| Other | 0.86 (0.79-0.93) | 0.0002 | 0.88 (0.81-0.96) | 0.0049 | 0.89 (0.74-1.06) | 0.1904 | |
|
| |||||||
| HS only %* | <20% | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||
| 20-29.9% | 1.02 (0.95-1.10) | 0.5241 | 0.99 (0.92-1.06) | 0.7290 | 1.02 (0.88-1.18) | 0.8027 | |
| ≥30% | 1.11 (1.03-1.20) | 0.0062 | 1.13 (1.05-1.23) | 0.0019 | 0.97 (0.83-1.13) | 0.6838 | |
|
| |||||||
| Median household income** | <$35,000 | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||
| $35,000-$59,999 | 0.90 (0.84-0.97) | 0.0041 | 1.03 (0.95-1.10) | 0.4829 | 0.84 (0.73-0.97) | 0.0161 | |
| ≥$60,000 | 1.16 (1.07-1.26) | 0.0005 | 1.09 (1.00-1.19) | 0.0419 | 1.02 (0.86-1.20) | 0.8537 | |
|
| |||||||
| Stage | Localized | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||
| Regional | 1.20 (1.13-1.27) | <.0001 | 2.65 (2.50-2.82) | <.0001 | 2.05 (1.82-2.30) | <.0001 | |
| Distant | 0.74 (0.66-0.83) | <.0001 | 2.12 (1.88-2.39) | <.0001 | 3.72 (3.03-4.56) | <.0001 | |
|
| |||||||
| Histology | Papillary | 1 [Ref] | 1 [Ref] | 1 [Ref] | |||
| Follicular | 0.89 (0.81-0.97) | 0.0121 | 1.14 (1.04-1.25) | 0.0070 | 0.84 (0.69-1.03) | 0.0879 | |
| Hurthle cell | 0.91 (0.81-1.03) | 0.1308 | 1.21 (1.07-1.36) | 0.0021 | 1.54 (1.24-1.91) | <.0001 | |
|
| |||||||
| Decade of Diagnosis | 1991-2000 | 1 [Ref] | 1 [Ref] | ||||
| 2001-2009 | 2.15 (2.02-2.28) | <.0001 | 1.44 (1.35-1.54) | <.0001 | |||
Percent of people ≥25 years in geographic area with only high school education.
Median Household Income by geographic region.
Includes patients diagnosed 2005-2009. OR, Odds Ratio; CI, Confidence Interval
SEER stage was significantly associated with the likelihood of having an imaging claim. Patients with regional disease were more likely to have a claim for neck ultrasound compared to those with localized disease (OR 1.20, 95% CI 1.13-1.27), whereas those with distant disease were less likely (OR 0.74, 95% CI 0.66-0.83). Patients with both regional DTC (OR 2.65, 95% CI 2.50-2.82) and distant disease (OR 2.12, 95% CI 1.88-2.39) were more likely to have an I-131 scan. Again, patients with regional disease (OR 2.05, 95% CI 1.82-2.30) and distant DTC (OR 3.72, 95% CI 3.03-4.56) were also more likely to undergo PET scan. Histology was not a clinically significant factor in utilization of imaging, except for an increased tendency to obtain PET scans (OR 1.54, 95% CI 1.24-1.91) in patients with Hurthle cell carcinomas. Controlling for other patient characteristics, patients diagnosed from 2001-2009 were more likely to have a neck ultrasound than those diagnosed from 1991-2000 (OR 2.15, 95% CI 2.02-2.28). Patients diagnosed after 2000 were also more likely to have an I-131 scan (OR 1.44, 95% CI 1.35-1.54).
Discussion
We hypothesized that imaging use after thyroid cancer diagnosis would decrease over time due to the rise in low-risk disease. Using the SEER-Medicare database, we unexpectedly found that there was a significant increase in the use of surveillance imaging studies over the past 2 decades across all stages of disease. This is true for neck ultrasound, I-131 scan and PET scan. The most dramatic increase was seen in the use of PET scan after 2004. SEER stage at diagnosis appears to have the largest impact on the likelihood of undergoing an imaging test. However, we still observed an increased use of imaging after controlling for other patient characteristics, including stage at diagnosis.
It has become apparent that there is more reliance on advanced imaging studies in the practice of medicine. Initially, this was felt to be confined to the fee-for-service model.2 However, an integrated health system saw an approximate doubling of CT scans and almost tripling of MRI from 1997-2006.1 Consistent with our findings, nuclear medicine studies have had slower growth, except for PET and PET/CT scans. A striking increase was observed in the number of PET scans performed after the year 2004 in a non-Medicare population.1 The greatest growth in imaging utilization has been seen in older enrollees, but was observed in all ages.1
Imaging studies in patients with malignancy are very commonly performed and increasing. Over 95% of patients with advanced malignancy will undergo a high-cost diagnostic imaging procedure.19 Studies have demonstrated a rise in imaging being performed in patients after treatment for malignancy. Patients treated for hepatocellular carcinoma saw a large increase in utilization of MRI scans from 1998 to 2005.5 In patients with pancreatic cancer, the number of CT, MRI and PET scans performed in the 5 years following surgical resection more than doubled from 1991 until 2005.20 Similar increases occurred in patients after resection of colorectal liver metastasis.4 PET scan is being rapidly adapted in the evaluation of malignancies; 65.3% of patients with non-small cell lung cancer received a PET scan from 2005-2007, compared to 6.3% from 1998-2000.21
Studies have not demonstrated a clear mortality benefit for surveillance imaging. CT scan after pancreatic cancer resection did not improve survival in patients receiving annual surveillance.20 Similar results were seen in patients with hepatocellular carcinoma and colorectal cancer with liver metastasis.4, 5 PET scan was not a useful tool to detect melanoma recurrence in patients at low risk, and did not confer any additional mortality benefit beyond yearly CT scan in patients at high risk. 22
There is often poor adherence to recommendations for surveillance imaging. Studies in early-stage breast cancer patients found a high incidence of non-recommended imaging tests (such as MRI) for surveillance; however, physicians often fail to obtain recommended mammograms.7 Patients with colorectal cancer have seen a sharp increase in the number of non-recommended CT and PET scans from 2001-2006.6 In men with low-risk prostate cancer, 34% had non-recommended bone scans or CT scans.23 Interestingly, only 60% of men with high-risk prostate cancer, in whom they are recommended, had these tests.
There is a variety of imaging modalities used for post-treatment surveillance in DTC. Neck ultrasound is useful to detect recurrent disease, but does not detect distant metastasis.24 I-131 scan can identify distant disease, but has been shown to have limited value in the setting of low thyroglobulin, even in high risk patients.13 Additionally, the sensitivity of diagnostic I-131 scan in patients with mildly elevated stimulated thyroglobulin is low.25 PET scan has shown the ability to detect small volume non-iodine avid disease. A significant portion of patients with a detectable thyroglobulin below 10ng/mL have evidence of disease on PET scan.26
However, the significance of the thyroid cancer detected by PET scan is debated. There is a lack of evidence regarding the effect of PET scan on progression free survival in DTC.27, 28 Others have questioned whether PET scan provides additional information beyond neck ultrasound and CT scan.14 While PET positive disease has been shown to be a poor prognostic indicator, this effect is strongest in patients with metastatic, large volume disease.29, 30 Patients with small volume, regional, PET-positive disease continue to have an excellent prognosis.
We found a large increase in the use of PET scan after the year 2004. Medicare approved use of PET scan for patients treated with thyroidectomy and radioactive iodine ablation with thyroglobulin >10ng/mL and a negative I-131 scan in October 2003. In January 2005, Medicare expanded its coverage of PET scans under the Coverage with Evidence Development program to include indications for “diagnosis, other staging, restaging and monitoring response to treatment.”31 Part of the increase in PET scan use may be related to this expanded Medicare coverage. However, the same increase in PET scan utilization has been demonstrated in patients without thyroid cancer or Medicare coverage, suggesting there may be additional factors, such as improved accessibility.2, 21
One inherent limitation of our study is the lack of patient-level data available in large databases. There is limited information on cancer recurrence, iodine-avidity, and patient preference, all of which influence imaging decisions. Another limitation is our restriction to three imaging modalities: ultrasound, I-131 scan, and PET. Billing information is not a reliable source for the indication for imaging tests. Many CT scans and MRIs are also being done in DTC patients. However, we were concerned they are more likely to be done for indications other than thyroid cancer. Therefore, we focused on three imaging modalities that we felt would be most specific to DTC surveillance. A third perceived limitation would be the absence of AJCC TNM staging. We used SEER stage in our study, because TNM stage is not available for patients diagnosed prior to 2004. Including patients diagnosed before 2004 is essential because of our emphasis on trends in imaging use over time.
Using SEER-Medicare, our cohort included patients diagnosed after 1991 that were Medicare enrollees, resulting in smaller numbers compared to the SEER database alone. Our cohort is primarily over age 65. Patients less than age 65 covered by Medicare are likely to have significant other co-morbidities and are not generalizable to a younger DTC population. Additionally, because the data is linked to Medicare billing records, there may be under-reporting of imaging tests performed. For example, if physicians perform bedside ultrasound or patients have secondary insurance covering the imaging test, there would be no documentation in the Medicare database. However, with regards to capturing I-131 scans, our percentage of patients with claims for an I-131 scan is similar to prior reported rates of radioactive iodine ablation following thyroidectomy.32-34
Despite these limitations, there is strong evidence that SEER-Medicare is a valid tool for evaluation of post-treatment surveillance imaging. Prior studies have confirmed the usefulness of the SEER-Medicare data to identify clinical events, such as a cancer treatment or surveillance after cancer treatment.35, 36 In fact, many studies of post-treatment surveillance in other malignancies have been performed using the SEER-Medicare database.4, 5, 20, 21, 37 The population in the SEER-Medicare dataset has also been found to be generalizable to the elderly American population.38 While our cohort is primarily over age 65, this is an important patient population to study, because the fastest increase in thyroid cancer incidence has been observed in this age group.39 In addition, the largest growth in general imaging utilization occurs in older patients. However, it has been demonstrated in younger patients as well.1 This is probably true for thyroid specific imaging also. Therefore, we believe a similar increase in imaging would be seen in a younger thyroid cancer population.
DTC is one of the fastest growing malignancies in the United States, resulting in a variety of providers delivering post-treatment surveillance, including endocrinologists, oncologists, surgeons, nuclear medicine specialists and primary care physicians.9 While there are specific guidelines and recommendations for post-treatment surveillance imaging, there is evidence that compliance with published guidelines is often low.6, 7 Work done evaluating surveillance imaging for other malignancies suggests small mortality benefits, if any.4, 5, 20, 22 We demonstrated increased utilization of imaging over time despite diagnosis of smaller, more limited, low-risk thyroid cancer. While many of the themes presented in this paper are true for other malignancies, the over-use of imaging is highlighted by the increased utilization in this relatively indolent malignancy. Greater imaging utilization clearly contributes to increased costs. Specific to thyroid cancer, increased imaging may identify low volume recurrent disease that is unlikely to be clinically significant, leading to heightened patient anxiety, and potentially unnecessary interventions. Growing healthcare costs underscore the importance of identifying those patients most likely to benefit from additional testing and determining when it can be avoided.
Figure 2. Imaging Claims in Patients with Regional Differentiated Thyroid Cancer by Year.
Acknowledgments
Research support: Dr. Haymart is supported by NIH 1K07CA154595-02. Dr. Banerjee is partially supported by 5 P30 CA 046592 from the National Cancer Institute. Support was provided by University of Michigan MCubed Seed Funding Program and the Punya Foundation for Thyroid Cancer Research. Brittany Gay assisted with creating the figures and tables.
Footnotes
Disclosures: The authors have no conflict of interest to disclose.
Contributor Information
Jaime Wiebel, Email: jwiebel@umich.edu, Fellow, Division of Metabolism, Endocrinology, & Diabetes, University of Michigan, Dominos Farms, Lobby C, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48106, Phone: 734-647-5871, Fax: 734-647-2145.
Mousumi Banerjee, Email: mousumib@umich.edu, Research Professor of Biostatistics, Department of Biostatistics, University of Michigan.
Daniel G. Muenz, Email: dmuenz@umich.edu, Department of Biostatistics, University of Michigan.
Francis P. Worden, Email: fworden@med.umich.edu, Professor of Medicine, Division of Hematology/Oncology, University of Michigan.
Megan R. Haymart, Email: meganhay@umich.edu, Assistant Professor of Medicine, Division of Metabolism, Endocrinology, & Diabetes, Hematology/Oncology, University of Michigan.
References
- 1.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307:2400–2409. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhargavan M, Sunshine JH. Utilization of radiology services in the United States: levels and trends in modalities, regions, and populations. Radiology. 2005;234:824–832. doi: 10.1148/radiol.2343031536. [DOI] [PubMed] [Google Scholar]
- 3.Gimbel RW, Fontelo P, Stephens MB, et al. Radiation exposure and cost influence physician medical image decision making: a randomized controlled trial. Med Care. 2013;51:628–632. doi: 10.1097/MLR.0b013e3182928fd5. [DOI] [PubMed] [Google Scholar]
- 4.Hyder O, Dodson RM, Mayo SC, et al. Post-treatment surveillance of patients with colorectal cancer with surgically treated liver metastases. Surgery. 2013;154:256–265. doi: 10.1016/j.surg.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyder O, Dodson RM, Weiss M, et al. Trends and patterns of utilization in post-treatment surveillance imaging among patients treated for hepatocellular carcinoma. J Gastrointest Surg. 2013;17:1774–1783. doi: 10.1007/s11605-013-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas GM, Sheffield KM, Parmar AD, Han Y, Brown KM, Riall TS. Physician follow-up and observation of guidelines in the post treatment surveillance of colorectal cancer. Surgery. 2013;154:244–255. doi: 10.1016/j.surg.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn EE, Hays RD, Kahn KL, Litwin MS, Ganz PA. Use of imaging and biomarker tests for posttreatment care of early-stage breast cancer survivors. Cancer. 2013;119:4316–4324. doi: 10.1002/cncr.28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 9.Institute NC. Cancer Trends Progress Report - 2011/2012 Update. [2014, July 15]; Available from URL: http://progressreport.cancer.gov.
- 10.American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C. Cooper DS, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 11.Cobin RH, Gharib H, Bergman DA, et al. AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid carcinoma. American Association of Clinical Endocrinologists American College of Endocrinology. Endocr Pract. 2001;7:202–220. [PubMed] [Google Scholar]
- 12.Pacini F, Castagna MG, Brilli L, Pentheroudakis G, Group EGW. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii110–119. doi: 10.1093/annonc/mds230. [DOI] [PubMed] [Google Scholar]
- 13.Rosario PW, Furtado Mde S, Mineiro Filho AF, Lacerda RX, Calsolari MR. Value of diagnostic radioiodine whole-body scanning after initial therapy in patients with differentiated thyroid cancer at intermediate and high risk for recurrence. Thyroid. 2012;22:1165–1169. doi: 10.1089/thy.2012.0026. [DOI] [PubMed] [Google Scholar]
- 14.Lal G, Fairchild T, Howe JR, Weigel RJ, Sugg SL, Menda Y. PET-CT scans in recurrent or persistent differentiated thyroid cancer: is there added utility beyond conventional imaging? Surgery. 2010;148:1082–1089. doi: 10.1016/j.surg.2010.09.015. discussion 1089-1090. [DOI] [PubMed] [Google Scholar]
- 15.Institute NC. Overview of the SEER Program. [July 7, 2014]; Available from URL: http://seer.cancer.gov/about/overview.html.
- 16.Greene FL, P DL, Fleming ID. AJCC Cancer Staging Manual. 6. New York, NY: Springer; 2002. [Google Scholar]
- 17.Percy C, F A, et al. International Classification of Diseases for Oncology. 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 18.Institute NC. SEER Stat Fact Sheets: Thyroid Cancer. [July 26, 2014]; Available from URL: http://seer.cancer.gov/statfacts/html/thyro.html.
- 19.Hu YY, Kwok AC, Jiang W, et al. High-cost imaging in elderly patients with stage IV cancer. J Natl Cancer Inst. 2012;104:1164–1172. doi: 10.1093/jnci/djs286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witkowski ER, Smith JK, Ragulin-Coyne E, Ng SC, Shah SA, Tseng JF. Is it worth looking? Abdominal imaging after pancreatic cancer resection: a national study. J Gastrointest Surg. 2012;16:121–128. doi: 10.1007/s11605-011-1699-z. [DOI] [PubMed] [Google Scholar]
- 21.Dinan MA, Curtis LH, Carpenter WR, et al. Variations in use of PET among Medicare beneficiaries with non-small cell lung cancer, 1998-2007. Radiology. 2013;267:807–817. doi: 10.1148/radiol.12120174. [DOI] [PubMed] [Google Scholar]
- 22.Rueth NM, Xing Y, Chiang YJ, et al. Is surveillance imaging effective for detecting surgically treatable recurrences in patients with melanoma? A comparative analysis of stage-specific surveillance strategies. Ann Surg. 2014;259:1215–1222. doi: 10.1097/SLA.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 23.Prasad SM, Gu X, Lipsitz SR, Nguyen PL, Hu JC. Inappropriate utilization of radiographic imaging in men with newly diagnosed prostate cancer in the United States. Cancer. 2012;118:1260–1267. doi: 10.1002/cncr.26416. [DOI] [PubMed] [Google Scholar]
- 24.Haber RS. Role of ultrasonography in the diagnosis and management of thyroid cancer. Endocr Pract. 2000;6:396–400. doi: 10.4158/EP.6.5.396. [DOI] [PubMed] [Google Scholar]
- 25.Mazzaferri EL, Kloos RT. Is diagnostic iodine-131 scanning with recombinant human TSH useful in the follow-up of differentiated thyroid cancer after thyroid ablation? J Clin Endocrinol Metab. 2002;87:1490–1498. doi: 10.1210/jcem.87.4.8338. [DOI] [PubMed] [Google Scholar]
- 26.Vera P, Kuhn-Lansoy C, Edet-Sanson A, et al. Does recombinant human thyrotropin-stimulated positron emission tomography with [18F]fluoro-2-deoxy-D-glucose improve detection of recurrence of well-differentiated thyroid carcinoma in patients with low serum thyroglobulin? Thyroid. 2010;20:15–23. doi: 10.1089/thy.2008.0416. [DOI] [PubMed] [Google Scholar]
- 27.Dennis K, Hay JH, Wilson DC. Effect of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography-guided management of suspected recurrent papillary thyroid carcinoma: long-term follow-up with tumour marker responses. Clin Oncol (R Coll Radiol) 2012;24:e168–172. doi: 10.1016/j.clon.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Palaniswamy SS, Subramanyam P. Diagnostic utility of PETCT in thyroid malignancies: an update. Ann Nucl Med. 2013;27:681–693. doi: 10.1007/s12149-013-0740-6. [DOI] [PubMed] [Google Scholar]
- 29.Creach KM, Nussenbaum B, Siegel BA, Grigsby PW. Thyroid carcinoma uptake of 18F-fluorodeoxyglucose in patients with elevated serum thyroglobulin and negative 131I scintigraphy. Am J Otolaryngol. 2013;34:51–56. doi: 10.1016/j.amjoto.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Larson SM, Fazzari M, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85:1107–1113. doi: 10.1210/jcem.85.3.6458. [DOI] [PubMed] [Google Scholar]
- 31.Services CfMaM. National Coverage Determination (NCD) for PET Scans. [July 8, 2014]; Available from URL: http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=211&ncdver=3&NCAId=104&NcaName=Positron+Emission+Tomography+(FDG)+and+Other+Neuroimaging+Devices+for+Suspected+Dementia&IsPopup=y&bc=AAAAAAAAAEAAAA%3d%3d&.
- 32.Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–4446. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin HW, Bhattacharyya N. Survival impact of treatment options for papillary microcarcinoma of the thyroid. Laryngoscope. 2009;119:1983–1987. doi: 10.1002/lary.20617. [DOI] [PubMed] [Google Scholar]
- 34.Goffredo P, Roman SA, Sosa JA. Have 2006 ATA practice guidelines affected the treatment of differentiated thyroid cancer in the United States? Thyroid. 2014;24:463–471. doi: 10.1089/thy.2013.0319. [DOI] [PubMed] [Google Scholar]
- 35.Butler Nattinger A, Schapira MM, Warren JL, Earle CC. Methodological issues in the use of administrative claims data to study surveillance after cancer treatment. Med Care. 2002;40:IV-69–74. doi: 10.1097/00005650-200208001-00010. [DOI] [PubMed] [Google Scholar]
- 36.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 37.Kowalczyk KJ, Harbin AC, Choueiri TK, et al. Use of surveillance imaging following treatment of small renal masses. J Urol. 2013;190:1680–1685. doi: 10.1016/j.juro.2013.05.109. [DOI] [PubMed] [Google Scholar]
- 38.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 39.Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23:885–891. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]


