Abstract
Background
Delta bilirubin (Bδ) forms when bilirubin conjugates covalently bind to albumin by way of non-enzymatic transesterification in patients with cholestasis. Cholestatic infants with biliary atresia form Bδ. The aim of this study was to investigate the factors determining serum Bδ concentrations in infants with biliary atresia.
Methods
Study subjects were infants enrolled into a prospective study (PROBE: Clinicaltrials.gov NCT00061828) of biliary atresia. We acquired data of concurrently measured serum bilirubin analytes (total bilirubin (TB), conjugated bilirubin (Bc), and unconjugated bilirubin (Bu)) and applied graphical methods and linear mixed effects model to study factors contributing to Bδ variability.
Results
Bδ level increased with increasing levels of Bc and TB. In addition, the length of time cholestasis persisted partially determined the level of Bδ. One mg/dL increase in Bc is related to approximately 0.36 mg/dL increase in Bδ (p < 0.0001); every 100 days of cholestasis is associated with an approximately 1.0 mg/dL increase in Bδ (p < 0.0001) given the same level of Bc. Serum albumin levels are not significantly related to Bδ (p=0.89).
Conclusions
Bδ levels in infants with BA increase with increasing levels of Bc and longer duration of cholestasis. Understanding the relationship between Bδ, Bc, total bilirubin and direct-reacting bilirubin levels can help in interpretation of the clinical extent of cholestasis in infants and children with biliary atresia assisting in the diagnosis and management of these infants.
Keywords: delta bilirubin, conjugated bilirubin, biliary atresia
Introduction
The measurement of serum bilirubin is perhaps the single most valuable tool in the clinical evaluation of infants with liver disease. The majority of circulating serum bilirubin consists of two species. First is unconjugated bilirubin (Bu), which is essentially insoluble in water and is tightly bound to albumin and other plasma proteins. The other is conjugated bilirubin (Bc), which consists of an array of sugar (mainly glucuronide) ester mono- and di-conjugates of bilirubin. Within the context of neonatology the clinical utility of measuring Bu comes in the management of infants with routine physiologic jaundice, those with increased bilirubin production and those with defective conjugation, and the accuracy of measurement specifically relates to assessing risk for bilirubin encephalopathy (1, 2). Serum Bc levels reflect the reflux of bilirubin from hepatocytes and perhaps bile after conjugation: thus the clinical utility of measuring Bc is as an indicator of cholestatic liver disease (3). Indeed, an elevated Bc (or direct bilirubin) is the entryway into the pathway leading to the diagnosis of biliary atresia (BA) (4).
Using slide-film technology it is possible in the clinical chemistry laboratory to precisely and independently measure total bilirubin (TB), Bu and Bc in serum samples (5–7). In doing so it may be found that certain sera contain a third bilirubin species referred to as delta-bilirubin (Bδ) because its value equals TB minus (Bu+Bc). Bδ consists of bilirubin conjugates covalently esterified to albumin and other serum proteins, so called bili-protein, and arises by spontaneous transesterification of bilirubin glucuronide esters to exposed carboxyl groups on various serum proteins (mainly albumin). As this only occurs when Bc is present in excess, significant amounts of circulating Bδ are only observed in patients with cholestasis. In studies involving children it was found that Bδ insignificantly contributed to TB in patients with predominantly Bu and in infants a month old or less; whereas, Bδ may contribute over 50% of TB in older children with cholestasis (8, 9).
BA is a liver disease of human infants, occurring with an incidence of 1 in 12–18,000 live births in populations around the world. Its etiology and pathogenesis of are unknown, but the characteristic endpoint of the process is fibro-inflammatory obliteration of part or all of the extrahepatic biliary system and obstructive cholestasis. Clinical cholestasis typically appears within 2 months of birth. The only known effective therapy for BA is the Kasai portoenterostomy (KPE), wherein the extrahepatic bile duct remnants are surgically removed and bile drainage is attempted by anastomosing the biliary limb of a Roux-en-Y to the liver hilum. Outcome after KPE is not good: about 50% of cases do not achieve adequate bile drainage and require liver transplantation for end-stage cirrhosis usually by 2 years of age. Cholestasis persists in patients with failure of drainage. While it is well known that the formation of Bδ is dependent upon the presence of Bc in plasma, the dynamics of the process over time are not as well understood. Since the transesterification process is bi-directional, we hypothesized that a dynamic equilibrium would be established over time, wherein the proportion of TB in serum comprising Bδ would become stable. The study of a very large and well characterized cohort of infants with BA and failed KPE permitted us to study the dynamics of Bδ formation over time.
Methods
Study subject and bilirubin assays
The subjects were infants with BA enrolled into the Prospective Study of Infants and Children with Cholestasis (PROBE: Clinicaltrials.gov NCT00061828) of the Childhood Liver Disease Research and Education Network (ChiLDREN) and its predecessor the Biliary Atresia Research Consortium (BARC) from Jun 1, 2004 to Mar 1, 2012. Informed consent was obtained from the study participant’s parents or guardians and the protocol was carried out under IRB approval. Baseline data was collected at the time of KPE, including demographics, medical history, physical exam, surgical information, and laboratory measurements. Follow-up visits for data collection occurred at 1, 2, 3, and 6 months, post KPE, then at ages 12 months and 18 months. Thereafter, annual follow-up was performed on each subject’s birth date, until age 9 or loss to follow-up. Serum bilirubin measurements obtained during the first two years of life before liver transplant (if any) in which TB, Bu and Bc were reported as being measured independently were used in this analysis. Each such measurement was considered as an “observation”; values from individual subjects obtained at different time points were considered to be repeat observations separated in time. The time interval was computed from the dates of the observations.
Measurements were made in the clinical chemistry laboratories of the various clinical centers. All centers but 1 utilized the Vitros Integrated Systems (Ortho Clinical Diagnostics, Rochester, NY), whereas the 1 center utilized Dimension Vista 3000T (Siemens, Malvern, PA).
Data analysis and statistics
Because the precision of bilirubin measurements is poor at low values and Bδ is considered to develop only in individuals with elevated Bc, observations with TB < 1.3 mg/dL, Bc = 0, or Bu = 0 were excluded from the analysis. Descriptive data were summarized as the mean, standard deviation (SD), and range for continuous variables and as percentages for categorical variables. For continuous variables with skewed distributions, median, quartiles, and range are reported. To characterize how Bδ level is related with TB, Bc, and serum albumin, LOESS (locally weighted scatterplot smoothing) (10) regression was used to explore function form of the relationship using all observations.
Non-enzymatic transesterification is a chemical reaction whose progress is determined by the amount of the reactants Bc and albumin, temperature and time. In this study of BA, Bc, serum albumin and time varied enough to contribute to variability in Bδ levels and were studied as independent variables. We computed the time interval between KPE and the date of the observation as an approximation of length of exposure to TB. To study how Bc and serum albumin contribute to Bδ variability over time, we constrained the analysis to observations from subjects with poor drainage after KPE (defined as no TB ≤ 2.0 had been reported before 3 months after KPE). The rationale for excluding observations from subjects with good drainage is because these infants achieved low level of Bc within three months after KPE and therefore did not have long-term exposure to cholestasis. A linear mixed effects model was used to test the association between Bδ and the factors of interest (i.e. Bc, time since KPE, and serum albumin). This model takes account of the correlation between repeated measures from the same subjects. Interaction terms between time and Bc were tested to access potential interaction between these two factors. Model diagnostics on residuals and influential points were examined to assure model fit and find final range of data to include in the analysis.
In addition, we also estimated mean time trajectories of Bδ as a fraction of TB (Bδ/TB) in the poor drainage groups. To adjust for potential bias caused by informative censoring of the repeated TB measurements due to transplant and death, a joint model of longitudinal and survival data with p-splines was used for this analysis (11) - All analyses were performed using SAS/STAT (SAS Institute Inc. 2008. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc.) and R (12).
Results
A total of 350 infants with BA were enrolled from Jun 1, 2004 until Mar 1, 2012. Among these 350 subjects, 140 subjects had one or more bilirubin measurements that were recorded as TB, Bu and Bc (total of 557 observations). After excluding observations with TB < 1.3 mg/dL (195 instances), Bc = 0 (18), and Bu = 0 (3), and (Bu+Bc) > TB (as being physiologically impossible and probably in error for technical or recording reasons) (5), 334 observations from 129 subjects were used for the analyses of the current study. The median of number of observations per subject is 2 (range: 1–10). Table 1 shows the demographics and other characteristics of this cohort of 129 subjects.
Table 1.
Characteristics of the study cohort.
| Demographic and Patient Characteristics | Summary Statistics | |
|---|---|---|
| Gender: Count (%) | Female | 63 (48.8%) |
| Male | 66 (51.2%) | |
| Race: Count (%) | Asian | 16 (12.4%) |
| Black or African American | 20 (15.5%) | |
| White | 79 (61.2%) | |
| Native Hawaiian or Other Pacific Islander | 1 (0.8%) | |
| Other | 8 (6.2%) | |
| Missing | 5 (3.9%) | |
| Ethnicity: Count (%) | Hispanic | 28 (21.7%) |
| Non-Hispanic | 101 (78.3%) | |
| Successful Drainage at 3 month after KPE: Count (%) | No | 66 (51.2%) |
| Yes | 63 (48.8%) | |
| Age at KPE (days): Mean (SD) (Min – Max) (n=129) | 65.7 (32.1) (9 – 193) | |
| TB at baseline: Mean (SD) (Min – Max) (n=127)# | 7.9 (2.8) (3.5 – 16.6) | |
Nineteen subjects do not have TB measurements at baseline visit, and their Bc+Bu value was used for this calculation.
The median (quartile, range) of the four bilirubin species among the 334 observations are 7.5 mg/dL (quartile: 4.7–10.5, range: 1.3–36.3) for TB, 3.6 mg/dL (quartile: 1.8–5.4, range: 0.1–27.0) for Bc, 1.1 mg/dL (quartile: 0.7–1.6, range: 0.1–11.0) for Bu, and 2.3 mg/dL (quartile: 1.5–3.7, range: 0.0–12.1) for Bδ.
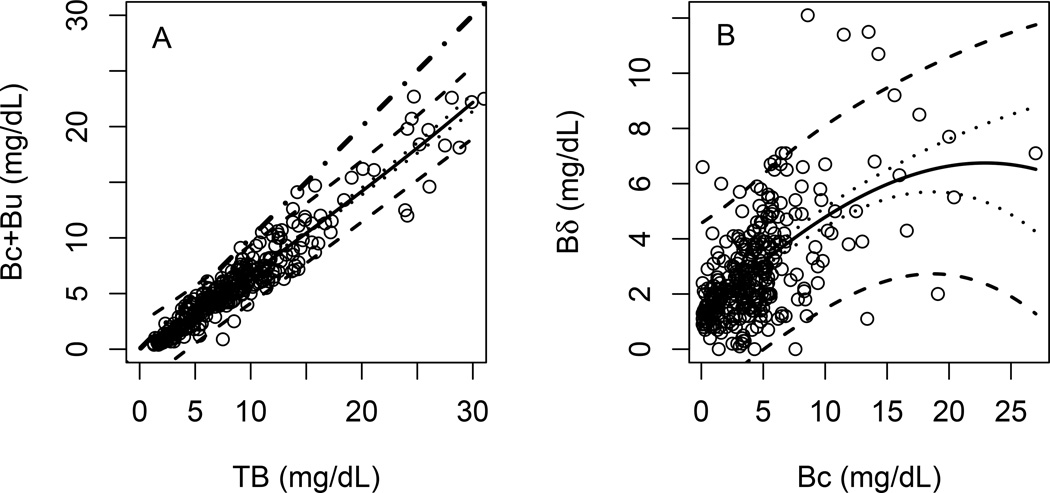
All observations are plotted as (Bu + Bc) versus TB in the left panel of Figure 1A. In this plot, Bδ for each observation equals the vertical distance from fitted line of (Bu + Bc) to the line of identity at each level of TB. This shows the general relationship between Bδ and TB expected in cholestasis, wherein (Bc+Bu) levels are elevated and give rise to Bδ. It can be seen that in this cohort of BA patients, the regression line for (Bu + Bc) observations falls increasingly below the line of identity with increasing TB, reflecting larger Bδ values at higher levels of TB.
Figure 1. Bδ and other bilirubin species.
A) Dot-dash line is the 45 degree line which indicates the complete identity of values on horizontal and vertical axes. Solid line is the estimated mean of Bc+Bu using LOESS regression. B) Solid line is the estimated mean of Bδ using LOESS regression. Dotted lines are the 95% confidence interval of the predicted mean. Dashed lines are the 95% prediction interval.
The relationship between Bδ and Bc is shown more clearly by plotting Bδ values calculated for each sample versus Bc, as shown in Figure 1B. These data show the expected relationship between these individual bilirubin analytes. Since Bδ is derived from Bc by spontaneous transesterification in plasma, Bδ tends to increase with increasing Bc. The data show this to be the case in BA. The regression line demonstrates that on average for every 1mg/dL increase in Bc between values of 2 – 10mg/dL, Bδ increases by 0.37mg/dL approximately. However, only about 60% of the variability in Bδ is explained by the variation of Bc (Pearson regression coefficient R2= 0.59, p<0.001), which suggests that there may be other factors that determine how much Bδ exists in plasma in BA patients. In addition, the change in Bδ with change in Bc above 10mg/dL flattens, suggesting saturation kinetics for the transesterification reaction. However, the determinants of steady state levels of Bδ are potentially numerous and cannot be gleaned from the current data.
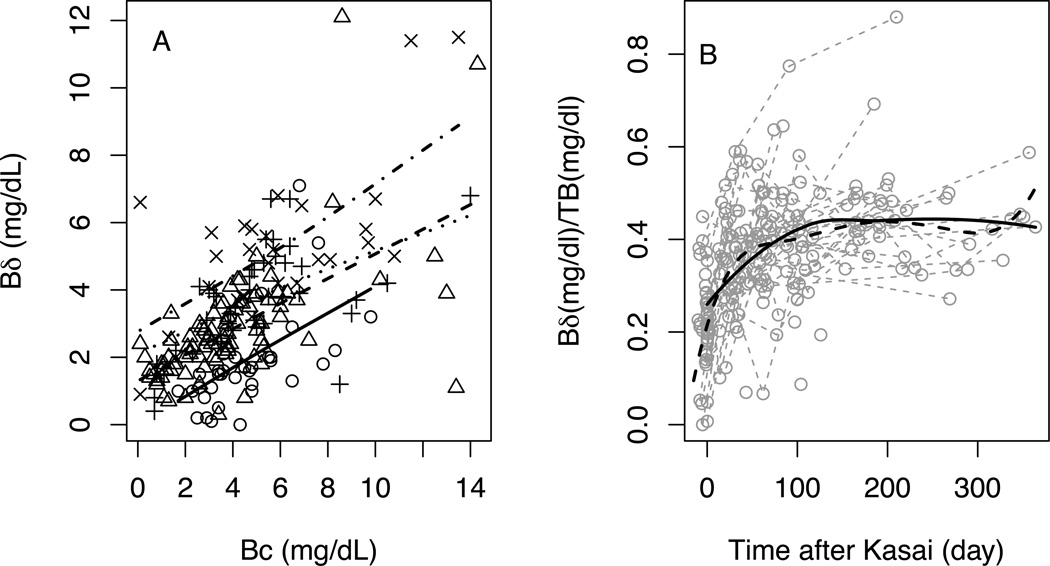
Among the 334 observations, there are only 15 observations from 9 subjects obtained between 1 year and 2 years after KPE. In addition, while 97% of the Bc values were between 0 to 15 mg/dL, there are 8 observations with Bc value between 15 – 27 mg/dL. Some of these observations were shown to be influential points when conducting model diagnostics with the initial model using all observations from all 214 observations from 65 subjects with poor drainage (one subject has missing serum albumin value and was excluded from the analysis). To avoid biased results driven by these extreme values we excluded observations with time > 365 days or Bc > 15 mg/dL. One hundred and ninety-five observations from 62 subjects with poor drainage were included in the final analysis. As demonstrated in Figure 2A, there is no significant interaction between time and Bc (p = 0.55), but both time and Bc are significantly associated with Bδ. One mg/dL increase in Bc is related to approximately 0.36 mg/dL increase in Bδ (p < 0.0001); every 100 days of exposure to Bc is associated with approximately 1.0 mg/dL increase in Bδ (p < 0.0001) given the same level of Bc. Albumin levels were not found to be significantly related to Bδ (p=0.89). However, the range of albumin concentrations in the cohort was narrow, which limits the conclusions that can be made from this observation.
Figure 2. Bδ and Bδ/TB and time in subjects with poor drainage after Kasai surgery.
Circles and solid line – around time of Kasai (2 weeks before to 1 days after Kasai); triangles and dashed line – 2 days to 3 months after Kasai; cross and dotted line – 3 months to 6 months after Kasai;×and dashed and dotted line – 6 months to 1 year after Kasai. All lines are estimated mean of Bδ using linear regression by time windows. B) Grey dotted shows individual trajectories. Solid line is the estimated mean of Bδ/TB using LOESS regression. Dashed line is the estimated mean of Bδ/TB using joint model of longitudinal and survival data for adjusting informative dropout.
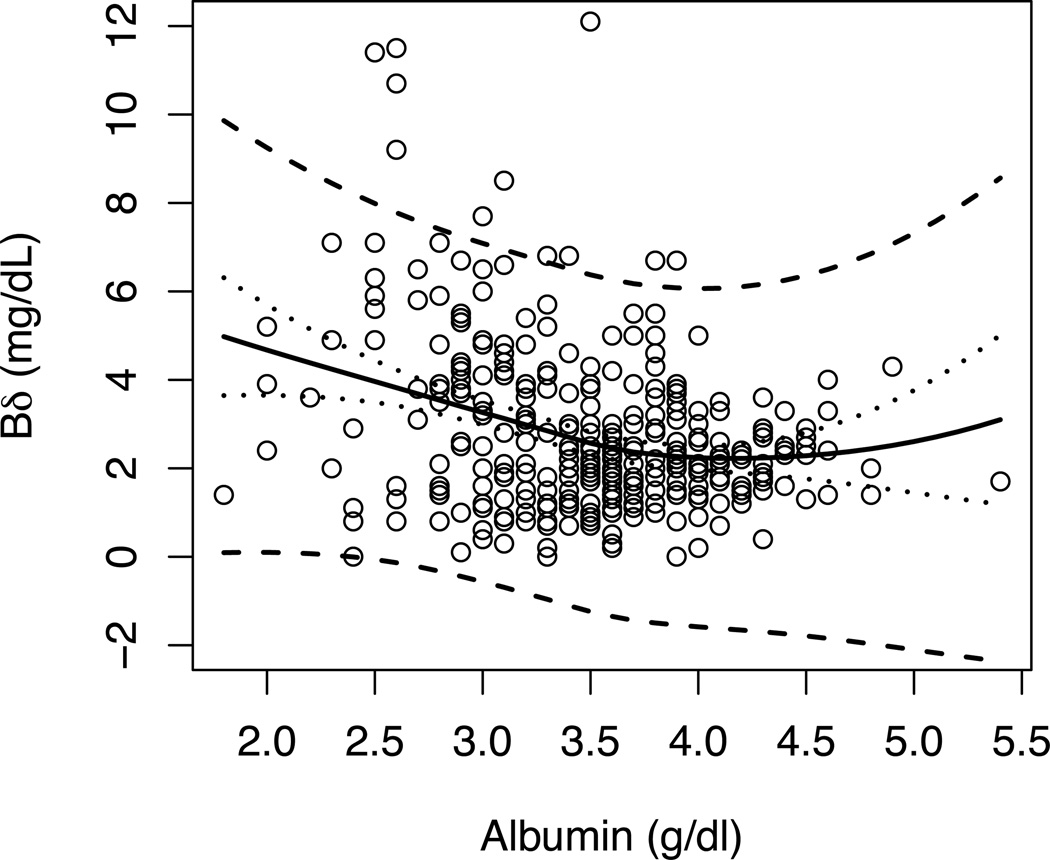
The data shown in Figure 2 demonstrate that in BA the length of time cholestasis exists in part determines the level of Bδ in subjects with poor drainage after KPE. In Figure 2A, Bδ values are plotted against Bc grouped for various windows of time. The data show that the regression lines for groups generally are displaced upward for later windows of time (i.e., longer exposure). Another way to look at the effect of time is to determine if the contribution of Bδ to TB increases with time in each individual subject (shown in Figure 2B). The data show that BA subjects with poor drainage have increasing amounts of Bδ, expressed as a fraction of TB, with time after KPE. The estimated mean trajectory using joint modeling, which adjusted for repeated measurements and informative dropout, shows a plateau somewhere around 50 days post-KPE and with Bδ around 40% of TB. Estimated mean trajectory using LOESS regression lead to a similar conclusion. Figure 3 demonstrates over the limited range of serum albumin levels, the albumin level does not influence the level of Bδ.
Figure 3. Bδ and Albumin.
Black solid line is the predicted mean value of Bδ using LOESS regression. Dotted lines are the 95% confidence intervals of the predicted mean of Bδ. Dashed lines are the 95% prediction interval of Bδ.
Discussion
We took the opportunity to study a large and well-characterized cohort of infants with BA and failed KPE whose serum bilirubin measurements were performed using slide technology, thus providing the ability to assess the dynamics of Bδ over time. This unique data set permitted us to establish the parameters that affect Bδ concentration with some certainty. We found that the concentration of Bc has the greatest influence on Bδ concentration, which is to be expected since Bδ is the product of Bc, the excretion of which is impaired in hepatobiliary disease. The prospective collection of observations over time permitted an accurate assessment of the effect of length of exposure on Bδ concentration, which has not been previously reported. We found that Bδ concentration as a fraction of TB increased with time of exposure up to about 50 days, after which a steady state is achieved. A model was constructed for Bδ dynamics in BA in which Bc and time are the important contributing factors to its formation and steady state concentration.
Our findings have relevance in regard to the clinical assessment of serum bilirubin in the follow-up in infants with BA. Many if not most clinical laboratories measure serum bilirubin as “total” and “direct” using chemical methodology, as opposed to the TB, Bu and Bc measurements provided by slide technology. The approaches are equally useful in clinical medicine, but the differences in output may be confusing to clinicians. Our findings may help clinicians’ understanding of serum bilirubin measurements obtained in infants with BA and perhaps other cholestatic diseases. TB is equivalent to “total” bilirubin from clinical analyzers using chemical methodology. However, Bc is not equivalent to “direct” bilirubin, which is actually roughly equivalent to Bc plus Bδ (or Bc plus TB − (Bu + Bc)). So, a clinician following an infant with BA using measurement of “total” and “direct” bilirubin will find that “direct” bilirubin will contribute a much greater percentage of “total” than a clinician using measurements from a laboratory employing slide technology will find Bc contributing to TB. And, if a clinician who typically follows “direct” bilirubin measurements gets results from a laboratory using slide technology and finds a Bc that is much lower than the last observed “direct” bilirubin, s/he should not interpret this as showing improved bilirubin excretion. Indeed, it has been shown that the fraction of TB constituted by Bδ increases considerably after relief of biliary obstruction in adults with obstructive jaundice (13), and thus the “direct” bilirubin level would remain elevated longer than would Bc. The two approaches are equally useful clinically, but not equivalent unless the differences in chemical species are understood. This study helps to explain the differences expected in follow-up of BA patients.
Ours is the most comprehensive study of the determinants of serum Bδ reported to date. Some studies have encompassed larger numbers of patients, but with an array of disorders including hemolysis, Gilbert syndrome, and hepatobiliary disease (8, 9, 14). None of the previous reports investigated a single population with a single disease as this study did. Focusing on infants with BA restricts any variability in Bδ related to the disease state and to age. Furthermore, the study cohort was prospectively acquired and followed for 2 years, with close ongoing monitoring of data quality. One shortfall of the study is the lack of knowledge as to when in the course of events cholestasis began. Hyperbilirubinemia may be present at birth in some BA patients (15). Thus, in a few BA patients cholestasis may have existed and Bδ may have been formed in utero (16). However, most patients with BA do not develop clinical cholestasis until well after birth, typically at 4–6 weeks of age. Our data did not provide a time for onset of cholestasis in individual subjects. Since we needed to select a consistent and secure start-time for our analysis and since no subject had serum bilirubin measurement prior to the time of KPE, we chose that time to begin our analysis. The data presented in Figure 2 support the choice of start-time in that they indicate the beginning of Bδ accumulation at or just before KPE.
Our data show that a linear increase in Bδ with increasing Bc up to a Bc of 10 mg/dL. Upwards of that point, however, Bδ concentration appeared to plateau, but this observation must be viewed cautiously because there were very few observations with Bc > 10 mg/dL. It seems unlikely that the transesterification reaction would have reached saturation at this Bc concentration given that the concentration of albumin did not affect Bδ concentration. More data in the Bc range > 10 mg/dL are required to make more confirmatory conclusions.
The increase in Bδ/TB with increasing time exposure appears to be linear up to an exposure time of 50 days, after which it plateaus. This is probably a true phenomenon as we have adjusted for the potential bias caused by informative dropout (subjects with high value of Bδ/TB tended to have transplants earlier and consequently have shorter follow-up and less number of observations). This makes physiologic sense as well. The plateau would occur where the rate of formation of Bδ equals the rate of degradation. The rate of formation is determined by the Bc concentration, whereas the rate of degradation is determined by the half-life of albumin, which is about 20 days. Whereas the Bc concentration varied substantially among subjects, the half-life of albumin would not be expected to vary among individuals. These determinants would lead to a plateau of Bδ (or steady state condition) that would vary somewhat in Bδ concentration but not in time of reaching steady state.
In conclusion, Bδ levels in infants with BA increase with increasing levels of Bc and longer duration of cholestasis. Understanding the relationship between Bδ, Bc, total bilirubin and direct-reacting bilirubin levels can help in interpretation of the clinical extent of cholestasis in infants and children with BA, assisting in the diagnosis and management of these infants.
What is Known
Conjugated bilirubin (Bc) in plasma of cholestatic patients reacts to form the bilirubin-albumin conjugate called delta-bilirubin (Bδ).
Bδ is one component of direct-reacting bilirubin.
Bδ has a long half-life in plasma; thus, Bδ and direct bilirubin may remain elevated long after biliary obstruction is cleared.
What is New
The level of Bδ increases for about 50 days after a failed Kasai operation, after which Bδ plateaus.
After Kasai operation, persistent elevated Bδ is accompanied by elevated Bc and is thus related to continuing cholestasis.
Acknowledgments
Source of Funding: This work was supported by U01 grants from the National Institute of Diabetes, Digestive and Kidney Diseases: DK 62503 (Baltimore), DK 62436 (Chicago), DK 62497 (Cincinnati), DK 62453 (Denver), DK 62445 (New York), DK 62481 (Philadelphia), DK 62466 (Pittsburgh), DK 62500 (San Francisco), DK 62452 (St. Louis), DK 84536 (Indianapolis), DK 84575 (Seattle), DK 62470 (Houston), DK 84538 (Los Angeles), DK 62470 (Atlanta), DK 62456 (Ann Arbor).
Footnotes
PROBE study: http://clinicaltrials.gov/show/NCT00061828
Conflicts of Interest and Source of Funding: None
Author contributions: PFW and PP conceived and designed the study. WY analyzed the data. PFW, WY, and PP wrote the paper. PFW, PP, and JCM were involved in acquisition of data. JCM critically revised the manuscript for important intellectual content. PFW supervised the study.
References
- 1.Flaherman VJ, Kuzniewicz MW, Escobar GJ, et al. Total serum bilirubin exceeding exchange transfusion thresholds in the setting of universal screening. J Pediatr. 2012;160:796–800. e1. doi: 10.1016/j.jpeds.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 2.Gartner LM, Herrarias CT, Sebring RH. Practice patterns in neonatal hyperbilirubinemia. Pediatrics. 1998;101:25–31. doi: 10.1542/peds.101.1.25. [DOI] [PubMed] [Google Scholar]
- 3.Arvan D, Shirey TL. Conjugated bilirubin: a better indicator of impaired hepatobiliary excretion than direct bilirubin. Ann Clin Lab Sci. 1985;15:252–259. [PubMed] [Google Scholar]
- 4.Moyer V, Freese DK, Whitington PF, et al. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39:115–128. doi: 10.1097/00005176-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal P, Keefe MT, Henton D, et al. Ektachem and unconjugated bilirubin measurements. J Clin Chem Clin Biochem. 1989;27:829. [PubMed] [Google Scholar]
- 6.Wu TW, Dappen GM, Powers DM, et al. The Kodak Ektachem clinical chemistry slide for measurement of bilirubin in newborns: principles and performance. Clin Chem. 1982;28:2366–2372. [PubMed] [Google Scholar]
- 7.Wu TW, Dappen GM, Spayd RW, et al. The Ektachem clinical chemistry slide for simultaneous determination of unconjugated and sugar-conjugated bilirubin. Clin Chem. 1984;30:1304–1309. [PubMed] [Google Scholar]
- 8.Brett EM, Hicks JM, Powers DM, et al. Delta bilirubin in serum of pediatric patients: correlations with age and disease. Clin Chem. 1984;30:1561–1564. [PubMed] [Google Scholar]
- 9.Rosenthal P, Henton D, Felber S, et al. Distribution of serum bilirubin conjugates in pediatric hepatobiliary diseases. J Pediatr. 1987;110:201–205. doi: 10.1016/s0022-3476(87)80154-3. [DOI] [PubMed] [Google Scholar]
- 10.Cleveland WS. Robust locally weighted regression and smoothing scatter-plots. J Amer Statist Assoc. 1979;74:827–836. [Google Scholar]
- 11.Rizopoulos D. In: Joint Models for Longitudinal and Time-to-Event Data, with Applications. R, editor. Boca Raton: Chapman & Hall/CRC Press; 2012. [Google Scholar]
- 12.Team RDC R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 13.Kozaki N, Shimizu S, Higashijima H, et al. Significance of serum delta-bilirubin in patients with obstructive jaundice. J Surg Res. 1998;79:61–65. doi: 10.1006/jsre.1998.5357. [DOI] [PubMed] [Google Scholar]
- 14.Weiss JS, Gautam A, Lauff JJ, et al. The clinical importance of a protein-bound fraction of serum bilirubin in patients with hyperbilirubinemia. N Engl J Med. 1983;309:147–150. doi: 10.1056/NEJM198307213090305. [DOI] [PubMed] [Google Scholar]
- 15.Harpavat S, Finegold MJ, Karpen SJ. Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics. 2011;128:e1428–e1433. doi: 10.1542/peds.2011-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrea EM, Jr, Ongtengco EA, Tolia VA, et al. The occurrence and significance of the bilirubin species, including delta bilirubin, in jaundiced infants. J Pediatr Gastroenterol Nutr. 1988;7:511–516. [PubMed] [Google Scholar]