Abstract
The cilium is the site of function for a variety of membrane receptors, enzymes and signal transduction modules critical to a spectrum of cellular processes. Through targeted transport and selective gating mechanisms, the cell localizes specific proteins to the cilium that equip it for the role of sensory antenna. This capacity of the cilium to serve as a specialized compartment where specific proteins can be readily concentrated for sensory reception also makes it an ideal organelle to employ for the regulated emission of specific biological material and information. In this review, we present and discuss an emerging body of evidence centered on ciliary ectosomes - bioactive vesicles released from the surface of the cilium.
Keywords: cilium, extracellular vesicles, intercellular signaling
Cilia and extracellular vesicles at a glance
The cilium is a slender, protrusive organelle characterized by a highly conserved, microtubule-based core architecture sheathed in a specialized extension of the cell membrane. Cilia manifest in a variety of forms across a wide range of diverse and evolutionarily distant organisms and are broadly classified as either motile (referred to traditionally as flagella in some contexts) or immotile (primary cilia); the latter of which can be expressed by a majority of cell types in the vertebrate body. Having been regarded classically as an organelle specializing in motile function, the cilium has undergone a renaissance of appreciation during the past decade touched off by research in the algal model system, Chlamydomonas, which revealed its role as a ubiquitous sensory antenna (1-4). The ensuing growth of a vibrant community of cilia researchers has given rise to what is now a significant literature on the role of cilia in sensing and transducing signals from the extracellular space.
The importance of cilia is underscored most potently by the growing list of ciliopathies - a classification of diseases and syndromes associated with abnormal formation or function of cilia (5-7). Despite its relatively small size compared with that of the cell types from which it protrudes, the cilium acts as the obligate site of action for a variety of membrane receptors and signal transduction modules critical to basic, cellular processes regulating growth, development and homeostasis (8). As cilia are ubiquitous throughout mammalian tissues, ciliopathies present with a diverse set of clinical features including cystic kidney, liver and pancreatic diseases, blindness, anosmia, cognitive defects, randomization of left-right body axis, polydactyly, and obesity (6, 7). Although cilia research has gained momentum over the past several years, many aspects of the basic cell biology of the organelle remain to be elucidated, and the field is ripe for more fundamental discoveries. Here we review a new area in the biology of cilia marked by a burgeoning literature on the active shedding of bona fide, bioactive vesicles from the ciliary membrane – ciliary ectosomes.
The release of membrane vesicles from the outer surfaces of cells into their surrounding environment is a phenomenon observed in a diversity of organisms from prokaryotes to multicellular eukaryotes. Extracellular vesicles were first described by Chargaff and West in 1946 as a precipitable factor in blood plasma (9). Since that time, the existence of actively released, extracellular membrane vesicles has become well established, and compelling evidence of their significance in a broad range of biological processes has accumulated (10-14). Extracellular vesicles have been shown to carry a wide array of biological effector molecules including signaling proteins, enzymes, DNA, mRNA and microRNA, and because they can bring these cargoes to the surfaces of other cells, they can serve as vehicles for cell-to-cell communication (15). Current research focuses chiefly on two distinct modes of vesicle release. The discharge of preformed vesicles via the fusion of an endosomal multivesicular body with the cell membrane gives rise to extracellular vesicles called exosomes (16). Vesicles may also bud in an outward direction from the cell membrane by an active process and the resulting extracellular vesicles are called ectosomes (17). (Figure 1)
Figure 1.
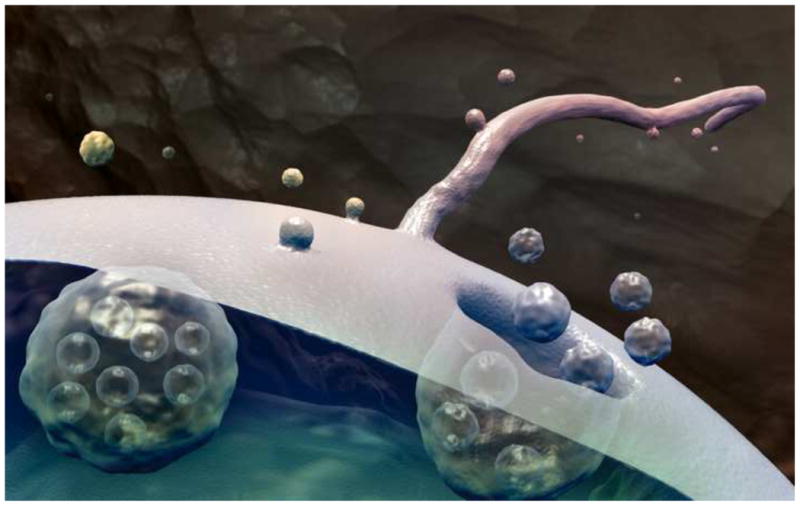
The release of extracellular vesicles. Exosomes are released when multivesicular bodies fuse with the cell membrane. Ectosomes are released by outward budding directly from the cell membrane or cilium membrane. (illustration by Christopher R. Wood)
Original observations of vesicle release from algal flagella
The cilium's capacity to release portions of its membrane into the extracellular space has been recognized or inferred in a variety of past studies. As with a number of breakthroughs in the understanding of cilia, such as the discovery of intraflagellar transport and work leading to the ciliary hypothesis of polycystic kidney disease (1, 18), it was research with the model organism, Chlamydomonas, that provided the earliest positive evidence of membrane vesicle release from cilia (19-21). Chlamydomonas reinhardtii, the most common laboratory strain, is a biflagellate, unicellular alga that uses its flagella for both locomotion and sexual adhesion. Gametogenesis in Chlamydomonas reinhardtii is accompanied by the display of sex-specific adhesion molecules, the agglutinins, on the outer membrane surfaces of flagella. During mating, the flagella of opposite mating type gametes adhere to one another by means of agglutinin binding, and this interaction triggers a signaling pathway that brings about cell fusion (22-24, 25). Early investigations into the nature of Chlamydomonas mating revealed that the adhesive material responsible for flagellar agglutination, referred to then as “gamone”, is released into the medium by gametes in a form sedimentable by high-speed centrifugation (19-21, 26,25). Analysis of this material by electron microscopy revealed it to be composed of membrane vesicles that carried an activity sufficient for stimulation of the mating reaction when added back to gametes of a single Chlamydomonas mating type (19, 27,25). These membrane vesicles were thought to derive from the flagellar membrane for two reasons. First, the cell body of Chlamydomonas reinhardtii is completely encased in a cell wall with the exception of two cylindrical holes through which the flagella project. Therefore, the only membrane surfaces directly exposed to the external milieu are those of flagella. Second, no membrane vesicles were obtained when flagella-less mutants of Chlamydomonas reinhardtii were subjected to the same membrane vesicle sedimentation procedure (19). These studies provided evidence that a ciliary membrane can be the source of extracellular membrane vesicles. What these studies did not resolve, however, is the extent to which such vesicles are of functional significance in vivo.
Extracellular vesicles and the algal mother cell wall
Remodeling of the extracellular matrix (ECM) is an important process in which extracellular vesicles may be functioning. Degradation and weakening of the ECM is a key aspect of tumor invasion, for example, and tumor cells have been shown to actively release vesicles carrying a variety of proteinases that have the capacity to degrade ECM such as matrix metalloproteinases (28-30), cathepsin B (31), urokinase-type plasminogen activator (29), and the adamalysins ADAM10 and ADAM17 (32, 33). Degradation of ECM is a critical step in the life cycle of some unicellular organisms as well, which may serve as potential model systems for such processes in higher organisms. Chlamydomonas reinhardtii produces two different proteolytic enzymes that function at specific stages of its life cycle to degrade the cell wall, a type of ECM unique to volvocine algae (34-38). Gamete lytic enzyme (GLE) is a zinc-containing matrix metalloprotease that mediates digestion of Chlamydomonas gamete cell walls in order to expose their plasma membranes for cell fusion during mating (39-41). Vegetative lytic enzyme (VLE) is a subtilase-like serine protease that mediates the digestion of the sporangial cell walls required for the liberation of Chlamydomonas daughter cells (referred to as “hatching”) after mitosis (34, 38, 42).
Recent studies on the role of daughter cell flagella in the post-mitotic hatching process of the Chlamydomonas life cycle have yielded another significant step forward in the recognition of the cilium as a source for extracellular vesicles (38). The lifecycle of Chlamydomonas reinhardtii is characterized by a gradual increase in cell size during a prolonged G1 phase followed by 2 - 4 rounds of S/M phase without an intervening G2 period. The result is a sporangial ball of 4 - 16 daughter cells trapped within the original, mother cell wall (38, 43, 44). Daughter cells are then liberated from the sporangium by digestion of the mother cell wall by means of secreted VLE protease (34, 35, 38). Recent studies have shown that Chlamydomonas cells accomplish this by packaging the VLE protease with its active site on the outer surface of ectosomes that bud directly from the membrane of daughter cell flagella (38) (Figure 2 and Figure 3). These ciliary ectosomes then diffuse through the interior space of the sporangium, transporting the protease from the daughter cell to the mother cell wall where it carries out its degradative function. In situ immunogold labeling and confocal fluorescence localization procedures, with an antibody specific for the catalytic region of VLE protease, enabled a definitive determination of the enzyme's localization to ciliary ectosomes released at the time of hatching (38). Isolation and immunogold labeling of intact, whole-mounted ciliary ectosomes demonstrated that the catalytic region of VLE protease is present on their outer-membrane surface. Immunoblot analysis of the protein content of isolated ciliary ectosomes verified the presence of VLE and other known flagellar membrane proteins, such as the flagellar membrane glycoprotein (FMG-1) and PKD2 (38).
Figure 2.
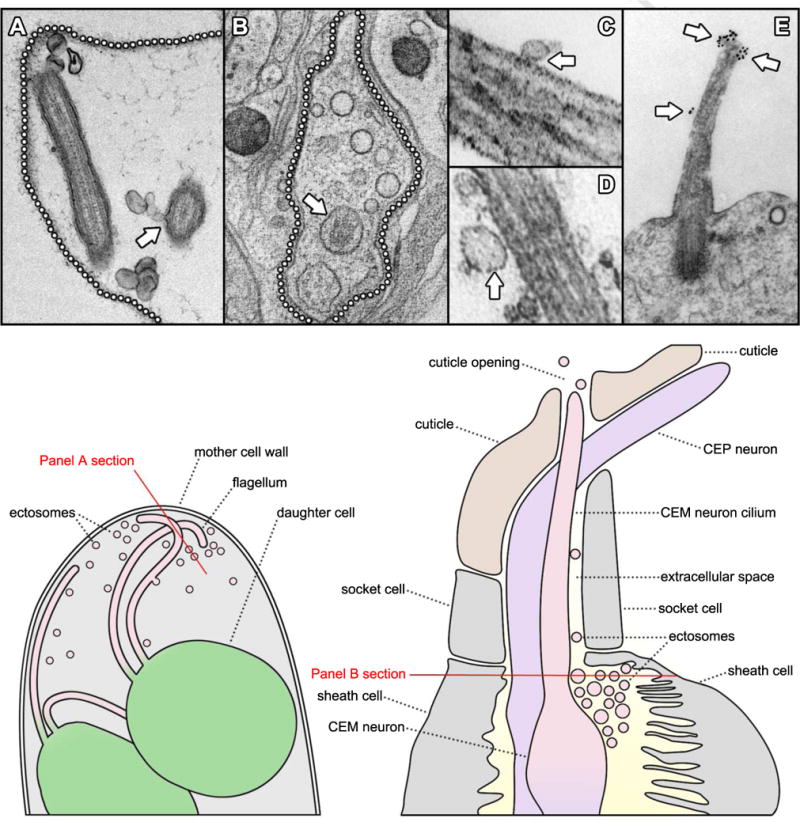
Observations of extracellular vesicles and cilia. A. TEM of an ultrathin section through a mature Chlamydomonas sporangium shows ectosomes in the extracellular space surrounding flagella. The location of the mother cell wall is indicated by a dotted line and an arrow indicates ectosomes caught in the process of budding directly from the flagellar membrane (38). B. A tomographic cross-section through a C. elegans cephalic sensillum shows numerous ectosomes (arrowheads) populating the extracellular space around a CEM neuronal cilium (arrow). The boundary of the sensillar lumen is indicated by a dotted line (51). C, D. TEMs of ultrathin sections through mouse biliary primary cilia show extracellular vesicles (arrows) closely apposed to the ciliary membranes. (67) E. TEM of an ultrathin section through the primary cilium of a mouse neuroepithelial cell shows prominin-1 specific gold particles decorating what appear to be regions of the ciliary membrane in the process of forming ectosomes (arrows) (69, courtesy of Michaela Wilsch-Bräuninger). A cartoon at the lower left illustrates a region of a Chlamydomonas sporangium like the one sectioned in panel A. Two flagellated daughter cells are shown releasing ciliary ectosomes into the extracellular space within the lumen of the mother cell wall. The red line indicates the orientation of the section depicted in panel A. A cartoon at the lower right illustrates a region of a cephalic male sensillum (CEM) like the one sectioned in panel B. Glial sheath and socket cells form a lumen surrounding the CEM neuron cilium. Ectosomes are found in the luminal space and may travel along the cilium to the cuticle opening where they are released into the worm's external environment. The red line indicates the orientation of the section depicted in panel B.
Figure 3.
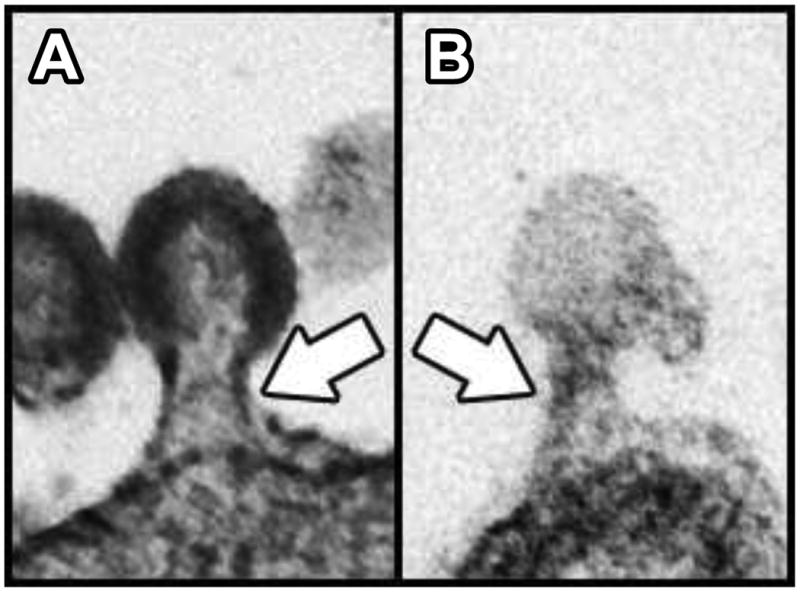
Comparison between a viral bud and a ciliary ectosomal bud. A. TEM of an ultrathin section through an HIV-1 particle arrested in the process of budding from the plasma membrane of a human cell in culture (112). B. TEM of an ultrathin section through a ciliary ectosome caught in the process of budding from the membrane of a Chlamydomonas flagellum (38). White arrows indicate the appearance of similar electron-dense structures within the two bud necks.
Failure of daughter cell release from the mother cell wall after mitosis is a common phenotypic deficiency expressed by the variety of known Chlamydomonas flagellar assembly mutants (45, 46). This fact enabled a functional assay in which sporangia of mutants defective in intraflagellar transport (IFT), that lack flagella and cannot hatch, were exposed to ciliary ectosomes isolated from a population of hatching wildtype sporangial daughter cells. The wildtype ciliary ectosomes were capable of inducing high efficiency hatching of the trapped flagella-less IFT mutants, verifying that the ciliary ectosomes are the carriers of the functional protease activity (38). This result suggested that the sporangial hatching difficulties typically observed with daughter cells of flagella-defective mutants are likely due to the necessity of the flagellum as an ectosome-producing organelle.
These studies served to advance our knowledge from the basic finding that the cilium has the potential to originate extracellular membrane vesicles to a full understanding that the cilium can behave as an emitter of biologically active ectosomes with a specific biological function. Are cilia functioning this way in any of the great variety of other systems in which they reside?
Extracellular vesicles and the ciliated sensory neurons of worms
Another model system that has provided fertile ground for progress in cilia biology is the nematode worm, Caenorhabditis elegans (47). As a free-living (nonparasitic) dweller of a complex water-soil environment, Caenorhabditis is equipped for navigation with a suite of chemotactic and avoidance behaviors mediated by a compact sensory nervous system that has been thoroughly mapped at single-cell resolution (48). Fundamental to the Caenorhabditis elegans nervous system is a diverse set of immotile, sensory cilia found at the dendritic endings of neurons located in the head and tail of the worm's body (49). The hermaphrodite female form is endowed with 60 ciliated sensory neurons, and an obligate male form possesses an additional ∼50 (48, 50). Simple sensory organs, called sensilla, are formed by groupings of glia-like sheath and socket cells that encapsulate the dendrites of ciliated neurons; in many cases arranged as a chemosensory unit with cilia exposed to the external environment. A variety of genes involved in formation, maintenance, and function of the morphologically diverse set of Caenorhabditis sensory cilia have human orthologs which, when mutated, result in ciliopathic disease (50).
Following the studies on ciliary ectosome release in Chlamydomonas, a relationship between extracellular vesicles and cilia was uncovered in Caenorhabditis elegans. The Caenorhabditis polycystin orthologs, PKD2 and LOV-1 (for location of vulva), are expressed exclusively in a subset of sensory neurons and, by GFP-tagging, were found localized to cilia, and to extracellular vesicles near to cilia that protrude into the environment through cuticular pores (51). Electron tomographic analysis revealed numerous extracellular vesicles in the lumen between sensory cilia and the glial sheath cells, socket cells, and cuticle that surrounds them (Figure 2). In one case, a vesicle was observed in the process of either budding from or fusing to the ciliary membrane (51). The ability to produce these polycystin-carrying extracellular vesicles is lost in mutants with defects in ciliary assembly, suggesting that intact cilia are required for their release (51). Production of the extracellular vesicles was unaffected in mutants of three different multivesicular body biogenesis components - STAM-1 (signal transducing adaptor molecule), MVB-12 (multivesicular body), and ALX-1 (apoptosis-linked gene 2 interacting protein X). This was consistent with the absence of multivesicular bodies in the distal dendrites as assessed by electron tomography, and supported an ectosomal derivation of polycystin-carrying extracellular vesicles rather than an exosomal one (51).
Following the observation that Caenorhabditis ciliated sensory neurons can release vesicles into the external environment, it was hypothesized that extracellular vesicles may be functioning in animal-animal communication. By washing the worms from culture plates, pelleting them, then subjecting the supernatant to filtration and differential centrifugation, the polycystin-carrying extracellular vesicles were isolated for use in worm behavioral assays. Two different behavioral responses, reversal and tail-curling, could be stimulated in male worms by exposure to the isolated extracellular vesicles. Both behaviors are relevant to the Caenorhabditis mating process in which the male worm responds to hermaphrodite contact by reversing his locomotive direction to scan the hermaphrodite's body surface with his tail. If the male reaches his partner's head or tail during this process, he executes a curling turn maneuver and continues scanning in a reverse direction until his tail contacts the vulva where mating is consummated by insertion of the copulatory spicules and ejaculation into the hermaphrodite uterus (52, 53). The tail-curling behavior induced by exposure to extracellular vesicles was dependent on their cargo content as evidenced by the fact that extracellular vesicles isolated from wildtype induced the tail-chasing behavior, while extracellular vesicles isolated from kinesin-3 mutants, which lack PKD2, did not (51). These findings suggest that extracellular vesicles released into the environment by ciliated sensory neurons have the capacity to modulate mating behaviors and play a role in animal-animal communication.
Extracellular vesicles and the ciliated renal epithelium of mammals
Extracellular membrane vesicles inhabit a variety of bodily fluids and have drawn increasing attention for their potential as carriers of diagnostic indicators relevant to development and disease (15, 54, 55). Membrane vesicles present in the urine of mammals, for example, have been the subjects of extensive analysis as a source of biomarkers for various renal disorders (56, 57). The most common hereditary renal disease, Autosomal Dominant Polycystic Kidney Disease (ADPKD), has two genetic loci, PKD1 and PKD2, that encode the proteins polycystin-1 (PC1) and polycystin-2 (PC2), respectively (58, 59). Autosomal Recessive Polycystic Kidney Disease (ARPKD), the most common form of hereditary PKD among children, is caused by mutations in PKHD1, the gene that encodes the protein fibrocystin (60, 61). All three of these proteins have been localized to cilia that extend into the lumen of kidney tubules where they are thought to function in the sensation of fluid flow (62-66). Electron microscopy of kidney tissue from patients with severe ARPKD revealed an abundance of small membrane vesicles associated with the surfaces of collecting duct cilia (67). Similar observations were made in the biliary tree of Pkhd1 mutant mice, where cilia were surrounded by seemingly attached membrane vesicles as numerous as 30 per micrometer of cilium length (67) (Figure 2). These urinary vesicles, which researchers termed exosome-like vesicles or ELVs, were obtained and subfractionated resulting in the isolation of a subpopulation (termed PKD-ELVs) enriched in PC1, PC2, fibrocystin, and other known ciliary proteins. The striking association of extracellular vesicles with cilia observed in vivo was reproduced in vitro, where, upon addition, PKD-ELVs rapidly interacted preferentially with primary cilia of cultured kidney and biliary epithelial cells (67).
These studies revealed a startling ability of the cilium to behave as an apparent receiver for extracellular vesicles in the renal and hepatic systems, but from where do these vesicles originate? Do the cilia themselves release some of these vesicles? The authors' use of the terminology “exosome-like” owes in part to the size and appearance of the vesicles when examined with the electron microscope, but proteomic analysis of the isolated PKD-ELVs also revealed proteins common to exosomes from other sources (56, 67, 68). The PKD-ELV proteome was reported to be enriched in proteins involved in the biogenesis of intraluminal vesicles, or ILVs, that form within multivesicular bodies and are released as exosomes upon fusion of multivesicular bodies with the cell membrane. Also, PC1 was observed on rat cholangiocyte multivesicular bodies by immunoelectron microscopy, suggesting an exosomal pathway for the origin of PC1-carrying PKD-ELVs (67). In considering a potential ciliary origin for extracellular vesicles, it should be noted that the membrane budding processes of exosomes and ectosomes likely share many of the same mechanistic components. Proteins of the ESCRT (endosomal sorting complexes require for transport) machinery, known to be involved in the formation of ILVs, were present in the PKD-ELV proteome and emphasized by the authors as indicative of a multivesicular body pathway origin. The ESCRT machinery, however, is also involved in ectosome formation (67), and its presence in cilia is currently being investigated in the context of ciliary ectosome release (67; Rosenbaum lab current work). It was also noted that PKD-ELVs carried only low levels of the exosome marker CD63, but were rich in prominin-1; a protein that has been shown to localize to both cilia and a population of extracellular vesicles thought to derive from cilia (69). One possible explanation for the mixture of prominent ciliary proteins and exosomal markers that characterizes the urinary ELV profile is that the membrane vesicle fraction subjected to proteomic analysis comprised material of both exosomal and ciliary ectosomal origin.
Extracellular vesicles and the ciliated neuroepithelium of mammals
In the lumen of the neural tube, extracellular vesicles bearing proteins of the neuroepithelial cell plasma membrane have been observed (70). One such protein, prominin-1, had been previously characterized as the defining constituent of an apical membrane microdomain found in plasmalemmal protrusions (71-73). Prominin-1-carrying extracellular vesicles lacked exosomal markers, and thus appeared to be distinct from multivesicular body-derived exosomes (70). In addition, prominin-1-carrying extracellular vesicles lacked actin, but showed a striking enrichment of alpha tubulin (69). These observations, together with the fact that neuroepithelial cells are known to bear a primary cilium on their apical surface (74, 75), lead researchers to examine the, both protrusive and tubulin-rich, cilium as a potential site of origin for extracellular vesicles. Immunogold labeling and transmission electron microscopy (TEM) analysis of neuroepithelium in the mouse forebrain revealed a striking ciliary membrane localization of prominin-1 that appeared temporally regulated in embryonic development (69). During the early stages of neurogenesis, prominin-1-specific gold particles were observed along the ciliary membrane, and occasionally, labeling was found preferentially on regions of membrane that appeared to be in the process of budding from the cilium (Figure 2). Prominin-1-specific gold particles were also observed on the surfaces of extracellular membrane vesicles in the immediate vicinity of cilia (69). These data are consistent with a ciliary origin of at least some of the prominin-1-carrying extracellular vesicles observed.
Why would neuroepithelial cells employ the cilium for membrane vesicle release, and what could be the function of such a process? The authors offered two lines of speculation. First is the notion that prominin-1-containing extracellular vesicles may function in intercellular signaling. The prominin-1-carrying extracellular vesicles appear in ventricular fluid at a developmental stage coincident with the period during which apical microvilli are most abundant in the floor plate (70). Given the role of the microvilli-carpeted floor plate as a major signaling center (76-78), the authors regard this apparent timing as a clue worthy of further investigation in light of cell-cell signaling. Their second train of thought pertains to the fact that the onset of neurogenesis is heralded by the transition of neuroepithelial cell division from a symmetric, proliferative mode to an asymmetric, neurogenerative mode (79-81). This transition in the orientation of neuroepithelial cell division and subsequent differentiations to radial glial cells and neuron-generating basal progenitors are accompanied by the reduction, or complete loss, of the apical plasma membrane (82, 83). These findings suggested the hypothesis that vesicle release could play a role in the depletion of a stem cell-characteristic apical membrane domain from neuroepithelial cells. As a mechanism of disposal, vesicle release could modify the composition of the cell membrane and in this way influences the switch to neurogenesis during development (69).
When the cilium contacts another cell directly
Considered at its most basic level, the phenomenon of ciliary ectosome release allows for a portion of the cilium's membrane to make indirect, functional contact with an external membrane surface of another cell some distance away (Figure 4). The uncovering of this phenomenon may shed new light on the fact that, within a range of microns, the cilium itself has the potential to reach out and make direct contact with a neighboring cell. To what extent do cilia make such contacts? Can these contacts function to mediate an exchange of biological material or information between cells?
Figure 4.
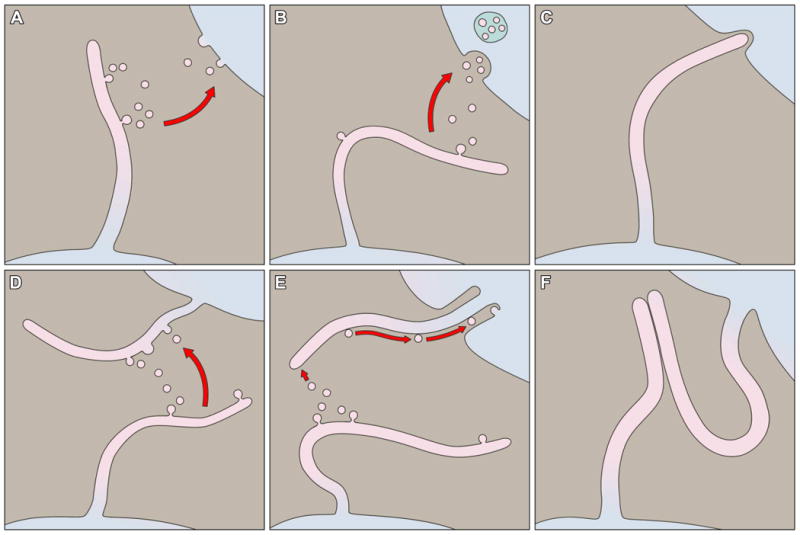
Modes of ciliary membrane interaction. A. Ciliary ectosomes may fuse with the plasma membrane of a recipient cell. B. Ciliary ectosomes may be endocytosed by a recipient cell. C. A cilium may interact with a recipient cell by direct membrane contact. D. Extracellular vesicles may fuse with the ciliary membrane of a recipient cell. E. Extracellular vesicles may adhere to the ciliary membrane of a recipient cell and be moved along the length of the cilium by sub-membrane motor activity. F. A cilium may interact with another cilium by direct membrane contact.
The best-studied occurrence of a cilium making contact with the surface of a neighboring cell takes place in the retina where the highly differentiated cilia of photoreceptor cells are involved in an intimate interaction with the overlying retinal pigment epithelium (RPE) cell layer (Figure 5). Vertebrate rod and cone cells have a distinctive form characterized by a cell body (the inner segment) that projects a ciliary axoneme (the connecting cilium) whose membrane gives way to an elongated, extensive array of disk-shaped elaborations (the outer segment). The membranous disks of the outer segment house the opsins, chromophores and other components comprising the light-harvesting machinery of photoreception. Because the phototransduction process involves the accumulation of potentially toxic photo-oxidative products, the outer segments undergo a daily renewal process wherein ∼10% of their several hundred membranous disks are shed from the distal tip and replaced by new disks that form continuously at the base (84, 85). In a process documented in fine detail with the electron microscope in seminal experiments dating from the late 1960s and early 1970s, groups of membranous disks separate from the tightly packed array and pinch off within an extracellular vesicle formed from the ciliary membrane at the tip of the outer segment. The distal-most regions of the outer segments are embedded in intimately apposed RPE cells, which immediately engulf the shed membranes (84, 86-88) (Figure 5). It is estimated that a single RPE cell phagocytizes hundreds of thousands of outer segment disks over the span of a human lifetime; a profound transfer of material between the ciliary compartment of one cell and the interior of another cell (84, 85). The synchronized collaboration between RPE cells and photoreceptor cells in the daily renewal of outer segments is crucial to the maintenance of retinal health, and perturbations in outer segment shedding and phagocytosis can lead to retinopathies (85, 89-91)
Figure 5.
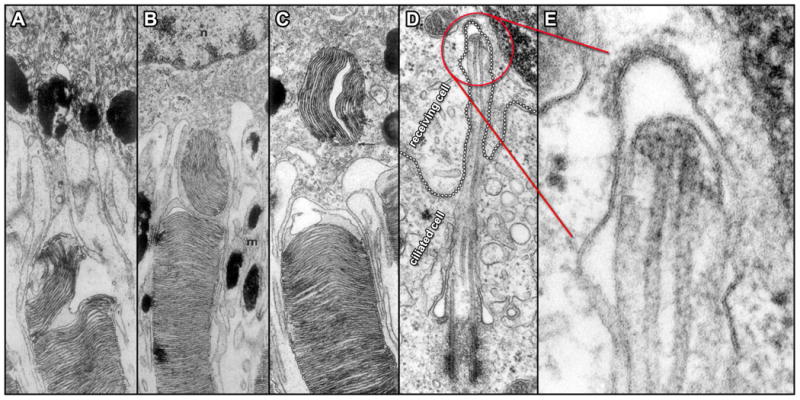
Direct interactions between cilia and neighboring cell surfaces. A – C. TEMs of ultrathin sections through three different rod outer segment tips illustrate the sequential events in the interaction with adjacent RPE cells in Rhesus monkey. A grouping of outer segment disks initially undergoes separation within the outer segment (A); followed by engulfment via RPE cell cytoplasmic extensions (B) and movement deeper into the cytoplasm of the RPE cell (C) (84). D. TEM of an ultrathin section through a fibroblast from the testes of rat shows the cell membrane of a receiving cell (emphasized by a dotted outline) forming what appears to be a coated pit in response to an intruding primary cilium extending from an adjacent ciliated cell. Panel E displays a higher magnification view of the ciliary tip region seen in D and indicated in red (125).
This example of a biological process conveyed through cilium-cell contact seems to be best characterized as a critical disposal pathway for outer segment disks. The close contact between photoreceptor cilia and the RPE cells, however, also facilitates a different intercellular exchange of material that is central to the biochemical events of the phototransduction process itself. Light harvesting that occurs in the photoreceptor cilium outer segment is achieved by opsin, a G protein coupled receptor that requires a bound chromophore, 11-cis-retinal, to absorb photons. Upon photon absorption, 11-cis-retinal undergoes an isomerization to all-trans-retinal, and this induces a conformational change in its associated opsin moiety, which stimulates activation of the phototransduction cascade (92). Restoration of the initial photosensitive receptor conformation requires the formation and return of 11-cis-retinal from all-trans-retinal by means of a process called the retinoid cycle. A remarkable feature of the retinoid cycle is that the processing and re-isomerization of 11-cis-retinal actually occurs not in the photoreceptor outer segment, but in a neighboring RPE cell. That is, the all-trans-retinal produced in the light harvesting reaction is transported across the ciliary membrane of the photoreceptor outer segment then imported across the plasma membrane of an adjacent RPE cell where final conversion to 11-cis-retinal takes place. The 11-cis-retinal is then transported back from the RPE cell and into the photoreceptor cell where it can re-join opsin for function in the outer segment (93-101). Thus, a cycle of secretion and uptake between a ciliary compartment and a neighboring cell lies at the very core of the vertebrate visual system, and disruption of this key process can result in a range of retinal diseases (102-106).
Evidence of the cilium's ability to communicate via ectosome release, taken together with the well-studied precedent of the photoreceptor cilium-RPE cell interaction, warrants a thorough survey of cilia in the context of short-range, direct cell-cell communication. This is a technically challenging enterprise requiring high-resolution imaging of ciliary contacts that may be transient in nature; one may have to determine when to look in addition to where. Despite these challenges some clues have surfaced in recent years. An electron microscopic examination of the trypanosomatid protozoan parasite, Leishmania, has revealed what appears to be an intimate and persistent interaction between the tip of the parasite's cilium and an aspect of its vertebrate host membrane (107). Instances in vertebrate tissues, where the plasma membrane of one cell seems to receive the tip of the cilium of a neighboring cell, have been observed by electron microscopy. Figure 5 includes an example in which the plasma membrane of a fibroblast from the testes of rat appears to be forming a coated pit in response to an intruding ciliary tip, suggesting a functional interaction of some sort (Figure 5, D, E). Another study has documented prolonged, direct contacts between mammalian, immotile cilia of adjacent cells in tissues of the eye and liver, and in cultured cells of the kidney (108). Though the functional significance of such cilium-cilium contacts is not presently known, the aforementioned Chlamydomonas mating process demonstrates that signal transduction initiated by ciliary adhesion is a mechanism in nature.
Concluding Remarks
Future efforts in the investigation of ciliary ectosomes should involve – i. identification of the machinery that mediates outward budding of the ciliary membrane, and ii. elucidation of the functional significance of vesicles derived from cilia in tissues of higher organisms. A good candidate mechanism for ciliary ectosome formation is membrane remodeling by ESCRT complexes (109-111). A wide variety of enveloped viruses recruit the ESCRT machinery to escape cells by outward budding from the plasma membrane (110, 111, 112) (Figure 3). The membrane topology characteristic of ciliary ectosome release is like that of viral budding and differs from that of other cellular vesiculation processes such as endocytosis, where budding occurs in the direction of the cytoplasm and is catalyzed by dynamin complexes positioned on the outside of the bud neck (113). The “reverse topology” of ectosomal budding, by contrast, requires the assembly of membrane fission machinery within the bud neck, and the ESCRT pathway represents the only well-characterized example of such a mechanism. Studies in worms have documented the involvement of ESCRT complexes in the formation of non-viral ectosomes (114) and proteomic analyses of Chlamydomonas reveal the presence of ESCRT proteins in purified flagellar membranes and flagellum-derived ectosomes (115). Future experiments in which ciliary ectosome release is monitored in the context of ESCRT disruption via mutation or knockdown is predicted to reveal a role for ESCRT proteins in the formation of ciliary ectosomes and possibly other ciliary membrane remodeling events such as ciliary resorption and severing (116).
The functional significance of ciliary ectosome release is likely to be manifold. Well known is the fact that the cilium, despite its relatively small surface area compared with that of the cell proper, is the site of function for a variety of receptors and signal transduction modules critical to basic cellular processes involved in growth, development, and homeostasis (8, 117). Via pathways that researchers are just now beginning to understand, the cilium is employed as a sensory platform where specific proteins are localized by targeted transport and selective gating (118-123). This capacity of the cilium to serve as a specialized region of the cell where specific proteins can be readily concentrated for sensory function also makes it an ideal organelle to employ for the regulated release of specific biological material and information. Studies in model systems have thus far provided important precedents for the role of ciliary ectosomes in mediating the release of enzymes and signals that carry out extracellular functions (38, 51). Alternatively, ciliary ectosome release employed as a means of disposal could serve as a key remodeling mechanism for both the lipid and protein content of the ciliary compartment as well as that of the cell generally. From the standpoint of homeostasis, the release of ciliary ectosomes could prove to be a mechanism integral to the stability of the ciliary membrane, whose formation and dynamic maintenance must be coordinated with that of the axoneme, and balanced with regard to membrane and protein flux at the ciliary base (119, 121, 123, 124).
Our perspective on cilia has progressed from a view centered on motility to one in which cilia, both motile and immotile, are recognized as highly functional instruments of sensory reception. With the phenomenon of ciliary ectosome release in view we have arrived at a fully mature concept of the cilium as the cell's antenna capable of both sending and receiving extracellular signals.
Highlights.
Cilia function as a sensory antennae
Cilia can release extracellular vesicles that carry out biological functions beyond the cell
Extracellular vesicles carry a wide range of cargoes and participate in cell-to-cell communication
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pazour GJ, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pazour GJ, Rosenbaum J. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–555. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- 3.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–10. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum JL, Witman GB. Intraflagellar Transport. Nature Rev Cell Mol Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 5.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 6.Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- 7.Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–56. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 9.Chargaff E, West R. The biological significance of the thromboplastic protein of blood. Journal of Biological Chemistry. 1946;166:189–97. [PubMed] [Google Scholar]
- 10.Candelario KM, Steindler DA. The role of extracellular vesicles in the progression of neurodegenerative disease and cancer. Trends Mol Med. 2014;20:368–74. doi: 10.1016/j.molmed.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyorgy B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cellular and Molecular Life Sciences. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadallah S, Eken C, Schifferli JA. Ectosomes as modulators of inflammation and immunity. Clinical and Experimental Immunology. 2011;163:26–32. doi: 10.1111/j.1365-2249.2010.04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tickner JA, Urquhart AJ, Stephenson SA, Richard DJ, O'Byrne KJ. Functions and therapeutic roles of exosomes in cancer. Front Oncol. 2014;4:127. doi: 10.3389/fonc.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature Reviews: Immunology. 2002;2:569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 17.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends in Cell Biology. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergman K, Goodenough UW, Goodenough DA, Jawitz J, Martin H. Gametic differentiation in Chlamydomonas reinhardtii. II. Flagellar membranes and the agglutination reaction. J Cell Biol. 1975;67:606–22. doi: 10.1083/jcb.67.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLean R, Laurendi CJ, Brown RM., Jr The relationship of gamone to the mating reaction in Chlamydomonas moewusii. Proc Natl Acad Sci U S A. 1974;71:2610–3. doi: 10.1073/pnas.71.7.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snell WJ, Kroop SA, Rosenbaum JL. Characterization of adhesive substances on the surface of Chlamydomonas gamete flagella. J Cell Bio. 1973;59:327. a. Abstr. [Google Scholar]
- 22.Goodenough U, Lin H, Lee JH. Sex determination in Chlamydomonas. Semin Cell Dev Biol. 2007;18:350–61. doi: 10.1016/j.semcdb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Goodenough UW, et al. The role of calcium in the Chlamydomonas reinhardtii mating reaction. J Cell Biol. 1993;121:365–74. doi: 10.1083/jcb.121.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan J, Misamore MJ, Wang Q, Snell WJ. Protein transport and signal transduction during fertilization in chlamydomonas. Traffic. 2003;4:452–9. doi: 10.1034/j.1600-0854.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 25.Goodenough UWH, JE Deep-etch analysis of adhesion complexes between gametic flagellar membranes of Chlamydomonas reinhardtii (Chlorophyceae) J Phycol. 1999;35:756–767. [Google Scholar]
- 26.Snell WJ. Mating in Chlamydomonas: a system for the study of specific cell adhesion. I. Ultrastructural and electrophoretic analyses of flagellar surface components involved in adhesion. J Cell Biol. 1976;68:48–69. doi: 10.1083/jcb.68.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demets R, Tomson AM, Homan WL, Stegwee D, Vandenende H. Cell-Cell Adhesion in Conjugating Chlamydomonas Gametes - a Self-Enhancing Process. Protoplasma. 1988;145:27–36. [Google Scholar]
- 28.Dolo V, et al. Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res. 1998;58:4468–74. [PubMed] [Google Scholar]
- 29.Ginestra A, et al. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. Journal of Biological Chemistry. 1997;272:17216–22. doi: 10.1074/jbc.272.27.17216. [DOI] [PubMed] [Google Scholar]
- 30.Taraboletti G, et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160:673–80. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giusti I, et al. Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles. Neoplasia. 2008;10:481–8. doi: 10.1593/neo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutwein P, et al. Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clinical Cancer Research. 2005;11:2492–501. doi: 10.1158/1078-0432.CCR-04-1688. [DOI] [PubMed] [Google Scholar]
- 33.Stoeck A, et al. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J. 2006;393:609–18. doi: 10.1042/BJ20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubo T, et al. The Chlamydomonas hatching enzyme, sporangin, is expressed in specific phases of the cell cycle and is localized to the flagella of daughter cells within the sporangial cell wall. Plant & Cell Physiology. 2009;50:572–83. doi: 10.1093/pcp/pcp016. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda Y, Koseki M, Shimada T, Saito T. Purification and characterization of a vegetative lytic enzyme responsible for liberation of daughter cells during the proliferation of Chlamydomonas reinhardtii. Plant & Cell Physiology. 1995;36:681–9. [PubMed] [Google Scholar]
- 36.Roberts K, Grief C, Hills GJ, Shaw PJ. Cell wall glycoproteins: structure and function. J Cell Sci Suppl. 1985;2:105–27. doi: 10.1242/jcs.1985.supplement_2.6. [DOI] [PubMed] [Google Scholar]
- 37.Sumper M, Hallmann A. Biochemistry of the extracellular matrix of Volvox. International Review of Cytology. 1998;180:51–85. doi: 10.1016/s0074-7696(08)61770-2. [DOI] [PubMed] [Google Scholar]
- 38.Wood CR, Huang K, Diener DR, Rosenbaum JL. The cilium secretes bioactive ectosomes. Curr Biol. 2013;23:906–11. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claes H. Autolysis of the cell wall of gametes of Chlamydomonas reinhardii. Arch Mikrobiol. 1971;78:180–8. [PubMed] [Google Scholar]
- 40.Kinoshita T, Fukuzawa H, Shimada T, Saito T, Matsuda Y. Primary structure and expression of a gamete lytic enzyme in Chlamydomonas reinhardtii: similarity of functional domains to matrix metalloproteases. Proc Natl Acad Sci U S A. 1992;89:4693–7. doi: 10.1073/pnas.89.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuda Y, Saito T, Yamaguchi T, Kawase H. Cell wall lytic enzyme released by mating gametes of Chlamydomonas reinhardtii is a metalloprotease and digests the sodium perchlorate-insoluble component of cell wall. Journal of Biological Chemistry. 1985;260:6373–7. [PubMed] [Google Scholar]
- 42.Schlösser UG. Release of reproduction cells by action of cell wall autolytic factors in Chlamydomonas and Germinella. Ber Deutsch Bot Ges. 1981;94:373–374. [Google Scholar]
- 43.Bisova K, Krylov DM, Umen JG. Genome-wide annotation and expression profiling of cell cycle regulatory genes in Chlamydomonas reinhardtii. Plant Physiol. 2005;137:475–91. doi: 10.1104/pp.104.054155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones RF. Physiological and biochemical aspects of growth and gametogenesis in Chlamydomonas reinhardtii. Ann NY Acad Sci. 1970;175:648–659. [Google Scholar]
- 45.Goodenough UW, StClair HS. BALD-2: a mutation affecting the formation of doublet and triplet sets of microtubules in Chlamydomonas reinhardtii. J Cell Biol. 1975;66:480–91. doi: 10.1083/jcb.66.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris EH. The Chlamydomonas Sourcebook. A Comprehensive Guide to Biology and Laboratory Use. Academic Press; San Diego, CA: 1989. [DOI] [PubMed] [Google Scholar]
- 47.Blacque OE, Sanders AA. Compartments within a compartment: what C. elegans can tell us about ciliary subdomain composition, biogenesis, function, and disease. Organogenesis. 2014;10:126–37. doi: 10.4161/org.28830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inglis PN, Ou G, Leroux MR, Scholey JM. The sensory cilia of Caenorhabditis elegans. WormBook. 2007:1–22. doi: 10.1895/wormbook.1.126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doroquez DB, Berciu C, Anderson JR, Sengupta P, Nicastro D. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. Elife. 2014;3:e01948. doi: 10.7554/eLife.01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bae YK, Barr MM. Sensory roles of neuronal cilia: cilia development, morphogenesis, and function in C. elegans. Front Biosci. 2008;13:5959–74. doi: 10.2741/3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, et al. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol. 2014;24:519–25. doi: 10.1016/j.cub.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barr MM, Garcia LR. Male mating behavior. WormBook. 2006:1–11. doi: 10.1895/wormbook.1.78.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherlekar AL, et al. The C. elegans male exercises directional control during mating through cholinergic regulation of sex-shared command interneurons. PLoS One. 2013;8:e60597. doi: 10.1371/journal.pone.0060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen WX, et al. MicroRNAs delivered by extracellular vesicles: an emerging resistance mechanism for breast cancer. Tumour Biol. 2014;35:2883–92. doi: 10.1007/s13277-013-1417-4. [DOI] [PubMed] [Google Scholar]
- 55.Lee TH, et al. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular ‘debris’. Semin Immunopathol. 2011;33:455–67. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 56.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–73. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou H, et al. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006;70:1847–57. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hughes J, et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10:151–60. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 59.Mochizuki T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–42. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 60.Ward CJ, et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–69. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 61.Onuchic LF, et al. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–17. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 63.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–80. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 64.Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003;12:517–20. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 65.Wang S, et al. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–52. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–16. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 67.Hogan MC, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009;20:278–88. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bakeberg JL, et al. Epitope-tagged Pkhd1 tracks the processing, secretion, and localization of fibrocystin. J Am Soc Nephrol. 2011;22:2266–77. doi: 10.1681/ASN.2010111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Brauninger M. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol. 2007;176:483–95. doi: 10.1083/jcb.200608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marzesco AM, et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci. 2005;118:2849–58. doi: 10.1242/jcs.02439. [DOI] [PubMed] [Google Scholar]
- 71.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94:12425–30. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2:582–92. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- 73.Corbeil D, Fargeas CA, Huttner WB. Rat prominin, like its mouse and human orthologues, is a pentaspan membrane glycoprotein. Biochem Biophys Res Commun. 2001;285:939–44. doi: 10.1006/bbrc.2001.5271. [DOI] [PubMed] [Google Scholar]
- 74.Hinds JW, Ruffett TL. Cell proliferation in the neural tube: an electron microscopic and golgi analysis in the mouse cerebral vesicle. Z Zellforsch Mikrosk Anat. 1971;115:226–64. doi: 10.1007/BF00391127. [DOI] [PubMed] [Google Scholar]
- 75.Cohen E, Binet S, Meininger V. Ciliogenesis and centriole formation in the mouse embryonic nervous system. An ultrastructural analysis. Biol Cell. 1988;62:165–9. [PubMed] [Google Scholar]
- 76.Dodd J, Jessell TM, Placzek M. The when and where of floor plate induction. Science. 1998;282:1654–7. doi: 10.1126/science.282.5394.1654. [DOI] [PubMed] [Google Scholar]
- 77.Marti E, Bovolenta P. Sonic hedgehog in CNS development: one signal, multiple outputs. Trends Neurosci. 2002;25:89–96. doi: 10.1016/s0166-2236(02)02062-3. [DOI] [PubMed] [Google Scholar]
- 78.Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of Sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995;121:2537–47. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- 79.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A. 2004;101:3196–201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–41. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 81.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–20. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 82.Kosodo Y, et al. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J. 2004;23:2314–24. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyata T, et al. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–45. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- 84.Young RW. The renewal of rod and cone outer segments in the rhesus monkey. J Cell Biol. 1971;49:303–18. doi: 10.1083/jcb.49.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Young RW, Droz B. The renewal of protein in retinal rods and cones. J Cell Biol. 1968;39:169–84. doi: 10.1083/jcb.39.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaitin MH, Hall MO. Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest Ophthalmol Vis Sci. 1983;24:812–20. [PubMed] [Google Scholar]
- 90.Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci U S A. 2003;100:6481–6. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gal A, et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–1. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 92.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–67. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hubbard R, Wald G. Cis-trans isomers of vitamin A and retinene in the rhodopsin system. J Gen Physiol. 1952;36:269–315. doi: 10.1085/jgp.36.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dowling JL, Jr, Smith TR. An ocular study of pulseless disease. Arch Ophthalmol. 1960;64:236–43. doi: 10.1001/archopht.1960.01840010238009. [DOI] [PubMed] [Google Scholar]
- 95.Zimmerman WF. The distribution and proportions of vitamin A compounds during the visual cycle in the rat. Vision Res. 1974;14:795–802. doi: 10.1016/0042-6989(74)90143-6. [DOI] [PubMed] [Google Scholar]
- 96.Bridges CD. Vitamin A and the role of the pigment epithelium during bleaching and regeneration of rhodopsin in the frog eye. Exp Eye Res. 1976;22:435–55. doi: 10.1016/0014-4835(76)90182-2. [DOI] [PubMed] [Google Scholar]
- 97.Deigner PS, Law WC, Canada FJ, Rando RR. Membranes as the energy source in the endergonic transformation of vitamin A to 11-cis-retinol. Science. 1989;244:968–71. doi: 10.1126/science.2727688. [DOI] [PubMed] [Google Scholar]
- 98.Redmond TM, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–51. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 99.Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–48. [PubMed] [Google Scholar]
- 100.McBee JK, et al. Isomerization of all-trans-retinol to cis-retinols in bovine retinal pigment epithelial cells: dependence on the specificity of retinoid-binding proteins. Biochemistry. 2000;39:11370–80. doi: 10.1021/bi001061c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen P, et al. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet. 2001;28:256–60. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- 102.Fletcher EL, et al. Animal models of retinal disease. Prog Mol Biol Transl Sci. 2011;100:211–86. doi: 10.1016/B978-0-12-384878-9.00006-6. [DOI] [PubMed] [Google Scholar]
- 103.Tsybovsky Y, Molday RS, Palczewski K. The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease. Adv Exp Med Biol. 2010;703:105–25. doi: 10.1007/978-1-4419-5635-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cai Z, Feng GS, Zhang X. Temporal requirement of the protein tyrosine phosphatase Shp2 in establishing the neuronal fate in early retinal development. J Neurosci. 2010;30:4110–9. doi: 10.1523/JNEUROSCI.4364-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shroyer NF, et al. The rod photoreceptor ATP-binding cassette transporter gene, ABCR, and retinal disease: from monogenic to multifactorial. Vision Res. 1999;39:2537–44. doi: 10.1016/s0042-6989(99)00037-1. [DOI] [PubMed] [Google Scholar]
- 106.Goodwin P. Hereditary retinal disease. Curr Opin Ophthalmol. 2008;19:255–62. doi: 10.1097/ICU.0b013e3282fc27fc. [DOI] [PubMed] [Google Scholar]
- 107.Gluenz E, Ginger ML, McKean PG. Flagellum assembly and function during the Leishmania life cycle. Curr Opin Microbiol. 2010;13:473–9. doi: 10.1016/j.mib.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Ott C, et al. Primary cilia utilize glycoprotein-dependent adhesion mechanisms to stabilize long-lasting cilia-cilia contacts. Cilia. 2012;1:3. doi: 10.1186/2046-2530-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Developmental Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 110.Jouvenet N. Dynamics of ESCRT proteins. Cell Mol Life Sci. 2012;69:4121–33. doi: 10.1007/s00018-012-1035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCullough J, Colf LA, Sundquist WI. Membrane fission reactions of the mammalian ESCRT pathway. Annu Rev Biochem. 2013;82:663–92. doi: 10.1146/annurev-biochem-072909-101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morita E, et al. ESCRT-III protein requirements for HIV-1 budding. Cell Host Microbe. 2011;9:235–42. doi: 10.1016/j.chom.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morlot S, Roux A. Mechanics of dynamin-mediated membrane fission. Annu Rev Biophys. 2013;42:629–49. doi: 10.1146/annurev-biophys-050511-102247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wehman AM, Poggioli C, Schweinsberg P, Grant BD, Nance J. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr Biol. 2011;21:1951–9. doi: 10.1016/j.cub.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Long H, Diener DR, Rosenbaum JL, Huang K. Comparative proteomic analysis of vesicles released from flagellar membrane with isolated flagellar membranes. Mol Biol Cell. 2013;24:1214. [Google Scholar]
- 116.Quarmby LM. Cellular deflagellation. Int Rev Cytol. 2004;233:47–91. doi: 10.1016/S0074-7696(04)33002-0. [DOI] [PubMed] [Google Scholar]
- 117.Seeger-Nukpezah T, Golemis EA. The extracellular matrix and ciliary signaling. Curr Opin Cell Biol. 2012;24:652–61. doi: 10.1016/j.ceb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. Journal of Cell Science. 2010;123:529–36. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Current Topics in Developmental Biology. 2008;85:115–49. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- 120.Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13:608–18. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sung CH, Leroux MR. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat Cell Biol. 2013;15:1387–97. doi: 10.1038/ncb2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang J, Deretic D. Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res. 2014;38:1–19. doi: 10.1016/j.preteyeres.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wood CR, Rosenbaum JL. Proteins of the ciliary axoneme are found on cytoplasmic membrane vesicles during growth of cilia. Curr Biol. 2014;24:1114–20. doi: 10.1016/j.cub.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dentler W. A role for the membrane in regulating Chlamydomonas flagellar length. PLoS One. 2013;8:e53366. doi: 10.1371/journal.pone.0053366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Diener DR, Burton PR. The Cell: An Image Library. 2014 available at http://www.cellimagelibrary.org.


