Abstract
Previous studies have shown that the highest incidence of acute chest syndrome (ACS) in sickle cell disease (SCD) occurs in children less than 4 years old, and a history of ACS at this age is a risk factor for future ACS episodes. However, the interval associated with the highest risk of subsequent ACS or severe pain is not known. Through this mixed retrospective-prospective observational study, the Sleep and Asthma Cohort, we sought to determine the interval after an initial ACS episode during which the majority of children <4 years old are re-hospitalized for ACS or severe pain. The cumulative prevalence of re-hospitalization for ACS or severe pain within 6 months, 1 years, and 2 years was calculated for children with an initial ACS episode <4 years old and compared to children with an initial ACS episode ≥4 years old. A total of 44.8% and 55.2% of participants had an initial ACS episode <4 years and ≥4 years old (Range: 4-17.7 years), respectively. At 1 year following the initial ACS episode, children <4 years old had a significantly higher cumulative prevalence of re-hospitalizations for ACS or pain as compared to children ≥4 years of age, 62.5% and 39.1%, respectively (P = 0.009). After initial ACS episodes, the majority of children <4 years old will be re-hospitalized for ACS or severe pain within one year, suggesting the need for a therapeutic intervention for this high-risk group.
Keywords: sickle cell disease, acute chest syndrome, children, pediatric, hematology/oncology, respiratory virus, infections
Introduction
Acute chest syndrome (ACS) is the leading cause of death1–3 and admissions to the pediatric intensive care unit in children and adolescents with sickle cell disease (SCD).4 In SCD, ACS has been implicated as a precipitating factor in chronic lung disease.5,6 The incidence of ACS in SCD is inversely correlated to age, with the highest incidence seen in early childhood.2,7
Many potential etiologies are associated with ACS, and the most commonly identified in young children is viral infection.3 Also, asthma, asthma symptoms, or asthma risk factors are associated with an increased rate of ACS.8–10 Further, children with sickle cell anemia (SCA) and asthma are younger at the time of their first ACS episode than those without asthma.11 However, distinguishing between ACS and an asthma exacerbation clinically can be difficult.12,13
Given that an initial ACS episode as a young child <4 years of age predicts future ACS episodes,8,9 we sought to determine whether there was a short window of time of particularly high risk for vaso-occlusive episodes, including ACS or pain, requiring hospitalization. Using data from the prospective observational study, the Sleep and Asthma Cohort (SAC) study, we tested the hypothesis that after an initial ACS episode as a young child (<4 years of age), these children are at an increased risk for a severe vaso-occlusive episode within a short period of time when compared to older children (≥4 years of age). Evidence demonstrating that there is a short window for a severe vaso-occlusive episode would provide a rationale for future intervention trials to decrease the proportion of those re-hospitalized.
Methods
The Sleep and Asthma Cohort (SAC)
SAC is a mixed retrospective-prospective observational cohort of children with SCD who were 4-18 years of age at time of enrollment. Recruitment took place at three pediatric hematology sites: St Louis, Missouri; Cleveland, Ohio; and London, United Kingdom. IRB approval was obtained at all three sites. All patients included were followed for at least 3 years following enrollment at the respective site. All study participants were prospectively observed until one of the following events occurred: death, start of regular blood transfusions, or final date of follow-up. Exclusion criteria included: receiving long-term blood transfusions or long-term continuous positive airway pressure therapy at the time of enrollment; participating in a clinical trial involving blood transfusion, oxygen, or hydroxyurea therapy; with a medical history of chronic lung disease other than asthma or HIV; or a personal history of smoking.14 Written informed parental consent and patient assent was obtained for all participants. Parents and primary caretakers completed questionnaires about demographics and past medical history.
Of the 433 eligible children and families approached, 283 (66%) agreed to participate and 273 (60%) enrolled between June 2006 and December 2009 and followed prospectively until June 2013. The analytic sample in this manuscript was limited to study participants with a confirmed diagnosis of homozygous sickle cell anemia (HbSS) or HbSβ0-thalassemia who were followed from birth and had at least one ACS episode after their first birthday, ultimately including 125 children. Nineteen children with an ACS episode before age 1 were excluded because of our clinical experience that ACS in infancy may be related to perinatal infections. This decision was made a priori. Within our final cohort, we grouped children into younger children and older children based on information from pervious studies.9,11 Younger children were defined as greater than or equal to 1 and less than 4 years of age, which we will refer to as, “<4 years of age”. Older children were defined as greater than or equal to 4 years of age, which we will refer to as, “≥4 years of age.”
Definition of vaso-occlusive episodes: ACS and pain
Acute chest syndrome (ACS) was defined as an acute respiratory distress episode associated with a new radiodensity(ies) on chest x-ray, temperature >38°C and increased respiratory effort with decreased oxygen saturation or increased respiratory rate. A pneumonia diagnosis was considered an ACS episode. Vasoocclusive pain was defined as a SCA-associated episode, requiring hospitalization and treated with opioids. This did not include headaches requiring hospitalization and opioids. To assure a consistent diagnosis of ACS and/or vaso-occlusive pain, one investigator at each site was trained by the PI and was required to review and confirm the diagnoses in the charts of all patients. All pain and ACS episodes in the first four years of the study were reviewed by one person to ensure consistency in the definition of ACS and pain across all three sites.
Definition of Asthma
Participants were classified as having asthma if they received a physician diagnosis of asthma and were on prescription asthma medications at the time of enrollment and during the course of the study. Participants were classified as having no asthma if they did not receive a physician diagnosis of asthma and/or were not currently on prescription asthma medications.15
Statistical analysis
Categorical data were analyzed using chi-square tests, including Fisher's exact test where appropriate. Continuous data were analyzed using Student t-tests with the Mann-Whitney-Wilcoxon test used for variables with unequal variance or a non-normal distribution. Incidence rates for ACS and pain were analyzed using a mid-P exact rate test. Analyses were conducted using IBM SPSS Statistics (Version 22, Chicago, IL, IBM). The primary analysis compared those participants with a first ACS between ages 1 and 4 (<4 years of age) to those with a first ACS at older ages.
Results
Re-hospitalization following initial ACS episode for study population
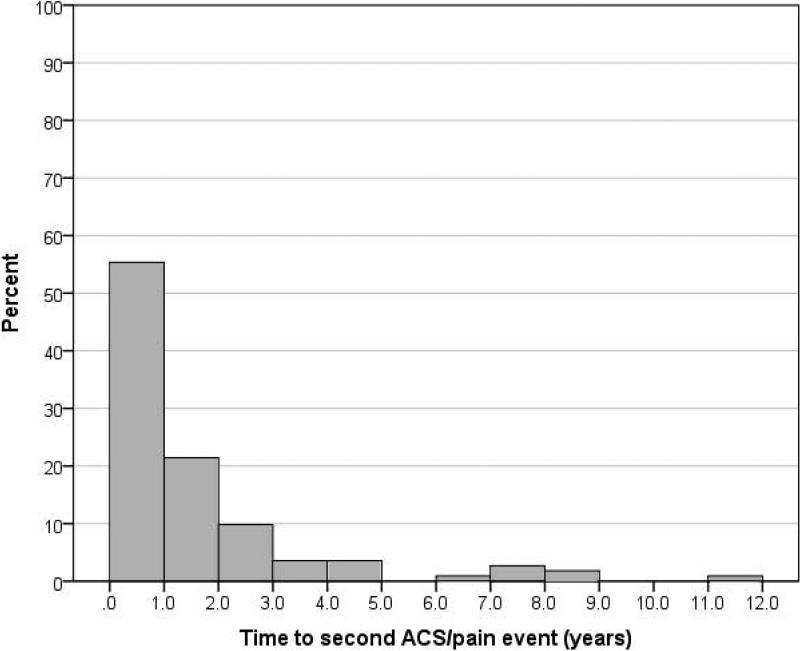
Of the 125 participants included in our analyses, 89.6% (112) had at least one additional severe vaso-occlusive episode (pain or ACS). The characteristics of this population are described in Table I. The median follow-up time was 15.0 years (Range: 6.4 – 24.7 years). The cumulative prevalence of re-hospitalization for an additional vaso-occlusive episode was 29.6% (37) within 6 months, 49.6% (62) within 1 year, and 68.8% (86) within 2 years. Table II. The median time to a subsequent episode was 0.86 years (Mean: 1.60 years, Range: 0.02 to 11.46 years). Figure 1.
Table I.
Population characteristics: total and by initial ACS age grouping
| Characteristic | Total Population* | Initial ACS <4 years (n = 56) | Initial ACS ≥4 years (n = 69) | P Value§ |
|---|---|---|---|---|
| Age | 5.78 (3.85) (Range: 1.02 - 17.67) | |||
| Male (%) | 55.2 | 67.9 | 44.9 | 0.010 |
| Time followed from age 1 | 14.07 (4.46) | 13.16 (4.33) | 14.82 (4.45) | 0.038 |
| Asthma diagnosis (%) | 34.4 | 41.1 | 29.0 | 0.157 |
| Mother has asthma (%) n=120 | 13.3 | 15.1 | 11.9 | 0.614 |
| Hemoglobin | 8.23 (1.17) | 8.12 (1.06) | 8.32 (1.25) | 0.338 |
| White blood cell count | 11.94 (3.98) | 12.78 (4.50) | 11.25 (3.38) | 0.033 |
| Reticulocyte | 11.31 (5.44) | 10.41 (4.49) | 12.04 (6.04) | 0.095 |
| Log Ige (n=118) | 4.07 (1.57) | 4.13 (1.67) | 4.02 (1.49) | 0.701 |
| ACS or pain recurrence (%) | ||||
| ACS recurrence after first ACS n (%) | 93 (74.4) | 47 (83.9) | 46 (66.7) | 0.028 |
| Pain after first ACS n (%) | 99 (79.2) | 46 (82.1) | 53 (76.8) | 0.465 |
| ACS rate per year after first ACS, mean [median] (std. dev.) | 0.29 [0.19] (0.25) | 0.40 [0.32] (0.29) | 0.20 [0.14] (0.17) | <0.001 |
| Pain rate per year after first ACS, mean [median] (std. dev.) | 0.65 [0.42] (0.82) | 0.67 [0.48] (0.80) | 0.64 [0.32] (0.84) | 0.415 |
| ACS and pain rate per year after first ACS, mean [median] (std. dev.) | 0.94 [0.70] (0.88) | 1.07 [0.88] (0.87) | 0.84 [0.59] (0.88) | 0.011# |
Mean (std. dev.) unless otherwise noted
Chi-square test for categorical variables; t test for continuous variables
Mann-Whitney U test
Table II.
Cumulative prevalence of re-hospitalization for severe vaso-occlusive episode (ACS or pain) following an initial ACS episode, by age of initial ACS episode.
| Time to next ACS/pain episode | % with subsequent ACS/pain episode | P Value | ||
|---|---|---|---|---|
| Total | <4 years | ≥4 years | ||
| 6 months | 29.6 (37/125) | 26.8 (15/56) | 31.9 (22/69) | 0.535 |
| 1 year | 49.6 (62/125) | 62.5 (35/56) | 39.1 (27/69) | 0.009 |
| 2 years | 68.8 (86/125) | 78.6 (44/56) | 60.9 (42/69) | 0.034 |
| 3 years | 77.6 (97/125) | 83.9 (47/56) | 72.5 (50/69) | 0.126 |
Figure 1.
Time to next severe vaso-occlusive episode (ACS or pain) following initial ACS episode for all children ≥1 year of age. (Please see file entitled “Figure 1”) ACS, acute chest syndrome; pain, severe pain requiring hospitalization
Re-hospitalization following initial ACS episode as a young child
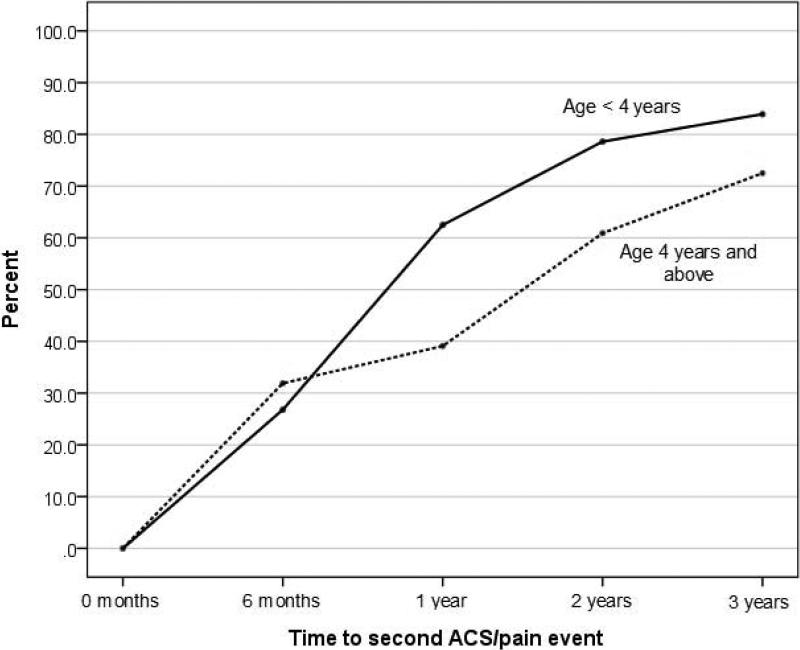
Of the 125 children with at least one initial ACS episode, 44.8% (n = 56) experienced the initial episode as a young child (<4 years of age), while 55.2% (n = 69) experienced an initial ACS episode ≥4 years of age (Range 4-17.7 years of age). After the initial ACS episode, the cumulative prevalence of re-hospitalization for a vasoocclusive episode for this <4 years of age group was 26.8% (15/56) within 6 months, 62.5% (35/56) within 1 year and 78.6% (44/56) within 2 years. Table II, Figure 2. The increase in the cumulative prevalence was rapid for the first 2 years before plateauing between the second and third years following the initial ACS episode. The median time to re-hospitalization within this age group was 0.80 years (Mean: 1.59 years, Range: 0.05 to 11.46 years).
Figure 2.
Cumulative prevalence of re-hospitalization for severe vaso-occlusive episode (ACS or pain) following an initial ACS episode, by age of initial ACS episode. (Please see file entitled “Figure 2”)
ACS, acute chest syndrome; pain, severe pain requiring hospitalization; Age <4 years includes children greater than or equal to 1 year and less than 4 years of age; Age 4 years and above includes children greater than or equal to 4 years of age (Range 4-17.7 years of age)
Age-dependent timing of re-hospitalization
At 1 and 2 years, those with an initial ACS episode <4 years of age had significantly higher cumulative prevalence of re-hospitalization for a vaso-occlusive episode when compared to children ≤4 years of age. At 1 year after the initial ACS episode 62.5 and 39.1% of the children were re-hospitalized, in the younger and older age groups respectively (P = 0.009,). Similarly two years after the initial ACS episodes, 78.6 and 60.9% were re-hospitalized in the younger and older age groups respectively (p= 0.034). However, the difference in cumulative prevalence at 6 months and 3 years between the two groups was not significant (P = 0.535, 0.126, respectively), Table II, Figure 2. Additionally, for those with an initial ACS as an older child, the median time to re-hospitalization was 1.19 years (Mean: 1.61 years, Range: 0.02 to 7.70 years). The median time to re-hospitalization was not different between the two groups. (P = 0.829).
Lifetime incidence of vaso-occlusive episodes by age of initial ACS
In children with an initial ACS episode <4 years of age, following the initial ACS episode, there was a significantly higher lifetime incidence of ACS but not severe pain, as compared to children ≥4 years of age. The mean lifetime incidence of ACS was 40 vs. 20 episodes/100 person-years in the younger and older age group respectively (P < 0.001). The mean lifetime incidence of severe pain was 67 vs. 64 episodes/100 person-years in the younger and older age group respectively (P = 0.415).
Discussion
Several studies have demonstrated that the highest incidence of ACS occurs in children less than 4 years of age2,7, and ACS in this group is predictive of future ACS episodes8,9. For the first time we have demonstrated that within this age group the majority of first subsequent severe vaso-occlusive episodes requiring hospitalization occurred within a year after the initial ACS episode. Further, the cumulative prevalence of those re-hospitalized increased over the first 2 years following the initial ACS episode, and to over 75% within 3 years. In the older age group, the pattern of severe vaso-occlusive episodes requiring hospitalization was similar to the younger age group within the first 6 months. However, younger children had a significantly higher cumulative prevalence of re-hospitalization at 1 and 2 years, as well as a significantly higher lifetime rate of ACS episodes. Our results suggest that regardless of age, following an initial ACS episode, there is a critical window of susceptibility for future severe vaso-occlusive episodes, with the peak onset being within the first 6 months. Additionally, children with an initial ACS episode at a young age are at a greater risk for future episodes for up to 2 years after the initial ACS episode.
The most important finding from this study was that a majority of children <4 years of age experienced a severe vaso-occlusive episode within one year of an initial ACS episode. We can only postulate why this is the case. In the general population, the highest incidence of viral respiratory infections is in early childhood.16 In certain children, viral respiratory infections present with wheezing, and children with virus-induced wheezing at this age are at risk of having future wheezing and/or asthma.17–20 In the general population, risk factors for virus-induced wheezing and its future sequelae include a genetic predisposition21,22 and an atopic profile, including aeroallergen sensitization17 and elevated total IgE23. Stensballe et al. demonstrated that in young children, the risk of asthma hospitalization after hospitalization for a viral respiratory infection is time-dependent, with the greatest risk being within the first 2 months and lasting for the first year.24 While not fully understood, the reason for this risk increase is thought to be due to short-term viralinduced inflammation and airway hyperresponsiveness24,25 which is cumulatively prolonged and more severe in the presence of atopy26 and which is more severe in those with asthma27. We believe that similar to asthma exacerbations, after an initial ACS episode, young children have airway inflammation for a period of time that predisposes them to airway hyperresponsiveness and susceptibility to future severe vaso-occlusive episodes following a second airway pathogen or aeroallergen exposure.
We believe based on the high rate of re-hospitalization, regardless of age, an intervention immediately following the initial ACS episode may help reduce the acute risk of re-hospitalization for a vaso-occlusive episode for all children, but with the additional morbidity observed in younger children the need for intervention in this population is especially important. The American Academy of Pediatrics recommends that every child over 1 year of age receive the annual influenza vaccination early in the same flu season, which is a reasonable precautionary measure.28 The National Heart Lung and Blood Institute Consensus panel recommends starting hydroxyurea therapy at 9 months of age as a reasonable primary and potential secondary prevention strategy for ACS episodes and rehospitalization, respectively.29 However, it takes approximately 3-6 months for hydroxyurea to take effect,30,31 and as we have demonstrated, a large proportion of ACS recurrences take place during that time period. Alternative strategies should also be considered for secondary prevention. Due to the finding that in children with SCA later diagnosed with asthma, approximately 80% experienced an initial ACS episode when they were <4 years of age,11 inhaled corticosteroids for a brief period of time may be further investigated. Regardless of the strategy, a clinical trial is warranted to determine if a therapy can reduce the high re-hospitalization rate temporally associated with an initial ACS episode as a young child.
There were several limitations in this study. Our sample size within SAC was small, potentially limiting the validity of our results. However, the cohort is one of the largest modern cohorts assembled and results from this cohort replicated those of an earlier one, namely that ACS in young children were predictive of future ACS events.8 Data from SAC was collected retrospectively before the age of 4, potentially introducing data collection bias to our results, though this potential bias was likely to be minimized as it is based on complete medical review rather than parental recall.
The majority of children with ACS <4 years of age will be re-hospitalized for severe vaso-occlusive episodes within a year of an initial ACS episode. We believe that the most likely reason is secondary to airway lability after an initial viral infection. Given the high rate of recurrence, an added effort should be made for children in this age group to receive the annual influenza immunization early in the season, and after the first ACS episode, a discussion about hydroxyurea initiation should take place. Additionally, future studies should address whether inhaled corticosteroids for a defined period of time can prevent the high risk of re-hospitalization for ACS or severe pain in the year after the initial ACS episode.
Acknowledgements
Leah Vance and Dr. DeBaun had full access to all of the data in the study and take responsibility for the integrity of the data, the accuracy of the data analysis, and the work as a whole. Leah Vance1 designed the concepts for the manuscript, interpreted the data, created the initial draft, and finalized the manuscript for submission; Dr. Rodeghier2 developed the statistical approach, performed those analyses, reviewed the integrity of those analyses, reviewed and revised the manuscript; Dr. Cohen3 helped in the interpretation of the data, and reviewed and revised the manuscript; Dr. Rosen4 helped develop the data collection instruments for sleep, supervised sleep data collection at 1 site, and reviewed and helped revise the manuscript; Dr. Kirkham5 was the principal investigator for 1 site, helped develop the concepts for this National Institutes of Health-sponsored Sleep and Asthma Cohort (SAC) project and reviewed/revised the manuscript; Dr. Strunk6 contributed to the development of the SAC project, study concepts, and procedures, supervised the quality assurance for pulmonary function testing, and reviewed and helped revise the manuscript; Dr. DeBaun7 was the principal investigator for the SAC project, helped develop the concepts for SAC and this manuscript, analyzed and interpreted the data, contributed to the development of the initial draft, and reviewed/revised the manuscript; and all authors approved the final manuscript as submitted.
The authors would like to acknowledge the Doris Duke Charitable Foundation.
Funding
All phases of this study were supported in part by the National Heart, Lung, and Blood Institute (NIH 1R01HL079937 [Dr. DeBaun], UL1 RR024989 Case Western Reserve University Clinical Research Unit and by Research and Development in the National Health Service (United Kingdom). Funded by the National Institutes of Health (NIH). This work was also supported by Grant 2014086 from the Doris Duke Charitable Foundation.
Footnotes
Potential Conflict of Interest
Dr. Rosen is a consultant for Jazz Pharmaceuticals and has been a consultant for Natus Medical and Advance-Scientific.
All other authors have indicated that they have no potential conflicts of interest to disclose.
Financial Disclosures
The authors have indicated they have no financial relationships relevant to this article to disclose.
Doris Duke Clinical Research Mentorship Program, Vanderbilt University School of Medicine, Vanderbilt University Medical Center, Nashville, TN;
Independent statistician, Chicago, Illinois;
Department of Pediatrics, Boston University School of Medicine, Boston, MA;
Department of Pediatrics, Case Western Reserve University School of Medicine, Rainbow Babies and Children's Hospitals, University Hospitals Case Medical Center, Cleveland, OH;
University College, London Institute of Child Health;
Division of Allergy, Immunology, and Pulmonary Medicine, Department of Pediatrics, Washington University School of Medicine, St Louis, MO;
Department of Pediatrics, Division of Hematology/Oncology, Vanderbilt and Meharry Center for Excellence in Sickle Cell Disease, Vanderbilt University Medical Center, Nashville, TN
References
- 1.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. doi:10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 2.Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84(2):643–649. [PubMed] [Google Scholar]
- 3.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N. Engl. J. Med. 2000;342(25):1855–1865. doi: 10.1056/NEJM200006223422502. doi:10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 4.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. doi: 10.1182/blood-2009-07-233700. doi:10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn CT, Buchanan GR. The acute chest syndrome of sickle cell disease. J. Pediatr. 1999;135(4):416–422. doi: 10.1016/s0022-3476(99)70162-9. doi:10.1016/S0022-3476(99)70162-9. [DOI] [PubMed] [Google Scholar]
- 6.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. The impact of recurrent acute chest syndrome on the lung function of young adults with sickle cell disease. Lung. 2010;188(6):499–504. doi: 10.1007/s00408-010-9255-2. doi:10.1007/s00408-010-9255-2. [DOI] [PubMed] [Google Scholar]
- 7.Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B. Acute chest syndrome in sickle cell disease: clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood. 1997;89(5):1787–1792. [PubMed] [Google Scholar]
- 8.Quinn CT, Shull EP, Ahmad N, Lee NJ, Rogers ZR, Buchanan GR. Prognostic significance of early vaso-occlusive complications in children with sickle cell anemia. Blood. 2007;109(1):40–45. doi: 10.1182/blood-2006-02-005082. doi:10.1182/blood-2006-02-005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBaun MR, Rodeghier M, Cohen R, et al. Factors predicting future ACS episodes in children with sickle cell anemia. Am. J. Hematol. 2014 doi: 10.1002/ajh.23819. doi:10.1002/ajh.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An P, Barron-Casella EA, Strunk RC, Hamilton RG, Casella JF, DeBaun MR. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. J. Allergy Clin. Immunol. 2011;127(6):1440–1446. doi: 10.1016/j.jaci.2010.12.1114. doi:10.1016/j.jaci.2010.12.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108(9):2923–2927. doi: 10.1182/blood-2006-01-011072. doi:10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anim SO, Strunk RC, DeBaun MR. Asthma morbidity and treatment in children with sickle cell disease. Expert Rev. Respir. Med. 2011;5(5):635–645. doi: 10.1586/ers.11.64. doi:10.1586/ers.11.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field JJ, DeBaun MR. Asthma and sickle cell disease: two distinct diseases or part of the same process? Hematol. Educ. Program Am. Soc. Hematol. Am. Soc. Hematol. Educ. Program. 2009:45–53. doi: 10.1182/asheducation-2009.1.45. doi:10.1182/asheducation-2009.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Rosen CL, Debaun MR, Strunk RC, et al. Obstructive Sleep Apnea and Sickle Cell Anemia. Pediatrics. 2014;134(2):273–281. doi: 10.1542/peds.2013-4223. doi:10.1542/peds.2013-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strunk RC, Cohen RT, Cooper BP, et al. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. J. Pediatr. 2014;164(4):821–826. e1. doi: 10.1016/j.jpeds.2013.11.034. doi:10.1016/j.jpeds.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monto AS. Epidemiology of viral respiratory infections. Am. J. Med. 2002;112(6, Supplement 1):4–12. doi: 10.1016/s0002-9343(01)01058-0. doi:10.1016/S0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 17.Jackson DJ, Evans MD, Gangnon RE, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am. J. Respir. Crit. Care Med. 2012;185(3):281–285. doi: 10.1164/rccm.201104-0660OC. doi:10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. doi:10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am. J. Respir. Crit. Care Med. 1999;159(3):785–790. doi: 10.1164/ajrccm.159.3.9801052. doi:10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 20.Kusel MMH, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 2007;119(5):1105–1110. doi: 10.1016/j.jaci.2006.12.669. doi:10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartlett NW, McLean GR, Chang Y-S, Johnston SL. Genetics and epidemiology: asthma and infection. Curr. Opin. Allergy Clin. Immunol. 2009;9(5):395–400. doi: 10.1097/ACI.0b013e32833066fa. doi:10.1097/ACI.0b013e32833066fa. [DOI] [PubMed] [Google Scholar]
- 22.Calışkan M, Bochkov YA, Kreiner-Møller E, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N. Engl. J. Med. 2013;368(15):1398–1407. doi: 10.1056/NEJMoa1211592. doi:10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jartti T, Kuusipalo H, Vuorinen T, et al. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2010;21(7):1008–1014. doi: 10.1111/j.1399-3038.2010.01059.x. doi:10.1111/j.1399-3038.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stensballe LG, Simonsen JB, Thomsen SF, et al. The causal direction in the association between respiratory syncytial virus hospitalization and asthma. J. Allergy Clin. Immunol. 2009;123(1):131–137. e1. doi: 10.1016/j.jaci.2008.10.042. doi:10.1016/j.jaci.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Dakhama A, Lee YM, Gelfand EW. Virus-induced airway dysfunction: pathogenesis and biomechanisms. Pediatr. Infect. Dis. J. 2005;24(11 Suppl):S159–169. doi: 10.1097/01.inf.0000188155.46381.15. discussion S166-167. [DOI] [PubMed] [Google Scholar]
- 26.Xepapadaki P, Papadopoulos NG, Bossios A, Manoussakis E, Manousakas T, Saxoni-Papageorgiou P. Duration of postviral airway hyperresponsiveness in children with asthma: effect of atopy. J. Allergy Clin. Immunol. 2005;116(2):299–304. doi: 10.1016/j.jaci.2005.04.007. doi:10.1016/j.jaci.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busse WW, Lemanske RF, Gern JE. The Role of Viral Respiratory Infections in Asthma and Asthma Exacerbations. Lancet. 2010;376(9743):826–834. doi: 10.1016/S0140-6736(10)61380-3. doi:10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diseases C on I. Recommendations for Prevention and Control of Influenza in Children, 2014–2015. Pediatrics. 2014 doi: 10.1542/peds.2014-2413. peds.2014-2413. doi:10.1542/peds.2014-2413. [DOI] [PubMed] [Google Scholar]
- 29.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517. doi:10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 30.Koren A, Segal-Kupershmit D, Zalman L, et al. Effect of hydroxyurea in sickle cell anemia: a clinical trial in children and teenagers with severe sickle cell anemia and sickle cell beta-thalassemia. Pediatr. Hematol. Oncol. 1999;16(3):221–232. doi: 10.1080/088800199277272. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea. Multicenter Study of Hydroxyurea. Blood. 1997;89(3):1078–1088. [PubMed] [Google Scholar]