Abstract
Background
Hemorrhage is the leading cause of survivable death in trauma. Resuscitation strategies including early red blood cell (RBC) transfusion have reduced this. Pre-trauma center (PTC) RBC transfusion is growing and preliminary evidence suggests improved outcomes. The study objective was to evaluate the association of PTC RBC transfusion with outcomes in air medical trauma patients.
Study Design
Retrospective cohort study of trauma patients transported by helicopter to a level-I trauma center, 2007—2012. Patients receiving PTC RBC transfusion were matched to control patients (receiving no PTC RBC transfusion during transport) in a 1:2 ratio using a propensity-score based on prehospital variables. Conditional logistic regression and mixed-effects linear regression were used to determine the association of PTC RBC transfusion with outcomes. Subgroup analysis was performed for scene transport patients.
Results
Two-hundred forty treatment patients were matched to 480 control patients receiving no PTC RBC transfusion. PTC RBC transfusion was associated with increased odds of 24-hour survival (adjusted odds ratio [AOR] 4.92; 95%CI 1.51, 16.04, p=0.01), lower odds of shock (AOR 0.28; 95%CI 0.09, 0.85, p=0.03), and lower 24-hour RBC requirement (Coef −3.6 RBC units; 95%CI −7.0, −0.2, p=0.04). Among matched scene patients, PTC RBC was also associated with increased odds of 24-hour survival (AOR 6.31; 95%CI 1.88, 21.14, p<0.01), lower odds of shock (AOR 0.24; 95%CI 0.07, 0.80, p=0.02), and lower 24-hour RBC requirement (Coef −4.5 RBC units; 95%CI −8.3, −0.7, p=0.02).
Conclusions
PTC RBC was associated with an increased probability of 24-hour survival, decreased risk of shock, and lower 24-hour RBC requirement. PTC RBC appears beneficial in severely injured air medical trauma patients and prospective study is warranted as PTC RBC transfusion becomes more readily available.
INTRODUCTION
Hemorrhage remains a major driver of early mortality in injured patients.(1, 2) Recent literature has focused on resuscitation in the trauma center to reduce this burden. Strategies include minimizing crystalloid use,(3, 4) high ratio of plasma and platelets to red blood cell (RBC) transfusion,(5–8) and massive transfusion protocols.(9) Approaches to early blood transfusion have received significant attention recently.(1, 10)
Prehospital resuscitation has also received considerable interest. To date, studies in the civilian population have focused on the use of crystalloids for resuscitation in the prehospital setting.(11–13) However, blood transfusion remains the mainstay of resuscitation for traumatic shock.(14) The capability to provide RBC transfusion during transport is generally limited to well-developed helicopter emergency medical service (HEMS) systems.(15, 16) Because of this, there is little evidence examining the use of prehospital RBC transfusion to mitigate early hemorrhage and shock in injured civilians.(17)
Military data have demonstrated that more than 90% of potentially survivable casualties die from hemorrhage, and medevac platforms from the United States (US) and United Kingdom (UK) have prehospital RBC transfusion capabilities that have been associated with improved survival.(18–21) Given the encouraging results from military and hospital based resuscitation strategies, applying the lessons learned to the civilian prehospital setting is appealing.
Our group presented the first preliminary civilian data documenting improved outcomes associated with pre-trauma center (PTC) RBC transfusion in the severely injured Glue Grant Cohort.(22) However, this study was limited as a secondary analysis and only a small number of patients underwent PTC RBC transfusion. A more recent larger study evaluated outcomes of prehospital RBC and plasma transfusion in HEMS patients, finding improved acid-base status and decreased transfusion requirements in patients receiving prehospital blood products.(23)
The objective of this study was to evaluate the association of PTC RBC transfusion with early outcomes in trauma patients undergoing air medical transport. Given mounting evidence in support of PTC RBC transfusion, we hypothesized that PTC RBC transfusion would be associated with improved 24-hour mortality, lower 24-hour RBC requirements, and lower risk of shock and coagulopathy on admission.
METHODS
Data Sources and Study Population
This is a retrospective cohort study conducted at the University of Pittsburgh Medical Center (UPMC) Presbyterian Hospital, an urban level I trauma center with the highest volume of trauma patients in the state of Pennsylvania. All injured patients age >15 years who underwent air medical transport by STAT MedEvac to UPMC Presbyterian Hospital between 2007 and 2012 were eligible for inclusion. STAT MedEvac is a large HEMS provider managed through the University of Pittsburgh's Center for Emergency Medicine, and accounts for approximately 40% of Pennsylvania's HEMS transports. During the study period STAT MedEvac was staffed by a paramedic/nurse team and carried 2 units of type O negative RBCs on each mission. A standing protocol was in place to allow RBC transfusion to trauma patients who have evidence of decreased tissue perfusion (Table 1).
Table 1.
Protocol for Helicopter Emergency Medical Services Red Blood Cell Transfusion
| RBC transfusion should be administered after 1–2L of crystalloid total has been received by an injured patient and any one of the following are present: |
|
| In cases of penetrating wounds or clinical evidence of active bleeding, RBC may be initiated earlier through consultation with a medical command physician. |
HEMS, helicopter emergency medical services; RBC, red blood cells; HR, hHeart rate; SBP, systolic blood pressure.
Data sources included emsCharts (Pittsburgh, PA), a prospectively collected prehospital database fed by electronic documentation of STAT MedEvac personnel, the UPMC trauma registry, and the UPMC electronic health record. Patients meeting inclusion criteria were identified through the emsCharts database. These patients' data were merged with the other data sources to obtain demographics, injury characteristics, time at referring facility, prehospital time and distance, prehospital and admission vital signs, crystalloid and RBC volumes, prehospital and admission laboratory values, intensive care unit (ICU) admission, emergency department (ED) disposition, surgical procedures, complications, and hospital disposition for each patient.
Missing Data
For analysis variables missing more than 1% but less than 25% of observations, multiple imputation was performed. Imputed variables included race, admission Glasgow Coma Scale (GCS), admission systolic blood pressure (SBP), and admission heart rate (HR). Multiple imputation using an iterative Markov chain Monte Carlo fully conditional specification model based on available demographics, injury severity, ICU admission, and survival was performed using five imputations. Outcome models were performed using the multiple imputation estimation techniques that combine model coefficients and standard errors from each imputed dataset while adjusting for the variability between imputed datasets.(24) Missing data for imputed variables ranged from 1.2% (admission HR) to 4.9% (admission SBP). Sensitivity analysis was performed using complete cases and no significant differences in outcomes were seen.
Propensity Score Matching
Among included patients, those receiving RBC transfusion during air medical transport were identified. These patients were classified as having undergone PTC RBC transfusion, reflecting both scene patients who received true prehospital RBC transfusion, as well as transfer patients who received RBC during air medical transport after being initially evaluated, and in some cases receiving RBC transfusion, at a non-trauma center hospital. Patients receiving any blood product other than RBC, such as plasma or platelets were excluded. As those receiving PTC RBC transfusion were not randomly assigned to this treatment, they were more severely injured with a higher risk of mortality. Because of this propensity score matching was performed. The propensity score model was developed to predict the likelihood of receiving PTC RBC transfusion based on several prehospital variables to allow matching of treated (PTC RBC transfused) and control (no PTC RBC transfusion) patients based on this probability of receiving a PTC RBC transfusion. Covariates in the propensity score model included transfer status, age, prehospital SBP, prehospital HR, RBC volume prior to HEMS arrival, crystalloid volume prior to HEMS arrival, crystalloid volume during HEMS transport, mechanism of injury (blunt or penetrating), and HEMS transport distance. These covariates were selected a priori based on information that would be available in the prehospital period that HEMS providers would evaluate to reasonably guide the decision to administer PTC RBC. Propensity score model performance was assessed using the C-statistic and Pearson goodness-of-fit test.
Matching was performed using a 1:2 ratio nearest neighbor algorithm with replacement and no caliper. Standardized differences and standardized percent bias were used to assess the balance of covariates used in propensity score estimation after matching. An absolute value for the standardized difference >0.2 was considered to indicate significant residual imbalance for a given variable between treatment groups.(25)
Statistical Analysis
The primary outcome was 24-hour survival. Secondary outcomes included shock on admission (defined as admission base deficit ≥6mEq/L or lactate >4mmol/L), 24-hour in-hospital RBC transfusion volume, trauma-induced coagulopathy (TIC; defined as admission international normalized ratio [INR]≥1.5), and in-hospital survival. Acute respiratory distress syndrome (ARDS) and transfusion reactions were evaluated as a safety outcome. Conditional logistic regression models were used to determine the association of PTC RBC transfusion compared to no PTC RBC transfusion with binary outcomes while accounting for matching. Mixed-effects linear regression was used to determine the association of PTC RBC transfusion with continuous outcomes with a random-effect for matched patients.
Model covariates were selected a priori for known prognostic significance to the outcomes of interest that were not accounted for in the propensity score matching procedure. Covariates were then confirmed to be associated with the outcomes in univariate analysis or change the model coefficient for PTC RBC transfusion by ≥10%. Covariates in the model for 24-hour survival included gender, race, injury severity score (ISS), admission SBP, admission HR, admission GCS, admission INR, alcohol intoxication, ICU admission, emergent abdominal, thoracic, or vascular operation, mechanical ventilation, and Trauma Mortality Prediction Model (TMPM) predicted mortality.(26) Covariates in the model for shock on admission included gender, race, ISS, admission SBP, admission GCS, admission INR, alcohol intoxication, admission hemoglobin, and TMPM predicted mortality. Covariates in the model for 24-hour in-hospital RBC transfusion volume included gender, ISS, admission SBP, admission HR, shock on admission, admission INR, admission hemoglobin, and emergent abdominal, thoracic, or vascular operation. Covariates in the model for TIC included gender, race, ISS, admission SBP, admission base deficit, alcohol intoxication, admission hemoglobin and TMPM predicted mortality. The model of in-hospital survival included covariates described above for 24-hour survival with the addition of in-hospital complications. Collinearity was assessed using variance inflation factors and any covariate with a value >10 was removed from final models.
Subgroup analysis was performed for patients undergoing air medical transport from the scene of injury. This subgroup of patients is of particular interest as they had no opportunity to receive treatment or blood products prior to air medical crew interventions and transport, and thus received true prehospital RBC transfusion. As transfer and scene trauma patients are frequently considered to be different populations for this reason, the propensity score matching procedures described above were performed separately among scene patients to re-estimate the likelihood of receiving RBC transfusion during transport and generate matched treatment and control air medical scene transport patients. Transfer status and RBC volume prior to HEMS arrival were omitted in estimation of the propensity score for scene patients. Similar conditional logistic regression and mixed-effects linear regression models described above were used to determine the association of PTC RBC transfusion among air medical scene transport patients with the outcomes of interest.
For univariate comparisons, Chi-square tests and Wilcoxon rank-sum tests were used for categorical and continuous variables respectively. A p value ≤0.05 was considered significant with 2-sided tests. Data analysis was conducted using STATA version 13 (College Station, TX). This study was approved by the Institutional Board Review at the University of Pittsburgh.
RESULTS
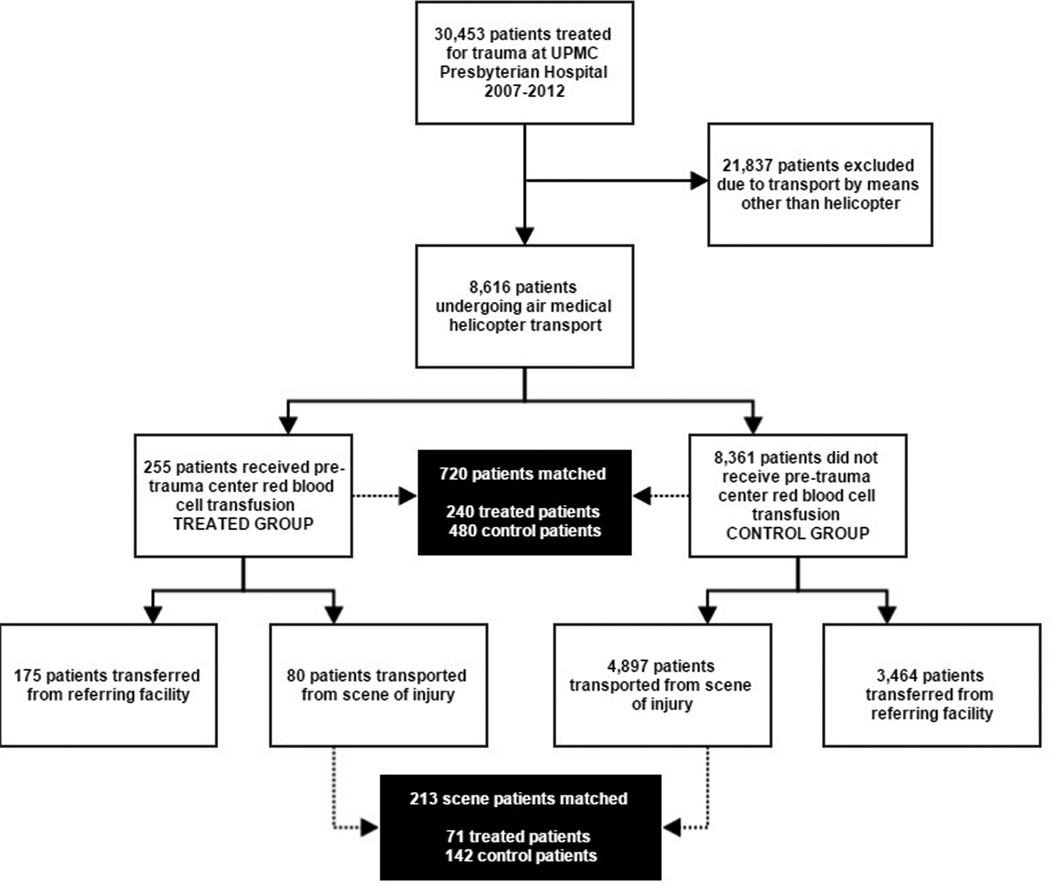
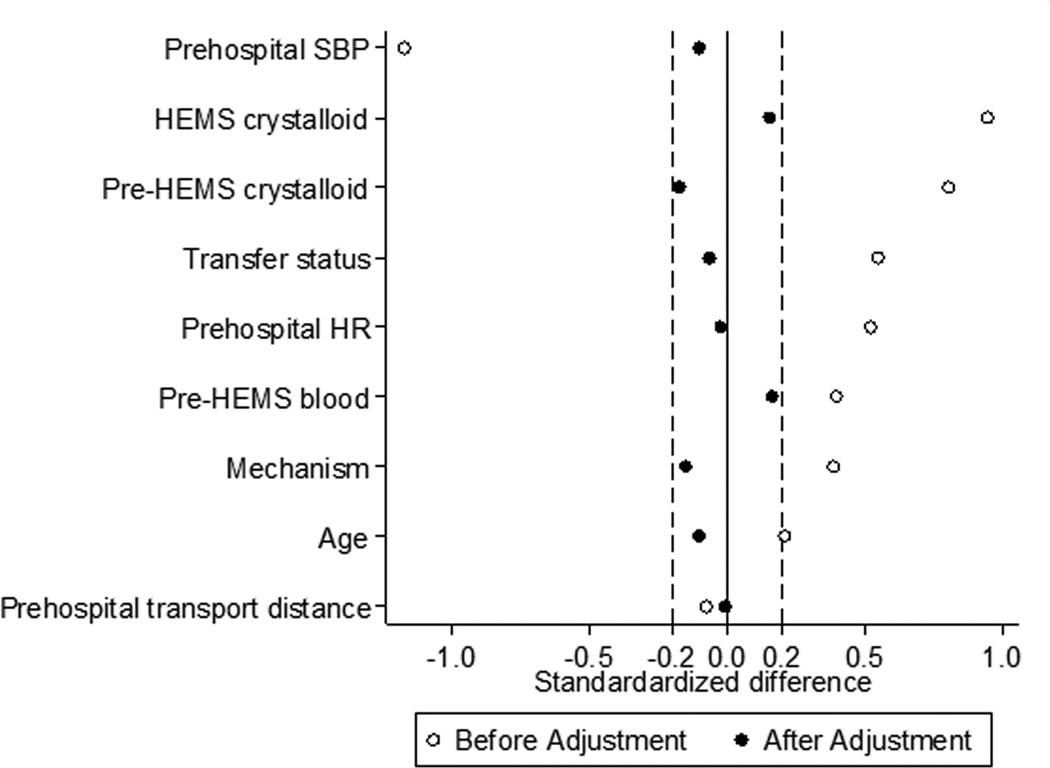
There were 8,616 air medical trauma patients identified (Figure 1), including 255 patients receiving PTC RBC transfusion during HEMS transport. During propensity score matching, 240 PTC RBC patients were matched to 480 control patients, giving 720 matched treatment and control patients for analysis. After matching, no variable included in the estimation of the propensity score remained unbalanced with an absolute value of the standardized difference >0.2 (Figure 2), and overall standardized bias was reduced from 63.8% to 6.3%. The propensity score model C-statistic was 0.915 indicating excellent discrimination, and the Pearson goodness-of-fit test was non-significant (p=0.99) indicating adequate data fit.
Figure 1.
Study participant selection and propensity score matching.
Figure 2.
Standardized differences for variables included in the propensity score estimation before and after the matching procedure for all air medical trauma patients. HEMS, helicopter emergency medical services; HR, heart rate; SBP, systolic blood pressure.
Table 2 summarizes and compares characteristics of treated and control patients. PTC RBC patients had higher admission INR, ISS, TMPM predicted mortality, and more emergent operations. Both groups exhibited signs of prehospital shock with hypotension and tachycardia. Time at the referring facility for transferred patients was similar between groups. Of transferred patients receiving PTC RBC, 44% received PTC RBC transfusion only from HEMS while the remaining 56% received RBC transfusion from both the referring facility and HEMS. PTC RBC patients had lower unadjusted 24-hour survival with higher unadjusted shock on admission and TIC. Unadjusted 24-hour RBC and plasma requirements were similar. For safety outcomes, ARDS was similar between groups (p=0.61). No transfusion reactions occurred during PTC RBC transfusion. Only one transfusion reaction overall occurred, which was in the PTC RBC group (0.4%) during an in-hospital RBC transfusion with no long-term sequelae.
Table 2.
Demographics, Injury Characteristics, and Outcomes in Pre-Trauma Center Red Blood Cell and No Pre-Trauma Center Red Blood Cell Groups
| Characteristic | PTC RBC n = 240 |
No PTC RBC n = 480 |
p Value |
SD* |
|---|---|---|---|---|
| Age, y, median (IQR) | 49 (28, 71.5) | 49 (31, 68) | 0.59 | −0.102 |
| Sex, male, n (%) | 165 (69) | 321 (67) | 0.61 | - |
| Race, n (%) | 0.02 | - | ||
| White | 211 (88) | 441 (92) | - | - |
| Black | 29 (12) | 30 (6) | - | - |
| Asian | 0 (0) | 6 (1) | - | - |
| Other | 0 (0) | 3 (1) | - | - |
| Transferred, n (%) | 163 (68) | 75 | 0.04 | −0.065 |
| Time at referring facility, min, median (IQR)† | 131 (76, 193) | 139 (76, 236) | 0.35 | - |
| Mechanism of injury, n (%) | 0.38 | −0.151 | ||
| Blunt | 191 (80) | 395 (82) | - | - |
| Penetrating | 49 (20) | 85 (18) | - | - |
| Prehospital transport time, min, median (IQR) | 23 (17, 40) | 23 (17, 38) | 0.99 | - |
| Transport distance, miles, median (IQR) | 35.4 (23.5, 64.9) | 36.5 (23.4, 67.1) | 0.72 | −0.008 |
| Prehospital SBP, mmHg, median (IQR) | 84 (66, 106) | 88 (73, 109) | 0.13 | −0.101 |
| Prehospital HR, beats/min, median (IQR) | 114 (93.5, 136) | 113 (96, 131) | 0.81 | −0.023 |
| Prehospital GCS, median (IQR) | 13 (3, 15) | 14 (3, 15) | 0.26 | - |
| Pre-HEMS crystalloid, mL, median (IQR) | 800 (200, 2000) | 1000 (200, 1950) | 0.50 | −0.175 |
| Pre-HEMS RBC, mL, median (IQR) | 0 (0, 150) | 0 (0, 0) | 0.01 | 0.166 |
| HEMS crystalloid, mL, median (IQR) | 500 (100, 1000) | 400 (100, 1000) | 0.31 | 0.154 |
| HEMS RBC, mL, median (IQR) | 300 (200, 500) | - | - | - |
| Prehospital lactate, mmol/L, median (IQR)‡ | 4.0 (2.3, 6.7) | 2.6 (1.7, 5.0) | <0.01 | - |
| Admission SBP, mmHg, median (IQR) | 106 (80, 132) | 110 (91, 130) | 0.07 | - |
| Admission INR, median (IQR) | 1.4 (1.2, 1.9) | 1.2 (1.1, 1.6) | <0.01 | - |
| Admission hemoglobin, g/dL, median (IQR) | 11.5 (9.8, 13.1) | 12.0 (9.9 , 13.4) | 0.19 | - |
| Injury severity score, median (IQR) | 18 (10, 29) | 17 (9, 27) | 0.05 | - |
| TMPM probability of death, median (IQR) | 0.12 (0.04, 0.34) | 0.11 (0.02, 0.31) | 0.05 | - |
| Shock on admission, n (%) | 139 (58) | 226 (47) | <0.01 | - |
| 24-hour RBC, U, median (IQR) | 5 (2, 11) | 4 (2, 9) | 0.06 | - |
| Emergent operation, n (%)§ | 84 (35) | 86 (18) | <0.01 | - |
| 24-h Plasma, U, median, (IQR) | 5 (3, 10) | 4 (3, 10) | 0.98 | - |
| 24-h Platelets, U, median, (IQR) | 1 (1, 3) | 1 (1, 2) | 0.01 | - |
| TIC, n (%) | 113 (47) | 149 (31) | <0.01 | - |
| ARDS, n (%) | 5 (2) | 14 (3) | 0.61 | - |
| 24-h Survival, n (%) | 187 (78) | 394 (82) | 0.16 | - |
| In-hospital survival, n (%) | 166 (69) | 365 (76) | 0.03 | - |
Represents the standardized difference between groups after matching for variables used in propensity score estimation. Differences in the absolute value for the standardized difference >0.2 are considered imbalance between groups after matching. Values only reported for variables in the propensity score; - indicates no value calculated.
Time in transfer patients only.
Prehospital lactate measurement began in 2009; n = 113 in PTC RBC group, n = 251 in No PTC RBC group
Includes emergent abdominal, thoracic, or vascular operations.
PTC RBC, Pre-trauma center red blood cells; SD, Standardized difference; SBP,Systolic blood pressure; HR, Heart rate; GCS, Glasgow Coma Scale; HEMS, Helicopter emergency medical services; INR, International normalized ratio; TMPM, Trauma mortality prediction model; TIC, Trauma induced coagulopathy; ARDS, Acute respiratory distress syndrome.
After adjustment, PTC RBC was independently associated with a nearly 5-fold increase in odds of 24-hour survival (Table 3). PTC RBC was also independently associated with a 72% reduction in the odds of shock on admission (Table 3). PTC RBC was independently associated with a more than 3unit lower 24-hour RBC transfusion volume (Coef −3.6 RBC units; 95%CI −7.0, −0.2, p=0.04). PTC RBC transfusion was not associated with TIC (p=0.17) or in-hospital survival (p=0.90).
Table 3.
Adjusted Odds Ratios from Conditional Logistic Regression for Pre-Trauma Center Red Blood Cells Transfusion in Primary and Secondary Outcomes among All Helicopter Emergency Medical Services Patients and Helicopter Emergency Medical Services Patients Transported from the Scene of Injury
| AOR for PTC RBC | 95%CI | p Value |
|
|---|---|---|---|
| All HEMS | |||
| 24-h survival | 4.91 | 1.51, 16.04 | 0.01 |
| Shock on admission | 0.28 | 0.09, 0.85 | 0.03 |
| TIC | 1.39 | 0.87, 2.24 | 0.17 |
| In-hospital survival | 1.06 | 0.42, 2.61 | 0.90 |
| Scene HEMS | |||
| 24-h survival | 6.31 | 1.88, 21.14 | <0.01 |
| Shock on admission | 0.24 | 0.07, 0.80 | 0.02 |
| TIC | 2.02 | 0.53, 7.71 | 0.30 |
| In-hospital survival | 4.32 | 0.76, 24.72 | 0.10 |
PTC RBC, Pre-trauma center red blood cells; HEMS, Helicopter emergency medical services; AOR, Adjusted odds ratio; 95%CI, 95% Confidence interval; TIC, Trauma induced coagulopathy.
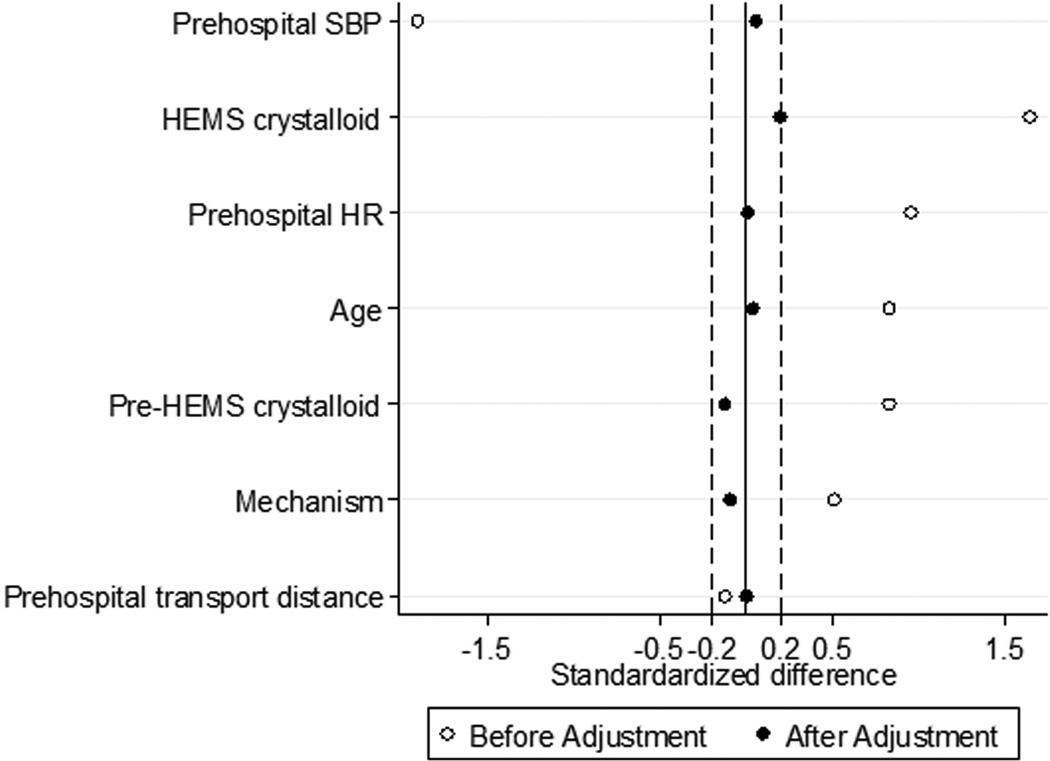
There were 4,977 air medical trauma patients transported from the scene of injury, including 80 patients receiving PTC RBC transfusion during HEMS transport. During propensity score matching, 71 PTC RBC patients were matched to 142 control patients, giving 213 matched treatment and control patients for analysis. After matching, no variable included in the estimation of the propensity score remained unbalanced with an absolute value of the standardized difference >0.2 (Figure 3), and overall standardized bias was reduced from 92.1% to 9.4%. The propensity score model C-statistic was 0.959 indicating excellent discrimination, and the Pearson goodness-of-fit test was non-significant (p=1.00) indicating adequate data fit.
Figure 3.
Standardized differences for variables included in the propensity score estimation before and after the matching procedure for air medical trauma patients transported from the scene of injury. HEMS, helicopter emergency medical services; HR, heart rate; SBP, systolic blood pressure.
PTC RBC scene patients were more likely to be male, had a higher admission INR, and more likely to undergo emergent operation (Table 4). Again, both groups exhibited signs of prehospital shock with hypotension and tachycardia. Unadjusted 24-hour survival as well as shock on admission, 24-hour RBC and plasma transfusion volume, and TIC were similar between groups. For safety outcomes, ARDS was again similar between groups (p=0.07). No transfusion reactions occurred in patients transported from the scene.
Table 4.
Demographics, Injury Characteristics, and Outcomes in Pre-Trauma Center Red Blood Cells and No Pre-Trauma Center Red Blood Cells Groups for Scene Air Medical Transport
| PTC RBC n = 71 |
No PTC RBC n = 142 |
p Value |
SD* | |
|---|---|---|---|---|
| Age, y, median (IQR) | 42 (24, 55) | 37 (25, 65) | 0.77 | 0.038 |
| Sex, male, n (%) | 59 (83) | 89 (68) | 0.02 | - |
| Race, n (%) | 0.18 | - | ||
| White | 62 (87) | 131 (92) | - | - |
| Black | 9 (13) | 9 (6) | - | - |
| Asian | 0 (0) | 0 (0) | - | - |
| Other | 0 (0) | 3 (2) | - | - |
| Mechanism of injury, n (%) | 0.67 | -0.094 | ||
| Blunt | 51 (72) | 98 (69) | - | - |
| Penetrating | 20 (28) | 44 (31) | - | - |
| Prehospital transport time, min, median (IQR) |
19 (15, 23) | 20 (16, 24) | 0.33 | - |
| Transport distance, miles, median (IQR) | 34.2 (22.3, 39.4) |
33.2 (26.8, 40.8) | 0.50 | 0.001 |
| Prehospital SBP, mmHg, median (IQR) | 72 (60, 91) | 80 (60, 92) | 0.47 | 0.054 |
| Prehospital HR, beats/min, median (IQR) | 131 (104, 146) | 120 (100, 140) | 0.27 | 0.009 |
| Prehospital GCS, median (IQR) | 13 (3, 15) | 12 (3, 15) | 0.95 | - |
| Pre-HEMS crystalloid, mL, median (IQR) | 500 (100, 1200) | 500 (100, 1000) | 0.95 | −0.125 |
| HEMS crystalloid, mL, median (IQR) | 1000 (500, 1800) |
1000 (500, 1500) |
0.91 | 0.193 |
| HEMS RBC, mL, median (IQR) | 300 (200, 500) | - | - | - |
| Prehospital lactate, mmol/L, median (IQR)† | 6.1 (4.7, 6.6) | 4.1 (2.7, 6.6) | 0.02 | - |
| Admission SBP, mmHg, median (IQR) | 82 (60, 92) | 104 (81, 126) | 0.01 | - |
| Admission INR, median (IQR) | 1.5 (1.2, 2.0) | 1.3 (1.2, 1.6) | 0.04 | - |
| Admission hemoglobin, g/dL, median (IQR) | 11.4 (10.1, 12.9) |
11.35 (9.75, 12.7) |
0.27 | - |
| Injury severity score, median (IQR) | 22 (10, 34) | 22 (13, 29) | 0.90 | - |
| TMPM probability of death, median (IQR) | 0.19 (0.05, 0.39) |
0.18 (0.07, 0.36) | 0.53 | - |
| Shock on admission, n (%) | 51 (72) | 99 (70) | 0.74 | - |
| Emergent operation, n (%)‡ | 34 (48) | 40 (28) | <0.01 | - |
| 24-h RBC, U, median (IQR) | 8 (2, 18) | 9 (3, 13) | 0.66 | - |
| 24-h Plasma, U, median (IQR) | 8 (4, 14) | 9 (5, 12) | 0.96 | - |
| 24-h Platelets, U, median (IQR) | 2 (1, 6) | 2 (1, 3) | 0.35 | - |
| TIC, n (%) | 37 (52) | 51 (36) | 0.06 | - |
| ARDS, n (%) | 3 (4) | 1 (1) | 0.07 | - |
| 24-h survival, n (%) | 48 (68) | 105 (74) | 0.33 | - |
| In-hospital survival, n (%) | 45 (63) | 94 (66) | 0.68 | - |
Represents the standardized difference between groups after matching for variables used in propensity score estimation. Differences in the absolute value for the standardized difference >0.2 are considered imbalance between groups after matching. Values only reported for variables in the propensity score; - indicates no value calculated.
Prehospital lactate measurement began in 2009; n = 37 in PTC RBC group, n = 77 in No PTC RBC group.
Includes emergent abdominal, thoracic, or vascular operations.
PTC RBC, Pre-trauma center red blood cells; SD, Standardized difference; SBP, Systolic blood pressure; HR, Heart rate; GCS, Glasgow Coma Scale; HEMS, Helicopter emergency medical services; INR, International normalized ratio; TMPM, Trauma mortality prediction model; TIC, Trauma induced coagulopathy; ARDS, Acute respiratory distress syndrome.
In patients transported by HEMS from the scene, PTC RBC was independently associated with a more than 6-fold increase in odds of 24-hour survival (Table 3). PTC RBC was also independently associated with a 76% reduction in the odds of shock on admission (Table 3). PTC RBC was independently associated with a more than 4unit lower 24-hour RBC transfusion volume (Coef −4.5 RBC units; 95%CI −8.3, −0.7, p=0.02). PTC RBC transfusion again was not associated with TIC (p=0.30). PTC RBC exhibited a trend towards an association with increased odds of in-hospital survival; however this did not reach statistical significance (Table 3; p=0.10).
DISCUSSION
This is the largest study to date examining outcomes associated with PTC RBC transfusion in civilian trauma patients. The current data demonstrate that PTC RBC transfusion is strongly associated with an increase in the odds of 24-hour survival among severely injured air medical trauma patients after accounting for confounding with propensity score matching methods. Additionally, PTC RBC transfusion is associated with less physiologic shock on admission, as evidenced by base deficit and lactate, and a lower 24-hour RBC transfusion requirement. PTC RBC transfusion was not associated with TIC or in-hospital survival in this study; however there was a trend towards improved in-hospital survival in scene patients receiving PTC RBC transfusion.
In terms of safety, no increase in ARDS was seen with PTC RBC transfusion, and no prehospital transfusion reactions occurred during study, suggesting PTC RBC transfusion is safe in a well-established HEMS transfusion program. Although transfusion is not without risk, most studies demonstrating increased organ failure or ARDS are in the setting of massive transfusion, whereas the volume of blood product that can be given in the prehospital phase is limited.(8, 27) Patients in this study received approximately 1 unit of PTC RBC which was associated with a lower overall transfusion requirement in the first 24 hours. This may actually reduce the chance of requiring massive transfusion that carries the risk of adverse outcomes. Thus, the benefit likely outweighs the risks of PTC RBC transfusion.
These findings were present in all air medical patients, as well as the subgroup of patients transported from the scene of injury. These results are similar to growing evidence examining prehospital blood product transfusion. The increase in probability of 24-hour survival likely represents improved physiologic status on admission, evidenced by less shock in the treatment group. RBC transfusion serves both as a volume expander and increases oxygen carrying capacity that is critical to cell metabolism during hypoperfusion. It has become increasingly evident that the earliest events in the post-injury inflammatory cascade can have profound detrimental effects, and intervening as early as possible in this cascade may improve outcomes.(7, 28–31) Prior practice calling for 2L of rapid crystalloid infusion in trauma has been shown to be associated with these inflammatory disturbances, resulting in systemic acquired coagulopathy with hemodilution, hypothermia, and hyperchloremic acidosis.(32) Prehospital crystalloid has also been associated with hyperfibrinolysis and increased mortality, leading to recommendations for a more balanced resuscitation limiting crystalloid in favor of early blood product administration.(11, 33, 34)
This evidence and internal review of our own program has led to more aggressive use of PTC RBC transfusion by our air medical providers over time, who frequently contact medical command for RBC transfusion orders early in trauma patients with evidence of ongoing hemorrhage. The initial protocol originally called for 2L of crystalloid and was revised to only 1L of crystalloid including any volume received prior to the air medical provider's arrival. Based on the cohort represented in the current study results, we believe patients with obvious shock as evidenced by significant hypotension, tachycardia, and more recently elevated lactate following injury would be better served with immediate initiation of RBC transfusion rather than giving crystalloid first. We are reviewing our current protocol for possible revision to allow immediate initiation of RBC transfusion by air medical providers with these signs of shock in trauma patients.
The military has pioneered the forward use of prehospital RBC transfusion. Both US and UK medevac platforms have implemented prehospital RBC transfusion capabilities for casualties at the point of wounding.(18, 19) Survival for these advanced platforms including prehospital RBC transfusion is greater than predicted.(35) An evaluation of advanced en-route prehospital capabilities demonstrated a 37% reduction in 30-day mortality among casualties with ISS>15.(20) In this study one-third of patients with ISS>15 received prehospital RBC transfusion. A recent review of the UK military experience matched 97 combat casualties who received prehospital blood products to control patients prior to the availability of prehospital transfusion, demonstrating an 11% lower in mortality in casualties receiving prehospital RBC and plasma transfusions.(21) This encouraging military experience has stimulated interest in the civilian arena.
Civilian evidence of effectiveness for prehospital RBC transfusion is less developed. Early studies demonstrated utilization of PTC RBC in a HEMS program required careful monitoring and defined arrangements with a blood bank, but overall was feasible and safe.(15, 16) As this practice became more common in HEMS programs, small studies emerged evaluating outcomes in trauma patients receiving PTC RBC transfusion. Sumida compared 17 patients receiving PTC RBC with 31 patients receiving more than 2L of crystalloid only.(36) The authors found no difference in unadjusted outcomes despite longer prehospital time and higher base deficit in the PTC RBC group.
Our group reviewed the experience with PTC RBC transfusion in the severely injury Glue Grant Cohort.(22) In that study we found a reduction in 24-hour mortality, 30-day mortality, and TIC among patient receiving RBC transfusion within 2hours prior to arriving at a trauma center. This study was limited by small numbers (50 patients in treatment group, 26 scene transport patients), inability to determine other blood products transfused, and inability to determine timing of RBC transfusion (at referring facility versus during transport). Further, we could not determine transport mode, and it was unclear what role HEMS may have played in the survival benefits given previous evidence supporting HEMS in trauma.(37–39) Given the encouraging results of this prior study despite the limitations, we undertook the current study with a larger and better defined population. In restricting the study population to air medical patients, we were able to ensure a consistent level of prehospital care while isolating the effects of RBC transfusion received specifically during transport, confirming significant early benefits of PTC RBC transfusion in air medical trauma patients.
Two groups have examined the effect of PTC RBC and plasma transfusion in HEMS programs. Kim and colleagues compared 9 patients receiving PTC RBC and plasma transfusion with 50 patients receiving only PTC RBC.(40) They noted improved coagulation status on admission and plasma:RBC ratios closer to 1:1 in the first 24-hours, but no differences in mortality. More recently, Holcomb et al reviewed their experience with prehospital RBC and plasma transfusion in 137 trauma patients.(23) The authors reported prehospital transfusion was associated with less shock on admission, decreased 24-hour blood requirements, and improved survival over the first 6-hours in patients requiring ICU admission, emergent operation, or who died in the ED. Importantly, this group also documented less than 2% of blood product was lost to expiration among nearly 1,000 units.
The current results support prior findings with improved admission physiology and lower 24-hour transfusion requirements. Our finding of improved 24-hour survival is not universal among previous work; however many prior studies looking at survival were small or did not adjust for confounding variables. Additionally, as no randomized trial exists, all studies are comparing prehospital RBC patients that are more severely injured with higher risk of death than those not receiving prehospital RBC transfusion. Our use of propensity score matching may account for the improved survival seen in our study. Propensity score matching is a more robust method than multivariable regression adjustment, reducing potential bias and confounding by matching treated and control patients based on their likelihood of being exposed to the treatment taking into account observed variables that would be expected to influence treatment assignment.(41)
We did not see improvements in TIC or longer term survival as in our prior study.(22) Although these findings were unexpected in that study, we postulated the attenuated endothelial activation associated with RBC transfusion coupled with lower crystalloid administration may reduce inflammation and coagulopathy through these mechanisms.(31) In turn less TIC and inflammation may mediate reduced 30 day mortality. However other factors in the prior study may have contributed to these observations. In addition to the limitations noted above, we had no information on plasma that may have been given prior to arrival at the trauma center and could not exclude patients that received plasma prior to trauma center arrival as done in this study. The cohort in the prior study was more severely injured with a median ISS 15–20 points higher which may influence differences in TIC and longer term survival. Finally, our prior study evaluated patients enrolled in the Inflammation and the Host Response to Injury Collaborative which included highly protocolized care and prospective collection of in-hospital data. These factors were not present in the current study and likely have some influence in assessing competing risks on mortality during hospitalization and evaluating in-hospital survival.
Damage control resuscitation emphasizing early blood product resuscitation has become standard of care in both military and civilian hospital settings.(6, 42, 43) The patients represented in the current study are very similar to the severely injured populations that have been shown to benefit from early high ratios of plasma to blood transfusion. Studies evaluating high ratios of plasma to RBC in-hospital report effect sizes ranging from 2.6 to 7.6 increased odds of survival, and the current results are within this range.(1, 5, 6, 8, 44) Thus, extending this concept into prehospital care is compelling, as the addition of plasma to PTC RBC transfusion may provide further opportunity to correct early coagulopathy and optimize prehospital resuscitation. This may portend even greater outcome benefits in patients in traumatic shock similar to those studied here.(23) The availability of prehospital blood products is increasing and as a result, the Department of Defense is funding 3 separate multicenter randomized trials to evaluate outcomes for the use of prehospital plasma transfusion in both air medical and ground transport trauma patients.(45–47) The multicenter Prehospital Air Medical Plasma (PAMPer) trial, coordinated by our group, will offer a unique opportunity to investigate the effects of PTC RBC and plasma. Of the 6 participating sites, 3 provide PTC RBC transfusion while 3 do not, allowing us to explore the effect of PTC plasma with and without PTC RBC transfusion to determine any additive or synergistic effects. The promising results of the current and prior studies of prehospital RBC transfusion, combined with the outcomes of these pending trials may usher damage control resuscitation to the forefront of prehospital trauma care.
These results may appear to be at odds with the current practice of permissive hypotension in the prehospital phase until definitive hemorrhage control is achieved, particularly in penetrating trauma.(17, 48) This concept, however, has evolved during a period in which the only prehospital resuscitation fluid available was crystalloid. Crystalloid itself has been shown to have many deleterious metabolic and inflammatory effects, and this may have contributed to the poor outcomes associated with large volumes of crystalloid.(4, 49, 50) A more contemporary analysis suggest judicious use of prehospital crystalloid may improve outcomes, particularly if not raising the blood pressure into the normal range.(12) Further evidence has shown that correction of hypotension in severely injured blunt trauma patients without pushing the blood pressure to normal levels may have some benefit.(11) In the current study the SBP was higher on admission than in the field, which may have restored or maintained perfusion without returning to normotensive levels that exacerbate hemorrhage. This is particularly evident in scene patients receiving PTC RBC.
The decisions of which patient and when to initiate blood transfusion in the field are not clear cut. Given the cohort studied here, those that appear to benefit from PTC RBC are patients with obvious shock demonstrated by profound hypotension (SBP ≤80mmHg, particularly in scene patients) and tachycardia (HR >110bpm) at least once during transport. More recently prehospital point of care lactate have become available and elevations >4mmol/L are characteristic of this cohort benefiting from PTC RBC. Thus these measures would likely be useful as criteria to identify patients who are most likely to benefit from this intervention.
This study has several limitations. First are those inherent to a retrospective design. As noted, observational data are subject to selection bias in treatment groups; however we used propensity score matching to mitigate this bias. Despite this, only observed confounders can be utilized in the propensity score and unmeasured confounding may remain. Second, this is a single center study utilizing a single HEMS program and the outcomes may not generalize to other settings with different prehospital and trauma system characteristics. We were limited to using INR to define TIC, as thromboelastography on admission was not routinely performed, but represents a better measure of TIC.(51) Potential survival bias is possible, as patients must survive long enough to receive a PTC RBC transfusion; however we were able to capture all patients for which STAT MedEvac was activated. Further, treatment and control cohorts were well matched, providing a similar likelihood to undergo PTC RBC transfusion. Missing data were present but minimal, and sensitivity analysis comparing imputation and complete cases were very similar. Finally, we focused on early outcomes rather than longer term survival or health-related quality of life outcomes, as it is difficult to establish associations between prehospital interventions and in-hospital or post-discharge outcomes given the presence of competing risks during admission, particularly in a retrospective study.
CONCLUSION
After propensity score matching, PTC RBC was independently associated with an increased probability of 24-hour survival, decreased risk of shock on admission, and lower 24-hour RBC transfusion requirement in severely injured air medical trauma patients with evidence of prehospital shock. These improved early outcomes were also present in patients transported from the scene of injury who truly received a prehospital RBC transfusion. These data further support the use of PTC RBC transfusion in severely injured air medical trauma patients. Prospective study of this issue is warranted as blood products become more readily available for prehospital resuscitation, and may lead to improved outcomes in the severely injured patient in shock.
ACKNOWLEDGMENT
Darrell J Triulzi, MD from the Institute for Transfusion Medicine and Division of Transfusion Medicine, Department of Pathology, University of Pittsburgh assisted with obtaining transfusion data for this study; Matthew D Neal, MD from the Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh assisted with obtaining transfusion data for this study.
Support: Dr Brown receives support from an institutional T32 Ruth L Kischstein National Research Service Award training grant (5T32GM008516-20) from the National Institutes of Health. Dr Sperry receives support from a career development award (K23GM093032) from the National Institute of General Medical Sciences.
ABBREVIATIONS
- PTC
pre-trauma center
- RBC
red blood cells
- HEMS
helicopter emergency medical services
- TIC
trauma induced coagulopathy
- ARDS
acute respiratory distress syndrome
- ICU
intensive care unit
- ED
emergency department
- INR
international normalized ratio
- SBP
systolic blood pressure
- HR
heart rate
- GCS
Glasgow Coma Scale
- TMPM
Trauma Mortality Prediction Model
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose
Presented at the National Association of Emergency Medical Service Physicians Annual Meeting, New Orleans, LA, January 2015.
No financial compensation was received by either Dr. Triulzi or Dr. Neal for their contributions.
REFERENCES
- 1.Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahbar E, Fox EE, del Junco DJ, et al. Early resuscitation intensity as a surrogate for bleeding severity and early mortality in the PROMMTT study. J Trauma Acute Care Surg. 2013;75:S16–S23. doi: 10.1097/TA.0b013e31828fa535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA. The use of lactated ringer's in shock resuscitation: the good, the bad and the ugly. J Trauma. 2011;70:S15–S16. doi: 10.1097/TA.0b013e31821a4d6e. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber MA. The use of normal saline for resuscitation in trauma. J Trauma. 2011;70:S13–S14. doi: 10.1097/TA.0b013e31821a4ba5. [DOI] [PubMed] [Google Scholar]
- 5.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 6.Brown JB, Cohen MJ, Minei JP, et al. Debunking the survival bias myth: characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J Trauma Acute Care Surg. 2012;73:358–364. doi: 10.1097/TA.0b013e31825889ba. discussion 364. [DOI] [PubMed] [Google Scholar]
- 7.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 8.Sperry JL, Ochoa JB, Gunn SR, et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 9.Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182. doi: 10.1097/TA.0b013e31816c5c80. discussion 1182–1183. [DOI] [PubMed] [Google Scholar]
- 10.Holcomb JB, Fox EE, Wade CE, Group PS. The PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study. J Trauma Acute Care Surg. 2013;75:S1–S2. doi: 10.1097/TA.0b013e3182983876. [DOI] [PubMed] [Google Scholar]
- 11.Brown JB, Cohen MJ, Minei JP, et al. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg. 2013;74:1207–1212. doi: 10.1097/TA.0b013e31828c44fd. discussion 12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampton DA, Fabricant LJ, Differding J, et al. Prehospital intravenous fluid is associated with increased survival in trauma patients. J Trauma Acute Care Surg. 2013;75:S9–S15. doi: 10.1097/TA.0b013e318290cd52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasotakis G, Sideris A, Yang Y, et al. Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant database. J Trauma Acute Care Surg. 2013;74:1215–1221. doi: 10.1097/TA.0b013e3182826e13. discussion 21-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Advanced Trauma Life Support. 8 ed. Chicago, IL: American College of Surgeons; 2008. [Google Scholar]
- 15.Berns KS, Zietlow SP. Blood usage in rotor-wing transport. Air Med J. 1998;17:105–108. doi: 10.1016/s1067-991x(98)90104-3. [DOI] [PubMed] [Google Scholar]
- 16.Higgins GL, 3rd, Baumann MR, Kendall KM, et al. Red blood cell transfusion: experience in a rural aeromedical transport service. Prehosp Disaster Med. 2012;27:231–234. doi: 10.1017/S1049023X12000659. [DOI] [PubMed] [Google Scholar]
- 17.Cotton BA, Jerome R, Collier BR, et al. Guidelines for Prehospital Fluid Resuscitation in the Injured Patient. J Trauma. 2009;67:389–402. doi: 10.1097/TA.0b013e3181a8b26f. [DOI] [PubMed] [Google Scholar]
- 18.Eastridge BJ, Mabry RL, Seguin P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73:S431–S437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 19.Malsby RF, 3rd, Quesada J, Powell-Dunford N, et al. Prehospital blood product transfusion by U.S. army MEDEVAC during combat operations in Afghanistan: a process improvement initiative. Mil Med. 2013;178:785–791. doi: 10.7205/MILMED-D-13-00047. [DOI] [PubMed] [Google Scholar]
- 20.Morrison JJ, Oh J, DuBose JJ, et al. En-route care capability from point of injury impacts mortality after severe wartime injury. Ann Surg. 2013;257:330–334. doi: 10.1097/SLA.0b013e31827eefcf. [DOI] [PubMed] [Google Scholar]
- 21.O'Reilly DJ, Morrison JJ, Jansen JO, et al. Prehospital blood transfusion in the en route management of severe combat trauma: A matched cohort study. J Trauma Acute Care Surg. 2014;77:S114–S120. doi: 10.1097/TA.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 22.Brown JB, Cohen MJ, Minei JP, et al. Pre-trauma center red blood cell transfusion is associated with reduced mortality and coagulopathy in severely injured blunt trauma patients. Ann Surg. 2014 Mar 25; doi: 10.1097/SLA.0000000000000674. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holcomb JB, Donathan DP, Cotton BA, et al. Prehospital Transfusion of Plasma and Red Blood Cells in Trauma Patients. Prehosp Emerg Care. 2014;19:1–9. doi: 10.3109/10903127.2014.923077. [DOI] [PubMed] [Google Scholar]
- 24.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 1987. [Google Scholar]
- 25.Yang D, JE D. [Accessed March 29, 2014];A unified approach to measuring the effect size between two groups using SAS®. Available at: http://support.sas.com/resources/papers/proceedings12/335-2012.pdf.
- 26.Glance LG, Osler TM, Mukamel DB, et al. TMPM-ICD9: a trauma mortality prediction model based on ICD-9-CM codes. Ann Surg. 2009;249:1032–1039. doi: 10.1097/SLA.0b013e3181a38f28. [DOI] [PubMed] [Google Scholar]
- 27.Watson GA, Sperry JL, Rosengart MR, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:221–227. doi: 10.1097/TA.0b013e3181ad5957. discussion 8–30. [DOI] [PubMed] [Google Scholar]
- 28.Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247:320–326. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 29.Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon's perspective. Hematology Am Soc Hematol Educ Program. 2013;2013:656–659. doi: 10.1182/asheducation-2013.1.656. [DOI] [PubMed] [Google Scholar]
- 30.Moore EE, Chin TL, Chapman MC, et al. Plasma First in the Field for Postinjury Hemorrhagic Shock. Shock. 2013;41:S35–S38. doi: 10.1097/SHK.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urner M, Herrmann IK, Buddeberg F, et al. Effects of Blood Products on Inflammatory Response in Endothelial Cells In Vitro. PLoS ONE. 2012;7:e33403. doi: 10.1371/journal.pone.0033403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen MJ, West M. Acute Traumatic Coagulopathy: From Endogenous Acute Coagulopathy to Systemic Acquired Coagulopathy and Back. The Journal of Trauma and Acute Care Surgery. 2011;70:S47–S49. doi: 10.1097/TA.0b013e31821a5c24. [DOI] [PubMed] [Google Scholar]
- 33.Advanced Trauma Life Support. 9 ed. Chicago, IL: American College of Surgeons; 2012. [Google Scholar]
- 34.Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73:365–370. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 35.Apodaca A, Olson CM, Jr, Bailey J, et al. Performance improvement evaluation of forward aeromedical evacuation platforms in Operation Enduring Freedom. J Trauma Acute Care Surg. 2013;75:S157–S163. doi: 10.1097/TA.0b013e318299da3e. [DOI] [PubMed] [Google Scholar]
- 36.Sumida MP, Quinn K, Lewis PL, et al. Prehospital blood transfusion versus crystalloid alone in the aire medical transport of trauma patients. Air Med J. 2000;19:140–143. doi: 10.1016/s1067-991x(00)90007-5. [DOI] [PubMed] [Google Scholar]
- 37.Brown JB, Stassen NA, Bankey PE, et al. Helicopters and the civilian trauma system: national utilization patterns demonstrate improved outcomes after traumatic injury. J Trauma. 2010;69:1030–1034. doi: 10.1097/TA.0b013e3181f6f450. discussion 4–6. [DOI] [PubMed] [Google Scholar]
- 38.Brown JB, Stassen NA, Bankey PE, et al. Helicopters improve survival in seriously injured patients requiring interfacility transfer for definitive care. J Trauma. 2011;70:310–314. doi: 10.1097/TA.0b013e3182032b4f. [DOI] [PubMed] [Google Scholar]
- 39.Galvagno SM, Jr, Haut ER, Zafar SN, et al. Association between helicopter vs ground emergency medical services and survival for adults with major trauma. JAMA. 2012;307:1602–1610. doi: 10.1001/jama.2012.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim BD, Zielinski MD, Jenkins DH, et al. The effects of prehospital plasma on patients with injury: a prehospital plasma resuscitation. J Trauma Acute Care Surg. 2012;73:S49–S53. doi: 10.1097/TA.0b013e31826060ff. [DOI] [PubMed] [Google Scholar]
- 41.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 42.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 43.Joint Theater Trauma System Clinical Practice Guideline. [Accessed March 13, 2014];Damage control resuscitation at level IIb/III treatment facilities. Available at: http://www.usaisr.amedd.army.mil/assets/cpgs/Damage%20Control%20Resuscitation%20-%201%20Feb%202013.pdf.
- 44.Holcomb JB, Wade CE, Michalek JE, et al. Increased Plasma and Platelet to Red Blood Cell Ratios Improves Outcome in 466 Massively Transfused Civilian Trauma Patients. Annals of Surgery. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 45.Prehospital Air Medical Plasma Trial (PAMPer) [Accessed July 13, 2014];Prehospital Air Medical Plasma Trial (PAMPer) Available at: http://clinicaltrials.gov/show/NCT01818427.
- 46.Control of Major Bleeding After Trauma Study (COMBAT) [Accessed July 21, 2014];Control of Major Bleeding After Trauma Study (COMBAT) Available at: http://clinicaltrials.gov/show/NCT01838863.
- 47. [Accessed July 14, 2014];Prehospital Use of Plasma for Traumatic Hemorrhage (PUPTH) Study. Available at: http://www.cctr.vcu.edu/news/feature/plasma.html.
- 48.Bickell WH, Wall MJ, Pepe PE, et al. Immediate versus Delayed Fluid Resuscitation for Hypotensive Patients with Penetrating Torso Injuries. New England Journal of Medicine. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 49.Watters JM, Brundage SI, Todd SR, et al. Resuscitation with Lactated Ringer???S Does Not Increase Inflammatory Response in a Swine Model of Uncontrolled Hemorrhagic Shock. Shock. 2004;22:283–287. doi: 10.1097/01.shk.0000135288.54535.8a. [DOI] [PubMed] [Google Scholar]
- 50.Watters JM, Tieu BH, Todd SR, et al. Fluid resuscitation increases inflammatory gene transcription after traumatic injury. J Trauma. 2006;61:300–308. doi: 10.1097/01.ta.0000224211.36154.44. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 51.Holcomb JB, Minei KM, Scerbo ML, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256:476–486. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]