Abstract
Mounting evidence suggests that individuals with schizophrenia have an underlying vulnerability to cardiovascular disease, and a recent study suggested that this vulnerability might be reflected in the retinal microvasculature. The purpose of this study was to test the hypothesis that the retinal microvessels reflect familial vulnerability to psychotic symptoms. Participants were 531 adolescent and young adult twins who took part in the Brisbane Longitudinal Twin Study and the Twins Eye Study in Tasmania. The twins had photographs taken of their retina when they were adolescents or young adults (M age=20.6 years), and retinal vessel diameter was assessed using computer software. The twins completed an assessment of psychosis symptoms approximately six years later. We compared retinal venular diameters of individuals with one or more symptoms of psychosis (n=45), their unaffected co-twins (n=24), and controls (n=462). Individuals with one or more symptoms of psychosis had wider venules (standardized mean=0.29) than controls (standardized mean=-0.04; p=.03), and unaffected co-twins had venular diameters that were intermediate (standardized mean=0.13) between the two groups, suggesting that wide venules may represent a proxy marker of familial vulnerability to psychosis symptoms. Consistent with previous work, there were no differences in arteriolar diameter between individuals with and without symptoms of psychosis. Findings suggest that wide retinal venules may serve as a proxy marker of familial liability to psychosis symptoms. The pathophysiological mechanisms linking psychosis and cardiovascular disease may be operative from early in life, possibly at the level of the microvasculature.
Keywords: Psychosis, retinal vessel diameter, twin
1. Introduction
Individuals diagnosed with schizophrenia are disproportionally affected by cardiovascular and cerebrovascular diseases (Bresee et al., 2010; Carney et al., 2006; Crump et al., 2013; Fan et al., 2013; Hennekens et al., 2005; Lahti et al., 2012; Lin et al., 2008), and their elevated rates of these diseases cannot be fully explained by lifestyle factors, such as smoking or use of antipsychotic medication (Fan et al., 2013; Osborn et al., 2007). Mounting evidence suggests that individuals with schizophrenia may have an underlying liability to cardiovascular disease (Andreassen et al., 2013; Correll et al., 2014; Kohen, 2004; Ryan et al., 2003; Spelman et al., 2007), and a recent study suggested that this liability might be reflected in the retinal microvasculature (Meier et al., 2013).
The retinal microvasculature offers a unique opportunity to visualize the microcirculation in vivo. The condition of the retinal microvessels can used to gauge the condition of the microvessels in the heart and brain, because the microvasculatures of the retina, heart, and brain are similar (Cheung et al., 2012; Patton et al., 2005). Prior research has shown that the retinal microvessels are sensitive to a range of cardiovascular risks (Ikram et al., 2012; Sun et al., 2009b), and wider retinal venules (veins) predict risk of coronary heart disease (McGeechan et al., 2009a; Wong et al., 2006b), stroke (Cheung et al., 2013; McGeechan et al., 2009b; Wong et al., 2006b; Yatsuya et al., 2010), cognitive impairment (Shalev et al., 2013), and dementia (de Jong et al., 2011) independently of other cardiovascular risks.
In an initial study of a population-representative cohort from New Zealand (the Dunedin Study), adults diagnosed with schizophrenia were distinguished from other cohort members by wider retinal venules, even after accounting for other cardiovascular risks (high blood pressure, pre-diabetes/diabetes, persistent tobacco dependence) (Meier et al., 2013). Further, adults with symptoms of psychosis and cohort children who exhibited symptoms of psychosis but did not develop schizophrenia had wider venules as adults (Meier et al., 2013), suggesting that wide retinal venules may be related to vulnerability to schizophrenia rather than the disease process itself (Malaspina, 2013). Based on these initial findings, we hypothesized that 1) wide venules may be apparent among adolescents and young adults who experience symptoms of psychosis, and 2) wide venules may be apparent among the unaffected relatives of adolescents and young adults with symptoms of psychosis. We tested these hypotheses in a sample of adolescent and young adult twins.
2. Materials and Methods
2.1. Participants
Participants were members of the Brisbane Longitudinal Twin Study, an ongoing study of adolescent and young-adult monozygotic (MZ) and dizygotic (DZ) twin pairs and their siblings (Gillespie et al., 2013; Wright and Martin, 2004). As described in detail elsewhere (Gillespie et al., 2013; Wright and Martin, 2004), twins were initially recruited to the study from primary and secondary schools in South East Queensland in 1992, with new twins added at various intervals. All schools in South East Queensland were approached, and all regions in South East Queensland were represented. Comparisons of this sample with other population-based samples suggest that this sample is representative with respect to a variety of traits, including, for example, personality (de Moor et al., 2012) and retinal vessel diameter (Ikram et al., 2010) (Mitchell et al., 2007).
The twins have undergone a variety of phenotypic assessments, including, most recently (and ongoing), an assessment of psychosis symptoms. A subset of participants also completed an extensive eye examination as part of the Twins Eye Study in Tasmania (Mackey et al., 2009; Sun et al., 2009c). Here we report on 531 participants for whom both eye exam and psychosis symptom data were available. These 531 participants included n=45 individuals with one or more symptoms of psychosis (probands), n=24 unaffected co-twins of the probands, and n=462 controls (participants with no symptoms of psychosis). Demographic information for these participants is presented in Table 1. The study was approved by the ethics committees of the Royal Victorian Eye and Ear Hospital, the Royal Hobart Hospital, and the QIMR Berghofer Medical Research Institute, as well as the Australian Twin Registry, and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all of the participants or their legal guardians, with the participants' assent before examination.
Table 1.
Means (standard deviations) on demographic variables and health risks for controls, unaffected co-twins, and probands.
| Controls N=462 | Unaffected Co-Twins N=24 | Probands N=45 | |
|---|---|---|---|
|
|
|||
| Sex (% Female) | 61 | 58 | 58 |
| Age at Eye Exam | 20.74 (3.56) | 19.67 (3.00) | 19.91 (3.09) |
| Age at Psychosis Symptom Assessment | 26.70 (3.53) | 26.13 (3.59) | 26.04 (3.27) |
| Smoker (%) | 16 | 25 | 27 |
| Body Mass Index | 22.51 (3.63) | 22.54 (4.06) | 23.43 (4.55) |
Note. All pairwise comparisons between groups were non-significant.
2.2. Measures
2.2.1. Psychosis Symptoms
Participants reported on six lifetime symptoms of psychosis during either a computer-assisted telephone interview or an online survey using the same items (Gillespie et al., 2013). Symptoms were taken from the modified Composite International Diagnostic Interview (CIDI) (Kessler et al., 2005). Several studies of community samples have shown that CIDI-assessed psychosis symptoms correlate with demographic factors and psychiatric symptoms in expected ways (Scott et al., 2006; Scott et al., 2008; van Os et al., 2001; Varghese et al., 2011). In our sample, CIDI-assessed psychosis symptoms were associated with mental health problems (r=0.19, p<.001), assessed with the Somatic and Psychological Health Report (Hickie et al., 2001a; Hickie et al., 2001b), and smoking (F=4.19, p=.040), similar to other measures of psychotic experiences given in community-based samples (Kelleher and Cannon, 2011; Kelleher et al., 2012). Table 2 shows the prevalence of each psychosis symptom, and the prevalence of each symptom matched the prevalence in the larger study from which participants were drawn. Because of the rarity of some of the symptoms, we collapsed the responses into a single categorical variable reflecting the presence versus absence of symptoms.
Table 2.
Prevalence of psychosis symptoms among adolescents and young adults (n=531).
| Symptom | N | % |
|---|---|---|
| Saw a vision that other people could not see. | 26 | 5 |
| Heard voices that other people could not hear. | 19 | 4 |
| Experienced mind control (thought insertion or extraction). | 3 | .6 |
| Felt that mind was being taken over by strange forces. | 3 | .6 |
| Experienced attempts at communication from strange forces. | 5 | 1 |
| Believed there was a plot to harm you. | 5 | 1 |
Note. ‘0’ symptoms: n=486; ‘1’ symptom: n=33; 2+ symptoms: n=12. The prevalence of each psychosis symptom matched the prevalence in the larger study from which participants were drawn.
2.2.2. Retinal vessel diameter
As previously described (Sun et al., 2009a; Sun et al., 2009c), all of the twins had 10° stereoscopic optic disc–centered photographs using a Nidek 3-Dx/F fundus camera (Nidek) after dilatation of the pupils with tropicamide 1% or cyclopentolate 1%. Photographs were digitalized, and retinal vascular diameter was measured with computer-assisted software (IVAN, University of Wisconsin, USA) according to a standardized protocol (Wong et al., 2004). Two trained graders, masked to participant characteristics, performed the vessel measurements on the optic disc–centered image for both eyes for all of the participants. The largest 6 arterioles and venules coursing through a zone between half to 1 disc diameter from the optic disc margin were measured. As in the Dunedin Study (Meier et al., 2013), estimates were summarized as the central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE), representing the average diameter of arterioles and venules of the eye, respectively, using a revised Knudtson-Parr-Hubbard formula (Knudtson et al., 2003). Intragrader variation was assessed in 67 randomly selected retinal photographs. The intragrader intraclass correlation coefficient was 0.95 for CRAE and 0.99 for CRVE. Intergrader reliability was assessed in 52 randomly selected retinal images, and the interclass correlation coefficient was 0.93 for CRAE and 0.98 for CRVE. The correlation between CRVE and CRAE was 0.51 (Sun et al., 2009c). Mean CRAE and CRVE values for the n=531 that we report on here were 163.88 μm (SD=12.50) and 249.69 μm (SD=17.55), respectively. These means match published means for the larger study from which participants were drawn (Sun et al., 2009c).
2.2.3. Covariates
Smoking status was included as a covariate, as smoking is associated with both wider venular diameter and psychosis symptoms (Degenhardt et al., 2001; Ikram et al., 2012; Kelleher and Cannon, 2011; Meier et al., 2014; Morgan et al., 2014; Sun et al., 2009b). Body mass index was considered as a covariate because higher body mass index is associated with wider venular diameter (Ikram et al., 2012; Sun et al., 2009b) and is seen in psychosis (Foley et al., 2013; Morgan et al., 2014). Smoking status and body mass index were each assessed at the time of the eye exam.
2.3. Statistical Analysis
We tested whether (a) probands had wider venules than controls and (b) unaffected co-twins had venular diameters intermediate between probands and controls. We also tested whether, as in the Dunedin Study (Meier et al., 2013), psychosis symptoms were unrelated to arteriolar diameter. Prior to analyses, we adjusted vessel diameter for the effects of age, sex, axial length, and sphericity, because vessel diameter varies depending on these factors (Sun et al., 2009b). Vessel diameter was then standardized (M=0, SD=1). To account for participants' familial relatedness (the non-independence of observations), robust standard errors were computed using the SURVEYREG procedure in SAS (SAS Institute, Inc., Cary, NC, USA). This procedure downwardly adjusts the degrees of freedom based on the number of twin pairs in the analysis, taking into account that information obtained about one twin is related to information obtained about the co-twin.
3. Results
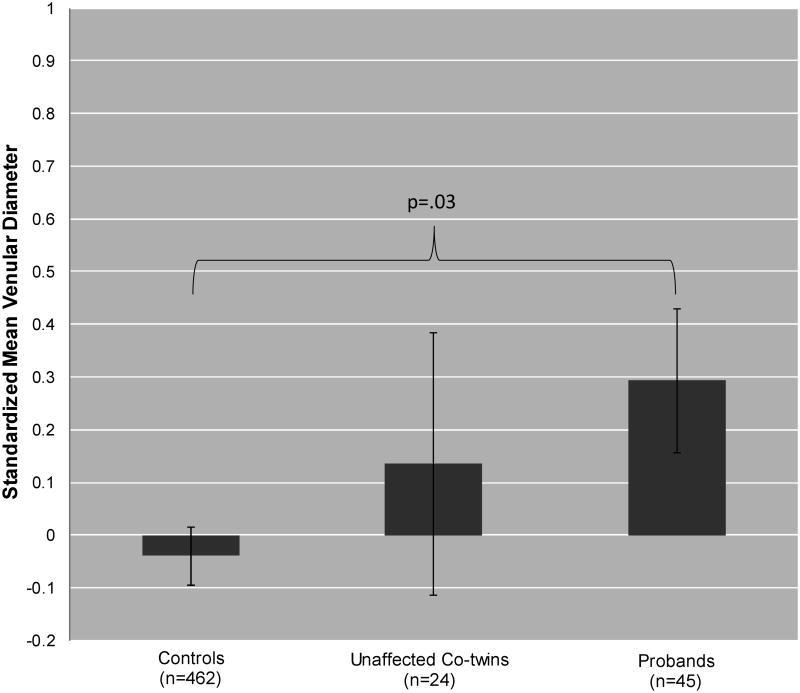
Figure 1 shows mean venular diameter for controls, unaffected co-twins, and probands Means were adjusted for age, sex, axial length, and sphericity and standardized. The standardized scores reflect effect sizes for how different each group is from the sample mean and from each other group. Effect sizes of 0.20, 0.50, and 0.80 reflect small, medium, and large effects, respectively (Cohen, 1992). Consistent with hypotheses, probands had wider venules (M=0.29) than controls (M=-0.04) (t=2.25, p=.03), and unaffected co-twins had venular diameters that were intermediate between probands and controls (M=0.13; linear trend t=2.16, p=.03).
Figure 1.
Standardized Mean Venular Diameter for Controls, Unaffected Co-twins, and Probands. Error bars=robust standard errors. Means were adjusted for age, sex, axial length, and sphericity and standardized. These standardized means translate to units of micrometers (μm) as follows: 248.99 (controls), 252.06 (unaffected co-twins), and 254.78 (probands).
Figure 2 shows the distribution of venular diameter (adjusted for age, sex, axial length, and sphericity and standardized) in each group. This figure shows that the distribution was shifted to the right in probands and unaffected co-twins. That is, 62% of probands and 67% of unaffected co-twins had venular diameters that were wider than the sample mean (Z=0.00), compared with 45% of controls. Results of a non-parametric test, which does not rely on outliers, showed that, compared with controls, a greater proportion of probands (χ2=4.61, p=.03) and unaffected co-twins (χ2=4.15, p=.04) had wider than average venules.
Figure 2.
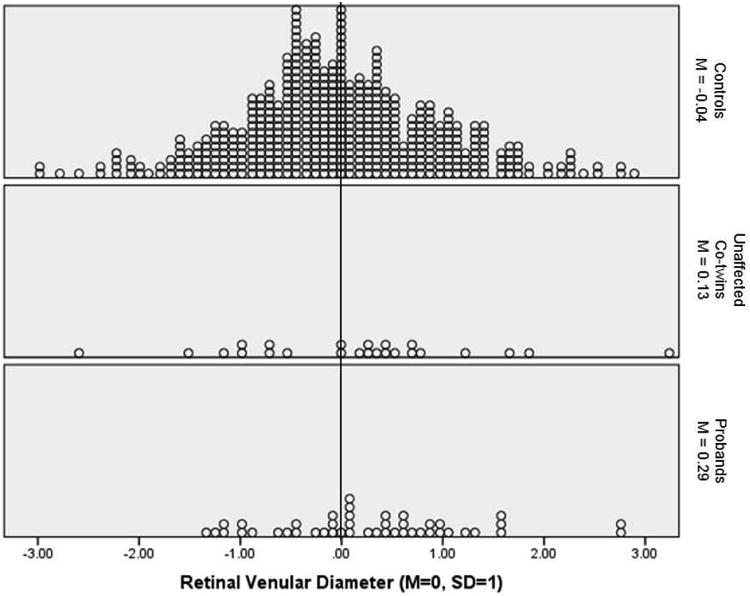
Distibution of retinal venular diameter in controls, unaffected-cotwins, and probands. Venular diameter was adjusted for age, sex, axial length, and sphericity and then standardized to M=0, SD=1 in the full sample. The vertical line shows the sample mean of 0.00. A greater proportion of probands (62%) and unaffected co-twins (67%) had wider than average venules compared with controls (45%).
We repeated analyses, controlling for smoking status, as there was some indication that smoking, but not body mass index, was elevated among probands and unaffected co-twins (Table 1). Results were virtually unchanged after controlling for smoking status. Smoking-adjusted means for controls, unaffected co-twins, and probands were -0.03, 0.12, 0.27, respectively. The difference in venular diameter between probands and controls remained statistically significant (t=2.15, p = .03), and the linear trend demonstrating that unaffected co-twins had venular diameters intermediate between probands and controls also remained statistically significant (t=2.06, p=.04).
There were no differences in arteriolar diameter between probands (M=0.11), unaffected co-twins (M=-0.02), and controls (M=-0.01), consistent with the previous report of no association between psychosis symptoms and arteriolar diameter (Meier et al., 2013).
4. Discussion
Here we demonstrate that adolescent and young adult twins with one or more symptoms of psychosis, and their unaffected co-twins, have wide retinal venules. Findings are consistent with the recent Dunedin Study report that adults with symptoms of psychosis and adults diagnosed with schizophrenia have wide retinal venules (Meier et al., 2013). Our findings advance knowledge in two main ways. First, they show that the novel Dunedin Study finding is reproducible; replication is recognized as the gold standard in biomarker research and is especially important given the poor track record of biomarker research in psychiatry. The current study was conducted by an almost entirely independent team of investigators using a different research design in a sample from another country, suggesting that the finding of wide venules in individuals with symptoms of psychosis may be robust. We know of no other attempts to replicate this very recent finding.
Second, our findings suggest that wide venules are related to familial liability to psychosis symptoms: A greater proportion of probands and unaffected co-twins had wider than average venules compared with controls, and unaffected co-twins had venular diameters that were intermediate between probands and controls. Thus, an intriguing possibility is that wide venules reflect susceptibility to psychosis symptoms as opposed to a consequence of psychosis symptoms. This finding bolsters the implication from the Dunedin Study that wide venules are associated with vulnerability to schizophrenia, as cohort children who exhibited symptoms of psychosis but did not develop schizophrenia had wider venules as adults (Malaspina, 2013; Meier et al., 2013).
An understanding of the mechanisms underlying wider venules might provide insight into the pathogenesis of psychosis symptoms. Genetic factors influence venular diameter (Fahy et al., 2011; Ikram et al., 2010; Xing et al., 2006), indicating that some people are genetically predisposed to having wider venules. Inflammation, endothelial dysfunction, and hypoxia have also been found to be associated with wider venules, in addition to traditional cardiovascular risks, including obesity, impaired fasting glucose, dyslipidemia, and smoking (De Jong et al., 2008; Ikram et al., 2012; Klein et al., 2006; Nguyen et al., 2010; Sun et al., 2009b; Wong et al., 2006a). All of these factors have been implicated in schizophrenia (Barnett et al., 2007; Israel et al., 2011; Kirkpatrick and Miller, 2013; Mitchell et al., 2013; Ryan et al., 2003; Schmidt-Kastner et al., 2012), and some of these factors occur at higher than expected rates in unaffected relatives of patients with schizophrenia (Lyons et al., 2002; Martinez-Gras et al., 2012; Mukherjee et al., 1989; Nunes et al., 2006; Spelman et al., 2007) and in individuals with symptoms of psychosis (Degenhardt et al., 2001; Foley et al., 2013; Kelleher and Cannon, 2011).
An interesting question is whether wide venules in individuals with symptoms of psychosis and their unaffected relatives simply reflect the cumulative effects of these risks, or whether wide venules might be causally related to psychosis symptoms. We showed that the association between wider venules and psychosis symptoms was independent of smoking and body mass index in this young and otherwise healthy sample. It is unlikely that blood pressure could explain the wide venules in the probands and unaffected co-twins, because higher blood pressure is most consistently associated with narrower arterioles (Ikram et al., 2012; Sun et al., 2009b), and there was no evidence of narrower arterioles in probands or unaffected co-twins. Concerns about the confounding effects of other cardiovascular risks are alleviated somewhat by the youth and health of the sample, but subclinical levels of risks, such as inflammation (Khandaker et al., 2014), should still be considered. We note that whether or not wide venules are causally related to psychosis symptoms, they might be especially useful indicators of cardiovascular risk in otherwise healthy adolescents and young adults with symptoms of psychosis, as conventional cardiovascular risk factors are difficult to detect in these populations.
Another question is whether wider retinal venules are specific to individuals with psychosis symptoms. In the Dunedin Study, individuals diagnosed with schizophrenia had wider venules than medical and psychiatric (i.e., persistent depression) comparison groups (Meier et al., 2013). In our sample of young adult twins, depression and anxiety symptoms were unrelated to venular diameter (Meier et al., 2014). Thus, initial findings suggest that wider venules may be specific to individuals with symptoms of psychosis, but further investigation is warranted.
This study should be interpreted in the context of limitations. First, it is possible that our measure of psychosis symptoms may not have captured ‘true’ or severe psychotic experiences. However, it is now clear that subclinical psychotic symptoms are common in the general population (Scott et al., 2006; van Os et al., 2009), and subclinical and clinical psychotic experiences vary along a continuum (Linscott and van Os, 2013; van Os et al., 2009). Moreover, etiological factors appear to be similar across this continuum (Kelleher and Cannon, 2011; van Os et al., 2009). Second, because of the small number of twin pairs discordant for psychosis symptoms, we had to combine discordant MZ and DZ pairs, precluding us from determining whether shared genetic factors or shared environmental factors account for the wide venules in the unaffected co-twins. Future follow-ups of our twins may allow us to parse genetic and environmental influences or, complementarily, to test the association between polygenic risk for schizophrenia and retinal venular diameter.
This study has a number of implications. First, as wide retinal venules predict cardiovascular and cerebrovascular diseases (Cheung et al., 2013; McGeechan et al., 2009a; McGeechan et al., 2009b; Wong et al., 2006b; Yatsuya et al., 2010), our findings add to the growing evidence base suggesting that individuals with psychosis may have an underlying vulnerability to these diseases (Andreassen et al., 2013; Kohen, 2004; Ryan et al., 2003; Spelman et al., 2007). Early intervention to improve cardiovascular health in individuals with symptoms of psychosis may be warranted. Second, we detected this vulnerability fairly early in life, attesting to the potential utility of retinal imaging for identifying cardiovascular risk in young people with symptoms of psychosis. Retinal venular diameter might be considered for addition to cardiovascular risk prediction models for individuals with psychosis (Osborn et al., 2014). Third, research on retinal venular diameter may help to clarify the pathophysiology of psychosis symptoms. Wide retinal venules may represent an endophenotype for psychosis and schizophrenia (Gottesman and Gould, 2003); wide venuels are heritable (Fahy et al., 2011; Sun et al., 2009c; Taarnhoj et al., 2006) and are apparent in individuals with symptoms of psychosis and their unaffected relatives, as well as in individuals with schizophrenia (Meier et al., 2013). Thus, genetic research on retinal venular diameter could potentially aid in the discovery of variants associated with psychosis and schizophrenia (Glahn et al., 2014).
Acknowledgments
The authors would like to thank Jane MacKinnon, Shayne Brown, Lisa Kearns, Jonathan Ruddle, Paul Sanfilippo, Fleur O'Hare, Sandra Staffieri, Johan Poulsen, Justin Sherwin, Robert Macmillan, Byoung Sung Chu, Katherine Smallcombe, Olivia Bigault, Colleen Wilkinson, Julie Barbour, Robin Wilkinson, Rachael Adams, Robyn Troutbeck, Jonathan Yeoh, Ya Ling Ma, Trent Roydhouse, Lindsey Scotter, Katarina Creese, Vischal Jhanji, Sonya Bennett, Christine Chen, Ann Eldridge, Marlene Grace, Yingfeng Zheng, Jian Zhang, Mingguang He and Amy Cohn for helping examine twins. In addition, we appreciate the assistance in recruiting twins from Thanuja Gunasekera, Allison McKenzie, Anne-Louise Ponsonby, Terry Dwyer, James Dilger, Palma Ragno, Jenny Boadle, Kim Dorrell, Shyamali Dharmage, John Hopper, Christopher Hammond, Terri Young and Jamie Craig.
Role of the Funding Source: This work was supported by grants from the Australian Research Council (ARC); the National Health & Medical Research Council (NHMRC); Beyond Blue, Australia; and by the United States National Institute on Drug Abuse (R00DA023549). We also thank the following organizations for their financial support: Clifford Craig Medical Research Trust, Ophthalmic Research Institute of Australia (ORIA), American Health Assistance Foundation (AHAF), Peggy and Leslie Cranbourne Foundation, Foundation for Children, National Health and Medical Research Foundation Project Grant (2005–2007), NEI Project Grant (2007–2010).
Footnotes
Conflicts of Interest: There are no conflicts of interest to report.
Contributors. M.H. Meier., N.A. Gillespie, N.G. Martin, and D.A. Mackey designed research. All authors performed research. M.H. Meier analyzed the data. All authors wrote the paper and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreassen OA, Djurovic S, Thompson WK, Schork AJ, Kendler KS, O'Donovan MC, Rujescu D, Werge T, van de Bunt M, Morris AP, McCarthy MI, Roddey JC, McEvoy LK, Desikan RS, Dale AM. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. American Journal of Human Genetics. 2013;92(2):197–209. doi: 10.1016/j.ajhg.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AH, Mackin P, Chaudhury I, Farooqi A, Gadsby R, Heald A, Hill J, Millar H, Peveler R, Rees A, Singh V, Taylor D, Vora J, Jones PB. Minimising metabolic and cardiovascular risk in schizophrenia: diabetes, obesity and dyslipidaemia. J Psychopharmacol. 2007;21(4):357–373. doi: 10.1177/0269881107075509. [DOI] [PubMed] [Google Scholar]
- Bresee LC, Majumdar SR, Patten SB, Johnson JA. Prevalence of cardiovascular risk factors and disease in people with schizophrenia: A population-based study. Schizophr Res. 2010;117(1):75–82. doi: 10.1016/j.schres.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: A population-based controlled study. J Gen Intern Med. 2006;21(11):1133–1137. doi: 10.1111/j.1525-1497.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CYL, Ikram MK, Sabanayagam C, Wong TY. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension. 2012;60(5):1094–+. doi: 10.1161/HYPERTENSIONAHA.111.189142. [DOI] [PubMed] [Google Scholar]
- Cheung CYL, Tay WT, Ikram MK, Ong YT, De Silva DA, Chow KY, Wong TY. Retinal microvascular changes and risk of stroke: the Singapore Malay Eye Study. Stroke. 2013;44(9):2402–2408. doi: 10.1161/STROKEAHA.113.001738. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, Marcy P, Addington J, Estroff SE, Robinson J, Penn DL, Azrin S, Goldstein A, Severe J, Heinssen R, Kane JM. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: Baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71(12):1350–1363. doi: 10.1001/jamapsychiatry.2014.1314. [DOI] [PubMed] [Google Scholar]
- Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiat. 2013;170(3):324–333. doi: 10.1176/appi.ajp.2012.12050599. [DOI] [PubMed] [Google Scholar]
- de Jong FJ, Schrijvers EMC, Ikram MK, Koudstaal PJ, de Jong PTVM, Hofman A, Vingerling JR, Breteler MMB. Retinal vascular caliber and risk of dementia: the Rotterdam Study. Neurology. 2011;76(9):816–821. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong FJ, Vernooij MW, Ikram MK, Ikram MA, Hofman A, Krestin GP, Van der Lugt A, De Jong PTVM, Breteler MMB. Arteriolar oxygen saturation, cerebral blood flow, and retinal vessel diameters - The Rotterdam Study. Ophthalmology. 2008;115(5):887–892. doi: 10.1016/j.ophtha.2007.06.036. [DOI] [PubMed] [Google Scholar]
- de Moor MHM, Costa PT, Terracciano A, Krueger RF, de Geus EJC, Toshiko T, Penninx BWJH, Esko T, Madden PAF, Derringer J, Amin N, Willemsen G, Hottenga JJ, Distel MA, Uda M, Sanna S, Spinhoven P, Hartman CA, Sullivan P, Realo A, Allik J, Heath AC, Pergadia ML, Agrawal A, Lin P, Grucza R, Nutile T, Ciullo M, Rujescu D, Giegling I, Konte B, Widen E, Cousminer DL, Eriksson JG, Palotie A, Peltonen L, Luciano M, Tenesa A, Davies G, Lopez LM, Hansell NK, Medland SE, Ferrucci L, Schlessinger D, Montgomery GW, Wright MJ, Aulchenko YS, Janssens ACJW, Oostra BA, Metspalu A, Abecasis GR, Deary IJ, Raikkonen K, Bierut LJ, Martin NG, van Duijn CM, Boomsma DI. Meta-analysis of genome-wide association studies for personality. Mol Psychiatr. 2012;17(3):337–349. doi: 10.1038/mp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96(11):1603–1614. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- Fahy SJ, Sun C, Zhu G, Healey PR, Spector TD, Martin NG, Mitchell P, Wong TY, Mackey DA, Hammond CJ, Andrew T. The relationship between retinal arteriolar and venular calibers is genetically mediated, and each is associated with risk of cardiovascular disease. Invest Ophth Vis Sci. 2011;52(2):975–981. doi: 10.1167/iovs.10-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan ZX, Wu YY, Shen J, Ji T, Zhan RY. Schizophrenia and the risk of cardiovascular diseases: A meta-analysis of thirteen cohort studies. J Psychiatr Res. 2013;47(11):1549–1556. doi: 10.1016/j.jpsychires.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Foley DL, Mackinnon A, Watts GF, Shaw JE, Magliano DJ, Castle DJ, McGrath JJ, Waterreus A, Morgan VA, Galletly CA. Cardiometabolic risk indicators that distinguish adults with psychosis from the general population, by age and gender. Plos One. 2013;8(12):e82606. doi: 10.1371/journal.pone.0082606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Henders AK, Davenport TA, Hermens DF, Wright MJ, Martin NG, Hickie IB. The Brisbane Longitudinal Twin Study: Pathways to cannabis use, abuse, and dependence project -- Current status, preliminary results, and future directions. Twin Research and Human Genetics. 2013;16(1):21–33. doi: 10.1017/thg.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Knowles EEM, Mckay DR, Sprooten E, Raventos H, Blangero J, Gottesman II, Almasy L. Arguments for the sake of endophenotypes: Examining common misconceptions about the use of endophenotypes in psychiatric genetics. Am J Med Genet B. 2014;165(2):122–130. doi: 10.1002/ajmg.b.32221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiat. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Davenport TA, Hadzi-Pavlovic D, Koschera A, Naismith SL, Scott EM, Wilhelm KA. Development of a simple screening tool for common mental disorders in general practice. Med J Australia. 2001a;175:S10–S17. doi: 10.5694/j.1326-5377.2001.tb143784.x. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Davenport TA, Scott EM, Hadzi-Pavlovic D, Naismith SL, Koschera A. Unmet need for recognition of common mental disorders in Australian general practice. Med J Australia. 2001b;175:S18–S24. doi: 10.5694/j.1326-5377.2001.tb143785.x. [DOI] [PubMed] [Google Scholar]
- Ikram MK, Ong YT, Cheung CY, Wong TY. Retinal vascular caliber measurements: Clinical significance, current knowledge and future perspectives. Ophthalmologica. 2012 doi: 10.1159/000342158. [DOI] [PubMed] [Google Scholar]
- Ikram MK, Xueling S, Jensen RA, Cotch MF, Hewitt AW, Ikram MA, Wang JJ, Klein R, Klein BEK, Breteler MMB, Cheung N, Liew G, Mitchell P, Uitterlinden AG, Rivadeneira F, Hofman A, de Jong PTVM, van Duijn CM, Kao L, Cheng CY, Smith AV, Glazer NL, Lumley T, McKnight B, Psaty BM, Jonasson F, Eiriksdottir G, Aspelund T, Harris TB, Launer LJ, Taylor KD, Li XH, Iyengar SK, Xi QS, Sivakumaran TA, Mackey DA, MacGregor S, Martin NG, Young TL, Bis JC, Wiggins KL, Heckbert SR, Hammond CJ, Andrew T, Fahy S, Attia J, Holliday EG, Scott RJ, Islam FMA, Rotter JI, McAuley AK, Boerwinkle E, Tai ES, Gudnason V, Siscovick DS, Vingerling JR, Wong TY, Consortium GB. Four novel loci (19q13, 6q24, 12q24, and 5q14) influence the microcirculation in vivo. Plos Genet. 2010;6(10) doi: 10.1371/journal.pgen.1001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel AK, Seeck A, Boettger MK, Rachow T, Berger S, Voss A, Bar KJ. Peripheral endothelial dysfunction in patients suffering from acute schizophrenia: A potential marker for cardiovascular morbidity? Schizophr Res. 2011;128(1-3):44–50. doi: 10.1016/j.schres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 2011;41(1):1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Keeley H, Corcoran P, Lynch F, Fitzpatrick C, Devlin N, Molloy C, Roddy S, Clarke MC, Harley M, Arseneault L, Wasserman C, Carli V, Sarchiapone M, Hoven C, Wasserman D, Cannon M. Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Brit J Psychiat. 2012;201(1):26–32. doi: 10.1192/bjp.bp.111.101543. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(7):709–709. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71(10):1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophrenia Bull. 2013;39(6):1174–1179. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BEK, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol-Chic. 2006;124(1):87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BEK. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- Kohen D. Diabetes mellitus and schizophrenia: Historical perspective. Brit J Psychiat. 2004;184:S64–S66. doi: 10.1192/bjp.184.47.s64. [DOI] [PubMed] [Google Scholar]
- Lahti M, Tiihonen J, Wildgust H, Beary M, Hodgson R, Kajantie E, Osmond C, Raikkonen K, Eriksson J. Cardiovascular morbidity, mortality and pharmacotherapy in patients with schizophrenia. Psychol Med. 2012;42(11):2275–2285. doi: 10.1017/S0033291712000396. [DOI] [PubMed] [Google Scholar]
- Lin HC, Hsiao FH, Pfeiffer S, Hwang YT, Lee HC. An increased risk of stroke among young schizophrenia patients. Schizophr Res. 2008;101(1-3):234–241. doi: 10.1016/j.schres.2007.12.485. [DOI] [PubMed] [Google Scholar]
- Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43(6):1133–1149. doi: 10.1017/S0033291712001626. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Kremen WS, Toomey R, Eisen SA, Goldberg J, Faraone SV, Tsuang M. Nicotine and familial vulnerability to schizophrenia: A discordant twin study. J Abnorm Psychol. 2002;111(4):687–693. doi: 10.1037//0021-843x.111.4.687. [DOI] [PubMed] [Google Scholar]
- Mackey DA, MacKinnon JR, Brown SA, Kearns LS, Ruddle JB, Sanfilippo PG, Sun C, Hammond CJ, Young TL, Martin NG, Hewitt AW. Twins Eye Study in Tasmania (TEST): Rationale and methodology to recruit and examine twins. Twin Research and Human Genetics. 2009;12(5):441–454. doi: 10.1375/twin.12.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D. Looking schizophrenia in the eye. Am J Psychiat. 2013;170(12):1382–1384. doi: 10.1176/appi.ajp.2013.13081136. [DOI] [PubMed] [Google Scholar]
- Martinez-Gras I, Garcia-Sanchez F, Guaza C, Rodriguez-Jimenez R, Andres-Esteban E, Palomo T, Rubio G, Borrell J. Altered immune function in unaffected first-degree biological relatives of schizophrenia patients. Psychiatry Res. 2012;200(2-3):1022–1025. doi: 10.1016/j.psychres.2012.05.036. [DOI] [PubMed] [Google Scholar]
- McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, Dejong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P, Wong TY. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Annals of Internal Medicine. 2009a;151(6):404–413. doi: 10.7326/0003-4819-151-6-200909150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BEK, Wang JJ, Mitchell P, Vingerling JR, de Jong PTVM, Witteman JCM, Breteler MMB, Shaw J, Zimmet P, Wong TY. Prediction of incident stroke events based on retinal vessel caliber: A systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009b;170(11):1323–1332. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Gillespie NA, Hansell NK, Hewitt AW, Hickie IB, Lu Y, MacGregor S, Medland SE, Sun C, Wong TY, Wright MJ, Zhu G, Martin NG, Mackey DA. Associations between depression and anxiety symptoms and retinal vessel caliber in adolescents and young adults. Psychosom Med. 2014;76(9):732–738. doi: 10.1097/PSY.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Shalev W, Moffitt TE, Kapur S, Keefe RSE, Wong TY, Belsky DW, Harrington HL, Hogan S, Houts R, Caspi A, Poulton R. Microvascular abnormality in schizophrenia as shown by retinal imaging. Am J Psychiat. 2013;170(12):1451–1459. doi: 10.1176/appi.ajp.2013.13020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu WP, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders-A systematic review and meta-analysis. Schizophrenia Bull. 2013;39(2):306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Cheung N, de Haseth K, Taylor B, Rochtchina E, Islam FMA, Wang JJ, Saw SM, Wong TY. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49(5):1156–1162. doi: 10.1161/HYPERTENSIONAHA.106.085910. [DOI] [PubMed] [Google Scholar]
- Morgan VA, McGrath JJ, Jablensky A, Badcock JC, Waterreus A, Bush R, Carr V, Castle D, Cohen M, Galletly C, Harvey C, Hocking B, McGorry P, Neil AL, Saw S, Shah S, Stain HJ, Mackinnon A. Psychosis prevalence and physical, metabolic and cognitive co-morbidity: data from the second Australian national survey of psychosis. Psychol Med. 2014;44(10):2163–2176. doi: 10.1017/S0033291713002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Schnur DB, Reddy R. Family history of type-2 diabetes in schizophrenic patients. Lancet. 1989;1(8636):495–495. doi: 10.1016/s0140-6736(89)91392-5. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Islam FMA, Farouque HMO, Klein R, Klein BEK, Cotch MF, Herrington DM, Wong TY. Retinal vascular caliber and brachial flow-mediated dilation the multi-ethnic study of atherosclerosis. Stroke. 2010;41(7):1343–1348. doi: 10.1161/STROKEAHA.110.581017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes SOV, Matsuo T, Kaminami MS, Watanabe MAE, Reiche EMV, Itano EN. An autoimmune or an inflammatory process in patients with schizophrenia, schizoaffective disorder, and in their biological relatives. Schizophr Res. 2006;84(1):180–182. doi: 10.1016/j.schres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Osborn DP, Hardoon S, Omar RZ, Holt RI, King M, Larsen J, Marston L, Morris RW, Nazareth I, Walters K, Petersen I. Cardiovascular risk prediction models for people with severe mental illness: Results from the prediction and management of cardiovascular risk in People with Severe Mental Illnesses (PRIMROSE) Research Program. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn DPJ, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom's General Practice Research Database. Arch Gen Psychiatry. 2007;64(2):242–249. doi: 10.1001/archpsyc.64.2.242. [DOI] [PubMed] [Google Scholar]
- Patton N, Aslam T, MacGillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206(4):319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MCM, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiat. 2003;160(2):284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HW, Rutten BP. An environmental analysis of genes associated with schizophrenia: Hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiatry. 2012;17(12):1194–1205. doi: 10.1038/mp.2011.183. [DOI] [PubMed] [Google Scholar]
- Scott J, Chant D, Andrews G, McGrath J. Psychotic-like experiences in the general community: The correlates of CIDI psychosis screen items in an Australian sample. Psychol Med. 2006;36(2):231–238. doi: 10.1017/S0033291705006392. [DOI] [PubMed] [Google Scholar]
- Scott J, Welham J, Martin G, Bor W, Najman J, O'Callaghan M, Williams G, Aird R, McGrath J. Demographic correlates of psychotic-like experiences in young Australian adults. Acta Psychiat Scand. 2008;118(3):230–237. doi: 10.1111/j.1600-0447.2008.01214.x. [DOI] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Wong TY, Meier MH, Houts RM, Ding J, Cheung CY, Ikram MK, Caspi A, Poulton R. Retinal vessel caliber and lifelong neuropsychological functioning: Retinal imaging as an investigative tool for cognitive epidemiology. Psychol Sci. 2013;24(7):1198–1207. doi: 10.1177/0956797612470959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabetic Med. 2007;24(5):481–485. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- Sun C, Ponsonby AL, Wong TY, Brown SA, Kearns LS, Cochrane J, MacKinnon JR, Ruddle JB, Hewitt AW, Liew G, Dwyer T, Scurrah K, Mackey DA. Effect of birth parameters on retinal vascular caliber: The Twins Eye Study in Tasmania. Hypertension. 2009a;53(3):487–493. doi: 10.1161/HYPERTENSIONAHA.108.125914. [DOI] [PubMed] [Google Scholar]
- Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: Systemic, environmental, and genetic associations. Surv Ophthalmol. 2009b;54(1):74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhu G, Wong TY, Hewitt AW, Ruddle JB, Hodgson L, Montgomery GW, Young TL, Hammond CJ, Craig JE, Martin NG, He MG, Mackey DA. Quantitative genetic analysis of the retinal vascular caliber: The Australian Twins Eye Study. Hypertension. 2009c;54(4):788–U200. doi: 10.1161/HYPERTENSIONAHA.109.132902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taarnhoj NCBB, Larsen M, Sander B, Kyvik KO, Kessel L, Hougaard JL, Sorensen TIA. Heritability of retinal vessel diameters and blood pressure: A twin study. Invest Ophth Vis Sci. 2006;47(8):3539–3544. doi: 10.1167/iovs.05-1372. [DOI] [PubMed] [Google Scholar]
- van Os J, Hanssen M, Bijl RV, Vollebergh W. Prevalence of psychotic disorder and community level of psychotic symptoms - An urban-rural comparison. Arch Gen Psychiatry. 2001;58(7):663–668. doi: 10.1001/archpsyc.58.7.663. [DOI] [PubMed] [Google Scholar]
- van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- Varghese D, Scott J, Welham J, Bor W, Najman J, O'Callaghan M, Williams G, McGrath J. Psychotic-like experiences in major depression and anxiety disorders: A population-based survey in young adults. Schizophrenia Bull. 2011;37(2):389–393. doi: 10.1093/schbul/sbp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Islam FMA, Klein R, Klein BEK, Cotch MF, Castro C, Sharrett AR, Shahar E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: The Multi-Ethnic Study of Atherosclerosis (MESA) Invest Ophth Vis Sci. 2006a;47(6):2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, Cushman M, Duncan BB. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons - The Cardiovascular Health Study. Arch Intern Med. 2006b;166(21):2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- Wong TY, Knudtson MD, Klein R, Klein BEK, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the beaver dam eye study -Methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Martin NG. Brisbane Adolescent Twin Study: Outline of study methods and research projects. Aust J Psychol. 2004;56(2):65–78. [Google Scholar]
- Xing C, Klein BEK, Klein R, Jun G, Lee KE, Iyengar SK. Genome-wide linkage study of retinal vessel diameters in the Beaver Dam Eye Study. Hypertension. 2006;47(4):797–802. doi: 10.1161/01.HYP.0000208330.68355.72. [DOI] [PubMed] [Google Scholar]
- Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BEK, Sharrett AR, Investigators AS. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke. 2010;41(7):1349–1355. doi: 10.1161/STROKEAHA.110.580837. [DOI] [PMC free article] [PubMed] [Google Scholar]