Abstract
Background
Pre-hospital resuscitation with crystalloid exacerbates fibrinolysis, which is associated with high mortality. We hypothesize that plasma compared to crystalloid resuscitation prevents hyperfibrinolysis in a tissue plasminogen activator (tPA) rich environment via preservation of proteins essential for regulation of fibrinolysis.
Study Design
Healthy individuals donated blood, which was assayed using a native (non activated) thrombelastography (TEG). Whole-blood (WB) was mixed with normal saline (NS) or platelet poor plasma (PPP) at progressive dilutions. TPA was added to promote a fibrinolytic environment. In a separate experiment PPP was run through 100 KD filter and liquid remaining on top of the filter (TFP) and below the filter (BFP) was obtained. Whole blood was diluted by 50% with TFP, BPF and NS and assayed with tPA TEG challenge. TFP and BFP were assayed for protein concentration and protein composition.
Results
NS and PPP dilution of WB with out tPA did not affect clot lysis at 30 minutes (LY30) (NS Spearman’s Rho 0.300 p=0.186 and PPP 0.294 p=0.288). When tPA was added NS dilution of whole blood increased LY30 in a percentage dependent manner (0.844 p<0.001) but did not significantly increase with PPP dilution (0.270 p=0.202). The difference in LY30 from WB to diluted WB with PPP (mean change −1.05 95% CI −9.42 to 7.33) was similar with TFP (1.23 95%CI −5.20 to 7.66 p=0.992). However, both BPF (37.65 95%CI 24.47 to 50.82 p=0.001) and NS (47.36 95%CI 34.3–60.45 p<0.001) showed large increases in fibrinolysis compared to PPP.
Conclusions
Crystalloid and plasma dilution of whole blood does not increase fibrinolysis. However NS dilution of WB, increases susceptibility to tPA mediated fibrinolysis. Plasma resuscitation, simulated by plasma dilution of whole blood, attenuates increased susceptibility to tPA mediate fibrinolysis. The benefits of plasma resuscitation are mediated through preservation of plasma proteins.
Introduction
The optimal pre hospital resuscitation strategy in trauma patients experiencing hemorrhagic shock remains unclear. Historically, crystalloids were administered to normalize blood pressure in all trauma patients, which was prompted by the Advanced Trauma Life Support (ATLS) guidelines (1). In the early 1990’s a clinical trial suggested that pre-hospital saline increased mortality in patients who were actively bleeding (2). Subsequently, the concept of permissive hypotension was adopted by many centers (3). The perceived beneficial mechanisms of limited pre-hospital crystalloid resuscitation were: 1) limiting the rise in blood pressure would prevent “popping” off blood clots on hemostatic injuries and 2) minimize hemodilution of coagulation factors. These concepts date back to the 1960’s (4). While permissive hypotension, to a degree, may benefit some trauma patients, a more recent analysis indicates that pre hospital resuscitation to correct severe shock is life-saving (5). On the other hand, normal saline (NS) infusion in humans not in shock has been associated with improved hemostasis measured by thrombelastography (TEG)(6), which brings into question the mechanism of saline dilution driving trauma induced coagulopathy (TIC).
Trauma-induced coagulopathy impairs both clot formation and promotes fibrinolysis (7) and the mechanism driving these two aspects of coagulation appear to be separate (8, 9). With the widespread use of TEG during trauma resuscitation, correlation has been observed between the volume of pre hospital crystalloid and increased levels of systemic fibrinolysis (10). Fibrinolysis is part of normal clot remodeling and is important physiologically to keep small vessels patent in a contained vascular bed (11). However, systemic over activation of fibrinolysis (hyperfibrinolysis) in trauma has mortality rates ranging from 40–90%(12–15). In our ongoing experience with pre hospital plasma resuscitation we observed an apparent reversal of hyperfibrinolysis. With mounting evidence that tPA elevation is the driver of post injury hyperfibrinolysis (16), the relationship of saline infusion and the development of hyperfibrinolysis needs to be established. We hypothesize that saline dilution of whole blood does not increase fibrinolysis, but in the presence of tPA, whole blood diluted by saline enhances fibrinolysis predominantly by decreasing the concentration of plasma proteins which contribute to regulation of the fibrinolytic system.
Methods
Subjects
After obtaining informed consent under an institution review board approved protocol (COMIRB # 14-0366) blood samples were obtained from healthy volunteers with no known abnormalities in the coagulation or fibrinolytic system; none of these individuals were taking salicylic acid or other non-steroidal anti-inflammatory medications within 120 hours of the experiment. Six subjects were recruited for each experiment. There were 4 men and 2 women with a median age of 30 years, range 26 – 67 years. The women were not taking oral contraceptive medications and were not pregnant.
Tissue Plasminogen Activator
Human single chain tPA from Molecular Innovation (Novi, MI) was diluted in 5% bovine serum albumin in phosphate buffered saline to a final concentration of 10 microgram per microliter. Individual aliquots of tPA (prepared for a final concentration of 75 ng/ml)were stored at −80 degrees C and thawed immediately prior to use. The 75 ng/ml concentration of of tPA was previously found to be an inflection point for which a citrated native TEG increased fibrinolytic activity compared to a non-tPA-challenged whole blood sample in healthy volunteers (data not shown).
Platelet Poor Plasma
Platelet poor plasma was employed to eliminate the known inhibitory effects of platelets on fibrinolysis. 3.3 ml of Blood was collected in 3% citrated tubes from healthy volunteers and was centrifuged at 6,000 g for 10 minutes at 4 degrees C. Plasma was removed and spun at 12,500 g for 10 minutes at the same temperature to remove contaminating platelets and a cellular debris. PPP was stored on ice (<2 hours), and an aliquot of PPP was flash frozen and stored at −80°C for later proteomic analysis.
Platelet Poor Plasma Filtration
A 100 kDa molecular weight filters were purchased from Sigma Adlrich (Product # Z677906 St. Louis, MO) and used to filter 500 μl of PPP. A 100 kDa cutoff was employed because the majority of SERPINs (serine protease inhibitors) known to impact fibrinolysis are below 100 kDa. PPP was centrifuged at 15,000 g for 45 minutes at 4°C. The liquid that passed through the filter was re-suspended to the original volume of 500 μL with NS. The fluid that did not pass through filter was also re-suspended to the original volume (500 μL) with NS. Aliquots of PPP from the top of the filter (TFP) and below the filter (BFP) were flash frozen in liquid nitrogen and stored at −80 Celsius for later proteomic experiments.
Proteomics
Sample preparation for mass spectrometric analysis
The samples were digested according to the FASP protocol using a 10 kDa molecular weight cutoff filter. In brief, samples were mixed in the filter unit with 8 M (molar) urea in 0.1 M Tris-HCl, pH 8.5 and centrifuged at 14,000 g for 15 min. The proteins were reduced by addition of 100 μL of 10 mM DTT in 8 M urea in 0.1 M Tris-HCl, pH 8.5, incubation for 30 min at RT and the device was centrifuged. Subsequently, 100 μl of 55 mM iodoacetamide in 8 M urea in 0.1 M Tris-HCl, pH 8.5 were added to the samples, incubation for 30 min at RT in dark followed by centrifugation. Afterward, three washing steps with 100 μL of 8 M urea in 0.1 M Tris-HCl, pH 8.5 solution were performed, followed by three washing steps with 100 μL of 50 mM ABC buffer. Proteins were digested with trypsin overnight at 37 °C. Peptides were recovered from the filter using 30% ACN. The volume of the eluted sample was reduced to ~2 μL in a vacuum centrifuge and reconstituted to 100 μL with 0.1% formic acid. Thermo Scientific Pierce 660 nm Protein Assay was used to quantify the total protein concentration in each sample.
Mass Spectrometry
Samples analysis and protein identification was used with a technique previously described.(17)
Macromolecule Replenishment in Normal Saline
There were a large number of proteins identified by proteomic analysis of plasma above and below the filter. A targeted approach for proteins, which were candidates for inhibiting fibrinolysis, was based on previous work using affinity chromatography with tPA and plasminogen as capture proteins (17). In this study platelet lysate, which inhibited fibrinolysis, had fibrinogen subunits and fibronectin that bound to tPA and plasminogen. Therefore these two proteins were likely candidates for fibrinolysis regulation. Purified Human fibrinogen and fibronectin were purchased from sigma Aldrich (Product # F3879 and F1056, St. Louis, MO), and both were solubilized in NS at 5,000 micrograms/ml and 300 micrograms/ml, respectively.
Thrombelastography Tissue Plasminogen Activator Challenge (TEG tPA Challenge)
Blood was collected in 3.3 ml citrated blood tubes from venous puncture. Citrated samples were kept at room temperature and assayed between 20 minutes and 2 hours after blood draw, in accordance with manufacturer recommendations. Whole blood was mixed with normal saline, PPP, and NS to reach a total volume of 500 microliter in individual Eppendorf tubes (Hamburg, Germany). The percent of whole blood replaced with the previously mentioned fluids was at a range of 5–50%. TPA was added to whole blood mixtures to reach a concentration of 75 ng/ml within 5 minutes of running the assay. Citrated native thrombelastography assays were re-calcified and run according to the manufacturer’s instructions on a TEG 5000 Thrombelastograph Hemostasis Analyzer (Haemonetics, Niles IL). The following parameters were recorded from the tracings of the TEG: R time (ACT, seconds), angle (α, degrees), maximum amplitude (MA, mm), and lysis 30 minutes after MA (LY30, %). The same tPA TEG challenge was used with whole blood contrasted to whole blood diluted by 50% with PPP, TFP, BFP and NS. This was also repeated with NS enriched with fibronectin and fibrinogen at a 25% replacement of whole blood. This percent replacement of whole blood with enriched saline was selected as replenishment of fibrinogen at this dilution brought clot strength back to baseline levels. Dilution at 50% with fibrinogen enriched saline were unable to obtain baseline clot strength, while in the previous experiments 50% dilution of WB with PPP did not reduce clot strength.
Statistical Analysis
SPSS software version 22 (IBM, Armonk, NY) was used for statistical analysis. The TEG value LY30 had a skewed, non-normal distribution. Spearman’s Rho was used to assess the correlation of LY30 values to the percent dilution of whole blood (0%, 5%, 25%,50%). Comparisons of the groups WB, WB with tPA, and WB diluted with NS and WB diluted with PPP at the 50% dilution level were performed with the non-parametric paired Friedman’s test with the Bonferroni adjustment for multiple comparisons. Filtration and saline enrich plasma were assessed for the differences in LY30 from whole blood with tPA to whole blood diluted samples. These values had a normal distribution. ANOVA with post hoc Dunnett test assuming unequal variance was used for analysis using PPP as the reference group for filtration experiments, and NS for saline enrichment studies. Protein concentration also had a normal distribution. The above the filter and below filter concentrations were tested for significance with a paired T test. Significance was set at two-tailed alpha of 0.05 for all statistical tests. Spectral counts from mass spectrometry analysis represents a relative concentration of protein. Descriptive statistics were used to summarize these data.
Results
Saline and PPP Dilution of Whole Blood Do Not Active Fibrinolysis
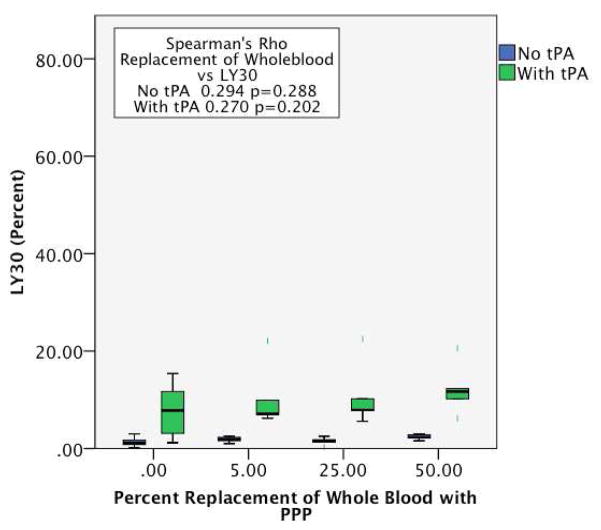
Lysis at 30 minutes was not affected by NS dilution of whole blood (0%, 5%, 25%, or 50%) as measured by TEG (Spearman Rho 0.300, p=0.186, Figure 1). Similarly, PPP dilutions of whole blood did not induce increases in LY30 (Spearman Rho =0.294, p=0.288, Figure 2). The LY30 of undiluted WB (median 1.1%, IQR: 0.35–1.75) whole blood diluted by 50% with NS (1.4%, IQR 0.8–2.3, p=0.314) and with PPP (2.3%, IQR 1.9–2.9, p=0.94) were not significantly different.
Figure 1.
NS dilution of whole blood increases sensitivity to tPA mediated fibrinolysis. The Y axis represents the percent fibrinolysis quantified by LY30 (Amount of blood clot lysed 30 minutes after reaching maximum amplitude). The X axis represents progressive dilution of whole blood with saline with largest dilution on the right. Blue bar, whole blood with no tPA added. Green bar, whole blood mixed with tPA.
Figure 2.
Plasma dilution of whole blood does not alter sensitivity to tPA mediated fibrinolysis. The Y axis represents the percent fibrinolysis quantified by LY30 (Amount of blood clot lysed 30 minutes after reaching maximum amplitude). The X axis represent progressive dilution of whole blood with platelet poor plasma with largest dilution on the right. Blue bar, whole blood with no tPA added. Green bar, whole blood mixed with tPA. PPP, platelet poor plasma.
Saline Dilution of Whole Blood Potentiates tPA Mediated Fibrinolysis
Whole blood with tPA induced a significant increase in LY30 compared to WB without tPA (7.8%, IQR 2.8–12.5, p=0.002). In comparison to unmodified WB, whole blood dilution with NS and added tPA positively correlated with LY30 (Spearman Rho 0.844, P<0.001 Figure 1). Identical PPP dilution of whole blood attenuated the tPA-mediated increase in LY30 seen with NS dilution (Spearman Rho =0.294 p=0.288 figure 2).
Filtration Reduced Protein Concentration and Increases tPA Mediated Fibrinolysis
Filtration of plasma reduced the concentration of plasma protein; the filtrate (BFP) had a mean protein concentration of 1.17 ug/ul, compared to above the filter (AFP) with a mean difference 59.18 ug/ul (95%CI 54.3–61.7 p<0.001). An increase was observed in LY30 from whole blood with tPA compared to 50% dilution using PPP, PAF, PBF, and NS (p<0.001 Figure 3). Post hoc analysis with PPP defined as the reference group showed had similar changes in LY30 compared to PAF (mean difference 2.3 95%CI −11.0 to 15.6 p=0.992), but increased in PBF (mean difference 38.7 95%CI 18.5 to 58.9 p=0.001), and NS (mean difference 48.4 95%CI 28.4 to 68.5 p<0.001).
Figure 3.
Plasma filtration results in increased sensitivity to tPA mediated fibrinolysis. Y axis represents the mean change in LY30 from whole blood to whole blood diluted by 50% of the dilutants listed on the X axis. All groups had the same final concentration of tPA. The plasma group represents the reference group for statically analysis. Plasma, platelet poor plasma. *p<0.05.
Proteomic Analysis of Filtered Plasma
Mass spectrometry analysis of BAF and BPFsmaples identified 263 proteins. The spectral counts provided semi-quantifications to support albumin as the most abundant protein in BAF and BPF. Table 1 lists proteins identified that are known to inhibit tPA or plasmin directly or indirectly. Table 2 lists proteins identified that impact fibrin clot formation and stability.
Table 1.
Inhibitors of tPA Mediated Fibrinolysis Identified by Mass Spectroscopy
| Rank (226) | Identified proteins | Molecular weight | Top filter | Bottom filter |
|---|---|---|---|---|
| 4 | Alpha-2-macroglobulin | 163 kDa | 436 | 7 |
| 16 | Alpha-1-antitrypsin | 47 kDa | 102 | 2 |
| 41 | Plasma protease C1 inhibitor | 55 kDa | 41 | 1 |
| 50 | Vitronectin | 54 kDa | 28 | 0 |
| 54 | Alpha-2-antiplasmin | 55 kDa | 22 | 0 |
| 97 | Plasma serine protease inhibitor | 46 kDa | 5 | 0 |
Proteins identified by mass spectroscopy (n=226) with relative rank based off of spectral count.
Top and bottom filter represent spectral count.
kDA, kilodalton.
Table 2.
Proteins Involved with Fibrin Clot Stabilization Identified by Mass Spectroscopy
| Rank (226) | Identified proteins | Molecular weight | Top filter | Bottom filter |
|---|---|---|---|---|
| 8 | Fibrinogen beta chain | 56 kDa | 248 | 2 |
| 12 | Fibrinogen gamma chain | 52 kDa | 183 | 1 |
| 17 | Fibrinogen alpha chain | 95 kDa | 138 | 1 |
| 23 | Prothrombin | 70 kDa | 60 | 1 |
| 29 | Fibronectin | 263 kDa | 59 | 1 |
| 109 | Coagulation factor XIII B chain | 76 kDa | 5 | 0 |
| 121 | Coagulation factor XIII A chain | 83 kDa | 3 | 0 |
| 134 | Fibulin-1 | 77 kDa | 3 | 0 |
| 157 | Thrombin activatable fibrinolysis inhibitor | 52 kDa | 2 | 0 |
Proteins identified by mass spectroscopy (n=226) with relative rank based off of spectral count.
Top and bottom filter represent spectral count.
kDA, kilodalton.
Enrichment of Saline With Clot Stabilizing Proteins Attenuates Fibrinolysis
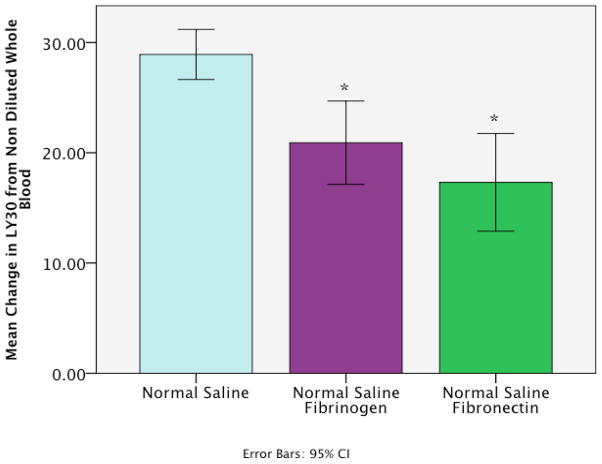
The change in LY30 from unmodified whole blood with tPA was different when contrasting 25% dilution using NS, and NS with physiologic concentrations of fibrinogen and fibronectin (p=0.001 Figure 3). Post hoc analysis with NS defined as the reference group showed the addition of the plasma proteins decreased LY30 (Fibrinogen mean difference −8.0 95%CI −13.0 to 3.0 p=0.004; Fibronectin mean difference-11.6 95%CI −17.4 to −5.8 p=0.001 Figure 4).
Figure 4.
Enrichment of saline with plasma proteins reduces dilutional sensitivity to tPA mediated fibrinolysis. Y axis represents the mean change in LY30 from whole blood to whole blood diluted by 25% of the experimental groups listed on the X axis. All groups had the same final concentration of tPA. The normal group represents the reference group for statically analysis. Normal Saline Fibrinogen; Fibrinogen dissolved in normal saline to reach a physiologic concentration, Normal saline fibronectin; fibrinonectin dissolved in normal saline to reach a physiologic level. *p<0.05.
Discussion
Saline dilution of whole blood does not activate fibrinolysis; however, saline dilution of whole blood increases susceptibility to tPA-mediated fibrinolysis. Conversely, PPP dilution of whole blood attenuates tPA mediated fibrinolysis (figure 2). Filtration of PPP resulted in a 1:50 dilution of plasma proteins but did not completely separate the high and low molecular weight proteins. Nevertheless, the PPP filtrate that passed through the filter has a similar increase in tPA mediated fibrinolysis as normal saline at a 50% dilution of whole blood, while PPP that remained on the top of the filter retained fibrinolysis attenuation similar to non filtrated PPP (figure 3). Enrichment of normal saline with large molecular weight proteins that were predominant in the upper filtrate partially attenuated the normal saline’s effect of increase susceptibility to tPA mediated fibrinolysis (figure 4). These data support the hypothesis that plasma contains multiple proteins that buffer the effects of tPA mediate fibrinolysis.
Our results are consistent with previous in vitro analysis demonstrating that whole blood dilution enhances tPA mediated fibrinolysis; however, these investigators concluded that plasma dilution of whole blood also exacerbated fibrinolysis (18). One of the major differences between these two studies is the concentration of tPA used in the TEG assay. In the Kostousov et al study 225 ng/ml of tPA was used compared to 75 ng/ml in our analysis. Brohi et al. reported patients with TIC have total tPA levels in the 30–50 ng/ml range (7). Our ongoing work has identified that patients with hyperfibrinolysis have total tPA levels at the upper end of this range (unpublished). Additionally, the Kostousov study used tissue factor in their TEG assay, and the role of tissue factor in trauma remains unclear. Many studies have demonstrated that this factor is a procoagulant, but these characteristics are dependent on its source and association with a cellular membrane (19). Injured patients with TIC had lower levels of microparticles bearing tissue factor after trauma than trauma patients without TIC and healthy controls (20). Adding tissue factor to healthy control blood is not applicable to all types of trauma. This addition may be a confounder of the Kostousov study, as they employed a supra physiologic levels of tPA to produce fibrinolysis. Recently, there appears to be a spectrum of fibrinolysis observed post-injury such that patients with impaired fibrinolysis (fibrinolysis shutdown) after injury were at an increased risk of death compared to patients with a moderate levels of fibrinolysis (21). Tissue factor may be contributory towards impairment of fibrinolysis, and warrants future investigation in patients with fibrinolysis shutdown.
In 1958 Ellison et al. demonstrated that plasma proteins were important for maintaining physiologic acid balance base and demonstrated that patients with disease processes causing loss of proteins have a reduction in plasma buffering capacity (22). In this historic paper they challenged the prevailing notion that bicarbonate was the major contributor to acid base control in the blood, and although not disagreeing that bicarbonate was an important component of acid base buffering, they insisted that proteins were also major contributors. To this end, the trauma literature may be at risk of repeating history with plasminogen activator -1 (PAI-1), as this protein has been implicated as the predominant regulator of post injury fibrinolysis (7, 16, 23). PAI-1 is a member of the SERPIN family, a group of proteases inhibitors that are involved in the regulation of coagulation, fibrinolysis, and inflammation (24). There are numerous other SERPINs that are in the systemic circulation which alter fibrinolytic function, and importantly this functionality is dependent on interactions with cofactors (25). The majority of SERPIN proteins have a molecular weight of less than 100 kDa, but their cofactor proteins can increase their relative weight and partially explain why filtration did not separate plasma proteins in their native form; ie, there were proteins with a molecular weight <100 kDa above the filter. PAI-1 was not identified in our analysis; however vitronectin, a cofactor of PAI-1, was one of the top 50 proteins identified and is known to increase PAI-1 activity (26). There are abundant SERPINs and coagulation cofactors in plasma that have not been studied in injured patients and may contribute to coagulation abnormalities.
Fibrinolysis activity also requires consideration of the intrinsic properties of the fibrin clot. Fibrinogen is a complex molecule that binds multiple proteins on different domains including tPA and plasminogen (27). It is therefore logical that fibrinogen concentration and its modification can alter sensitivity to fibrinolysis. For example, inhibition of factor XIII, which cross links fibrin polymers, renders clots more susceptible to fibrinolysis (28). A recent study evaluating the effect of whole blood dilution also indicated that low concentrations of fibrinogen increase susceptibility to fibrinolysis with no impairment on clot formation (29). Fibrinogen enrichment of normal saline to a physiologic level attenuates the exacerbation of fibrinolysis from dilution of whole blood. A fibrinogen modifier and adhesion protein, fibronectin, also improved resistance to fibrinolysis when normal saline was enriched to a physiologic level. Elevated plasma levels of fibronectin have been associated with thrombotic disorders, and have been shown to integrate into and alter the formation of fibrin clot (30).
Limitations of this study are the in vitro environment and artificial alteration of whole blood with tPA. There is evidence that endothelial damage and release of intracellular content, such as histones, contribute to trauma-induced coagulopathy which are not accounted for in the reported experiments, although their role in fibrinolysis remains undefined (31). However, these series of experiments are noteworthy to demonstrate that saline does not cause fibrinolysis in the absence of tPA. The observed fibrinolytic effect is from the dilution of plasma proteins increasing whole blood susceptibility to tPA-mediated fibrinolysis. This is confirmed by filtration of plasma proteins, in which plasma that has proteins diluted by 50 fold causes a similar dilutional susceptibility to tPA as seen with normal saline dilution. While enrichment of saline with selected plasma proteins attenuates the dilutional effects of saline, they were unable to completely suppress the process. With a list of more than 100 protein candidates it is likely that multiple proteins act synergistically to regulate tPA-mediated fibrinolysis; furthermore, plasma proteins are important buffers in the circulation. This in vitro experiment further supports the concepts of our plasma first resuscitation study (COMBAT, Clinicaltrials.gov NCT01838863) and provides a potential mechanism for therapeutic benefit.
Acknowledgments
Support: Supported in part by National Institute of General Medical Sciences grants: T32-GM008315, P50-GM049222 1, UM 1HL120877 and CCTSI supported in part by Colorado CTSA Grant UL1 TR001082 from NCATS/NIH.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS, NHLBI or National Institutes of Health.
Abstract presented at the American College Surgeons 100th Annual Clinical Congress, Surgical Forum, San Francisco, CA, October 2014.
Disclosure Information: Haemonetics provide research support in the form of providing the reagents for experiments. No money was paid to the authors or to their institutions. All authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collicott PE. Advanced Trauma Life Support (ATLS): past, present, future--16th Stone Lecture, American Trauma Society. J Trauma. 1992;33:749–753. [PubMed] [Google Scholar]
- 2.Bickell WH, Wall MJ, Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 3.Waibel BH, Rotondo MM. Damage control surgery: it’s evolution over the last 20 years. Revista do Colegio Brasileiro de Cirurgioes. 2012;39:314–321. doi: 10.1590/s0100-69912012000400012. [DOI] [PubMed] [Google Scholar]
- 4.Shaftan GW, Chiu CJ, Dennis C, Harris B. Fundamentals of physiologic control of arterial hemorrhage. Surgery. 1965;58:851–856. [PubMed] [Google Scholar]
- 5.Brown JB, Cohen MJ, Minei JP, et al. Goal-directed resuscitation in the prehospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg. 2013;74:1207–1212. doi: 10.1097/TA.0b013e31828c44fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruttmann TG. Haemodilution enhances coagulation. Br J Anaesthesia. 2002;88:470–472. doi: 10.1093/bja/88.4.470. [DOI] [PubMed] [Google Scholar]
- 7.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 8.Chin TL, Moore EE, Moore HB, et al. A principal component analysis of postinjury viscoelastic assays: Clotting factor depletion versus fibrinolysis. Surgery. 2014 doi: 10.1016/j.surg.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74:1223–1229. doi: 10.1097/TA.0b013e31828b7fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotton BA, Harvin JA, Kostousouv V, Minei KM, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73:365–370. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 11.Fearnley GR. Fibrinolysis. Ann Royal Coll Surg Engl. 1967;41:51–54. [PMC free article] [PubMed] [Google Scholar]
- 12.Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67:125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 13.Chapman MP, Moore EE, Ramos CR, et al. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75:961–967. doi: 10.1097/TA.0b013e3182aa9c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashuk JL, Moore EE, Sawyer M, et al. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252:434–442. doi: 10.1097/SLA.0b013e3181f09191. [DOI] [PubMed] [Google Scholar]
- 15.Ives C, Inaba K, Branco BC, et al. Hyperfibrinolysis elicited via thromboelastography predicts mortality in trauma. J Am Coll Surg. 2012;215:496–502. doi: 10.1016/j.jamcollsurg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Cardenas JC, Matijevic N, Baer LA, et al. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41:514–521. doi: 10.1097/SHK.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 17.Moore HB, Moore EE, Gonzalez E, et al. Hemolysis exacerbates hyperfibrinolysis while platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 2015;43:39–46. doi: 10.1097/SHK.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostousov V, Wang YW, Cotton BA, et al. Influence of resuscitation fluids, fresh frozen plasma and antifibrinolytics on fibrinolysis in a thrombelastography-based, in-vitro, whole-blood model. Blood Coag Fibrinolysis. 2013;24:489–497. doi: 10.1097/MBC.0b013e32835e4246. [DOI] [PubMed] [Google Scholar]
- 19.Key NS, Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Sem Thrombosis Hemostasis. 2010;36:865–875. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- 20.Matijevic N, Wang YW, Wade CE, et al. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thrombosis Res. 2014;134:652–658. doi: 10.1016/j.thromres.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–817. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellison G, Straumfjord JV, Jr, Hummel JP. Buffer capacities of human blood and plasma. Clin Chem. 1958;4:452–461. [PubMed] [Google Scholar]
- 23.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huntington JA. Serpin structure, function and dysfunction. J Thrombosis Haemostasis. 2011;9:26–34. doi: 10.1111/j.1538-7836.2011.04360.x. [DOI] [PubMed] [Google Scholar]
- 25.Schaller J, Gerber SS. The plasmin-antiplasmin system: structural and functional aspects. Cellular Molecular Life Sci. 2011;68:785–801. doi: 10.1007/s00018-010-0566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, hemostasis and fibrinolysis. J Thrombosis Haemostasis. 2007;5:102–115. doi: 10.1111/j.1538-7836.2007.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin J Biological Chem. 1982;257:2912–2919. [PubMed] [Google Scholar]
- 28.Mutch NJ, Koikkalainen JS, Fraser SR, et al. Model thrombi formed under flow reveal the role of factor XIII-mediated cross-linking in resistance to fibrinolysis. J Thrombosis Haemostasis. 2010;8:2017–2024. doi: 10.1111/j.1538-7836.2010.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S, Johnsson H, Zabczyk M, et al. Fibrinogen depletion after plasma-dilution: impairment of proteolytic resistance and reversal via clotting factor concentrates. Thrombosis Haemostasis. 2014;111:417–428. doi: 10.1160/TH13-06-0497. [DOI] [PubMed] [Google Scholar]
- 30.Ramanathan A, Karuri N. Fibronectin alters the rate of formation and structure of the fibrin matrix. Biochem Biophys Res Comm. 2014;443:395–399. doi: 10.1016/j.bbrc.2013.11.090. [DOI] [PubMed] [Google Scholar]
- 31.Johansson PI, Windelov NA, Rasmussen LS, et al. Blood levels of histone-complexed DNA fragments are associated with coagulopathy, inflammation and endothelial damage early after trauma. J Emerg Trauma Shock. 2013;6:171–175. doi: 10.4103/0974-2700.115327. [DOI] [PMC free article] [PubMed] [Google Scholar]