Abstract
Background
Reward learning has been postulated as a critical component of hedonic functioning that predicts depression risk. Reward learning deficits have been established in adults with current depressive disorders, but no prior studies have examined the relationship of reward learning and depression in children. The present study investigated reward learning as a function of familial depression risk and current diagnostic status in a pediatric sample.
Method
The sample included 204 children of parents with a history of depression (n=86 high-risk offspring) or parents with no history of major mental disorder (n=118 low-risk offspring). Semi-structured clinical interviews were used to establish current mental diagnoses in the children. A modified signal detection task was used to assess reward learning. We tested whether reward learning was impaired in high-risk offspring relative to low-risk offspring. We also tested whether reward learning was impaired in children with current disorders known to blunt hedonic function (depression, social phobia, PTSD, GAD, n=13) compared to children with no disorders and to a psychiatric comparison group with ADHD.
Results
High- and low-risk youth did not differ in reward learning. However, youth with current anhedonic disorders (depression, social phobia, PTSD, GAD) exhibited blunted reward learning relative to nondisordered youth and those with ADHD.
Conclusions
Our results are a first demonstration that reward learning deficits are present among youth with disorders known to blunt anhedonic function and that these deficits have some degree of diagnostic specificity. We advocate for future studies to replicate and extend these preliminary findings.
Keywords: mood disorders, anxiety disorders, emotions, affect, anhedonia, behavior, child behavior, adolescent behavior, reinforcement, risk factors
Anhedonia, a hallmark symptom of depression, is characterized by reduced pleasure and motivation to pursue rewards.1 Depressed adults show blunted emotion to various hedonic laboratory stimuli relative to nondepressed persons,2,3,4 and exhibit reduced willingness to expend effort to obtain rewards on laboratory tasks.5 Although previously neglected, there is now growing interest in hedonic deficits in depressed youth6 and youth at high risk for depression.7 The domain of hedonic functioning is broad, however, and empirical studies have only begun to identify the aspects most critical to depression (e.g., diminished anticipation of reward value;8,9,10 reward seeking deficits11,12). One aspect of hedonic function that may be pivotal in explaining depression risk is reward learning, or the ability to learn reward value of environmental contingencies and modulate behavior appropriately to maximize reinforcement.
Recent advances in objective assessment of reward learning involve modified signal detection techniques.13 Subjects are presented with one of two similar stimuli very briefly (100 ms) and must provide a speedy stimulus identification (<1500 ms) Correct responses are rewarded more frequently for one stimulus than the other. The critical metric is the extent to which subjects develop a response bias toward the more frequently rewarded stimulus. Unlike other reward-relevant decision making tasks (e.g., gambling tasks), reward learning paradigms index responses to a single, consistent reward contingency in the absence of punishment or “risky” choices. Thus, a key advantage of the reward learning paradigm is that it cleanly isolates the ability to learn reward value.
Studies utilizing reward learning paradigms have documented deficits in depressed14,15 and dysphoric adults,16,13 and these deficits predict worse prospective outcomes.13,15 Recent work in adult depression suggests this paradigm is also sensitive to enhancements in reward learning due to substances thought to act on the reward system.17 In sum, this paradigm has been successfully used to illuminate various aspects of reward functioning in adult depression.
Surprisingly, reward learning has not been investigated in pediatric depression. Depression often begins during adolescence, and is likely to recur in adulthood.18 Recent commentary has advocated for studies characterizing reward functioning during youth, when deficits may begin to increase future depression risk.6,19 Some have suggested that reward dysfunction may be a mechanism by which parental depression renders youth more vulnerable to psychopathology.20 Studies examining children of depressed parents, who are at particularly high risk for mood and anxiety disorders,21,22,23 are well designed to examine pediatric reward learning.
Hedonic Functioning in High-Risk Youth
Although no studies examined reward learning in high-risk youth, deficits in other aspects of hedonic functioning have been documented. In particular, studies utilizing behavioral assessments found that children at high familial risk of depression exhibit lower positive affect, less positive emotional behavior, and lower positive emotion expression than those at low risk.24,25,26,27 Studies assessing neural responses to pleasant or rewarding stimuli have also documented differences between youth at high and low familial depression risk, even in the absence of differences in reported affect.9,28 These findings suggest that hedonic deficits in high risk youth may be more discernable using implicit or objective assessment methodology.
Hedonic Functioning in Disordered Youth
No studies of reward learning have been performed in depressed youth. However, a growing literature documents deficits in other aspects of hedonic functioning.6 For instance, differences in neural responses to rewards have been documented in depressed youth29 and shown to predict future symptoms and treatment responses.30 Behavioral studies also reveal deficits, including impaired reward-related decision-making. On a computerized decision making task that varied reward magnitude and probability, depressed boys were less able than controls to modulate behavior to maximize reward in the face of changing reward contingencies.12 Under high reward probability conditions, controls maximized payoff by more frequently choosing the high magnitude reward, while depressed boys were equally likely to choose a low magnitude reward. These findings suggest that depression impairs behavioral modulation to maximize reward in the face of changing contingencies.
The Question of Diagnostic Specificity
Research on the various aspects of hedonic functioning, including reward learning, has focused mainly on depression. However, affective models increasingly recognize that hedonic deficits are not unique to depression,31 and some anxiety disorders may also impact hedonic functioning. Specifically, marked hedonic impairments has been found in social phobia,32 posttraumatic stress disorder (PTSD),33,34 and generalized anxiety disorder (GAD).35 By contrast, specific phobias, panic disorder, and obsessive- compulsive disorder (OCD) appear to have a limited, if any, impact on hedonic functioning.36,37 Depression in youth is often accompanied or preceded by anxiety symptoms, likely reflecting overlap in risk mechanisms,38 and anxiety disorders continue to exhibit high comorbidity with depression throughout adulthood.39 Studies examining the effect of anxiety on youth's hedonic functioning have thus been advocated by pediatric researchers.6
Other disorders may enhance rather than diminish hedonic functioning. Attention-deficit hyperactivity disorder (ADHD) has been associated with increased sensitivity to opportunities for immediate gratification, which may be related to the impulsivity commonly seen in ADHD.40 Two previous studies have found behavioral differences on reward learning tasks between ADHD and control youth,41,42 suggesting that reward learning paradigms are well-suited to examine reward learning across disorders.
The Present Study
The first aim of the present study was to test whether high-risk youth exhibit deficient reward learning relative to youth at low depression risk. High-risk youth were the offspring of proband parents with a history of depression; low-risk youth were the offspring of control parents with no prior history of major mental disorder. A second aim was to examine whether reward learning deficits were present in youth with current depression or anxiety disorders that have been shown to impact hedonic functioning (social phobia, PTSD, GAD), referred to collectively as the “anhedonic” disorders group. This group of currently disordered youth was compared to youth with no current disorders and to a psychiatric comparison group with ADHD (and no mood or anxiety disorders).
We hypothesized that (a) high-risk proband offspring would exhibit lower levels of reward learning relative to low-risk control offspring, and that (b) youth with anhedonic disorders would exhibit lower levels of reward learning than those with no disorder or with ADHD. To better isolate the effects of risk status and current disorders, our tests controlled for age, ethnicity, and the number of stressful life events in the last year. High-risk offspring were likely to experience more stressors than controls, and stress has been associated with impaired reward learning.43,44
Method
Participants
Participants were 204 youth ages 8-19 (M=12.30, SD=3.97). Participants were recruited based on being the offspring of a parent who, between 1997 and 2007, participated in a previous study either as a proband with a history of childhood onset depression or a control with no history of major mental disorder. Our sample included 86 high-risk offspring of 59 proband parents and 118 low-risk offspring of 48 control parents,).
Measures
Diagnosis of Psychiatric Disorders
Psychiatric diagnoses of offspring were determined via the semi-structured Interview Schedule for Children-Diagnostic version (ISCA-D), which covers the major DSM-IV psychiatric disorders and was based on the ISCA.45 The ISCA-D has been used previously with acceptable inter-rater reliability.46 Diagnostic procedures have been described elsewhere.46 Trained clinicians (master's or doctoral level) interviewed the parent about the child and then the child about him/herself. The interviewing clinician derived the initial diagnosis, which was routinely checked by a second clinician. The diagnoses were subsequently verified by consensus among senior research clinicians.
Depression symptoms
The Children's Depression Inventory-II Self Report (CDI-II)47 is a 28-item assessment of depression symptoms experienced in the last 2 weeks.
Anxiety symptoms
The Revised Children's Manifest Anxiety Scale (RCMAS)48 is a 37 item self report assessment of current anxiety symptoms.
Stressful Life Events
Parents were asked about 34 life events occurring during the last year, including events involving family members (e.g., hospitalization, job loss of a parent), or the child directly (e.g., contact with juvenile court). Scores were the total number of adverse events endorsed.
Reward Learning
Our method drew upon previous work with this task,13,41,44 and involved briefly presenting one of two stimulus versions (e.g., a short line or long line) and asking participants to identify which stimulus was seen. The two stimulus versions were presented equally often. Only some correct responses were followed by a monetary reward (10 cents)—one stimulus version was scheduled to be rewarded for correct responses three times as often as the other stimulus. Creating an unbalanced reward schedule between the two types of correct responses produces a systematic preference—or response bias—for the stimulus most often followed by the reward.49 Conceptually, individuals with higher reward learning capacity exhibit more of a response bias because they modulate their responses to increase the chances of receiving the reward (i.e., more often report seeing the stimulus that is more frequently paired with a reward). Individuals with lower reward learning exhibit less response bias, but still perform adequately on the task.13,41 Response bias on the signal detection task, therefore, was used to index reward learning. To enable use with a pediatric sample, we adapted the original task in the following ways: 1) fixation times and inter-trial intervals were shortened to improve task pacing, 2) rewarding stimuli were made more entertaining, 3) practice trials were expanded.
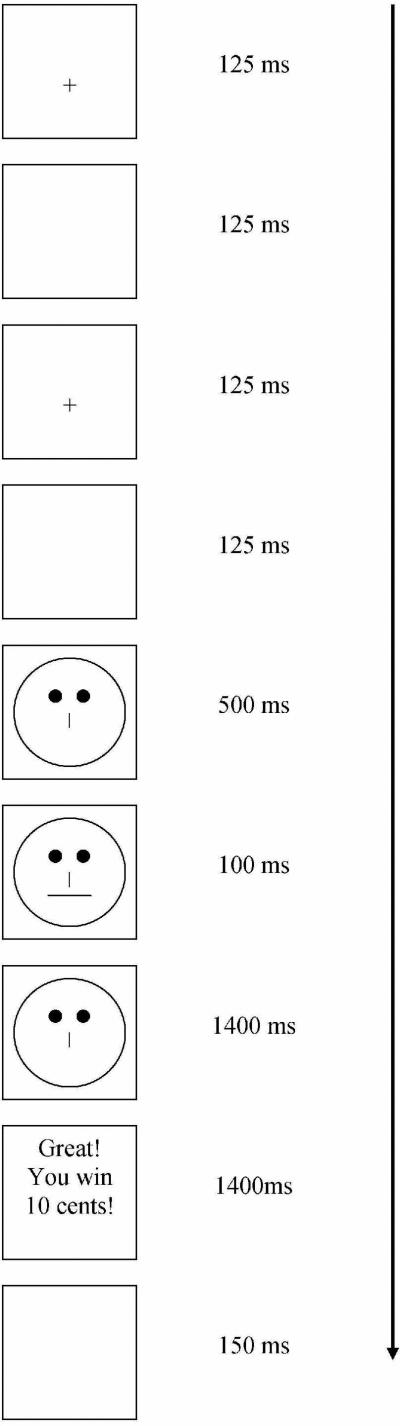
The task was presented on a PC via E-prime software (version 2.0, Psychological Software Tools, Inc., Pittsburgh, PA), in 3 blocks of 100 trials. Each trial began with a flashing fixation point, presented twice in the middle of the screen (125ms) followed by a blank screen (125ms). The fixation point was then replaced with a schematic face missing a mouth (500ms). The mouth then appeared either as a long (13mm) or short (12mm) version for 100ms and then disappeared, leaving the mouthless face on the screen (1400ms). Upon seeing the mouth, participants responded as to whether they saw the long or short mouth by pressing either the ‘z’ or ‘m’ key. Participants were instructed to keep their index fingers on the z and m keys, which were marked with brightly colored stickers with schematic faces on them to represent which key corresponded to which stimuli (long or short). Short and long stimuli were presented equally often (50 trials each per 100-trial block) in a quasi-randomized order (neither version was presented more than 3 times in a row). Participants were instructed that not all correct identifications would be followed by a reward. Indeed, only 40 correct identifications per each 100-trial block were scheduled to receive a reward. All other correct responses were unrewarded. When correct identifications were rewarded the words “Great! You win 10 cents!” appeared in bold white letters along with a brightly colored fireworks display on a black screen (1400ms) accompanied by audio praise (cheering), followed by a blank screen (150ms). All other trials offered no feedback and a blank screen (1550ms). Figure 1 displays a schematic of a representative trial.
Figure 1.
Trial Schematic. This figure illustrates the length of each screen seen during a reward trial with a correct response. Actual reward trials included a colorful fireworks display and an audio praise clip following a correct response.
The stimulus version scheduled to be rewarded most often (30 out of 40 potential reward trials) is referred to as the “rich” stimulus, and the version associated with reward less often (10 out of 40 potential reward trials) is the “lean” stimulus. The assignment of each stimulus to be “rich” or “lean” was counterbalanced along with the corresponding key to create 4 task versions. Some previous studies utilizing this methodology have implemented a controlled reinforcement procedure, or missed reward replacement (i.e., offering additional reward opportunities until a fixed ratio of received rewards was met). Following our prior work utilizing this task in adults,50 we controlled only the potential for receiving 3 rich rewards for every 1 lean reward. The advantage of this design is that the participant's reward ratio is contingent upon his/her own performance, allowing for individual variation in the exact ratio of rich to lean rewards, and serving as a more stringent test of response bias hypotheses. Prior work using this procedure in adults has successfully yielded the desired reward ratio of 3:1.50
Procedure
All sessions were monitored by trained post-Bachelor's level research assistants who remained in the experimental room. The child was seated approximately 20 inches from the computer monitor and was told the task was a “Faces Game.” In the demonstration trial, the researcher walked the child through each stimulus screen he/she would see during a trial, including the rewarding stimuli screen. The researcher then guided participants through 6 slowly paced practice trials. This was followed by 20 practice trials, performed independently. The child was then told that the game would not always tell if he/she responded correctly or incorrectly, but that some correct responses would be rewarded with 10 cents and he/she could win up to $12. To establish credibility and enhance salience of the monetary reward, the $12 remained visible to the child. After each trial block, the amount won so far was presented and the experimenter verbally praised participants by name. After the third and final block, the experimenter distributed all winnings. Children received breaks between trial blocks.
Data Reduction and Analysis
Excluded cases
Following prior work,50,51 we excluded cases based on the number of reward received in each trial block. Participants who received fewer than 20 of 40 potential rewards (including both rich and lean reward trials) were excluded (n=47). This strategy, although more liberal than a previous adult study requiring receipt 75% of potential rewards,51 has been shown to ensure included participants receive adequate numbers of rewards to create the desired 3:1 rich to lean reward ratio.50
Deleted trials
Consistent with prior work,44 trials with reaction times <100 ms or >1500 ms were excluded. Trials with non-allowed key presses (i.e., keys other than z or m) were also excluded. The total number of deleted trials ranged from 0 to 35 (M=4.30, SD = 6.12).
Response bias calculations
Response bias was calculated following past work. Calculation formulas were derived from signal detection theory.49 For clarity, components of the formulas are defined below in both traditional signal detection terms (e.g., hits, misses) and in terms specific to our task:
H = Hits = Correct identification of the rich stimulus (rich = rewarded more often)
F = False alarms = Choosing the rich stimulus when the lean stimulus was presented
M = Misses = Choosing the lean stimulus when the rich stimulus was presented
C = Correct Rejections = Correct identification of the lean stimulus
Response bias was defined as the tendency to systematically prefer the rich stimulus over the lean stimulus and was represented by the following formula:
Following previous work, 0.5 was added to each cell of the decision matrix to allow calculations where cells contain zeros.43
Task Performance
Consistent with previous studies,44 we conducted preliminary analyses of discriminability and accuracy to ensure adequate overall task performance. Mean accuracy on the task across the sample was 69% (SD= 10.0), indicating acceptable performance. Discriminability refers to the ability to discriminate between the two stimuli and was calculated as in previous studies.44
Covariates
In considering task performance, we also screened the data for age and sex effects. Sex did not affect accuracy rates, discriminability, or response bias (ps>.05). Age was positively associated with accuracy, F(1,203)=68.09, p<.001, and discriminability, F(1,203)=69.32, p<.001, but was unrelated to response bias, p>.05. Age was included as a covariate in the main statistical analyses. We also tested for effects of task version (rich versus lean stimuli version and accompanying key press), and no task version effects were evident in accuracy or discriminability, ps>.05. Task version had an unexpected effect on response bias, F(3,203)=7.53, p<.01, with one version yielding lower response bias scores than the other three. Task version was thus included as a covariate in subsequent analyses.
Familial Dependence
Finally, because 48% of parents had 2 or more offspring in the study, we initially used mixed models to test for effects of familial dependence on reward task performance. Because results of these analyses found no significant family-level random effects, we applied standard repeated measures general linear model approaches for subsequent data analyses.
Results
Sample Characteristics
Proband and control offspring did not differ in terms of age, sex, or handedness, but differed in ethnicity (see Table 1). Proband offspring had significantly higher self-rated depression (CDI-II) and anxiety symptoms (RCMAS) than control offspring. Proband offspring also experienced a higher rate of stressful life events in the last year than controls. Proband and control offspring did not differ in rates of lifetime depression (n=8 proband offspring, n=10 control offspring), p>.05. Fourteen children (7 proband offspring, 7 controls) met criteria for current anhedonic disorders (depression, social phobia, PTSD, GAD). Twenty-three children met criteria for current ADHD (17 proband offspring, 6 controls). Proband offspring were more likely than control offspring to be diagnosed with ADHD, χ2 (1, N=203) = 11.10,p=.001, but did not differ in their rates of current anhedonic disorders, p<.05. One participant met criteria for both ADHD and a current anhedonic disorder and was excluded from analyses.
Table 1.
Sample Characteristics by Proband Status
| Variable | Proband Offspring n=86 | Control Offspring n=118 | Test Statistic |
|---|---|---|---|
| Sex (% females) | n=46 (53%) | n=55 (47%) | χ2 (2, N=204) = .94, ns |
| Mean Age (SD) | 11.98 (3.04) | 12.53 (3.08) | t(202) = 1.27, ns |
| Handedness* (% right-handed) | 83% | 90% | Cramer's V=.11, ns |
| Race | χ2 (2, N=204) = 12.48, p <.01 | ||
| Caucasian | n=58 (67%) | n=67 (57%) | |
| African-American | n=11 (13%) | n=39 (33%) | |
| Biracial | n=17 (20%) | n=12 (10%) | |
| Mean Number of Stressful Life Events (past year) | 3.72 (2.96) | 2.02 (1.89) | F(1,203)=25.13, p<.001 |
| CDI-II mean (SD) | 9.83(7.76) | 7.03 (7.40) | F(1,192)=6.49, p=.012 |
| RCMAS mean (SD) | 9.88 (6.39) | 7.89 (6.59) | F(1, 181)=4.17, p<.05 |
CDI-II = Children's Depression Inventory-Self Report. RCMAS = Revised Manifest Anxiety Scale.
Comparison of three handedness groups: right, left, or both.
Diagnostic group differences in anhedonia and depression symptoms were verified with one-way ANOVAs. An anhedonia symptom composite score was first created with CDI-II items #11 (have fun), #18 (like being with people), #21 (push self to do schoolwork), #27 (fun at school). As expected, there were group differences in anhedonia symptoms, F(2,140)=7.50, p=.001, with the anhedonic disorders group reporting higher anhedonia scores (M=3.25, SD=2.05) than the ADHD (M=1.00, SD=.89) and the nondisordered (M=1.39, SD=1.66) groups, ps>.05. Likewise, the diagnostic groups differed in CDI total scores, F(2,139)=10.48, p<.001, with the anhedonic disorders group reporting higher CDI scores (M=16.67, SD=10.36) than the ADHD (M=6.00, SD=4.90) and the nondisordered groups (M=7.41, SD=6.58), ps<.001.
Preliminary Analyses of Reward Learning Task
Proband and control offspring did not differ in number of deleted trials (overall M=4.30, SD=6.12), task accuracy rates (overall M=.69, SD=.10) or number of rewards received (overall M=28.86, SD=3.82). When participants were compared based on current diagnostic status (anhedonic disorders, ADHD, non-disordered), there were also no differences in deleted trials, accuracy, or rewards received, all ps>.05. For the full sample, the mean number of rewards received did not differ across blocks, p>.05. A ratio of lean to rich rewards received was calculated for each participant (computed as lean divided by rich). The ratio of potential rewards was 10 lean to 30 rich (1:3 or .33). The actual mean ratio of rewards received for the sample was .26, which acceptably exceeds the desired 1:3 ratio and corresponds to a ratio of receiving 1 lean reward for every 3.8 rich rewards. There was a trend level difference between proband and control offspring on reward ratio, F=(1, 155)=3.48, p=.06, and a significant difference among diagnostic groups, F(1,155)=3.57, p=.03. Reward ratio was thus included as a covariate in all main analyses.
Response Bias as a Function of Familial Risk
A repeated measures ANCOVA was performed with offspring status (proband offspring, control) as a between subjects variable and trial block (block 1, block 2, block 3) as a within-subjects repeating variable. Effects of reward ratio, task version, age, ethnicity, and number of life stressors were covariates. Results revealed a main effect of trial block, F(2, 294)=15.86, p<.001, and planned within-subjects polynomial contrasts showed that the trial block effect was linear, F(1,147)=21.73, p<.001, with means increasing across blocks (M block1 =.13, SD=.20; M block2 =.22, SD=.25; M block3 =.26, SD=.29). The linear effect indicates response bias, or successful reward learning. No main effect, F(1,147)=.642, p=.42, or interaction with block, F(2,294)=.069, p=.93, emerged for offspring status. Thus, contrary to our hypothesis, high- and low-risk offspring did not differ in response bias. a
Discriminability, accuracy, and reaction time
A repeated measures ANCOVA with offspring status (proband offspring, control) as the between subjects variable and block as the within subjects variable was performed for discriminability. There were no significant main effects or interactions of offspring status on discriminability, all ps>.05. Identical analyses were performed for accuracy and reaction time, revealing no significant main effects or interaction effects of offspring status on accuracy or reaction time, all ps>.05.
Response Bias as a Function of Current Mood and Anxiety Disorders
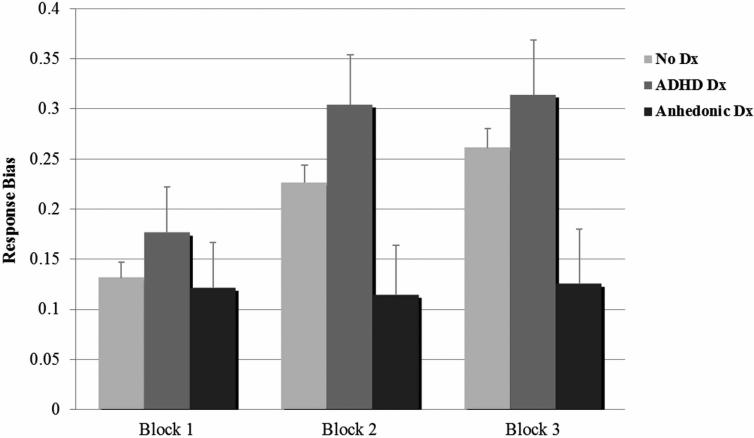
To test the effects of anhedonic disorders on reward learning, current diagnostic status (anhedonic disorders n=13, ADHD n=12, non-disordered n=131) was added into the model above. There remained a main effect of block, F(2, 288)=10.43, p<.001, and within-subjects contrasts showed the effect was linear, F(1,144)=14.39, p<.001. As in the above analyses, there was no main effect for proband status and no block x proband status interaction, all ps>.05. The block x diagnostic group interaction was also not significant, F(2,288)=.99, p=.41. There was a main effect of diagnostic group, F(2,144)=5.48,p=.005. Pairwise Bonferroni-corrected comparisons revealed that youth with anhedonic disorders exhibited lower overall response bias than nondisordered youth (p=.042) and youth with ADHD (p=.004). Nondisordered and ADHD youth did not differ significantly in response bias (p>.05). See Figure 2.
Figure 2.
Estimated Marginal Means for Response Bias by Diagnostic Group. The group with current anhedonic disorders (n=13) shows blunted reward learning compared to those with current ADHD (n=12) and no disorders (n=131), who show the normative reward learning pattern.
Discriminability, accuracy, and reaction time
Repeated measures ANCOVAs were performed to test for effects of current diagnostic on discriminability, accuracy, and reaction time across blocks. There were no significant main effects or interaction effects for diagnostic status on discriminability, accuracy, or reaction time, all ps>.05.
Discussion
Reward learning is a key aspect of hedonic functioning and its impairment may increase depression risk. This study was the first to investigate reward learning as a function of depression risk in youth. Using a modified signal detection task, we assessed reward learning in offspring of parents with a history of depression. Unexpectedly, we found no association between reward learning and familial depression risk. The literature on hedonic deficits in such high-risk groups has been mixed, with no prior reward learning studies. One possibility is that the association between reward learning and other aspects of hedonic functioning is weaker than one might assume. A second possibility is that reward learning deficits are present in high-risk children, but less detectable in laboratory performance than in other indices (e.g., neural activity). A final possibility is that such deficits emerge only when more complex decision-making processes are called upon (e.g., intentional risk decisions11). Multi-method assessment of neural, behavioral, and subjective components of reward function will be critical for future clarification.29
We also assessed the impact of current diagnoses on reward learning. We found that youth with anhedonic disorders exhibited blunted reward learning in comparison to the non-disordered and ADHD groups. This finding is the first documentation of reward learning deficits in youth with pediatric depression and anxiety. The finding of blunted reward learning in a pediatric sample suggests the possibility that reward learning deficits observed in adult studies cannot be attributed to a long history of depressive episodes and associated changes in behaviors. Rather, our results support reward learning as a state dependent variable, which is more related to a current anhedonic state than any generic impacts of having a depressed parent. Considering the links between reward learning deficits and a worse symptomatic course in adults,13,15 it is an open question whether impaired reward learning increases risk for future depressive episodes among those with early onsets. Future research may have implications for early interventions such as behavioral activation,51 which targets low rates of reinforcement long seen by behavioral models as central to the development and maintenance of adult depression.52
Our study was also novel in examining diagnostic specificity of reward learning deficits. We found partial evidence of diagnostic specificity: youth with anhedonic disorders differed not only from controls but also from youth with ADHD. Prior studies have found similar levels of overall response bias in ADHD and controls,41 but have identified more subtle group differences suggestive of enhanced response bias toward immediate versus delayed rewards.42 Future studies with more fine-grained analyses of reward functioning among ADHD youth are needed.
Our study's findings should also be appreciated in light of some limitations. For theoretical and practical reasons, we examined reward learning among youth with either a current depressive disorder and/or an anxiety disorder shown previously to diminish hedonic functioning. Reinforcing our decision, the anhedonic disorders group indeed reported increased anhedonic symptoms compared to the ADHD and non-disordered groups. Unfortunately, we lacked statistical power to compare reward learning between depressive and anxiety disorders. Because our sample was recruited based on offspring status rather than current diagnoses, our group sizes for diagnostic groups were small (anhedonic disorders n=13, ADHD n=12). Therefore, replication as well as specific group contrasts using larger sample sizes would be useful. Finally, because the study was cross-sectional, we cannot comment on the causal status of reward deficits with respect to current or future mood and anxiety disorders. Longitudinal studies are needed to elucidate the etiological significance of reward learning deficits in youth.
Acknowledgments
This work was supported by National Institute of Health (NIH) grant RO1 MH085722. The Children's Depression Inventory is published by Multi-Health Systems, Inc, from which Dr. Kovacs receives royalties.
Footnotes
Exploratory analyses examined the potential impact of stress and risk status in predicting reward learning. Prior work suggests stress has a blunting effect on reward learning in healthy adults and can interact with depression risk,44,50 but no studies have examined the impact of stress in youth. Using the same model as the main analyses, we reran the ANCOVA including an interaction term representing the number of life stressors x offspring status, and including current diagnostic status as a control variable. As in the main analysis, the effect of block was significant, F(2,286)=10.94, p<.001, as was the main effect of diagnostic status, F(2,143)=5.37, p=.006. A three way interaction between block, life stressors, and offspring status emerged, F(2,286)=3.03, p=.050. To decompose this interaction, two groups were formed using the median number of life stressors in the sample: those with <2 stressors formed the low stress group (n=58) and those with 2 or more stressors formed the high stress group (n=99). Separate ANOVAs were performed examining each offspring group. Among control offspring, life stress was unrelated to response bias in block 1, 2, or 3 (all ps>.05). Among proband offspring, high stress proband offspring tended to exhibit higher response bias in block 1 (M=.19, SD=.20) than those with low stress (M=.09, SD=.16), F(1,65)=3.74, p=.058,. In block 2, the difference was significant, F(1,65)=7.57, p=.008, with high stress proband offspring again showing greater response bias (M=.33, SD=.28) than those with low stress (M=.14, SD=.22). Again in block 3, high stress proband offspring had significantly greater response bias (M=.36, SD=.30) than those with low stress (M=.18, SD=.24), p=.023. These results held when univariate analyses were repeated controlling for current diagnostic status. We found no evidence that life stress impacts reward learning in low risk youth. Among high risk youth, however, increased stress was related to heightened reward learning. Interestingly, this effect was similar to our recent findings in adults at high and low depression risk, where high risk adults displayed heightened reward learning under stress. In that study, we found those who exhibited heightened reward learning under stress had lower depression one month later, prompting us to question whether enhanced reward learning under stress might indicate depression resistance.50 Taken together, these findings suggest stress may impact reward learning differently at varying depression risk levels, and future research is needed to determine how stress-induced heightening of reward learning relates to prospective functioning in high risk youth.
The other authors report no potential conflicts of interest.
Contributor Information
Bethany H. Morris, University of South Florida
Lauren M. Bylsma, University of Pittsburgh Medical School
Ilya Yaroslavsky, University of Pittsburgh Medical School.
Maria Kovacs, University of Pittsburgh Medical School.
Jonathan Rottenberg, University of South Florida.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. APA; Arlington, VA: 2013. [Google Scholar]
- 2.Dunn BD, Dalgleish T, Lawrence AD, et al. Categorical and dimensional reports of experienced affect to emotion-inducing pictures in depression. J Abnorm Psychol. 2004;113(4):654–660. doi: 10.1037/0021-843X.113.4.654. [DOI] [PubMed] [Google Scholar]
- 3.Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2(2):135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- 4.Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. J Abnorm Psychol. 1992;101(1):37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. J Abnorm Psychol. 2012;121(3):553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forbes EE, Dahl RE. Research review: Altered reward function in adolescent depressionWhat, when and how?. J Child Psychol Psychiatry. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert KE. The neglected role of positive emotion in adolescent psychopathology. Clin Psychol Rev. 2012;32:467–481. doi: 10.1016/j.cpr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Forbes EE, Dahl R. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Dev and Psychopathol. 2012;17(3):827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotlib IH, Hamilton J, Cooney RE, et al. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2012;67(4):380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121(1):51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawal AA, Collishaw SS, Thapar AA, Rice FF. 'The risks of playing it safe': A prospective longitudinal study of response to reward in the adolescent offspring of depressed parents. Psychol Med. 2012;43(1):27–38. doi: 10.1017/S0033291712001158. [DOI] [PubMed] [Google Scholar]
- 12.Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry. 2007;61(5):633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzagalli DA, Iosifescu D, Hallett LA, et al. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2009;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vrieze E, Pizzagalli DA, Demyttenaere K, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol. 1994;103(3):460–466. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- 17.Liverant GI, Sloan DM, Pizzagalli DA, et al. Associations among smoking, anhedonia, and reward learning in depression. Behav Ther. doi: 10.1016/j.beth.2014.02.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler RC, Avenevoli S, Merikangas K. Mood disorders in children and adolescents: An epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs M, Lopez-Duran N. Prodromal symptoms and atypical affectivity as predictors of major depression in juveniles: Implications for prevention. J Child Psychol Psychiatry. 2010;51(4):472–496. doi: 10.1111/j.1469-7610.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beardslee WR, Gladstone TRG, O'Conner EE. Transmission and prevention of mood disorders among children of affectively ill parents: a review. J Am Amer Acad Child Adolesc Psychiatry. 2011;50(11):1098–1107. doi: 10.1016/j.jaac.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Beardslee WR, Versage EM, Gladstone TRG. Children of affectively ill parents: A review of the past 10 years. J Am Acad Child Psychiatry. 1998;37(11):1134–1141. doi: 10.1097/00004583-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Goodman SH, Rouse MH, Connell AM, et al. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- 23.Weissman MM, Wickramaratne P, Nomura Y, et al. Families at high and low risk for depression: A 3-generation study. Arch Gen Psychiatry. 2005;62(1):29–36. doi: 10.1001/archpsyc.62.1.29. [DOI] [PubMed] [Google Scholar]
- 24.Durbin C, Klein DN, Hayden EP, et al. Temperamental emotionality in preschoolers and parental mood disorders. J Abnorm Psychol. 2005;114(1):28–37. doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- 25.Feng X, Shaw DS, Skuban EM, Lane T. Emotional exchange in mother-child dyads: Stability, mutual influence, and associations with maternal depression and child problem behavior. J Fam Psychol. 2007;21(4):714–725. doi: 10.1037/0893-3200.21.4.714. [DOI] [PubMed] [Google Scholar]
- 26.McMakin DL, Burkhouse KL, Olino TM, et al. Affective functioning among early adolescents at high and low familial risk for depression and their mothers: A focus on individual and transactional processes across contexts. J Abnorm Child Psychol. 2011;39(8):1213–1225. doi: 10.1007/s10802-011-9540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olino TM, Klein DN, Dyson MW, et al. Temperamental emotionality in preschool-aged children and depressive disorders in parents: Associations in a large community sample. J Abnorm Psychol. 2010;119(3):468–478. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCabe C, Woffindale C, Harmer CJ, Cowen PJ. Neural processing of reward and punishment in young people at increased familial risk of depression. Biol Psychiatry. 2012;72(7):588–594. doi: 10.1016/j.biopsych.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 29.Forbes EE. Where's the fun in that? Broadening the focus on reward function in depression. Biol Psychiatry. 2009;66(3):199–200. doi: 10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forbes EE, Olino TM, Ryan ND, et al. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect & Behav Neurosci. 2010;10:107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Ann Rev Psychol. 1998;49(1):377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- 32.Kashdan TB, Weeks JW, Savostyanova A. Whether, how, and when social anxiety shapes positive experiences and events: A self-regulatory framework and treatment implications. Clin Psychol Rev. 2011;31:786–799. doi: 10.1016/j.cpr.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Hopper JW, Pitman RK, Su Z, Heyman GM, et al. Probing reward function in posttraumatic stress disorder: Expectancy and satisfaction with monetary gains and losses. J Psychiatr Res. 2008;42(10):802–807. doi: 10.1016/j.jpsychires.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litz BT, Orsillo SM, Kaloupek D, Weathers F. Emotional processing in posttraumatic stress disorder. J Abnorm Psychol. 2000;109(1):26–39. doi: 10.1037//0021-843x.109.1.26. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava S, Sharma HO, Mandal MK. Mood induction with facial expressions of emotion in patients with generalized anxiety disorder. Depress Anxiety. 2003;18(3):144–148. doi: 10.1002/da.10128. [DOI] [PubMed] [Google Scholar]
- 36.Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol. 1998;107(2):179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- 37.Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. J Abnormal Psychol. 1988;97:346–353. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs M, Devlin B. Internalizing disorders in childhood. J Child Psychol Psychiatry. 1998;39(1):47–63. [PubMed] [Google Scholar]
- 39.Wittchen HU, Kessler RC, Pfister H, Lieb R. Why do people with anxiety disorders become depressed? A prospective longitudinal community study. Acta Psychiatr Scand. 2000;102:14–23. [PubMed] [Google Scholar]
- 40.Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clin Psychol Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28(3):366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- 42.Tripp G, Alsop BB. Sensitivity to reward delay in children with attention deficit hyperactivity disorder (ADHD). J Child Psychol Psychiatry. 2001;42(5):691–698. [PubMed] [Google Scholar]
- 43.Pizzagalli DA, Bogdan R, Ratner KG, Jahn AL. Increased perceived stress is associated with blunted hedonic capacity: Potential implications for depression research. Behav Res Ther. 2007;45(11):2742–2753. doi: 10.1016/j.brat.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: Implications for depression. Biol Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherrill JT, Kovacs M. Interview Schedule for Children and Adolescents (ISCA). J Amer Acad Child & Adolesc Psychiatry. 2000;39(1):67–75. doi: 10.1097/00004583-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Kiss E, Gentzler AM, George C, et al. Factors influencing mother-child reports of depressive symptoms and agreement among clinically referred depressed youngsters in Hungary. J Affect Disord. 2007;100(1-3):143–151. doi: 10.1016/j.jad.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs M, MHS Staff . The Children’s Depression Inventory. 2nd Ed. Multi-Health Systems, Inc.; North Tonawanda NY: 2011. [Google Scholar]
- 48.Reynolds CR, Richmond BO. What I think and feel: A revised measure of children' manifest anxiety. J Abnorm Child Psychol. 1978;6(2):271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- 49.Macmillan NA, Creelman CD. Detection theory: A user's guide. Cambridge University Press; New York, NY, US: 1991. [Google Scholar]
- 50.Morris BH, Rottenberg J. Heightened reward learning under stress in generalized anxiety disorder: A predictor of depression resistance? J Abnorm Psychol. doi: 10.1037/a0036934. in press. [DOI] [PubMed] [Google Scholar]
- 51.Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: Implications for development of dependence. Biological Psychiatry. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: Returning to contextual roots. Clin Psychol: Sci Pract. 2001;8(3):255–270. [Google Scholar]
- 53.Lewinsohn PM, Sullivan JM, Grosscup SJ. Changing reinforcing events: An approach to the treatment of depression. Psychother: Theory Res Pract. 1980;17(3):322–334. [Google Scholar]